Abstract
Purpose
To investigate the clinical and microbiological characteristics of invasive and hypervirulent Klebsiella pneumoniae (HvKP) in a teaching hospital in Southern China.
Patients and Methods
A total of 495 non-repetitive K. pneumoniae strains were isolated from Dongguan People’s Hospital affiliated to Southern Medical University in 2018. Multivariate analysis was performed using the patients’ clinical data to identify the risk factors for HvKP.
Results
Eighty-one isolates were HvKP (16.4%, 81/495), of which 43 (53.1%) were invasive HvKP, whereas 38 (46.9%) were non-invasive HvKP. The incidence of extended spectrum beta-lactamases (ESBLs) in HvKP and classic K. pneumoniae (cKP) were 7.4% (6/81) and 28.0% (116/414), respectively (p<0.05). Multivariate analysis indicated that diabetes mellitus (odds ratio [OR]=12.849, 95% confidence interval [CI]: 1.494–110.511, P=0.020) was an independent risk factor for invasive HvKP infection. Altogether, 51.2% (22/43) of invasive HvKP infections were treated with antimicrobial therapy combined with surgical drainage, and achieved good prognosis. K1-ST23 HvKP accounted for a higher proportion of invasive infections than non-invasive infections (P<0.05), but there was no statistical difference in the prognosis between the two groups (P>0.05). The most prevalent virulence genes in HvKP were rmpA 98.7% (80/81), followed by rmpA2 (82.7%, 67/81), iroN (98.7%, 80/81), and iutA 90.1% (70/81). There was no significant difference in the distribution of virulence genes between invasive HvKP and non-invasive HvKP isolates (P>0.05).
Conclusion
Invasive HvKP infection in this study was positively associated with diabetes as independent risk factors. Antibiotic therapy combined with surgical drainage is one of the most effective treatment measures of HvKP infection. Adequate attention should be paid to HvKP infection in clinical and microbiological laboratories.
Keywords: nosocomial infection, surgical drainage, pneumonia, mortality, serotype, MLST
Introduction
Klebsiella pneumoniae is the main pathogen causing nosocomial infections, such as bloodstream infections, pneumonia, and urinary tract infections.1,2 Since the first report of hypervirulent K. pneumoniae (HvKP) in Taiwan in 1986,3 which is a new variant of K. pneumoniae that differs from the classic K. pneumoniae (cKP), it has been gradually attracting great attention. Various characteristics of HvKP, such as, can cause serious infections not only in immunosuppressed patients but also in healthy people; HvKP can quickly cause invasive infections, such as endophthalmitis, meningitis, and neuritis.4 Infections caused by HvKP are prone to spread to multiple organs; the appearance of the colony has high mucus characteristics.5 HvKP infections have gradually attracted increased attention in the scientific literature and have been mainly reported in Asia, but several cases have now been reported worldwide in recent years. A multi-center research report on HvKP showed that, from the data obtained from 10 teaching hospitals in mainland China, 37.8% of the 230 K. pneumoniae isolates were HvKP, indicating a higher incidence of HvKP in mainland China.6 In 2017, Chinese scholars discovered a kind of K. pneumoniae with both high drug resistance and high virulence in Hangzhou, Zhejiang Province, which infected intensive care unit (ICU) patients with a poor prognosis.7 However, so far, there is almost no research on HvKP infection in Dongguan, Guangdong. However, there are still many patients with K. pneumoniae infection in the hospital ward of the research institute. The virulence factors, genotype characteristics, and clinical data of infection have not been extensively studied. Some earlier studies have shown that diabetes may be an important host factor for liver abscess caused by K. pneumoniae.8,9 Some special capsular serotypes, such as K1, K2, and ST23, in multilocus sequence typing (MLST) are highly related to HvKP.10,11
This study aimed to systematically analyze the risk factors, molecular characteristics, and patient mortality rate of HvKP-induced Bloodstream infections (BSIs). Specially, we analyzed the clinical characteristics of patients with invasive HvKP infection in the Dongguan area of Guangdong, China, and explored the relationship between the genotype, resistance phenotype, and HvKP infection.
Patients and Methods
Isolates and Antimicrobial Susceptibility Test
A total of 495 isolates of K. pneumoniae from various clinical specimens were collected from Dongguan People’s Hospital from January to December 2018. Only the first strain was collected from duplicate isolates from the same patient. All isolates were identified by matrix-associated laser desorption ionization-time of flight mass spectrometry (Vitek MS, Biomerieux, France) and stored at −80°C.
The antimicrobial susceptibility test was performed using the Vitek 2 Compact system (Biomerieux, France). The quality control isolates were Escherichia coli ATCC25922 and K. pneumoniae ATCC700603.
Hypervirulent Phenotype Determination and Serotype as Well as Virulence Gene Detection
Isolates were inoculated onto Columbia Blood Agar and incubated at 35°C for 24 h. The inoculation loop was used to contact the surface of a single colony. The results were considered positive if the colony with a viscous mucilage string with a length of ≥5 mm could be extracted; otherwise, the results were considered negative. Serum capsule genes (K1, K2, K20, K54, and K57) and virulence genes (rmpA, rmpA2, iroN, and iutA) were amplified as previously described.12
Sequence Type Detection
MLST was performed following the protocol described on the Pasteur Institute MLST website (https://bigsdb.pasteur.fr/klebsiella/klebsiella.html).
Clinical Data and Definition
A standard medical record form was designed. The clinical information of 81 HvKP infected patients was obtained from their medical records. Invasive infections are defined as infections with K. pneumoniae isolated from the bloodstream or abscesses (liver abscess, brain abscess, lung abscess, and endophthalmitis). Other infections, such as urinary system infections and skin and soft tissue infections, are referred to as non-invasive infections.
Statistical Analysis
Continuous variables were compared with the Student’s t-test (for normally distributed variables) or the Mann–Whitney U-test (for non-normally distributed variables) and presented as the mean ± standard deviation or median. Categorical variables were evaluated using the χ2 test or two-tailed Fisher’s exact test. Logistic regression models were used to analyze the risk factors. All variables with a P-value<0.10 in the univariate analysis were included in the multivariate analysis. All tests were two-tailed, with a significance level set at p<0.05. SPSS 20 (SPSS Inc., Chicago, IL, USA) software was used for data analysis.
Results
Microbiological Characteristics of HvKP Isolates
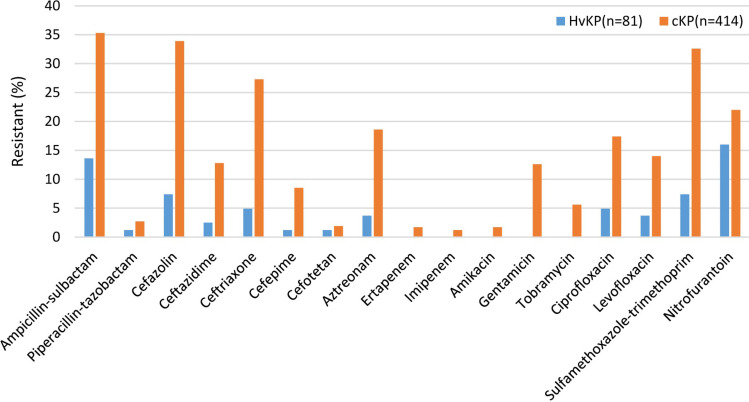
As shown in Figure 1, the resistance ratios of HvKP isolates against ampicillin/sulbactam, cefazolin, ceftazidime, ceftriaxone, cefepime, aztreonam, gentamicin, tobramycin, levofloxacin, and sulfamethoxazole-trimethoprim were significantly lower than that of cKP isolates (all P < 0.05). The extended spectrum beta-lactamase (ESBL)-producing rates of HvKP isolates were (7.4%, 6/81) lower than those of (28.0%, 116/414) (P<0.05). No carbapenem-resistant HvKP was isolated.
Figure 1.
Resistance rate of hypervirulent Klebsiella pneumoniae and classic Klebsiella pneumoniae.
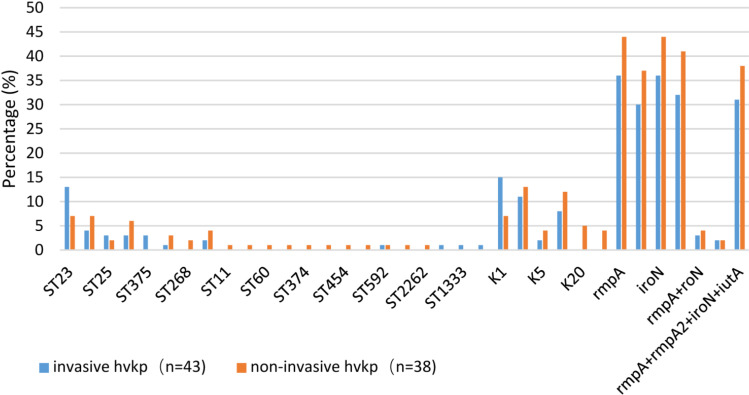
The predominant sequence types of the 81 HvKP isolates were ST23 (20/81, 21.9%), followed by ST86 (11/81, 12.1%) and ST218 (9/81, 9.9%). The frequency of ST23 in invasive HvKP isolates (34.9%, 15/43) was significantly higher than that of non-invasive HvKP isolates (13.2%, 5/38) (p<0.05). Three new ST types, ST1049, ST1333, and ST3816, of invasive HvKP isolates were identified.
The main capsular serotype was K2 (27.5%, 25/81), followed by K1 (24.2%, 22/81), K20 (5.5%, 5/81), K54 (14.3%, 13/81), K57 (21.9%, 20/81), and K5 (6.6%, 6/81). The frequency of K1 type identified in invasive HvKP isolates was significantly higher than that of non-invasive isolates, whereas those of the K20 and K54 serotypes were opposite (p <0.05). Among the 81 HvKP isolates, 90.12% (73/81) of the isolates showed positive results in the wire drawing test; the positive rate of virulence genes was 87.7%, and the virulence gene detection rates for rmpA, rmpA2, iroN, and iucA were 98.7% (80/81), 82.7% (67/81), 98.7% (80/81), and 90.1% (70/81), respectively. If the wire drawing test was negative, 8 isolates carried virulence genes, accounting for 9.8% (8/81) of all HvKP isolates, whereas if the wire drawing test was positive, 3 isolates did not carry virulence genes, accounting for 3.7% (3/81). K1 is highly correlated with ST23, and both contain 4 virulence genes, followed by K2 ST86; the distribution of virulence genes is rmpA+rmpA2+iroN+iucA, accounting for 72.2 (8/11), and rmpA/iroN accounting for 27.8%, 3/11, invasive HvKP, and non-invasive HvKP isolates were in the total virulence gene distribution. No significant difference (p>0.05) (Table 1 and Figure 2).
Table 1.
Hypervirulent K. pneumoniae Capsule Serotype and Virulence Gene Spectrum Distribution
| K-Type | MLST | Number | The wire drawing test | Virulence Gene |
|---|---|---|---|---|
| K1 | ST23 | 22 | + | rmpA+rmpA2+iroN+iucA (100%) |
| K2 | ST25 | 5 | -/+ | rmpA+rmpA2+iroN+iutA (2 isolates);rmpA/iroN/iucA (2 isolates)rmpA/iroN (1 strain) |
| ST65 | 4 | -/+ | rmpA+rmpA2+iroN+iucA (100%) | |
| ST86 | 11 | -/+ | rmpA+rmpA2+iroN+iucA (8 isolates); rmpA/iroN (3 isolates) | |
| ST374 | 1 | -/+ | rmpA+rmpA2+iroN+iucA (100%) | |
| ST375 | 3 | -/+ | rmpA+rmpA2+iroN+iucA (100%) | |
| K57 | ST218 | 9 | -/+ | rmpA+rmpA2+iroN+iucA (100%) |
| ST412 | 6 | + | rmpA+rmpA2+iroN+iucA (100%) | |
| ST592 | 2 | + | ||
| ST182 | 1 | + | rmpA+rmpA2+iroN+iucA (100%) | |
| unknown | 2 | -/+ | rmpA+rmpA2+iroN+iucA (100%) | |
| K20 | ST420 | 1 | _ | rmpA+rmpA2+iroN+iucA (100%) |
| ST268 | 2 | + | rmpA+rmpA2+iroN+iucA (100%) | |
| 11 | 1 | + | rmpA+rmpA2+iroN+iucA (100%) | |
| 893 | 1 | + | rmpA+rmpA2+iroN+iucA (100%) | |
| K5 | 60 | 1 | + | rmpA/iroN/iucA |
| 485 | 1 | - | rmpA+rmpA2+iroN+iucA (100%) | |
| 1049 | 1 | + | rmpA/iroN | |
| 1333 | 1 | + | rmpA/iroN | |
| unknown | 2 | + | rmpA+rmpA2+iroN+iucA (100%) | |
| K54 | ST23 | 1 | + | rmpA+rmpA2+iroN+iucA (100%) |
| ST45 | 1 | + | rmpA+rmpA2+iroN+iucA (100%) | |
| ST454 | 1 | - | iucA | |
| ST2262 | 1 | + | rmpA/iroN |
Figure 2.
Comparison of the molecular characteristics between invasive and non-invasive hypervirulent Klebsiella pneumoniae (HvKP) infections.
Clinical Features of HvKP Infection
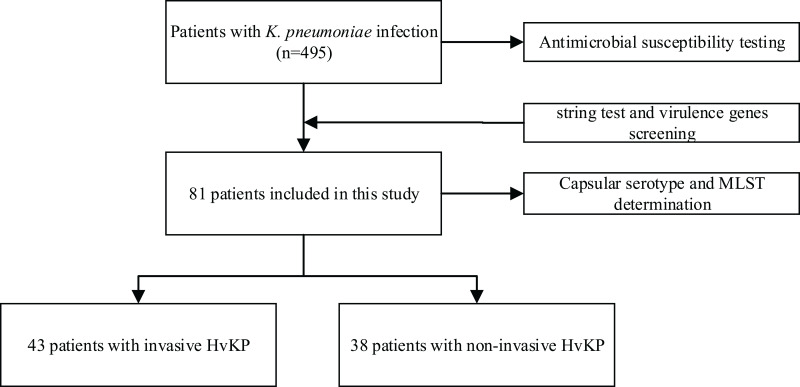
A total of 81 out of 495 isolates (16.4%) of K. pneumoniae collected were determined to be HvKP through a positive wire drawing test and a positive virulence gene from January 1, 2018 to December 31, 2018. The 81 HvKP isolates were mainly from the sputum (39.6%, 36/81), followed by blood (24.8%, 20/81), liver abscess drainage (16%, 13/18), urine (8.7%, 7/81), pus (4.9%, 4/81), wound secretions (2.5%, 4/81), alveolar lavage fluid (1.2%, 1/81), pleural fluid (1.2%, 1/81), and ear secretions (1.2%, 1/81). The bloodstream infections caused by HvKP mainly occurred in ICU (20.9%, 9/43) and general surgery (27.9%, 12/43) patients. Among the 81 HvKP isolates, 53.08% (43/81) were classified as invasive and highly virulent K. pneumoniae, and 46.92% (38/81) were classified as non-invasive HvKP (Figure 3).
Figure 3.
Flowchart of the included patients with hypervirulent Klebsiella pneumoniae (HvKP).
According to the clinical characteristics of the 81 HvKP isolates shown in Table 2, there were 57 male and 24 female patients, with an average age of 59±17.03 years. Regarding the patients’ general diseases, a statistical difference was found in the distribution of hypertension, lung disease, nervous system disease, and cardiovascular disease between invasive HvKP and non-invasive HvKP patients (P<0.05). The frequency of invasive HvKP was significantly higher than that of non-invasive HvKP in patients with liver disease (51.16% vs 13.16%), with diabetes (48.83% vs 26.32%), and undergoing invasive procedures (55.81% vs 10.52%). Fifty percent (22/43) of invasive HvKP infected patients were mainly treated with abscess drainage combined with antibiotics, especially carbapenems. There were significant differences in the treatment outcomes between the two groups (P<0.05). The frequency of K1-ST23 was higher in invasive HvKP isolates than in non-invasive HvKP isolates (P<0.05), but there was no significant difference in the prognosis between the two groups (P>0.05).
Table 2.
Univariate Analysis for Identifying the Predictors of Invasive HvKP Infection
| Variables | Invasive HvKP (43) | Non-Invasive HvKP (38) | P-value |
|---|---|---|---|
| Demographics | |||
| Male sex | 30 (69.76) | 27 (71.05) | 0.229 |
| Age, >60 years | 17 (39.53) | 20 (52.63) | 0.238 |
| Length of hospital stay at the time of infection (day) | 18 (18.00) | 22 (35.75) | 0.424 |
| Underlying disease | |||
| Hypertension | 5 (11.63) | 20 (52.63) | <0.001 |
| Urinary tract infection | 11 (25.58) | 10 (26.32) | 0.94 |
| Lung disease | 15 (34.88) | 23 (60.52) | 0.021 |
| Smoking and drinking history | 4 (9.3) | 5 (13.16) | 0.728 |
| Neurologic disease | 3 (6.98) | 17 (44.74) | <0.001 |
| Cardiovascular disease | 1 (2.32) | 10 (26.32) | 0.002 |
| Agranulocytosis | 1 (2.32) | 0 | 1.0 |
| Splenectomy | 2 (4.65) | 0 | 0.496 |
| Peritonitis | 6 (13.95) | 1 (2.63) | 0.114 |
| Liver disease | 22 (51.16) | 5 (13.16) | <0.001 |
| Diabetes mellitus | 21 (48.83) | 10 (26.32) | 0.037 |
| Tumor disease | 10 (23.25) | 6 (15.79) | 0.4 |
| Invasive procedures | |||
| Mechanical ventilation | 10 (23.25) | 16 (42.11) | 0.07 |
| Catheter drainage | 24 (55.81) | 4 (10.52) | <0.001 |
| Thoracentesis | 10 (23.25) | 9 (23.68) | 0.964 |
| Lumbar puncture | 3 (6.97) | 13 (30.23) | 0.002 |
| Tracheotomy | 10 (23.25) | 12 (31.58) | 0.401 |
| Therapy | |||
| Hormone therapy | 7 (16.27) | 2 (5.26) | 0.162 |
| Monotherapy | 9 (20.93) | 5 (13.15) | 0.356 |
| Combination therapy | 34 (79) | 33 (86.84) | 0.356 |
| Antibiotic therapy combined with puncture drainage | 22 (50%) | 2 (4.4%) | <0.001 |
| Antibiotic therapy before cultivation | 4 (9.3) | 0 | 0.119 |
| Antibiotics | |||
| Enzyme inhibitor complex | 14 | 18 | 0.174 |
| Quinolones | 6 | 6 | 0.816 |
| Carbapenems | 17 | 3 | 0.001 |
| Cephalosporins | 6 | 5 | 0.917 |
| Prognosis | |||
| Death | 3 (6.97) | 4 (10.52) | 0.701 |
| Improved | 33 (76.74) | 26 (68.42) | 0.401 |
| Abandoned treatment | 7 (16.28) | 8 (21.05) | 0.581 |
| Capsular serotype | |||
| K1 | 18 (41.86) | 4 (10.52) | 0.002 |
| K2 | 13 (30.23) | 11 (28.95) | 0.899 |
| K57 | 10 (23.25) | 10 (26.32) | 0.75 |
| MLST | |||
| ST23 | 15 (34.88) | 5 (13.16) | 0.04 |
| ST86 | 5 (11.63) | 6 (15.79) | 0.585 |
| ST218 | 5 (11.63) | 4 (10.52) | 1.0 |
| Virulence positive (rmpA/rmpA2/iroN/iutA) | |||
| Positive for the 4 virulence genes | 38 (88.37) | 31 (81.58) | 0.39 |
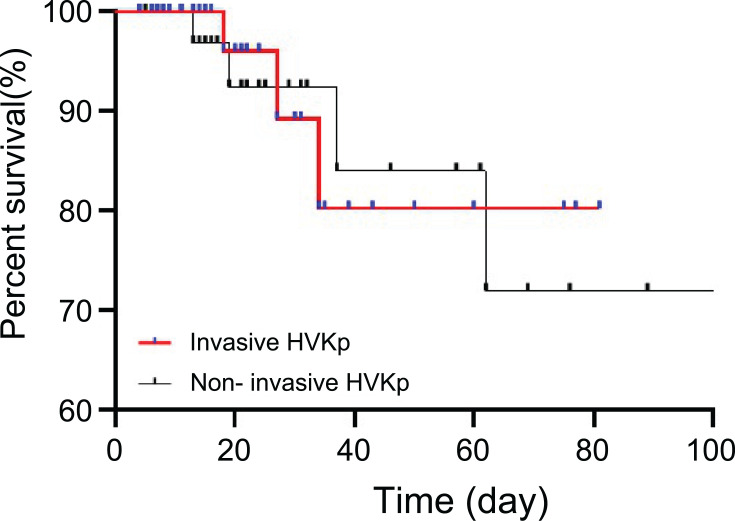
In the Kaplan–Meier survival curve in Figure 4, there was no difference in the hospital fatality rate between bloodstream infection cases with HvKP and non-bloodstream infection cases with HvKP (P=0.7303).
Figure 4.
Kaplan–Meier curve showing the effect of invasive and non-invasive HvKP infections on mortality.
Risk Factor Analysis
On univariate analysis, capsular serotype K1, hypertension, diabetes, lung disease, nervous system disease, cardiovascular system disease, liver disease, mechanical ventilation, and catheter drainage were significant risk factors for invasive HvKP infection (P<0.1). On multivariate regression analysis, hypertension (odds ratio [OR]=0.041, 95% confidence interval [CI]: 0.004–0.385, P=0.005)and cardiovascular disease (OR=0.025, 95% CI: 0.001–0.94, P=0.046)were negatively correlated with invasive HvKP, diabetes (OR=12.849, 95%CI: 1.494-110.511, P=0.02) was independently associated with invasive HvKP infection (Table 3).
Table 3.
Univariate and Multivariate Logistic Regression Analysis of Invasive HV K. pneumoniae
| Variables | Univariate Analysis | Multivariable Analysis | ||
|---|---|---|---|---|
| OR (95% CI) | P-value | OR (95% CI) | P-value | |
| K1 | 6.120 (1.843–20.323) | 0.003 | 11.537 (0.992–134.114) | 0.051 |
| Hypertension | 0.118 (0.038–0.366) | <0.001 | 0.041 (0.004–0.385) | 0.005 |
| Lung disease | 0.349 (0.142–0.862) | 0.023 | 0.25 (0.041–1.511) | 0.131 |
| Neurologic disease | 0.093 (0.024–0.352) | <0.001 | 0.139 (0.013–1.544) | 0.108 |
| Cardiovascular disease | 0.067 (0.008–0.55) | 0.012 | 0.025 (0.001–0.94) | 0.046 |
| Liver Disease | 6.914 (2.268–21.076) | 0.001 | 1.282 (0.202–8.119) | 0.792 |
| Diabetes mellitus | 2.673 (1.047–6.825) | 0.04 | 12.849 (1.494–110.511) | 0.02 |
| Mechanical Ventilation | 0.417 (0.16–1.085) | 0.073 | 0.424 (0.045–3.949) | 0.451 |
| Catheter drainage | 10.737 (3.24–35.582) | <0.001 | 8.877 (0.945–83.43) | 0.056 |
Discussion
Recently, many countries have reported infectious diseases due to HvKP, which had attracted worldwide attention. Epidemiological data indicate that HvKP is highly prevalent in Asian populations,13 suggesting that there may be differences in host gene susceptibility or different levels of antibiotic exposure among different regions. Given that cases of HvKP causing bloodstream infections have increased, in this retrospective study, we systematically analyzed 81 HvKP cases in a tertiary teaching hospital in southern China and further compared the clinical and microbiological characteristics between HvKP-BSI and non-HvKP-BSI. Owing to the regional differences in HvKP-BSI, this study systematically analyzed the risk factors of aggressive HvKP, its molecular characteristics, and patient mortality, and provided evidence for the prediction and diagnosis of HvKP infection in the southern region.
Compared with the antibiotic resistance rates of cKP, the rates of HvKP were much lower. Although no carbapenem-resistant HvKP was found in our samples, 6 out of 81 HvKP isolates produced ESBLs (7.4%). Thus, more attention should be paid to ESBLs and KPC-HvKP, which also appeared in China.14 ESBL-producing HvKP may originate from isolates colonized in the host’s respiratory tract or intestine, or acquired resistant plasmid from other ESBL-producing Enterobacteriaceae. Infection with these highly invasive and resistant gene-producing isolates will increase the difficulty and cost of treatment. Therefore, antibacterial drugs should be used rationally, surveillance should be strengthened, and control measures should be implemented to reduce the prevalence in the hospital. Contrarily, new antibacterial drugs may be a good treatment option.15 Some new products of natural origin against K. pneumoniae were reported to be useful in hospital settings.16–20
According to the clinical characteristics of invasive and non-invasive HvKP, fifty percent (22/43) of patients with invasive HvKP infection were mainly treated with antibiotics combined with abscess drainage, and their prognosis was good. Additionally, although the overall drug susceptibility of HvKP was lower than that of cKP, the clinical symptoms of HvKP-induced infections were very serious, especially bloodstream infections. This suggests the need for timely application of carbapenem drugs and puncture drainage to improve patient prognosis.
A previous study on risk factors for HvKP infections reported that patients with community-acquired infections and underlying diseases, such as diabetes, cancer, and hypertension, are more likely to develop HvKP infections. Men may be slightly more likely to be infected than women are.21 Poor blood sugar control impairs the phagocytic ability of neutrophils and promotes the growth of pathogens in the tissues, whereas metabolic disorders damage the liver.21,22 However, in this study, diabetes mellitus as an independent risk factor for invasive HvKP, hypertension and cardiovascular disease was negatively correlated with invasive HvKP. This indicates that clinicians should pay attention to patients with the above-mentioned underlying diseases, as they are susceptible to HvKP bloodstream infections.
As an essential substance of K. pneumoniae, the capsular polysaccharide has 82 serotypes (K1-K82).11,23 Previous studies have reported that the dominant serotypes of HvKP are mainly K1 and K2.6,24 The majority of serotypes of HvKP in this study were K2 (27.5%, 25/81), followed by K1 (24.2%, 22/81), and K57 (21.9%, 20/81). The prevalence rate of K57 was equivalent to that of K1. Although other studies reported that the detection rates of K57 varied across regions (9.6% (8/84) in Wenzhou,25 13.6% (3/22) in Changsha,26 10.4% (10/96) in Beijing,27 and 18.9% (7/37) in Shanghai28). Additionally, the K1 serotype was mainly related to liver abscess disease, whereas the K2 serotype was related to aggressive or bloodstream infection,29 although these findings had not been confirmed, and further research is needed.
ST23 or its single-point variation is closely related to K1 serotype isolates and pyogenic liver abscess, whereas ST65 is related to K2 isolates.30 The sequence type of invasive HvKP in this study was mainly K1-ST23, and there may be a certain epidemic clone dissemination. K2 was predominant in ST86, which is inconsistent with the abovementioned research. Thus, clinical departments must pay attention to infections caused by HvKP, especially to prevent metastasis caused by the invasive HvKP bloodstream infection.
Screening of HvKP found that a total of 8 isolates (9.8%, 8/81) were negative in the wire drawing test and positive in the presence of virulence genes, whereas 3 isolates (3.7%, 3/81) without virulence genes were positive in the wire drawing test. This suggested that the drawing experiment had limitations, which was consistent with the findings of previous studies.31,32 Hence, in this study, the definition of HvKP was based on the following aspects: positive wire drawing test, positivity of rmpA/rmpA2/iroN genes, and/or positivity of the aerobactin gene. Although virulence factors were not always HvKP, they served as a warning for invasive HvKP bloodstream infection, which requires long-term follow-up of treatment, maximizing the possibility of successful treatment and minimizing the recurrence rate.31,33 The total positive rate of virulence genes in this study was as high as 87.7% (71/81), and the detection rates of the four virulence genes were 98.7% (80/81), 82.7% (67/81), 98.7% (80/81), and 90.1% (70/81) for rmpA, rmpA2, iroN, and iucA, respectively. The rmpA gene regulates the synthesis of extracellular polysaccharide capsules and is responsible for their high viscosity. The ablation of rmpA may cause loss or thinning of the capsule, thereby weakening the ability to evade the immune responses, which leads to reduced virulence of the bacteria.33,34 Although there was no significant difference in the distribution of virulence genes between invasive and non-invasive HvKP isolates (p>0.05), the high positive rate in virulence genes greatly increased the virulence of invasive HvKP and made its clinical treatment more difficult. A recent study31 showed that the iroB, iucA, rmpA, rmpA2, peg-344, and peg-589 genes on virulence plasmids can be used to distinguish HvKP and cKP isolates with high accuracy. These genes can also accurately predict mortality in mouse sepsis models. This is also a shortcoming of this study. The present investigation was a retrospective study, and a mouse model was not performed to confirm the true virulence of HvKP. Future research should analyze a larger sample and number of virulence genes for testing.
In conclusion, although this study did not isolate carbapenem-resistant HvKP, despite the annual increase in HvKP cases with carbapenem-resistant genes, such as blaKPC among hospitals in China,35 carbapenem-resistant HvKP would become a serious problem in nosocomial infections, and more control measures should be applied to prevent its dissemination. HvKP acquiring antibiotic-resistant genes, a highly virulent and highly resistant organism will become another “super bacteria”.36 This study focused on the analysis of the clinical characteristics and homology of invasive HvKP isolated from patients with bacteremia. Our findings may guide clinicians to pay more attention to infections caused by HvKP. Moreover, laboratory personnel should also pay special attention to the detection of the high-virulence, high-mucus phenotype of K. pneumoniae and timely communication with the clinical staff to reduce the adverse prognosis of patients and prevent its dissemination in the hospital.
Funding Statement
This research is supported by General Project of Dongguan Social Science and Technology Development in 2019 (No. 201950715001884).
Ethics Approval and Informed Consent
This study was approved by the research ethics board of Dongguan People’s Hospital, Southern Medical University. The study was observational and retrospective and was reviewed and granted ethical exemption by the medical ethics committee of the Affiliated Dongguan People’s Hospital, Southern Medical University. Obtaining informed consent was not needed because the medical records and patient information were anonymously reviewed and collected in this observational study.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Gajdacs M, Batori Z, Abrok M, Lazar A, Burian K. Characterization of resistance in gram-negative urinary isolates using existing and novel indicators of clinical relevance: a 10-year data analysis. Life. 2020;10(2):16. doi: 10.3390/life10020016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Oliveira D, Forde B, Kidd T, et al. Antimicrobial resistance in ESKAPE pathogens. Clin Microbiol Rev. 2020;33(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheng D, Liu Y, Yen M, Liu C, Wang R. Septic metastatic lesions of pyogenic liver abscess. Their association with Klebsiella pneumoniae bacteremia in diabetic patients. Arch Intern Med. 1991;151(8):1557–1559. doi: 10.1001/archinte.1991.00400080059010 [DOI] [PubMed] [Google Scholar]
- 4.Shon AS, Bajwa RP, Russo TA. Hypervirulent (hypermucoviscous) Klebsiella pneumoniae: a new and dangerous breed. Virulence. 2013;4(2):107–118. doi: 10.4161/viru.22718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harada S, Doi Y, Diekema DJ. Hypervirulent Klebsiella pneumoniae: a call for consensus definition and international collaboration. J Clin Microbiol. 2018;56(9):9. doi: 10.1128/JCM.00959-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang Y, Zhao C, Wang Q, et al. High prevalence of hypervirulent Klebsiella pneumoniae infection in China: geographic distribution, clinical characteristics, and antimicrobial resistance. Antimicrob Agents Chemother. 2016;60(10):6115–6120. doi: 10.1128/AAC.01127-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gu D, Dong N, Zheng Z, et al. A fatal outbreak of ST11 carbapenem-resistant hypervirulent Klebsiella pneumoniae in a Chinese hospital: a molecular epidemiological study. Lancet Infect Dis. 2018;18(1):37–46. [DOI] [PubMed] [Google Scholar]
- 8.Wang JH, Liu YC, Lee SS, et al. Primary liver abscess due to Klebsiella pneumoniae in Taiwan. Clin Infect Dis. 1998;26(6):1434–1438. doi: 10.1086/516369 [DOI] [PubMed] [Google Scholar]
- 9.Chan K-S, Chen C-M, Cheng K-C, Hou -C-C, Lin H-J, Yu W-L. Pyogenic liver abscess: a retrospective analysis of 107 patients during a 3-year period. Jpn J Infect Dis. 2005;58(6):366–368. [PubMed] [Google Scholar]
- 10.Liu C, Guo J. Hypervirulent Klebsiella pneumoniae (hypermucoviscous and aerobactin positive) infection over 6 years in the elderly in China: antimicrobial resistance patterns, molecular epidemiology and risk factor. Ann Clin Microbiol Antimicrob. 2019;18(1). doi: 10.1186/s12941-018-0302-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Russo TA, Marr CM. Hypervirulent Klebsiella pneumoniae. Clin Microbiol Rev. 2019;32(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Y, Zeng J, Liu W, et al. Emergence of a hypervirulent carbapenem-resistant Klebsiella pneumoniae isolate from clinical infections in China. J Infect. 2015;71(5):553–560. doi: 10.1016/j.jinf.2015.07.010 [DOI] [PubMed] [Google Scholar]
- 13.Li W, Sun G, Yu Y, et al. Increasing occurrence of antimicrobial-resistant hypervirulent (hypermucoviscous) Klebsiella pneumoniae isolates in China. Clin Infect Dis. 2014;58(2):225–232. doi: 10.1093/cid/cit675 [DOI] [PubMed] [Google Scholar]
- 14.Struve C, Bojer M, Nielsen E, Hansen D, Krogfelt K. Investigation of the putative virulence gene magA in a worldwide collection of 495 Klebsiella isolates: magA is restricted to the gene cluster of Klebsiella pneumoniae capsule serotype K1. J Med Microbiol. 2005;54:1111–1113. [DOI] [PubMed] [Google Scholar]
- 15.Usai D, Donadu M, Bua A, et al. Enhancement of antimicrobial activity of pump inhibitors associating drugs. J Infect Dev Ctries. 2019;13(2):162–164. doi: 10.3855/jidc.11102 [DOI] [PubMed] [Google Scholar]
- 16.Trong Le N, Viet Ho D, Quoc Doan T, et al. Biological activities of essential oils from leaves of paramignya trimera (Oliv.) Guillaum and Limnocitrus littoralis (Miq.) Swingle. Antibiotics. 2020;9(4):207. doi: 10.3390/antibiotics9040207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trong Le N, Viet Ho D, Quoc Doan T, et al. In vitro antimicrobial activity of essential oil extracted from leaves of Leoheo domatiophorus Chaowasku, D.T. Ngo and H.T. Le in Vietnam. Plants. 2020;9(4):453. doi: 10.3390/plants9040453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Le NT, Donadu MG, Ho DV, et al. Biological activities of essential oil extracted from leaves of Atalantia sessiflora Guillauminin Vietnam. J Infect Dev Ctries. 2020;14(9):1054–1064. doi: 10.3855/jidc.12469 [DOI] [PubMed] [Google Scholar]
- 19.Donadu MG, Trong Le N, Viet Ho D, et al. Phytochemical compositions and biological activities of essential oils from the leaves, rhizomes and whole plant of Hornstedtia bella Škorničk. Antibiotics. 2020;9(6):334. doi: 10.3390/antibiotics9060334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bua A, Usai D, Donadu MG, et al. Antimicrobial activity of Austroeupatorium inulaefolium (H.B.K.) against intracellular and extracellular organisms. Nat Prod Res. 2018;32(23):2869–2871. doi: 10.1080/14786419.2017.1385014 [DOI] [PubMed] [Google Scholar]
- 21.Foo N, Chen K, Lin H, Guo H. Characteristics of pyogenic liver abscess patients with and without diabetes mellitus. Am J Gastroenterol. 2010;14(09):328–335. doi: 10.1038/ajg.2009.586 [DOI] [PubMed] [Google Scholar]
- 22.Lin J, Siu L, Fung C, et al. Impaired phagocytosis of capsular serotypes K1 or K2 Klebsiella pneumoniae in type 2 diabetes mellitus patients with poor glycemic control. J Clin Endocrinol Metab. 2006;91(8):3084–3087. doi: 10.1210/jc.2005-2749 [DOI] [PubMed] [Google Scholar]
- 23.Behzadi P, Urbán E, Matuz M, Benkő R, Gajdács M. The role of gram-negative bacteria in urinary tract infections: current concepts and therapeutic options. Adv Exp Med Biol. 2020:1–35. [DOI] [PubMed] [Google Scholar]
- 24.Yan Q, Zhou M, Zou M, Liu W. Hypervirulent Klebsiella pneumoniae induced ventilator-associated pneumonia in mechanically ventilated patients in China. Eur J Clin Microbiol Infect Dis. 2016;35(3):387–396. doi: 10.1007/s10096-015-2551-2 [DOI] [PubMed] [Google Scholar]
- 25.Guo Y, Wang S, Zhan L, et al. Klebsiella pneumoniae microbiological and clinical characteristics of hypermucoviscous isolates associated with invasive infections in China. Front Cell Infect Microbiol. 2017;7:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu Y, Li B, Zhang Y, et al. Clinical and molecular characteristics of emerging hypervirulent Klebsiella pneumoniae bloodstream infections in Mainland China. Antimicrob Agents Chemother. 2014;58(9):5379–5385. doi: 10.1128/AAC.02523-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu C, Shi J, Guo J. Klebsiella pneumoniae high prevalence of hypervirulent infection in the genetic background of elderly patients in two teaching hospitals in China. Infect Drug Resist. 2018;11:1031–1041. doi: 10.2147/IDR.S161075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun Y, Wu H, Shen D. Clinical and molecular analysis of Klebsiella pneumoniae causing liver abscess in China. J Mol Microbiol Biotechnol. 2016;26(4):245–251. doi: 10.1159/000444367 [DOI] [PubMed] [Google Scholar]
- 29.Walker K, Miner T, Palacios M, et al. A Klebsiella pneumoniae regulatory mutant has reduced capsule expression but retains hypermucoviscosity. mBio. 2019;10(2). doi: 10.1128/mBio.00089-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liao C, Huang Y, Chang C, Hsu H, Hsueh P. Capsular serotypes and multilocus sequence types of bacteremic Klebsiella pneumoniae isolates associated with different types of infections. Eur J Clin Microbiol Infect Dis. 2014;33(3):365–369. doi: 10.1007/s10096-013-1964-z [DOI] [PubMed] [Google Scholar]
- 31.Russo T, Olson R, Fang C, et al. Identification of biomarkers for differentiation of hypervirulent Klebsiella pneumoniae from Classical K. pneumoniae. J Clin Microbiol. 2018;56(9). doi: 10.1128/JCM.00776-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tan T, Ong M, Cheng Y, Ng L. Hypermucoviscosity, rmpA, and aerobactin are associated with community-acquired Klebsiella pneumoniae bacteremic isolates causing liver abscess in Singapore. J Microbiol Immunol Infect. 2019;52(1):30–34. [DOI] [PubMed] [Google Scholar]
- 33.Yu W, Ko W, Cheng K, et al. Association between rmpA and magA genes and clinical syndromes caused by Klebsiella pneumoniae in Taiwan. Clin Infect Dis. 2006;42(10):1351–1358. doi: 10.1086/503420 [DOI] [PubMed] [Google Scholar]
- 34.Paczosa M, Mecsas J. Klebsiella pneumoniae: going on the offense with a strong defense. Microbiol Mol Biol Rev. 2016;80(3):629–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Q, Wang X, Wang J, et al. Phenotypic and genotypic characterization of carbapenem-resistant enterobacteriaceae: data from a longitudinal large-scale CRE study in China (2012–2016). Clin Infect Dis. 2018;67(suppl_2):S196–S205. doi: 10.1093/cid/ciy660 [DOI] [PubMed] [Google Scholar]
- 36.Shon A, Russo T. Hypervirulent Klebsiella pneumoniae: the next superbug? Future Microbiol. 2012;7(6):669–671. doi: 10.2217/fmb.12.43 [DOI] [PubMed] [Google Scholar]