Abstract
Plant pathogens cause severe losses or damage to crops worldwide and thereby significantly reduce the quality and quantity of agricultural commodities. World tendencies are shifting towards reducing the usage of chemically synthesized pesticides, while various biocontrol methods, strategies and approaches are being used in plant disease management. Fungal antagonists play a significant role in controlling plant pathogens and diseases and they are used as Biocontrol Agents (BCAs) throughout the world. This review provides a comprehensive list of fungal BCAs used against fungal plant pathogens according to modern taxonomic concepts, and clarifies their phylogenetic relationships because thewrong names are frequently used in the literature of biocontrol. Details of approximately 300 fungal antagonists belonging to 13 classes and 113 genera are listed together with the target pathogens and corresponding plant diseases. Trichoderma is identified as the genus with greatest potential comprising 25 biocontrol agents that have been used against a number of plant fungal diseases. In addition to Trichoderma, nine genera are recognized as significant comprising five or more known antagonistic species, namely, Alternaria, Aspergillus, Candida, Fusarium, Penicillium, Pichia, Pythium, Talaromyces, and Verticillium. A phylogenetic analysis based on partial sequences of the 28S nrRNA gene (LSU) of fungal antagonists was performed to establish their phylogenetic relationships.
Keywords: biocontrol agents, disease control, fungicides, plant diseases, plant pathogens, phylogeny, Trichoderma
Introduction
Plant pathogens including fungi, bacteria, viruses and nematodes cause serious losses or damage to crops worldwide and significantly reduce the quality and quantity of agricultural commodities. These losses pose a major threat to global food production annually (El Ghaouth et al., 2002; Dean et al., 2012; Singh, 2014; O’Brien, 2017). Moreover, pathogenic infection in the field or in post-harvest storage can affect the health of humans and livestock, especially if the pathogen produces toxins in or on consumable products (Brimner and Boland, 2003; Menzler-Hokkanen, 2006).
Various methods, strategies, and approaches are used in the management of plant diseases. These encompass the development of resistant varieties through plant breeding, genetically engineered plants, use of agrochemicals and physical methods (i.e., heat treatments, UV irradiation, modified or controlled atmosphere, cold storage, and inducing resistance by applying elicitors), application of biological control agents and good agronomic and horticultural practices (Stevens et al., 1997; Wisniewski et al., 2000; Droby, 2006; Singh and Chawla, 2012; Gupta and Sharma, 2014; Singh, 2014; O’Brien, 2017). These approaches have contributed significantly to the remarkable improvements in crop productivity and quality over the past few decades (Punja, 1997; Droby, 2006; Chandrashekara et al., 2012).
Biological Control: Overview and Significance
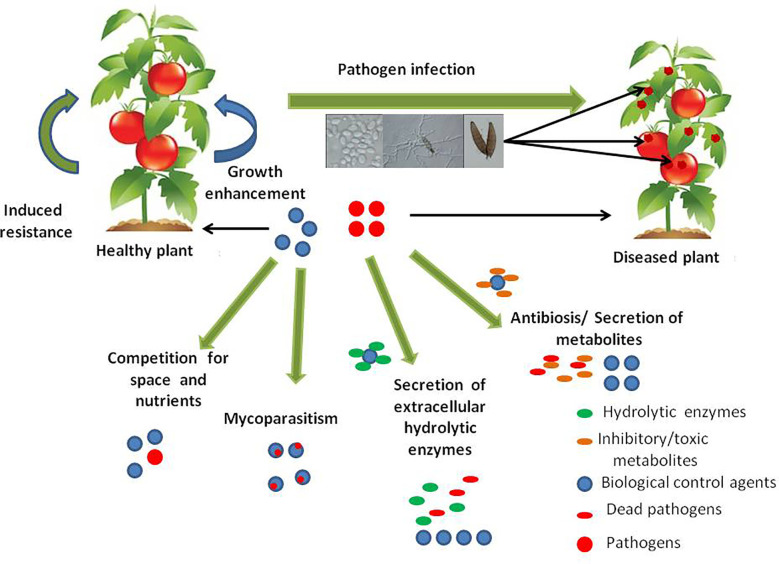
Biological control approaches of plant diseases include any reduction in the amount or the effect of pathogens (disease-producing activity) that is achieved through the induction of biological mechanisms or the action of naturally occurring or introduced antagonists, that occurs by manipulating the microenvironment to favour the activity of antagonists (Baker, 1987; Stirling and Stirling, 1997). Microbial biocontrol agents (BCAs) for plant diseases are usually fungal or bacterial strains isolated from the phyllosphere, endosphere or rhizosphere and they play an important role in controlling plant-pathogenic organisms. Biocontrol agents or microbial antagonists prevent infection of the host plant by the pathogen, or establishment of the pathogen in the host plant. The principal mechanisms for the control have been assumed to be those that act primarily upon the pathogens. The antagonists can exhibit several direct or indirect mechanisms of action involved in biological disease control. These mechanisms include; antibiosis (where an inhibitory metabolite or antibiotic is produced by the antagonist), mycoparasitism (where the antagonist derives some or all of its nutrients from the fungal host), induced resistance (induction of plant defense response against plant pathogens) and growth enhancement (BCAs promote plant growth while the effects of the disease are being reduced and also through microbial hormones such as indoleacetic acidand gibberellic acid). Secretion of extracellular hydrolytic enzymes by the antagonist, competition for space and nutrients between organisms and detoxification of virulence factors are other actions involved in biological disease control (Wilson et al., 1991; Punja, 1997; Heydari and Pessarakli, 2010; Chandrashekara et al., 2012; Singh, 2014; Zhang et al., 2014; Deketelaere et al., 2017). Recent studies have demonstrated that effects such as induced systemic or localized resistance by microbial BCAs on plants are also crucial. These fungi or bacteria can colonize the root epidermis and outer cortical layers and release bioactive molecules that cause walling-off of the fungal thallus or bacterial colonies (Harman, 2006). Consequently, they will alter the transcriptome and the proteome machinery of plants substantially. This alteration in the plant’s genetic material will provide certain additional advantages to the plant, such as increased plant growth and nutrient uptake in addition to induction of pathways for resistance in plants.
World trends are shifting towards reducing the use of agrochemicals in the management of plant diseases. Considerable research effort today is focused on seeking safe, eco-friendly and effective alternatives to synthetic, chemical fungicides to reduce the decay loss in harvested commodities and to control crop diseases in the field that lead to significant economic losses (Stirling and Stirling, 1997; Wisniewski et al., 2000; Droby, 2006; Chandrashekara et al., 2012). Due to the aforementioned mechanisms, biological control agents for plant diseases are gaining stature as viable alternatives to synthetic pesticides given their perceived increased level of safety and minimal negative environmental impacts. It is imperative to continue this line of research, since regulations on the use of new and existing fungicides are becoming more and more stringent. In particular, this has led to extensive researches on the use of microbial antagonists as protective agents and many fungal diseases can now be controlled by microbial antagonists. As a result, commercial products containing microbial BCAs have been successfully exploited in modern agriculture (e.g., Trichoderma based products and biopesticides based on Bacillus thuringiensis) (Menzler-Hokkanen, 2006).
A significant amount of harvested fruits and vegetables is lost annually due to microbial spoilage and this loss can range from 10%–50% depending on the commodity and country (El Ghaouth et al., 2002; Janisiewicz and Korsten, 2002). Developing countries experience greater losses due to inadequate storage and transportation facilities, and improper handling methods that are employed during harvesting and transit (Pathak, 1997; El Ghaouth et al., 2002; Nabi et al., 2017). The harvested yield might have been infected by one or several pathogens prior to harvest or they may become infected during transit and storage. (Punja, 1997; Janisiewicz and Korsten, 2002; Nabi et al., 2017). Several researches have been carried out to identify effective biocontrol agents for post-harvest disease management and as a result, biocontrol antagonists are now employed to control postharvest diseases worldwide. A few examples of these applications are indicated here. Mohamed and Saad (2009) found that the application of specific strains of Pichia anomala was a safe and effective biocontrol agent against Diplodia postharvest rot of guava fruit caused by Lasiodiplodia theobromae (Pat.) Griffon & Maubl. Alvindia and Natsuaki (2008) and Sangeetha et al. (2009) demonstrated the superior biocontrol potential of Trichoderma species for the management of the postharvest crown rot complex of banana caused by a variety of fungal pathogens including Colletotrichum musae, Fusarium verticillioides, and Lasiodiplodia theobromae. The search for suitable biological-control systems has largely taken place in the last fifty years and there has been considerable interest in the use of antagonistic microorganisms for the control of postharvest diseases. (Droby, 2006; Heydari and Pessarakli, 2010; Wisniewski et al., 2016). Bacteria associated with plants are known to develop biofilms on plant surfaces and within intercellular spaces of plant tissues, which act as microniches. It has been reported that the conditions within these microniches created because of the biofilm formation are markedly different from those of the ambient environment, which will eventually lead the microbial cells to effect functions that are not possible alone. This may influence the ecology of the bacteria they harbor and the relationship of bacteria with plants, which directly influence the development of strategies for biological control of plant disease and for assuring food safety (Morris and Monier, 2003).
Fungal Antagonists
The potential for the application of fungal biological control agents against plant pathogens has largely increased because fungi have a comparatively high reproductive rate (sexually as well as asexually), a short generation time and they are target specific. Furthermore, in the absence of the host, they can survive in the environment shifting their mode of parasitism to saprotrophism thus maintaining sustainability. Many fungal species possess mechanisms that allow them to efficiently protect plants from diseases caused by plant pathogenic fungi ( Figure 1 ).
Figure 1.
Key mechanisms of action involved in biological control of plant fungal diseases by fungal antagonists.
History of Fungal Biological Control Applications
Since ancient times man has attempted to increase crop production and control disease severity of crop plants by altering cultivation practices, which reduce both initial inoculum as well as infection rate (Singh and Chawla, 2012; Gupta and Sharma, 2014). With the finding of microorganisms and their interactions, many methods have been employed to control pathogens through the use of fungal antagonists.
Roberts (1874) showed the antagonistic action of microorganisms in liquid cultures between Penicillium glaucum and bacteria, introducing the term antagonism as used in microbiology. Hartley (1921) made the first attempt at direct application of biological control of plant pathogens by inoculating soil with microorganisms that were thought to have antagonistic potential. He inoculated forest nursery soils with thirteen antagonistic fungi to control damping-off caused by Pythium debaryanum. (Baker, 1987; Gupta and Sharma, 2014). Weindling (1932, 1934) described the potential of Trichoderma lignorum (T. viride) to control plant-pathogenic fungi by mycoparasitism and reported the first use of a known antimycotic-producing antagonist in plant disease control (Baker, 1987). Later, Weindling (1941) noted that Trichoderma species excrete an antimycotic that was toxic to plant pathogens including Rhizoctonia solani and Sclerotinia americana, and named it gliotoxin. This was the first record of the use of a known antimycotic-producing antagonist in plant disease control (Baker, 1987; Howell, 2003). The discovery of penicillin by A. Fleming in 1928, and its purification and use in pharmaceutical production, significantly stimulated studies on antagonists of plant pathogens (Baker, 1987).
Development of modern biotechnological approaches lead to increase the potential usage of fungal antagonists against a wide range of plant diseases. Numerous researches and experiments have been carried out during the past few decades to identify new fungal BCAs and evaluate their effectiveness under different environmental conditions.
Commercialization of Fungal Biological Control Agents
Commercial uses and applications of biological control of plant diseases have been slow mainly due to their variable performances under different environmental conditions in the field as well as due to their host specificity. To overcome this problem, it is essential to develop new formulations of BCAs with a higher degree of stability, efficiency and survival using new biotechnological practices (Heydari and Pessarakli, 2010). Several criteria have to be satisfied for upscaling a particular BCA to reach the stage of commercialization (Punja, 1997). Commercialization of biological control agents is expensive as it involves many steps such as isolation in pure culture or enrichment of the microorganism, identification and characterization, the development of a suitable formulation, mass production, efficacy testing of the product, inspection of storage stability, finding an industrial partner, attention to human and environmental safety matters, registration and marketing (Punja, 1997; Stirling and Stirling, 1997; Janisiewicz and Korsten, 2002; Montesinos, 2003). A number of biologically based products are being sold worldwide for the control of fungal plant pathogens and generally they are produced as granules, wettable powders, dusts, and aqueous or oil-based liquid products using different mineral and organic carriers (Ardakani et al., 2009; Nega, 2014). Several microbial antagonists have been patented and evaluated for commercial uses (Wisniewski et al., 2000; El Ghaouth et al., 2002; Schena et al., 2004; Nabi et al., 2017) and these agents are frequently recommended for plants (Albajes et al., 2000; Fravel, 2005; O’Brien, 2017). Some commercialized fungal BCAs used to control plant fungal diseases and their particulars are listed in Table 1 .
Table 1.
Some commercialized fungal Biocontrol Agents (BCAs) for plant fungal diseases and their specifications.
| Biocontrol agent | Product | Target Pathogen(s) or crop disease | Manufacturer or distributor |
|---|---|---|---|
| Ampelomyces quisqualis | AQ10® Bio Fungicide | Powdery mildew | Ecogen Inc, USA, Israel |
| Anthracocystis flocculosa (Pseudozyma flocculosa) | Sporodex L | Powdery mildew | Plant Products Co., Canada |
| Candida oleophila | Aspire | Post-harvest diseases | Ecogen Inc, USA, Israel |
| Paraphaeosphaeria minitans (Coniothyrium minitans) | Contans WG; KONI |
Sclerotinia sclerotiorum and S. minor | Prophyta Biologischer Pflanzenschutz GmbH; Germany, Bioved Ltd, Hungary |
| Clonostachys rosea (Gliocladium catenulatum) | Primastop | Damping-off, seed rot, root and stem rot, and wilt diseases | Kemira Agro OY, Finland; RegWest Co., USA |
| Prestop | Soil-Borne and foliar diseases of greenhouse vegetables, herbs and ornamentals | Danstar Ferment Ag., Switzerland; AgBio, Inc., USA | |
| Fusarium oxysporum (non-pathogenic) | Fusaclean; Biofox C | Wilt diseases | SIAPA, Italy; Natural Plant Protection, France |
| Phlebiopsis gigantea | Rotstop® | Root rot diseases | Kemira Agro Oy, Finland |
| Trichoderma virens (Gliocladium virens) | Soilgard® | Soil-borne pathogens; Rhizoctonia and Pythium species | Certis USA |
| Trichoderma harzianum | RootShield® | Root rot diseases; Pythium, Fusarium, Rhizoctonia, Thielaviopsis and Cylindrocladium species | BioWorks, Inc., USA |
| Trichoderma harzianum | Trichodex | Grey mould (Botrytis cinerea); Rhizoctonia, Sclerotinia and Colletotrichum species | Makhteshim Agan Industries, Israel |
| Trichoderma harzianum and T. polysporum | Binab T | Root rot diseases, pruning wounds in ornamental, shade, and forest trees | BINAB Bio-Innovation AB, Sweden |
| Trichoderma viride | Trichoderma Viride Trieco | Soil-borne fungal diseases | Ecosense Lab (I) Pvt. Ltd., India |
Integrated Applications of BCAs With Synthetic Fungicides for the Control of Plant Fungal Pathogens
Synthetic fungicides consisting of inorganic or organic compounds are commonly used in developed agricultural systems to control plant diseases, including post-harvest diseases, and to safeguard crop yield and quality mainly due to their relatively low cost, ease of application, and effectiveness. Chemical agents such as Captan, dithiocarbamates, thiabendazole (TBZ) and imazalil (IMZ) are widely used in the control of plant fungal pathogens (Lucas et al., 2015; Perez et al., 2016; Gupta, 2018). However, the massive and indiscriminate use of synthetic fungicides in crop protection and post-harvest food preservation has resulted in resistance to some fungicides and also led to severe effects on humans, animals, and wildlife resulting in widespread adverse ecological effects (Gupta, 2018; Gupta, 2019). Significant biocontrol of postharvest diseases of fruits and vegetables can be achieved with both field and postharvest applications (Janisiewicz and Korsten, 2002). The combined or integrated applications of a BCA with a synthetic fungicide or physical additives, either simultaneously or in rotation, would be expected to result in an enhanced degree of disease suppression, provided that the biocontrol agent is compatible with the fungicide used (Punja, 1997; Janisiewicz and Korsten, 2002; Droby, 2006; Eshel et al., 2009).
Modern Biotechnological Approaches Used in Plant Fungal Pathogen Biocontrol
Biological control of plant diseases using fungal BCAs has developed considerably in recent years with the application of genomics, genetic engineering and recombinant DNA techniques. These techniques have been developed to a high degree of precision and have been applied to the improvement of fungal strains for agro-industrial processes. Development of new crop varieties or clones that are resistant to plant pathogens offers a commonly acceptable and potentially long-term control option. Furthermore, many studies have been conducted to identify genetic traits of fungal antagonists and determine their potential to enhance biocontrol activity (Janisiewicz and Korsten, 2002; Droby, 2006; O’Brien, 2017). A few examples are pointed out here; (a). the introduction of multiple lytic enzyme‐encoding genes into Trichoderma virens genome resulted in a strain that secreted a mixture of glucanases and showed greatly enhanced inhibition of the pathogens Pythium ultimum (Oomycota, Chromista), Rhizoctonia solani, and Rhizopus oryzae (Djonovic et al., 2007); (b). McDougal et al. (2012) presented a method for the genetic transformation of Cyclaneusma minus, the causal agent of Cyclaneusma needle-cast, using protoplasts generated by incubation with Glucanex™ enzyme. Cyclaneusma minus was transformed with a gene encoding green fluorescent protein (GFP), which was allowed to identify several Trichoderma strains with potential for biocontrol of the disease. The interaction between C. minus and the Trichoderma strains, in the interaction zone where GFP expression was lost, was determined by a dual culture technique to be fungicidal; (c). Yakoby et al. (2001) generated reduced-pathogenicity mutants of the avocado fruit pathogen Colletotrichum gloeosporioides using insertional mutagenesis by restriction enzyme mediated integration (REMI) transformation and these isolates can be used for the biological control of anthracnose caused by C. gloeosporioides.
Aims of This Study
The present study aims to provide a comprehensive list of fungal BCAs that are used against fungal pathogens of crop plants, and clarify their phylogenetic relationships as these are often wrongly mentioned and interpreted in the literature of biocontrol. In this review, the main researches conducted during past the fifty years to evaluate the interactions between fungal antagonists and fungal plant pathogens were highlighted. Thus, this review is meant to serve as an updated database for applications of potential fungal antagonists against particular plant fungal diseases employed by different researchers worldwide. This can also serve as a useful tool to select and compare suitable and most applicable fungal antagonistic applications for fungal disease management in ongoing practices and researches. Many fungal antagonists and causative agents of plant diseases are presently identified and described based on the traditional classification and taxonomic systems. This review is the first comprehensive study of potential fungal antagonists applied against fungal plant pathogens based on a phylogenetic analysis and provides an extensive list of fungal BCAs used against fungal plant pathogens according to modern taxonomic concepts.Therefore, in this review the fungal antagonists and fungal pathogens are presented and listed using updated taxonomic nomenclature, which helps to avoid complications and misunderstandings. Also, the phylogenies of potential fungal BCAs are shown and discussed.
A variety of biological control methods are available nowadays, however they should be further developed before they can be effectively adopted. Therefore, this study makes a significant contribution for a greater understanding in developing such approaches with the help of the detailed information presented.
Materials and Methods
Data Collection and Presentation
Research data on fungal antagonists used against fungal plant pathogens were collected from resources published during the past fifty years. The collected data are summarized in the Supplementary Table 1 , which includes information on Fungal biocontrol agent, Disease and host as well as Pathogen. The disease or the pathogen suspension/control rate equal to or greater than 50% in each case is indicated with an asterisk (*) after the pathogen. Several potential fungal-like taxa causing plant diseases have also been taken into account to show the broad spectrum of activity of fungal BCAs and they are indicated with @. Different applications and treatment methods (in vitro and in vivo or both) have often been used when evaluating the effect of fungal BCAs in the research papers we considered. Thus, the disease/pathogen suppression percentage was accounted based on the maximum inhibition shown (if different conditions were applied).
Phylogenetic Analysis of Fungal Antagonists
Sequence data of the 28S nrRNA gene (LSU) from ex-type, ex-epitype, or ex-neotype strains of fungal antagonists listed in this study were downloaded from the NCBI’s GenBank nucleotide database ( Supplementary Table 2 ). If no ex-type strains were available, sequences from voucher, authentic or reference strains were included in the analysis. Hyphochytrium catenoides (EF594059) was chosen as the outgroup taxon.
Sequences were aligned with Bioedit 7.1.3.0 (Hall, 1999), and the consensus sequences were further improved with MUSCLE implemented in MEGA 5v (Tamura et al., 2011). Alignments were checked and optimized manually when necessary. The phylogenetic tree was generated by maximum likelihood (ML) criterion using RAxML-HPC2 BlackBox (8.2.10) (Stamatakis, 2006; Stamatakis et al., 2008) on the CIPRES Science gateway portal V 3.3 (Miller et al., 2010) and a Bayesian analysis was performed with MrBayes v. 3.2.6 (Ronquist and Huelsenbeck, 2003). The general time-reversible model of evolution including estimation of invariable sites and assuming a discrete gamma distribution with default parameters was used for the ML analysis. The model of evolution (GTR + I + G) was determined with MrModeltest 2.2 (Nylander, 2004) under the Akaike Information Criterion (AIC) implemented in PAUP v. 4.0b10. Bayesian inference (Rannala and Yang, 1996; Zhaxybayeva and Gogarten, 2002) was determined by Markov Chain Monte Carlo sampling (MCMC) in MrBayes v. 3.0b4 (Huelsenbeck and Ronquist, 2001). Six simultaneous Markov chains were run for 7,000,000 generations and trees were sampled every 100th generation. The first 20% of the trees (14000), representing the burn-in phase of the analysis, were discarded, while the remaining trees were used to calculate posterior probabilities (PP) in the majority rule consensus tree. The best scoring RAxML tree was selected and visualized with MEGA v. 5 (Tamura et al., 2011) and the graphical layout of the tree was created using PowerPoint 2010 version. ML Bootstrap support values (MLBS) greater than or equal to 50% and Bayesian posterior probabilities (PP) greater than or equal to 0.90 are indicated at the nodes of the branches. Alignments were deposited in TreeBASE (www.treebase.org) under the submission number 26869.
Results
Phylogenetic Analysis and Taxonomy of Fungal Antagonists
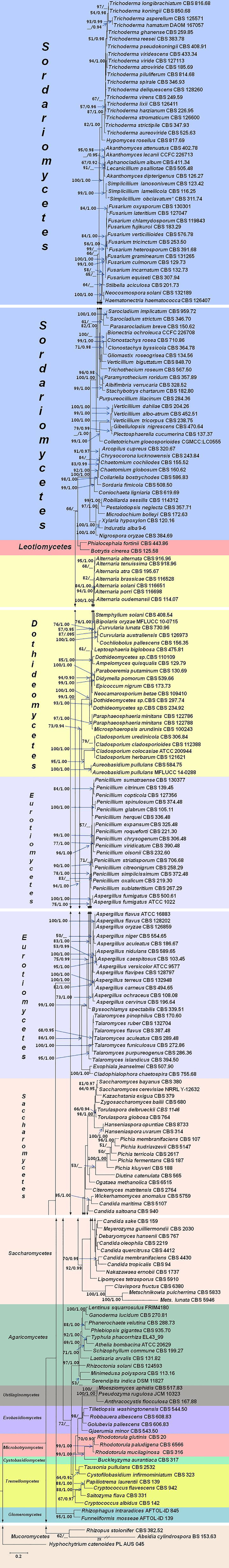
After alignment the LSU dataset consisted of 1,030 characters (including alignment gaps) for 218 ingroup taxa and the outgroup taxon. The Bayesian tree had had a topology identical to the ML tree presented. (data not shown). Fungal BCAs are distributed in four phyla (Ascomycota, Basidiomycota, Glomeromycota and Mucoromycota) and thirteen classes viz. Sordariomycetes, Dothideomycetes, Eurotiomycetes, Leotiomycetes, Saccharomycetes (Ascomycota), Agaricomycetes, Exobasidiomycetes, Ustilaginomycetes, Microbotryomycetes, Cystobasidiomycetes, Tremellomycetes (Basidiomycota), Glomeromycetes (Glomeromycota), Mucoromycetes (Mucoromycota) in the kingdom fungi ( Figure 2 ). The results show the phylogenetic placement of the various fungal BCAs within the kingdom fungi and confirm the current taxonomic placement of those BCAs. Most of the fungal BCAs belong to the class Sordariomycetes.
Figure 2.
Phylogram resulting from maximum likelihood (RAxML) analysis of sequence alignment of the 28S nrRNA gene (LSU) sequences of fungal antagonists. ML bootstrap values (MLBS) ≥ 50% and Bayesian posterior probabilities (PP) ≥ 0.90 are at each node. The tree was rooted to Hyphochytrium catenoides (PL AUS 045). Classes are indicated with coloured blocks to the left of the tree.
Fungal Antagonists and Their Potential Against Plant Pathogens
Approximately 300 species or varieties belonging to 113 fungal genera are identified as BCAs for plant fungal pathogens based on the previous studies ( Supplementary Table 1 ). Nine genera are recognized as potential genera, which consist of five or more known antagonistic species ( Table 2 ). They are Alternaria, Aspergillus, Candida, Fusarium, Penicillium, Pichia, Talaromyces, Trichoderma, and Verticillium. Trichoderma is the most prominent genus comprising 25 BCAs and they are widely used in controlling plant diseases caused by fungi. Alternaria, Botrytis, Colletotrichum, Fusarium, Lasiodiplodia, Penicillium, Phytophthora, Sclerotinia, and Verticillium are identified as common and most abundant plant pathogenic genera, to which frequently BCAs are applied for disease control.
Table 2.
Number of known fungal species in each genus with a potential Biocontrol Agent (BCA) activity against plant fungal pathogens.
| Phylum | Class | Genus | Number of known species with a potential BC activity against plant fungal pathogens |
|---|---|---|---|
| Ascomycota | Dothideomycetes | Alternaria | 08 |
| Ampelomyces | 01 | ||
| Aureobasidium | 01 | ||
| Bipolaris | 01 | ||
| Cladosporium | 04 | ||
| Curvularia | 03 | ||
| Didymella | 01 | ||
| Epicoccum | 01 | ||
| Leptosphaeria | 01 | ||
| Microsphaeropsis | 02 | ||
| Neocamarosporium | 01 | ||
| Paraboeremia | 01 | ||
| Paraphaeosphaeria | 01 | ||
| Phaeotheca | 01 | ||
| Stemphylium | 01 | ||
| Eurotiomycetes | Aspergillus | 16 | |
| Cladophialophora | 01 | ||
| Exophiala | 01 | ||
| Paecilomyces | 01 | ||
| Penicillium | 17 | ||
| Talaromyces | 08 | ||
| Incertae sedis | Gonatobotryum | 01 | |
| Teratosperma | 01 | ||
| Leotiomycetes | Botrytis | 01 | |
| Phialocephala | 01 | ||
| Cadophora | 01 | ||
| Saccharomycetes | Candida | 08 | |
| Citeromyces | 01 | ||
| Debaryomyces | 01 | ||
| Diutina | 01 | ||
| Hanseniaspora | 02 | ||
| Kazachstania | 01 | ||
| Lipomyces | 01 | ||
| Metschnikowia | 03 | ||
| Meyerozyma | 02 | ||
| Nakazawaea | 01 | ||
| Ogataea | 01 | ||
| Pichia | 05 | ||
| Saccharomyces | 02 | ||
| Torulaspora | 02 | ||
| Wickerhamomyces | 01 | ||
| Zygosaccharomyces | 01 | ||
| Sordariomycetes | Acremonium | 02 | |
| Akanthomyces | 03 | ||
| Albifimbria | 01 | ||
| Aphanocladium | 01 | ||
| Arcopilus | 01 | ||
| Bionectria | 01 | ||
| Coniochaeta | 01 | ||
| Chaetomium | 03 | ||
| Clonostachys | 02 | ||
| Collariella | 01 | ||
| Colletotrichum | 02 | ||
| Fusarium | 12 | ||
| Gibellulopsis | 01 | ||
| Gliomastix | 01 | ||
| Haematonectria | 01 | ||
| Hypomyces | 01 | ||
| Lecanicillium | 01 | ||
| Metapochonia | 01 | ||
| Metarhizium | 01 | ||
| Microdochium | 01 | ||
| Muscodor | 03 | ||
| Neocosmospora | 01 | ||
| Nigrospora | 01 | ||
| Paramyrothecium | 01 | ||
| Parasarocladium | 01 | ||
| Pestalotiopsis | 01 | ||
| Plectosphaerella | 01 | ||
| Purpureocillium | 01 | ||
| Robillarda | 01 | ||
| Sarocladium | 01 | ||
| Simplicillium | 02 | ||
| Sordaria | 01 | ||
| Stachybotrys | 01 | ||
| Stilbella | 01 | ||
| Trichoderma | 25 | ||
| Trichothecium | 01 | ||
| Verticillium | 05 | ||
| Xylaria | 01 | ||
| Basidiomycota | Agaricomycetes | Athelia | 01 |
| Ganoderma | 01 | ||
| Laetisaria | 01 | ||
| Lentinus | 01 | ||
| Minimedusa | 01 | ||
| Phlebiopsis | 01 | ||
| Rhizoctonia | 01 | ||
| Schizophyllum | 01 | ||
| Serendipita | 01 | ||
| Trametes | 01 | ||
| Typhula | 01 | ||
| Waitea | 01 | ||
| Cystobasidiomycetes | Buckleyzyma | 01 | |
| Exobasidiomycetes | Gjaerumia | 01 | |
| Robbauera | 01 | ||
| Tilletiopsis | 02 | ||
| Microbotryomycetes | Rhodotorula | 03 | |
| Tremellomycetes | Cystofilobasidium | 01 | |
| Naganishia | 01 | ||
| Papiliotrema | 02 | ||
| Saitozyma | 01 | ||
| Tausonia | 01 | ||
| Ustilaginomycetes | Anthracocystis | 01 | |
| Moesziomyces | 02 | ||
| Glomeromycota | Glomeromycetes | Claroideoglomus | 01 |
| Diversispora | 01 | ||
| Funneliformis | 01 | ||
| Gigaspora | 01 | ||
| Rhizophagus | 03 | ||
| Septoglomus | 01 | ||
| Simiglomus | 01 | ||
| Mucoromycota | Mucoromycetes | Absidia | 01 |
| Rhizopus | 01 |
Potential antagonistic genera are highlighted in boldface.
Discussion
The present study provides a comprehensive list of fungal BCAs used against a wide range of fungal plant pathogens, and establishes their phylogenetic relationships using a phylogenetic analysis based on the available authentic 28S nrRNA gene (LSU) sequence data. This will help clarify the currently correct names for the species since they have often been wrongly quoted and interpreted in the literature of biocontrol. It will further help with searches for the species in earlier literature where the old names were used.
In the phylogenetic analysis presented in this review, fungal BCAs were distributed in thirteen classes viz. Sordariomycetes, Dothideomycetes, Eurotiomycetes, Leotiomycetes, Saccharomycetes (Ascomycota), Agaricomycetes, Exobasidiomycetes, Ustilaginomycetes, Microbotryomycetes, Cystobasidiomycetes, Tremellomycetes (Basidiomycota), Glomeromycetes (Glomeromycota), Mucoromycetes (Mucoromycota) in the kingdom fungi ( Figure 2 ). The results confirmed the taxonomic placement of the various fungal BCAs and most of the fungal BCAs belong to the class Sordariomycetes. However, DNA sequences for some of the species are not currently available and therefore it is essential to have sequence data from authentic strains to confirm their taxonomy.
The main and common issue that we found when preparing this review was the method used by the authors to identify the fungal antagonists. Most of the publications have not used an appropriate identification method and they mainly followed the traditional identification methods and resources. However, molecular DNA sequencing and phylogenetic tools have been used in some of the recent publications to identify the antagonists and the pathogen correctly (Grondona et al., 1997; Rubini et al., 2005; Sriram and Poornadchanddra, 2013; Hung et al., 2015; Kheireddine et al., 2018). In addition, the nomenclature and classification of several fungal species have been subjected to change during the past few decades due to the modern taxonomic approaches and views. Therefore, corrections on those changes are mandatory in order to avoid misinterpretations. In this study, we have given the current names of the fungal species and the names commonly used in each particular research paper are mentioned in the brackets where applicable ( Table 2 ).
Trichoderma is a species rich, asexual genus in the family Hypocreaceae (Hypocreales, Sordariomycetes) and currently includes 433 species epithets in Index Fungorum, but DNA sequence data for most of the species are not available in GenBank. The sexual morphs of Trichoderma species are linked to Hypocrea. Members of the genus Trichoderma are biotrophic, hemibiotrophic, saprobic or hypersaprobic on various plants, or other fungi (Maharachchikumbura et al., 2016) and have been identified as the antagonists with greatest potential. They have been employed in several applications in plant fungal disease control. Twenty-five known Trichoderma species are included in Table 2 and these species have great potential for significantly controlling more than 100 fungal plant pathogens worldwide. Out of these species Trichoderma harzianum can be considered as the most common and commercially developed BCA used for a wide range of plant fungal diseases. Trichoderma species produce a number of metabolites and these metabolites play a major role in biological control mechanisms (Reino et al., 2008). Apart from that, Aspergillus and Penicillium species also play a vital role as BCAs next to Trichoderma ( Supplementary Table 1 and Table 2 ).
Some fungus-like genera (Globisporangium, Hyphochytrium and Pythium) belonging to Oomycota, Chromista, are also important as BCAs and have been used to control plant fungal diseases ( Table 3 ). Considering the activity of fungus-like BCAs, Pythium oligandrum may be considered as the one with the greatest potential as a BCA.
Table 3.
Fungal-like species (Oomycota, Chromista) used as Biocontrol Agents (BCAs) in the past few decades against fungal pathogens/diseases of different host plants.
| Biocontrol agent | Disease and host | Pathogen | References |
|---|---|---|---|
| Hyphochytrium catenoides | Phytophthora root rot of soybean | Phytophthora megasperma f. sp. glycines | Filonow and Lockwood, 1985 |
| Globisporangium ultimum (Pythium ultimum) | Barley powdery mildew | Blumeria graminis f. sp. hordei | Haugaard et al., 2001 |
| Pythium acanthicum | Damping-off of cucumber | Globisporangium ultimum (Pythium ultimum) | Ali-Shtayeh and Saleh, 1999 |
| Fusarium ear blight of wheat | Fusarium culmorum & Microdochium nivale | Diamond and Cooke, 2003 | |
| Pythium nunn | Damping-off disease of cucumber | Globisporangium ultimum (Pythium ultimum) | Paulitz and Baker, 1987 |
| Pythium oligandrum | Seedling and taproot diseases of sugar beet | Aphanomyces cochlioides | Takenaka and Ishikawa, 2013 |
| Seedling disease of sugar beet | Aphanomyces cochlioides | Takenaka et al., 2006 | |
| Grey mould of grapevine | Botrytis cinerea | Mohamed et al., 2007 | |
| Grey mould of tomato | Botrytis cinerea | Le Floch et al., 2003 | |
| Grey mould of strawberry | Botrytis cinerea | Meszka and Bielenin, 2010 | |
| Cercospora leaf spot in sugar beet | Cercospora beticola | Takenaka and Tamagake, 2009 | |
| Foot rot pathogens of pea | Didymella pinodella (Phoma medicaginis var. pinodella) | Bradshaw-Smith et al., 1991 | |
| Fusarium head blight | Fusarium graminearum | Takenaka et al., 2003 | |
| Fusarium crown and root rot of tomato | Fusarium oxysporum f. sp. radicis-lycopersici | Benhamou et al., 1997; Gerbore et al., 2014 | |
| Seed rot of tomato | Globisporangium ultimum (Pythium ultimum) | He et al., 1992; Gerbore et al., 2014 | |
| Pythium damping-off in cress and sugar-beet | Globisporangium ultimum (Pythium ultimum) | Vesely, 1977; McQuilken et al., 1990; McQuilken et al., 1992 | |
| Damping-off disease of cress | Globisporangium ultimum (Pythium ultimum) | Al-Hamdani et al., 1983 | |
| Damping-off of cucumber | Globisporangium ultimum (Pythium ultimum) | Ali-Shtayeh and Saleh, 1999 | |
| Seedling diseases of cotton | Globisporangium ultimum (Pythium ultimum) | Martin and Hancock, 1986; Gerbore et al., 2014 | |
| Pre-emergence damping-off disease of sugar beet | Globisporangium ultimum (Pythium ultimum) | Martin and Hancock, 1987 | |
| Pythium root rot of tomato | Pythium dissotocum | Vallance et al., 2009; Gerbore et al., 2014 | |
| Crown and root rot of tomato | Phytophthora parasitica (Phytophthora nicotianae) | Picard et al., 2000 | |
| Damping-off of wheat | Pythium ultimum var. ultimum | Abdelzaher et al., 1997 | |
| Leaf spot of strawberry | Ramularia grevilleana (Mycosphaerella fragariae) | Meszka and Bielenin, 2010 | |
| Damping off disease of tomato | Rhizoctonia solani | He et al., 1992; Gerbore et al., 2014 | |
| Black scurf of potato | Rhizoctonia solani | Ikeda et al., 2012 | |
| Seedling disease of sugar beet | Rhizoctonia solani | Takenaka et al., 2003 | |
| Powdery mildew of strawberry | Sphaerotheca macularis (Podosphaera aphanis) | Meszka and Bielenin, 2010 | |
| Verticillium wilt of pepper | Verticillium dahliae | Al-Rawahi and Hancock, 1998; Rekanovic et al., 2007 | |
| Verticillium wilt of olive | Verticillium dahliae | Varo et al., 2016 | |
| Pythium periplocum | Grey mould of grape-vine | Botrytis cinerea | Paul, 1999a |
| Damping-off of cucumber | Globisporangium ultimum (Pythium ultimum) | Ali-Shtayeh and Saleh, 1999 | |
| Pythium radiosum | Grey mould of grape-vine | Botrytis cinerea | Paul, 1999b |
Application of one or more biocontrol agents combined with physical and/or chemical control treatments can be considered as a useful strategy in achieving an enhanced performance against plant diseases. Sometimes, it is difficult to select individual strains with a broad spectrum of activity against several plant pathogens that cause severe damages and infections on plants or fruits. Mixed cultures of the microbial antagonists appear to provide better control of plant diseases over individual strains (Guetsky et al., 2001; Freeman et al., 2004; Xu et al., 2011) and these BCAs should be compatible and applied properly with a correct formulation. The application of antagonist mixtures improves the efficacy of biocontrol and are used in many agricultural fields including post-harvest disease control systems. (Datnoff et al., 1995; Nagtzaam et al., 1998; Guetsky et al., 2001; Janisiewicz and Korsten, 2002; Freeman et al., 2004; Droby, 2006; Xu et al., 2011; Doley et al., 2017; Nabi et al., 2017). In a study of the biological control of Armillaria root rot of strawberry plants by Raziq and Fox (2005), all the strawberry plants treated with Trichoderma harzianum isolates or T. viride isolate alone died by the end of the experiment, while 50% of them survived when treated with a combination of any of the antagonists with Dactylium dendroides. Calvo et al. (2003) improved the biocontrol efficiency of postharvest diseases of apples (Penicillium expansum and Botrytis cinerea) by using mixtures of yeasts (Rhodotorula glutinis, Cryptococcus albidus and C. laurentii) without increasing the amount of antagonists applied. Haggag and Nofal (2006) revealed that the multi-biocontrol agents namely Trichoderma koningii, T. hamatum, Pseudomonas fluorescens, P. putida, Tilletiopsis minor, and T. washingtonensis were more effective against Botryodiplodia disease (Lasiodiplodia theobromae = Botryodiplodia theobromae) on some Annona cultivars when applied in combination than when applied individually or even when applied in any combination of two agents. Furthermore, applications of multi-biocontrol agents resulted in a significant increase of fruit yield. Doley et al. (2017) showed that the incidence of stem-rot in groundnut caused by Sclerotium rolfsii was significantly lowered by combined inoculation of both Glomus fasciculatum along with Trichoderma viride as compared to when either G. fasciculatum or T. viride was applied alone.
Application of BCAs with inorganic and organic substances and chemicals such as bicarbonates and chlorides of alkali metals, minerals and other elements (Piano et al., 1997; Droby et al., 1997; Tian et al., 2002a; Gamagae et al., 2003; Tian et al., 2005; Karabulut et al., 2005; Cao et al., 2012) or low dosage of fungicides (Chand-Goyal and Spotts, 1996; Spotts et al., 1998; Qin and Tian, 2004; De Cal et al., 2009) are widely used to control plant pathogenic diseases including post-harvest diseases. A few potential examples of these applications are indicated here. Thus Tian et al. (2005) investigated the synergistic biocontrol effects of Cryptococcus laurentii and Rhodotorula glutinis combined with silicon (Si) against Alternaria alternata and Penicillium expansum moulds in jujube fruit stored at different temperatures and found that combinations of these two biocontrol agents with 2% Si is most effective in controlling the diseases on jujube fruit stored at 20°C. The studies of Karabulut et al. (2005) demonstrated that postharvest applications of sodium bicarbonate within a hydrocooler significantly controlled postharvest diseases of sweet cherries. Liu et al. (2010) revealed that tea polyphenol alone or in combination with biocontrol agents has great potential in commercial management of postharvest diseases in fruits. Larena et al. (2010) enhanced the adhesion of Epicoccum nigrum conidia to peach surfaces by adding 2.5% methylcellulose to the conidial formulation of E. nigrum and this improved the biocontrol of brown rot caused by Monilinia laxa. Cao et al. (2012) found that Boron improves biocontrol activity of Cryptococcus laurentii against Penicillium expansum in jujube fruit. The study by Gamagae et al. (2003) showed that the use of sodium bicarbonate at 2% with the biocontrol agent Candida oleophila reduces anthracnose caused by Colletotrichum gloeosporioides on papaya during storage. Sometimes, these integrated applications increase the efficiency of the particular BCA and sometimes indirectly improve plant productivity. Ordentlich et al. (1990) showed that integrated treatment of the fungicide captan and the biocontrol agent Trichoderma harzianum resulted in a reduction of Verticillium dahlia colonization of potato stems (Verticillium wilt), increasing marketable and total potato yield of cultivars Draga by 84% and 46% respectively, and total yield of cultivar Desiree by 80%. However, only BCA alone applications (without combining with organic or inorganic chemical substances) are considered in this review.
Fungal biological control agents act through several mechanisms (see the introduction) when controlling plant diseases. However, these mechanisms may cause risks to non-target species including mycorrhizal and saprophytic fungi, soil bacteria, other plants, insects, aquatic and terrestrial animals, and humans. Possible non-target effects of any BCAs or method used in the field should be determined before their applications. Continuous monitoring and the use of molecular techniques to identify and follow the movement of BCAs are also necessary and negative biological impacts can then be avoided (Brimner and Boland, 2003). Cross-protection can be defined as the protection conferred on a host by infection with one strain of a microorganism that prevents infection by a closely related strain of that microorganism. This method has been widely used to control the plant diseases caused by Fusarium and Verticillium species (Matta and Garibaldi, 1977; Larkin and Fravel, 1998; Nel et al., 2006; Zhu et al., 2013).
The efficiency of a particular BCA against plant diseases can be altered by many factors such as; environmental factors, time of treatment, season of the application, nature or technique of the treatment and the frequency of the application (McLaughlin et al., 1990; Wu and Hsiang, 1998; Ali-Shtayeh and Saleh, 1999; Schisler et al., 2002; Bhagat and Pan, 2007; Lal et al., 2009; Perelló et al., 2009; Padder and Sharma, 2011). The same BCA can show different efficiencies at in vitro, in vivo, and greenhouse/field conditions (Wokocha et al., 1986; Larena et al., 2003; Campanile et al., 2007; Padder and Sharma, 2011; Ommati et al., 2013). The dual culture method is the most widely used and simplest in vitro technique to determine the activity of BCAs against pathogens and sometimes the results obtained from this method may differ from results found in the in vivo, field or greenhouse conditions (Padder and Sharma, 2011; Zheng et al., 2011; Kheireddine et al., 2018). Therefore, it is hard to expect the same result for the same BCA in the field compared to the laboratory or greenhouse applications and it is essential to carry out tests in both in vitro and in vivo before scaling up the particular BCA. However, in this review, the maximum disease control situations are stipulated in Table 2 , in case where different environmental conditions have been used in the respective publication.
Future Aspects
An assay based on modern taxonomic approaches is highly recommended to identify the antagonists and the pathogens correctly. Also, verified antagonistic cultures must be obtained from reputable culture collections in a case when researchers are planning to use known strains. These practices will largely minimize the confusion related to this field in the future. Moreover, modern biotechnological and genetic engineering tools have contributed immensely towards the development of new fungal strains with high capacity and efficiency of biocontrolling. Last, but not least, fungal extracts and secondary metabolites produced by various fungal species (Mathivanan et al., 1998; Park et al., 2005; Pal and McSpadden Gardener, 2006; Koitabashi and Tsushima, 2007; Reino et al., 2008; Stoppacher et al., 2010; Lou et al., 2011; Cimmino et al., 2013) will also play a significant role in future bio-control methods of plant pathogens.
Author Contributions
KT contributed to conception and design of the study. DD and KT performed the phylogenetic analysis. AP, SK, and IP wrote sections of the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank Kevin D. Hyde, Key Laboratory for Plant Diversity and Biogeography of East Asia, Kunming Institute of Botany, Chinese Academy of Science, Kunming 650201, Yunnan, P.R. China and Levente Kiss, University of Southern Queensland, Centre for Crop Health. University of Southern Queensland for critical review of the manuscript. AP acknowledges the support from UIDB/04046/2020 and UIDP/04046/2020 Centre grants from FCT, Portugal (to BioISI). IP acknowledges Chiang Mai University for partial support of this work. DD would like to thank the financial support from grant RP/03/02/01/02/2020 awarded from the University of Kelaniya.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcimb.2020.604923/full#supplementary-material
References
- Abdelzaher H. M., Elnaghy M. A., Fadl-Allah E. M. (1997). Isolation of Pythium oligandrum from Egyptian soil and its mycoparasitic effect on Pythium ultimum var. ultimum the damping-off organism of wheat. Mycopathologia. 139 (2), 97–106. 10.1023/A:1006836703594 [DOI] [PubMed] [Google Scholar]
- Albajes R, Gullino M. L., van Lenteren J. C., Elad Y. (Eds.) (2000). Integrated pest and disease management in greenhouse crops (Vol. 14) (Dordrecht, The Netherlands: Springer Science & Business Media; ). [Google Scholar]
- Al-Hamdani A. M., Lutchmeah R. S., Cooke R. C. (1983). Biological control of Pythium ultimum–induced damping-off by treating cress seed with the mycoparasite Pythium oligandrum . Plant Pathol. 32 (4), 449–454. 10.1111/j.1365-3059.1983.tb02860.x [DOI] [Google Scholar]
- Ali-Shtayeh M. S., Saleh A. S. (1999). Isolation of Pythium acanthicum, P. oligandrum, and P. periplocum from soil and evaluation of their mycoparasitic activity and biocontrol efficacy against selected phytopathogenic Pythium species. Mycopathologia 145 (3), 143–153. 10.1023/A:1007065010931 [DOI] [Google Scholar]
- Al-Rawahi A. K., Hancock J. G. (1998). Parasitism and biological control of Verticillium dahliae by Pythium oligandrum . Plant Dis. 82 (10), 1100–1106. 10.1094/PDIS.1998.82.10.1100 [DOI] [PubMed] [Google Scholar]
- Alvindia D. G., Natsuaki K. T. (2008). Evaluation of fungal epiphytes isolated from banana fruit surfaces for biocontrol of banana crown rot disease. Crop Prot. 27 (8), 1200–1207. 10.1016/j.cropro.2008.02.007 [DOI] [Google Scholar]
- Ardakani S., Heydari A., Khorasani N., Arjmandi R., Ehteshami M. (2009). Preparation of new biofungicides using antagonistic bacteria and mineral compounds for controlling cotton seedling damping-off disease. J. Plant Prot. Res. 49 (1), 49–56. 10.2478/v10045-009-0007-3 [DOI] [Google Scholar]
- Baker K. F. (1987). Evolving concepts of biological control of plant pathogens. Annu. Rev. Phytopathol. 25 (1), 67–85. 10.1146/annurev.py.25.090187.000435 [DOI] [Google Scholar]
- Benhamou N., Rey P., Chérif M., Hockenhull J., Tirilly Y. (1997). Treatment with the mycoparasite Pythium oligandrum triggers induction of defense-related reactions in tomato roots when challenged with Fusarium oxysporum f. sp. radicis-lycopersici. Phytopathol. 87 (1), 108–122. 10.1094/PHYTO.1997.87.1.108 [DOI] [PubMed] [Google Scholar]
- Bhagat S., Pan S. (2007). Mass multiplication of Trichoderma harzianum on agricultural byproducts and their evaluation against seedling blight (Rhizoctonia solani) of mungbean and collar rot (Sclerotium rolfsii) of groundnut. Indian J. Agric. Sci. 77 (9), 583–588. [Google Scholar]
- Bradshaw-Smith R. P., Whalley W. M., Craig G. D. (1991). Interactions between Pythium oligandrum and the fungal footrot pathogens of peas. Mycol. Res. 95 (7), 861–865. 10.1016/S0953-7562(09)80050-6 [DOI] [Google Scholar]
- Brimner T. A., Boland G. J. (2003). A review of the non-target effects of fungi used to biologically control plant diseases. Agric. Ecosyst. Environ. 100 (1), 3–16. 10.1016/S0167-8809(03)00200-7 [DOI] [Google Scholar]
- Calvo J., Calvente V., de Orellano M. E., Benuzzi D., de Tosetti M. I. S. (2003). Improvement in the biocontrol of postharvest diseases of apples with the use of yeast mixtures. Biocontrol 48 (5), 579–593. 10.1023/A:1025738811204 [DOI] [Google Scholar]
- Campanile G., Ruscelli A., Luisi N. (2007). Antagonistic activity of endophytic fungi towards Diplodia corticola assessed by in vitro and in planta tests. Eur. J. Plant Pathol. 117 (3), 237–246. 10.1007/s10658-006-9089-1 [DOI] [Google Scholar]
- Cao B., Li H., Tian S., Qin G. (2012). Boron improves the biocontrol activity of Cryptococcus laurentii against Penicillium expansum in jujube fruit. Postharvest Biol. Technol. 68, 16–21. 10.1016/j.postharvbio.2012.01.008 [DOI] [Google Scholar]
- Chand-Goyal T., Spotts R. A. (1996). Control of postharvest pear diseases using natural saprophytic yeast colonists and their combination with a low dosage of thiabendazole. Postharvest Biol. Technol. 7 (1-2), 51–64. 10.1016/0925-5214(95)00031-3 [DOI] [Google Scholar]
- Chandrashekara K. N., Manivannan S., Chandrashekara C., Chakravarthi M. (2012). Biological Control of Plant Diseases. Eco-friendly Innovative Approaches in Plant Disease Management (Uttarakhand, India: International Book Distributors; ), 147–166. [Google Scholar]
- Cimmino A., Andolfi A., Zonno M. C., Avolio F., Santini A., Tuzi A., et al. (2013). Chenopodolin: a phytotoxic unrearranged ent-pimaradiene diterpene produced by Phoma chenopodicola, a fungal pathogen for Chenopodium album biocontrol. J. Natural Prod. 76 (7), 1291–1297. 10.1021/np400218z [DOI] [PubMed] [Google Scholar]
- Datnoff L. E., Nemec S., Pernezny K. (1995). Biological control of Fusarium crown and root rot of tomato in Florida using Trichoderma harzianum and Glomus intraradices . Biol. Control l5 (3), 427–431. 10.1006/bcon.1995.1051 [DOI] [Google Scholar]
- De Cal A., Larena I., Liñán M., Torres R., Lamarca N., Usall J., et al. (2009). Population dynamics of Epicoccum nigrum, a biocontrol agent against brown rot in stone fruit. J. Appl. Microbiology. J. Appl. Microbiol. 106 (2), 592–605. 10.1111/j.1365-2672.2008.04030.x [DOI] [PubMed] [Google Scholar]
- Dean R., Van Kan J. A., Pretorius Z. A., Hammond-Kosack K. E., Di Pietro A., Spanu P. D., et al. (2012). The Top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 13 (4), 414–430. 10.1111/j.1364-3703.2011.00783.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deketelaere S., Tyvaert L., França S. C., Höfte M. (2017). Desirable traits of a good biocontrol agent against Verticillium wilt. Front. Microbiol. 8, 1186. 10.3389/fmicb.2017.01186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond H., Cooke B. M. (2003). Preliminary studies on biological control of the Fusarium ear blight complex of wheat. Crop Prot. 22 (1), 99–107. 10.1016/S0261-2194(02)00117-5 [DOI] [Google Scholar]
- Djonovic S., Vittone G., Mendoza-Herrera A., Kenerley C. M. (2007). Enhanced biocontrol activity of Trichoderma virens transformants constitutively coexpressing beta-1,3- and beta-1,6-glucanase genes. Mol. Plant Pathol. 8, 469–480. 10.1111/j.1364-3703.2007.00407.x [DOI] [PubMed] [Google Scholar]
- Doley K., Dudhane M., Borde M. (2017). Biocontrol of Sclerotium rolfsii in groundnut by using microbial inoculants. Notulae Sci. Biol. 9 (1), 124–130. 10.15835/nsb919992 [DOI] [Google Scholar]
- Droby S., Wisniewski M. E., Cohen L., Weiss B., Touitou D., Eilam Y., et al. (1997). Influence of CaCl2 on Penicillium digitatum, grapefruit peel tissue, and biocontrol activity of Pichia guilliermondii . Phytopathology 87 (3), 310–315. 10.1094/PHYTO.1997.87.3.310 [DOI] [PubMed] [Google Scholar]
- Droby S. (2006). Biological control of postharvest diseases of fruits and vegetables: difficulties and challenges. Phytopathol. Pol. 39, 105–117. [Google Scholar]
- El Ghaouth A., Wilson C., Wisniewski M., Droby S., Smilanick J. L., Korsten L. (2002). “Biological control of postharvest diseases of fruits and vegetables,” in Applied mycology and biotechnology, vol. Vol. 2 (Amsterdam, the Netherlands: Elsevier; ), 219–238. [Google Scholar]
- El-Ghaouth A., Droby S. (2001). Nonchemical approaches to postharvest disease control. ActaHorticulture 553, 407–412. 10.17660/ActaHortic.2001.553.93 [DOI] [Google Scholar]
- Eshel D., Regev R., Orenstein J., Droby S., Gan-Mor S. (2009). Combining physical, chemical and biological methods for synergistic control of postharvest diseases: A case study of Black Root Rot of carrot. Postharvest Biol. Technol. 54 (1), 48–52. 10.1016/j.postharvbio.2009.04.011 [DOI] [Google Scholar]
- Filonow A. B., Lockwood J. L. (1985). Evaluation of several actinomycetes and the fungus Hyphochytrium catenoides as biocontrol agents for Phytophthora root rot of soybean. Plant Dis. 69 (12), 1033–1036. [Google Scholar]
- Fravel D. R. (2005). Commercialization and implementation of biocontrol. Annu. Rev. Phytopathol. 43, 337–359. 10.1146/annurev.phyto.43.032904.092924 [DOI] [PubMed] [Google Scholar]
- Freeman S., Minz D., Kolesnik I., Barbul O., Zveibil A., Maymon M., et al. (2004). Trichoderma biocontrol of Colletotrichum acutatum and Botrytis cinerea and survival in strawberry. Eur. J. Plant Pathol. 110 (4), 361–370. 10.1023/B:EJPP.0000021057.93305.d9 [DOI] [Google Scholar]
- Gamagae S. U., Sivakumar D., Wijeratnam R. W., Wijesundera R. L. C. (2003). Use of sodium bicarbonate and Candida oleophila to control anthracnose in papaya during storage. Crop Prot. 22 (5), 775–779. 10.1016/S0261-2194(03)00046-2 [DOI] [Google Scholar]
- Gerbore J., Benhamou N., Vallance J., Le Floch G., Grizard D., Regnault-Roger C., et al. (2014). Biological control of plant pathogens: advantages and limitations seen through the case study of Pythium oligandrum . Environ. Sci. Pollut. Res. 21 (7), 4847–4860. 10.1007/s11356-013-1807-6 [DOI] [PubMed] [Google Scholar]
- Grondona I., Hermosa R., Tejada M., Gomis M. D., Mateos P. F., Bridge P. D., et al. (1997). Physiological and biochemical characterization of Trichoderma harzianum, a biological control agent against soilborne fungal plant pathogens. Appl. Environ. Microbiol. 63 (8), 3189–3198. 10.1128/AEM.63.8.3189-3198.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guetsky R., Shtienberg D., Elad Y., Dinoor A. (2001). Combining biocontrol agents to reduce the variability of biological control. Phytopathology 91, 621–627. 10.1094/PHYTO.2001.91.7.621 [DOI] [PubMed] [Google Scholar]
- Gupta S. K., Sharma M. (2014). Approaches and trends in plant disease management (India: Scientific Publishers; ), Pg. 429. [Google Scholar]
- Gupta P. K. (2018). “Toxicity of fungicides,” in Veterinary Toxicology (Cambridge, USA: Academic Press; ), 569–580. [Google Scholar]
- Gupta P. K. (2019). “Toxic Effects of Pesticides and Agrochemicals,” in Concepts and Applications in Veterinary Toxicology (Cham: Springer; ), 59–82. [Google Scholar]
- Haggag W. M., Nofal M. A. (2006). Improving the biological control of Botryodiplodia disease on some Annona cultivars using single or multi-bioagents in Egypt. Biol. Control 38 (3), 341–349. 10.1016/j.biocontrol.2006.02.010 [DOI] [Google Scholar]
- Hall T. A. (1999). “BioEdit: a user-friendly biological sequence alig nment editor and analysis program for Windows 95/98/NT,” in Nucleic Acids Symposium Series (London: Retrieval Ltd; ), 95–98. [Google Scholar]
- Harman G. E. (2006). Overview of mechanisms and uses of Trichoderma spp. Phytopathology. 96 (2), 190–194. 10.1094/PHYTO-96-0190 [DOI] [PubMed] [Google Scholar]
- Hartley C. (1921). Damping-off in forest nurseries. U. S. Dept. Agr. Bul. 934, 99 pp. 10.5962/bhl.title.108260 [DOI] [Google Scholar]
- Haugaard H., Lyngs Jørgensen H. J., Lyngkjær M. F., Smedegaard-Petersen V., Collinge D. B. (2001). Control of Blumeria graminis f. sp. hordei by treatment with mycelial extracts from cultured fungi. Plant Pathol. 50 (5), 552–560. 10.1046/j.1365-3059.2001.00595.x [DOI] [Google Scholar]
- He S. S., Zhang B. X., Ge Q. X. (1992). On the antagonism by hyperparasite Pythium oligandrum . Acta Phytopathol. Sin. 22 (1), 77–82. [Google Scholar]
- Heydari A., Pessarakli M. (2010). A review on biological control of fungal plant pathogens using microbial antagonists. J. Biol. Sci. 10 (4), 273–290. 10.3923/jbs.2010.273.290 [DOI] [Google Scholar]
- Howell C. R. (2003). Mechanisms employed by Trichoderma species in the biological control of plant diseases: the history and evolution of current concepts. Plant Dis. 87 (1), 4–10. 10.1094/PDIS.2003.87.1.4 [DOI] [PubMed] [Google Scholar]
- Huelsenbeck J. P., Ronquist F. (2001). MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 17, 754–755. 10.1093/bioinformatics/17.8.754 [DOI] [PubMed] [Google Scholar]
- Hung P. M., Wattanachai P., Kasem S., Poaim S. (2015). Biological Control of Phytophthora palmivora Causing Root Rot of Pomelo Using Chaetomium spp. Mycobiology 43 (1), 63–70. 10.5941/MYCO.2015.43.1.63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda S., Shimizu A., Shimizu M., Takahashi H., Takenaka S. (2012). Biocontrol of black scurf on potato by seed tuber treatment with Pythium oligandrum . Biol. Control 60 (3), 297–304. 10.1016/j.biocontrol.2011.10.016 [DOI] [Google Scholar]
- Janisiewicz W. J., Korsten L. (2002). Biological control of postharvest diseases of fruits. Annu. Rev. Phytopathol. 40 (1), 411–441. 10.1146/annurev.phyto.40.120401.130158 [DOI] [PubMed] [Google Scholar]
- Karabulut O. A., Arslan U., Ilhan K., Kuruoglu G. (2005). Integrated control of postharvest diseases of sweet cherry with yeast antagonists and sodium bicarbonate applications within a hydrocooler. Postharvest Biol. Technol. 37 (2), 135–141. 10.1016/j.postharvbio.2005.03.003 [DOI] [Google Scholar]
- Kheireddine A., Essghaier B., Hedi A., Dhieb C., Zouaoui N. S. (2018). New epiphytic yeasts able to reduce grey mold disease on apples. Plant Prot. Sci. 54 (4), 248–257. 10.17221/103/2017-PPS [DOI] [Google Scholar]
- Koitabashi M., Tsushima S. (2007). Studies on biocontrol of air-borne plant disease by a filamentous fungus producing antifungal volatiles. Japan Agric. Res. Q. 41 (4), 261–265. 10.6090/jarq.41.261 [DOI] [Google Scholar]
- Lal R. J., Sinha O. K., Bhatnagar S., Lal S., Awasthi S. K. (2009). Biological control of sugarcane smut (Sporisorium scitamineum) through botanicals and Trichoderma viride . Sugar Tech. 11 (4), 381–386. 10.1007/s12355-009-0065-x [DOI] [Google Scholar]
- Larena I., Sabuquillo P., Melgarejo P., De Cal A. (2003). Biocontrol of Fusarium and Verticillium wilt of tomato by Penicillium oxalicum under greenhouse and field conditions. J. Phytopathol. 151 (9), 507–512. 10.1046/j.1439-0434.2003.00762.x [DOI] [Google Scholar]
- Larena I., De Cal A., Melgarejo P. (2010). Enhancing the adhesion of Epicoccum nigrum conidia to peach surfaces and its relationship to the biocontrol of brown rot caused by Monilinia laxa . J. Appl. Microbiol. 109 (2), 583–593. 10.1111/j.1365-2672.2010.04681.x [DOI] [PubMed] [Google Scholar]
- Larkin R. P., Fravel D. R. (1998). Efficacy of various fungal and bacterial biocontrol organisms for control of Fusarium wilt of tomato. Plant Dis. 82 (9), 1022–1028. 10.1094/PDIS.1998.82.9.1022 [DOI] [PubMed] [Google Scholar]
- Larran S., Simon M. R., Moreno M. V., Siurana M. S., Perelló A. (2016). Endophytes from wheat as biocontrol agents against tan spot disease. Biol. Control. 92, 17–23. 10.1016/j.biocontrol.2015.09.002 [DOI] [Google Scholar]
- Le Floch G., Rey P., Déniel F., Benhamou N., Picard K., Tirilly Y. (2003). Enhancement of development and induction of resistance in tomato plants by the antagonist, Pythium oligandrum . Agronomie 23 (5-6), 455–460. 10.1051/agro:2003018 [DOI] [Google Scholar]
- Liu H. M., Guo J. H., Liu P., Cheng Y. J., Wang B. Q., Long C. A., et al. (2010. b). Inhibitory activity of tea polyphenol and Candida ernobii against Diplodia natalensis infections. J. Appl. Microbiol. 108 (3), 1066–1072. 10.1111/j.1365-2672.2009.04511.x [DOI] [PubMed] [Google Scholar]
- Lou B., Wang A., Lin C., Xu T., Zheng X. (2011). Enhancement of defense responses by oligandrin against Botrytis cinerea in tomatoes. Afr. J. Biotechnol. 10 (55), 11442–11449. 10.5897/AJB11.618 [DOI] [Google Scholar]
- Lucas J. A., Hawkins N. J., Fraaije B. A. (2015). The evolution of fungicide resistance. Adv. Appl. Microbiol. 90, 29–92. 10.1016/bs.aambs.2014.09.001 [DOI] [PubMed] [Google Scholar]
- Maharachchikumbura S. S., Hyde K. D., Jones E. G., McKenzie E. H. C., Bhat J. D., Dayarathne M. C., et al. (2016). Families of sordariomycetes. Fungal Divers. 79 (1), 1–317. 10.1007/s13225-016-0369-6 [DOI] [Google Scholar]
- Martin F. N., Hancock J. G. (1986). Association of chemical and biological factors in soils suppressive to Pythium ultimum . Phytopathology 76 (11), 1221–1231. 10.1094/Phyto-76-1221 [DOI] [Google Scholar]
- Martin F. N., Hancock J. G. (1987). The use of Pythium oligandrum for biological control of preemergence damping-off caused by P. Ultimum. Phytopathol. 77 (7), 1013–1020. 10.1094/Phyto-77-1013 [DOI] [Google Scholar]
- Mathivanan N., Kabilan V., Murugesan K. (1998). Purification, characterization, and antifungal activity of chitinase from Fusarium chlamydosporum, a mycoparasiteto groundnut rust, Puccinia arachidis . Can. J. Microbiol. 44 (7), 646–651. 10.1139/w98-043 [DOI] [PubMed] [Google Scholar]
- Matta A., Garibaldi A. (1977). Control of Verticillium wilt of tomato by preinoculation with avirulent fungi. Netherlands J. Plant Pathol. 83 (1), 457–462. 10.1007/BF03041463 [DOI] [Google Scholar]
- McDougal R., Stewart A., Bradshaw R. (2012). Transformation of Cyclaneusma minus with green fluorescent protein (GFP) to enable screening of fungi for biocontrol activity. Forests 3 (1), 83–94. 10.3390/f3010083 [DOI] [Google Scholar]
- McLaughlin R. J., Wisniewski M. E., Wilson C. L., Chalutz E. (1990). Effect of inoculum concentration and salt solutions on biological control of postharvest diseases of apple with Candida sp. Phytopathology 80 (5), 456–461. 10.1094/Phyto-80-456 [DOI] [Google Scholar]
- McQuilken M. P., Whipps J. M., Cooke R. C. (1990). Control of damping-off in cress and sugar-beet by commercial seed-coating with Pythium oligandrum . Plant Pathol. 39 (3), 452–462. 10.1111/j.1365-3059.1990.tb02521.x [DOI] [Google Scholar]
- McQuilken M. P., Whipps J. M., Cooke R. C. (1992). Use of Oospore Formulations of Pythium oligandrum for Biological Control of Pythium Damping-off in Cress. J. Phytopathol. 135 (2), 125–134. 10.1111/j.1439-0434.1992.tb01259.x [DOI] [Google Scholar]
- Menzler-Hokkanen I. (2006). “Socioeconomic significance of biological control,” in An ecological and societal approach to biological control (Dordrecht: Springer; ), 13–25. [Google Scholar]
- Meszka B., Bielenin A. (2010). Polyversum WP a new biological product against strawberry grey mould. Phytopathologia 58, 13–19. [Google Scholar]
- Miller M. A., Pfeiffer W., Schwartz T. (2010). “Creating the CIPRES Science Gateway for inference of large phylogenetic trees,” in Proceedings of the Gateway Computing Environments Workshop (GCE), (New Orleans, LA), 1–8. 10.1109/GCE.2010.5676129 [DOI] [Google Scholar]
- Mohamed H., Saad A. (2009). The biocontrol of postharvest disease (Botryodiplodia theobromae) of guava (Psidium guajava L.) by the application of yeast strains. Postharvest Biol. Technol. 53 (3), 123–130. [Google Scholar]
- Mohamed N., Lherminier J., Farmer M. J., Fromentin J., Beno N., Houot V., et al. (2007). Defense responses in grapevine leaves against Botrytis cinerea induced by application of a Pythium oligandrum strain or its elicitin, oligandrin, to roots. Phytopathology 97 (5), 611–620. 10.1094/PHYTO-97-5-0611 [DOI] [PubMed] [Google Scholar]
- Montesinos E. (2003). Development, registration and commercialization of microbial pesticides for plant protection. Int. Microbiol. 6 (4), 245–252. 10.1007/s10123-003-0144-x [DOI] [PubMed] [Google Scholar]
- Morris C. E., Monier J. M. (2003). The ecological significance of biofilm formation by plant-associated bacteria. Annu. Rev. Phytopathol. 41 (1), 429–453. 10.1146/annurev.phyto.41.022103.134521 [DOI] [PubMed] [Google Scholar]
- Nabi S. U., Raja W. H., Kumawat K. L., Mir J. I., Sharma O. C., Singh D. B. (2017). Post harvest diseases of temperate fruits and their management strategies-a review. Int. J. Pure App. Biosci. 5 (3), 885–898. 10.18782/2320-7051.2981 [DOI] [Google Scholar]
- Nagtzaam M. P. M., Bollen G. J., Termorshuizen A. J. (1998). Efficacy of Talaromyces flavus alone or in combination with other antagonists in controlling Verticillium dahliae in growth chamber experiments. J. Phytopathol. 146 (4), 165–173. 10.1111/j.1439-0434.1998.tb04674.x [DOI] [Google Scholar]
- Nega A. (2014). Review on concepts in biological control of plant pathogens. J. Biol. Agric. Healthcare 4 (27), 33–54. [Google Scholar]
- Nel B., Steinberg C., Labuschagne N., Viljoen A. (2006). The potential of nonpathogenic Fusarium oxysporum and other biological control organisms for suppressing fusarium wilt of banana. Plant Pathol. 55 (2), 217–223. 10.1111/j.1365-3059.2006.01344.x [DOI] [Google Scholar]
- Nylander J. (2004). MrModeltest v2. Program distributed by the author (Uppsala, Sweden: Evolutionary Biology Centre, Uppsala University; ). [Google Scholar]
- Ommati F., Zaker M., Mohammadi A. (2013). Biological control of Fusarium wilt of potato (Fusarium oxysporum f. sp. tuberosi) by Trichoderma isolates under field condition and their effect on yield. J. Crop Prot. 2 (4), 435–442. [Google Scholar]
- O’Brien P. A. (2017). Biological control of plant diseases. Australas. Plant Pathol. 46 (4), 293–304. 10.1007/s13313-017-0481-4 [DOI] [Google Scholar]
- Ordentlich A., Nachmias A., Chet I. (1990). Integrated control of Verticillium dahlia in potato by Trichoderma harzianum and captan. Crop Prot. 9 (5), 363–366. 10.1016/0261-2194(90)90008-U [DOI] [Google Scholar]
- Padder B. A., Sharma P. N. (2011). In vitro and in vivo antagonism of biocontrol agents against Colletotrichum lindemuthianum causing bean anthracnose. Arch. Phytopathol. Plant Protect. 44 (10), 961–969. 10.1080/03235400903460619 [DOI] [Google Scholar]
- Pal K. K., McSpadden Gardener B. (2006). Biological Control of Plant Pathogens. Plant Health Instructor. 2, 1117–1142. 10.1094/PHI-A-2006-1117-02 [DOI] [Google Scholar]
- Park J. H., Choi G. J., Jang K. S., Lim H. K., Kim H. T., Cho K. Y., et al. (2005). Antifungal activity against plant pathogenic fungi of chaetoviridins isolated from Chaetomium globosum . FEMS Microbiol. Lett. 252 (2), 309–313. 10.1016/j.femsle.2005.09.013 [DOI] [PubMed] [Google Scholar]
- Pathak V. N. (1997). Postharvest fruit pathology: present status and future possibilities. Indian Phytopath. 50, 161–185. [Google Scholar]
- Paul B. (1999. a). Pythium periplocum, an aggressive mycoparasite of Botrytis cinerea causing the gray mould disease of grape-vine. FEMS Microbiol. Lett. 181 (2), 277–280. 10.1111/j.1574-6968.1999.tb08855.x [DOI] [PubMed] [Google Scholar]
- Paul B. (1999. b). Suppression of Botrytis cinerea causing the grey mould disease of grape-vine by an aggressive mycoparasite, Pythium radiosum . FEMS Microbiol. Lett. 176 (1), 25–30. 10.1111/j.1574-6968.1999.tb13637.x [DOI] [PubMed] [Google Scholar]
- Paulitz T. C., Baker R. (1987). Biological control of Pythium damping-off of cucumbers with Pythium nunn: Population dynamics and disease suppression. Phytopathology 77 (2), 335–340. 10.1094/Phyto-77-335 [DOI] [Google Scholar]
- Perelló A. E., Moreno M. V., Mónaco C., Simón M. R., Cordo C. (2009). Biological control of Septoria tritici blotch on wheat by Trichoderma spp. under field conditions in Argentina. Bio Control 54 (1), 113–122. 10.1007/s10526-008-9159-8 [DOI] [Google Scholar]
- Perez M. F., Contreras L., Garnica N. M., Fernández-Zenoff M. V., Farías M. E., Sepulveda M., et al. (2016). Native killer yeasts as biocontrol agents of postharvest fungal diseases in lemons. PLoS One 11 (10), p.e0165590. 10.1371/journal.pone.0165590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piano S., Neyrotti V., Migheli Q., Gullino M. L. (1997). Biocontrol capability of Metschnikowia pulcherrima against Botrytis postharvest rot of apple. Postharvest Biol. Technol. 11 (3), 131–140. 10.1016/S0925-5214(97)00022-7 [DOI] [Google Scholar]
- Picard K., Ponchet M., Blein J. P., Rey P., Tirilly Y., Benhamou N. (2000). Oligandrin. A Proteinaceous Molecule Produced by the Mycoparasite Pythium oligandrum Induces Resistance to Phytophthora parasitica Infection in Tomato Plants. Plant Physiol. 124 (1), 379–396. 10.1104/pp.124.1.379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Punja Z. K. (1997). Comparative efficacy of bacteria, fungi, and yeasts as biological control agents for diseases of vegetable crops. Can. J. Plant Pathol. 19 (3), 315–323. 10.1080/07060669709500531 [DOI] [Google Scholar]
- Qin G. Z., Tian S. P. (2004). Biocontrol of postharvest diseases of jujube fruit by Cryptococcus laurentii combined with a low dosage of fungicides under different storage conditions. Plant Dis. 88 (5), 497–501. 10.1094/PDIS.2004.88.5.497 [DOI] [PubMed] [Google Scholar]
- Rannala B., Yang Z. (1996). Probability distribution of molecular evolutionary trees: a new method of phylogenetic inference. J. Mol. Evol. 43, 304–311. 10.1007/BF02338839 [DOI] [PubMed] [Google Scholar]
- Raziq F., Fox R. T. V. (2005). Combinations of fungal antagonists for biological control of Armillaria root rot of strawberry plants. Biol. Agric. Horticult. 23 (1), 45–57. 10.1080/01448765.2005.9755307 [DOI] [Google Scholar]
- Reino J. L., Guerrero R. F., Hernández-Galán R., Collado I. G. (2008). Secondary metabolites from species of the biocontrol agent Trichoderma . Phytochem. Rev. 7 (1), 89–123. 10.1007/s11101-006-9032-2 [DOI] [Google Scholar]
- Rekanovic E., Milijasevic S., Todorovic B., Potocnik I. (2007). Possibilities of biological and chemical control of Verticillium wilt in pepper. Phytoparasitica 35 (5), 436. 10.1007/BF03020601 [DOI] [Google Scholar]
- Roberts W. (1874). Studies on biogenesis. Philos. Trans. R. Soc Lond. 164, 466. [Google Scholar]
- Rubini M. R., Silva-Ribeiro R. T., Pomella A. W., Maki C. S., Araújo W. L., Dos Santos D. R., et al. (2005). Diversity of endophytic fungal community of cacao (Theobroma cacao L.) and biological control of Crinipellis perniciosa, causal agent of Witches’ Broom Disease. Int. J. Biol. Sci. 1 (1), 24–33. 10.7150/ijbs.1.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangeetha G., Usharani S., Muthukumar A. (2009). Biocontrol with Trichoderma species for the management of postharvest crown rot of banana. Phytopathol. Mediterr. 48 (2), 214–225. 10.14601/Phytopathol_Mediterr-2741 [DOI] [Google Scholar]
- Schena L., Nigro F., Soleti Ligorio V., Yaseen T., Ippolito A., El Ghaouth A. (2004). “June. Biocontrol activity of bio-coat and biocure against postharvest rots of table grapes and sweet cherries,” in V International Postharvest Symposium, vol. 682 (Leuven, Belgium: International Society for Horticultural Science; ), 2115–2120. [Google Scholar]
- Schisler D. A., Khan N. I., Boehm M. J., Slininger P. J. (2002). Greenhouse and field evaluation of biological control of Fusarium head blight on durum wheat. Plant Dis. 86 (12), 1350–1356. 10.1094/PDIS.2002.86.12.1350 [DOI] [PubMed] [Google Scholar]
- Singh V. K., Chawla S. (2012). Cultural Practices: An Eco-friendly Innovative Approaches in Plant Disease Management Publisher (New Delhi: International Book Distributors and Publisher; ). [Google Scholar]
- Singh H. B. (2014). Management of plant pathogens with microorganisms. Proc. Indian Natl. Sci. Acad. 80 (2), 443–454. 10.16943/ptinsa/2014/v80i2/55120 [DOI] [Google Scholar]
- Spotts R. A., Cervantes L. A., Facteau T. J., Chand-Goyal T. (1998). Control of brown rot and blue mold of sweet cherry with preharvest iprodione, postharvest Cryptococcus infirmo-miniatus, and modified atmosphere packaging. Plant Dis. 82 (10), 1158–1160. 10.1094/PDIS.1998.82.10.1158 [DOI] [PubMed] [Google Scholar]
- Sriram S., Poornadchanddra S. R. (2013). Biological control of postharvest mango fruit rot caused by Colletotrichum gloeosporioides and Diplodia natalensis with Candida tropicalis and Alcaligenes feacalis . Indian Phytopathol. 66 (4), 375–380. [Google Scholar]
- Stamatakis A., Hoover P., Rougemont J. (2008). A rapid bootstrap algorithm for the RAxML Web servers. Syst. Biol. 57, 758–771. 10.1080/10635150802429642 [DOI] [PubMed] [Google Scholar]
- Stamatakis A. (2006). RAxML-VI-HPC: Maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22, 2688–2690. 10.1093/bioinformatics/btl446 [DOI] [PubMed] [Google Scholar]
- Stevens C., Khan V. A., Lu J. Y., Wilson C. L., Pusey P. L., Igwegbe E. C. K., et al. (1997). Integration of ultraviolet (UV-C) light with yeast treatment for control of postharvest storage rots of fruit and vegetables. Biol. Control 10, 98–103. 10.1006/bcon.1997.0551 [DOI] [Google Scholar]
- Stirling M., Stirling G. (1997). “Disease Management: Biological Control,” in Plant Pathogens and Plant Diseases. Eds. Brown J., Ogle H., 427–439. [Google Scholar]
- Stoppacher N., Kluger B., Zeilinger S., Krska R., Schuhmacher R. (2010). Identification and profiling of volatile metabolites of the biocontrol fungus Trichoderma atroviride by HS-SPME-GC-MS. J. Microbiol. Methods 81 (2), 187–193. 10.1016/j.mimet.2010.03.011 [DOI] [PubMed] [Google Scholar]
- Takenaka S., Ishikawa S. (2013). Biocontrol of sugar beet seedling and taproot diseases caused by Aphanomyces cochlioides by Pythium oligandrum treatments before transplanting. Japan Agric. Res. Quarterly. 47 (1), 75–83. 10.6090/jarq.47.75 [DOI] [Google Scholar]
- Takenaka S., Tamagake H. (2009). Foliar spray of a cell wall protein fraction from the biocontrol agent Pythium oligandrum induces defence-related genes and increases resistance against Cercospora leaf spot in sugar beet. J. Gen. Plant Pathol. 75 (5), 340. 10.1007/s10327-009-0186-9 [DOI] [Google Scholar]
- Takenaka S., Nishio Z., Nakamura Y. (2003). Induction of defense reactions in sugar beet and wheat by treatment with cell wall protein fractions from the mycoparasite Pythium oligandrum . Phytopathology 93 (10), 1228–1232. 10.1094/PHYTO.2003.93.10.1228 [DOI] [PubMed] [Google Scholar]
- Takenaka S., Nakamura Y., Kono T., Sekiguchi H., Masunaka A., Takahashi H. (2006). Novel elicitin-like proteins isolated from the cell wall of the biocontrol agent Pythium oligandrum induce defence-related genes in sugar beet. Mol. Plant Pathol. 7 (5), 325–339. 10.1111/j.1364-3703.2006.00340.x [DOI] [PubMed] [Google Scholar]
- Tamura K., Peterson D., Peterson N., Stecher G., Nei M., Kumar S. (2011). MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 28, 2731–2739. 10.1093/molbev/msr121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian S. P., Fan Q., Xu Y., Jiang A. L. (2002). Effects of calcium on biocontrol activity of yeast antagonists against the postharvest fungal pathogen Rhizopus stolonifer . Plant Pathol. 51 (3), 352–358. 10.1046/j.1365-3059.2002.00711.x [DOI] [Google Scholar]
- Tian S., Qin G., Xu Y. (2005). Synergistic effects of combining biocontrol agents with silicon against postharvest diseases of jujube fruit. J. Food Protect. 68 (3), 544–550. 10.4315/0362-028X-68.3.544 [DOI] [PubMed] [Google Scholar]
- Vallance J., Le Floch G., Déniel F., Barbier G., Lévesque C. A., Rey P. (2009). Influence of Pythium oligandrum biocontrol on fungal and oomycete population dynamics in the rhizosphere. Appl. Environ. Microbiol. 75 (14), 4790–4800. 10.1128/AEM.02643-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varo A., Raya-Ortega M. C., Trapero A. (2016). Selection and evaluation of micro-organisms for biocontrol of Verticillium dahliae in olive. J. Appl. Microbiol. 121 (3), 767–777. 10.1111/jam.13199 [DOI] [PubMed] [Google Scholar]
- Vesely D. (1977). Potential biological control of damping-off pathogens in emerging sugar beet by Pythium oligandrum Drechsler. Phytopathol. Zeitschrift. 90 (2), 113–115. 10.1111/j.1439-0434.1977.tb03225.x [DOI] [Google Scholar]
- Weindling R. (1932). Trichoderma lignorum as a parasite of other soil fungi. Phytopathology 22, 837–845. [Google Scholar]
- Weindling R. (1934). Studies on a lethal principle effective in the parasitic action of Trichoderma lignorum on Rhizoctonia solani and other soil fungi. Phytopathology 24, 1153–1179. [Google Scholar]
- Weindling R. (1941). Experimental consideration of the mold toxins of Gliocladium and Trichoderma . Phytopathology 31 (11), 991. [Google Scholar]
- Wilson C. L., Wisniewski M. E., Biles C. L., McLaughlin R., Chalutz E., Droby S. (1991). Biological control of post-harvest diseases of fruits and vegetables: alternatives to synthetic fungicides. Crop Protect. 10 (3), 172–177. 10.1016/0261-2194(91)90039-T [DOI] [Google Scholar]
- Wisniewski M., Droby S., Norelli J., Liu J., Schena L. (2016). Alternative management technologies for postharvest disease control: the journey from simplicity to complexity. Postharvest Biol. Technol. 122, 3–10. 10.1016/j.postharvbio.2016.05.012 [DOI] [Google Scholar]
- Wokocha R. C., Ebenebe A. C., Erinle I. D. (1986). Biological control of the basal stem rot disease of tomato caused by Corticium rolfsii (Sacc.) Curzi in Northern Nigeria. Int. J. Pest Manage 32 (1), 35–39. [Google Scholar]
- Xu X. M., Jeffries P., Pautasso M., Jeger M. J. (2011). Combined use of biocontrol agents to manage plant diseases in theory and practice. Phytopathology 101 (9), 1024–1031. 10.1094/PHYTO-08-10-0216 [DOI] [PubMed] [Google Scholar]
- Yakoby N., Zhou R., Kobiler I., Dinoor A., Prusky D. (2001). Development of Colletotrichum gloeosporioides restriction enzyme-mediated integration mutants as biocontrol agents against anthracnose disease in avocado fruits. Phytopathology 91 (2), 143–148. 10.1094/PHYTO.2001.91.2.143 [DOI] [PubMed] [Google Scholar]
- Zhang Q., Zhang J., Yang L., Zhang L., Jiang D., Chen W., et al. (2014). Diversity and biocontrol potential of endophytic fungi in Brassica napus . Biol. Control. 72, 98–108. 10.1016/j.biocontrol.2014.02.018 [DOI] [Google Scholar]
- Zhaxybayeva O., Gogarten J. P. (2002). maximum likelihood mapping: exploring new tools for comparative genome analyses. BMC Genomics 3, 4. 10.1186/1471-2164-3-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y., Xue Q. Y., Xu L. L., Xu Q., Lu S., Gu C., et al. (2011). A screening strategy of fungal biocontrol agents towards Verticillium wilt of cotton. Biol. Control 56 (3), 209–216. 10.1016/j.biocontrol.2010.11.010 [DOI] [Google Scholar]
- Zhu H. Q., Feng Z. L., Li Z. F., Shi Y. Q., Zhao L. H., Yang J. R. (2013). Characterization of two fungal isolates from cotton and evaluation of their potential for biocontrol of Verticillium wilt of cotton. J. Phytopathol. 161 (2), 70–77. 10.1111/jph.12027 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.