Abstract
The aim of this study is to compare the effectiveness of carbon ion radiation therapy (CIRT), proton radiation therapy (PRT), and photon‐based intensity‐modulated radiation therapy (IMRT) in the treatment of sinonasal malignancies. We identified studies through systematic review and divided them into three cohorts (CIRT group/PRT group/IMRT group). Primary outcomes of interest were overall survival (OS) and local control (LC). We pooled the outcomes with meta‐analysis and compared the survival difference among groups using Chi2 (χ 2) test. A representative sample of 2282 patients with sinonasal malignancies (911 in the CIRT group, 599 in the PRT group, and 772 in the IMRT group) from 44 observation studies (7 CIRT, 16 PRT, and 21 IMRT) was included. The pooled 3‐year OS, LC, distant metastasis–free survival, and progression‐free survival rates were 67.0%, 72.8%, 69.4%, and 52.8%, respectively. Through cross‐group analysis, the OS was significantly higher after CIRT (75.1%, 95% CI: 67.1%‐83.2%) than PRT (66.2%, 95% CI: 57.7%‐74.6%; χ 2 = 13.374, P < .0001) or IMRT (63.8%, 95% CI: 55.3%‐72.3%; χ 2 = 23.814, P < .0001). LC was significantly higher after CIRT (80.2%, 95% CI: 73.9%‐86.5%) than PRT (72.9%, 95% CI: 63.7%‐82.0%; χ 2 = 8.955, P = .003) or IMRT (67.8%, 95% CI: 59.4%‐76.2%; χ 2 = 30.955, P < .0001). However, no significant difference between PRT and IMRT for OS and LC was observed. CIRT appeared to provide better OS and LC for patients with malignancies of nasal cavity and paranasal sinuses. A prospective randomized clinical trial is needed to confirm the superiority of CIRT in the treatment of sinonasal tumors.
Keywords: carbon ion radiation therapy, intensity‐modulated radiation therapy, meta‐analysis, proton radiation therapy, sinonasal malignancy, survival
Carbon‐ion radiation therapy achieved higher overall survival and local control rates as compared to both proton radiation therapy and photon based intensity‐modulated radiation therapy through meta‐analysis of 2, 282 patients with sinonasal malignancies from the real world. CIRT appeared to provide better OS and LC for patients with malignancies of nasal cavity and paranasal sinuses.

1. INTRODUCTION
Primary tumors originating from the nasal cavity and paranasal sinuses are rare but represent a heterogeneous group of histologies with substantially diverse biological behaviors. In total, sinonasal malignances (SNM) account for approximately 3%‐5% of cancers in the head and neck region. 1 Due to the inconspicuous anatomic location, SNM are usually asymptomatic at early stages and diagnosed with extensive direct invasion to adjacent vital organs at risk (OARs). Surgery with negative margin is usually not feasible except for a minority of patients with early T‐disease, and a multimodality strategy with surgery and radiotherapy with or without chemotherapy is often needed to achieve long‐term local control (LC) for locally advanced SNM. Radiotherapy is also the mainstay treatment for unresectable and inoperable diseases. Nevertheless, like in surgery, radiation dose is also limited by the presence of critical OARs adjacent to or tethered with the tumor. As such, treatment outcomes historically reported in the literature for unresected SNM have been suboptimal.
The prevailing use of three‐dimensional conformal radiotherapy (3D‐CRT) has shed light to patients with SNM. The 5‐year overall survival (OS) rate improved from less than 30% in the two‐dimensional radiotherapy (2DRT) era to 50%‐60% with the use of 3D‐CRT. 2 , 3 , 4 Nevertheless, whether the more advanced intensity‐modulated radiotherapy (IMRT) could further improve OS is controversial, although it significantly reduces radiation‐induced toxicities. 3 , 5 , 6 , 7
The unique physical properties of accelerated charged particle (such as proton and carbon ion) beams including small lateral scattering and a dose‐focusing Bragg peak followed by a rapid fall‐off thereby promise a more conformal dose distribution than photon‐based IMRT. 8 , 9 The higher linear energy transfer (LET) and greater relative biological effectiveness (RBE) of carbon ion as compared with photon or proton may further improve disease control and OS especially for radio‐resistant histologies such as mucosal melanoma (MM), sarcoma, and adenoid cystic carcinoma (ACC). In 2014, a meta‐analysis 10 that included a few observational studies using proton therapy (10 studies with 218 patients) or heavy‐ion radiotherapy (3 studies with 68 patients) revealed that charged‐particle therapy significantly improved the 5‐year and/or long‐term OS, disease‐free survival (DFS), and potentially LC rates as compared with photon radiotherapy (30 studies including IMRT, 3D‐CRT, 2DRT, or brachytherapy in 1186 patients). However, there were not enough carbon ion studies to compare the survival outcomes sufficiently, and direct comparison among IMRT, carbon ion radiation therapy (CIRT), and proton radiation therapy (PRT) has not been attempted in view of the rarity of SNM and the scarce particle beam radiotherapy facilities. During the past 5 years, the prevailing use of advanced radiotherapy worldwide has made conventional 2DRT and 3D‐CRT obsolete in the treatment of tumors with complex anatomy such as SNM. In addition, the clinical efficacy of CIRT or PRT has been suggested by the results of a few more recently published 11 , 12 , 13 , 14 , 15 , 16 retrospective studies, although many of them had a limited sample size. 15 , 17 , 18 However, it is highly improbable to initiate and complete a randomized clinical trial to confirm superior outcomes of CIRT and PRT over IMRT. Therefore, we conducted this study to estimate the worldwide survival status and compare the treatment outcomes of SNM patients who received CIRT or PRT versus photon‐based IMRT.
2. MATERIALS AND METHODS
2.1. Study design
We conducted a meta‐analysis of SNM patients who received CIRT (including an unpublished online cohort from our institute), PRT, and photon‐based IMRT. In addition, we compared the survival difference among combined cohorts and identified potential prognostic factors of SNM using Chi2 (χ 2) test. The meta‐analysis was performed according to a defined protocol (Appendix S1) and reported adhering to the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses Statement (PRISMA) (Appendix S2). 19 The unpublished online cohort from Shanghai Proton and Heavy Ion Center (SPHIC) has signed a patient consent form and has been approved by the ethics committee of SPHIC. This cohort study has been accepted by the Journal of Cancer Medicine 20 but has not been published online yet. The rest of the cohorts were from published studies online; thus, no patient consent or ethical approval was required.
2.2. Search strategy and selection criteria
We conducted systematic literature searches to identify studies of interest with two search queries in PubMed, Web of Science, and the Cochrane library (last search updated in November 2019). The first search query was for identification of studies with CIRT or PRT in SNM, and the second one was for studies using IMRT. We used the PICO (patient, intervention, comparison, and outcome) framework 21 to structure the search queries in PubMed with MeSH (Medical Subject Headings) terms if possible. The full search strategy is classified and reported in Appendix S3.
We included studies after 1990 that met all of the following criteria: (a) patients with primary SNM (ie, the maxillary, ethmoid, sphenoid, and frontal sinus or the nasal cavity); (b) treatment by radiotherapy technology of IMRT, PRT, or CIRT; (c) reported outcomes of interest including survival, LC, and complications. CIRT has been an emerging radiation technology in the past 10 years. Many patients were treated using CIRT in combination with proton or photon to explore the toxicity, efficacy as well as the optimal dose through dose escalation. Because of the potential additional biologic effects of the beam with higher LET and RBE, patients who received combined CIRT + PRT, CIRT + photon, and CIRT alone were defined as a single group (CIRT group) to be compared with other cohorts. For the same reasons, we grouped patients who received PRT and those who received PRT + photon together as “PRT group.” Patients who received only photon‐based IMRT were defined as “IMRT group” to get relatively accurate estimate rates.
We excluded studies if they met one of the following criteria: (a) review, comment, or other nonoriginal study; (b) sample size less than five patients; (c) patient sample only included lymphoma; (d) survival data of interest not extractable (note 22 : Mohr et al reported inconsistent and illogical survival data in the abstract and different parts of the result section in 2015 and thus were excluded due to unreliable data); (e) median follow‐up time <6 months; (f) overlapped population (note: Dagan et al published two articles 23 , 24 and one poster 25 with overlapped study population between 2016 and 2019; we display their basic information in Table 1 but only included the article published in 2019 and the survival data not reported in the article published in 2019; we dealt with the other three articles published by Zenda et al 26 , 27 , 28 between 2010 and 2014 in the same way). When patients in the study received IMRT and other conventional radiotherapy such as 2DRT or 3D‐CRT, we excluded the study if the survival of the IMRT part was not extractable. We did not limit our study by language, country, or other conditions.
Table 1.
Characteristics of the eligible studies
| Study | Country | No. | Inclusion period | Naive/recurrent no. | Median age (y) | Median follow‐up time (mo) | Pathology (no.) | Survival outcome | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MM | ACC | SCC | ONB | AC | UC | Other | OS | LC | DMFS | PFS/DFS | Year | |||||||
| CIRT | ||||||||||||||||||
| Demizu 2013‐C | Japan | 29 | 2003‐2011 | Naive | 72 (33‐89) | 14.2 (6.3‐28.9) | 29 | – | – | – | – | – | – | 75.0% | 57.1% | 28.6% | – | 1 |
| Koto 2014 | Japan | 22 | 1997‐2010 | 18/4 | 61 (26‐73) | 43 (4‐126) | – | – | – | – | 22 | – | – | 59.1% | 76.9% | – | 45.5% | 3 |
| Ohkubo 2016 | Japan | 5 | 2006‐2015 | Naive | 56 (43‐78) | 27 (9‐91) | 2 | 1 | – | 1 | – | – | 1 | 60.0% | 80.0% | – | – | 2 |
| Koto 2018 | Japan | 458 | 2003‐2014 | 393/65 | 63 (21‐91) | 25.2 (1.4‐132.3) | 221 | 122 | 31 | 30 | 21 | 6 | 27 | 79.6% | 84.1% | – | 52.8% | 2 |
| Toyomasu 2018 | Japan | 59 | 2001‐2012 | Naive | 60 (35‐92) | 30 (8‐127) | – | – | 59 | – | – | – | – | 56.2% | 54.0% | – | 42.9% | 3 |
| Akbaba 2019‐ Cohort1 | Germany | 90 | 2009‐2019 | Naive | 56 (21‐80) | 50 (3‐109) | – | 90 | – | – | – | – | – | 64.0% | 79.0% | 67.0% | – | 3 |
| Akbaba 2019‐ Cohort2 | Germany | 137 | 2009‐2019 | Naive | 53 (17‐77) | 50 (3‐109) | – | 137 | – | – | – | – | – | 79.0% | 82.0% | 74.0% | – | 3 |
| SPHIC‐ unpublished | China | 111 | 2015‐2019 | 76/35 (30re‐RT) | 49 (14‐85) | 18.5 (2.7‐49) | 11 | 39 | 19 | 9 | 3 | 30 | 82.0% | b 80.5% | 85.9% | 66.0% | 2 | |
| PRT | ||||||||||||||||||
| Fitzek 2002 | USA | 19 | 1992‐1998 | Naive | 44 (26‐67) | 45 (20‐92) | – | – | – | 9 | – | – | 10 | 74.0% | 88.0% | – | – | 5 |
| Trong 2009 | USA | 20 | 1991‐2005 | Naive | 53 (17‐78) | 21 | – | 7 | 10 | – | 1 | – | 2 | 53.0% | 86.0% | 50.0% | 31.0% | 2 |
| Okano 2012 | Japan | 13 | 2006‐2012 | Naive | 47 (28‐60) | 56.5 (0.6‐63.5) | – | – | 3 | 7 | 1 | 1 | 1 | 75.5% | 75.5% | – | 33.8% | 5 |
| Fukumitsu 2012 | Japan | 17 | 2001‐2007 | 15/2 | 62 (30‐83) | 23 | – | 2 | 11 | – | 2 | 1 | 1 | 47.1% | 35.0% | – | – | 2 |
| Demizu 2013‐P | Japan | 33 | 2003‐2011 | Naive | 70 (39‐86) | 25.9 (5.2‐82.7) | 33 | – | – | – | – | – | – | 58.0% | 83.0% | – | 30.0% | 2 |
| Herr 2013 | USA | 22 | 1997‐2013 | Naive | 45.5 (11‐77) | 73 (24‐183) | – | – | – | 22 | – | – | – | 95.2% | – | – | 86.4% | 5 |
| Fuji 2014 | Japan | 20 | 2006‐2012 | 11/9 | 74 (55‐81) | 35 (6‐77) | 20 | – | – | – | – | – | – | 54.0% | 62.0% | – | 38.0% | 5 |
| Zenda 2014 | Japan | 90 | 1999‐2008 | Naive | 57 (17‐84) | 57.5 (12.4‐162.7) | 14 | 15 | 22 | 27 | – | – | 12 | 64.2% | – | – | 44.5% | 5 |
| Zenda 2010‐study1 | Japan | 39 | 1999‐2006 | Naive | 57 (22‐84) | 45.4 (1.3‐90.9) | 6 | 5 | 11 | 9 | – | 3 | 5 | 59.3% | – | – | 49.1% | 3 |
| Zenda 2010‐study2 | Japan | 14 | 2004‐2007 | Naive | 73 (56‐79) | 36.7 | 14 | – | – | – | – | – | – | 58.0% | – | – | ‐ | 3 |
| Zenda 2015 | Japan | 32 | 2008‐2012 | Naive | 73 (36‐89) | 36.2 | 32 | – | – | – | – | – | – | 46.1% | – | – | 36.4% | 3 |
| Saito 2015 | Japan | 7 | 1997‐2012 | Naive | 63 (46‐79) | 43 (12‐62) | – | – | 7 | – | – | – | – | 28.6% | 38.1% | – | 14.3% | 4 |
| Lucas 2015 | USA | 8 | 2000‐2013 | 7/1 | 10 (4‐21) | 55.2 (9.6‐112.8) | – | – | – | 8 | – | – | – | 87.5% | 100% | 75.0% | 75.0% | 5 |
| Russo 2016 | USA | 54 | 1991‐2008 | Naive | 56 (18‐82) | 82 (36‐219) | – | – | 54 | – | – | – | – | 47.0% | 80.0% | 78.0% | 48.0% | 5 |
| Nakamura 2016 | Japan | 26 | 2009‐2011 | Naive | 66 (26‐85) | ‐ | 1 | 6 | 15 | – | 1 | 3 | 57.0% | 74.0% | – | 74.0% | 3 | |
| Nakamura 2017‐ Cohort1 | Japan | 5 | 1999‐2012 | Naive | 51 (20‐87) | 69 (7‐186) | – | – | – | 5 | – | – | – | 100% | – | – | 80.0% | 5 |
| Nakamura 2017‐ Cohort2 | 9 | – | – | – | 9 | – | – | – | 86.0% | – | – | 65.0% | 5 | |||||
| Nakamura 2017‐ Cohort3 | 28 | – | – | – | 28 | – | – | – | 76.0% | – | – | 39.0% | 5 | |||||
| Yu 2019‐ Cohort1 | USA | 42 | 2010‐2016 | Naive | 53.1 (15.7‐82.1) | 26.4 (3.5‐220.5) | – | 8 | 15 | 10 | 5 | 2 | 2 | 100% | b 92.9% | 84.0% | 77.3% | 3 |
| Yu 2019‐ Cohort2 | 27 | re‐RT | 57.4 (31.3‐88) | – | 6 | 11 | 4 | 4 | 1 | 1 | 76.2% | b 33.8% | 47.4% | 32.1% | 3 | |||
| Mimica 2019 | USA | 7 | 2000‐2018 | Naive | 66 (38‐86) | 11.6 (3.3‐33.4) | – | – | 7 | – | – | – | – | 100% | 75.0% | 100% | 75.0% | 1 |
| Dagan 2019 | USA | 120 | 2007‐2016 | 113/7 | 58 (21‐82) | 38.4 | – | 20 | 35 | 31 | 9 | 9 | 16 | – | 80.0% | 73.0% | 63.0% | 3 |
| Dagan 2016‐study1 | USA | 24 | ‐ | 22/2 | ‐ | 28.8 (4.8‐80.4) | – | 8 | 9 | 2 | 1 | 1 | 2 | 71.0% | 65.0% | 96.0% | 61.0% | 2 |
| Dagan 2016‐study2 | USA | 84 | 2007‐2013 | 77/7 | 59 (28‐71) | 28.8 (4.8‐86.4) | – | 14 | 22 | 23 | 8 | 7 | 7 | 68.0% | 83.0% | 73.0% | 63.0% | 3 |
| IMRT | ||||||||||||||||||
| Uchida 2004 | Japan | 25 | 1976‐2002 | Naive | 58 (35‐78) | 16.8 (7.2‐171.6) | – | – | 20 | – | – | 5 | – | 34.0% | 48.9% | – | – | 3 |
| Daly 2006 | USA | 36 | 1998‐2004 | Naive | 57 (27‐84) | 39 (6‐82) | – | 5 | 12 | 7 | 5 | 5 | 2 | 45.0% | 58.0% | – | 55.0% | 5 |
| Combs 2007 | Germany | 8 | 1999‐2004 | 2/6 | 44.5 (18‐74) | 27 (12‐71) | 8 | – | – | – | – | – | – | 75.0% | 57.1% | 28.6% | – | 3 |
| Hoppe 2008 | USA | 37 | 2000‐2006 | Naive | 55 (15‐88) | 28 (11‐57) | – | 4 | 17 | 3 | 3 | 3 | 7 | 80.0% | b 75.0% | – | – | 2 |
| Dirix 2009 | Belgium | 40 | 2003‐2008 | Naive | 66 (37‐84) | 30 (4‐74) | – | – | 2 | 2 | 31 | 1 | 4 | 89.0% | 76.0% | 89.0% | 72.0% | 2 |
| Duprez 2011 | Belgium | 130 | 1998‐2009 | 113/17 | 62 (24‐80) | 52 (15‐121) | – | 6 | 23 | 10 | 82 | 8 | 1 | 52.0% | 59.0% | – | 39.0% | 5 |
| Luo 2011 a | China | 52 | 2006‐2008 | Naive | 50 (17‐72) | 32 (6‐48) | 6 | – | 31 | 8 | 19 | ‐ | 20 | 46.7% | – | – | – | 3 |
| Al‐mamgani 2012 a | Netherland | 57 | 1999‐2010 | Naive | 62 (28‐86) | 51 (4‐132) | – | 12 | 40 | – | 10 | 19 | 1 | – | 80.0% | – | – | 5 |
| Wiegner 2012 | USA | 52 | 2000‐2009 | Naive | 63 (11‐87) | 26.6 (2.9‐118.4) | – | 5 | 28 | 7 | 1 | 7 | 4 | 66.0% | b 64.0% | 71.0% | – | 2 |
| Kaur 2013 | USA | 6 | 1995‐2009 | Naive | 56 (40‐63) | 53.4 (12.3‐68.2) | – | – | – | 6 | – | – | – | 80.0% | 100% | – | – | 3 |
| Suh 2015 a | Korea | 19 | 2001‐2012 | Naive | 61 (29‐73) | 34 | – | 4 | 9 | – | – | – | 6 | 75.3% | b 89.2% | 94.7% | – | 3 |
| Burt 2015 | USA | 11 | 1998‐2010 | Naive | 61 (28‐75) | 41 (2‐114) | – | – | 3 | 2 | – | 1 | 5 | – | 72.7% | – | 45.5% | 2 |
| Mukai 2016 | Switzerland | 36 | 2008‐2015 | 26/8 | 66 (34‐94) | 33 (5‐92) | 1 | – | 25 | – | 3 | – | 7 | 88.0% | 91.0% | – | – | 3 |
| Askoxylakis 2016 | Germany | 122 | 1999‐2009 | 82/40 | 56 (21‐79) | 36 (1‐124) | 6 | 47 | 26 | – | 17 | 4 | 22 | 70.0% | 60.0% | – | 57.0% | 3 |
| Chopra 2017 a | USA | 17 | 1998‐2009 | Naive | 31‐88 | 50.9 | – | 6 | 10 | – | 1 | 4 | 2 | 73.0% | – | – | 41.2% | 3 |
| Gamez 2017 a | USA | 24 | 1990‐2014 | Naive | 56.7 (29‐82) | 82.8 (13.2‐204) | – | – | – | – | – | 24 | – | 59.0% | – | – | – | 5 |
| Debonnecaze 2018 a | France | 31 | 2007‐2014 | Naive | 54 (27‐81) | 43 (29‐48) | – | – | – | – | – | 31 | – | – | 73.8% | – | – | 2 |
| Debacker 2018 | Belgium | 20 | 1998‐2016 | Naive | 64.5 (36‐78) | 13.2 (2.5‐128.9) | – | – | – | – | 20 | – | – | 55.0% | – | – | 45.0% | 1 |
| Sas‐Korczvnska 2018 | Poland | 6 | 2008‐2016 | Naive | 65.5 (56‐72) | 20 (8‐60) | 6 | – | – | – | – | – | – | 33.3% | – | – | – | 2 |
| Thierauf 2018 a | Germany | 9 | 1993‐2014 | 8/13 | 63 | 51 (2‐202) | 9 | – | – | – | – | – | – | 74.2% | – | – | – | 4 |
| Ferella 2019 | Italy | 34 | 2007‐2015 | Naive | 53 (34‐81) | 73 | – | – | 16 | – | – | 13 | 5 | 42.9% | b 33.3% | 29.4% | 37.3% | 5 |
Abbreviations: AC, adenocarcinoma; ACC, adenoid cystic carcinoma; CIRT, carbon ion radiation therapy; DFS, disease‐free survival; DMFS, distant‐metastasis–free survival; IMRT, intensity‐modulated radiation therapy; LC, local control; MM, mucosal melanoma; ONB, olfactory neuroblastoma; OS, overall survival; PFS, progress‐free survival; PRT, proton radiation therapy; Re‐RT, reirradiation radiotherapy; SCC, squamous cell carcinoma; SPHIC, Shanghai Proton and Heavy Ion Center; UC, undifferentiated carcinoma.
The sample size of these studies we displayed were those who received IMRT.
Local‐regional control survival.
2.3. Data collection
Data of eligible studies were extracted by two independent reviewers, and in case of discrepancies, consensus was reached involving a third reviewer. We recorded general characteristics: the first author, year of publication, radiotherapy type, country, study period, sample size, follow‐up time, treatment phase, treatment strategy, radiation dose, age, sex, lymph node status, T4, histological type, tumor location, grade 3‐5 adverse event rate (AER) and outcomes of interest. Primary outcomes of interest were defined as OS and LC. Secondary outcomes of interest were defined as distant metastasis–free survival (DMFS) and progression‐free survival (PFS). We also divided and reported the histological types in detail: squamous cell carcinoma (SCC), adenocarcinoma (AC), MM, ACC, undifferentiated carcinoma (UC), mucoepidermoid carcinoma (MEC), olfactory neuroblastoma/esthesioneuroblastoma (ONB), neuroendocrine carcinoma (NEC), and other patterns except lymphoma. Tumor locations were displayed as follows: nasal cavity, maxillary sinus, ethmoid sinus, sphenoid sinus, frontal sinus, and other or mixed locations.
2.4. Data analysis
We performed the meta‐analysis with STATA 15.0 (Stata Corp). Survival rate available or calculated through individual data with Statistical Package for the Social Science version 19.0 (SPSS) were pooled with a random‐effects model. We calculated the mean follow‐up time, mean age, and variance according to the methods detailed by Hozo et al. 29 We also did meta‐analysis of the follow‐up time, toxicities, and age with a random‐effects model. Heterogeneity across cohorts was evaluated by χ 2 test and expressed as I 2 statistic (<50% indicating obvious heterogeneity). In addition, we performed subgroup analysis and metaregression to test the heterogeneity. Potential publication bias was inspected with the symmetry of a funnel plot. Sensitivity analysis was performed to detect the reliability and stability of our results. We compared the survival difference among cohorts using χ 2 test. P value <.05 was defined as statistically significant and <.01 when doing repeated analysis.
3. RESULTS
3.1. Eligible studies
Forty‐four (44) eligible studies (7 carbon ion, 11 , 12 , 13 , 14 , 15 , 16 16 proton, 17 , 41 and 21 IMRT 5 , 6 , 7 , 59 ) including the unpublished online data from our hospital 20 were identified from the database (see Figure 1). A representative sample of 2282 real‐world patients from 49 cohorts (8 CIRT cohorts, 20 PRT cohorts, and 21 IMRT cohorts) was included. Characteristics of the included studies are shown in Table 1.
FIGURE 1.
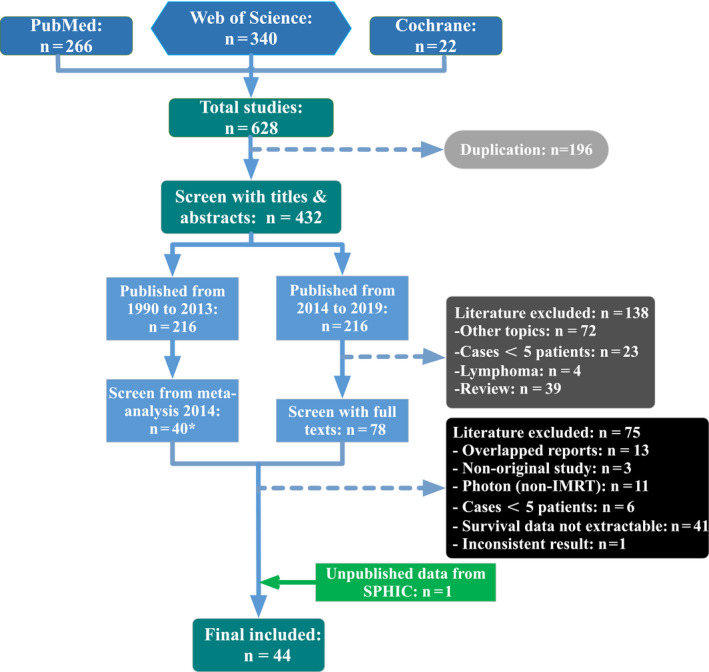
Flow chart. *One study published before 1990 which met the exclusion criteria was excluded and meta‐analysis 2014 referred to the article published in lancet oncology in 2014 by Patel, S. H. et al
We pooled baseline information from the eligible studies and displayed it in Table 2. Overall, there were 911 patients in the CIRT group, 599 patients in the PRT group, and 772 patients in the IMRT group. The ratios of male patients in the CIRT, PRT, and IMRT group were 50.3%, 56.4%, and 63.1%, respectively. The mean age for the CIRT group was 58.1 (range 53.4‐62.9) years, for the PRT group 54.8 (range 48.4‐61.2) years, and for the IMRT group 58.2 (range 55.7‐60.7) years. Most patients presented with T4 and N0 disease. The majority of the patients were treatment‐naive, but there were 104 (11.4%), 46 (7.7%), and 84 (10.9%) recurrent patients in each group, respectively. We also calculated the distribution of different histological types and primary sites. In the CIRT group, MM (28.9%) and ACC (42.7%) were the main constituents. In the PRT group, the top three pathological types were SCC (31.7%), ONB (26.7%), and MM (16.7%). SCC (31.3%), AC (23.0%), UC (15.0%), and ACC (10.6%) covered two‐third of the IMRT group.
Table 2.
Pooled baseline information of the eligible studies
| CIRT group | PRT group | IMRT group | |
|---|---|---|---|
| Cohorts (n) | 8 | 20 | 21 |
| Total patients (n) | 911 | 599 | 772 a |
| Sex (n, %) | |||
| Male | 458 (50.3%) | 338 (56.4%) | 559 (63.1%) b |
| Female | 453 (49.7%) | 261 (43.6%) | 327 (36.9%) b |
| Mean age (range) (year) | 58.1 (53.4‐62.9) | 54.8 (48.4‐61.2) | 58.2 (55.7‐60.7) |
| T4 (n, %) | 625 (68.6%) | 309 (51.6%) | 538 (60.7%) b |
| N status (n, %) | |||
| N0 | 822 (90.2%) | 370 (61.8%) | 572 (64.6%) b |
| N+ | 54 (5.9%) | 85 (14.2%) | 72 (8.1%) b |
| NA | 35 (3.9%) | 144 (24.0%) | 242 (27.3%) b |
| Treatment status (n, %) | |||
| Naive | 807 (88.6%) | 553 (92.3%) | 688 (89.1%) |
| Recurrent | 104 (11.4%) | 46 (7.7%) | 84 (10.9%) |
| Dose range (cGy/GyE) | 57.6‐80.0 | 12.0‐89.6 | 50.4‐79.0 |
| Pathology (n, %) | |||
| MM | 263 (28.9%) | 100 (16.7%) | 36 (4.3%) |
| ACC | 389 (42.7%) | 64 (10.7%) | 89 (10.6%) |
| SCC | 109 (12.0%) | 190 (31.7%) | 262 (31.3%) |
| NEC | 1 (0.1%) | 22 (3.7%) | 13 (1.6%) |
| ONB | 40 (4.4%) | 160 (26.7%) | 45 (5.4%) |
| AC | 46 (5.0%) | 23 (3.8%) | 192 (23.0%) |
| UC | 6 (0.6%) | 14 (2.4%) | 125 (15.0%) |
| MEC | 6 (0.6%) | 5 (0.8%) | 5 (0.6%) |
| Sarcoma | 24 (2.6%) | 0 | 23 (2.8%) |
| NA | 27 (3.0%) | 21 (3.5%) | 45 (5.4%) |
| Total | 911 (100%) | 599 (100%) | 835 (100%) |
| Primary site (n, %) | |||
| Nasal cavity | 299 (32.8%) | 186 (31.1%) | 116 (15.0%) |
| Maxillary sinus | 270 (29.6%) | 108 (18.0%) | 85 (11.0%) |
| Ethmoid sinus | 108 (11.9%) | 50 (8.3%) | 190 (24.6%) |
| Sphenoid sinus | 31 (3.4%) | 20 (3.3%) | 15 (2.0%) |
| Frontal sinus | 9 (1.0%) | 1 (0.2%) | 1 (0.1%) |
| Mixed sites | 81 (8.9%) | 96 (16.0%) | 22 (2.9%) |
| NA | 113 (12.4%) | 138 (23.1%) | 343 (44.4%) |
Abbreviations: AC, adenocarcinoma; ACC, adenoid cystic carcinoma; CIRT, carbon ion radiation therapy; IMRT, intensity‐modulated radiation therapy; MEC, Mucoepidermoid carcinoma; MM, mucosal melanoma; NA, not available; NEC, neuroendocrine carcinoma; ONB, olfactory neuroblastoma; PRT, proton radiation therapy; SCC, squamous cell carcinoma; UC, undifferentiated carcinoma.
This number only included patients who received IMRT.
The total sample size was 886.
3.2. Data synthesis and comparison
We calculated the mean follow‐up time of different survival outcomes and compared the pooled survival (Table 3). For all the cohorts, the mean follow‐up time was 36.8 (range 32.5‐41.0) months for OS, 37.3 (range 32.1‐42.5) months for LC, 38.9 (range 27.5‐50.4) months for DMFS, and 40.4 (range 34.0‐46.8) months for PFS. The pooled 3‐year OS was 67% (95% CI: 62.0%‐71.9%), 3‐year LC was 72.8% (95% CI: 68.0%‐77.5%), 3‐year DMFS was 69.4% (95% CI: 60.8%‐78.0%), and 3‐year PFS was 52.8% (95% CI: 47.1%‐58.5%) for all the SNM patients included, regardless of radiotherapy technology or histological types. The corresponding funnel plots of OS and LC are shown in Figure S1. We did not perform further quantitative tests such as Begg's test or Egger's test in view of the very high heterogeneity (I 2 > 80%).
Table 3.
Summary of meta‐analysis results
| Outcome | Cohorts no. | Sample size | Mean follow‐up time (mo) | Survival, 95% CI | I 2, % | χ 2 (comparison) | P value* |
|---|---|---|---|---|---|---|---|
| OS | |||||||
| CIRT | 8 | 911 | 34.6 (24.4‐44.8) | 75.1% (67.1%‐83.2%) | 85.4% | 13.374 (CIRT vs PRT) | <.0001 |
| PRT | 20 | 563 | 41.6 (35.5‐47.7) | 66.2% (57.7%‐74.6%) | 77.8% | 0.846 (PRT vs IMRT) | .358 |
| IMRT | 18 | 673 | 39.2 (31.1‐47.4) | 63.8% (55.3%‐72.3%) | 81.9% | 23.814 (CIRT vs IMRT) | <.0001 |
| Total | 46 | 2183 | 36.8 (32.5‐41.0) | 67.0% (62.0%‐71.9%) | 83.6% | 26.489 (among groups) | <.0001 |
| LC | |||||||
| CIRT | 8 | 911 | 34.6 (24.4‐44.8) | 80.2% (73.9%‐86.5%) | 77.4% | 8.955 (CIRT vs PRT) | .003 |
| PRT | 14 | 413 | 36.0 (29.8‐42.2) | 72.9% (63.7%‐82.0%) | 80.1% | 3.014 (PRT vs IMRT) | .083 |
| IMRT | 15 | 644 | 39.7 (29.9‐49.5) | 67.8% (59.4%‐76.2%) | 81.9% | 30.955 (CIRT vs IMRT) | <.0001 |
| Total | 37 | 1968 | 37.3 (32.1‐42.5) | 72.8% (68.0%‐77.5%) | 83.3% | 31.432 (among groups) | <.0001 |
| DMFS | |||||||
| CIRT | 3 | 338 | 39.5 (15.3‐63.6) | 76.1% (65.2%‐86.9%) | 83.0% | 5.379 (CIRT vs PRT) | .02 |
| PRT | 7 | 278 | 37.0 (23.9‐50.1) | 67.6% (56.2%‐79.1%) | 73.5% | 0.378 (PRT vs IMRT) | .539 |
| IMRT | 5 | 153 | 41.0 (13.0‐69.0) | 64.7% (40.9%‐88.4%) | 93.8% | 6.782 (CIRT vs IMRT) | .009 |
| Total | 15 | 769 | 38.9 (27.5‐50.4) | 69.4% (60.8%‐78.0%) | 87.2% | 8.573 (Among groups) | .014 |
| PFS a | |||||||
| CIRT | 5 | 679 | 25.1 (19.2‐30.9) | 54.8% (46.3%‐63.2%) | 66.5% | 0.163 (CIRT vs PRT) | .687 |
| PRT | 18 | 563 | 42.6 (36.0‐49.1) | 53.7% (43.7%‐63.8%) | 84.0% | 1.435 (PRT vs IMRT) | .231 |
| IMRT | 8 | 410 | 43.3 (29.1‐57.6) | 49.8% (40.4%‐59.2%) | 68.4% | 2.596 (CIRT vs IMRT) | .107 |
| Total | 31 | 1652 | 40.4 (34.0‐46.8) | 52.8% (47.1%‐58.5%) | 79.5% | 2.681 (Among groups) | .262 |
Abbreviations: CI, confidence interval; CIRT, carbon ion radiation therapy; DMFS, distant‐metastasis–free survival; IMRT, intensity‐modulated radiation therapy; LC, local control; OS, overall survival; PFS, progress‐free survival; PRT, proton radiation therapy.
We pooled PFS and disease‐free survival together as PFS.
P < .05 has statistical significance for comparison among groups and P < .01 has statistical significance for repeating analysis between groups.
All the eight cohorts in the CIRT group reported OS and LC, but only three cohorts reported DMFS, and five cohorts reported PFS. The mean follow‐up time was 34.6 (range 24.4‐44.8) months for OS and LC, 39.5 (range 15.3‐63.6) months for DMFS and 25.1 (range 19.2‐30.9) months for PFS. The pooled 3‐year OS was 75.1% (95% CI: 67.1%‐83.2%), 3‐year LC was 80.2% (95% CI: 73.9%‐86.5%), 3‐year DMFS was 76.1% (95% CI: 65.2%‐86.9%), and 2‐year PFS was 54.8% (95% CI: 46.3%‐63.2%) for SNM patients who received CIRT. The forest plots of OS and LC for the CIRT group are displayed in Figure 2.
FIGURE 2.
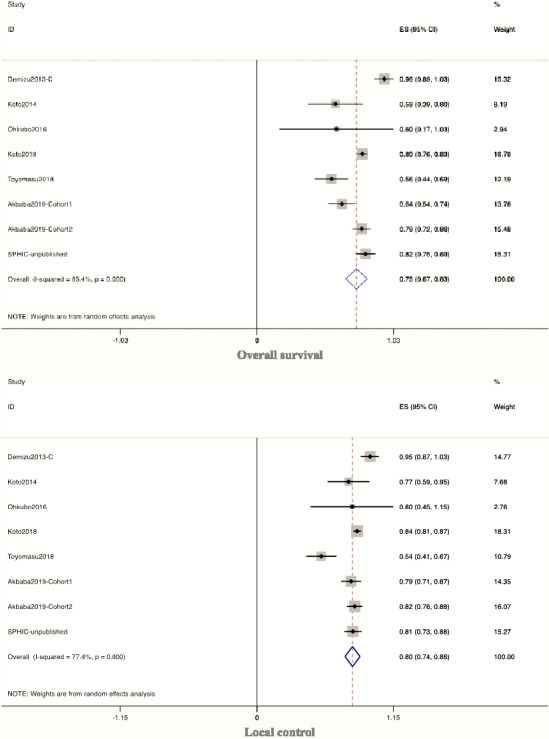
Forest plots of overall survival and local control for the CIRT group. CIRT, carbon ion radiation therapy; IMRT, intensity‐modulated radiotherapy; SPHIC, Shanghai Proton and Heavy Ion Center
All the 20 cohorts in the PRT group reported OS, and 14 cohorts reported LC. There were 7 cohorts that reported DMFS, and 18 cohorts reported PFS. The mean follow‐up time was 41.6 (range 35.5‐47.7) months for OS, 36.0 (range 29.8‐42.2) months for LC, 37.0 (range 23.9‐50.1) months for DMFS, and 42.6 (range 36.0‐49.1) months for PFS. The pooled 3‐year OS was 66.2% (95% CI: 57.7%‐74.6%), 3‐year LC was 72.9% (95% CI: 63.7%‐82.0%), 3‐year DMFS was 67.6% (95% CI: 56.2%‐79.1%), and 3‐year PFS was 53.7% (95% CI: 43.7%‐63.8%) for SNM patients who received PRT.
There were 18 cohorts of the IMRT group that reported OS, and 15 cohorts reported LC. The mean follow‐up time was 39.2 (range 31.1‐47.4) months for OS and 39.7 (range 29.9‐49.5) months for LC. The pooled 3‐year OS was 63.8% (95% CI: 55.3%‐72.3%), and 3‐year LC was 67.8% (95% CI: 59.4%‐76.2%) for SNM patients receiving photon‐based IMRT.
Through cross‐group analysis, the OS was significantly higher for CIRT than for PRT (χ 2 = 13.374, P < .0001) or IMRT (χ 2 = 23.814, P < .0001), but there was no significant difference between PRT and IMRT (χ 2 = 0.846, P = .358). LC was significantly higher for CIRT than for PRT (χ 2 = 8.955, P = .003) or IMRT (χ 2 = 30.955, P < .0001), but there was no significant difference between PRT and IMRT (χ 2 = 3.014, P = .083). There was improved DMFS for CIRT compared with IMRT (χ 2 = 6.782, P = .009), but there was no significant difference between CIRT and PRT or between PRT and IMRT. There was also no significant difference among the groups for PFS.
3.3. Subgroup analysis
We also performed a subgroup analysis based on the following aspects: treatment‐naive or recurrent, reirradiated, surgically resected or not, chemotherapy or not, pathological types, primary sites, T4, and N0 if possible (Table 4). Compared with different subgroups in the table, the pooled OS (χ 2 = 14.028, P < .0001) and LC (χ 2 = 10.14, P = .001) rates of the studies in which all patients underwent surgical resection (regardless of extent) appeared to be better than those without surgery. Similarly, the pooled LC rate of the studies in which all patients received chemotherapy was better than those without chemotherapy (χ 2 = 5.688, P = .017), although there was no significant difference for OS (χ 2 = 0.614, P = .433). Moreover, ACC and ONB patients had relatively good prognosis. The 3‐year OS for ACC (mean follow‐up time: 34.9 months) was 83.3%, and 4‐year OS for ONB (mean follow‐up time: 48.1 months) was 88.6%. The 3‐year LC for ACC and ONB was 84.1% and 90.3%, respectively. Though the 2‐year OS for MM was 66.6%, the 2‐year LC could be as good as 81.4%. SCC patients had both poor OS and poor LC. As for the primary site, tumors originating from the nasal cavity and maxillary sinus had relatively good 3‐year OS (nasal cavity: 79.3%; maxillary sinus: 73.2%) and 3‐year LC (nasal cavity: 86.1%; maxillary sinus: 81.7%). However, tumors originating from the ethmoid sinus may have relatively poor prognosis. Although we could not collect the entire T‐ and N‐category–related survival, we extracted and pooled the survival of T4 and N0 subgroup patients, who covered 51.6%‐90.2% of the entire population. The 3‐year OS and LC of T4 patients was 60.3% and 65.2%, respectively. The 3‐year OS and LC of N0 patients was 67.0% and 78.1%, respectively. For the recurrent patients, the 2‐year OS (n = 132) was 71.2% and the 2‐year LC (n = 92) was 66.5%. When treated with reirradiation (n = 57), the OS was 77.9%, and the LC was 55.2% with a mean follow‐up time of 18.6 (range 16.0‐21.4) months.
Table 4.
Summary of subgroup analysis results
| Outcome/subgroup | CIRT cohort no. | PRT cohort no. | IMRT cohort no. | Total cohort no. | Sample size | Mean follow‐up time (mo) | Survival, 95% CI | I 2, % |
|---|---|---|---|---|---|---|---|---|
| OS | ||||||||
| Naive | 6 | 15 | 14 | 35 | 1570 | 40.3 (34.7‐45.9) | 65.9% (59.9%‐75.4%) | 85.2% |
| Recurrent | 1 | 1 | 1 | 3 | 132 | 29.9 (21.2‐38.6) | 71.2% (52.4%‐89.9%) | 83.0% |
| Reirradiation | 1 | 1 | 0 | 2 | 57 | 18.6 (16.0‐21.4) | 77.9% (67.1%‐88.6%) | 0% |
| Post operation | 2 | 2 | 9 | 13 | 426 | 38.6 (28.8‐48.4) | 72.1% (62.2%‐82.1%) | 83.7% |
| Without operation | 6 | 11 | 4 | 21 | 938 | 40.4 (32.3‐48.5) | 61.6% (53.7%‐69.5%) | 79.4% |
| Received chemo. | 0 | 5 | 0 | 5 | 73 | 36.4 (21.3‐51.6) | 72.4% (59.8%‐85.0%) | 29.7% |
| Without chemo. | 1 | 0 | 2 | 3 | 119 | 29.8 (25.6‐34.0) | 67.6% (42.6%‐92.6%) | 89.8% |
| T4 | 2 | 4 | 3 | 9 | 507 | 36.6 (21.8‐51.3) | 60.3% (48.4%‐72.3%) | 83.0% |
| N0 | 4 | 5 | 4 | 13 | 789 | 35.5 (29.4‐41.8) | 67.0% (58.0%‐76.0%) | 82.5% |
| MM | 3 | 4 | 3 | 10 | 383 | 26.3 (19.0‐33.5) | 66.6% (53.7%‐79.4%) | 84.9% |
| SCC | 3 | 5 | 3 | 11 | 277 | 34.3 (26.2‐42.4) | 56.8% (46.6%‐66.9%) | 66.4% |
| ACC | 4 | 0 | 2 | 6 | 440 | 34.9 (21.1‐48.6) | 83.3% (71.9%‐94.6%) | 93.5% |
| ONB | 2 | 6 | 2 | 10 | 143 | 48.1 (34.0‐62.1) | 88.6% (81.2%‐96.1%) | 43.6% |
| UC | 1 | 0 | 3 | 4 | 42 | 37.3 (17.6‐53.4) | 64.4% (50.2%‐78.6%) | 0% |
| AC | 2 | 0 | 1 | 3 | 63 | 35.4 (21.3‐51.6) | 69.2% (45.1%‐93.2%) | 80.8% |
| Nasal cavity | 1 | 1 | 1 | 3 | 325 | 36.8 (22.9‐50.7) | 79.3% (70.6%‐87.9%) | 54.4% |
| Ethmoid sinus | 1 | 1 | 1 | 3 | 103 | 31.9 (22.6‐41.3) | 51.6% (7.9%‐95.4%) | 94.4% |
| Maxillary sinus | 1 | 1 | 1 | 3 | 154 | 31.4 (22.3‐40.4) | 73.2% (58.6%‐87.8%) | 65.2% |
| LC | ||||||||
| Naive | 6 | 10 | 11 | 27 | 1314 | 36.9 (30.6‐43.2) | 75.4% (70.4%‐80.4%) | 77.1% |
| Recurrent | 1 | 1 | 0 | 2 | 92 | 25.3 (20.1‐30.4) | 66.5% (3.7%‐129.3%) | 97.9% |
| Reirradiation | 1 | 1 | 0 | 2 | 57 | 18.6 (16.0‐21.4) | 55.2% (13.8%‐96.5%) | 91.9% |
| Post operation | 2 | 1 | 7 | 10 | 351 | 36.1 (25.1‐47.1) | 77.0% (68.6%‐82.1%) | 83.7% |
| Without operation | 6 | 7 | 4 | 17 | 874 | 37.1 (28.4‐45.9) | 67.7% (68.6%‐82.1%) | 82.0% |
| Received chemo. | 0 | 5 | 0 | 5 | 73 | 36.4 (21.3‐51.6) | 80.3% (70.8%‐89.8%) | 0% |
| Without chemo. | 1 | 0 | 2 | 3 | 119 | 30.0 (25.6‐34.4) | 64.9% (43.4%‐86.5%) | 81.9% |
| T4 | 2 | 3 | 1 | 6 | 443 | 37.7 (18.9‐56.6) | 65.2% (48.7%‐81.7%) | 88.8% |
| N0 | 4 | 3 | 3 | 10 | 730 | 34.6 (28.3‐41.0) | 78.1% (71.7%‐84.6%) | 62.2% |
| MM | 3 | 3 | 1 | 7 | 354 | 26.1 (18.4‐33.7) | 81.4% (73.8%‐88.9%) | 58.2% |
| SCC | 3 | 4 | 1 | 8 | 220 | 32.6 (22.4‐42.7) | 61.9% (49.0%‐74.7%) | 74.9% |
| ACC | 4 | 0 | 1 | 5 | 393 | 34.6 (18.6‐50.6) | 84.1% (80.1%‐88.1%) | 14.9% |
| ONB | 2 | 2 | 2 | 6 | 79 | 35.3 (21.5‐49.1) | 90.3% (83.2%‐97.5%) | 0% |
| UC | 1 | 0 | 2 | 3 | 44 | 24.0 (22.4‐25.7) | 73.5% (60.6%‐86.4%) | 0% |
| AC | 2 | 0 | 0 | 2 | 43 | 39.2 (11.0‐67.5) | 77.4% (64.9%‐89.9%) | 0% |
| Nasal cavity | 1 | 0 | 1 | 2 | 299 | 32.7 (17.3‐48.2) | 86.1% (79.1%‐93.1%) | 50.2% |
| Ethmoid sinus | 1 | 1 | 1 | 3 | 103 | 31.9 (22.6‐41.3) | 61.1% (26.9%‐95.4%) | 89.9% |
| Maxillary sinus | 1 | 1 | 1 | 3 | 154 | 31.4 (22.3‐40.4) | 81.7% (75.6%‐87.8%) | 0% |
Abbreviations: AC, adenocarcinoma; ACC, adenoid cystic carcinoma; Chemo., Chemotherapy; CI, confidence interval; CIRT, carbon ion radiation therapy; IMRT, intensity‐modulated radiation therapy; LC, local control; MM, mucosal melanoma; ONB, olfactory neuroblastoma; OS, overall survival; PRT, proton radiation therapy; SCC, squamous cell carcinoma; UC, undifferentiated carcinoma.
3.4. Metaregression
We performed a metaregression analysis of the overall OS and LC based on the sample size, year of publication, and technology of radiotherapy, respectively. We found that the sample size and year of publication had no obvious effect on the heterogeneity of the OS or LC. Although the technology of radiotherapy had no significant relationship with the heterogeneity of OS (P = .16) or LC (P = .106) either, it might contribute to 4.84% and 7.7% heterogeneity, respectively. We display the metaregression results of the OS based on the year of publication and radiotherapy technology in Figure S2.
3.5. Sensitivity analysis
We performed a sensitivity analysis of the overall OS (46 cohorts), LC (37 cohorts), and DMFS (15 cohorts) including the cohorts with 100% survival (Figure S3). The combined survival rates were 67.0% (95% CI: 62.0%‐71.9%), 72.8% (95% CI: 68.0%‐77.5%), and 69.4% (95% CI: 60.8%‐78.0%) for the OS, LC, and DMFS, respectively. The results were in concordance with our previous data listed in Table 3. When taking out of each cohort from the population to analyze the rest cohorts, the remaining studies had similar combined results of OS, LC, and DMFS (Appendix S4). As such, we considered the results in this study reliable and stable.
3.6. Toxicities
We recorded the occurrence of grade 3‐5 adverse effects and calculated the corresponding AER. Seven of the eight CIRT cohorts covering 99.4% of the CIRT‐treated population reported acute grade 3‐5 AER. Only one case series of five patients did not report AER. 12 The rates were substantially lower in the PRT group (11/20 cohorts, 45.6% of the population) and the IMRT group (12/21 cohorts, 75.8% of the population). The overall acute AER of the CIRT group was 31.1% (95% CI: 22.9%‐39.3%), which was higher than the IMRT group (23.9%, 95% CI: 10.8%‐36.9%). In the CIRT group, the percentages of the severe acute AER (ie, grade 3‐5) ranged from 0% to 41.6%, including three cohorts 14 , 16 reporting > 40% severe acute AER. Two 16 of these three cohorts divided from one article studied IMRT followed‐by CIRT boost to treat patients, and another cohort 14 focused on malignant melanoma originated from the nasal cavity treated with CIRT alone. The PRT group had the slightest acute toxic reaction (14.4%, 95% CI: 10.0%‐18.7%) with reported percentages ranging from 0% to 40%. The reported severe acute AER of the IMRT group ranged from 0% to 52% across different cohorts. The late toxic reactions were similar among the three groups, ranging from 10.8% to 13.4%.
4. DISCUSSION
Our study of 2282 patients from the real world revealed that CIRT might be the optimal radiotherapy technology for malignancies of the nasal cavity and paranasal sinus. This was the first direct and effective comparison among CIRT, PRT, and photon‐based IMRT with sufficient available population. Our analysis indicated improved 3‐year OS and LC rates for patients who received CIRT as compared with those treated with PRT or IMRT. CIRT might also have improved effects on DMFS, which warrants further investigation for its mechanism.
The efficacy of particle beam radiotherapy (PBRT) versus photon‐based radiotherapy has been previously addressed in a few retrospective studies and a well‐conducted meta‐analysis published in 2014. 10 However, the technology of radiotherapy used in many of the historical studies was conventional (eg, 2DRT and 3D‐CRT). Its results 10 suggested an advantage of PBRT over conventional photon radiotherapy (including 2DRT, 3D‐CRT, and IMRT), and the subgroup analysis indicated PRT (n = 191) had higher DFS at 5 years and locoregional control at longest follow‐up than IMRT. However, the insufficient number of studies on CIRT limited further subgroup analysis that compared different PBRT technologies. In the past 5 years, several publications using more advanced and precision radiation technologies such as IMRT and PBRT have been published for SNM owing to the constantly updated radiotherapy equipment worldwide. In addition, IMRT has replaced 3D‐CRT and 2DRT and became a standard technology of radiotherapy for SNM. After a stringent screening, seven studies on CIRT published after 2014 including 911 patients with SNM (along with 111 unpublished online cases from the authors' own institute) were analyzed 11 , 12 , 13 , 14 , 15 , 16 , 20 . Moreover, 12 studies on PRT (with 508 patients) and 11 studies on photon‐based IMRT (with 329 patients) that have been published since 2014 were accrued to our analysis. 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 28 , 30 , 31 , 32 , 33 , 34 , 35 , 36 With this surge of publications on CIRT and PRT in the management of SNM in the past several years, we consider that an update that directly compares the effectiveness of CIRT, PRT, and photon‐based IMRT is feasible and necessary.
In our study, we combined the reported year of survival adjacent to the median follow‐up time of each included study and got the estimated month of pooled survival through meta‐analysis of the median follow‐up time and its variance instead of extracting outcomes at 5 years and at the longest follow‐up as performed in the previously published meta‐analysis. Through efficient analysis, we concluded that there were no significant differences between PRT and IMRT in OS, LC, DMFS, and PFS rates at 3 years but demonstrated the superiority of CIRT over both PRT and X‐ray–based IMRT in detail. Most patients in our study were treatment‐naive and presented with T4 or N0 disease. The overall 3‐year OS, LC, DMFS, and PFS of SNM patients were 67%, 72.8%, 69.4%, and 52.8%, respectively. When treated with CIRT, the treatment‐naive patients could achieve a 3‐year OS of 75% and a 3‐year LC of 79.6%. We also found that patients who received surgery followed by adjuvant radiotherapy appeared to have better OS and LC than those who had radiotherapy alone. However, as the majority of patients presented with inoperable T4 disease with extensive invasion, the data set is skewed, and the seemingly improved outcome of combined modality over radiotherapy alone is most likely due to the favorable presenting stage of the disease. Nevertheless, our finding may explain, at least in part, why patients with malignancies of nasal cavity had better prognosis than those with ethmoid sinus, as surgery is more feasible for nasal cavity tumors.
The more commonly diagnosed histological types of SNM are of epithelial origin and include SCC (51.6%), AC (12.6%), ONB (6.3%), ACC (6.2%), MM (6.6%), and UC (3.1%). 10 Soft‐tissue and bone sarcomas of the head and neck commonly originate from the nasal cavity as well as paranasal sinuses. Due to their inconspicuous anatomic location, SNM are usually asymptomatic at early stages and diagnosed with extensive direct invasion to adjacent vital OARs such as skull base, orbit, optic nerve/chiasm, brain, and/or brain stem. This reduces the opportunity of surgery and increases the difficulty of radiation dose distribution sufficient for disease control. Thus, the most common treatment failure pattern of SNM is local recurrence. The characteristics of carbon ion beams such as higher LET and greater RBE as well as their precise dosimetric distribution provide advantages for treating resistant histologies close to critical OARs. In the current analysis, patients with ACC and ONB achieved relatively good prognosis in terms of disease control. However, more acute grade 3‐5 toxicities were observed after CIRT treatment (31.1%) in the literature as compared with proton and photon therapy. This finding is highly different from what we had observed from our patients (0%). 20 Several cohorts exhibited particularly high probability of acute severe toxicity in our study. The following four reasons could be considered for such findings. First, seven out of eight studies on CIRT reported acute adverse effects. However, the studies on PRT and IMRT reported acute adverse effects for only 45.6% and 75.8%, respectively. Thus, the pooled rates of acute adverse effects from the PRT and IMRT groups could be underestimated and inaccurate. Second, the high rates of acute adverse events reported by Akbaba et al could be partly attributed to the high radiotherapy dose delivered. 16 Patients in that study received combined photon‐based IMRT (48‐56 Gy in 1.8 or 2 Gy fractions) followed by carbon ion radiation boost (18‐24 Gy RBE in 3 Gy RBE fractions), with or without surgery, to a median combined dose of 80 Gy (range 71‐80 Gy), whereas most photon‐based IMRT cases reported in other studies used 70 Gy or less. Third, the use of surgery may complicate the analysis of adverse effects. For patients who received complete resection (eg, maxillectomy) before radiation, oral mucosa might be easily excluded from high‐dose radiation field. On the other hand, surgery may affect the blood supply to the surgical bed which is usually encompassed by adjuvant radiation. In the study reported by Akbaba et al, 16 the acute and late grade 3‐5 reactions were 34.4% and 6.2% after CIRT, and 41.6% and 17.2% after surgery followed by adjuvant CIRT. Fourth, most of the CIRT patients received treatment in Japan, and the biological model used for dose calculation (ie, the micro kinetic model [MKM]) is different from that used in Germany and our institution (ie, the local effect model [LEM]). The daily per fraction equivalent dose could be higher after conversion for patients treated using MKM. 60 , 61 In addition, hypofractioned CIRT is commonly practiced in Japan, and all patients treated at the Heidelberg Ion Therapy Center and our institute used 3.0 Gy (RBE) per fraction.
The present study has some limitations. First and most importantly, all the eligible studies were observation studies and 10 of them had less than 10 patients. Some variables and survival data might not be available or accurate. This may influence the reliability of our results to a limited extent. However, given the limited number of publications on the subject, these studies were of the highest quality available in the most updated literature search. With the prevailing application of proton and carbon ion radiotherapy worldwide, it is important to update the knowledge with the most recent available data to guide the clinical practice and decisions on public health endeavors. Second, three studies 17 , 18 , 36 that reported 100% survival rate in the PRT group (2 for OS, 1 for LC, and 1 for DMFS) and one study 59 that reported 100% LC rate in the IMRT group were excluded when pooled survival rate was calculated in Stata. However, these studies had quite small sample size and the influence could be ignored to some extent. We also performed sensitivity analysis including these four studies, which resulted in similar findings, as displayed in Appendix S4. Third, the results could have selection bias. SNM consists of many kinds of histological types and each radiotherapy group had a different pathological constituent ratio. However, the proportion of patients with high‐risk pathologies such as MM in the CIRT group was much higher than in the PRT or IMRT group, which in turn confirmed the better tumor control and survival in the CIRT group. Fourth, the between‐study heterogeneity should not be neglected. We performed subgroup analysis to test the heterogeneity and listed the results in detail. We also performed metaregression and sensitivity analysis to detect the heterogeneity and its influence on the results. However, metaregression, sample size, year of publication, and radiotherapy technology were not the main reasons for heterogeneity. In stead, the different histological types and primary sites should be considered. After sensitivity analysis, the heterogeneity among cohorts did not affect the stability and reliability of the results.
Despite the pitfalls mentioned above, the study was conducted to evaluate the effectiveness of conventional and novel radiation technologies head to head at the appropriate time. Our study firstly highlights promising treatment outcomes for SNM patients by CIRT in contrast to both PRT and photon‐based IMRT. A prospective clinical trial is being evaluated at the SPHIC to confirm our results with much evidence of higher quality. As more and more particle therapy centers are expected to spring up all over the world in the following decade, prospective data on long‐term effect, quality of life, and cost effectiveness of PBRT will be useful for effective utilization of the novel technology in SNM treatment.
DISCLOSURE
The authors have declared no conflicts of interest.
Supporting information
Fig S1‐S3
App S1
App S2
App S3
App S4
ACKNOWLEDGEMENTS
This study was supported by grants from the National Key Research and Development Program of China (2018YFC0115700), Program of Shanghai Academic/Technology Research Leader (18XD1423000), and Science and Technology Commission of Shanghai Municipality (19411951000).
Zhang W, Hu W, Hu J, et al. Carbon ion radiation therapy for sinonasal malignancies: Promising results from 2282 cases from the real world. Cancer Sci. 2020;111:4465–4479. 10.1111/cas.14650
Contributor Information
Lin Kong, Email: lin.kong@sphic.org.cn.
Jiade J. Lu, Email: jiade.lu@sphic.org.cn.
REFERENCES
- 1. Muir CS, Nectoux J. Descriptive epidemiology of malignant neoplasms of nose, nasal cavities, middle ear and accessory sinuses. Clin Otolaryngol Allied Sci. 1980;5:195‐211. [DOI] [PubMed] [Google Scholar]
- 2. Dulguerov P, Jacobsen MS, Allal AS, Lehmann W, Calcaterra T. Nasal and paranasal sinus carcinoma: are we making progress? A series of 220 patients and a systematic review. Cancer. 2001;92:3012‐3029. [DOI] [PubMed] [Google Scholar]
- 3. Siddiqui F, Smith RV, Yom SS, et al. ACR appropriateness criteria (R) nasal cavity and paranasal sinus cancers. Head Neck. 2017;39:407‐418. [DOI] [PubMed] [Google Scholar]
- 4. Turner JH, Reh DD. Incidence and survival in patients with sinonasal cancer: a historical analysis of population‐based data. Head Neck. 2012;34:877‐885. [DOI] [PubMed] [Google Scholar]
- 5. Daly ME, Chen AM, Bucci MK, et al. Intensity‐modulated radiation therapy for malignancies of the nasal cavity and paranasal sinuses. Int J Radiat Oncol Biol Phys. 2007;67:151‐157. [DOI] [PubMed] [Google Scholar]
- 6. Duprez F, Madani I, Morbée L, et al. IMRT for sinonasal tumors minimizes severe late ocular toxicity and preserves disease control and survival. Int J Radiat Oncol Biol Phys. 2012;83:252‐259. [DOI] [PubMed] [Google Scholar]
- 7. Dirix P, Vanstraelen B, Jorissen M, Vander Poorten V, Nuyts S. Intensity‐modulated radiotherapy for sinonasal cancer: improved outcome compared to conventional radiotherapy. Int J Radiat Oncol Biol Phys. 2010;78:998‐1004. [DOI] [PubMed] [Google Scholar]
- 8. Mock U, Georg D, Bogner J, Auberger T, Pötter R. Treatment planning comparison of conventional, 3D conformal, and intensity‐modulated photon (IMRT) and proton therapy for paranasal sinus carcinoma. Int J Radiat Oncol Biol Phys. 2004;58:147‐154. [DOI] [PubMed] [Google Scholar]
- 9. Mazeron J‐J, Noel G, Feuvret L, Calugaru V, Racadot S. Clinical complementarities between proton and carbon therapies. Radiother Oncol. 2004;73:S50‐S52. [DOI] [PubMed] [Google Scholar]
- 10. Patel SH, Wang Z, Wong WW, et al. Charged particle therapy versus photon therapy for paranasal sinus and nasal cavity malignant diseases: a systematic review and meta‐analysis. Lancet Oncology. 2014;15:1027‐1038. [DOI] [PubMed] [Google Scholar]
- 11. Koto M, Hasegawa A, Takagi R, et al. Feasibility of carbon ion radiotherapy for locally advanced sinonasal adenocarcinoma. Radiother Oncol. 2014;113:60‐65. [DOI] [PubMed] [Google Scholar]
- 12. Ohkubo JI, Hohchi N, Takeuchi S, et al. Treatment outcome of ion beam therapy in eight patients with head and neck cancers. Eur Arch Otorhinolaryngol. 2016;273:4397‐4402. [DOI] [PubMed] [Google Scholar]
- 13. Koto M, Demizu Y, Saitoh JI, et al. Definitive carbon‐ion radiation therapy for locally advanced sinonasal malignant tumors: subgroup analysis of a multicenter study by the Japan Carbon‐Ion Radiation Oncology Study Group (J‐CROS). Int J Radiat Oncol Biol Phys. 2018;102:353‐361. [DOI] [PubMed] [Google Scholar]
- 14. Demizu Y, Fujii O, Terashima K, et al. Particle therapy for mucosal melanoma of the head and neck. A single‐institution retrospective comparison of proton and carbon ion therapy. Strahlenther Onkol. 2014;190:186‐191. [DOI] [PubMed] [Google Scholar]
- 15. Toyomasu Y, Demizu Y, Matsuo Y, et al. Outcomes of patients with sinonasal squamous cell carcinoma treated with particle therapy using protons or carbon ions. Int J Radiat Oncol Biol Phys. 2018;101:1096‐1103. [DOI] [PubMed] [Google Scholar]
- 16. Akbaba S, Ahmed D, Mock A, et al. Treatment outcome of 227 patients with sinonasal adenoid cystic carcinoma (ACC) after intensity modulated radiotherapy and active raster‐scanning carbon ion boost: a 10‐year single‐center experience. Cancers. 2019;11:E1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mimica X, Yu Y, McGill M, et al. Organ preservation for patients with anterior mucosal squamous cell carcinoma of the nasal cavity: rhinectomy‐free survival in those refusing surgery. Head Neck. 2019;41:2741‐2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nakamura N, Zenda S, Tahara M, et al. Proton beam therapy for olfactory neuroblastoma. Radiother Oncol. 2017;122:368‐372. [DOI] [PubMed] [Google Scholar]
- 19. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. Open Med. 2009;3:e123‐e130. [PMC free article] [PubMed] [Google Scholar]
- 20. Hu W, Hu J, Huang Q, et al. Particle beam radiation therapy for sinonasal malignancies: single institutional experience at the Shanghai proton and heavy ion certer. Cancer Med. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Richardson WS, Wilson MC, Nishikawa J, Hayward RS. The well‐built clinical question: a key to evidence‐based decisions. ACP J Club. 1995;123:A12‐A13. [PubMed] [Google Scholar]
- 22. Mohr A, Chaudhri N, Hassel JC, et al. Raster‐scanned intensity‐controlled carbon ion therapy for mucosal melanoma of the paranasal sinus. Head Neck. 2016;38(Suppl 1):E1445‐E1451. [DOI] [PubMed] [Google Scholar]
- 23. Dagan R, Bryant C, Li Z, et al. Outcomes of sinonasal cancer treated with proton therapy. Int J Radiat Oncol Biol Phys. 2016;95:377‐385. [DOI] [PubMed] [Google Scholar]
- 24. Dagan R, Bryant CM, Mendenhall WM, et al. Isolated leptomeningeal progression from sinonasal carcinomas: Implications for staging workup and treatment. Head Neck. 201941(8):2647–2654. [DOI] [PubMed] [Google Scholar]
- 25. Dagan R, Bryant CM, Mendenhall WM. Improving local control for unresectable/incompletely resected sinonasal cancer with hyperfractionated proton therapy and concurrent Chemotherapy. Int J Radiat Oncol Biol Phys. 2016;94:951. [Google Scholar]
- 26. Zenda S, Kawashima M, Nishio T, et al. Proton beam therapy as a nonsurgical approach to mucosal melanoma of the head and neck: a pilot study. Int J Radiat Oncol Biol Phys. 2011;81:135‐139. [DOI] [PubMed] [Google Scholar]
- 27. Zenda S, Kohno R, Kawashima M, et al. Proton beam therapy for unresectable malignancies of the nasal cavity and paranasal sinuses. Int J Radiat Oncol Biol Phys. 2011;81:1473‐1478. [DOI] [PubMed] [Google Scholar]
- 28. Zenda S, Kawashima M, Arahira S, et al. Late toxicity of proton beam therapy for patients with the nasal cavity, para‐nasal sinuses, or involving the skull base malignancy: importance of long‐term follow‐up. Int J Clin Oncol. 2015;20:447‐454. [DOI] [PubMed] [Google Scholar]
- 29. Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fuji H, Yoshikawa S, Kasami M, et al. High‐dose proton beam therapy for sinonasal mucosal malignant melanoma. Radiat Oncol. 2014;9:162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lucas JT Jr, Ladra MM, MacDonald SM, et al. Proton therapy for pediatric and adolescent esthesioneuroblastoma. Pediatr Blood Cancer. 2015;62:1523‐1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Saito T, Ishikawa H, Ohnishi K, et al. Proton beam therapy for locally advanced and unresectable (T4bN0M0) squamous cell carcinoma of the ethmoid sinus: a report of seven cases and a literature review. Oncol Lett. 2015;10:201‐205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nakamura T, Azami Y, Ono T, et al. Preliminary results of proton beam therapy combined with weekly cisplatin intra‐arterial infusion via a superficial temporal artery for treatment of maxillary sinus carcinoma. Jpn J Clin Oncol. 2016;46:46‐50. [DOI] [PubMed] [Google Scholar]
- 34. Russo AL, Adams JA, Weyman EA, et al. Long‐term outcomes after proton beam therapy for sinonasal squamous cell carcinoma. Int J Radiat Oncol Biol Phys. 2016;95:368‐376. [DOI] [PubMed] [Google Scholar]
- 35. Zenda S, Akimoto T, Mizumoto M, et al. Phase II study of proton beam therapy as a nonsurgical approach for mucosal melanoma of the nasal cavity or para‐nasal sinuses. Radiother Oncol. 2016;118:267‐271. [DOI] [PubMed] [Google Scholar]
- 36. Yu NY, Gamez ME, Hartsell WF, et al. A multi‐institutional experience of proton beam therapy for sinonasal tumors. Adv Radiat Oncol. 2019;4:689‐698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Truong MT, Kamat UR, Liebsch NJ, et al. Proton radiation therapy for primary sphenoid sinus malignancies: treatment outcome and prognostic factors. Head Neck. 2009;31:1297‐1308. [DOI] [PubMed] [Google Scholar]
- 38. Fukumitsu N, Okumura T, Mizumoto M, et al. Outcome of T4 (International Union Against Cancer Staging System, 7th edition) or recurrent nasal cavity and paranasal sinus carcinoma treated with proton beam. Int J Radiat Oncol Biol Phys. 2012;83(2):704‐711. [DOI] [PubMed] [Google Scholar]
- 39. Okano S, Tahara M, Zenda S, et al. Induction chemotherapy with docetaxel, cisplatin and S‐1 followed by proton beam therapy concurrent with cisplatin in patients with T4b nasal and sinonasal malignancies. Jpn J Clin Oncol. 2012;42:691‐696. [DOI] [PubMed] [Google Scholar]
- 40. Herr M, Lin A, Curry W, et al. Esthesioneuroblastoma: an update on the Massachusetts eye and ear infirmary and Massachusetts General Hospital Experience with craniofacial resection, proton beam radiation, and chemotherapy. J Neurol Surg B Skull Base. 2013;75(1):58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fitzek MM, Thornton AF, Varvares M, et al. Neuroendocrine tumors of the sinonasal tract. Results of a prospective study incorporating chemotherapy, surgery, and combined proton‐photon radiotherapy. Cancer. 2002;94:2623‐2634. [DOI] [PubMed] [Google Scholar]
- 42. Burt LM, Orlandi RR, Hunt JP, et al. Function preservation and optimal outcomes—definitive chemoradiotherapy with multi‐phase treatment planning for locally advanced sinonasal cancer. J Radiat Oncol. 2015;5:47‐54. [Google Scholar]
- 43. Askoxylakis V, Hegenbarth P, Timke C, et al. Intensity modulated radiation therapy (IMRT) for sinonasal tumors: a single center long‐term clinical analysis. Radiat Oncol. 2016;11:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Suh YG, Lee CG, Kim H, et al. Treatment outcomes of intensity‐modulated radiotherapy versus 3D conformal radiotherapy for patients with maxillary sinus cancer in the postoperative setting. Head Neck. 2016;38(Suppl 1):E207‐E213. [DOI] [PubMed] [Google Scholar]
- 45. Chopra S, Kamdar DP, Cohen DS, et al. Outcomes of nonsurgical management of locally advanced carcinomas of the sinonasal cavity. Laryngoscope. 2017;127:855‐861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gamez ME, Lal D, Halyard MY, et al. Outcomes and patterns of failure for sinonasal undifferentiated carcinoma (SNUC): the mayo clinic experience. Head Neck. 2017;39:1819‐1824. [DOI] [PubMed] [Google Scholar]
- 47. Mukai Y, Janssen S, Glanzmann C, Holzmann D, Studer G. Local control and intermediate‐term cosmetic outcome following IMRT for nasal tumors: an update. Strahlenther Onkol. 2017;193:295‐304. [DOI] [PubMed] [Google Scholar]
- 48. de Bonnecaze G, Verillaud B, Chaltiel L, et al. Clinical characteristics and prognostic factors of sinonasal undifferentiated carcinoma: a multicenter study. Int Forum Allergy Rhinol. 2018;8:1065‐1072. [DOI] [PubMed] [Google Scholar]
- 49. Debacker J, Huvenne W, Bonte K, et al. Open surgery versus primary radiotherapy in T4b sinonasal carcinoma. B‐Ent. 2018;14:93‐99. [Google Scholar]
- 50. Sas‐Korczynska B, Reinfuss M, Mitus JW, Pluta E, Patla A, Walasek T. Radiotherapy alone as a method of treatment for sinonasal mucosal melanoma: a report based on six cases and a review of current opinion. Rep Pract Oncol Radiother. 2018;23:402‐406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ferella L, Cavallo A, Miceli R, et al. Prognostic role of primary tumor, nodal neck, and retropharyngeal GTVs for unresectable sinonasal cancers treated with IMRT and chemotherapy. Tumori. 2019;106(1):39‐46. [DOI] [PubMed] [Google Scholar]
- 52. Thierauf J, Gluck AM, Plinkert P, et al. Mucosal melanoma of the cranio‐facial region: Surgical challenges and therapeutic options. Auris Nasus Larynx. 2019;46:252‐259. [DOI] [PubMed] [Google Scholar]
- 53. Uchida D, Shirato H, Onimaru R, et al. Long‐term results of ethmoid squamous cell or undifferentiated carcinoma treated with radiotherapy with or without surgery. Cancer J. 2005;11:152‐156. [DOI] [PubMed] [Google Scholar]
- 54. Combs SE, Konkel S, Thilmann C, Debus J, Schulz‐Ertner D. Local high‐dose radiotherapy and sparing of normal tissue using intensity‐modulated radiotherapy (IMRT) for mucosal melanoma of the nasal cavity and paranasal sinuses. Strahlenther Onkol. 2007;183:63‐68. [DOI] [PubMed] [Google Scholar]
- 55. Hoppe BS, Wolden SL, Zelefsky MJ, et al. Postoperative intensity‐modulated radiation therapy for cancers of the paranasal sinuses, nasal cavity, and lacrimal glands: technique, early outcomes, and toxicity. Head Neck. 2008;30:925‐932. [DOI] [PubMed] [Google Scholar]
- 56. Prognostic analysis of patients with locally advanced nasal cavity and sino‐nasal carcinoma treated by radiotherapy. 2011.
- 57. Al‐Mamgani A, Monserez D, Rooij P, Verduijn GM, Hardillo JA, Levendag PC. Highly‐conformal intensity‐modulated radiotherapy reduced toxicity without jeopardizing outcome in patients with paranasal sinus cancer treated by surgery and radiotherapy or (chemo)radiation. Oral Oncol. 2012;48:905‐911. [DOI] [PubMed] [Google Scholar]
- 58. Wiegner EA, Daly ME, Murphy JD, et al. Intensity‐modulated radiotherapy for tumors of the nasal cavity and paranasal sinuses: clinical outcomes and patterns of failure. Int J Radiat Oncol Biol Phys. 2012;83:243‐251. [DOI] [PubMed] [Google Scholar]
- 59. Kaur G, Kane AJ, Sughrue ME, et al. The prognostic implications of Hyam's subtype for patients with Kadish stage C esthesioneuroblastoma. J Clin Neurosci. 2013;20:281‐286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Fossati P, Molinelli S, Matsufuji N, et al. Dose prescription in carbon ion radiotherapy: a planning study to compare NIRS and LEM approaches with a clinically‐oriented strategy. Phys Med Biol. 2012;57:7543‐7554. [DOI] [PubMed] [Google Scholar]
- 61. Wang W, Huang Z, Sheng Y, et al. RBE‐weighted dose conversions for carbon ionradiotherapy between microdosimetric kinetic model and local effect model for the targets and organs at risk in prostate carcinoma. Radiother Oncol. 2019;144:30‐36. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1‐S3
App S1
App S2
App S3
App S4


