Abstract
Study Design:
Randomized control trial.
Objective:
The purpose of the study is to evaluate the safety and efficacy of tranexamic acid in reducing blood loss when administered through various routes in instrumented spine surgeries.
Methods:
A total of 104 patients undergoing instrumented spine surgery were randomly assigned to 4 groups (n = 26 in each group). Groups included (1) ivTXA—intravenous administration of tranexamic acid (TXA) 1 hour prior to surgery, (2) loTXA—local infiltration of TXA bilaterally into the paraspinal musculature prior to incision, (3) tTXA—topical application of TXA just before wound closure, and (4) control group. Outcome measures included intraoperative blood loss, postoperative blood loss, need for blood transfusion, length of hospital stay, and hematological parameters.
Results:
All the 3 different modes of TXA administration were found to be effective in reducing blood loss in the treated groups compared with the control group. Intraoperative blood loss was significantly reduced in ivTXA (223.6 ± 40.1 mL, P < .0001) and loTXA (256.07 ± 119 mL, P = .0039) groups when compared with controls (344 ± 88.5 mL).The postoperative blood loss was least in tTXA followed by ivTXA, loTXA, and controls. There was 67% reduction in need for blood transfusion in tTXA group, 55.5% reduction in ivTXA group, and 33% reduction in loTXA group when compared with the control group.
Conclusion:
In instrumented spine surgery, ivTXA and loTXA were found to be equally effective in reducing the intraoperative blood loss. The tTXA has better postoperative blood conserving effects. This is the first study to detail about safety and efficacy on local infiltration of TXA in spine surgery, which is an effective and safe method for reducing intraoperative blood loss.
Keywords: tranexamic acid, spine surgery, local application, topical wash, blood loss
Introduction
Excessive blood loss in spine surgery is a complication often encountered in multilevel fusions and still remains a challenge to be overcome.1 It may lead to undesirable consequences such as anemia, prolonged hospital stay, transfusion requirement, and increased incidences of wound infection, thereby negatively affecting patient’s outcomes.2 Causes for significant blood loss in spine surgery are attributed to lengthy operative times, extensive dissection, and decortication of bone for achieving fusion. Osteotomies in complex deformities and preparation of endplates during interbody fusions are additional factors responsible for massive blood loss. Blood conservation strategies such as cell saver, preoperative autologous blood donation, and acute haemodilution reduce perioperative blood loss resulting in decreased requirement for blood transfusion. However, these strategies are believed not to have an impact on bleeding from surgical wound.3 Despite controlled hypotensive anesthesia, allogenic blood transfusions are often required in complex spine surgeries leading to well-known complications such as blood-borne disease transmission, infections, and more commonly, transfusion reactions.4
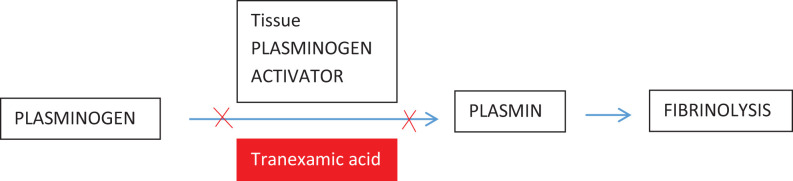
Tranexamic acid (TXA) is antifibrinolytic drug (synthetic lysine analogue) acting by competitive blocking the lysine binding site of plasminogen, plasmin, and tissue plasminogen activator.5 It primarily works by impairing the binding capacity of plasminogen and tissue plasminogen activator resulting in decreased conversion of plasminogen to plasmin, which causes dissolution of fibrin clots.6 Apart from preventing clot breakdown, TXA also improves the impaired platelet function thereby contributing to the blood conservation in 2 ways.7
TXA was essentially popularized through CRASH-2 (Clinical Randomization of an Anti-fibrinolytic in Significant Haemorrhage) trauma trial. Since then, the number of articles examining the peri- and postoperative blood saving effects of TXA have increased by more than 10-fold.8 Intravenous TXA (ivTXA), although extensively used in cardiac surgeries, is now frequently being used in all the orthopedic procedures such as total hip arthroplasty, total knee arthroplasty, trauma, and spine surgeries.8-12 However, systemic side effects of intravenous tranexamic acid are rare but not uncommon. The concept of acute fibrinolysis resulting from surgical trauma and being persisted in the postoperative period has incited authors to investigate the effects of topical tranexamic acid (tTXA). The effect of tTXA is widely established in joint replacement procedures where it is applied via intra-articular injection after closure of the wound or through irrigation into the wound just before closure.13-16 More recently, tTXA has also been used in spinal surgeries.3,17,18 However, the consensus regarding the usage of tTXA in spine surgeries have not been clearly established due to inconclusive results. Local infiltration of tranexamic acid (loTXA) has also been studied in trauma surgeries where intramuscular or subfascial infiltration was given before wound closure.19 Present study evaluates the efficacy of Intravenous, Local infiltration and topical administration of TXA in reducing blood loss in elective spine surgery.
Materials and Methods
The study design is a prospective randomized control trial done in a single institution from October 2017 to August 2018. Approval from the Institutional Ethics Committee was obtained for the study. After age and body mass index (BMI) were matched, 104 cases of single or dual level lumbar fixation with interbody fusions were included. Patients with low preoperative hemogram values (ie, hemoglobin <10 mg/dL in females; hemoglobin <11 mg/dL in males), low platelet counts (<100 × 109/L), bleeding disorders, coagulopathies, on drugs (like aspirin, clopidogrel, and nonsteroidal anti-inflammatory drugs since 1 week prior to surgery), international normalized ratio (INR) >1.4, prolonged activated partial thromboplastin time (APTT; 1.4 × normal), hepatorenal, or cardiopulmonary dysfunction were excluded from the study. General anesthesia was given for all the procedures and were performed by a surgeon with significant expertise in spine surgery. Patients with intraoperative surgical complications like dural tears were excluded from the study to obtain a homogenous group.
Surgical technique did not vary, and the protocol was similar in all the cases with no major variations. All the cases included in the study were diagnosed with degenerative grade 1 or 2 spondylolisthesis. All the cases were treated with pedicle screw fixations and single level interbody fusions. Laminectomy, complete removal of unilateral facet joint for interbody fusion, and bilateral foraminotomy were performed in all the cases. Single-level interbody fusion was performed in all the cases with additional posterolateral fusion in the adjacent level (in case of dual-level fixations).
All cases have been randomly divided into 4 groups.
Intravenous tranexamic acid (ivTXA): Single dose of 1 g TXA was administered intravenously 1 hour prior to surgery (n = 26 cases).
Topical tranexamic acid (tTXA): After securing hemostasis with radiofrequency bipolar and excluding the possible incidental dural tears by Valsalva maneuver, 1 g of TXA diluted in 100 mL normal saline was poured into surgical wound followed by a wait time of 5 minutes. Then solution was sucked off completely followed by drain insertion and wound closure (n = 26 cases).
Local infiltration of tranexamic acid (loTXA): Prior to the incision, 1 g of TXA was divided equally and administered bilaterally into the paraspinal muscles (n = 26 cases).
Control group: 10 ml of 2% lignocaine with adrenaline (1 in 200 000 dilution) mixed in 10 ml normal saline was divided equally and administered bilaterally into the paraspinal muscles (n = 26 cases).
Estimation of intraoperative (IO) blood loss was done by applying the following formula:
where WSS is the weight of surgical sponges, WES, is the weight of dry sponges, VSC is the volume of suction canisters, and VIF is the volume of irrigation fluids. (Note: 1 mL of blood approximately weighs 1 g.)
At the end of the surgery, 12 size closed vacuum suction drain was placed deep to the fascia before closure. Amount of total drainage at 24, 48, and 72 hours postsurgery was recorded. Suction drain was removed when output was less than 50 mL/24-hour period after postoperative day 3. Other clinical data includes age, sex, height, weight, BMI, packed cell volume (PCV), prothrombin time (PT), APTT, INR, hemoglobin levels at 24, 48, and 72 hours, requirement of blood transfusions, length of stay, and duration of surgery.
Transfusion was carried out in the patients with hemoglobin <8 g/dL and for patients with clinical symptoms of anemia such as hypotension and persistent tachycardia.
Statistical Analysis
Pearson chi-square test was used to analyze categorical variables. Differences were analyzed using 1-way analysis of variance with level of statistical significance set at P < .05. Data was analyzed using SPSS version 24.0 (IBM COrp, Armonk, NY).
Results
Demographics
A total of 104 cases (55 females and 49 males) who met the inclusion criteria participated in the study. After applying block randomization scheme, 26 patients were assigned to each group. All patients underwent posterior spinal instrumentation and single level interbody fusion. There were no statistical differences in age, gender, and BMI in all 4 groups. Surgical components (length of incision, duration of surgery, number of levels operated) also showed no statistical differences between the groups (Table 1).
Table 1.
Age, Gender, and BMI of 4 Groups.a
| Control | loTXA | ivTXA | tTXA | |
|---|---|---|---|---|
| Age (years) | 50.8 ± 3.4 | 48.0 ± 2.3 | 50.3 ± 3.2 | 51.9 ± 2.8 |
| Sex (male:female), n | 14:12 | 10:16 | 12:14 | 13:13 |
| BMI (kg/m2) | 27.6 ± 1.4 | 26.1 ± 1.9 | 25.8 ± 2.3 | 25.6 ± 2.1 |
| Duration of surgery (min) | 130 ± 10.3 | 128 ± 9.4 | 142 ± 12.4 | 135 ± 9.7 |
| Length of incision (cm) | 8.3 ± 1.2 | 9.2 ± 2.1 | 8.7 ± 1.4 | 8.7 ± 1.4 |
Abbreviations: BMI, body mass index; TXA, tranexamic acid; ivTXA, intravenous administration of TXA; loTXA, local infiltration of TXA; tTXA, topical application of TXA.
a All values are expressed as mean ± SD unless mentioned otherwise.
Hematological Parameters
Hemoglobin and PCV values were taken preoperatively, on postoperative days 1, 2, and 3. PT, APTT, and INR were documented on preoperative and discharge days (Table 2). There were no statistical differences in hemoglobin, PCV, PT, APTT, and INR values between and within the treatment groups.
Table 2.
Preoperative and Postoperative Blood Parameters of All Groups.
| Control | loTXA | ivTXA | tTXA | |
|---|---|---|---|---|
| Preop Hb (g/L) | 130.2 ± 4.5 | 130.0 ± 4.8 | 130.4 ± 3.2 | 120.8 ± 5.3 |
| Preop PCV (%) | 40.3 ± 2.1 | 39.1 ± 2.4 | 39.2 ± 2.3 | 40.6 ± 2.3 |
| Preop PT (s) | 14.6 ± 0.9 | 14.5 ± 1.1 | 14.9 ± 0.7 | 14.6 ± 1.1 |
| Preop APTT (s) | 31.4 ± 2.2 | 31.8 ± 1.7 | 32.7 ± 2.1 | 32.1 ± 2.2 |
| Preop INR | 1.06 ± 0.2 | 1.06 ± 0.2 | 1.06 ± 0.2 | 1.1 ± 0.3 |
| Postop Hb (g/L)-24 h | 100.5 ± 5.5 | 100.9 ± 6.3 | 105.6 ± 4.1 | 100.5 ± 4.3 |
| Postop PCV (%)-24 h | 33.1 ± 2.1 | 33.5 ± 1.9 | 34.9 ± 2.0 | 34.1 ± 2.2 |
| Postop Hb (g/L)-48 h | 90.5 ± 4.8 | 100.9 ± 5.8 | 110.4 ± 3.3 | 90.9 ± 7.3 |
| Postop PCV (%)-48 h | 29.2 ± 2.9 | 31.2 ± 2.1 | 33.5 ± 2.0 | 32.9 ± 1.9 |
| Postop Hb (g/L)-72 h | 90.8 ± 6.4 | 100.6 ± 4.3 | 110.8 ± 3.3 | 90.8 ± 5.9 |
| Postop PCV (%)-72 h | 30.6 ± 1.8 | 33.04 ± 1.9 | 34.4 ± 2.3 | 32.0 ± 2.0 |
| Postop PT-72 h (s) | 14.9 ± 0.9 | 15.5 ± 1.2 | 15.4 ± 1.1 | 15.7 ± 1.3 |
| Postop APTT-72 h (s) | 33.98 ± 2.7 | 34.8 ± 2.1 | 36.3 ± 3.2 | 36.9 ± 3.2 |
| Postop INR-72 h | 1.06 ± 0.2 | 1.1 ± 0.3 | 1.15 ± 0.2 | 1.1 ± 0.3 |
Abbreviations: TXA, tranexamic acid; ivTXA, intravenous administration of TXA; loTXA, local infiltration of TXA; tTXA, topical application of TXA; preop, preoperative; postop, postoperative; Hb, hemoglobin; PCV, packed cell volume; PT, prothrombin time; APTT, activated partial thromboplastin time; INR, international normalized ratio.
Intraoperative Blood Loss
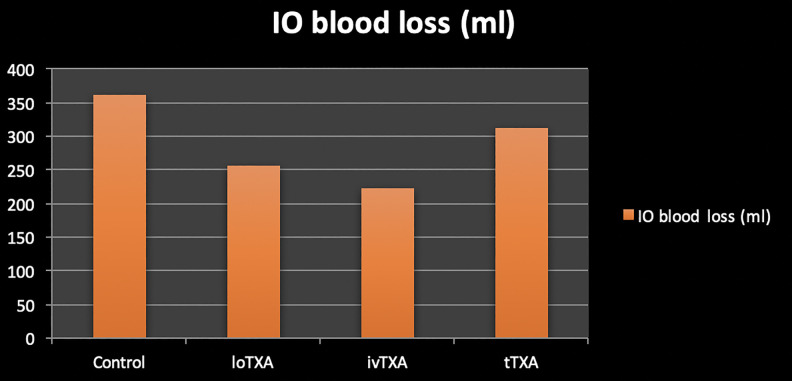
Outcomes of IO blood loss are summarized in Table 3 and Figure 1. IO blood loss (mL) in control group was 344 ± 88.5, in loTXA was 256.07 ± 119, in ivTXA was 223.6 ± 40.1, and in tTXA was 311.4 ± 84.67.
Table 3.
Summary of Intraoperative Blood Loss in All Groups.
| Control | loTXA | ivTXA | tTXA | |
|---|---|---|---|---|
| N | 26 | 26 | 26 | 26 |
| Sum of drain collection (mL) | 8944 | 6656 | 5814 | 8094 |
| Mean | 344 | 256.0714 | 223.6364 | 311.3636 |
| SD | 88.4631 | 119.0051 | 40.1305 | 84.6785 |
| P (vs control) | .0039* | <.0001* | .1803 | |
Abbreviations: TXA, tranexamic acid; ivTXA, intravenous administration of TXA; loTXA, local infiltration of TXA; tTXA, topical application of TXA.
* Indicates that P value is significant in the TXA groups when compared with the control group.
Figure 1.
Intraoperative blood loss of all 4 groups.
Drain Levels
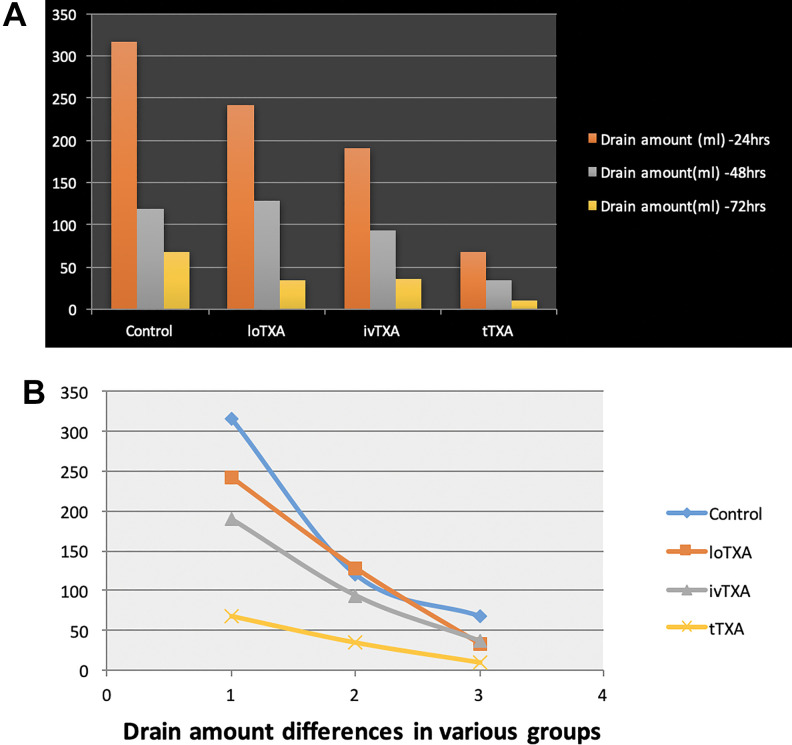
Drain amount (mL) of all patients was calculated on postoperative days 1, 2, and 3 (summarized in Table 4 and Figure 2a and b). Drain was constantly kept in suction mode and was removed when drain amount was less than 50 mL/24 h after the third day. As expected, trends of drain collection were declining from postoperative day 1 to 3 in all groups with control group having the highest amount of collection at any given time. On postoperative day 1, control group had 316.3 ± 110.1, loTXA had 241.4 ± 110.9, ivTXA had 190 ± 112.7, and tTXA had 67.3 ± 32.6 mL of average drain collection. Drain amount of loTXA and ivTXA was not significantly different on postoperative day 3. However, significant difference was noted in tTXA, which recorded lowest amount of collection on all postoperative days when compared with other groups.
Table 4.
Summary of Drain Amount Collected in All Groups.a
| Drain Amount | Control | loTXA | ivTXA | tTXA | F Ratio | P |
|---|---|---|---|---|---|---|
| Postoperative day 1 | 316.3 ± 110.1 | 241.4 ± 110.9 | 190 ± 112.7 | 67.3 ± 32.6 | 14.00 | <.00 001* |
| Postoperative day 2 | 119.4 ± 78.9 | 128.2 ± 65.5 | 93.6 ± 44.8 | 34.5 ± 18.6 | 5.85 | .001 627* |
| Postoperative day 3 | 67.1 ± 32.2 | 33.5 ± 29.3 | 36.3 ± 18.0 | 10 ± 10 | 12.45 | <.00 001* |
Abbreviations: TXA, tranexamic acid; ivTXA, intravenous administration of TXA; loTXA, local infiltration of TXA; tTXA, topical application of TXA.
a F ratios and P values are highly significant for between- and within-treatment groups. *Indicates significant difference.
Figure 2.
Drain amount differences in all groups.
Postoperative Blood Transfusions
Nine patients in the control group required (35%) blood transfusion, 6 patients (23%) in loTXA, 4 patients (15%) in ivTXA, and 3 patients (11.5%) in the tTXA group. No intraoperative blood transfusions were given. All the blood transfusions were done within 24 to 48 hours after surgery.
Duration of Hospitalization
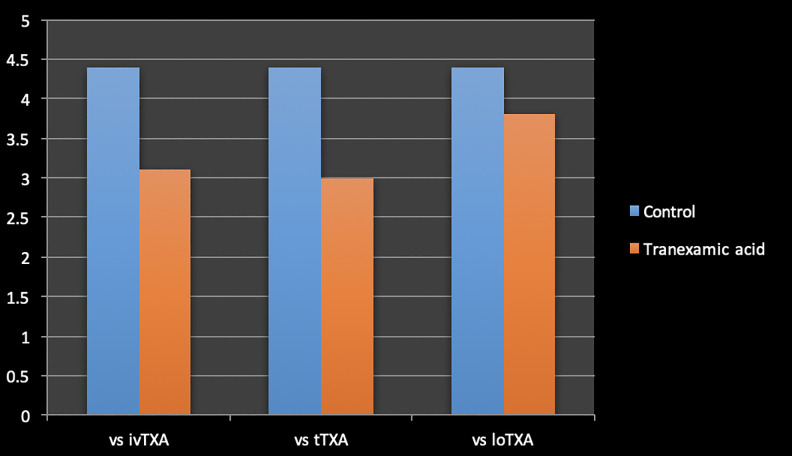
Average length of stay was 4.4 ± 1.6 days in the control group, 3.8 ± 1.2 days in the loTXA group, 3.1 ± 0.5 days in the ivTXA group, and 3 ± 0.5 days in the tTXA group. There was no statistical difference in duration of hospitalization between the groups receiving TXA, but patients receiving ivTXA and tTXA observed shorter duration of stay when compared with the control group (Table 5 and Figure 3).
Table 5.
Comparision of Length of Stay Between Tranexamic Receiving Groups and Control Group.
| Duration of Hospitalization—Comparison | P |
|---|---|
| 3.1 ± 0.5 (ivTXA) vs 4.4 ± 1.6 (control) | <.0001* |
| 3.0 ± 0.6 (tTXA) vs 4.4 ± 1.6 (control) | .0002* |
| 3.8 ± 1.2 (loTXA) vs 4.4 ± 1.6 (control) | .1324 |
Abbreviations: TXA, tranexamic acid; ivTXA, intravenous administration of TXA; loTXA, local infiltration of TXA; tTXA, topical application of TXA. *Indicates significant difference.
Figure 3.
Comparison of length of stay between tranexamic receiving groups and control group.
Discussion
TXA has been the first choice among hemostatic agents to decrease peri- and postoperative bleeding in various surgical cases.20-26 In spine surgery, resultant decorticated bony surfaces cannot be addressed by standard hemostatic maneuvers used for soft tissue bleeding. The blood loss from the exposed bony surfaces may continue even after wound closure. TXA can reduce this type of bone bleeding through its attenuation of enhanced fibrinolytic activity. Over the past decade, efficacy of TXA administered through different routes in spine surgery has also been established.3,8,9,17,18 Intravenous administration of TXA has been confirmed to be effective in decreasing blood loss during spine surgery.8,9 According to Hillman et al,27 ivTXA reduces the need for allogenic blood transfusion by one-third. As the pharmacology has been well understood, intravenous dosing protocols for the clinical usage of the drug have also been well established. Although the advantages of ivTXA are undeniable, its association with the risk of systemic complications are also not uncommon. It possesses potential risk of thrombosis in predisposed individuals with other rare systemic side effects such as visual disturbances, orthostatic symptoms, headaches, and myoclonus. To negate these undesirable systemic effects, topical route has been introduced and is being studied extensively over the recent past.13-16 According to Astedt et al,28 TXA acts directly at active bleeding and clot formation sites and not within the circulation. In a systematic review by Winter et al,29 the role of tTXA has been very well established in arthroplasty surgeries providing at least equal benefits of reducing blood loss especially in the postoperative period and minimizing the risk of systemic complications compared with ivTXA. The plasma concentration of tranexamic acid required to inhibit fibrinolysis is 5 to 10 μg/mL.30,31 Intravenous administration of 1 g TXA results in plasma concentrations >10 μg/mL for more than 2.5 hours.32 In a study by Ausen et al32 on pharmacokinetics of TXA, they observed a mean peak serum concentration of 4.9 to 5.2 µg/mL after topical administration in massive weight loss skin reducing surgery.
The recommended protocol is to irrigate the wound with tTXA solution (1 g in 100 mL saline) and soak for 5 minutes followed by complete suction and closure of the wound. More recently, local infiltration of TXA is being studied in trauma surgeries where TXA is being given intramuscularly (deep to the fascia) before closure of the wound. Two studies have been identified where loTXA is given in peritrochanteric fractures.19,33 Interestingly, results of these studies are contrast to each other with one reporting up to 43% reduction in transfusion requirement with loTXA and other observing no differences between loTXA and control groups. The optimal dosing and timing of the infiltration is unknown or not clearly established. In both the identified studies, 2 to 3 g of loTXA was given into vastus lateralis just before closure of the wound. However, this timing of application will not show any effect on intraoperative bleeding. Hence in our study, we preferred 500 mg of TXA each into bilateral paraspinal muscles (subfascial) prior to the incision.
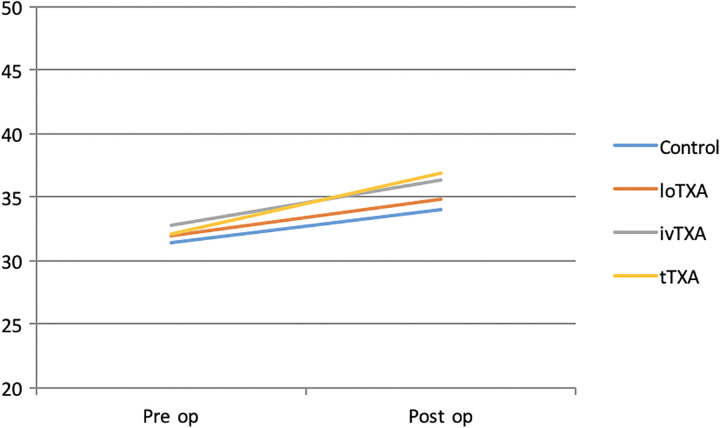
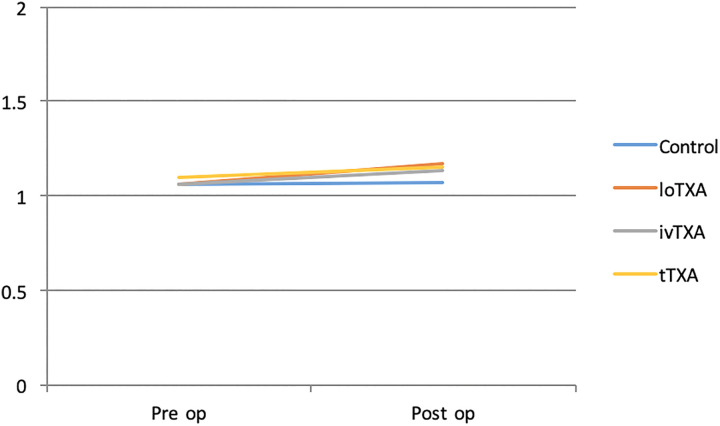
In our study, there were no statistical differences in demographics between the groups. Hemoglobin percentage has been well maintained on all postoperative days in ivTXA group when compared with other groups receiving TXA. In our study, postoperative coagulation profile within groups was assessed by PT, APTT, and INR. We observed no differences between controls and groups receiving TXA, which confirms that the route of TXA administration does not alter patient’s blood coagulation function (Figures 4 –6).
Figure 4.
Preoperative and postoperative Prothrombin time (PT) of all groups.
Figure 5.
Preoperative and postoperative activated partial thromboplastin time (APTT) of all groups.
Figure 6.
Preoperative and postoperative international normalized ratio (INR) of all groups.
IO blood loss has been observed to be the lowest in ivTXA among other groups followed by loTXA. Resultant IOBL was similar between tTXA and control group. This is an expected result as ivTXA and loTXA are given before incision whereas tTXA was given at the time of closure, which has no effect on IOBL.

The amount of drain collection has been one of the significant findings of our study. As expected, drain amount declined from postoperative day 1 to 3in all groups. Among all groups, the control group recorded highest amount of drain collection on all postoperative days. tTXA demonstrated very low amount of drain collection at any given time when compared to other groups receiving TXA and control group. Even though drain amount in ivTXA was lower than loTXA on postoperative days 1 and 2, resultant collection on postoperative day 3 was observed to be similar. Finally, the sum of drain collections until tube removal was found to be the lowest in tTXA followed by ivTXA, loTXA, and control (Table 3).

There was 33% reduction in need for blood transfusion in loTXA group, 55.5% reduction in ivTXA group, and 67% reduction in tTXA group when compared with the control group. This confirms that TXA irrespective of route of administration decreases the requirement of blood transfusions. However, need for postoperative transfusions is less in tTXA followed by ivTXA and loTXA.
Shorter duration of stay was observed to be a significant finding in ivTXA and tTXA when compared to loTXA and control (Table 5 and Figure 3). This finding was similar to other studies examining the efficacy of ivTXA and tTXA.3,18,34,35 This is attributed to the concept that blood volume loss is associated with increased rate of postoperative complications and delayed healing process.36 There were no systemic (eg, thromboembolic events) or local (eg, wound dehiscence) complications in any of the patients.
The findings of this study open up the field on further research as the efficacy of local TXA is to be studied in complex spine procedures where the blood loss is expected to be even more. Till date there is no literature on loTXA in spine surgery that has evaluated, through a prospective cohort, with larger sample size and observed its long-term effects related to fusion. Dose-related response of TXA also needs to be studied with low and high dosing of loTXA and tTXA to help in determining the optimal timing and adequate dosing of the drug. Whether combination therapies like using loTXA/ivTXA prior to incision and tTXA before closure will maximize benefits of reducing blood loss remains unknown and needs further research.
Conclusion
Intravenous and local methods of TXA administration are found to be equally effective in reducing the IO blood loss. The topical TXA has better postoperative blood conserving effects. This is the first study to detail about safety and efficacy on local infiltration of TXA in spine surgery, which is an effective and safe method for reducing IO blood loss.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Viswanadha Arun-Kumar, MBBS, MS  https://orcid.org/0000-0002-6322-4357
https://orcid.org/0000-0002-6322-4357
References
- 1. Nuttall GA, Horlocker TT, Santrach PJ, Oliver WC, Jr, Dekutoski MB, Bryant S. Predictors of blood transfusions in spinal instrumentation and fusion surgery. Spine (Phila Pa 1976). 2000;25:596–601. [DOI] [PubMed] [Google Scholar]
- 2. Zollo RA, Eaton MP, Karcz M, Pasternak R, Glance LG. Blood transfusion in the perioperative period. Best Pract Res Clin Anaesthesiol. 2012;26:475–484. [DOI] [PubMed] [Google Scholar]
- 3. Ren Z, Li S, Sheng L, et al. Efficacy and safety of topical use of tranexamic acid in reducing blood loss during primary lumbar spinal surgery: a retrospective case control study. Spine (Phila Pa 1976). 2017;42:1779–1784. [DOI] [PubMed] [Google Scholar]
- 4. Marik PE, Corwin HL. Efficacy of red blood cell transfusion in the critically ill: a systematic review of the literature. Crit Care Med. 2008;36:2667–2674. [DOI] [PubMed] [Google Scholar]
- 5. McCormack PL. Tranexamic acid: a review of its use in the treatment of hyperfibrinolysis. Drugs. 2012;72:585–617. [DOI] [PubMed] [Google Scholar]
- 6. Dunn CJ, Goa KL. Tranexamic acid: a review of its use in surgery and other indications. Drugs. 1999;57:1005–1032. [DOI] [PubMed] [Google Scholar]
- 7. Weber C, Görlinger K, Byhahn C, et al. Tranexamic acid partially improves platelet function in patients treated with dual antiplatelet therapy. Eur J Anaesthesiol. 2011;28:57–62. [DOI] [PubMed] [Google Scholar]
- 8. Shi H, Ou Y, Jiang D, Quan Z, Zhao Z, Zhu Y. Tranexamic acid reduces perioperative blood loss of posterior lumbar surgery for stenosis or spondylolisthesis: a randomized trial. Medicine (Baltimore). 2017;96:e5718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yu CC, Gao WJ, Yang JS, et al. Can tranexamic acid reduce blood loss in cervical laminectomy with lateral mass screw fixation and bone grafting: a retrospective observational study. Medicine (Baltimore). 2017;96:e6043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yuan QM, Zhao ZH, Xu BS. Efficacy and safety of tranexamic acid in reducing blood loss in scoliosis surgery: a systematic review and meta-analysis. Eur Spine J. 2017;26:131–139. [DOI] [PubMed] [Google Scholar]
- 11. Patel JN, Spanyer JM, Smith LS, Huang J, Yakkanti MR, Malkani AL. Comparison of intravenous versus topical tranexamic acid in total knee arthroplasty: a prospective randomized study. J Arthroplasty. 2014;29:1528–1531. [DOI] [PubMed] [Google Scholar]
- 12. Georgiadis AG, Muh SJ, Silverton CD, Weir RM, Laker MW. A prospective double blind placebo controlled trial of topical tranexamic acid in total knee arthroplasty. J Arthroplasty. 2013;28(8 suppl):78–82. [DOI] [PubMed] [Google Scholar]
- 13. Konig G, Hamlin BR, Waters JH. Topical tranexamic acid reduces blood loss and transfusion rates in total hip and total knee arthroplasty. J Arthroplasty. 2013;28:1473–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chang CH, Chang Y, Chen DW, Ueng SW, Lee MS. Topical tranexamic acid reduces blood loss and transfusion rates associated with primary total hip arthroplasty. Clin Orthop Relat Res. 2014;472:1552–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gilbody J, Dhotar HS, Perruccio AV, Davey JR. Topical tranexamic acid reduces transfusion rates in total hip and knee arthroplasty. J Arthroplasty. 2014;29:681–684. [DOI] [PubMed] [Google Scholar]
- 16. Alshryda S, Sukeik M, Sarda P, Blenkinsopp J, Haddad FS, Mason JM. A systematic review and meta-analysis of the topical administration of tranexamic acid in total hip and knee replacement. Bone Joint J. 2014;96-B:1005–1015. [DOI] [PubMed] [Google Scholar]
- 17. Liang J, Liu H, Huang X, et al. Using tranexamic acid soaked absorbable gelatin sponge following complex posterior lumbar spine surgery: a randomized control trial. Clin Neurol Neurosurg. 2016;147:110–114. [DOI] [PubMed] [Google Scholar]
- 18. Ren Z, Li S, Sheng L, et al. Topical use of tranexamic acid can effectively decrease hidden blood loss during posterior lumbar spinal fusion surgery: a retrospective study. Medicine (Baltimore). 2017;96:e8233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Virani SR, Dahapute AA, Panda I, Bava SS. Role of local infiltration of tranexamic acid in reducing blood loss in peritrochanteric fracture surgery in the elderly population. Malays Orthop J. 2016;10:26–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zufferey P, Merquiol F, Laporte S, et al. Do antifibrinolytics reduce allogeneic blood transfusion in orthopaedic surgery. Anesthesiology. 2006;105:1034–1046. [DOI] [PubMed] [Google Scholar]
- 21. Koster A, Faraoni D, Levy JH. Antifibrinolytic therapy for cardiac surgery: an update. Anesthesiology. 2015;123:214–221. [DOI] [PubMed] [Google Scholar]
- 22. Choi W, Irwin MG, Samman N. The effect of tranexamic acid on blood loss during orthognathic surgery: a randomized controlled trial. J Oral Maxillofac Surg. 2009;67:125–133. [DOI] [PubMed] [Google Scholar]
- 23. Dadure C, Sauter M, Bringuier S, et al. Intraoperative tranexamic acid reduces blood transfusion in children undergoing craniosynostosis surgery: a randomized double-blind study. Anesthesiology. 2011;114:856–861. [DOI] [PubMed] [Google Scholar]
- 24. Gluud LL, Klingenberg SL, Langholz SE. Systematic review: tranexamic acid for upper intestinal bleeding. Aliment Pharmacol Ther. 2008;27:752–758. [DOI] [PubMed] [Google Scholar]
- 25. George A, Kumar R, Kumar S, Shetty S. A randomized control trial to verify the efficacy of pre-operative intra venous tranexamic acid in the control of tonsillectomy bleeding. Indian J Otolaryngol Head Neck Surg. 2011;63:20–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Crescenti A, Borghi G, Bignami E, et al. Intraoperative use of tranexamic acid to reduce transfusion rate in patients undergoing radical retropubic prostatectomy: double blind, randomised, placebo controlled trial. BMJ. 2011;343:d5701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hillman J, Fridriksson S, Nilsson O, Yu Z, Säveland H, Jakobsson KE. Immediate administration of tranexamic acid and reduced incidence of early rebleeding after aneurysmal subarachnoid hemorrhage: a prospective randomized study. J Neurosurg. 2002;97:771–778. [DOI] [PubMed] [Google Scholar]
- 28. Astedt B, Liedholm P, Wingerup L. The effect of tranexamic acid on the fibrinolytic activity of vein walls. Ann Chir Gynaecol. 1978;67:203–205. [PubMed] [Google Scholar]
- 29. Winter SF, Santaguida C, Wong J, Fehlings MG. Systemic and topical use of tranexamic acid in spinal surgery: a systematic review. Global Spine J. 2016;6:284–295. doi:10.1055/s-0035-1563609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rozen L, Faraoni D, Torres CS, et al. Effective tranexamic acid concentration for 95% inhibition of tissue-type plasminogen activator induced hyperfibrinolysis in children with congenital heart disease: a prospective, controlled, in-vitro study. Eur J Anaesthesiol. 2015;32:844–850. [DOI] [PubMed] [Google Scholar]
- 31. Yee BE, Wissler RN, Zanghi CN, Feng C, Eaton MP. The effective concentration of tranexamic acid for inhibition of fibrinolysis in neonatal plasma in vitro. Anesth Analg. 2013;117:767–772. [DOI] [PubMed] [Google Scholar]
- 32. Ausen K, Pleym H, Liu J, et al. Serum concentrations and pharmacokinetics of tranexamic acid after two means of topical administration in massive weight loss skin-reducing surgery. Plast Reconstr Surg. 2019;143:1169e–1178e. doi:10.1097/PRS.0000000000005620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Drakos A, Raoulis V, Karatzios K, et al. Efficacy of local administration of tranexamic acid for blood salvage in patients undergoing intertrochanteric fracture surgery. J Orthop Trauma. 2016;30:409–414. [DOI] [PubMed] [Google Scholar]
- 34. Xu D, Zhuang Q, Li Z, Ren Z, Chen X, Li S. A randomized controlled trial on the effects of collagen sponge and topical tranexamic acid in posterior spinal fusion surgeries. J Orthop Surg Res. 2017;12:166 doi:10.1186/s13018-017-0672-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tse EY, Cheung WY, Ng KF, Luk KD. Reducing perioperative blood loss and allogeneic blood transfusion in patients undergoing major spine surgery. J Bone Joint Surg Am. 2011;93:1268–1277. [DOI] [PubMed] [Google Scholar]
- 36. Elgafy H, Bransford RJ, McGuire RA, Dettori JR, Fischer D. Blood loss in major spine surgery: are there effective measures to decrease massive hemorrhage in major spine fusion surgery? Spine (Phila Pa 1976). 2010;35(9 suppl):S47–S56. [DOI] [PubMed] [Google Scholar]