Abstract
Metachromatic leukodystrophy (MLD) is a glycosphingolipid storage disease caused by deficiency of the lysosomal enzyme arylsulfatase A (ASA) or its activator protein saposin B. MLD can affect all age groups in severity varying from a severe fatal form to milder adult onset forms. Diagnosis is usually made by measuring leukocyte ASA activity. However, this test can give false negative or false positive laboratory results due to pseudodeficiency of ASA and saposin B deficiency, respectively. Therefore, we aimed to evaluate patients with suspected MLD in a Turkish population by comprehensive clinical, biochemical, radiological, and genetic analyses for molecular and phenotypic characterization. We analyzed 28 suspected MLD patients and 41 relatives from 24 families. ASA activity was found to be decreased in 21 of 28 patients. Sixteen patients were diagnosed as MLD (11 late infantile, 2 juvenile and 3 adult types), 2 MSD, 2 pseudodeficiency (PD) and the remaining 8 patients were diagnosed as having other leukodystrophies. Enzyme analysis showed that the age of onset of MLD did not correlate with residual ASA activity. Sequence analysis showed 11 mutations in ARSA, of which 4 were novel (p.Trp195GlyfsTer5, p.Gly298Asp, p.Arg301Leu, and p.Gly311Asp), and 2 mutations in SUMF1 causing multiple sulfatase deficiency, and confirmed the diagnosis of MLD in 2 presymptomatic relatives. All individuals with confirmed mutations had low ASA activity and urinary sulfatide excretion. Intra- and inter-familial variability was high for the same ARSA missense genotypes, indicating the contribution of other factors to disease expression. Imaging findings were evaluated through a modified brain MRI scoring system which indicated patients with protein-truncating mutations had more severe MRI findings and late-infantile disease onset. MRI findings were not specific for the diagnosis. Anti-sulfatide IgM was similar to control subjects, and IgG, elevated in multiple sulfatase deficiency. In conclusion, the knowledge on the biochemical, clinical and genetic basis of MLD was expanded, a modified diagnostic laboratory algorithm for MLD based on integrated evaluation of ASA activity, urinary sulfatide excretion and genetic tests was devised.
Keywords: Metachromatic leukodystrophy, Multiple sulfatase deficiency, Arylsulfatase A, Urinary sulfatide, Antibody, MRI, ARSA, SUMF1
1. Introduction
Metachromatic leukodystrophy (MLD, OMIM #250100, #249900) is a glycosphingolipid storage disease caused by deficiency of the lysosomal enzyme arylsulfatase A (ASA) encoded by ARSA, or of its activator saposin B encoded by PSAP. MLD is an autosomal recessive disorder with an estimated global incidence of 1/40,000‐1/100,000 [1], and 1/69,000 in Turkey [2]. To date, around 200 ARSA mutations have been described. ASA (E.C.3.1.6.8) hydrolyses sulfated glycosphingolipids (sulfatides), which are primarily located in myelin sheaths of the peripheral and central nervous systems (CNS), as well as in such visceral organs as the kidneys, gallbladder, and liver. In cases of ASA deficiency, progressive accumulation of undegraded sulfatides such as galactosylceramide sulfate in lysosomes and related organelles in sulfatide-abundant tissues causes clinical symptoms [1]. Experimental studies suggest an immune response against sulfatides which may contribute to pathogenesis of MLD [3,4].
Multiple sulfatase deficiency (MSD, OMIM #272200) is a similar autosomal recessive disorder caused by insufficient activity of sulfatase-modifying factor 1, encoded by SUMF1. MSD is rarer than MLD; however, it results in deficiency of several sulfatase enzymes including ASA and usually manifests as a more diverse phenotype with ichthyosis, skeletal deformities, and cardiomyopathy in addition to features of MLD [5].
The characteristic findings of MLD are progressive demyelination and neurodegeneration, which can appear at different ages, defining the late infantile, juvenile, and adult forms of the disease. The common signs of the late infantile and juvenile forms are spasticity, neuropathy, ataxia, seizures, and mental regression. Dementia, as well as gait and behavioral disturbances are characteristic of the adult form [1].
Laboratory diagnosis of MLD is, in practice, usually based on low ASA activity in leukocytes. Two drawbacks of leukocyte ASA activity measurement are low activity due to pseudodeficiency and normal activity despite saposin B deficiency. Urinary sulfatide analysis can tell apart saposin B deficient from healthy and pseudodeficient individuals, and thus is required for diagnosis of MLD [1]. In certain cases, arylsulfatase isoenzymes may need to be differentiated [6]. Genetic testing is particularly helpful for genetic counseling and screening of asymptomatic family members. The study evaluated individuals with suspected MLD in a Turkish cohort from a single institution by comprehensive clinical, biochemical, radiological, and genetic analyses for molecular and phenotypic characterization. Additionally, a novel MRI scoring system for comprehensive evaluation of disease severity in MLD is presented.
2. Materials and methods
2.1. Participants
Symptomatic individuals with clinically suspected MLD aged 20 months-49 years (n = 28) who applied to Hacettepe University Hospital's Pediatric Neurology and Neurology clinics and 41 clinically unaffected family members from 24 families were evaluated for this study. Suspicion of MLD was based on either existence of at least one clinical finding (gait disturbance, motor weakness/hypotonia, spasticity in the lower extremities, intellectual disability and gradual loss of speech in younger patients; cognitive and psychiatric symptoms, spastic paraparesis and/or polyneuropathy in adult patients) and MRI findings compatible with MLD (hyperintensity in periventricular and subcortical white matter). Asymptomatic individuals with a family history suspected MLD were also included. A total of 23 cranial MRI scans, 16 initial and 7 follow-up, obtained in various centers from MLD patients were reviewed by an experienced neuroradiologist, provided they comprised a minimum set of axial and coronal T2-weighted images. Contrast-enhanced T1-weighted images were examined when available. A modified MRI scoring system (0–49) was constructed based on Eichler et al. [7]. Segments of the corpus callosum and corticospinal tract were included [8]. Thalamus atrophy and T2-hypointensity, reported as imaging findings associated with MLD, were also added to the scoring system [9]. Additionally, any cranial nerve involvement and diffusion features were assessed in available scans. Electrophysiological nerve conduction studies were reviewed when available.
Immunological assays were performed in MLD/MSD patients (n = 18), and comparison groups consisting of autoimmune demyelinating disorders of the CNS: multiple sclerosis or neuromyelitis optica (MS-NMOSD group) (n = 15), healthy parents of affected individuals (n = 25), and age-matched healthy individuals (n = 10). All participants and/or parents provided written informed consent. The study protocol was approved by the Hacettepe University Ethics Committee (25.10.2016, GO 16/658–24).
2.2. Plasma and leukocyte lysosomal enzyme assays
Plasma and leukocytes were isolated from 10 ml of whole EDTA-blood. The activity of 12 lysosomal enzymes (plasma chitotriosidase, β-mannosidase, hexosaminidase A and total hexosaminidase, leukocyte galactocerebrosidase, α-mannosidase, α-fucosidase, β-glucuronidase, β-glycosidase, α-galactosidase, β-galactosidase, and arylsulfatase A) were measured using spectrophotometric and fluorometric substrates (Glycosynth, UK and Sigma, Germany) [[10], [11], [12], [13], [14], [15], [16]]. For ASA activity, we specifically used the protocol established in the Willink Biochemical Genetics Unit by Alan Cooper, adapted from the method of Baum et al. [10]. ASA enzyme analysis was performed using synthetic colorimetric substrate p-nitrocathecol sulfate in leukocytes. p-nitrocathecol sulfate, which has a high affinity for the enzyme, is also hydrolyzed by the other isoenzyme arylsulfatase B (ASB). In Baum's method ASB is inhibited at a high NaCl concentration [1,10]. Additionally, the enzyme activity was measured in relevant fractions of DEAE cellulose ion-exchange chromatography by fluorometric substrate 4-MU sulfate in the presence and absence of ASA inhibitor silver nitrate to confirm the activity measurements by p-nitrocathecol method which is described under Section 2.4. Protein levels in leukocyte supernatants were measured by the Lowry method [17].
2.3. Molecular analysis
In all individuals with ASA activity <50 μmol/g protein/h, all exons of ARSA including pseudodeficiency alleles at c.1055A > G (p.Asn352Ser) (PD1) and c.*96A > G (PD2) were sequenced using Big Dye Terminator v3.1 and ABI Prism 3500 Genetic Analyzer according to the manufacturer's protocols, and primers designed by Bognar et al. [18] and Ben Halim et al. [19]. Sanger sequencing of all exons of SUMF1 was performed when MSD was suspected. NM_000487.6, NM_182760.4, and NM_002778.4 RefSeq sequences were referred to for annotating variations in ARSA, SUMF1, and PSAP, respectively.
2.4. Diethylaminoethyl (DEAE) cellulose chromatography
Leukocytes resuspended in 10 mM TRIS/HCl buffer at pH 7.5 containing 0.1% Nonidet P-40 were homogenized by 5 freeze-thaw cycles. Total protein in supernatant fractions was measured using the Bicinchoninic acid (BCA) method. Supernatants of homogenized samples were loaded into 50% DEAE cellulose matrix columns and elutions were collected to determine ASA and ASB activities. Further elutions with 0.05–0.25 M NaCl gradient were obtained to collect matrix-bound fractions for determining ASA and ASB activities, as previously described [20]. The fluorogenic substrate 4-methylumbelliferyl sulfate (17 mM) was utilized for measuring isoenzyme activity in the fractions in the presence or absence of 125 μM AgNO3, a specific inhibitor of ASA [6,21,22].
2.5. Urine sulfatide determination via thin-layer chromatography (US-TLC)
First-void morning urine samples (20 ml), were adjusted to pH = 5 using glacial acetic acid, incubated for 16 h at 4 °C, and then centrifuged. The supernatant was discarded and glycolipids were extracted from the precipitate using chloroform/methanol (2:1, v/v). Next, the samples were applied to silica gel plates (20 × 20 cm) for TLC and run in chloroform/methanol/water (144:56:7, v/v/v) as the mobile phase, using 20 μmol C-18 sulfatide (Matreya, USA) as the standard. Following chromatography, glycolipids were visualized via orcinol spraying, followed by incubation at 110 °C for 5 min [23,24].
2.6. Immunological assays
Serum anti-sulfatide IgM and IgG levels were measured using ELISA kits (Mybiosource Inc., CA, USA), according to manufacturers' instructions.
3. Results
3.1. ASA activity and urinary sulfatide levels for identifying MLD and MSD patients
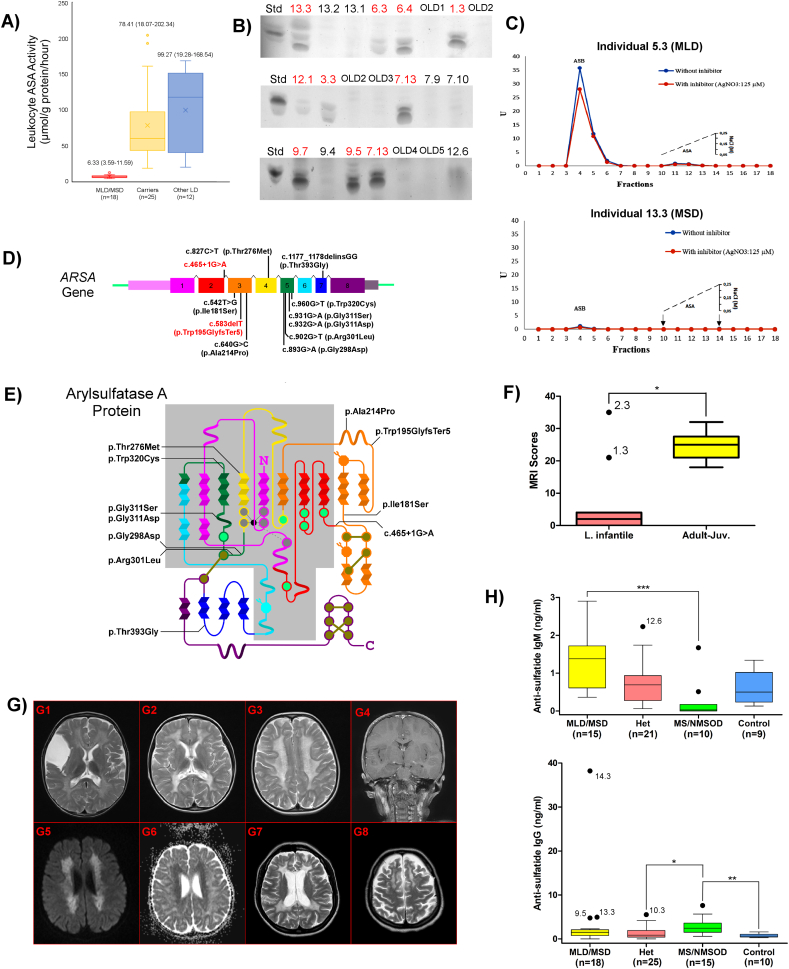
The activity of 12 leukocyte lysosomal enzymes were measured and US-TLC was performed in 28 suspected MLD patients and 41 of their relatives (Fig. 1A and B). ASA activity was <50 μmol/g protein/h in 21/69 participants: of these, 16 patients had homozygous or compound heterozygous ARSA mutations, and 2 patients had homozygous SUMF1 mutations (Table 1 and Fig. S1). All of these patients (n = 18) had ASA activity ≤11.6 μmol/g protein/h and tested positive for urinary sulfatides. In all, 11 disease-causing ARSA mutations, of which 4 are novel, were identified in exons 2–5, and 7; as well as 2 disease-causing SUMF1 mutations were found in exons 6 and 9 (Table 1, Fig. 1D and, E, and Fig. S1). All novel mutations affect evolutionarily conserved amino acid residues (Fig. S2).
Fig. 1.
Biochemical test results, genetic findings, and MRI evaluations in individuals with MLD/MSD. (A) Boxplots of ASA activity measurements in affected individuals, carriers, and individuals with other causes of leukodystrophy. Median and range values are indicated above each boxplot. (B) US-TLC results in affected individuals (red) and others (black). (C) DEAE cellulose chromatography for differential measurement of arylsulfatase A (ASA) and arylsulfatase B (ASB) activity in an individual with MLD (above) and in an individual with MSD (below). Note that only ASA activity is low in MLD, while both ASA and ASB activity are low in MSD. (D) The ARSA gene containing 8 exons with mutations discovered in this study. Protein-truncating mutations are shown in red. (E) The secondary structure of ASA adopted from Lukatela et al. [25]. Helices indicate α helices, pleated sheets indicate β-strands, dark yellow dots indicate cysteine residue forming disulfide bridges, octagonal amino acid residue indicates glycosylation targets. Grey and green dots show active site residues bonding and not bonding to Mg++ (represented by the black dot), respectively. Residues in the grey area lie in a conserved protein structure. Note that the color palette of the protein backbone matches the exons shown in (D). Mutations in this study are indicated on the protein. (F) Boxplots showing MRI scores in 2 MLD age groups. Comparison of 2 groups by Mann–Whitney U test showed a significant difference (*p < 0.05). Note that individuals with protein-truncating mutations (1.3 and 2.3) have higher MRI scores, being infantile-onset. (G) A collection from cranial MRI studies of MLD and MSD patients. Extensive bilateral white matter involvement is seen as T2 hyperintensity in (G1) to (G3). White matter involvement is prominent in the periventricular area, corpus callosum, internal capsules, and centrum semiovale. Tigroid pattern formed from T2 hypointense stripes and dots in the background of diffuse hyperintensity are easily seen in (G3). Bilateral thalami in (G1) and (G2) show T2 hypointensity and atrophy, which are more profound in (G2). Arachnoid cyst is an incidental finding in (G1). Coronal T1-weighted contrast-enhanced image shows bilateral linear enhancement along the cranial nerves (G4). Involvement of the centrum semiovale shows restricted diffusion as hyperintensity on trace diffusion image (G5) and low values on the corresponding ADC map (G6). Axial T2-weighted images in a 44-year old patient (individual 12.11) show severe white matter involvement accompanied by prominent volume loss (G7-8). Please note the preferential involvement of frontal white matter in the convexity (G8). (H) Serum anti-sulfatide IgM (above) and IgG (below) levels in the MLD/MSD patients, heterozygous carriers, individuals with MS-NMOSD, and controls. For all group comparisons, Kruskal-Wallis tests were performed, in case a significant difference were found then non-parametric multiple comparison test was conducted. (*p < 0.05, **p < 0.005, ***p < 0.001).
Table 1.
Genetic, clinical and biochemical findings in individuals with MLD/MSD
| Family ID | Individual ID | Type of mutation | Disease causing mutation | PD1 | PD2 | Reference | Type | Gender | Age of Onset | Age Evaluated for This Study | Consanguinity | Family History | First symptom | Gait Disorder/ Ataxia | Tendon Reflexes | Spasticity | Psychiatric/Behavioral | Cognitive Decline | Polyneuropathy | ASA activity (μmol/g protein/h) | Anti-sülfatid IgM | Anti-sülfatid IgG | Urine Sulfatide |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1.3 | Protein truncating | ARSA: c.583delT (p.Trp195GlyfsTer5) hom | NP | NP | nov. | L. Inf. | F | 12 mo | 29 mo | Rem. | No | Motor delay | + / - | ↓ | + | − | Severe | + | 4.47 | 2.13 | 2.28 | (+++) |
| 2 | 2.3 | Protein truncating | ARSA: c.465 + 1G > A (p.?) hom | NP | NP | 26 | L. Inf. | M | 13 mo | 24 mo | 2° | No | Imbalance | +/ + | ↓ | + | Irritable | Severe | + | 5.6 | NA | 1.03 | (+++) |
| 3 | 3.3 | Missense | ARSA: c.827C > T (p.Thr276Met) hom | NP | NP | 27 | L. Inf. | M | 13 mo | 24 mo | 1° | No | Frequent falls | + / - | ↑ | + | Irritable | Mod. | + | 7.3 | 1 | 0 | (++) |
| 4 | 4.3 | Missense | ARSA: c.640G > C (p.Ala214Pro) hom | NP | NP | 28 | L. Inf. | M | 14 mo | 20 mo | 1° | Sibling | Motor regression | + / + | ↓ | + | − | Mod. | NA | 5.91 | 0.36 | 1.57 | (++) |
| 5 | 5.3 | Missense | ARSA: c.932G > A (p.Gly311Asp) hom | Hom | NP | nov. | L. Inf. | F | 14 mo | 34 mo | 1° | No | Wide based walking, Imbalance | + / - | ↑ | + | − | Severe | NA | 5.05 | 0.5 | 0.57 | (+++) |
| 6 | 6.4 | Missense | ARSA: c.640G > C (p.Ala214Pro) het; c.960G > T (p.Trp320Cys) het | NP | NP | 28, 29 | L. Inf. | F | 14 mo | 8 mo | No | Sibling | Spasticity of legs, Frequent falls | - / - | N | − | − | − | NA | 8.35 | 0.97 | 1.4 | (+++) |
| 6.3 | Missense | ARSA: c.640G > C (p.Ala214Pro) het; c.960G > T (p.Trp320Cys) het | NP | NP | 28, 29 | L. Inf. | F | 18 mo | 24 mo | No | Sibling | Spasticity of legs, Irritability | + / - | ↑ | + | Irritable | Mod. | NA | 4.33 | 0.58 | 1.12 | (++) | |
| 7 | 7.11 | Missense | ARSA: c.931G > A (p.Gly311Ser) hom | NP | NP | 30 | L. Inf. | F | 24 mo | 30 mo | 1° | Cousin, Sibling | Imbalance | + / - | ↓ | + | − | None/ Mild | + | 4.91 | 2.46 | 0.89 | (+++) |
| 7.13 | Missense | ARSA: c.931G > A (p.Gly311Ser) hom | NP | NP | 30 | L. Inf. | F | 30 mo | 40 mo | 1° | Cousin, Sibling | Imbalance, Fatigue | + / - | ↓ | + | − | None/ Mild | + | 3.59 | 0.61 | 1.79 | (+++) | |
| 8 | 8.3 | Missense | ARSA: c.893G > A (p.Gly298Asp) hom | Hom | Hom | nov. | L. Inf. | M | 30 mo | 70 mo | 1° | No | Frequent falls | + / NA | ↓ | + | Stereotypic movements | Severe | + | 6.18 | 1.39 | 2.00 | (+++) |
| 9 | 9.7 | Missense | ARSA: c.931G > A (p.Gly311Ser) hom | NP | NP | 30 | L. Inf. | M | 30 mo | 26 mo | 1° | Siblings | Imbalance, Hypertrichosis | - / - | N | − | − | No | + | 11.6 | 1.49 | 0.86 | (+++) |
| 9.5 | Missense | ARSA: c.931G > A (p.Gly311Ser) hom | NP | NP | 30 | Juv. | M | 78 mo | 89 mo | 1° | Siblings | Speech impairment, Hypertrichosis | + / + | ↑ | + | Diminished interaction | Severe | + | 7.74 | 1.72 | 4.75 | (+++) | |
| 10 | 10.4 | Missense | ARSA: c.1177_1178delinsGG (p.Thr393Gly) hom | NP | NP | p. els. (31) | Juv. | M | 72 mo | 25 y | 2° | Siblings | Intellectual dysability, Motor retardation | + / - | ↑ | + | Irritable | Severe | + | 4.35 | 1.52 | 1.96 | (+) |
| 11 | 11.3 | Missense | ARSA: c.542 T > G (p.Ile181Ser) het; c.960G > T (p.Trp320Cys) het | NP | NP | 29 | Adult | M | 28 y | 38 y | No | No | Bile stone, Forgetfulness | - / - | ↑ | − | Depressive | Mild | NA | 7.19 | NA | 0.43 | (+++) |
| 12 | 12.12 | Missense | ARSA: c.902G > T (p.Arg301Leu) hom | Hom | Hom | nov. | Adult | M | 36 y | 49 y | 1° | Sibling | Polyneuropathy | - / - | ↓ | − | Personality change | Severe | + | 5.32 | 1.38 | 0 | (+) |
| 12.11 | Missense | ARSA: c.902G > T (p.Arg301Leu) hom | Hom | Hom | nov. | Adult | F | 44 y | 46 y | 1° | Sibling | Forgetfullness | - / - | ↓ | − | Inappropriate affect | Mild | + | 8.5 | 1.38 | 1.76 | (++) | |
| 13 | 13.3 | Missense | SUMF1: c.1045C > T (p.Arg349Trp) hom | NP | NP | 32 | MSD | F | 2 mo | 39 mo | 1° | No | Motor regression, Hypertrichosis, Dry skin | + / - | ↑ | + | − | Severe | NA | 6.15 | 2.9 | 4.94 | (+++) |
| 14 | 14.3 | Missense | SUMF1: c.739G > C (p.Gly247Arg) hom | Het | Het | 33 | MSD | F | 12 mo | 56 mo | 2° | No | Ichthyosis, Hypertrichosis, Coarse face, Malnutrition, Developmental delay | + / - | ↑ | + | Vegetative state | Severe | NA | 1.8 | NA | 38.2 | (+++) |
PD1 pseudodeficiency 1 allele, PD2 pseudodeficiency 2 allele, NP Not present, Hom Homozygous, Het Heterozygous, nov. novel, L inf. late infantile, Juv. Juvenile, F Female, M Male, y years, mo months, Mod. moderate, SM dem. Sensorimotor demyelination, NA Not Available, p.els. published elsewhere.
Among the 16 patients with ARSA mutations, 2 were identified during the asymptomatic phase of MLD based on evaluation of relevant pedigrees (individuals 6.4 and 9.7 shown in Table 1) and subsequently became symptomatic. Therefore, measurement of ASA activity and US-TLC clearly differentiated between these asymptomatic cases and non-MLD/MSD individuals. However, neither ASA activity nor US-TLC differentiated between individuals according to age of onset. DEAE-cellulose chromatography was instrumental in differentiating MSD from MLD (Fig. 1C).
ASA activity in the remaining 3 of the 21 patients with ASA activity <50 μmol/g protein/h was 19.3–33.5 μmol/g protein/h. These 3 subjects did not have ARSA, PSAP (exons 6–8), or SUMF1 mutations; however, they all carried homozygous PD2 alleles as well as heterozygous or homozygous PD1 alleles. These findings showed that in this cohort ASA activity <11.6 μmol/g protein/h was diagnostic for MLD/MSD, while ASA activity between 19.3 and 33.5 μmol/g protein/h can be due to pseudodeficiency.
3.2. Clinical findings in patients with MLD/MSD
The MLD patients from 12 families, of which 10 had parental consanguinity, were aged 8 months-49 years. MLD patients were categorized according to age at onset as late infantile (6–48 months [n = 11]), juvenile (4–16 years [n = 2]), and adult (>16 years [n = 3]) [1]. The most common manifestations were ataxia/gait impairment (9/11, 81%), and motor regression (2/11, 18%) in late infantile-onset MLD, versus speech (especially expressive, 1/2, 50%) and cognitive impairment (1/2, 50%) in juvenile-onset MLD. Adult-onset MLD presented most commonly with cognitive and behavioral problems (2/3, 66%). Spasticity, especially in the lower extremities, was noted prior to motor regression in 9 of the 11 late infantile-onset patients (81%), while overt spasticity was not observed and deep tendon reflexes were either normal or diminished in adult-onset patients.
Uncommon but interesting findings were noted in 3 families. Individual 11.3 with adult-onset MLD had gallbladder stones at age 25 years, a decade before neurological symptoms. Individual 3.3 with late infantile-onset MLD had congenital sacrococcygeal hypoplasia and hemivertebra, which were considered as incidental findings. In family 9, there were 4 affected individuals, 2 of which are included in this study, who had hypertrichosis as the first symptom observed by their parents. Individuals 13.3 and 14.3, both with MSD, exhibited developmental, motor, and skin findings of varying clinical severity: although coarse facies, hypertrichosis, and dry skin were present in both, they were barely noticeable in individual 13.3. Marked ichthyosis, malnutrition, and developmental delay were noted at age 2 months in individual 14.3, versus at age 12 months in individual 13.3.
Nerve conduction studies had been performed in 11 MLD patients (6 late infantile-onset, 3 juvenile-onset, 2 adult-onset) within 6 months of the appearance of initial symptoms; independent of age, all had moderate to severe demyelinating sensory and motor polyneuropathy with or without secondary axonal degeneration. During his asymptomatic period, individual 9.7 had severe demyelinating polyneuropathy. Deep tendon reflexes were increased in 3 of these 11 patients (27%, aged 24 months, 6 years, and 6.5 years) despite electrophysiological findings of polyneuropathy.
3.3. MRI findings of individuals with MLD/MSD
In general, MRI scores did not correlate with disease duration in MLD patients but increased with age on follow-up as expected (Table 2). Initial MRI findings were normal in 4 patients with late infantile-onset MLD (individuals 2.3, 4.3, 6.4 and 7.13) despite the presence of clinical symptoms (mild gait impairment/spasticity/genu recurvatum). Tigroid MRI pattern, a finding suggestive of MLD, was observed in only 3/16 MLD patients (19%, 2 late infantile-onset and 1 juvenile-onset) (Fig. 1G). MRI scores of the symptomatic patients with juvenile-onset and adult-onset MLD were higher than those with late infantile-onset MLD (18–32 vs. 0–35, respectively, p < 0.005) (Fig. 1F). Interestingly, 2 outliers with late infantile-onset MLD had protein-truncating mutations (individuals 1.3 and 2.3).
Table 2.
MRI findings in patients with MLD.
| Individual ID |
Clinical Form |
Number of MRI studies |
MRI age (months) |
White Matter (SI) |
Atrophy |
Basal Ganglia |
Cortico-spinal Tract (SI) |
Corpus Callosum (SI) |
Total |
Other |
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Frontal |
Par-occ. |
Temporal |
Cerebellum |
|||||||||||||||||||||||||||||
| PV | C | U | PV | C | U | PV | C | U | Cerebrum | Thalamus | Cerebellum | MPC gyrus | LPC gyrus | Cor. Radiata | Posterior IC | Midbrain | Pons | Medulla obl. | Genu | Truncus | Isthmus | Splenium | CN Gd enhancement | Diffusion | Tigroid Pat. | |||||||
| 1.3 | L. Inf. | 1 | 48 | 1 | 1 | 1 | 2 | 2 | − | 1 | − | − | 1 | 1 | 2 | 2 | − | − | − | 1 | 1 | 1 | 1 | 1 | − | − | − | 2 | 21 | NA | ↓⁎ | − |
| 2.3 | L. Inf. | 1 | 21 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | 0 | NA | ↑ | − |
| 2 | 23 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | 1 | 1 | NA | ↑ | − | ||
| 3 | 36 | 2 | 2 | − | 2 | 2 | − | 1 | 1 | − | 1 | 1 | 2 | 1 | − | 2 | 2 | 2 | 2 | 2 | 2 | − | 2 | 2 | 2 | 2 | 35 | NA | ↑ | + | ||
| 3.3 | L. Inf. | 1 | 19 | − | − | − | 1 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | 1 | NA | ↑ | − |
| 4.3 | L. Inf. | 1 | 20 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | 0 | NA | ↑ | − |
| 5.3 | L. Inf. | 1 | 29 | − | − | − | 1 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | 1 | 2 | NA | NA | − |
| 6.4 | L. Inf. | 1 | 16 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | 0 | NA | NA | − |
| 6.3 | L. Inf. | 1 | 23 | − | − | − | 1 | 1 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | 1 | 3 | NA | ↑ | + |
| 7.11 | L. Inf. | 1 | 42 | − | − | − | 2 | − | − | 1 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | 3 | NA | ↑ | − |
| 2 | 45 | − | − | − | 2 | − | − | 2 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | 4 | 3,5,7,8 | ↑ | − | ||
| 3 | 51 | − | − | − | 2 | 1 | − | 2 | − | − | − | − | − | − | − | − | − | 1 | − | − | − | − | 1 | − | − | 1 | 8 | 3,5,7,8 | ↑ | − | ||
| 7.13 | L. Inf. | 1 | 37 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | 0 | NA | ↑ | − |
| 2 | 41 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | 0 | − | ↑ | − | ||
| 3 | 58 | − | − | − | − | 1 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | 1 | 3,5,7,8 | ↑ | − | ||
| 8.3 | L. Inf. | 1 | 35 | − | 1 | − | 1 | − | − | − | − | − | − | − | − | − | − | − | − | 1 | − | − | − | − | − | − | − | 1 | 4 | NA | ↑ | − |
| 9.7 | L. Inf. | 1 | 35 | − | − | − | 1 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | 1 | NA | ↑ | − |
| 9.5 | Juv. | 1 | 88 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | − | − | 1 | 2 | 1 | − | 2 | 2 | 2 | 2 | − | 2 | − | 1 | − | − | 1 | 32 | NA | ↓⁎ | + |
| 10.4 | Juv. | 1 | 124 | 2 | 2 | − | 2 | 2 | − | 2 | − | − | − | 1 | 2 | 1 | − | − | − | − | − | − | − | − | 1 | 1 | 1 | 1 | 18 | NA | ↑ | − |
| 11.3 | Adult | 1 | 444 | 2 | 2 | 2 | 2 | 2 | − | 2 | 2 | − | − | 2 | 1 | 1 | SWI + | − | − | − | 1 | − | − | − | 2 | 1 | 1 | 1 | 25 | NA | ↑ | − |
| 2 | 456 | 2 | 2 | 2 | 2 | 2 | − | 2 | 2 | − | − | 2 | 1 | 1 | SWI + | − | − | − | 1 | − | − | − | 2 | 1 | 1 | 1 | 25 | NA | ↑ | − | ||
| 12.11 | Adult | 1 | 528 | 2 | 2 | 2 | 2 | 2 | − | 2 | 2 | − | − | 2 | 2 | 1 | − | − | − | − | 1 | − | − | − | 2 | 2 | 1 | 1 | 26 | − | NA | − |
| 12.12 | Adult | 1 | 564 | 2 | 2 | 2 | 2 | 2 | 1 | 2 | − | − | − | 2 | 2 | 1 | − | − | − | − | − | − | − | − | 1 | 1 | 1 | 1 | 22 | NA | NA | − |
L. Inf. Late infantile, Juv. Juvenile, SI Signal intensity abnormality on T2-weighted MRI, PV (periventricular), C (Central), U U-fibers, MPC (medial precentral), LPC (lateral precentral), IC Internal Capsule, CN Cranial Nerves, SWI (Susceptibility Weighted Image), NA Not Available.
Diffusion restriction in frontal periventricular white matter (individual 1.3); frontoparietal white matter and centrum semiovale (individual 9.5).
Affected brain regions were predominantly periventricular and central regions of parieto-occipital white matter in late infantile-onset MLD (Table 2), while leukodystrophy in frontal white matter and corpus callosum (CC) were more pronounced in juvenile-onset and adult-onset MLD. In contrast to other late infantile-onset MLD patients, the two patients with protein-truncating mutations (1.3 and 2.3) exhibited frontal white matter involvement and severe thalamic atrophy as seen in juvenile-onset and adult-onset MLD patients. Decreased thalamic intensity on T2-weighted MRI and atrophy, observed in all the juvenile-onset and adult-onset MLD patients (n = 5), were present in only the 2 patients with protein-truncating mutations in the late infantile-onset group. In all, 7 individuals with thalamic findings had “dark thalami”, indicating severe thalamic damage, except for 1 adult-onset patient (individual 11.3). Furthermore, thalamic atrophy accompanied generalized brain atrophy, reflected by total MRI score (Table 2).
The CC showed a T2-hyperintensity gradient from splenium to genu in late infantile-onset MLD patients, as observed in the three consecutive MRIs of individual 2.3. In juvenile-onset and adult-onset patients, diffuse involvement of CC was noted. The connecting fibers of the CC were involved together with the corresponding fibers of white matter. Atrophy was observed at later stages, or in patients with more severe phenotypes of MLD: in contrast, early prominent CC atrophy (at 21 and 40 months) was notable in the 2 MSD patients.
Rare MRI findings included: T2-hyperintensity in the superior cerebellar peduncle (individual 8.3), dentate nuclei (individual 1.3), basal ganglia atrophy and paramagnetic substance accumulation (individual 11.3). In total, 2 MLD patients (individuals 1.3 and 9.5) had diffusion restriction, whereas mild to moderately elevated diffusion was observed in all others (Table 2) (individual 9.5; Fig. 1G5, 1G6). Of the 3 MLD patients whose contrast-enhanced MRIs had been obtained, two had bilateral cranial nerve involvement (individuals 7.11 and 7.13, cousins with late infantile-onset MLD). One MSD patient who underwent contrast-enhanced MRI also showed bilateral involvement of the 3rd, 5th, 7th, and 8th cranial nerves (data not shown).
3.4. Anti-sulfatide immune response
Plasma anti-sulfatide IgM and anti-sulfatide-IgG were measured in 55 and 68 participants, respectively. Anti-sulfatide IgM was higher in MLD and MSD patients compared to the MS-NMOSD group (p < 0.001) but similar to heterozygous and healthy control subjects (Fig. 1H). Anti-sulfatide IgG was lower than the MS-NMOSD group but similar to healthy control and heterozygous carrier groups (Fig. 1H). Individual 14.3 with severe MSD had high-titer anti-sulfatide IgG; the other MSD patient (individual 13.3) and one juvenile MLD patient (individual 9.5) with extensive white matter lesions also had elevated anti-sulfatide IgG (Fig. 1H).
4. Discussion
Definitive diagnosis of leukodystrophies can be complicated in the absence of extensive laboratory support. Here, we performed comprehensive clinical, biochemical, radiological and genetic analyses to differentiate MLD from other leukodystrophies.
The present findings stress that both ASA activity and urinary sulfatide analysis should be included in the first-line tests for diagnosing MLD. Mean normal ASA activity in our laboratory is considered 150 μmol/g protein/h (range: 50–250 μmol/g protein/h). Independent of the age of onset of symptoms, ASA activity <10% (<15 μmol/g protein/h) is indicative of MLD/MSD (Fig. 1A). Pseudodeficiency challenges the biochemical diagnosis, reducing the ASA activity to as low as 19.28 μmol/g protein/h in an individual with double homozygous PD1/PD2 alleles. This level, although close to 10% of normal ASA activity, did not fall below 10% in our cohort. However, ASA activities less than 10% has been reported in some cases of ARSA PD homozygotes, mutation heterozygotes, PD/mutation compound heterozygotes in other laboratories previously [23,34]. Double PD homozygotes may be encountered more frequently in countries with a high rate of consanguineous marriage, such as Turkey. Given the difficulty created by pseudodeficiency, urinary sulfatide analysis is essential for confirming the diagnosis. Additionally, since heterozygotes have both ASA activity and US-TLC comparable to healthy controls, they could be differentiated from MLD or MSD patients by the biochemical workup in our cohort. Interestingly, a slightly increased urinary sulfatide excretion in a PD/mutation compound heterozygote has been reported in only one study [34]. However, even this study clearly shows at least a 2-fold difference between that compound heterozygote and MLD patients [34]. Therefore, urinary sulfatide excretion is a reliable marker for MLD.
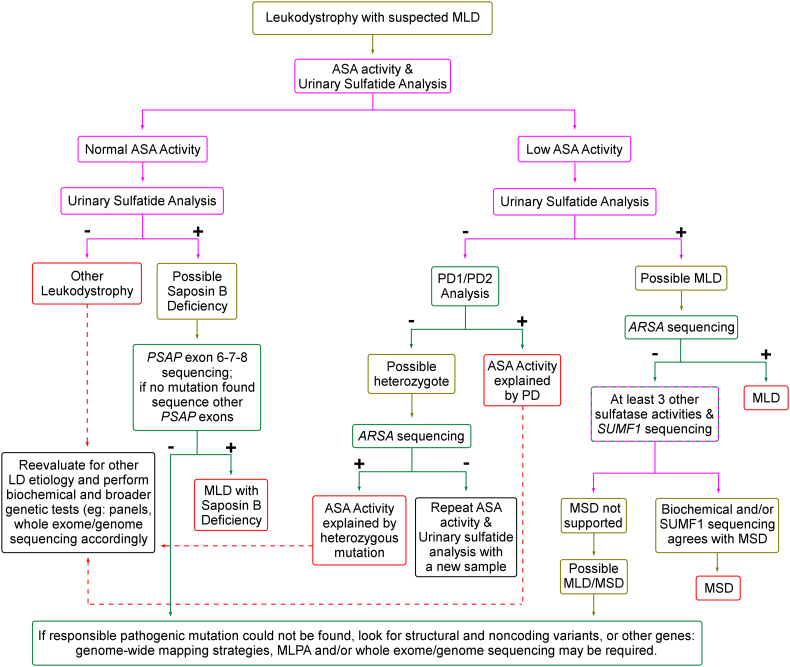
Based on the findings presented herein, we can modify a previously existing diagnostic workflow for individuals with suspected MLD as shown in Fig. 2 [35]. In this workflow, molecular genetic techniques serve to ascertain biochemical findings and allow for genetic counseling at every possible step. With easy access to molecular genetic techniques, genetic diagnosis of MLD is being widely adopted but variants with unknown significance and/or those undetectable by conventional sequencing techniques create a challenge for definitive diagnosis [36]. In order to avoid misdiagnosis, biochemical functional analyses should be adopted as first-line tests to determine the most accurate molecular genetic studies in later steps. Our results show that 50 μmol/g protein/h provides a safe cut-off value to discriminate normal and low ASA activities in our laboratory. However, it should be kept in mind that this value is tailor-fit for one laboratory and other laboratories should make the effort to determine the right cut-off value in accordance with their methods. When levels are below this cut-off value, urinary sulfatide excretion allows for a reliable distinction between MLD patients and others (Fig. 2). High urinary sulfatide excretion is important for saposin B deficient individuals who have normal ASA activity. In contrast, MLD-suspected individuals with normal ASA activity and without urinary sulfatide excretion should be reevaluated for other diagnoses, and considered a candidate for broader molecular genetic and biochemical analyses. MSD should also be considered on the differential diagnosis when biochemical tests are suggestive of MLD but there is no ARSA mutation as observed in a mild MSD patient in our cohort (individual 13.3).
Fig. 2.
Diagnostic flowchart for biochemical and genetic tests of individuals with suspected MLD. Purple boxes refer to biochemical tests and green boxes indicate genetic tests. Cut-off values for ASA activity measurement, which is 50 μmol/g protein/h for our laboratory, must be determined locally. PD1/PD2 analysis can be performed either by restriction fragment length polymorphism assay [19] or by sequencing of relative exons.
The relationship between the residual ASA activity level and age of onset remains controversial [1,37]; however, in the present study residual ASA activity did not correlate with age of onset (Table 1). In contrast to ASA activity, genotype hints at phenotypic expression in some cases. Two of our patients with protein-truncating ARSA mutations had rapid disease progression, high MRI scores, and the lowest age of onset. Their affected brain regions were typical for adult-onset MLD. In contrast, missense mutations of ARSA are not correlated with phenotypic outcome, exemplified by the intra- and inter-familial phenotypic variability (ages-of-onset and MRI findings) in individuals with the same genotype in families 7 and 9 having p.Gly311Ser mutation.
Hypertrichosis as the initial finding of MLD in every affected individual from family 9 is remarkable. Hypertrichosis is a common finding in MSD but not described in MLD [38]. Interestingly, despite the common ARSA genotype in families 7 and 9, hypertrichosis was not observed in family 7 which suggests the presence of modifier genes. On the other hand, considering consanguinity between parents, hypertrichosis might also be related to another genetic cause independently segregating in the family, resulting in blended phenotypes [39]. However, cosegregation of both hypertrichosis and MLD in all affected individuals, sparing the unaffected individual, suggests that hypertrichosis is somehow linked to MLD in this family. Based on the present findings, we think hypertrichosis may be a finding overlooked in MLD and should be part of the physical examination.
MRI is an important tool for diagnosing MLD. In this study, we improved upon previously existing MRI scoring systems to better compare MRI severity with clinical outcomes and genetic mutations in our cohort. Scoring proposed by Eichler is limited by analysis of projection fibers at the level of internal capsule and pons, and corpus callosum at its genu and splenium [7]. Being inspired by Cousyn et al.'s MRI scoring for Krabbe disease, another lysosomal storage disease affecting corticospinal tracts, we included all 7 sections that corticospinal tracts run through from the vertex down to medulla oblongata and all 4 segments of corpus callosum [8]. Also other imaging features including atrophy of the thalamus, gadolinium enhancement of cranial nerves and presence of diffusion restriction as well as the well-known ‘tigroid pattern’ were added to our scoring table and led us to a more detailed analysis which might reflect tissue abnormalities in MLD [9,40].
The earliest lesions identified via MRI in the present study involved the parieto-occipital region of the cortex, and then spread to other brain regions, seemingly following the sequence of developmental myelination [41]. The tigroid pattern of demyelination, considered as indicative of MLD, was a rare finding in the present cohort. The extent of MRI findings appeared to correlate with age within the late-infantile-onset form. On the other hand, severity or duration of disease did not correlate with MRI scores: patients with adult-onset MLD have late disease onset but high MRI scores. Serial MRI studies were available for only few patients: longitudinal follow-up would be useful to assess the evolution of the disease and serve as basis for treatment trials. Nevertheless, the present observations show the characteristic features of MRI as well as the less known ones, like cranial nerve enhancement and diffusion restriction indicating inflammation. The limitations of MR imaging in MLD were also observed, justifying biochemical and genetic studies in cases with high clinical suspicion even in the absence of suggestive MRI features.
Sulfatide accumulation in MLD might induce an autoimmune response. In the present study, a strong anti-sulfatide IgG response was apparent in MSD patients, especially in individual 14.3 who has severe phenotype, and one juvenile MLD patient with extensive white matter changes and diffusion restriction. Autoantibodies might have contributed to, or resulted from, rapid progression of the disease. The cellular immune response against accumulated sulfatides may also play a role in MLD, as suggested by mouse models [3,4].
In conclusion, integrated biochemical and genetic analyses are essential for overcoming the diagnostic challenges of MLD. Although ASA activity is frequently the main diagnostic test, it is critical to combine it with urinary sulfatide analysis as a first-line test for accurate diagnosis, especially in populations with parental consanguinity. MRI does not lead to definitive diagnosis, but MRI scores may indicate disease severity within the late-infantile-onset group.
The following are the supplementary data related to this article.
Pedigrees for each family and relevant mutations shown by Sanger sequencing
Evolutionary conservation of mutated amino acid residues by MutationTaster
Funding
This study was supported by the Hacettepe University Scientific Research Coordination Unit (TSA-2017-12891). The authors confirm independence from the sponsors and that the content of the article was not influenced by the sponsor.
Ethics approval
The study protocol was approved by the Hacettepe University Ethics Committee (25.10.2016, GO 16/658-24).
Patient consent statement
All participants or their parents provided written informed consent.
Availability of data
Anonymized data not presented herein are available upon request.
Author declarations
FP performed biochemical and PCR-restriction enzyme assays; this study was used for his medical specialty training thesis. NGEE, CEBK, SEO, ET, BK, MT, and BA performed physical examinations and evaluations, and provided biological samples. AÇ and NA performed DNA sequencing, and evaluated the genetic results. EK performed statistical analysis. KKO evaluated the participants' MRI studies. HAÖ was FP's thesis advisor, and also designed the study, set up the biochemical tests, and evaluated the results. FP, NGEE, AÇ, NAA, BA, HAÖ drafted the manuscript and all of the authors contributed to the preparation of the manuscript for publication.
Declaration of Competing Interest
All the authors declare there are no conflicts of interest—financial or otherwise—related to the material presented herein.
Acknowledgements
This work was produced from the medical specialty training thesis of Dr. Faruk Pekgül. It was supported by the Hacettepe University Scientific Research Coordination Unit, TSA-2017-12891. The authors wish to thank Deniz Ceylan for his technical support of DNA sequencing analysis. We also thank Scott B. Evans, MSW for professional language editing and proofreading services. We are also indebted to the patients and their families for their kind participation in the study.
References
- 1.Gieselmann V., Ingeborg K.M. Metachromatic leukodystrophy. In: Valle D., Antonarakis S., Ballabio A., Beaudet A.L., Mitchell G.A., editors. The Online Metabolic and Molecular Bases of Inherited Disease. The McGraw-Hill Companies; New York, NY: 2014. p. 1. [Google Scholar]
- 2.Ozkara H.A., Topcu M. Sphingolipidoses in Turkey. Brain Dev. 2004;26(6):363–366. doi: 10.1016/j.braindev.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 3.Stein A., Stroobants S., Gieselmann V., D'Hooge R., Matzner U. Anti-inflammatory therapy with simvastatin improves neuroinflammation and CNS function in a mouse model of metachromatic leukodystrophy. Mol. Ther. 2015;23(7):1160–1168. doi: 10.1038/mt.2015.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barrette B., Nave K.A., Edgar J.M. Molecular triggers of neuroinflammation in mouse models of demyelinating diseases. Biol. Chem. 12, 2013;394:1571–1581. doi: 10.1515/hsz-2013-0219. [DOI] [PubMed] [Google Scholar]
- 5.Hopwood J.J., Ballabio A. Multiple sulfatase deficiency and the nature of the sulfatase family. In: Scriver C.R., Sly W.S., Childs B., Beaudet A.L., Valle D., Kinzler K.W., Vogelstein B., editors. The Online Metabolic and Molecular Bases of Inherited Disease. The McGraw-Hill Companies; New York, NY: 2014. p. 1. [Google Scholar]
- 6.Lorioli L., Cesani M., Regis S., Morena F., Grossi S., Fumagalli F., Acquati S., Redaelli D., Pini A., Sessa M., Martino S., Filocamo M., Biffi A. Critical issues for the proper diagnosis of metachromatic leukodystrophy. Gene. 2014;537(2):348–351. doi: 10.1016/j.gene.2013.11.062. [DOI] [PubMed] [Google Scholar]
- 7.Eichler F., Grodd W., Grant E., Sessa M., Biffi A., Bley A., Kohlschuetter A., Loes D.J., Kraegeloh-Mann I. Metachromatic leukodystrophy: a scoring system for brain MR imaging observations. Am. J. Neuroradiol. 10, 2009;30:1893–1897. doi: 10.3174/ajnr.A1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cousyn L., Law-Ye B., Pyatigorskaya N., Debs R., Froissart R., Piraud M., Federico A., Salvatore S., Cerase A., Macário M.C., Durães J., Kim S.H., Adachi H., Audoin B., Ayrignac X., Da Y., Henderson R., La Piana R., Laule C., Nakamagoe K., Raininko R., Schols L., Sirrs S.M., Viader F., Jastrzębski K., Leclercq D., Nadjar Y. Brain MRI features and scoring of leukodystrophy in adult-onset Krabbe disease. Neurology. 2019;93(7):e647–e652. doi: 10.1212/WNL.0000000000007943. [DOI] [PubMed] [Google Scholar]
- 9.Martin A., Sevin C., Lazarus C., Bellersme C., Aubourg P., Adamsbaum C. Toward a better understanding of brain lesions during metachromatic leukodystrophy evolution. AJNR Am. J. Neuroradiol. 2012;33(9):1731–1739. doi: 10.3174/ajnr.A3038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baum H., Dodgson K.S., Spencer B. The assay of arylsulphatases A and B in human urine. Clin. Chim. Acta. 1959;4(3):453–455. doi: 10.1016/0009-8981(59)90119-6. [DOI] [PubMed] [Google Scholar]
- 11.Bayleran J., Hechtman P., Saray W. Synthesis of 4-methylumbelliferyl-beta-D-N-acetylglucosamine-6-sulfate and its use in classification of GM2 gangliosidosis genotypes. Clin. Chim. Acta. 1894;143(2):73–89. doi: 10.1016/0009-8981(84)90215-8. [DOI] [PubMed] [Google Scholar]
- 12.Cooper A., Hatton C., Thornley M., Sardharwalla I.B. Human β-mannosidase deficiency: biochemical findings in plasma, fibroblasts, white cells and urine. J. Inherit. Metab. Dis. 1988;11(1):17–29. doi: 10.1007/BF01800054. [DOI] [PubMed] [Google Scholar]
- 13.Desnick R.J., Allen K.Y., Desnick S.J., Raman M.K., Bernlohr R.W., Krivit W. Fabry's disease: enzymatic diagnosis of hemizygotes and heterozygotes. Alpha-galactosidase activities in plasma, serum, urine, and leukocytes. J. Lab. Clin. Med. 1973;81(2):157–171. [PubMed] [Google Scholar]
- 14.Hollak C.E., van Weely S., van Oers M.H., Aerts J.M. Marked elevation of plasma chitotriosidase activity. A novel hallmark of Gaucher disease. J. Clin. Invest. 1994;93(3):1288–1292. doi: 10.1172/JCI117084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peters S.P., Lee R.E., Glew R.H. A microassay for Gaucher's disease. Clin. Chim. Acta. 1975;60(3):391–396. doi: 10.1016/0009-8981(75)90083-2. [DOI] [PubMed] [Google Scholar]
- 16.Wiederschain G., Raghavan S., Kolodny E. Characterization of 6-hexadecanoylamino-4-methylumbelliferyl-beta-d-galactopyranoside as fluorogenic substrate of galactocerebrosidase for the diagnosis of Krabbe disease. Clin. Chim. Acta. 1992;205(1–2):87–96. doi: 10.1016/s0009-8981(05)80003-8. [DOI] [PubMed] [Google Scholar]
- 17.Lowry O.H., Rosebrough N.J., Farr A.L., Randall R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193(1):265–275. [PubMed] [Google Scholar]
- 18.Bognar S.K., Furac I., Kubat M., Cosović C., Demarin V. Croatian population data for arylsulfatase a pseudodeficiency-associated mutation in healthy subjects, and in patients with Alzheimer-type dementia and Down syndrome. Arch. Med. Res. 2002;33(5):473–477. doi: 10.1016/s0188-4409(02)00392-2. [DOI] [PubMed] [Google Scholar]
- 19.Ben Halim N., Dorboz I., Kefi R., Kharrat N., Eymard-Pierre E., Nagara M., Romdhane L., Ben Alaya-Bouafif N., Rebai A., Miladi N., Boespflug-Tanguy O., Abdelhak S. Determination of arylsulfatase A pseudodeficiency allele and haplotype frequency in the Tunisian population. Neurol. Sci. 2016;37(3):403–409. doi: 10.1007/s10072-015-2417-5. [DOI] [PubMed] [Google Scholar]
- 20.Fluharty A.L., Stevens R.L., Sanders D.L., Kihara H. Arylsulfatase B deficiency in Maroteaux-Lamy syndrome cultured fibroblasts. Biochem. Biophys. Res. Commun. 1976;59(2):455–461. doi: 10.1016/s0006-291x(74)80001-x. [DOI] [PubMed] [Google Scholar]
- 21.Chang P.L., Rosa N.E., Davidson R.G. Differential assay of arylsulfatase A and B activities: a sensitive method for cultured human cells. Anal. Biochem. 1981;117(2):382–389. doi: 10.1016/0003-2697(81)90795-8. [DOI] [PubMed] [Google Scholar]
- 22.Martino S., Consiglio A., Cavalieri C., Tiribuzi R., Costanzi E., Severini G.M., Emiliani C., Bordignon C., Orlacchio A. Expression and purification of a human, soluble arylsulfatase A for metachromatic leukodystrophy enzyme replacement therapy. J. Biotechnol. 2005;117(3):243–251. doi: 10.1016/j.jbiotec.2005.01.018. [DOI] [PubMed] [Google Scholar]
- 23.Rafi M.A., Coppola S., Liu S.L., Rao H.Z., Wenger D.A. Disease-causing mutations in cis with the common arylsulfatase A pseudodeficiency allele compound the difficulties in accurately identifying patients and carriers of metachromatic leukodystrophy. Mol. Genet. Metab. 2003;79(2):83–90. doi: 10.1016/s1096-7192(03)00076-3. [DOI] [PubMed] [Google Scholar]
- 24.Poorthuis B.J.H.M., Aerts J.M.F.G. Glycosphingolipids. In: Blau N., Duran M., Gibson K.M., editors. Laboratory Guide to the Methods in Biochemical Genetics. Springer-Verlag; Berlin Heidelberg: 2008. pp. 351–378. [Google Scholar]
- 25.Lukatela G., Krauss N., Theis K., Selmer T., Gieselmann V., von Figura K., Saenger W. Crystal structure of human arylsulfatase A: the aldehyde function and the metal ion at the active site suggest a novel mechanism for sulfate ester hydrolysis. Biochemistry. 11, 1998;37:3654–3664. doi: 10.1021/bi9714924. [DOI] [PubMed] [Google Scholar]
- 26.Polten A., Fluharty A.L., Fluharty C.B., Kappler J., von Figura K., Gieselmann V. Molecular basis of different forms of metachromatic leukodystrophy. N. Engl. J. Med. 1991;324(1):18–22. doi: 10.1056/NEJM199101033240104. [DOI] [PubMed] [Google Scholar]
- 27.Harvey J.S., Nelson P.V., Carey W.F., Robertson E.F., Morris C.P. An arylsulfatase A (ARSA) missense mutation (T274M) causing late-infantile metachromatic leukodystrophy. Hum. Mutat. 1993;2(4):261–267. doi: 10.1002/humu.1380020405. [DOI] [PubMed] [Google Scholar]
- 28.Biffi A., Cesani M., Fumagalli F., Del Carro U., Baldoli C., Canale S., Gerevini S., Amadio S., Falautano M., Rovelli A., Comi G., Roncarolo M.G., Sessa M. Metachromatic leukodystrophy - mutation analysis provides further evidence of genotype-phenotype correlation. Clin. Genet. 2008;74(4):349–357. doi: 10.1111/j.1399-0004.2008.01058.x. [DOI] [PubMed] [Google Scholar]
- 29.Onder E., Sinici I., Sonmez F.M., Topcu M., Ozkara H.A. Identification of two novel arylsulfatase A mutations with a polymorphism as a cause of metachromatic leukodystrophy. Neurol. Res. 2009;31(1):60–66. doi: 10.1179/016164108X323762. [DOI] [PubMed] [Google Scholar]
- 30.Kreysing J., Bohne W., Bösenberg C., Marchesini S., Turpin J.C., Baumann N., von Figura K., Gieselmann V. High residual arylsulfatase A (ARSA) activity in a patient with late-infantile metachromatic leukodystrophy. Am. J. Hum. Genet. 1993;53(2):339–346. [PMC free article] [PubMed] [Google Scholar]
- 31.Anlar B., Waye J.S., Eng B., Oguz K.K. Atypical clinical course in juvenile metachromatic leukodystrophy involving novel arylsulfatase a gene mutations. Dev. Med. Child Neurol. 2006;48(5):383–387. doi: 10.1017/S001216220600082X. [DOI] [PubMed] [Google Scholar]
- 32.Cosma M.P., Pepe S., Annunziata I., Newbold R.F., Grompe M., Parenti G., Ballabio A. The multiple sulfatase deficiency gene encodes an essential and limiting factor for the activity of sulfatases. Cell. 2003;113(4):445–456. doi: 10.1016/s0092-8674(03)00348-9. [DOI] [PubMed] [Google Scholar]
- 33.Dierks T., Dickmanns A., Preusser-Kunze A., Schmidt B., Mariappan M., von Figura K., Ficner R., Rudolph M.G. Molecular basis for multiple sulfatase deficiency and mechanism for formylglycine generation of the human formylglycine-generating enzyme. Cell. 2005;121(4):541–552. doi: 10.1016/j.cell.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 34.Lugowska A., Tylki-Szymańska A., Berger J., Molzer B. Elevated sulfatide excretion in compound heterozygotes of metachromatic leukodystrophy and ASA-pseudodeficiency allele. Clin. Biochem. 1997;30(4):25–31. doi: 10.1016/s0009-9120(97)00033-7. [DOI] [PubMed] [Google Scholar]
- 35.Artigalas O., Lagranha V.L., Saraiva-Percira M.L., Burin M.G., Lourenco C.M., van der Linden Jr H., Santos M.L.F., Rosemberg S., Steiner C.E., Kok F., Moura de Souza M., Giugliani R., Schwartz I.V. Clinical and biochemical study of 29 Brazilian patients with metachromatic leukodystrophy. J. Inherit. Metab. Dis. 2010;33(Suppl. 3):S257–S262. doi: 10.1007/s10545-010-9140-4. [DOI] [PubMed] [Google Scholar]
- 36.Eng B., Heshka T., Tarnopolsky M.A., Nakamura L.M., Nowaczyk M.J., Waye J.S. Infantile metachromatic leukodystrophy (MLD) in a compound heterozygote for the c.459 + 1G > A mutation and a complete deletion of the ARSA gene. Am. J. Med. Genet. A. 2004;128A(1):95–97. doi: 10.1002/ajmg.a.30085. [DOI] [PubMed] [Google Scholar]
- 37.Kumperscak H.G., Plesnicar B.K., Zalar B., Gradisnik P., Seruga T., Paschke E. Adult metachromatic leukodystrophy: A new mutation in the schizophrenia-like phenotype with early neurological signs. Psychiatr. Genet. 2007;17(2):85–91. doi: 10.1097/YPG.0b013e3280298280. [DOI] [PubMed] [Google Scholar]
- 38.Schlotawa L., Adang L.A., Radhakrishnan K., Ahrens-Nicklas R.C. Multiple Sulfatase Deficiency: A disease comprising mucopolysaccharidosis, shingolipidosis, and more caused by a defect in posttranslational modification. Int. J. Mol. Sci. 10, 2020;21:3448. doi: 10.3390/ijms21103448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Karaca E., Posey J.E., Coban Akdemir Z., Pehlivan D., Harel T., Jhangiani S.N., Bayram Y., Song X., Bahrambeigi V., Yuregir O.O., Bozdogan S., Yesil G., Isikay S., Muzny D., Gibbs R.A., Lupski J.R. Phenotypic expansion illuminates multilocus pathogenic variation. Genet Med. 12, 2018;20:1528–1537. doi: 10.1038/gim.2018.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morana G., Biancheri R., Dirocco M., Filocamo M., Marazzi M.G., Pessagno A., Rossi A. Enhancing cranial nerves and cauda equine: an emerging magnetic resonance imaging pattern in metachromatic leukodystrophy and krabbe disease. Neuropediatrics. 2009;40(6):291–294. doi: 10.1055/s-0030-1249654. [DOI] [PubMed] [Google Scholar]
- 41.Kinney H.C., Volpe J.J. Modeling the encephalopathy of prematurity in animals: the important role of translational research. Neurol. Res. Int. 2012;2012:295389. doi: 10.1155/2012/295389. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Pedigrees for each family and relevant mutations shown by Sanger sequencing
Evolutionary conservation of mutated amino acid residues by MutationTaster
Data Availability Statement
Anonymized data not presented herein are available upon request.