Abstract
The Japanese larch, (Larix kaempferi) is known to contain abundant taxifolin (dihydroquercetin) in its xylem. In this study, to assess the bioactivities of taxifolin rich methanol extract of L. kaempferi (LK-ME), anti-inflammatory effect, and the anti-lipid accumulation effect of LK-ME were investigated. The results showed that nitric oxide (NO) and reactive oxygen species (ROS) were reduced after treatment with LK-ME, and that lipid accumulation in adipocyte differentiated 3T3-L1 cells was inhibited after the cells were grown in medium containing LK-ME. Taxifolin, the major compound contained in LK-ME, and its related compounds, quercetin and luteolin also exhibited similar effects with LK-ME. The LK-ME exhibits relatively strong anti-inflammatory and anti-lipid accumulation activities compared with that of similar amounts of taxifolin contained in LK-ME, suggesting that other minor compounds contained in LK-ME is involved in the effects. These results indicate the potential of taxifolin-rich L. kaempferi extract for use as a supplement to prevent excess inflammation and obesity.
Keywords: Japanese larch, Larix kaempferi, Taxifolin, Inflammation, Lipid accumulation, Adipocytes, Macrophages, Food science, Bioactive compound, Biochemical characterization of food, Plant products, Cell culture, Cytotoxicity, Polyphenol, Flavonoid, Alternative medicine
Japanese larch, Larix kaempferi, Taxifolin, Inflammation, Lipid accumulation, Adipocytes, Macrophages; Food science; Bioactive compound; Biochemical characterization of food; Plant products; Cell culture; Cytotoxicity; Polyphenol; Flavonoid; Alternative medicine.
1. Introduction
A deciduous conifer, the Japanese larch (Larix kaempferi) is a Japanese endemic species and widely planted in Hokkaido, Japan. The same genus of larches, the Dahurian larch (Larix gmelinii), and the Siberian or Russian larch (Larix sibirica) contain abundant taxifolin (dihydroquercetin) in the xylems [1, 2]. Taxifolin is known to be an antioxidant agent [3], and is known for the many beneficial effects of taxifolin, such as improvement of microcirculation [4], hepatoprotective effects [5], anti-viral activity [6], and prevention of diabetic nephropathy [7] as well as diabetic cardiomyopathy [8]. In vitro studies have demonstrated that taxifolin exhibits anti-bacterial [9], anti-fungal [10], and anti-parasitic [11] effects, and also inhibitory activities against acetylcholinesterase and carbonic anhydrase isoenzymes [12]. Further, oligomer formation of amyloid β proteins is significantly inhibited by taxifolin in mice, suggesting that taxifolin is effective to prevent Alzheimer's disease [13]. The European Food Safety Authority (EFSA) assessed safety of supplements containing L. gmelinii extract, and concluded that the taxifolin rich Dahurian larch extract is safe under the proposed conditions of use [14, 15]. Due to this, taxifolin rich L. gmelinii and L. sibirica extracts are included in supplements to maintain human health, and its safety as a supplement is well validated. However, L. kaempferi extract has not so far been used in supplements to maintain human health.
Previously, we have demonstrated that the L. kaempferi methanol extract (LK-ME) exhibits anti-glycation activity [16]. High performance liquid chromatography (HPLC) analysis has indicated that taxifolin is the main compound in LK-ME, and most of the flavonoids contained in LK-ME are assumed to be taxifolin. Anti-glycation of the activity of taxifolin is equivalent to other taxifolin related flavonoids, quercetin and luteolin. At the same time, the cytotoxicity and inflammatory cytokine induction by taxifolin have been found lower than quercetin and luteolin in a human monocyte derived cell line, THP-1 cells.
In this study, for a further assessment of the biological activities of taxifolin-rich L. kaempferi extract, the anti-inflammatory activity of taxifolin on lipopolysaccharide (LPS) induced nitric oxide (NO) production in a mouse macrophage like cell line, RAW264.7 cells [17], was investigated. In addition, the effects of LK-ME on reactive oxygen species (ROS) production, and on LPS induced IL-1β and TNF-α mRNA expressions were examined. The effects of LK-ME on the expression of lipid metabolism related genes in macrophage differentiated THP-1 cells [18] were also investigated. Further, the effects of LK-ME on the lipid accumulation in adipocytes differentiated 3T3-L1 cells [19], which are known to be a mouse pre-adipocyte cell line and widely used in the study of lipid metabolism [20], were assessed.
2. Results
2.1. The effects of methanol extract of L. kaempferi (LK-ME) on NO and ROS production
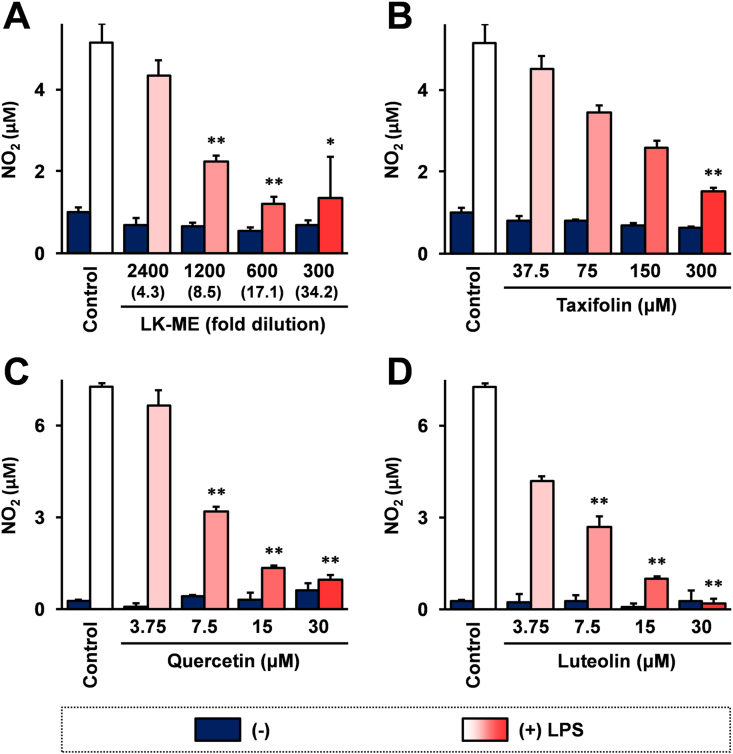
To investigate the anti-inflammatory activity of LE-ME, the effects of LK-ME on LPS induced NO production in a mouse macrophage like cell line, RAW264.7 cells, were examined. The NO generation after stimulation with LPS was monitored by measuring the accumulation of NO2, a stable degradation product of NO in culture supernatants using Griess reagent. The results show that LK-ME effectively reduces NO2 in culture supernatants (Figure 1A). Taxifolin, a main compound in LK-ME also reduces NO2 levels in the culture supernatant suggesting that taxifolin inhibits LPS induced NO production (Figure 1B). The taxifolin related flavonoids, quercetin and luteolin, also reduced NO2 concentrations in the culture supernatant of LPS stimulated RAW264.7 cells, and the reduction was stronger than that of taxifolin treated cells (Figure 1C and D).
Figure 1.
Effect of taxifolin-rich methanol extract of L. kaempferi extract (LK-ME) on LPS induced NO production in RAW264.7 cells. RAW264.7 cells were stimulated with 100 ng/ml LPS and cultured in medium containing the indicated amounts of LK-ME (A), taxifolin (B), quercetin (C), or luteolin (D) for 24 h. The NO2 concentrations in the culture supernatants were measured by Griess assay as described in the Materials and Methods section. The taxifolin concentrations at the indicated dilution of LK-ME are given in parentheses. Error bars indicate standard deviation calculated from three independent experiments. The asterisks (∗) and the double asterisks (∗∗) indicate that the difference is larger than two-fold compared with that of the control, and statistically significant; ∗: p < 0.05, ∗∗: p < 0.01.
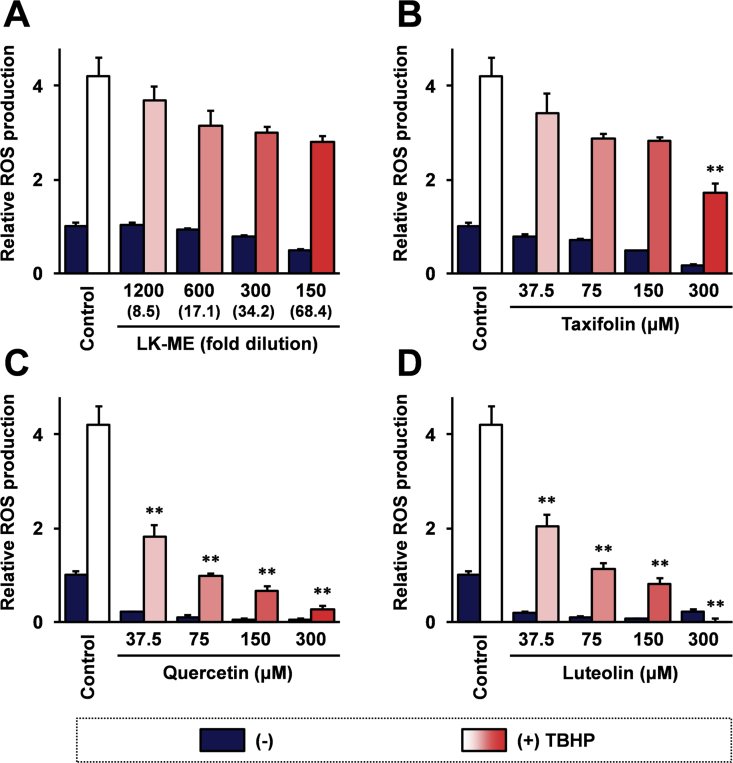
Next, the effects of LK-ME on ROS production in RAW264.7 cells were investigated. As shown in Figure 2A, LK-ME suppressed ROS production in the tert-Butyl hydroperoxide (TBHP) treated RAW264.7 cells in a dose-dependent manner. Taxifolin, quercetin and luteolin also suppressed the ROS production (Figure 2B-D). The ROS suppression activities of LK-ME and taxifolin were weaker than that of quercetin and luteolin.
Figure 2.
Effect of taxifolin-rich methanol extract of LK-ME on TBHP induced ROS production in RAW264.7 cells. RAW264.7 cells were cultured in medium containing the indicated amounts of LK-ME (A), taxifolin (B), quercetin (C), or luteolin (D) for 24 h. Then, the cells were stimulated with 150uM TBHP and concentrations of ROS in the culture supernatants were measured using DCFDA Cellular ROS Detection Assay Kit (abcam). Data represent relative ROS production values versus basal ROS production in the unstimulated control cells. The taxifolin concentrations at the indicated dilution of LK-ME are given in parentheses. Error bars indicate standard deviation calculated from three independent experiments. The double asterisks (∗∗) indicate that the difference is larger than two-fold compared with that of the control, and statistically significant (p < 0.01).
2.2. The effects of LK-ME on IL-1β and TNF-α mRNA expression in LPS stimulated RAW264.7 cells
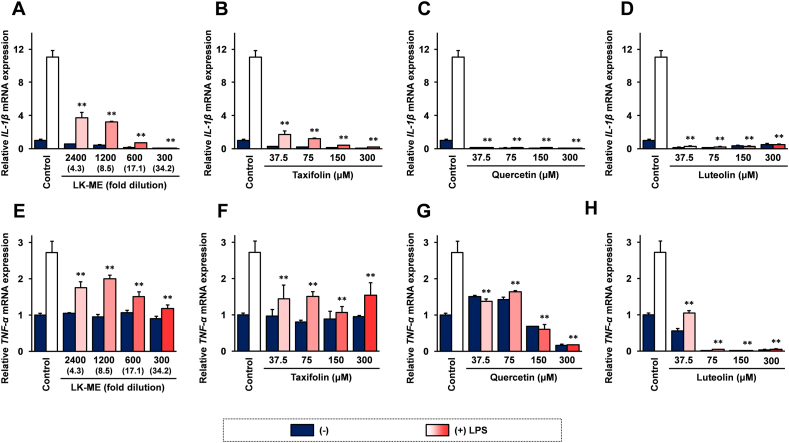
To further evaluation of anti-inflammatory effects of LK-ME, the effects of LK-ME on LPS-induced expression of IL-1β and TNF-α mRNAs were investigated. As shown in Figure 3, the expression of IL-1β mRNA as well as TNF-α mRNA were increased after stimulation with LPS. The increased expression of IL-1β and TNF-α mRNAs were significantly suppressed after treatment with LK-ME in a dose-dependent manner (Figure 3A and Figure 3E). Taxifolin, quercetin and luteolin also effectively suppressed LPS induced expression of IL-1β and TNF-α mRNAs in RAW264.7 cells (Figure 3B-D and Figure 3F-H).
Figure 3.
The Effects of LK-ME on IL-1β and TNF-α mRNA expression in LPS stimulated RAW264.7 cells. RAW264.7 cells were stimulated with 100 ng/ml LPS and cultured in medium containing the indicated amounts of LK-ME (A), taxifolin (B), quercetin (C), or luteolin (D) for 24 h. Then, the cells were harvested and total RNAs isolated from the cells were subjected to real-time RT-PCR analysis using specific primer set for IL-1β (A–E) and TNF-α (F–H) mRNAs. Data represent relative expression values versus mRNA expression in the unstimulated control cells after normalization with the GAPDH mRNA expression. The taxifolin concentrations at the indicated dilution of LK-ME are given in parentheses. Error bars indicate standard deviation calculated from three independent experiments. The double asterisks (∗∗) indicate that the difference is statistically significant compared with that of the LPS-stimulated control cells (p < 0.01).
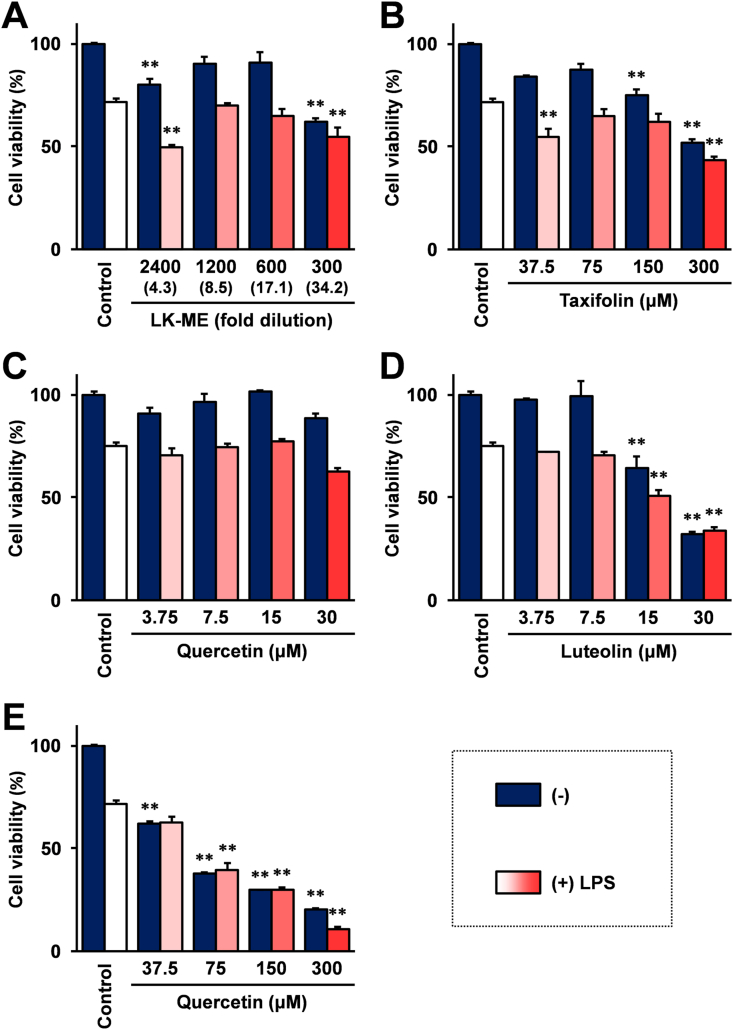
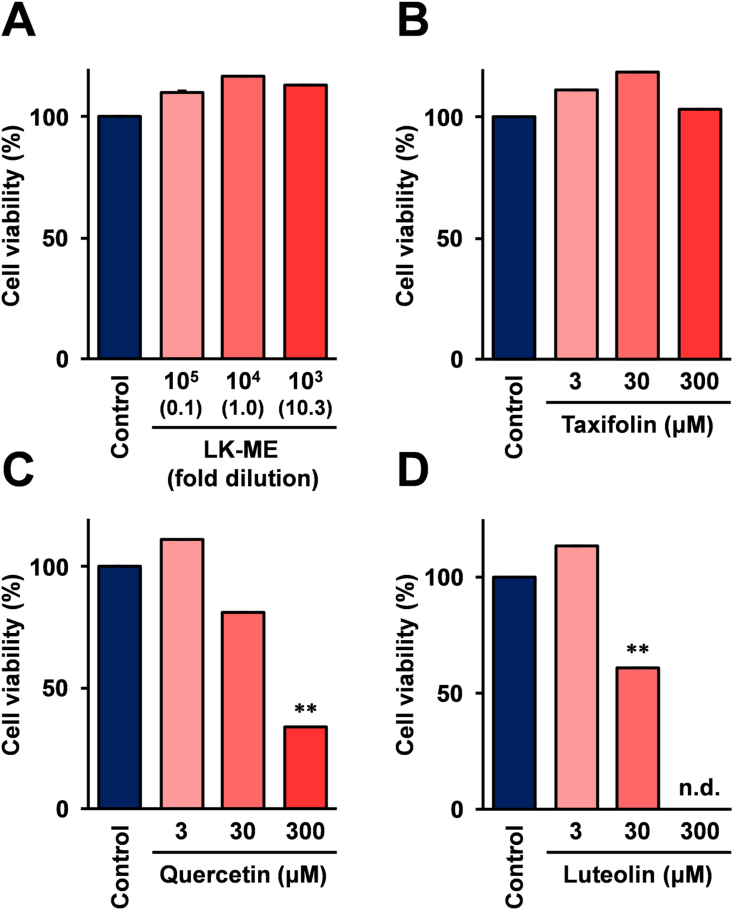
To further evaluate the influence of the treatment with taxifolin and its related compounds on the cell viability was monitored. As shown in Figure 4A and B, the results demonstrated that LK-ME and its main compound, taxifolin, strongly inhibited cell viability at high concentrations, a 300 fold dilution and 300 μM, respectively. The cell viability is not strongly inhibited after treatment with quercetin at 30 μM (Figure 4C), while it is strongly inhibited when the cells were grown in the same concentration of luteolin containing medium (Figure 4D). To compare the effect on the cell viability of taxifolin and quercetin, the cell viability after treatment with quercetin at the same concentrations as taxifolin shown in Figure 4B was monitored. The results showed that quercetin strongly inhibited cell viability at concentrations above 37.5 mM (Figure 4E).
Figure 4.
Effect of LK-ME and taxifolin related compounds on the growth of RAW264.7 cells. RAW264.7 cells were stimulated with 100 ng/ml LPS and cultured in medium containing the indicated amounts of LK-ME (A), taxifolin (B), quercetin (C, E) or luteolin (D). After 24 h, the cell viability was monitored with a modified MTT assay kit (Cell Counting Kit 8, Dojindo). The cell viability (%) was expressed as a percentage relative to the unstimulated control cells. The taxifolin concentrations at the indicated dilution of LK-ME are given in parentheses. Error bars indicate standard deviation calculated from three independent experiments. The double asterisks (∗∗) indicate that the cell viability is lower than 80% compared with the control cells, and the difference is statistically significant (p < 0.01).
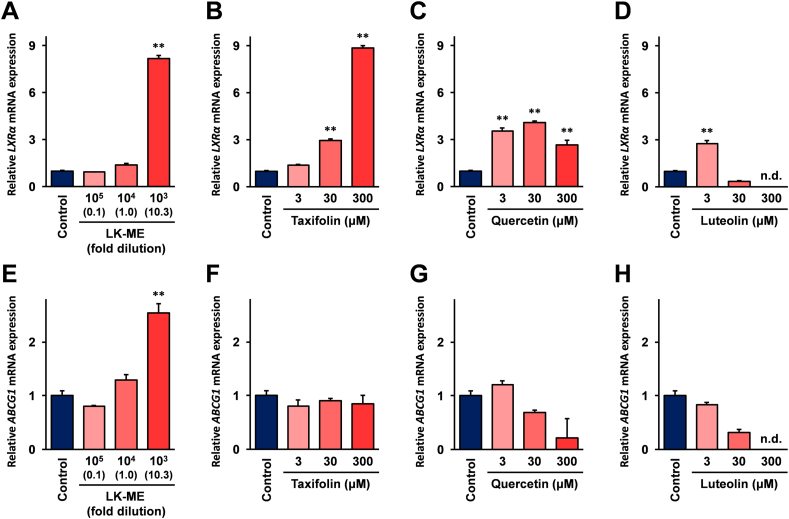
2.3. Effects of LK-ME on the expressions of liver X receptor β (LXRβ) and the ATP-binding cassette sub-family G member 1 (ABCG1) mRNA in macrophage differentiated THP-1 cells
The effects of LK-ME on the gene expressions which are involved in the lipid metabolism were investigated, and it was found that expressions of liver X receptor β (LXRβ) and the ATP-binding cassette sub-family G member 1 (ABCG1) mRNAs were markedly induced after stimulation with LK-ME in the macrophage differentiated THP-1 cells (Figure 5A and E). The LXRβ mRNA expression was also significantly increased after stimulation with taxifolin, the major compounds of LK-ME (Figure 5B), while the ABCG1 mRNA expression was not increased (Figure 5F). The expression of LXRβ mRNA was more weakly induced after stimulation with quercetin and luteolin than with taxifolin, and stimulation with quercetin and luteolin did not increase the expression of ABCG1 mRNA (Figure 5C, D, G, and H). These lower induction activities of quercetin and luteolin on the expressions of LXRβ and ABCG1 mRNAs are thought to be involved in cell cytotoxicity. Our previous study demonstrated that cell cytotoxicity of taxifolin is significantly lower than that of quercetin and luteolin in undifferentiated (monocyte like) THP-1 cells [16]. As shown in Figure 6, taxifolin cytotoxicity is also weaker in quercetin and luteolin in macrophage differentiated THP-1 cells.
Figure 5.
Effect of LK-ME on mRNA expression of lipid metabolism related genes in the macrophage differentiated THP-1 cells. THP-1 cells were differentiated into macrophage like cells using PMA. The cells were stimulated with the indicated concentrations of LK-ME (A, E), taxifolin (B, F), quercetin (C, G), and luteolin (D, H). After 6 h, the cells were harvested, and LXRα and ABCG1 mRNA expressions were monitored by real-time RT-PCR analysis. Data represent relative expression values versus mRNA expression in the unstimulated control cells after normalization with the GAPDH mRNA expression. The taxifolin concentrations at the indicated dilution of LK-ME are given in parentheses. Error bars indicate standard deviations calculated from three independent experiments, and the double asterisks (∗∗) indicate that the difference is statistically significant (p < 0.01) and larger than two-fold, compared with that of the control. n.d.: no data.
Figure 6.
Effect of LK-ME on the cell viability of macrophage differentiated THP-1 cells. THP-1 cells were differentiated into macrophage like cells using PMA. The cells were grown in medium containing the indicated concentrations of LK-ME (A), taxifolin (B), quercetin (C), and luteolin (D). After 24 h, the cell viability was monitored using Cell Counting Kit 8. The cell viability was expressed as a percentage relative to the unstimulated control cells. The taxifolin concentrations at the indicated dilution of LK-ME are given in parentheses. Error bars indicate standard deviations calculated from three independent experiments. The double asterisks (∗∗) indicate that the cell viability is lower than 80% compared with the control cells, and the difference is statistically significant (p < 0.01). n.d.: no data.
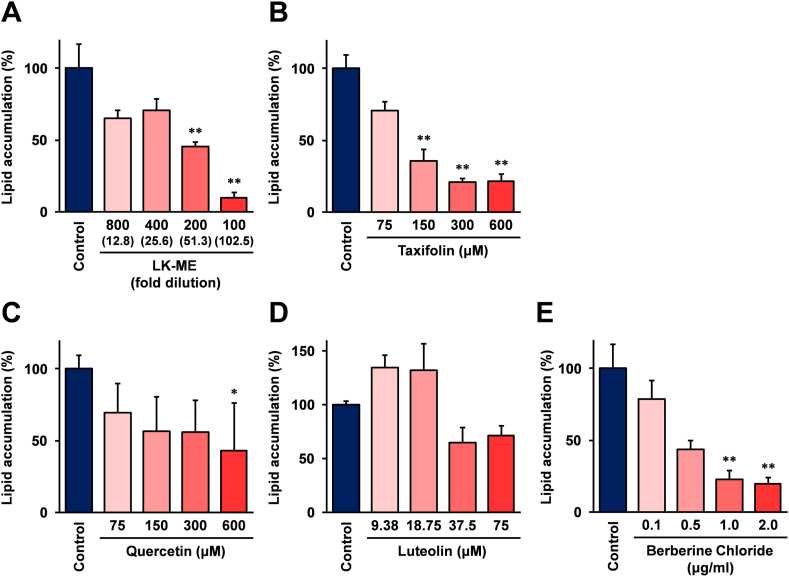
2.4. The treatment with LK-ME effectively reduced lipid accumulation in adipocyte differentiated 3T3-L1 cells
As shown in Figure 5, our results show that LK-ME and its main compound taxifolin exhibit induction activity of genes related to the lipid metabolism in macrophage differentiated THP-1 cells. This finding suggests that stimulation with LK-ME modulates the lipid metabolism, especially lipid accumulation. To verify this, and using adipocyte differentiated 3T3-L1 cells, the effects of LK-ME on the lipid accumulation in the cells were investigated. The adipocyte differentiated 3T3-L1 cells were grown for 3 days in medium containing LK-ME, taxifolin, quercetin, and luteolin, and the accumulated lipids were monitored by Oil Red O staining. Berberine chloride, a well known inhibitor for lipid accumulation, was used as a positive control. The results show that the lipid accumulation in the adipocyte differentiated 3T3-L1 cells were reduced after treatment with LK-ME to be similar to that with berberine chloride (Figure 7). Quercetin also indicates inhibition activity of the lipid accumulation (Figure 7C), and luteolin exhibited inhibition activity at lower concentrations than taxifolin and quercetin (Figure 7D). However, the maximum inhibition activity after treatment with taxifolin (21.6% at 600μM) was stronger than that of quercetin (42.8% at 600μM) and luteolin (65.0% at 37.5μM).
Figure 7.
Effects of LK-ME on lipid accumulation in adipocyte differentiated 3T3-L1 cells. Adipocyte differentiated 3T3-L1 cells were grown in medium containing the indicated dilutions of LK-ME (A) or indicated concentrations of taxifolin (B), quercetin (C), and luteolin (D). Berberine chloride (E) was used as positive controls. After 3 days, the cells were fixed and the accumulated lipids stained with Oil Red O. Then the stained Oil Red O was extracted, and measured by spectrophotometer as described in the Materials and Methods section. Lipid accumulations are shown as percent versus lipid accumulation in the untreated control cells after normalization with the protein concentration of cell lysate. The taxifolin concentrations at the indicated dilution of LK-ME are given in parentheses. Error bars indicate standard deviation calculated from three independent experiments. The asterisks (∗) and the double asterisks (∗∗) indicate that the difference is larger than two-fold compared with that of the control, and statistically significant; ∗: p < 0.05, ∗∗: p < 0.01.
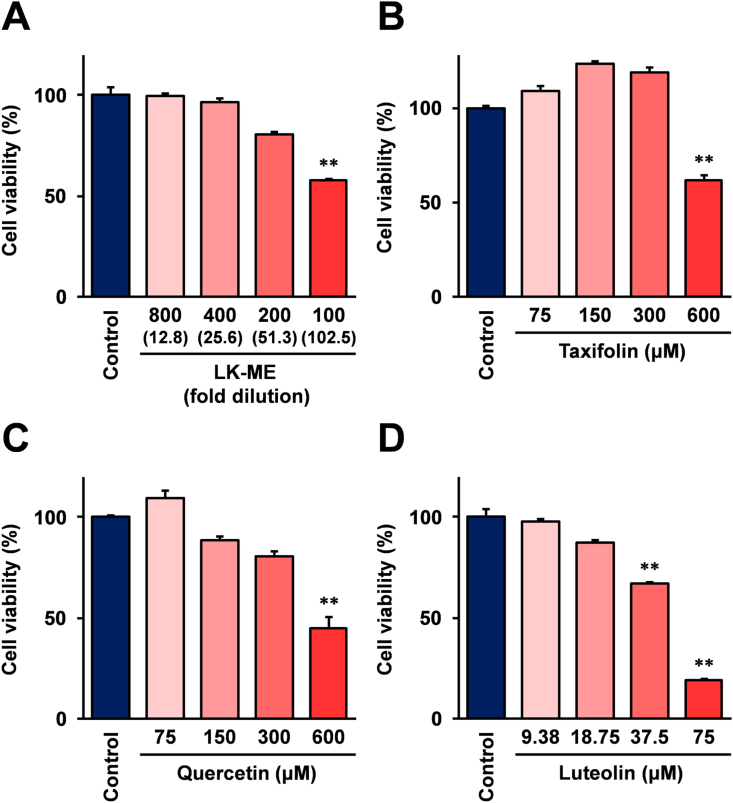
The data for the inhibition activity of lipid accumulation shown in Figure 7 were normalized with the protein concentration of cell lysate, and the results of the lipid accumulation assay suggest that the cell viability is influenced. To elucidate this further, the effects of LK-ME and the taxifolin related compounds on the cell viability of adipocyte differentiated 3T3-L1 cells were investigated. The results demonstrated that LK-ME and taxifolin inhibit the cell growth of adipocyte differentiated 3T3-L1 cells at high concentrations, however they did not inhibit the cell growth at concentrations which exhibit significant inhibition effects on lipid accumulation (Figure 8A and B). Quercetin exhibits a moderate inhibition activity of the cell growth of adipocyte differentiated 3T3-L1 cells (Figure 8C), while luteolin strongly inhibits the cell growth at lower concentrations than those of other compounds (Figure 8D). At the high concentrations, above those in the figure, the cell growth was completely inhibited after treatment with luteolin, and could not be determined by the assay.
Figure 8.
Effect of LK-ME on cell viability of adipocyte differentiated 3T3-L1 cells. Adipocytes differentiated 3T3-L1 cells were grown in medium containing the indicated dilution of LK-ME (A) or indicated concentration of taxifolin (B), quercetin (C), and luteolin (D). After 3 days, the cell viability was monitored using a Cell Counting Kit-8. The cell viability was expressed as a percentage relative to the unstimulated control cells. The taxifolin concentrations at the indicated dilution of LK-ME are given in parentheses. Error bars indicate standard deviation calculated from three independent experiments. The double asterisks (∗∗) indicate that the cell viability is lower than 80% compared with the control cells, and the difference is statistically significant (p < 0.01).
3. Discussion
In this study, the anti-inflammatory activity and effects on lipid metabolism of LK-ME were investigated, and the results demonstrate that LK-ME inhibits NO and ROS production in RAW264.7 cells, suppressed LPS-induced expression of IL-1β and TNF-α mRNAs in RAW264.7 cells, increases LXRβ and ABCG1 mRNA expressions in Macrophage differentiated THP-1 cells, and reduces lipid accumulation in adipocyte differentiated 3T3-L1 cells.
NO and ROS are produced by immune cells, such as monocytes, macrophages, and neutrophils after stimulation with extracellular pathogens. These molecules are important for the deactivation of pathogens through its cytotoxic activity. However, excess amounts of NO and ROS exacerbate inflammation and causes severe tissue damage [21, 22]. In this study, to assess the anti-inflammatory activity of LK-ME, the effects of LK-ME on LPS induced NO production was investigated by monitoring the concentration of NO2, a stable degradation product of NO, in a culture supernatant. The results show that the concentration of NO2 in the culture supernatant of LPS-stimulated RAW264.7 cells decreased after treatment with LK-ME, suggesting that LK-ME effectively suppresses LPS induced NO production (Figure 1). The half maximal inhibitory concentration (IC50) calculated from the data obtained in this study is shown in Table 1. The anti-inflammatory activity of taxifolin against LPS induced NO production in RAW264.7 cells is substantially lower than that provided by quercetin and luteolin. At the same time, LK-ME exhibits strong inhibitory activity against the LPS induced NO production at lower taxifolin concentrations than with the IC50 of taxifolin. Our previous study demonstrated that taxifolin is the major compound of LK-ME by the reverse phage chromatography using high performance liquid chromatography (HPLC) with a UV-detector (A280nm). The HPLC analysis suggested that there are unknown minor compounds in LK-ME. The several minor compounds in a methanol extract of L. kaempferi were reported as dihydrokaempferol, naringenin, 4-Hydroxybenzaldehyde, and p-Coumaryl aldehyde [23], and these minor compounds or other undetectable compounds by HPLC analysis may be involved in the stronger inhibition activities of LK-ME on LPS induced NO production. On the other hand, IC50 value of LK-ME against ROS production is equivalent to taxifolin, suggesting that minor compounds in LK-ME do not influence ROS production.
Table 1.
Half maximal inhibitory concentration (IC50) of LK-ME in the experiments performed in this study.
| LK-ME | Taxifolin | Quercetin | Luteolin | |
|---|---|---|---|---|
|
Biological activity | ||||
| NO production | ×1333.3 dil. | 123.8 μM | 6.9 μM | 4.6 μM |
| (7.7 μM) | ||||
| ROS production | ×41.5 dil | 239.5 μM | 28.4 μM | 36.3 μM |
| (247.1 μM) | ||||
| Lipid accumulation |
×319.5 dil. | 118.4 μM | 361.9 μM | 74.8 μM |
| (32.1 μM) |
||||
|
Cell cytotoxicity | ||||
| RAW264.7 cells (-LPS) | ×237.9 dil. | 360.5 μM | 54.7 μM | 20.6 μM |
| (43.1 μM) | ||||
| RAW264.7 cells (+LPS) | ×147.6 dil. | 358.4 μM | 101.3 μM | 25.7 μM |
| (69.5 μM) | ||||
| THP-1 cells (Macrophage differentiated) | n.d. | 662.9 μM | 124.5 μM | 31.5 μM |
| 3T3-L1 cells (Adipocyte differentiated) | ×73.5 dil. | 615.0 μM | 574.5 μM | 57.3 μM |
| (139.5 μM) | ||||
The taxifolin concentrations at the indicated dilution of LK-ME are given in parentheses. n.d.: not determined.
As shown in Figure 5, the LXRα mRNA is effectively induced after treatment with LK-ME and taxifolin. The LXRs are important to maintain lipid homeostasis. Originally, Liver X receptors (LXRs) were determined as orphan nuclear receptors [24, 25], and later, oxygenated derivatives of cholesterols (oxysterols) have been identified as ligands for LXRs [26, 27, 28]. There are two isoforms in LXRs, LXRα and LXRβ: The LXRα is restrictively expressed in cells with high cholesterol turnover, such as hepatocytes and macrophages, whereas LXRβ is expressed in various tissues. A previous report demonstrated that treatment with synthetic LXR ligands ameliorate the development of atherosclerosis in mice [29], suggesting that activation of LXR mediated signaling is important for the prevention of atherosclerosis. Further, macrophages derived from LXRα and LXRβ deficient mice have been shown to cause lipid accumulation and increases in aortic lesions in ApoE deficient mice which are known to develop atherosclerosis spontaneously [30]. This may suggest that L. kaempferi extract is effective to prevent the development of atherosclerosis. In addition, ABCG1 mRNA expression is increased after stimulation with LK-ME in THP-1 cells differentiated to macrophage-like cells (Figure 5E). The ABCG1 is functioning to export cellular cholesterol to high-density lipoproteins (HDL), and involved in atherosclerosis [31, 32]. Also, ABCG1 mRNA is not induced after stimulation with taxifolin (Figure 5F). These results suggest that compounds other than taxifolin in LK-ME would be involved in the induction of ABCG1 mRNA.
Analysis of the effects of LK-ME on lipid accumulation using adipocyte differentiated 3T3-L1 cells demonstrates that treatment with LK-ME effectively inhibit lipid accumulation (Figure 7A). The calculated IC50 values indicate that taxifolin, the major compound abundant in LK-ME exhibits strong inhibition activity of lipid accumulation in adipocyte differentiated 3T3-L1 cells, and it is comparable to that of luteolin (Table 1). Taxifolin exhibits the weakest cell cytotoxicity when compared with quercetin and luteolin. In the inhibition activity of LPS induced NO production, the IC50 value of taxifolin is two orders of magnitude higher than that of quercetin and luteolin. The IC50 value of taxifolin in cell growth of RAW264.7 cells is one order of magnitude higher than that of other compounds, suggesting a weak cytotoxicity of taxifolin, but it is difficult to determine any advantage of taxifolin on the inhibition effects of LPS induced NO production. However, the IC50 values of taxifolin on lipid accumulation in adipocyte differentiated 3T3-L1 cells and on the inhibition activity of the cell growth demonstrate the advantages of taxifolin as an agent for prevention of lipid accumulation.
Like in other cell lines, the inhibition activity of taxifolin with cell growth of adipocyte differentiated 3T3-L1 cells is lower than that of quercetin and luteolin (Figure 8 and Table 1). The sensitivity of adipocyte differentiated 3T3-L1 cells against these compounds is lower than RAW264.7 cells and macrophage differentiated THP-1 cells, and the differences in sensitivity between the cell lines are strongest in quercetin. The IC50 values shown in Table 1 indicate that the growth inhibition activity of quercetin in adipocyte differentiated 3T3-L1 cells are 5–10 fold weaker than that in the other cell lines used in this study. Here, the growth inhibition activity of taxifolin and luteolin in adipocyte differentiated 3T3-L1 cells is only around 2 fold weaker than that in the other cell lines. Quercetin and the other compounds used in this study are known to activate nuclear receptors, such as peroxisome proliferator-activated receptor γ (PPARγ) and the vitamin D receptor [33, 34], and part of the effects of these compounds are exhibited through its nuclear receptors. The differences in the nuclear receptor expressions between the cell lines used in this study may play a role in the differences in quercetin sensitivity.
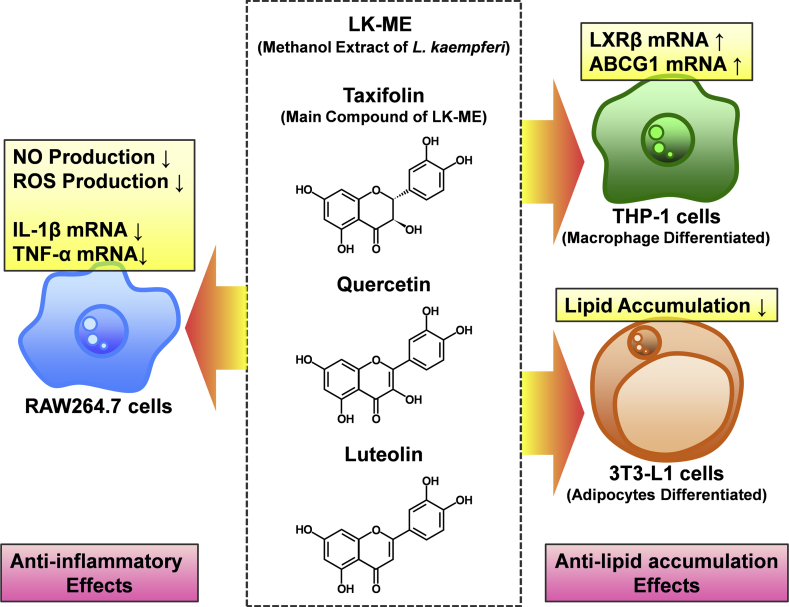
In conclusion, the data shown in this study demonstrated that LK-ME effectively inhibits NO and ROS production in RAW264.7 cells, suppresses LPS-induced expression of IL-1β and TNF-α mRNAs in RAW264.7 cells, induces genes for lipid metabolism in macrophage differentiated THP-1 cells, and suppresses lipid accumulation in adipocyte differentiated 3T3-L1 cells. The summary of findings in this study is shown in Figure 9. These findings suggest a potential for taxifolin rich extract of Larix kaempferi to be used as a supplement for the prevention of excess inflammation and obesity.
Figure 9.
The summary of findings in this study.
4. Materials and Methods
4.1. Preparation of L. kaempferi extract (LK-ME)
The methods for the preparation of methanol extract from saw dust of L. kaempferi used in this study was described previously [16]. Saw dust of L. kaempferi was obtained from the Forestry cooperative of Shimokawa town, Hokkaido, Japan. The dimethyl sulfoxide (DMSO) solution of the dried methanol extract of L. kaempferi was used as LK-ME in this study.
4.2. Cell culture and monitoring of cell viability
A human monocyte-derived cell line, THP-1 cells (ATCC TIB-202) [18], and a mouse macrophage-like cell line, RAW 264.7 cells (ATCC TIB-71) [17] were grown in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS), 100 U/ml penicillin, 100 mg/ml streptomycin (Life Technologies, Carlsbad, CA, USA). A mice preadipose cell line, 3T3-L1 cells (ATCC CL-173) [19] were obtained from the Japanese Collection of Research Bioresources (JCRB), National Institutes of Biomedical Innovation, Health and Nutrition (Osaka, Japan), and cultured in the D-MEM supplemented with 10% FBS, 100 U/ml penicillin, 100 mg/ml streptomycin. These cells were grown at 37 °C in 5% CO2 in a humidified incubator.
The cell viability was monitored using a Cell Counting Kit-8 (Dojindo, Kumamoto, Japan) according to the manufacturer's instructions.
The half maximal inhibitory concentration (IC50) values were calculated using the IC50 calculator (AAT Bioquest, Sunnyvale, CA, USA; https://www.aatbio.com/tools/ic50-calculator).
4.3. Real-time reverse transcriptase polymerase chain reaction (RT-PCR) analysis
Total RNA of the cultured cells was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA, USA). The isolated total RNA was treated with DNaseI (Takara, Otsu, Shiga, Japan), and oligo-dT- and random-primed reverse transcription was performed using ReverTra Ace (Toyobo, Osaka, Japan). Real-time PCR was performed on the CFX96 Real-Time PCR Detection System (Bio-Rad, Hercules, CA, USA) using Thunderbird SYBR qPCR Mix (Toyobo). Each procedure was performed according to the manufacturer's protocols. The following listed specific primer sets for IL-1β, TNF-α, LXRβ, ABCG1, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNAs were used in this study: IL-1β sense primer 5′- GCCTCGTGCTGTCGGACC -3’; IL-1β antisense primer 5′- TGTCGTTGCTTGGTTCTCCTTG -3’; TNF-α sense primer 5′- GACCCTCACACTCAGATCATCTTCT -3’; TNF-α antisense primer 5′- CCACTTGGTGGTTTGCTACGA -3’; LXRβ sense primer 5′- ACAACCACGAGACAGAGTGT -3’; LXRβ antisense primer 5′- CGAGAACTCGAAGATGGGGT -3’; ABCG1 sense primer 5′- GTGTACTGGATGACGTCGCA -3’; ABCG1 antisense primer 5′- GTCACTGGGCCCACGAAAG -3’; GAPDH sense primer 5′- TTCTTTTGCGTCGCCAGCCG -3’; and GAPDH antisense primer 5′- GGTGACCAGGCGCCCAATACG -3’.
4.4. Quantification of NO2
NO production from the cells was evaluated by monitoring NO2 production in the culture supernatant using Griess reagent. RAW264.7 cells were seeded onto 96 well plates at 2.0 × 104/well. After growing overnight, the cells were stimulated with 100 ng/ml LPS and grown in various concentrations of LE-ME, taxifolin, quercetin, or luteolin containing medium. After a 24 h incubation period, the culture supernatants were collected, and the cell debris were removed by centrifugation. 50 μl of the culture supernatants were diluted with ultrapure water up to 100 μl, and added to 50 μl of 2% sulfanilamide in 5% phosphoric acid. After a brief mixing, 50 μl of N-(1-Naphthyl) ethylenediamine dihydrochloride was added and mixed in. After 10 min incubation, the absorbance at 570 nm was monitored using a micro plate reader. The NO2 concentrations were calculated using NaNO2 solution as the standard.
4.5. Monitoring ROS production
ROS production in RAW264.7 cells were monitored using DCFDA Cellular ROS Detection Assay Kit (Abcam, Cambridge, MA) according to the manufacturer instructions. Briefly, RAW264.7 cells were seeded onto 96 well plates at 2.0 × 104/well and grown in various concentrations of LE-ME, taxifolin, quercetin, or luteolin containing medium. After growing overnight, the medium was removed, and incubate with DCFDA solution for 45 min at 37 °C in the dark. Then the cells were washed with PBS, and treated with 150 μM TBHP. After 3 h incubation, the fluorescence at 570 nm was monitored using a fluorescence plate reader at Ex/Em = 475/500–550 nm.
4.6. Monitoring lipid accumulation in adipocyte differentiated 3T3-L1 cells
Differentiation of 3T3-L1 cells into adipocyte-like cells was performed as previously described [17]. The 3T3-L1 cells were grown in D-MEM supplemented 10% FBS, 0.1 μM dexamethasone, 0.5 mM 3-isobutyl-1-methylxanthine (IBMX), and 10 μg/ml Insulin for 2 days, and cultured in D-MEM supplemented 10% FBS, 10 μg/ml Insulin for additional 3 days. Then the cells were used as the adipocyte differentiated 3T3-L1 cells in this study.
For the measurement of lipid accumulation in the adipocyte differentiated 3T3-L1 cells, the cells were grown in LK-ME, taxifolin or berberine chloride (positive control) containing medium (D-MEM containing 10% FCS) for 3 days. The cells were washed twice with PBS and fixed in 10% formaldehyde for 10 min at room temperature. Then the cells were washed three times with ultrapure water, and stained with Oil Red O for 15 min at room temperature. After removal of the staining solution, the cells were washed three times with 60% isopropanol, and once with ultrapure water. The lipid stained Oil Red O was extracted using 100% isopropanol, and quantified by the measurement of absorbance 492 nm.
4.7. Statistical analysis
To determine statistically significant differences between data pairs, a two-tailed unpaired Student's t-test was performed in this study.
Declarations
Author contribution statement
Daisuke Muramatsu: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Hirofumi Uchiyama: Conceived and designed the experiments; Performed the experiments.
Hiroshi Kida: Contributed reagents, materials, analysis tools or data; Wrote the paper.
Atsushi Iwai: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This study was funded by Aureo Co., Ltd., Kimitsu, Japan and Aureo-Science Co., Ltd., Sapporo, Japan. The funders had no role in the study design, data collection, or analysis, decision to publish, or preparation of the manuscript.
Declaration of interests statement
The authors declare the following conflicts of interest: Daisuke Muramatsu, Hirofumi Uchiyama and Atsushi Iwai are employees of Aureo-Science Co., Ltd., and Atsushi Iwai is an employee of Aureo Co., Ltd.
Additional information
No additional information is available for this paper.
References
- 1.Wang Y., Zu Y., Long J., Fu Y., Li S., Zhang D., Li J., Wink M., Efferth T. Enzymatic water extraction of taxifolin from wood sawdust of Larix gmelini (Rupr.) Rupr. and evaluation of its antioxidant activity. Food Chem. 2011;126:1178–1185. [Google Scholar]
- 2.Khlupova M.E., Vasil’Eva I.S., Shumakovich G.P., Morozova O.V., Chertkov V.A., Shestakov A.K., Kisin A.V., Yaropolov A.I. Enzymatic polymerization of dihydroquercetin using bilirubin oxidase. Biochem. 2015;80:233–241. doi: 10.1134/S0006297915020108. [DOI] [PubMed] [Google Scholar]
- 3.Topal F., Nar M., Gocer H., Kalin P., Kocyigit U.M., Gülçin İ., Alwasel S.H. Antioxidant activity of taxifolin: an activity-structure relationship. J. Enzym. Inhib. Med. Chem. 2016;31:674–683. doi: 10.3109/14756366.2015.1057723. [DOI] [PubMed] [Google Scholar]
- 4.Plotnikov M.B., Aliev O.I., Sidekhmenova A.V., Shamanaev A.Y., Anishchenko A.M., Fomina T.I., Chernysheva G.A., Smol’yakova V.I., Arkhipov A.M. Dihydroquercetin improves microvascularization and microcirculation in the brain cortex of SHR rats during the development of arterial hypertension. Bull. Exp. Biol. Med. 2017;163:57–60. doi: 10.1007/s10517-017-3737-7. [DOI] [PubMed] [Google Scholar]
- 5.Chiu Y.J., Chou S.C., Chiu C.S., Kao C.P., Wu K.C., Chen C.J., Tsai J.C., Peng W.H. Hepatoprotective effect of the ethanol extract of Polygonum orientale on carbon tetrachloride-induced acute liver injury in mice. J. Food Drug Anal. 2017;26:369–379. doi: 10.1016/j.jfda.2017.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Galochkina A.V., Anikin V.B., Babkin V.A., Ostrouhova L.A., Zarubaev V.V. Virus-inhibiting activity of dihydroquercetin, a flavonoid from Larix sibirica, against coxsackievirus B4 in a model of viral pancreatitis. Arch. Virol. 2016;161:929–938. doi: 10.1007/s00705-016-2749-3. [DOI] [PubMed] [Google Scholar]
- 7.Ding T., Wang S., Zhang X., Zai W., Fan J., Chen W., Bian Q., Luan J., Shen Y., Zhang Y., Ju D., Mei X. Kidney protection effects of dihydroquercetin on diabetic nephropathy through suppressing ROS and NLRP3 inflammasome. Phytomedicine. 2018;41:45–53. doi: 10.1016/j.phymed.2018.01.026. [DOI] [PubMed] [Google Scholar]
- 8.Sun X., Chen R., Yang Z., Sun G., Wang M., Ma X., Yang L., Sun X. Taxifolin prevents diabetic cardiomyopathy in vivo and in vitro by inhibition of oxidative stress and cell apoptosis. Food Chem. Toxicol. 2014;63:221–232. doi: 10.1016/j.fct.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 9.Kuspradini H., Mitsunaga T., Ohashi H. Antimicrobial activity against Streptococcus sobrinus and glucosyltransferase inhibitory activity of taxifolin and some flavanonol rhamnosides from kempas (Koompassia malaccensis) extracts. J. Wood Sci. 2009;55:308–313. [Google Scholar]
- 10.Mishra S., Singh S., Misra K. Restraining pathogenicity in Candida albicans by taxifolin as an inhibitor of ras1-pka pathway. Mycopathologia. 2017;182:953–965. doi: 10.1007/s11046-017-0170-4. [DOI] [PubMed] [Google Scholar]
- 11.Abugri D.A., Witola W.H., Russell A.E., Troy R.M. In vitro activity of the interaction between taxifolin (dihydroquercetin) and pyrimethamine against Toxoplasma gondii. Chem. Biol. Drug Des. 2018;91:194–201. doi: 10.1111/cbdd.13070. [DOI] [PubMed] [Google Scholar]
- 12.Gocer H., Topal F., Topal M., Küçük M., Teke D., Gülçin İ., Alwasel S.H., Supuran C.T. Acetylcholinesterase and carbonic anhydrase isoenzymes I and II inhibition profiles of taxifolin. J. Enzym. Inhib. Med. Chem. 2015;31:1–7. doi: 10.3109/14756366.2015.1036051. [DOI] [PubMed] [Google Scholar]
- 13.Saito S., Yamamoto Y., Maki T., Hattori Y., Ito H., Mizuno K., Harada-Shiba M., Kalaria R.N., Fukushima M., Takahashi R., Ihara M. Taxifolin inhibits amyloid-β oligomer formation and fully restores vascular integrity and memory in cerebral amyloid angiopathy. Acta Neuropathol. Commun. 2017;5:26. doi: 10.1186/s40478-017-0429-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Turck D., Bresson J., Burlingame B., Dean T., Fairweather-Tait S., Heinonen M., Hirsch-Ernst K.I., Mangelsdorf I., McArdle H.J., Naska A., Neuhäuser-Berthold M., Nowicka G., Pentieva K., Sanz Y., Siani A., Sjödin A., Stern M., Tomé D., Vinceti M., Willatts P., Engel K., Marchelli R., Pöting A., Poulsen M., Schlatter J., Gelbmann W., Van Loveren H. Scientific opinion on taxifolin-rich extract from dahurian larch (Larix gmelinii) EFSA J. 2017;15 [Google Scholar]
- 15.Turck D., Bresson J., Burlingame B., Dean T., Fairweather-Tait S., Heinonen M., Hirsch-Ernst K.I., Mangelsdorf I., McArdle H.J., Naska A., Neuhäuser-Berthold M., Nowicka G., Pentieva K., Sanz Y., Siani A., Sjödin A., Stern M., Tomé D., Vinceti M., Willatts P., Engel K., Marchelli R., Pöting A., Poulsen M., Schlatter J., Gelbmann W., Van Loveren H. Statement on the safety of taxifolin-rich extract from Dahurian Larch (Larix gmelinii) EFSA J. 2017;15 doi: 10.2903/j.efsa.2017.5059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muramatsu D., Uchiyama H., Kida H., Iwai A. Cell cytotoxity and anti-glycation activity of taxifolin-rich extract from Japanese larch, Larix kaempferi. Heliyon. 2019;5 doi: 10.1016/j.heliyon.2019.e02047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ralph P., Nakoinz I. Antibody-dependent killing of erythrocyte and tumor targets by macrophage-related cell lines: enhancement by PPD and LPS. J. Immunol. 1977;119:950–954. [PubMed] [Google Scholar]
- 18.Tsuchiya S., Yamabe M., Yamaguchi Y., Kobayashi Y., Konno T., Tada K. Establishment and characterization of a human acute monocytic leukemia cell line (THP-1) Int. J. Cancer. 1980;26:171–176. doi: 10.1002/ijc.2910260208. [DOI] [PubMed] [Google Scholar]
- 19.Green H., Meuth M. An established pre-adipose cell line and its differentiation in culture. Cell. 1974;3:127–133. doi: 10.1016/0092-8674(74)90116-0. [DOI] [PubMed] [Google Scholar]
- 20.Rubin C.S., Hirsch A., Fung C., Rosen O.M. Development of hormone receptors and hormonal responsiveness in vitro. J. Biol. Chem. 1978;253:7570–7578. [PubMed] [Google Scholar]
- 21.Guzik T.J., Korbut R., Adamek-Guzik T. Nitric oxide and superoxide in inflammation and immune regulation. J. Physiol. Pharmacol. 2003;54:469–487. [PubMed] [Google Scholar]
- 22.Bergamini C., Gambetti S., Dondi A., Cervellati C. Oxygen, reactive oxygen species and tissue damage. Curr. Pharmaceut. Des. 2005;10:1611–1626. doi: 10.2174/1381612043384664. [DOI] [PubMed] [Google Scholar]
- 23.Kawanishi Y., Bito N., Nakada R., Imai T. Visualization of the distribution of flavonoids in Larix kaempferi wood by fluorescence microscopy. Mokuzai Gakkaishi. 2015;61:297–307. [Google Scholar]
- 24.Apfel R., Benbrook D., Lernhardt E., Ortiz M.A., Salbert G., Pfahl M. A novel orphan receptor specific for a subset of thyroid hormone-responsive elements and its interaction with the retinoid/thyroid hormone receptor subfamily. Mol. Cell Biol. 1994;14:7025–7035. doi: 10.1128/mcb.14.10.7025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Willy P.J., Umesono K., Ong E.S., Evans R.M., Heyman R.A., Mangelsdorf D.J. LXR, a nuclear receptor that defines a distinct retinoid response pathway. Genes Dev. 1995;9:1033–1045. doi: 10.1101/gad.9.9.1033. [DOI] [PubMed] [Google Scholar]
- 26.Janowski B.A., Willy P.J., Devi T.R., Falck J.R., Mangelsdorf D.J. An oxysterol signalling pathway mediated by the nuclear receptor LXRα. Nature. 1996;383:728–731. doi: 10.1038/383728a0. [DOI] [PubMed] [Google Scholar]
- 27.Lehmann J.M., Oliver B.B., Su J.-L., Winegar D.A., Kliewer S.A., Willson T.M., Smith-Oliver T.A., Spencer T.A., Moore L.B., Sundseth S.S., Blanchard D.E. Activation of the nuclear receptor LXR by oxysterols defines a new hormone response pathway. J. Biol. Chem. 1997;272:3137–3140. doi: 10.1074/jbc.272.6.3137. [DOI] [PubMed] [Google Scholar]
- 28.Forman B.M., Ruan B., Chen J., Schroepfer G.J., Evans R.M. The orphan nuclear receptor LXR is positively and negatively regulated by distinct products of mevalonate metabolism. Proc. Natl. Acad. Sci. 1997;94:10588–10593. doi: 10.1073/pnas.94.20.10588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Joseph S.B., McKilligin E., Pei L., Watson M.A., Collins A.R., Laffitte B.A., Chen M., Noh G., Goodman J., Hagger G.N., Tran J., Tippin T.K., Wang X., Lusis A.J., Hsueh W.A., Law R.E., Collins J.L., Willson T.M., Tontonoz P. Synthetic LXR ligand inhibits the development of atherosclerosis in mice. Proc. Natl. Acad. Sci. 2002;99:7604–7609. doi: 10.1073/pnas.112059299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tangirala R.K., Bischoff E.D., Joseph S.B., Wagner B.L., Walczak R., Laffitte B.A., Daige C.L., Thomas D., Heyman R.A., Mangelsdorf D.J., Wang X., Lusis A.J., Tontonoz P., Schulman I.G. Identification of macrophage liver X receptors as inhibitors of atherosclerosis. Proc. Natl. Acad. Sci. 2002;99:11896–11901. doi: 10.1073/pnas.182199799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang X., Collins H.L., Ranalletta M., Fuki I.V., Billheimer J.T., Rothblat G.H., Tall A.R., Rader D.J. Macrophage ABCA1 and ABCG1, but not SR-BI, promote macrophage reverse cholesterol transport in vivo. J. Clin. Invest. 2007;117:2216–2224. doi: 10.1172/JCI32057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Münch G., Bültmann A., Li Z., Holthoff H.P., Ullrich J., Wagner S., Ungerer M. Overexpression of ABCG1 protein attenuates arteriosclerosis and endothelial dysfunction in atherosclerotic rabbits. Heart Int. 2012;7:57–64. doi: 10.4081/hi.2012.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Avior Y., Bomze D., Ramon O., Nahmias Y. Flavonoids as dietary regulators of nuclear receptor activity. Food Funct. 2013;4:831–844. doi: 10.1039/c3fo60063g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee K.Y., Choi H.S., Choi H.S., Chung K.Y., Lee B.J., Maeng H.J., Seo M.D. Quercetin directly interacts with vitamin D Receptor (VDR): structural implication of VDR activation by quercetin. Biomol. Ther. 2016;24:191–198. doi: 10.4062/biomolther.2015.122. [DOI] [PMC free article] [PubMed] [Google Scholar]