ABSTRACT
Chronic wasting disease (CWD) affects a broad array of cervid species and continues to be detected in an expanding geographic range. Initially introduced into the Republic of Korea through the importation of CWD-infected elk (Cervus canadensis), additional cases of CWD were subsequently detected in farmed Korean elk and sika deer (Cervus nippon). Wild and farmed sika deer are found in many regions of Asia, North America, and Europe, although natural transmission to this species has not been detected outside of the Republic of Korea. In this study, the oral transmission of CWD to sika deer was investigated using material from CWD-affected elk. Pathological prion (PrPCWD) immunoreactivity was detected in oropharyngeal lymphoid tissues of one sika deer at 3.9 months post-inoculation (mpi) and was more widely distributed in a second sika deer examined at 10.9 mpi. The remaining four sika deer progressed to clinical disease between 21 and 24 mpi. Analysis of PrPCWD tissue distribution in clinical sika deer revealed widespread deposition in central and peripheral nervous systems, lymphoreticular tissues, and the gastrointestinal tract. Prion protein gene (PRNP) sequences of these sika deer were identical and consistent with those reported in natural sika deer populations. These findings demonstrate the efficient oral transmission of CWD from elk to sika deer.
KEYWORDS: chronic wasting disease, CWD, prion, sika deer, susceptible, transmission
Introduction
Chronic wasting disease (CWD) is an infectious neurodegenerative prion disease of cervids, occurring in farmed and free-ranging cervid species of North America, the Republic of Korea, and Scandinavia. Naturally susceptible species include white-tailed deer (Odocoileus virginianus), mule deer (Odocoileus hemionus), elk or wapiti (Cervus canadensis), and moose (Alces alces), with cases more recently described in reindeer (Rangifer tarandus tarandus) [1], red deer (Cervus elaphus) [2,3] and sika deer (Cervus nippon) [4]. Consistent with other transmissible spongiform encephalopathies, the pathogenesis of CWD hinges on the conversion of a normal host-encoded prion protein (PrPC) to an abnormal disease-associated isoform (PrPCWD) which accumulates in multiple tissues eventually resulting in clinical disease [5]. The host PrPC gene (PRNP) sequence and resultant tertiary structure of PrPC influence susceptibility to conversion by PrPCWD, thereby underpinning host susceptibility to infection [6]. Nonetheless, the transmission of CWD within and between cervid species occurs relatively efficiently, ostensibly due to the dissemination of infectivity through saliva, urine, and feces [7,8], and persistence of infectivity in the environment [9]. The risk of CWD transmission to non-cervid species appears to be low under natural circumstances, although the risk of cross-species transmission continues to be assessed [10].
Introduced into the Republic of Korea through the inadvertent importation of asymptomatic but infected elk [11], CWD was subsequently detected in farmed elk populations in 2001, 2004 and 2005 [12]. Additional cases were later observed in farmed red deer, sika deer, and several cross-bred deer during investigations in 2010 and 2016 [4]. Farmed and feral sika deer exist in other regions of Asia, North America, and Europe [13], although natural CWD transmission to this species has not yet been documented beyond the Republic of Korea. In several regions of the world, the range of feral sika deer overlaps with other cervids, and hybridization with congeneric species such as red deer is known to occur [13,14]. Additionally, sika deer may be farmed to maintain broodstock for game ranches or for the production of venison and antler velvet, and may be cross-bred or housed with CWD-susceptible cervid species in this context.
The sustained prevalence of CWD in North America and Korea, and recent cases in Europe, highlights the continued need to characterize disease transmission in the range of cervid species which may be exposed. Understanding the pathogenesis of CWD in sika deer is important for the development of diagnostic and disease control strategies. Accordingly, we describe the clinical, pathological, and genetic findings associated with the oral transmission of CWD from elk to sika deer.
Results
Incubation period and clinical disease
Two (cases 1 and 2) of the six inoculated sika deer were removed from study at 3.9 and 10.9 months post-inoculation (mpi) due to intercurrent disease and neither animal displayed clinical signs consistent with CWD (Table 1). The remaining four sika deer progressed to terminal disease, with clinical signs beginning around 20 mpi, and a mean survival time of 22.2 mpi. Clinical signs were similar to those observed in other CWD-infected cervids, initially including subtle changes in hair coat and weight loss, but progressing to include ataxia, bruxism, sialorrhea and tremors. Two sika deer (cases 3 and 4) were subjected to recto-anal mucosa-associated lymphoid tissue (RAMALT) biopsy at 20 mpi, just prior to the onset of clinical disease, and samples from both showed marked PrPCWD deposition in follicular germinal centres by immunohistochemistry (Figure 1(c)). Gross lesions were not evident during the necropsy of sika deer with terminal CWD. Primary oral transmission occurred in all inoculated sika deer, and was detected in standard lymphoid and nervous tissues using routine diagnostic tests (ELISA, western blot, immunohistochemistry).
Table 1.
Summary of results from CWD-inoculated sika deer
| ELISA (OD)c |
IHCd |
|||||||
|---|---|---|---|---|---|---|---|---|
| Animal ID | Survival time (mpi)a | Clinical signs | RAMALTb | Obex | RPLN | Obex | RPLN | Western blote |
| 1 | 3.9 | - | ND | - (0.018) | +(1.666) | - | + | - |
| 2 | 10.9 | - | ND | +(1.960) | +(3.247) | ++ | + | + |
| 3 | 21.0 | + | 20.6 | +(>3.5) | +(3.184) | ++++ | + | + |
| 4 | 21.3 | + | 20.6 | +(>3.5) | +(>3.5) | ++++ | + | + |
| 5 | 22.3 | + | ND | +(>3.5) | +(2.477) | ++++ | + | + |
| 6 | 24.4 | + | ND | + (>3.5) | +(1.446) | ++++ | + | + |
aTime in months postinoculation (mpi).
bmpi when recto-anal mucosa-associated lymphoid tissue (RAMALT) biopsy positive, ND – not determined.
cELISA (Bio-Rad TeSeE) optical density (OD) value of the obex or retropharyngeal lymph node (RPLN).
dImmunohistochemistry (IHC) of obex graded (- to ++++) or RPLN (±).
eBio-Rad TeSeE western blot conducted on obex (±).
Figure 1.
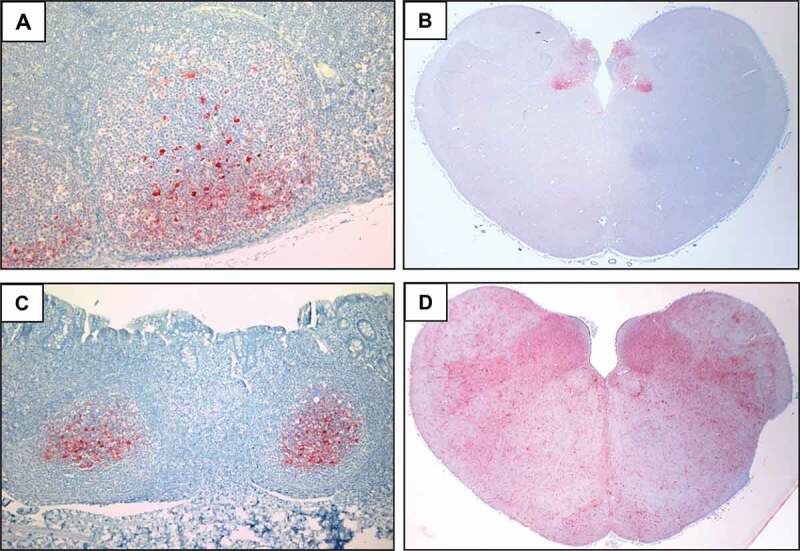
Distribution of PrPCWD deposition in the retropharyngeal lymph node (RPLN), obex and recto-anal mucosa-associated lymphoid tissue (RAMALT) of sika deer orally inoculated with CWD. Tissue sections were stained with anti-PrP mAb F99 and show the presence of PrPCWD (red deposits) in the (a) RPLN of sika deer 1 at 3.9 mpi; (b) obex of sika deer 2 at 10.9 mpi; and the (c) RAMALT and (d) obex of sika deer 3 at 20.6 and 21 mpi, respectively
Histopathology and immunohistochemistry
Brainstem histopathology of clinically affected sika deer identified notable spongiform change, particularly prominent in the dorsal vagal nucleus, and characterized by vacuolation of the grey matter neuropil and occasional neuronal perikarya. The distribution of PrPCWD was catalogued in a range of tissues using immunohistochemistry (Table 2). At 3.9 mpi, PrPCWD was restricted to follicular germinal centres of the retropharyngeal lymph node (RPLN) (Figure 1(a)) and tonsil, but was more widely distributed in lymphoid tissues of the sika deer examined at 10.9 mpi. Moreover, central nervous system involvement was evident in the sika deer at 10.9 mpi, with moderate PrPCWD deposits restricted to the dorsal nucleus of the vagus nerve (DMNV) and the area postrema, but no staining in the adjacent nuclei of the obex (Figure 1(b)). Also, apparent by 10.9 mpi was the affiliation of PrPCWD staining with lymphoid follicles, and neurons of the submucosal and myenteric plexuses throughout the gastrointestinal tract (Table 2).
Table 2.
Distribution of PrPCWD in tissues of CWD-infected sika deer
| Animal ID |
Animal ID |
||||||
|---|---|---|---|---|---|---|---|
| Tissue | 1 | 2 | 3,4,5,6 | Tissue | 1 | 2 | 3,4,5,6 |
| Cerebral cortex | -a | - | ++ | Palatine tonsil | + | +++ | +++ |
| Striatum | - | - | +++ | Retropharyngeal LN | + | +++ | +++ |
| Thalamus | - | - | ++++ | Mediastinal LN | - | +++ | +++ |
| Midbrain | - | - | ++++ | Ileocecocolic LN | - | +++ | +++ |
| Rostral medulla | - | - | ++++ | Spleen | - | + | ++ |
| Obex | - | ++ | ++++ | Duodenum | - | ++ | ++ |
| Cerebellum | - | - | +++ | Jejunum | - | ++ | ++ |
| Spinal cord | - | - | +++ | Ileum | - | ++ | +++ |
| Celiac ganglia | - | - | ++ | Colon | - | ++ | ++ |
| Sympathetic chain | - | - | ++ | Rectum | - | + | +++ |
aThe intensity of immunohistochemical staining (mAb F99) in each tissue was qualitatively scored as follows: – not detected; + mild focal staining; ++ moderate multifocal staining; +++ heavy multifocal staining; ++++ intense widespread staining. Scores from clinically affected sika deer (3–6) were averaged.
In the four sika deer reaching clinical disease, heavy PrPCWD deposits filled the DMNV, surrounding nuclei and white matter tracts of the entire obex section (Figure 1(d)). Although widely distributed throughout the central nervous system, PrPCWD staining intensity was highest in the medulla, with relatively lower levels in the striatum and cerebral cortex (Table 2). PrPCWD deposits were also widespread in the germinal centres of lymphoid follicles of the tonsil and all lymph nodes examined in clinical animals. Tissues from the musculoskeletal system, including tongue, masseter, diaphragm, trapezius, triceps, semitendinosus and psoas major did not contain detectable PrPCWD, nor did sections of skin. The tissue distribution and intensity of PrPCWD accretion were generally consistent among the four sika deer reaching clinical disease, and closely resembled the patterns observed in other cervid species with terminal CWD.
Western immunoblot
The obex region of each CWD-inoculated sika deer was analyzed by western immunoblot and proteinase K-resistant PrP (PrPres) was detected in five of the six sika deer (Figure 2). The electrophoretic migration patterns and glycoform ratios of PrPres in these sika deer were comparable to each other, and indistinguishable from the original elk inoculum.
Figure 2.
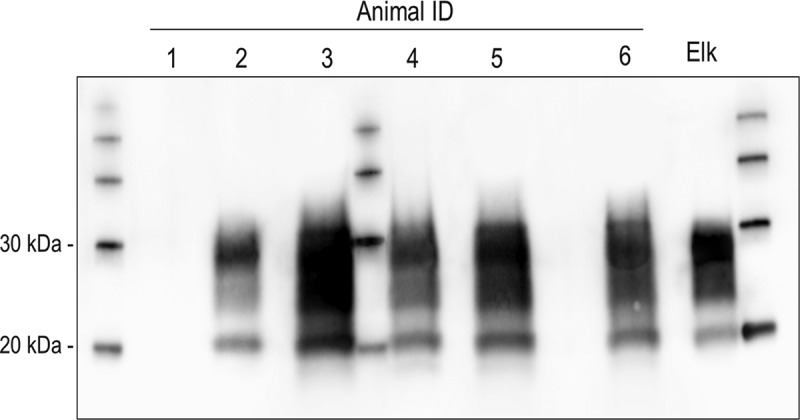
Western blot (mAb Sha31) demonstrating PrPCWD molecular profiles in the obex region of five of the six sika deer and the elk CWD inoculate (Elk). Molecular weight markers are present in the lane between silka deer 3 and 4 and flanking lanes on either side of the gel
PRNP sequence analysis
Sequencing the PRNP open reading frame from all six sika deer revealed an absence of variability among in this group, and consistency with the sika deer (C. nippon) PRNP sequence published in Genbank (accession no. MK103018). All animals were homozygous for glutamine (Q) at codon 95, glycine (G) at codon 96, methionine (M) at codon 132 and serine (S) at codon 225. These findings are consistent with PRNP sequences reported in Chinese [15], Japanese [16] and Korean sika deer [17], as well as a recent study involving European sika deer [18].
Discussion
Oral transmission of CWD from the brain tissues of infected elk to sika deer (Cervus nippon) occurred efficiently in all six sika deer inoculated in this study. PrPCWD was detected in tissues routinely collected for surveillance purposes (retropharyngeal lymph node and tonsil), using commercially available test methods, as early as 3.9 months after inoculation. Four of the six animals developed relatively comparable clinical symptoms and succumbed to disease within a mean period of 22 months. This incubation period is comparable to what has been reported in oral CWD inoculation studies in red deer [19] and elk homozygous for methionine at codon 132 [20], both species which are phylogenetically closely related to sika deer [21]. The progressive accumulation of PrPCWD in lymphoreticular and nervous system tissues was generally consistent with disease progression in other cervids infected naturally or experimentally with CWD [22].
The early accumulation of PrPCWD in oropharyngeal lymphoid tissues typically precedes broader lymphoid tissue involvement as well as the deposition of PrPCWD in central and peripheral nervous systems and other organs [23–25]. Our earliest tissue collection occurred at 3.9 mpi, at which point tonsil and retropharyngeal lymph node contained detectable PrPCWD, and by 10.9 mpi, PrPCWD was present in an array of peripheral lymph nodes and lymphoid follicles of the gastrointestinal tract. Other studies have found initial PrPCWD accumulations are detectable by IHC in oropharyngeal lymphoid tissues within a few weeks following the oral inoculation of mule deer [26] or white-tailed deer [25].
The progression from initial lymphoid deposition to eventual CNS involvement is a consistent feature of CWD pathogenesis in white-tailed deer and mule deer, with surveys detecting a proportion of early cases which are positive in lymph nodes but not the CNS [27–30]. This progression was also observed in our early incubation stage sika deer, but a more variable PrPCWD tissue distribution pattern appears to exist in elk. Surveys of CWD-infected elk populations detect cases which are positive in lymph nodes and not the CNS, but they also find a small percentage of animals to be positive in the obex, without detectable PrPCWD in retropharyngeal lymph nodes or tonsils [31–34] suggesting that limited lymphoid deposition may occur prior to CNS infection in this species. A study of PrPres in lymphoid tissues of CWD-infected deer and elk found comparatively higher levels could be detected in deer tonsil and lymph node samples [35], further supporting species differences in the tissue distribution of PrPCWD. Reasons for these varying tissue accumulation patterns are not clear but may relate to host genetics or conformational differences in the infectious agent. Given the close phylogenetic relationship between sika deer, elk and red deer [21], it seems reasonable to predict that surveys of CWD-exposed sika deer may identify a small proportion of CWD cases which have detectable PrPCWD in obex but not oropharyngeal lymphoid tissues.
In the four sika deer reaching clinical end point in this study, PrPCWD was widely disseminated throughout multiple organ systems. Although immunohistochemistry did not detect PrPCWD in some tissues such as skeletal muscle and skin, we cannot exclude the possibility that infectivity exists in these tissues below the detection levels of the methods used here. Amplification assays such as PMCA and RT-QuIC have the potential to characterize low PrPCWD tissue levels earlier in disease than the methods used in our study [36,37] and further analysis using these assays will refine our understanding of the progression of PrPCWD distribution in sika deer.
The PRNP sequence was identical in all six sika deer in our study and had been previously identified in studies of Asian [15–17] and European sika deer [18]. Many of the alleles present in our sika deer have been associated with CWD susceptibility in other cervid species (95Q, 96G, 132M, 225S) [6]. Sequence variation at codons 100 (S100G) and 226 (Q226E) has been identified in Chinese and Korean sika deer populations [15,17] although the relative significance of these polymorphisms on CWD susceptibility in sika deer remains unknown. Variation at codon 226 (Q226E) is common in red deer [18,38,39], although an oral transmission study found all genotypes (EE, EQ and QQ) were susceptible to CWD [19]. Notwithstanding an effect on CWD susceptibility, Q and E amino acid differences at codon 226 appear to influence CWD strain selection and propagation during disease progression, with potential repercussions on transmissibility within and between animal species [40]. Further study on the influence of codon 226 polymorphisms is relevant given the current presence of codon 226 variability in some sika deer populations, and the potential for increased variability in sika deer following hybridization with red deer.
Studies associating PRNP polymorphisms with resistance to CWD have largely been conducted in CWD-exposed populations of farmed or feral cervids in North America. Reduced CWD susceptibility has been associated with common polymorphisms such as M132L in elk and G96S in deer [6], but rarer polymorphisms may also convey resistance or alter disease progression in some cervid populations [41]. Numerous subspecies of sika deer exist in different regions of the world [13], and hybridization with other cervids has been demonstrated [14], so a more comprehensive assessment of PRNP polymorphisms in sika deer may reveal additional influential variants. The extent to which PRNP polymorphisms in sika deer convey resistance to different CWD isolates from North America, Korea or Scandinavia remains to be elucidated, and it is unknown if sika deer are susceptible to the novel type of CWD recently described in Europe [42].
Widespread detection of PrPCWD by IHC suggests that infectivity is distributed throughout a broad range of tissues in sika deer with clinical CWD, indicating the potential for transmission to other cervid species, and human exposure during the processing and consumption of infected animals. The early presence of PrPCWD in peripheral lymphoid tissues reflects the progressive accumulation pattern observed in other cervids and it seems reasonable to expect infectivity to be shed through similar routes such as feces, urine and saliva. PRNP polymorphisms associated with CWD resistance in other cervids were not present in the sika deer of this study, and the influence of other sika deer PRNP polymorphisms remains to be determined. Farmed and feral sika deer populations are distributed throughout the world and should be considered susceptible to CWD during the application of surveillance and control strategies.
Materials and methods
Ethics statement
All experimental procedures involving animals were performed under strict accordance with the Canadian Council on Animal Care guidelines in such a manner as to minimize the impact on animal wellbeing. Protocols were approved and monitored by the Animal Care Committee at the Canadian Food Inspection Agency, Ottawa Laboratory (Fallowfield).
Experimental challenge
The transmissibility of a Canadian elk CWD isolate was investigated in six, six-month-old sika deer sourced from a CWD-free farmed herd in a region of Canada not known to contain CWD-infected cervids. Animals were group housed in isolation barns at the Canadian Food Inspection Agency, Ottawa Laboratory (Fallowfield) (Ottawa, Ontario). The inocula were prepared as pooled brain homogenates containing 5 g of brain material in 20 ml of physiological saline and were comprised of brain material from a single infected farmed elk herd in western Canada. The pooled material was derived from 12 elk with clinical CWD of genotype 132 MM, confirmed positive by ELISA, western blot and IHC. Sika deer were orally inoculated with a total of 5 g of tissue equivalent on two separate days, 1 week apart, by directing a syringe towards the oropharynx and slowly depositing the brain homogenate. Inoculated sika deer were monitored daily for evidence of disease.
Tissue collection
At one time point, two animals were sedated with a mixture of ketamine and xylazine for biopsy of recto-anal mucosa-associated lymphoid tissue (RAMALT). RAMALT biopsies were obtained and evaluated by IHC as previously described [43]. If an animal displayed evidence of discomfort which was not responsive to treatment, or clinical signs consistent with terminal CWD, they were euthanized with sodium pentobarbital. A broad range of neural and non-neural tissues from all major organ systems was collected and adjacent tissue samples were frozen at −80°C or fixed in 10% neutral buffered formalin.
Immunohistochemical testing
Formalin-fixed, paraffin-embedded tissues were sectioned at 5-um thickness and stained with hematoxylin and eosin or by IHC as previously described [43]. Antigen retrieval was achieved by autoclaving in citrate buffer solution (DAKO Target Antigen Retrieval) and IHC for PrPCWD was performed on an automated immunostainer (Ventana Medical Systems) using the monoclonal antibody F99/97.6.1 (VMRD) and Ultraview Red detection kit (Ventana Medical Systems). Positive and negative staining were differentiated based on comparisons with positive and negative deer control tissues included in each run, and negative control tissues showed no immunoreactivity. The extent of PrPCWD deposition in obex sections was graded (- to ++++) using previously published criteria [31].
ELISA and western blot
ELISA and western blot were conducted on fresh or previously frozen tissue samples using commercially available detection kits (TeSeE ELISA and TeSeE Western blot, Bio-Rad Laboratories) according to manufacturer’s instructions as previously described [19]. Western blots were run with molecular mass markers and ELISA and western blot runs included CWD positive and negative control brain homogenates from elk and sika deer. Negative control samples did not show detectable immunoreactivity.
Genotyping
Genomic DNA was extracted from blood with the DNeasy Blood and Tissue kit (Qiagen). The PRNP open reading frame was amplified using Platinum PCR SuperMix High Fidelity (ThermoFisher Scientific) and the following primers: forward 5ʹ- ACATGGGCATATGATGCTGACACC-3ʹ and reverse 5ʹ- GCCAAGAAATGAGACACCACCACTACAGGG-3ʹ. PCR reaction conditions were as follows: 3 min at 94⁰C, followed by 40 cycles of 30 s at 94⁰C, 30 s at 58⁰C and 90 s at 68⁰C, followed by a final elongation for 5 min at 68⁰C. Sequencing was performed using the following PCR primers: forward 5ʹ-GGGCTCGAGGTCATCATGGTGAAAAGCCACATAGG–3ʹ and reverse 5ʹ- CCCACGCGTCTATCCTACTATGAGAAAAATGAGG–3ʹ and the BigDye Terminator v3.1 Cycle Sequencing kit (ThermoFisher Scientific). Sequence of both strands was generated using a 3730XL DNA Analyzer (ThermoFisher Scientific) and analyzed using MEGA7 software, and was considered valid only if forward and reverse results were in agreement.
Acknowledgments
This work was supported by the Animal and Plant Quarantine Agency, Republic of Korea, under project N-1543085-2018-22-01 and the Canadian Food Inspection Agency under project RPS-NTSE-1005. We thank Patricia Shaffer for IHC technical expertise and the diligent staff supporting the TSE Unit.
Funding Statement
This work was supported by the Animal and Plant Quarantine Agency [N-1543085-2018-22-01]; Canadian Food Inspection Agency [RPS-NTSE-1005].
Disclosure statement
The authors declare no conflict of interest.
References
- [1].Benestad SL, Mitchell G, Simmons M, et al. First case of chronic wasting disease in Europe in a Norwegian free-ranging reindeer. Vet Res. 2016. September 15;47(1):88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Vikoren T, Vage J, Madslien KI, et al. First detection of chronic wasting disease in a wild red deer (Cervus elaphus) in Europe. J Wildl Dis. 2019. March 28;55(4):970-972. [PubMed] [Google Scholar]
- [3].Schwabenlander MD, Culhane MR, Hall SM, et al. A case of chronic wasting disease in a captive red deer (Cervus elaphus). J Vet Diagn Invest. 2013. September;25(5):573–576. [DOI] [PubMed] [Google Scholar]
- [4].Sohn HJ, Roh IS, Kim HJ, et al., editors. Epidemiology of chronic wasting disease in Korea. Prion. Philadelphia, PA, USA: Taylor & Francis Inc.; 2016. [Google Scholar]
- [5].Prusiner SB. Novel proteinaceous infectious particles cause scrapie. Science. 1982;216(4542):136–144. [DOI] [PubMed] [Google Scholar]
- [6].Robinson SJ, Samuel MD, O’Rourke KI, et al. The role of genetics in chronic wasting disease of North American cervids. Prion. 2012. Apr-Jun;6(2):153–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Plummer IH, Wright SD, Johnson CJ, et al. Temporal patterns of chronic wasting disease prion excretion in three cervid species. J Gen Virol. 2017. July;98(7):1932–1942. [DOI] [PubMed] [Google Scholar]
- [8].Mathiason CK, Hays SA, Powers J, et al. Infectious prions in pre-clinical deer and transmission of chronic wasting disease solely by environmental exposure. PLoS ONE. 2009;4(6):e5916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Miller MW, Williams ES, Hobbs NT, et al. Environmental sources of prion transmission in mule deer. Emerg Infect Dis. 2004. June;10(6):1003–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Osterholm MT, Anderson CJ, Zabel MD, et al. Chronic wasting disease in cervids: implications for prion transmission to humans and other animal species. mBio. 2019;10:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Sohn HJ, Kim JH, Choi KS, et al. A case of chronic wasting disease in an elk imported to Korea from Canada. J Vet Med Sci. 2002. September;64(9):855–858. [DOI] [PubMed] [Google Scholar]
- [12].Kim TY, Shon HJ, Joo YS, et al. Additional cases of chronic wasting disease in imported deer in Korea. J Vet Med Sci. 2005. August;67(8):753–759. [DOI] [PubMed] [Google Scholar]
- [13].McCullough DR, Takatsuki S, Kaji K. Sika deer: biology and management of native and introduced populations. Tokyo: Springer; 2009. [Google Scholar]
- [14].Iacolina L, Corlatti L, Buzan E, et al. Hybridisation in European ungulates: an overview of the current status, causes, and consequences. Mammal Rev. 2019;49(1):45–59. [Google Scholar]
- [15].Meng LP, Zhao DM, Liu HX, et al. Polymorphisms of the prion protein gene (PRNP) in Chinese domestic sika deer (Cervus nippon hortulorum). Anim Genet. 2005. June;36(3):266–267. [DOI] [PubMed] [Google Scholar]
- [16].Kataoka N, Nishimura M, Horiuchi M, et al. Surveillance of chronic wasting disease in sika deer, Cervus nippon, from Tokachi district in Hokkaido. J Vet Med Sci. 2005. March;67(3):349–351. [DOI] [PubMed] [Google Scholar]
- [17].Jeong HJ, Lee JB, Park SY, et al. Identification of single-nucleotide polymorphisms of the prion protein gene in sika deer (Cervus nippon laiouanus). J Vet Sci. 2007. September;8(3):299–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Robinson AL, Williamson H, Guere ME, et al. Variation in the prion protein gene (PRNP) sequence of wild deer in Great Britain and mainland Europe. Vet Res. 2019. July 31;50(1):59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Balachandran A, Harrington NP, Algire J, et al. Experimental oral transmission of chronic wasting disease to red deer (Cervus elaphus elaphus): early detection and late stage distribution of protease-resistant prion protein. Can Vet J. 2010. February;51(2):169–178. [PMC free article] [PubMed] [Google Scholar]
- [20].Hamir AN, Gidlewski T, Spraker TR, et al. Preliminary observations of genetic susceptibility of elk (Cervus elaphus nelsoni) to chronic wasting disease by experimental oral inoculation. J Vet Diagn Invest. 2006;18(1):110–114. [DOI] [PubMed] [Google Scholar]
- [21].Hu P, Shao Y, Xu J, et al. Genome-wide study on genetic diversity and phylogeny of five species in the genus Cervus. BMC Genomics. 2019;20:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Williams ES. Chronic wasting disease. Vet Pathol. 2005;42(5):530–549. [DOI] [PubMed] [Google Scholar]
- [23].Fox KA, Jewell JE, Williams ES, et al. Patterns of PrPCWD accumulation during the course of chronic wasting disease infection in orally inoculated mule deer (Odocoileus hemionus). J Gen Virol. 2006. November;87(Pt 11):3451–3461. [DOI] [PubMed] [Google Scholar]
- [24].Sigurdson CJ, Spraker TR, Miller MW, et al. PrP(CWD) in the myenteric plexus, vagosympathetic trunk and endocrine glands of deer with chronic wasting disease. J Gen Virol. 2001. October;82(Pt 10):2327–2334. [DOI] [PubMed] [Google Scholar]
- [25].Hoover CE, Davenport KA, Henderson DM, et al. Pathways of Prion spread during early chronic wasting disease in deer. J Virol. 2017. May 15;91:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Sigurdson CJ, Williams ES, Miller MW, et al. Oral transmission and early lymphoid tropism of chronic wasting disease PrPres in mule deer fawns (Odocoileus hemionus). J Gen Virol. 1999. October;80(Pt 10):2757–2764. [DOI] [PubMed] [Google Scholar]
- [27].Hibler CP, Wilson KL, Spraker TR, et al. Field validation and assessment of an enzyme-linked immunosorbent assay for detecting chronic wasting disease in mule deer (Odocoileus hemionus), white-tailed deer (Odocoileus virginianus), and Rocky Mountain elk (Cervus elaphus nelsoni). J Vet Diagn Invest. 2003. July;15(4):311–319. [DOI] [PubMed] [Google Scholar]
- [28].Miller MW, Williams ES. Detection of PrP(CWD) in mule deer by immunohistochemistry of lymphoid tissues. Vet Rec. 2002. November 16;151(20):610–612. [DOI] [PubMed] [Google Scholar]
- [29].Thomsen BV, Schneider DA, O’Rourke KI, et al. Diagnostic accuracy of rectal mucosa biopsy testing for chronic wasting disease within white-tailed deer (Odocoileus virginianus) herds in North America: effects of age, sex, polymorphism at PRNP codon 96, and disease progression. J Vet Diagn Invest. 2012;24(5):878–887. [DOI] [PubMed] [Google Scholar]
- [30].Keane DP, Barr DJ, Keller JE, et al. Comparison of retropharyngeal lymph node and obex region of the brainstem in detection of chronic wasting disease in white-tailed deer (Odocoileus virginianus). J Vet Diagn Invest. 2008;20(1):58–60. [DOI] [PubMed] [Google Scholar]
- [31].Spraker TR, Balachandran A, Zhuang D, et al. Variable patterns of distribution of PrP(CWD) in the obex and cranial lymphoid tissues of Rocky Mountain elk (Cervus elaphus nelsoni) with subclinical chronic wasting disease. Vet Rec. 2004. September 4;155(10):295–302. [DOI] [PubMed] [Google Scholar]
- [32].Haley NJ, Siepker C, Hoon-Hanks LL, et al. Seeded amplification of chronic wasting disease prions in nasal brushings and recto-anal mucosa-associated lymphoid tissues from elk by real-time quaking-induced conversion. J Clin Microbiol. 2016;54(4):1117–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Spraker TR, Gidlewski T, Powers JG, et al. Progressive accumulation of the abnormal conformer of the prion protein and spongiform encephalopathy in the obex of nonsymptomatic and symptomatic Rocky Mountain elk (Cervus elaphus nelsoni) with chronic wasting disease. J Vet Diagn Invest. 2015;27(4):431–441. [DOI] [PubMed] [Google Scholar]
- [34].Monello RJ, Powers JG, Hobbs NT, et al. Efficacy of antemortem rectal biopsies to diagnose and estimate prevalence of chronic wasting disease in free-ranging cow elk (Cervus elaphus nelsoni). J Wildl Dis. 2013;49(2):270–278. [DOI] [PubMed] [Google Scholar]
- [35].Race BL, Meade-White KD, Ward A, et al. Levels of abnormal prion protein in deer and elk with chronic wasting disease. Emerg Infect Dis. 2007;13(6):824–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Haley NJ, Richt JA. Evolution of diagnostic tests for chronic wasting disease, a naturally occurring prion disease of cervids. Pathogens. 2017;6:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Henderson DM, Denkers ND, Hoover CE, et al. Progression of chronic wasting disease in white-tailed deer analyzed by serial biopsy RT-QuIC and immunohistochemistry. PLoS One. 2020;15(2):e0228327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Peletto S, Perucchini M, Acín C, et al. Genetic variability of the prion protein gene (PRNP) in wild ruminants from Italy and Scotland. J Vet Sci. 2009;10(2):115–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Pitarch JL, Raksa HC, Arnal MC, et al. Low sequence diversity of the prion protein gene (PRNP) in wild deer and goat species from Spain. Vet Res. 2018;49:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Bian J, Christiansen JR, Moreno JA, et al. Primary structural differences at residue 226 of deer and elk PrP dictate selection of distinct CWD prion strains in gene-targeted mice. Proc Natl Acad Sci. 2019;116(25):12478–12487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Haley NJ, Merrett K, Buros Stein A, et al. Estimating relative CWD susceptibility and disease progression in farmed white-tailed deer with rare PRNP alleles. PLoS One. 2019;14(12):e0224342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Pirisinu L, Tran L, Chiappini B, et al. Novel type of chronic wasting disease detected in Moose (Alces alces), Norway. Emerg Infect Dis. 2018. December;24(12):2210–2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Mitchell GB, Sigurdson CJ, O’Rourke KI, et al. Experimental oral transmission of chronic wasting disease to reindeer (Rangifer tarandus tarandus). PLoS One. 2012;7(6):e39055. [DOI] [PMC free article] [PubMed] [Google Scholar]


