Abstract
Study Design:
Retrospective analysis.
Objectives:
The objective of this study was to analyze the feasibility of correcting double-curve scoliosis using dynamic scoliosis correction (DSC, also known as vertebral body tethering), which requires a bilateral anterior approach with deflation of both lungs. Typically, this approach falls under the exclusionary criteria for the eligibility for anterior scoliosis surgery. No data exists on the feasibility of single-staged bilateral DSC.
Methods:
A retrospective analysis was performed utilizing the data from 25 patients who underwent a bilateral anterior thoracic approach and instrumentation. Thirty-day postoperative complication rates were analyzed. A learning curve subanalysis was also performed to compare the first 12 patients to the remainder of the 13 patients, with a T-test (P ≤ .05).
Results:
Of the 25 patients treated, there was 1 intraoperative event: After performing lumbar DSC, the contralateral DSC was abandoned due to unexpected pleural scarring and staged selective thoracic fusion was performed. We observed 4 postoperative complications: 2 patients had recurrent pleural effusions, 1 patient was diagnosed with pneumonia, and 1 patient had a minor pulmonary embolism without cardiopulmonary consequences (after an international 24 hour flight). All patients recovered well. We observed a significant influence of learning curve on surgical time (328 vs 280 min, P = .03) and blood loss (480 vs 197 mL, P = .03).
Conclusion:
Data suggests that bilateral, single-stage surgery for DSC is feasible albeit with an elevated complication rate that may partially attributable to the learning curve. Future research should focus on the cause of pulmonary complications and include a matched comparative analysis with traditional posterior fusion.
Keywords: dynamic scoliosis correction, vertebral body tethering, idiopathic scoliosis, bilateral approach, complication rate, feasibility
Introduction
Anterior surgical approaches to the treatment of scoliosis have been largely replaced by posterior techniques.1,2 This shift can be explained by the technical improvements of posterior systems, which represent valid alternatives to anterior fusion. Another contributing factor in this shift, revolves around a lack of training in anterior techniques. Together, these factors led many to believe that anterior approaches to scoliosis treatment will soon be a thing of the past.
However, the implementation of new and evolving techniques has the power to redirect and ultimately revive anterior scoliosis surgery. An example of one of these techniques is dynamic scoliosis correction (DSC), also known as vertebral body tethering (VBT). The role of DSC is currently under investigation, as further research is needed to identify the ideal candidate for this technique and to understand its relevance in the therapy of scoliosis.
DSC requires an anterior approach to the spine from the convex side. For this reason, a double-curve scoliosis would require a bilateral approach, which is typically included in the exclusion criteria for eligibility for anterior scoliosis surgery.3 Lowe et al4 summarized that anterior fusion is only recommended for curves Lenke type 1 and 5, opting for posterior fusion for double curves. As a result, a bilateral anterior approach was not deemed feasible, and many subsequent studies failed to include perioperative data on these patients. The question remains if single-staged anterior surgical treatment for scoliosis is feasible. Finding the answer to this question may have a profound impact not only on patient education and counseling but on surgical planning as well.
In June 2017, we began performing single-staged anterior DSC for patients with double-curve scoliosis. We analyzed the perioperative data as well as the 30-day complication rate for our first 25 patients.
Methods
A retrospective analysis was performed utilizing the data obtained from our first 25 patients who underwent a bilateral anterior thoracic approach and instrumentation of the spine. Only immature patients (Risser 0-4, Sanders 1-7) who had been operated at our institution were included in this study. Off note, the last author has operated on a few more patients with other surgeons at other hospitals, but these cases were excluded due to a lack of complete perioperative data and ultimate variations in postoperative care. The 30-day postoperative complication rates of these 25 patients were carefully analyzed, paying close attention to a subanalysis for a potentially confounding learning curve by comparing our first 12 versus our next 13 patients with a T test (P ≤ .05). The statistical analysis was conducted utilizing Microsoft Excel 2017.
Nomenclature
In the United States, this surgical technique has been popularized under the name of vertebral body tethering (VBT), a modification of vertebral body stapling (VBS).5,6 However, it is our belief that VBT is limited in that it only describes a growth modulation process, neglecting the potential for intraoperative correction. Therefore, in Germany we introduced the term dynamic scoliosis correction (DSC) and will use DSC instead of VBT. In addition, we use the term double-curve rather than double major curve.
We are in the process of analyzing our data to identify the ideal candidate for DSC, but currently, we instrument (in selected patients) all vertebrae that cross the midline (Lenke type C modifier), irrespective of whether the curve is structural or not.
Surgical Technique
Patients are positioned in a strict lateral position. We routinely start with instrumentation of the lumbar/thoracolumbar curve. The mid lumbar spine is exposed from L2 to L4 using a mini-open retroperitoneal approach. L1 and the levels above are instrumented through a mini-open intercostal, transpleural approach. To instrument L1, we perform a diaphragm split, through which the cord is tunneled following screw placement. A chest tube is inserted, and the patient is then turned to the opposite lateral position after wound closure. The instrumentation of the opposite side takes place entirely in the thoracic cavity, therefore not requiring a diaphragm split. Two to 3 mini-open intercostal incisions plus 1 to 3 thoracoscopic portals are required to visualize and instrument the curve.
A double lumen tube is used in every patient and the lung on the side that is being addressed is collapsed during almost the entire instrumentation. The peripheral neural activity is controlled through evoked motoric potentials after screw placement and after performing the correction of the curve.
Results
The average number of instrumented vertebrae was 11.7. Upper instrumented vertebra (UIV) on the right was T4 in 2 cases, T5 in 21 cases, T6 in 1 case, and T9 in 1 case. Lower instrumented vertebra (LIV) on the right was T10 in 2 cases, T11 in 18 cases, T12 in 4 cases, and L1 in 1 case. LIV on the right side corresponded to UIV on the left side. LIV on the left side was L3 in 20 cases and L4 in 5 cases. Mean anesthesia time was 425 ± 50 minutes, the mean surgical time was 297 ± 63 minutes, including repositioning and redraping (time from incision of the first side to suture of the second side). Mean blood loss was 358 ± 260 mL. Four patients received autologous blood transfusion after cell salvage. Three patients required a heterologous blood transfusion.
All patients were monitored at the intermediate care unit (ICU) for 3.5 ± 1.3 days. None of our patients required cardiocirculatory or pulmonary support during this time. All patients were able to stand up and walk in the room within the second postoperative day and were able to climb stairs 4 to 6 days after surgery. The intravenous pain medication could be discontinued 3 to 5 days after surgery. Patients were hospitalized an average of 9.0 ± 1.8 days. Perioperative data is listed in Table 1.
Table 1.
Summary of the Demographic and Perioperative Data of Our Patients (n = 25 Patients).
| Characteristic | Average |
|---|---|
| Age (years) | 14.5 |
| Sex, n | 8% male, 92% female |
| Risser | 2.5 |
| Sanders | 5.3 |
| Instrumented level | 11.7 |
| Anesthesia time (minutes) | 425 |
| Surgical time from incision of first side to suture of second side (minutes) | 297 |
| Blood loss (mL) | 358 |
| Intermediate care unit stay (days) | 3.5 |
| Inpatient stay (days) | 9.0 |
Of the 25 patients treated, we observed 1 intraoperative complication: After successfully performing lumbar DSC, the opposite side thoracic DSC had to be abandoned due to the unexpected presence of pleural scarring, that would not allow pulmonary deflation. The patient therefore received staged selective thoracic fusion. The patient recovered well, and postoperative x-rays showed a good correction of both curves and a satisfactory coronal balance.
Within the 30-day interval, we observed 4 postoperative complications (16%). Two patients had recurrent pleural effusions, 1 patient was diagnosed with pneumonia, and 1 patient had a minor pulmonary embolism without cardiopulmonary consequences (after an international 24-hour flight). Another patient also developed pleural effusion at 6 weeks postsurgery (not within the 30-day interval). For all the patients whose symptoms began after discharge (4), hospitalization at a local institution was required. The patients presenting with recurrent pleural effusions required invasive treatment (2 reinsertions of the chest tube, 1 explorative thoracoscopy with reinsertion of the chest tube). Postoperative complications are listed in Table 2.
Table 2.
Summary of the Postoperative Complications.
| Complication | Onset | Therapy |
|---|---|---|
| Right side recurring pleural effusion | Fifth postoperative day | Reinsertion of the chest tube for 2 days |
| Right side recurring pleural effusion due to minor lesion of the pulmonary ligament | Ninth postoperative day | Revision thoracic surgery, reinsertion of the chest tube. No repair of the pulmonary ligament required |
| Pneumonia | 2 weeks postoperatively | Inpatient antibiotic therapy |
| Minor pulmonary embolism | 3 weeks postoperatively— after a 24-hour flight | Enoxaparin for 1 month |
When comparing our first 12 with our next 13 patients, we observed a significant reduction of intubation time (first 12 patients = 453 minutes, next 13 patients = 397 minutes, P = .02), surgical time (first 12 patients = 328 minutes, next 13 patients = 280 minutes, P = .03), and blood loss (first 12 patients = 480 mL, next 13 patients = 197 mL, P = .03). Notably, all patients receiving autologous or heterologous blood transfusions were within the early phase of our learning curve. The length of inpatient stay also decreased significantly (first 12 patients = 10.3 days, next 13 patients = 8.1 days, P = .01).
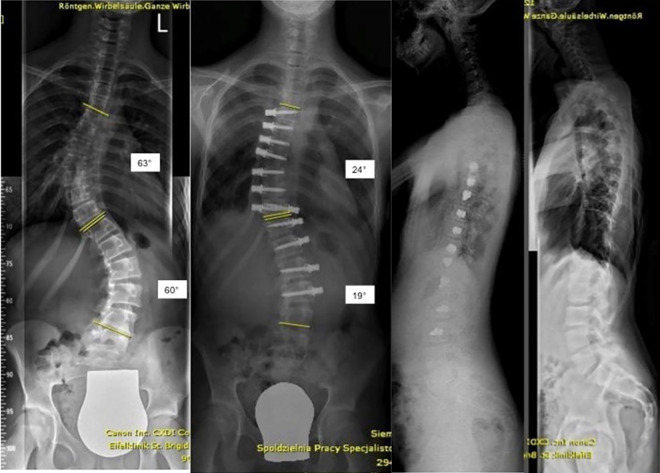
As a clinical example, in September 2018 we performed single-staged bilateral DSC on a 12-year-old girl who was skeletally immature (Risser 0). We instrumented 12 vertebrae in 257 minutes (anesthesia time 403 minutes), blood loss was 150 mL. Right lung deflation was insufficient and additional intraoperative manual lung retraction was required. The patient remained in the ICU for 3 days. During her stay, she required intravenous pain medication for 3 days and was able to climb stairs on the fifth postoperative day. The patient did not present any complication and could be discharged on the seventh postoperative day. The patient had an international 3-hour flight on the day of discharge. She was admitted for feeling sick on postoperative day 9 to a local hospital and was diagnosed with pleural effusion on the right. Explorative thoracoscopy was performed by a thoracic surgeon who found minor bleeding close to pulmonary attachment. A chest drain was inserted, and the patient recovered well afterward. Figure 1 shows her preoperative and 6 weeks postoperative radiographs.
Figure 1.
Preoperative and 6 weeks postoperative radiographs after bilateral dynamic scoliosis correction (details listed in “Results” section).
Discussion
This is the first study that analyzes perioperative data and the feasibility of bilateral thoracotomy for anterior scoliosis correction—a surgery that until recently was not commonly recommended or deemed possible. While anterior fusions for scoliosis have been increasingly displaced by posterior techniques, the introduction of DSC and VBT provides an additional option within the field of surgical treatment for scoliosis. There is a paucity of studies on DSC and VBT and, to the best of our knowledge, these studies only analyzed patients undergoing unilateral surgery.7-9 This study addresses patients with double curves who may be candidates for DSC. It helps establish a baseline of data to compare with patients undergoing posterior spinal fusion. Assuming that DSC remains a viable surgical approach, further research would have to be conducted to determine whether the treatment should be scheduled in stages or in a single session. Our data suggests that bilateral, single-stage surgery for dynamic scoliosis correction is possible, but the complication rate is still high. The comparison between the first 12 and next 13 operated patients shows that the learning curve of the surgeon allows for a decrease in anesthesia and surgical time, as well as a decrease in blood loss and need for transfusion.
We had 4 complications in the form of recurring pleural effusion, pneumonia, and pulmonary embolism. We believe that manual retraction of an insufficiently deflated lung may be the most probable cause of recurring pleural effusion. Therefore, we strongly recommend double lumen intubation. Routinely, we remove the chest tubes when the output is less than 200 mL over 24 hours. In case of insufficient intraoperative lung deflation that requires significant manual lung retraction, we suggest removal of the chest tubes when the output is less than 150 mL in 24 hours. Pleural incision should also be limited to the area of screw placement.
The duration of ICU time may seem long. At our institution, we cannot discharge patients to the ward until removal of both chest tubes. Without this restriction, the ICU stay could be reduced to the first postoperative day or the removal of the first chest tube. Intermediate care would also suffice. The average inpatient stay may also appear to be long. That is because most of our patients come from abroad and the inpatient stay is prolonged for safety reasons and social support reasons. As shown by the comparison between the first 12 and next 13 patients, a better understanding of the technique and the postoperative care required, leads to a reduction in inpatient stay. Notably, the average stay after DSC is shorter than the average inpatient stay following spondylodesis for scoliosis correction in Germany, which is 13 days (InEK GmbH, Siegburg, updated 2019). Discussions regarding the length of stay after these DSC procedures should be placed within the context of other spine procedures performed in Germany.
This study does have limitations. We did not provide statistically comparable radiographic data, nor is the aim of this article to promote DSC as a standard treatment for scoliosis. We are aware that additional data needs to be analyzed and continue to work toward that venture. These studies include the analysis of radiographic and clinical data and will be evaluated once our patients meet the minimal follow-up time of 24 months. However, given the demand from clinicians to report outcomes from DSC, we felt it was valid to report early outcomes from this select cohort.
In conclusion, the findings of our study add important information to the orthopedic literature, showing that single-session bilateral thoracotomy for scoliosis surgery is feasible and does not require staging. The complication rate is high. Further research is required to understand the causes of the complications reported and minimize extraneous factors that can increase the risk of these complications.
Footnotes
Authors’ Note: This retrospective review has been approved by the local ethics committee (University of Aachen).
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Alice Baroncini, MD  https://orcid.org/0000-0002-4383-3470
https://orcid.org/0000-0002-4383-3470
References
- 1. Lonner BS, Ren Y, Yaszay B, et al. Evolution of surgery for adolescent idiopathic scoliosis over 20 years: have outcomes improved? Spine (Phila Pa 1976). 2018;43:402–410. [DOI] [PubMed] [Google Scholar]
- 2. Trobisch PD, Ducoffe AR, Lonner BS, Errico TJ. Choosing fusion levels in adolescent idiopathic scoliosis. J Am Acad Orthop. 2013;21:519–528. [DOI] [PubMed] [Google Scholar]
- 3. Lenke LG, Betz RR, Haher TR, et al. Multisurgeon assessment of surgical decision-making in adolescent idiopathic scoliosis: curve classification, operative approach, and fusion levels. Spine (Phila Pa 1976). 2001;26:2347–2353. [DOI] [PubMed] [Google Scholar]
- 4. Lowe TG, Betz RR, Lenke LG, et al. Anterior single-rod instrumentation in the thoracic and lumbar spine: saving levels. Spine (Phila Pa 1976). 2003;28:S208–S216. [DOI] [PubMed] [Google Scholar]
- 5. Trobisch PD, Samdani A, Cahill P, Betz RR. Vertebral body stapling as an alternative in the treatment of idiopathic scoliosis. Oper Orthop Traumatol. 2011;23:227–231. [DOI] [PubMed] [Google Scholar]
- 6. Jain V, Lykissas M, Trobisch P, et al. Surgical aspects of spinal growth modulation in scoliosis correction. Instr Course Lect. 2014;63:335–344. [PubMed] [Google Scholar]
- 7. Samdani AF, Ames RJ, Kimball JS, et al. Anterior vertebral body tethering for immature adolescent idiopathic scoliosis: one-year results on the first 32 patients. Eur Spine J. 2015;24:1533–1539. [DOI] [PubMed] [Google Scholar]
- 8. Samdani AF, Ames RJ, Kimball JS, et al. Anterior vertebral body tethering for idiopathic scoliosis: two-year results. Spine (Phila Pa 1976). 2014;39:1688–1693. [DOI] [PubMed] [Google Scholar]
- 9. Newton PO, Kluck DG, Saito W, Jaszay B, Bartley CE, Bastrom TP. Anterior spinal growth tethering for skeletally immature patients with scoliosis: a retrospective look two to four years postoperatively. J Bone Joint Surg Am. 2018;100:1691–1697. [DOI] [PubMed] [Google Scholar]