Abstract
The majority of estrogen receptor positive (ER+) breast cancer (BC) maintain the ER at metastatic sites. Despite anti-estrogen therapy, almost 30% of ER+ BC patients relapse. Thus, new therapeutic targets for ER+ BC are needed. Amino acids (AAs) may affect the metastatic capacity by affecting inflammatory cells. Essential AAs (EAAs) cannot be produced by human cells and might therefore be targetable as therapeutics. Here we sampled extracellular EAAs in vivo by microdialysis in human BC. Mass spectrometry-based proteomics was used to identify proteins affected after EAA and estradiol (E2) exposure to BC cells. Proteins relevant for patient survival were identified, knocked down in BC cells, and metastatic capability was determined in vivo in the transgenic zebrafish model. We found that lysine was the most utilized EAA in human ER+BC in vivo. In zebrafish, lysine in presence of E2 increased neutrophil-dependent dissemination of ER+ BC cells via upregulation of U2AF1 and RPN2 proteins, which both correlated with poor prognosis of ER+ BC patients in clinical databases. Knockdown of U2AF1 and RPN2 decreased the expression of several cell-adhesion molecules resulting in diminished dissemination. Dietary lysine or its related metabolic pathways may be useful therapeutic targets in ER+ BC.
Keywords: microdialysis, dissemination, lysine, zebrafish, essential amino acids, breast cancer
Introduction
Amino acids (AAs) participate in all aspects of the pathophysiological processes that occur in tissues and organs (1). One hallmark of cancer is an increased demand for proteinogenic AAs due to extensive proliferation and metabolic reprogramming (2). Moreover, the metastatic capacity of cancer cells might depend on the redirection of AA use (3). The metabolic reprogramming of cancer cells is coupled to inflammation in the tumor microenvironment, leading to increased immune cell infiltration, which further enhances the metastatic capacity of cancer cells (4). Protein biosynthesis requires the local availability of AAs in the extracellular microenvironment, which can also act as signaling molecules that regulate the expression and activity of metastasis-related proteins, such as epithelial-to-mesenchymal transition proteins, matrix metalloproteinases, and focal adhesion kinase phosphorylation (5–8). Thus, AAs in the tissue microenvironment play an important role in controlling the progression of cancer. Essential AAs (EAAs) are AAs that cannot be synthesized by human cells and must be acquired through dietary intake (9). Because of that, EAAs might be useful as potential targets in cancer therapies. Promising results in experimental cancer models have shown that dietary restriction of EAAs can reduce tumor growth and metastasis (8, 10, 11).
Breast cancer (BC) is the most common cancer in women worldwide (12). More than 75% of all BC are estrogen receptor-positive (ER+) (13). Anti-estrogen therapy is the gold standard for patients with ER+ BC; however, approximately 30% of patients that receive anti-estrogen therapy experience relapse, making distant metastasis the leading cause of death among patients with ER+ BC (13). Estrogen stimulates proliferation of ER+ BC leading to an increased demand of AAs. Additionally, estrogen may also affect the influx of innate immune cells into BC tissues and promote a pro-tumoral polarization of these cells (14–16). Patients with various types of cancer, including BC, display altered levels of free proteinogenic AAs in their plasma and saliva (17) but it is not known whether EAA availability is altered in human ER+ BC microenvironment.
We aimed to determine whether EAAs availability is altered in human ER+ BC in vivo and, to investigate if the most consumed EAA affected BC cell dissemination. We further aimed to determine what mechanisms are involved in EAA mediated BC cell dissemination.
We found that consumption of five EAAs was increased in human ER+ BC; the levels were lower in BC than in normal breast tissue, with lysine displaying the most pronounced change. In an in vivo zebrafish model, exposure to lysine and estradiol (E2) increased the dissemination of human ER+ BC cells. Using mass spectrometry-based proteomic approach, we found that in ER+ BC mammospheres, exposure to lysine and E2 upregulated the splicing factor U2AF 35 kDa subunit (U2AF1) and ribophorin-2 (RPN2) proteins, which both were related to poor prognosis in BC patients. Knockdown of these proteins, achieved by using pre-designed silencing RNAs (siRNA), affected cell proliferation and migration, expression of cell adhesion molecules, and focal adhesion formation resulting in an inhibition of neutrophil-mediated dissemination of ER+ BC cells in vivo.
Strategies targeting lysine depletion, uptake, or lysine related pathways might be effective targets in ER+ BC.
Materials and Methods
Reagents
DMEM, Opti-MEM, DMEM/F12, DPBS glucose, MEM Vitamins 100 X, MEM Non-essential amino acids (NEAA) 100 X, Glutamine, Penicillin-G/Streptomycin and fetal bovine serum (FBS) were purchased from Gibco™, MA, USA. Bovine serum albumin (BSA) from Merck, NJ, USA. Apo-transferrin, ECM gel, 17-β-Estradiol, Tricaine, hydrocortisone, L-lysine hydrochloride, insulin, urea, iodoacetamide, collagen type I solution and recombinant trypsin from Sigma, MO, USA. Lipofectamine RNAi MAX transfection reagent, heat inactivated FBS and Ethylenediaminetetraacetic acid (EDTA) from Invitrogen, MA, USA. MammoCult kit and heparin from StemCell Technologies Inc., VBC, Canada. Silencer select negative control (#4390843) and silencer pre-designed siRNAs - U2AF1 (siRNA ID#s14553), and - RPN2 (siRNA ID#s230095), restore™ plus Western blot stripping buffer and Fast DiI™ oil red dye from ThermoFisher Scientific, MS, USA. Ficoll-Paque Plus from GE Healthcare, IL, USA.
Microdialysis of Patients
The regional ethical review board of Linköping approved the study. All participants gave informed consent, and all procedures were performed according to the Declaration of Helsinki. Eleven women (52–78 years of age), who were diagnosed with BC, underwent microdialysis before surgery. Before insertion, 0.5 ml lidocaine (10 mg/ml) was administrated intracutaneously. Microdialysis catheters with 10 mm membrane (#71, M Dialysis AB, Stockholm, Sweden) were inserted intratumorally into the BC and into adjacent normal breast tissue and connected to a pump (M Dialysis AB) and perfused with 154 mM NaCl and 60 g/l hydroxyethyl starch (Voluven; Fresenius Kabi AB, Uppsala, Sweden) at a perfusion rate of 0.5 µl/min. After a 60-min equilibration period, the outgoing perfusate was stored at −80°C.
Quantifications of Extracellular EAAs in Microdialysis Samples
Ultra-high-performance liquid chromatography with tandem mass spectroscopy and electrospray ionization (UHPLC-ESI-MS/MS) were used for EAAs quantification as previously described (18). Microdialysis samples from all patients were collected at the same time point after the insertion of the microdialysis catheters. 5 µl microdialysis samples were diluted with 20 µl LC-MS grade water (Sigma-Aldrich) and stored at −80°C. When analyzed, samples were thawed at +4°C, then 10 µl of the sample or the EAAs standard were prepared in 20% Voluven and mixed with 8 µl of 0.5 mM perfluoroheptanoic acid (Sigma-Aldrich) in water containing internal standards (Cambridge Isotope Laboratories, Tewksbury, MA, USA). 10 μl of sample was injected and standard curves for each EAA were created and concentrations were calculated.
Cell Lines
ER+ HER-2 negative BC can be divided into the molecular luminal A and luminal B subgroups where luminal B cancers are associated with increased relapse rates and decreased overall survival (19). To distinguish luminal A from luminal B BC, Ki67 and p53 can be used as determinants (20). Here we used the MCF-7 cells as a model for luminal A BC and the more aggressive p53 mutated T47D cells as a model for ER+ luminal B BC. MCF-7 (ATCC Cat# HTB-22, RRID : CVCL_0031) and T47D (ATCC Cat# HTB-133, RRID : CVCL_0553) were purchased from ATCC. MCF-7 were maintained in DMEM with 10% FBS, 2 mM glutamine and 50 IU/ml/50 µg/ml Penicillin-G/Streptomycin and T47D in Opti-MEM with 4% FBS.
Isolation of Human Neutrophils
Neutrophils were isolated from venous blood from healthy female donors. Blood samples were diluted 1:3 in PBS with 2 mM EDTA and 0.1% heat-inactivated FBS, mononuclear cells were separated by Ficoll-Paque gradient and discarded, pellets containing neutrophils were diluted in 0.9% NaCl and sedimentated 15 to 20 min with 3% dextran T-500/0.9% NaCl. Residual red blood cells were lysed with hypotonic saline solutions. For in vivo analysis, neutrophils were re-suspended in DMEM/F12 complete medium (DMEM/F12 with 0.02% of BSA, 10 µg/ml apo-transferrin and 1 μg/ml insulin) or DPBS glucose medium (DPBS glucose with 1× MEM Vitamins, 1× MEM-NEAA, 0.1% BSA, 2 mM glutamine and Penicillin-G/Streptomycin 50 IU/ml/50 µg/ml) and placed on ice until injections.
Lysine Treatment in ER+ Breast Cancer Cell Mammospheres
MCF-7 and T47D cells were cultured in low attachment 96-well plates (Sigma) at 37°C in MammoCult medium with heparin 4 µg/ml, hydrocortisone 0.48 µg/ml, Penicillin-G/Streptomycin 50 IU/ml/50 µg/ml and 2.5% ECM gel until mammosphere formation. l-lysine 0.146 mg/ml (concentration matched to that found in DMEM medium) ± E2 1 nM treatment was added in DPBS glucose medium free of EAAs to MCF-7 and T47D mammospheres during 5 or 3 days, respectively. Thereafter, mammospheres were lysed with RIPA buffer for proteome analysis. Total protein concentration was measured using Pierce™ BCA protein assay kit (ThermoFisher Scientific).
Proteome Analysis by Liquid Chromatography-Mass Spectrometry (LC-MS)
Equal amount of protein was loaded onto 10 kDa Microcon centrifugal filters (Merck) and processed according to FASP method as previously reported (21). Trypsin digestion was performed overnight at 37°C at 1:100 to protein ratio. Peptides were desalted by using Pierce C18 tips (ThermoFisher Scientific), resuspended in 0.1% formic acid and quantified by A280 using NanoDrop ND-1000 (Thermo Fisher Scientific, San Jose, CA, USA).
Peptides were separated by reverse phase chromatography at a flow rate of 300 nl/min on EASY-nLC II (Thermo Scientific). Automated online analyses were performed in positive mode by LTQ Orbitrap Velos Pro hybrid mass spectrometer (Thermo Scientific) as described previously (22). Generated files were analyzed using Sequest HT in Proteome Discoverer (Thermo Fisher Scientific, San Jose, USA, v. 1.4.0.288) using Uniprot database for Homo sapiens and searched with a fragment ion mass tolerance of 0.50 Da and parent ion tolerance of 6.0 ppm. Carbamidomethylation of cysteine was specified as fixed modification and oxidation of methionine, acetyl of the N-terminus and phosphorylation of serine, threonine and tyrosine as variable modifications. Scaffold (version 4.9.0; Proteome Software Inc., Portland, OR) was used to validate and quantify identified proteins. Identifications were based on a minimum of 2 peptides, 90% peptide identification probability, and minimum 99% protein identification probability (23). Proteins containing similar peptides were grouped into clusters. The label-free quantitative analysis was performed using total number of spectral counts.
Bioinformatics Analysis
Significantly altered proteins were analyzed by String database (STRING, RRID : SCR_005223) where Reactome pathways were obtained.
Transfection Experiments With Silencing RNAs
MCF-7 and T47D were seeded at 5 × 105 cells/well in 6-well plates in DMEM with 10% FBS and 2 mM glutamine. After 24 h incubation, fresh medium with Opti-MEM containing lipofectamine RNAiMAX transfection reagent and 25 pmol of pre-designed silencing RNAs (siRNAs) targeting the genes U2AF1, RPN2, or negative control (siRNA-C) was added. After 72 h incubation at 37°C, cells were used for migration assay, lysed in RIPA buffer for Western blot or labeled with Fast DiI™ oil red dye, 4 µg/ml, for zebrafish experiments as previously described (24).
Migration and Proliferation Assays
MCF-7 and T47D were transfected with siRNA-C, siRNA-U2AF1 or siRNA-RPN2 respectively, then seeded at 3 × 104 cells/well in CytoSelect 96-well migration assay plate (Cell Biolabs) in DMEM/F12 complete medium. DMEM with 10% FBS and 2 mM glutamine was used as chemoattractant in the lower chamber. After 24 h incubation at 37°C, cell migration was analyzed using the Spark 10M microplate reader (Tecan Trading AG, Switzerland). For proliferation, 3 x 103 cells were seeded/well in 96-well plates and transfected as above. BrdU cell proliferation assay (Roche Cat#11 647 229 001) was used and proliferation was measured with VersaMax microplate reader (Molecular Devices, California, USA).
Western Blot
Cell lysates were loaded onto 4% to 15% SDS-PAGE gels (BioRad), and separated proteins were transferred to PVDF membranes (BioRad), which were incubated with primary antibodies; mouse anti-human VCAM-1 1:1000 (Novus Cat# NBP1-28404, RRID : AB_1852860), mouse anti-human ICAM-1 1 µg/ml (Novus Cat# NBP2-22541), mouse anti-human MUC-1 1 µg/ml (Novus Biologicals Cat# NBP2-34737), mouse anti-human RPN2 1:300 (Santa Cruz Biotechnology Cat# sc-166421, RRID : AB_2238716) and mouse anti-human U2AF1 1:200 (Nordic BioSite Cat# LS-C751868). Goat anti-rabbit HRP 1:2000 (Agilent Cat# P0448, RRID : AB_2617138) and goat anti-mouse HRP 1:2500 (Agilent Cat# P0447, RRID : AB_2617137) were used as secondary antibodies. Rabbit anti-human GAPDH 1:2500 (Abcam Cat# ab9485, RRID : AB_307275) was used as loading control. Proteins were detected with ECL prime Western blotting detection reagent (GE Healthcare), visualized by Chemidoc™ MP Imaging System and analyzed with Image Lab™ Software version 5.2.1 (Image Lab Software, RRID : SCR_014210). Western blot images in the main manuscript were created by cropping and pasting from the original images shown in full in Supplementary Figure 1.
Immunocytochemistry
Cells were cultured at 2 × 105 cells/well on collagen-coated cover glasses in 6-well plates, transfected with siRNAs as described above and fixed in 4% PFA. Mouse anti-human vinculin 2 µg/ml (Thermo Fisher Scientific Cat# MA5-11690, RRID : AB_10976821) was used as primary antibody and goat anti-mouse Alexa Fluor-488 1:500 (Abcam Cat# ab150113, RRID : AB_2576208) as secondary antibody. Glasses were mounted with SlowFade Gold antifade reagent with DAPI (Invitrogen) and visualized by confocal microscopy using a Zeiss LSM700 upright confocal microscope (Carl Zeiss AG, Oberkochen, Germany). Focal adhesions area at the invasive front was quantified by using Fiji software version 1.52n (Fiji, RRID : SCR_002285) (25).
Zebrafish Tumor Xenograft Model
Zebrafish experiments were approved by the animal ethics committee at Linköping University. Transgenic Tg(fli1:EGFP)y1 zebrafish embryos, were kept at 28°C in E3 embryo medium with 0.2 mM 1-phenyl-2-thiourea (PTU).
For lysine experiments, MCF-7 and T47D were treated ± E2 1 nM for 48 h, Dil-labeled and treated ± lysine 0.146 mg/ml ± E2 1 nM in DPBS glucose medium for 5 h. Cells were injected in two days old embryos ± lysine 0.146 mg/ml ± E2 1 nM ± 50% neutrophils.
For experiments with transfected cells, MCF-7 and T47D were transfected with siRNAs and cultured in complete media containing lysine in presence of E2 1 nM and Dil-labeled. Cells were injected in two days old embryos + E2 1 nM ± 50% neutrophils.
T47D were treated for 48 h with E2 1 nM, Dil-labeled and injected + E2 1 nM ± isotype antibody control 1 µg/ml (BioLegend Cat# 400166, RRID : AB_11146992) ± mouse anti-human MUC-1 antibody 1 µg/ml as previously described (24).
After 24 h incubation at 28°C in E3/PTU medium ± E2 1 nM, cell dissemination to the tail region was quantified and embryos were photographed in Olympus BX43 light/fluorescence microscope (10×/0.30 magnification) with excitation filters BP460–495 and BP530–550, using Olympus DP72 CCD camera. Images were acquired with the Olympus CellSens Imaging software version 1.16 (Olympus cellSens Software, RRID : SCR_016238).
Breast Cancer Database Analysis
Kaplan Meier-plotter (KMP) database (Kaplan Meier Plotter, RRID : SCR_018753) (26) was used to analyze overall survival of ER+ BC patients. Patient’s data was split by Q1 vs Q4, overall survival, 180 months of follow up threshold and ER+ BC luminal A and luminal B options were selected, ER+ status derived from gene expression data was included. All other options were left as default.
Statistical Analysis
Data are presented as mean ± SEM. For multiple group testing ANOVA was used and if significant followed by two-tailed Student t test otherwise two-tailed Student t test was used. The human data was analyzed with Wilcoxon signed-rank test for paired observations. A P value < 0.05 was considered statistically significant. Statistics were performed with Prism 7.0 (GraphPad Software, San Diego, CA).
Analysis of proteomic data was performed with two-tailed Student’s t-test and stack of P values were analyzed with Benjamini-Hochberg correction to assess false discovery rate (FDR), which was set to 5%.
Results
Significantly Increased Consumption of Lysine in ER+ BC in Patients
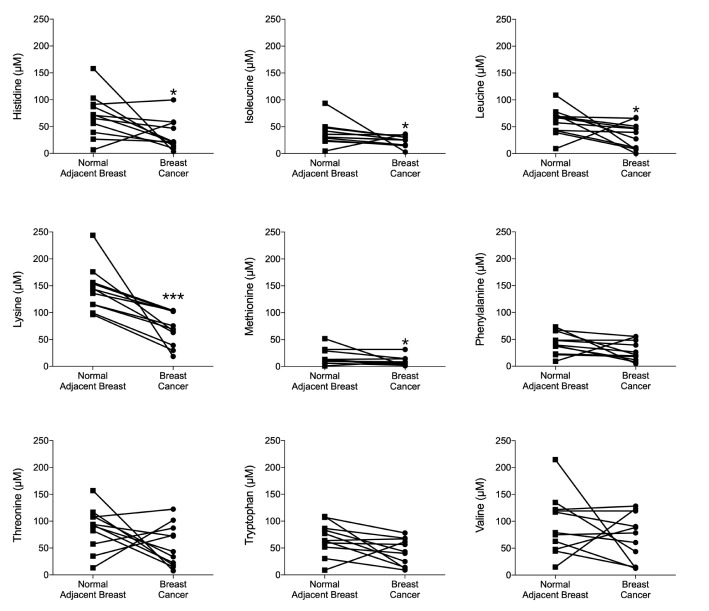
Extracellular EAAs from live ER+ BC were sampled using microdialysis in women before surgery. Cancer characteristics are shown in Table 1. As shown in Figure 1, histidine, isoleucine, leucine, methionine and lysine exhibited significant decreased levels in the cancerous breast tissue compared to normal adjacent breast tissue. Lysine was the most abundant EAA in the extracellular space. Additionally, all BC exhibited decreased levels of lysine whereas the results of the other EAA were more mixed; increased, decreased as well as no change of the levels were detected. Because of this, the effects of lysine were further investigated experimentally.
Table 1.
Characteristics of the breast cancers of patients subjected to intratumoral microdialysis.
| Patient | Age | Tumor size | Grade (NHG) | ER (%) | PR (%) |
|---|---|---|---|---|---|
| 1 | 69 | 21 | 2 | >50 | 10–50 |
| 2 | 70 | 22 | 2 | >50 | >50 |
| 3 | 68 | 24 | 2 | >50 | >50 |
| 4 | 52 | 25 | 3 | >50 | 10–50 |
| 5 | 78 | 28 | 2 | >50 | >50 |
| 6 | 62 | 21 | 2 | >50 | >50 |
| 7 | 48 | 30 | 2 | >50 | >50 |
| 8 | 73 | 30 | 2 | >50 | <5 |
| 9 | 57 | 27 | 1 | >50 | >50 |
| 10 | 66 | 60 | 2 | >50 | >50 |
| 11 | 65 | 50 | 2 | >50 | 0 |
All cancers were HER-2 negative.
NHG, Nottingham grade; ER, estrogen receptor; PR, progesterone receptor.
Figure 1.
Lysine was the most consumed essential amino acid (EAA) in vivo in ER+ breast cancer patients. Microdialysis was used for sampling of extracellular essential AAs (EAAs) from the live tissue of ER+ BC patients before surgery. One catheter was inserted within the breast cancer and another in normal adjacent breast tissue. EAAs were quantified by UHPLC-ESI-MS/MS as reported in materials and methods. Wilcoxon signed-rank test, *P < 0.05 and ***P < 0.001.
Lysine Increased the Neutrophil-Dependent BC Cell Dissemination in Presence of E2 in Luminal A and Luminal B Breast Cancer
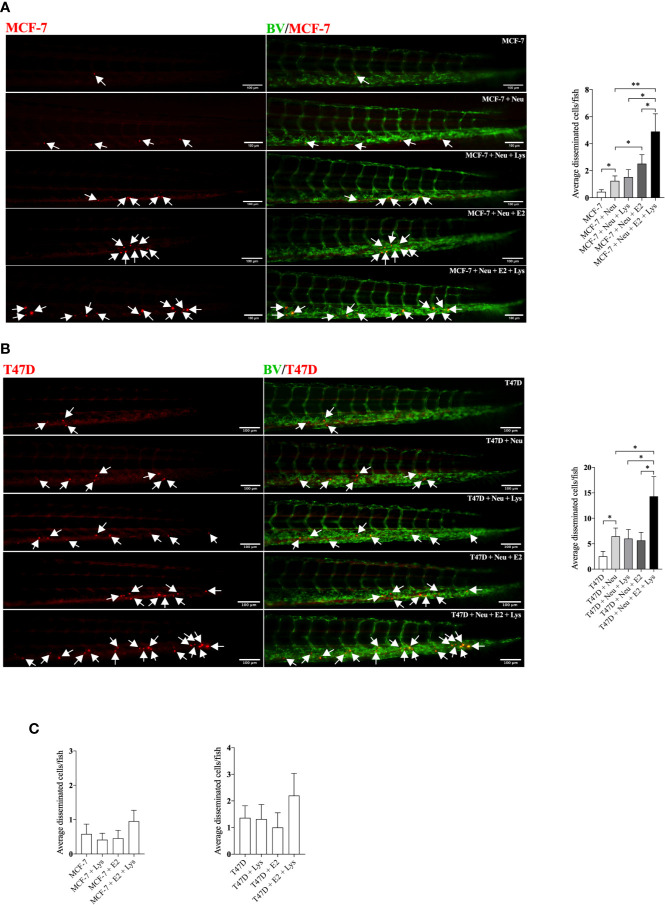
As neutrophils are the first responders in the inflammatory process and have been reported as key mediators of BC metastasis (27, 28), we injected BC cells in presence of neutrophils in zebrafish and exposed the embryos to E2, lysine or their combination. As shown in Figures 2A, B, both the low-metastatic ER+ luminal A MCF-7 cells and the more aggressive ER+ T47D luminal B cells showed significantly increased dissemination with lysine exposure in presence of E2, compared to untreated controls, lysine or E2 alone.
Figure 2.
Lysine in combination with estradiol increased breast cancer cell dissemination in presence of neutrophils in vivo. ER+ BC cells were pre-treated ± estradiol (E2) ± lysine (Lys). Zebrafish transgenic embryos, with green fluorescent blood vessels, were injected with BC cells ± Lys ± E2 ± neutrophils (Neu) and analyzed as described in materials and methods. (A) In vivo dissemination of co-injected MCF-7 ± Neu in presence of Lys, E2 or their combination E2+Lys. One-way ANOVA (P < 0.0001) followed by two-tailed Student’s t-test, (n = 15–33), *P < 0.05 and **P < 0.01. (B) In vivo dissemination of co-injected T47D ± Neu in presence of Lys, E2 or their combination E2+Lys. One-way ANOVA (p = 0.0014) followed by two-tailed Student’s t-test, (n = 15–22) *P < 0.05. (C) In vivo dissemination of MCF-7 and T47D cells alone in presence of Lys, E2 or their combination E2+Lys. No significant changes were detected. Representative images of zebrafish embryos are shown, arrows show disseminated BC cells. BV = blood vessels. Scale bar = 100 µm. Data are presented as Mean ± SEM. Data are represented of at least two independent experiments.
Lysine in Presence of E2-Induced Expression of Splicing Factor U2AF 35 kDa Subunit in Luminal A BC Cells
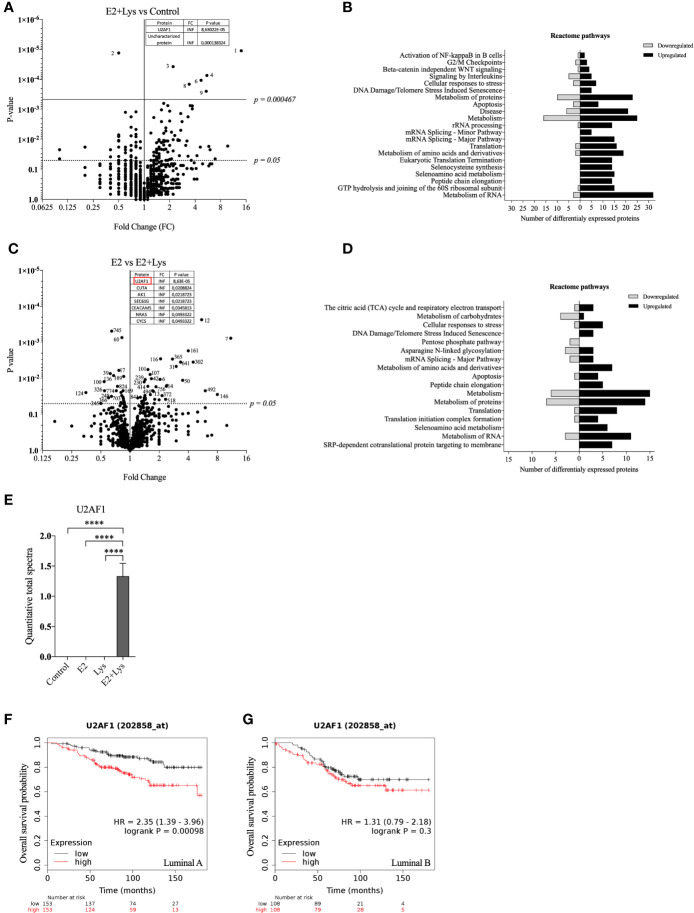
To further elucidate by which mechanisms lysine in combination with E2 increased cancer cell dissemination we next compared the proteome of ER+ BC mammospheres cultured in presence or absence of E2, lysine, and their combination by using LC-MS. As shown in Figure 3A and Supplementary Data 1 a total of 973 single proteins and protein clusters were identified in the luminal A BC cell line MCF-7.
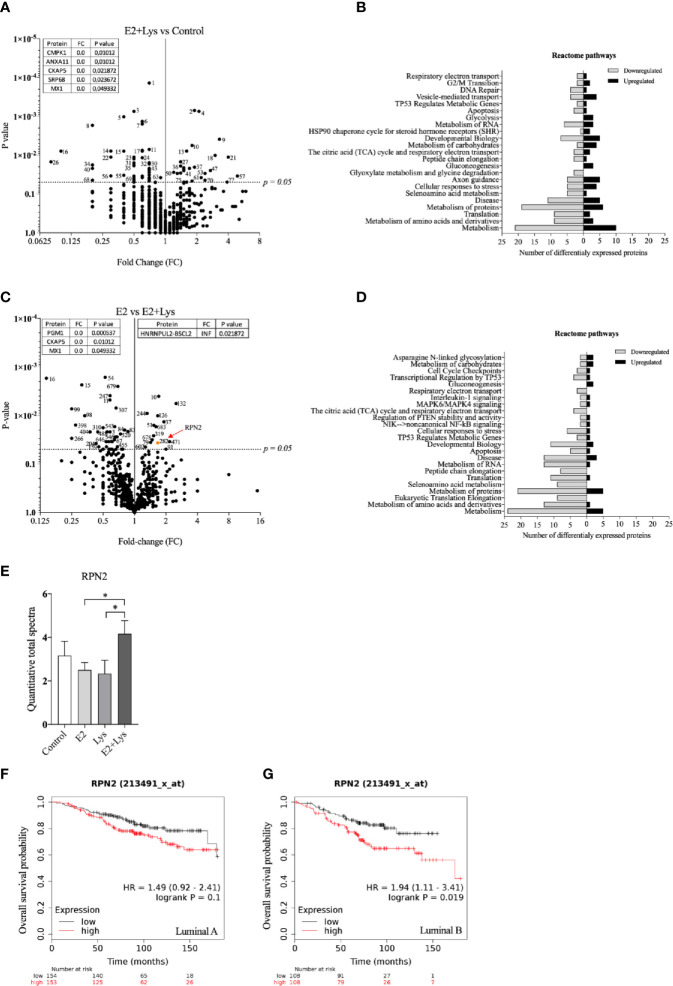
Figure 3.
Lysine in presence of estradiol induced expression of U2AF1 protein in luminal A ER+ breast cancer cells. Proteome analysis was performed in non-metastatic luminal A ER+ MCF-7 mammospheres, treated ± lysine (Lys) ± estradiol (E2), using LC-MS as described in materials and methods. (A) Volcano plot of identified proteins in E2+Lys vs control treated mammospheres (n = 6). Proteins with infinite (INF) fold change are shown in a separate table. Two-tailed Student’s t-test (P < 0.05) followed by False discovery rate method of Benjamini and Hochberg (P = 0.000467). (B) Reactome analysis of significantly down- and upregulated proteins (P < 0.05) by E2+Lys treatment compared to control in mammospheres. (C) Volcano plot of identified proteins in E2 vs. E2+Lys treated mammospheres (n = 6). Proteins with infinite (INF) fold change are shown in a separate table, U2AF1 protein is highlighted in a red rectangle. Two-tailed Student’s t-test analysis (P < 0.05). (D) Reactome analysis of significantly down- and upregulated proteins (P < 0.05) by E2+Lys treatment compared to E2 in mammospheres. (E) Quantitative total spectra of U2AF1 protein (n = 6). Data are presented as mean ± SEM. Two-tailed Student’s t-test ****P < 0.0001. The Kaplan-Meier plotter (KMP) database was used to analyze the effect of U2AF1 expression in overall survival of ER+ BC patients as described in materials and methods. (F) mRNA expression of U2AF1 in ER+ luminal A (n = 306) and (G) ER+ luminal B BC (n = 216). HR = hazard ratio. Volcano plots show code numbers corresponding to differentially affected proteins, protein names with their respective code number are shown in Supplementary Data 1.
As the most significant changes in cancer cell dissemination occurred when lysine was added in presence of E2 (E2+lysine) compared to untreated control cells we first compared these two groups. Exposure of E2+lysine compared to untreated control mammospheres resulted in significantly changed levels (P <0.05 before FDR correction) of 135 single proteins or protein clusters (Figure 3A). The Reactome analysis revealed that E2+lysine treatment increased the expression of proteins involved in metabolism including biological pathways related to translation, mRNA splicing and inflammation compared to control treatment (Figure 3B).
To clarify the role of lysine, E2+lysine treated mammospheres were compared to mammospheres treated with E2 only. As shown in Figure 3C and Supplementary Data 1, a total of 80 proteins or protein clusters were significantly changed (P <0.05 before FDR correction). Reactome analysis revealed that E2+lysine, increased the expression of proteins involved in mRNA splicing, the TCA cycle and most of BC cell metabolic pathways such as protein, AAs, and RNA metabolism whereas metabolism of carbohydrates was decreased (Figure 3D).
FDR correction of the first set of data revealed 9 significantly altered proteins or clusters of proteins in the E2+lysine treated mammospheres compared to controls without treatment (Figure 3A). Out of these, 3 remained significantly upregulated in E2+lysine treated mammospheres when they were compared to mammospheres treated with E2 alone; the splicing factor U2AF 35 kDa subunit (U2AF1), the cluster of neuroblast differentiation-associated protein AHNAK, and one uncharacterized protein (Figure 3C) suggesting that the changes of these proteins were dependent on lysine.
Figure 3E shows that U2AF1 was expressed in the presence of E2+lysine and not in any other treatment groups or control group. To elucidate whether U2AF1 affected BC prognosis in patients the publicly available database “Kaplan-Meier plotter” (KMP) was used. Increased expression of U2AF1 was associated with worse overall survival in luminal A BC but not luminal B BC (Figures 3F, G). As clusters of proteins have inherently lower probability of defining a specific protein, U2AF1, as the only identified specific protein, was selected for further experimental studies of luminal A BC dissemination.
Volcano plots and Reactome analysis of lysine vs. control and E2 vs. control groups are provided in Supplementary Figures 2A to D.
U2AF1-Mediated Migration and Dissemination of Luminal A ER+ Breast Cancer Cells
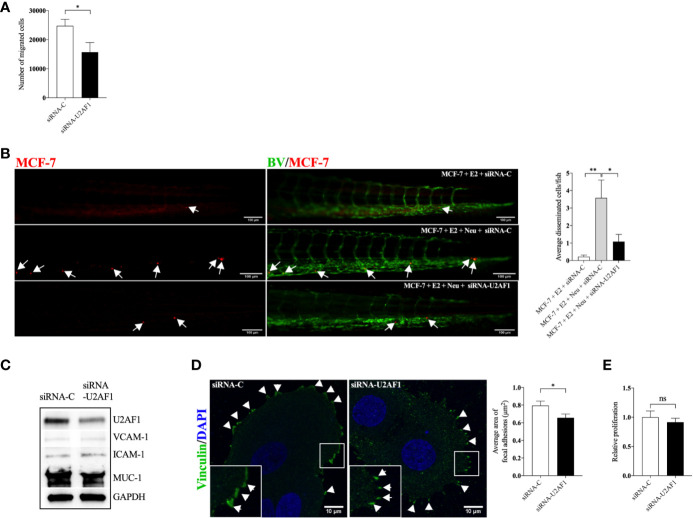
Next, we set up experiments to evaluate if, and by which mechanisms, U2AF1 protein affected growth and dissemination capacities of ER+ luminal A BC cells, MCF-7 cells. As shown in Figures 4A, B, knockdown of U2AF1 significantly decreased MCF-7 cell migration in vitro and in vivo. In zebrafish, a significant decrease in neutrophil-induced dissemination was observed. As cancer cell and neutrophil interactions are mediated by the expression of cell adhesion proteins, several of these were analyzed in transfected cells. As shown in Figure 4C, knockdown of U2AF1 decreased mucin-1 (MUC-1) expression whereas vascular cell adhesion molecule-1 (VCAM-1) or intercellular adhesion molecule-1 (ICAM-1) were unaffected. Overexpression of MUC-1 in MCF-7 cells has previously been shown to be important for invasiveness and metastatic behavior of these cells (29, 30).
Figure 4.
Knockdown of U2AF1 decreased luminal A BC cell dissemination in vivo. Luminal A ER+ MCF-7 cells, transfected with negative control siRNA (siRNA-C) or siRNA targeting U2AF1 (siRNA-U2AF1) were injected in presence of estradiol (E2) ± neutrophils (Neu) into zebrafish transgenic embryos, with green fluorescent blood vessels, and analyzed as described in materials and methods. (A) Migration in vitro (n = 6–12). (B) In vivo dissemination in presence of E2 ± Neu (n = 38–41). Scale bar = 100 µm. (C) Western blot analysis for confirmation of siRNA-U2AF1 transfection and ICAM-1, VCAM-1, and MUC-1 expression. (D) Focal adhesion area (n = 7). Scale bar = 10 µm. (E) Proliferation in vitro (n = 12). Representative images of zebrafish embryos with disseminated MCF-7 cells and immunocytochemistry analysis of vinculin expression are shown. Arrows show disseminated MCF-7 cells and arrowheads show focal adhesions. BV = blood vessels. Data are presented as mean ± SEM. Two-tailed Student’s t-test *P < 0.05, **P < 0.01, ns, not significant. Data are represented of at least two independent experiments.
Focal adhesions formation has been shown to mediate cell migration, which is key for cancer metastasis (31). Figure 4D shows that siRNA-U2AF1 transfection significantly decreased the focal adhesions area at the migratory front of MCF-7 cells. siRNA mediated knockdown of U2AF1 had no effect in MCF-7 proliferation (Figure 4E).
Lysine in Presence of E2 Exposure Increased Ribophorin-2 Protein in Luminal B BC Cells
In the luminal B ER+ T47D mammospheres, a total of 765 proteins or protein clusters were identified (Supplementary Data 2) and out of these, 83 were significantly altered between the E2+lysine group compared to control mammospheres without treatment (Figure 5A). The Reactome analysis revealed that E2+lysine treatment decreased the expression of proteins related to most metabolic pathways whereas it increased the expression of proteins that participate in carbohydrate metabolism and gluconeogenesis (Figure 5B). To elucidate which proteins that were affected by lysine, we compared E2+lysine treated mammospheres vs. E2 alone and found 65 proteins or protein clusters that were significantly altered (Figure 5C). The Reactome analysis showed decreased expression of proteins related to metabolic pathways while the gluconeogenesis pathway was upregulated (Figure 5D). Surprisingly, after FDR analysis no proteins remained significant. Therefore, out of the 65 significantly changed proteins, we identified proteins having an expression level ±50% change in the E2+lysine group compared to E2 alone. Thereafter we search in the KMP database if any of these proteins correlated with poor overall survival in patients with luminal B BC but not with luminal A BC. By this approach the ribophorin-2 (RPN2) protein was identified. As shown in Figure 5E, E2+lysine exposure increased RPN2 levels compared to E2 and lysine alone. In Figures 5F, G, the role of RPN2 in survival of luminal B BC patients but not for luminal A BC is shown.
Figure 5.
Lysine in presence of estradiol increased RPN2 protein in luminal B ER+ breast cancer cells. Proteome analysis was performed in mammospheres with luminal B ER+ T47D cells, treated ± lysine (Lys) ± estradiol (E2), using LC-MS as described in materials and methods. (A) Volcano plot of identified proteins in E2+Lys vs control treated mammospheres (n = 6). Proteins with zero-fold change are shown in a separate table. Two-tailed Student’s t-test (P < 0.05). (B) Reactome analysis of significantly down- and upregulated proteins (P < 0.05) by E2+Lys treatment compared to control mammospheres. (C) Volcano plot of identified proteins in E2 vs. E2+Lys treated mammospheres (n = 6). Proteins with zero and infinite (INF) fold change are shown in separate tables. RPN2 protein is highlighted in orange and red arrow. Two-tailed Student’s t-test analysis (P < 0.05). (D) Reactome analysis of significantly down- and upregulated proteins (P < 0.05) by E2+Lys treatment compared to E2. (E) Quantitative total spectra of RPN2 protein (n = 6). Data are presented as mean ± SEM. Two-tailed Student’s t-test *P < 0.05. The Kaplan-Meier plotter (KMP) database was used to analyze the effect of RPN2 expression in overall survival of ER+ BC patients as described in materials and methods. (F) mRNA expression of RPN2 in ER+ luminal A (n = 307) and (G) ER+ luminal B BC (n = 216). HR = hazard ratio. Volcano plots show code numbers corresponding to differentially affected proteins, protein names with their respective code number are shown in Supplementary Data 2.
Volcano plots and Reactome analysis of lysine vs. control and E2 vs. control are provided in the Supplementary Figures 2E to H.
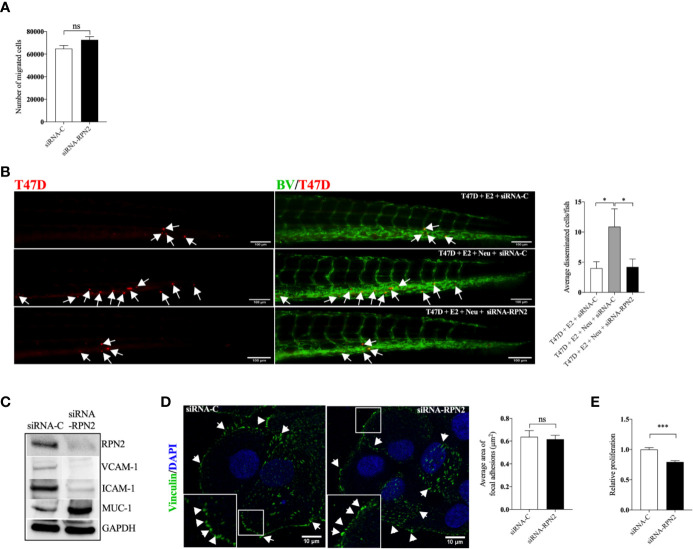
RPN2-Mediated Proliferation and Dissemination of Luminal B ER+ Breast Cancer Cells
In the more aggressive ER+ T47D, siRNA mediated knockdown of RPN2 had no effect in T47D migration in vitro, however, it significantly inhibited the neutrophil-induced dissemination of T47D cells in vivo in zebrafish (Figures 6A, B). Figure 6C shows that knockdown of RPN2 reduced the expression of ICAM-1 and VCAM-1 integrins whereas it increased the expression of MUC-1 in T47D. However, MUC-1 seems to be less important for dissemination of these cells as antibody neutralization of MUC-1 in T47D cells did not affect the dissemination in vivo in zebrafish (Supplementary Figure 3). Figure 6D shows that focal adhesions area was not affected when RPN2 was knocked down in T47D. siRNA mediated knockdown of RPN2 decreased significantly T47D proliferation in vitro (Figure 6E).
Figure 6.
Knockdown of RPN2 decreased luminal B BC cell dissemination in vivo. Luminal B ER+ T47D cells, transfected with negative control siRNA (siRNA-C) or siRNA targeting RPN2 (siRNA-RPN2) were injected + estradiol (E2) ± neutrophils (Neu) into zebrafish transgenic embryos with green fluorescent blood vessels and analyzed as described in materials and methods. (A) Migration in vitro (n = 6). (B) In vivo dissemination of transfected T47D in presence of E2 ± Neu (n = 23–26). Scale bar = 100 µm. (n = 23–26). (C) Western blot analysis for confirmation of siRNA-RPN2 transfection and VCAM-1, ICAM-1, and MUC-1 expression. (D) Focal adhesions area (n = 5–6). Scale bar = 10 µm. (E) Proliferation (n = 6). Representative images of zebrafish embryos with disseminated luminal B T47D BC cells and immunocytochemistry analysis of vinculin expression are shown. Arrows show disseminated T47D and arrowheads show focal adhesions. BV = blood vessels. Data are presented as mean ± SEM. Two-tailed Student’s t-test *P < 0.05, ***P < 0.001, ns, not significant. Data are represented of at least two independent experiments.
Discussion
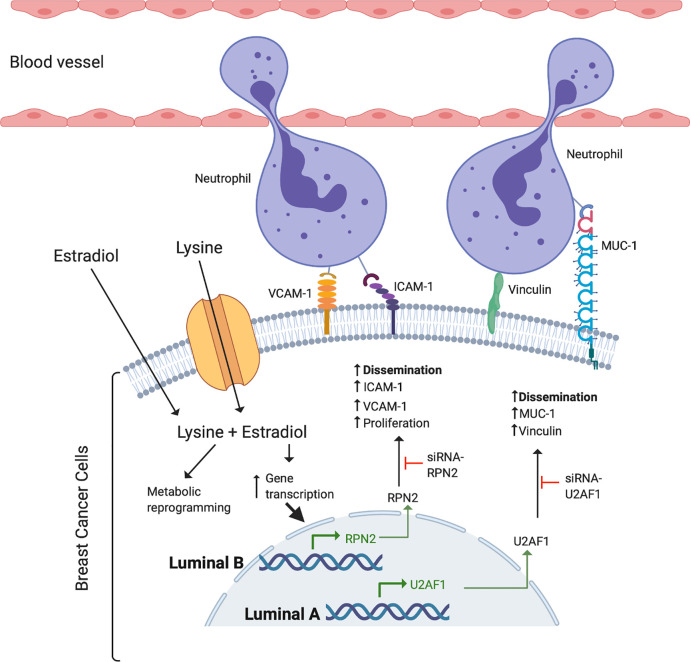
Despite the key role of EAAs in the pathophysiology of many diseases including BC, the consumption preference of BCs in vivo and their role in the metastatic process of BC has been poorly studied. Here we show that, compared to normal adjacent breast tissue, human ER+ BC exhibited significantly decreased levels of five EAAs with lysine being the most utilized in vivo. Lysine, in presence of E2, significantly increased neutrophil-induced dissemination of ER+ BC cells in vivo in zebrafish. Proteomic data revealed that lysine, in presence of E2, induced metabolic reprogramming including upregulation of U2AF1 and RPN2 in 3D cultured ER+ BC mammospheres. Knockdown of U2AF1 decreased migration in MCF-7 cells and knockdown of RPN2 decreased proliferation in T47D cells in vitro, and both proteins affected neutrophil-mediated dissemination in vivo in the zebrafish model of metastasis via effects of integrin expression and focal adhesion area as summarized in Figure 7. In clinical data-sets, these proteins decrease overall survival of ER+ luminal A and luminal B BC patients, respectively, suggesting clinical relevance of our findings.
Figure 7.
Schematic summary of the effects of lysine and estradiol on mechanisms involved in the regulation of ER+ cancer cells and neutrophil interactions resulting in increased ability of cancer cell dissemination.
Lysine is one of the most abundant EAA in human blood and one of the most important proteinogenic EAAs (32, 33). We show here that lysine also was the most abundant EAA extracellularly in human breast tissue corroborating previous results in human blood. In cancer, lysine modifications and lysine metabolites have been reported to be key drivers in the carcinogenic process and to enhance tumor aggressiveness in vitro (34, 35). Additionally, lysine has been shown to be important in the progression of murine BC as a low lysine diet retarded tumor growth at early stages (36). Our present results support these deleterious effects of lysine as the aggressiveness of both luminal A and luminal B ER+ BC increased after lysine exposure. However, lysine exposure per se exhibited limited effects but in presence of estradiol the dissemination capacity increased significantly compared to either treatment alone. Our data also support previous findings that metabolic reprogramming may depend on several intrinsic cues such as breast cancer subtype, ERα/β expression and p53 mutation state as lysine exposure affected different mechanism leading to increased dissemination in luminal A and luminal B BC cells respectively (37).
The metabolic reprogramming in cancer cells might also have implications for other cell types in the tumor microenvironment including infiltrated immune cells. A nutrient depleted microenvironment may facilitate infiltration and phenotypically changes of innate immune cells in cancers (37). Once in the cancer tissue, the innate immune cells are key in increasing the metastatic capacity of the cancer cells (38). Attachment of otherwise low metastatic BC cells to innate immune cells via integrins such as ICAM-1, VCAM-1, MUC-1, and focal adhesion enhance their dissemination ability and thereby the metastatic capability (15, 39–41). Our present data support these findings as lysine exposure, in presence of E2, upregulated the expression of several proteins, which in turn affected the expression of integrins and focal adhesion leading to altered dissemination of the cells. Lysine exposure affected luminal A and luminal B BC cells differently. In luminal A BC lysine in presence of estradiol upregulated the expression of U2AF1 protein. U2AF1 has been reported to be one of the most common spliceosome factors affected by somatic point mutations that induce alternative mRNA splicing and contribute to breast cancer development and metastases (42, 43). Knockdown of U2AF1 decreased cell migration in vitro supporting previous studies of this protein in cancer progression (42). Additionally, knockdown of U2AF1 also decreased the neutrophil dependent cancer cell dissemination in vivo, which has not been previously reported, supporting its role in the metastatic process. Increased expression of U2AF1 was associated with decreased survival of luminal A BC in clinical databases further supporting a clinical relevance of our data.
In luminal B BC cells, exposure to E2 and lysine resulted in increased expression of RPN2, which also correlate with poor overall survival of ER+ luminal B BC patients. In line with our results, RPN2 has been shown to be highly expressed in BC stem cells, promoting BC initiation and metastasis by stabilization of mutant p53, and participating in EMT transition and drug resistance (44, 45). Knockdown of RPN2 counteracted neutrophil-mediated dissemination in vivo by decreasing the expression of ICAM-1 and VCAM-1 integrins. Surprisingly, knockdown of RPN2 resulted in increased expression of MUC-1 and it would be expected that this in turn would have led to increased dissemination. However, our data suggest that this integrin does not play a role in the dissemination of the cells.
To be able to determine how an individual EAA affected the proteome of the BC cells, we first cultured the mammospheres in EAA deprived culture medium with added lysine. Under these conditions we determined that lysine in presence of E2 induced metabolic reprogramming including upregulation of U2AF1 and RPN2. To evaluate whether these proteins were relevant in a more physiological environment, complete nutrient cell culture media was used in the subsequent experiments. Indeed, we found that also during complete nutrient conditions U2AF1 and RPN2 affected proliferation, migration in vitro and neutrophil-mediated dissemination in vivo in the zebrafish via effect of integrin expression and focal adhesion area.
Metastatic BC is an incurable disease. The risk of metastatic disease is higher for ER- BC in the short-term perspective. However, 10 to 15 years after initial diagnosis the risk of relapse and death are higher in ER+ BC (46). It has previously been assumed that ER+ BC will change its phenotype into ER- during metastases but recent data clearly show that in human BC, the majority of ER+ primary BC maintains ER expression at the metastatic site (47). Consequently, models that allow for studies of ER+ BC dissemination are key. In all murine model of spontaneously metastatic BC, ER+ primary tumors will become ER- at the metastatic site. Because of this we used the zebrafish for the metastatic studies as this model allows for investigations of mechanisms involved in ER+ BC metastasis (27, 28, 48).
We conclude that lysine is the most utilized EAA in human ER+ BC in vivo. Lysine, in presence of E2, induced the expression of U2AF1 and RPN2, which contributed significantly to ER+ BC cells dissemination via interactions with neutrophils. According to the KMP online BC database, U2AF1 and RPN2 proteins correlate with poor overall survival in ER+ luminal A and luminal B BC patients, respectively, but not in ER- BC patients, confirming their clinical relevance and subtype specificity. Restricting dietary intake of lysine, blocking its uptake in cancer cells or targeting overexpressed proteins by lysine may be a potential therapeutic approach in decreasing the risk of metastases of ER+ BC.
Data Availability Statement
The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE (49) partner repository with the dataset identifier PXD022008.
Ethics Statement
The studies involving human participants were reviewed and approved by The regional ethical review board of Linköping. The patients/participants provided their written informed consent to participate in this study. The animal study was reviewed and approved by Animal ethics committee at Linköping University.
Author Contributions
GR performed experiments, wrote manuscript and analyzed data. AA performed experiments and wrote manuscript. MT analyzed data and wrote manuscript and CD performed the microdialysis investigations, analyzed data, conceived, designed, supervised the study and wrote manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by grants to CD from the Swedish Cancer Society (2018/464), the Swedish Research Council (2018–02584), LiU-Cancer, and ALF of Linköping University Hospital.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to thank Dr. Lasse Jensen, associate professor at Linköping University, for all the facilities provided to perform the zebrafish experiments. Mass spectrometry analysis was carried out at the Mass Spectrometry Core Facility of Faculty of Medicine and Health Sciences, Linköping University. The illustration was created with BioRender.com
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2020.598684/full#supplementary-material
References
- 1. Wu G. Functional amino acids in growth, reproduction, and health. Adv Nutr (2010) 1:31–7. 10.3945/an.110.1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pavlova NN, Thompson CB. The Emerging Hallmarks of Cancer Metabolism. Cell Metab (2016) 23:27–47. 10.1016/j.cmet.2015.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tsun ZY, Possemato R. Amino acid management in cancer. Semin Cell Dev Biol (2015) 43:22–32. 10.1016/j.semcdb.2015.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Neagu M, Constantin C, Popescu ID, Zipeto D, Tzanakakis G, Nikitovic D, et al. Inflammation and Metabolism in Cancer Cell-Mitochondria Key Player. Front Oncol (2019) 9:1–15. 10.3389/fonc.2019.00348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kimball SR, Jefferson LS. Regulation of protein synthesis by branched-chain amino acids. Curr Opin Clin Nutr Metab Care (2001) 4:39–43. 10.1097/00075197-200101000-00008 [DOI] [PubMed] [Google Scholar]
- 6. Jousse C, Averous J, Bruhat A, Carraro V, Mordier S, Fafournoux P. Amino acids as regulators of gene expression: molecular mechanisms. Biochem Biophys Res Commun (2004) 313:447–52. 10.1016/j.bbrc.2003.07.020 [DOI] [PubMed] [Google Scholar]
- 7. Knott SRV, Wagenblast E, Khan S, Kim SY, Soto M, Wagner M, et al. Asparagine bioavailability governs metastasis in a model of breast cancer. Nature (2018) 554:378–81. 10.1038/nature25465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jeon H, Kim JH, Lee E, Jang YJ, Son JE, Kwon JY, et al. Methionine deprivation suppresses triple-negative breast cancer metastasis in vitro and in vivo. Oncotarget (2016) 7:67223–34. 10.18632/oncotarget.11615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Eagle H. Nutrition needs of mammalian cells in tissue culture. Science (1955) 122:501–14. 10.1126/science.122.3168.501 [DOI] [PubMed] [Google Scholar]
- 10. Gao X, Sanderson SM, Dai Z, Reid MA, Cooper DE, Lu M, et al. Dietary methionine influences therapy in mouse cancer models and alters human metabolism. Nature (2019) 572:397–401. 10.1038/s41586-019-1437-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Abdallah RM, Starkey JR, Meadows GG. Dietary restriction of tyrosine and phenylalanine: inhibition of metastasis of three rodent tumors. J Natl Cancer Inst (1987) 78:759–69. [PubMed] [Google Scholar]
- 12. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2018) 68:394–424. 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 13. Early Breast Cancer Trialists’ Collaborative G. Davies C, Godwin J, Gray R, Clarke M, Cutter D, et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet (2011) 378:771–84. 10.1016/S0140-6736(11)60993-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bhat HK, Vadgama JV. Role of estrogen receptor in the regulation of estrogen induced amino acid transport of System A in breast cancer and other receptor positive tumor cells. Int J Mol Med (2002) 9:271–9. 10.3892/ijmm.9.3.271 [DOI] [PubMed] [Google Scholar]
- 15. Vazquez Rodriguez G, Abrahamsson A, Jensen LD, Dabrosin C. Estradiol Promotes Breast Cancer Cell Migration via Recruitment and Activation of Neutrophils. Cancer Immunol Res (2017) 5:234–47. 10.1158/2326-6066.CIR-16-0150 [DOI] [PubMed] [Google Scholar]
- 16. Svensson S, Abrahamsson A, Rodriguez GV, Olsson AK, Jensen L, Cao Y, et al. CCL2 and CCL5 Are Novel Therapeutic Targets for Estrogen-Dependent Breast Cancer. Clin Cancer Res (2015) 21:3794–805. 10.1158/1078-0432.CCR-15-0204 [DOI] [PubMed] [Google Scholar]
- 17. Miyagi Y, Higashiyama M, Gochi A, Akaike M, Ishikawa T, Miura T, et al. Plasma free amino acid profiling of five types of cancer patients and its application for early detection. PLoS One (2011) 6:e24143. 10.1371/journal.pone.0024143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Abrahamsson A, Rzepecka A, Dabrosin C. Increased nutrient availability in dense breast tissue of postmenopausal women in vivo. Sci Rep (2017) 7:42733. 10.1038/srep42733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yamamoto M, Hosoda M, Nakano K, Jia S, Hatanaka KC, Takakuwa E, et al. p53 accumulation is a strong predictor of recurrence in estrogen receptor-positive breast cancer patients treated with aromatase inhibitors. Cancer Sci (2014) 105:81–8. 10.1111/cas.12302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cancer Genome Atlas N Comprehensive molecular portraits of human breast tumours. Nature (2012) 490:61–70. 10.1038/nature11412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wisniewski JR, Zougman A, Nagaraj N, Mann M. Universal sample preparation method for proteome analysis. Nat Methods (2009) 6:359–62. 10.1038/nmeth.1322 [DOI] [PubMed] [Google Scholar]
- 22. Molinas A, Turkina MV, Magnusson KE, Mirazimi A, Vikstrom E. Perturbation of Wound Healing, Cytoskeletal Organization and Cellular Protein Networks during Hazara Virus Infection. Front Cell Dev Biol (2017) 5:98. 10.3389/fcell.2017.00098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nesvizhskii AI, Keller A, Kolker E, Aebersold R. A statistical model for identifying proteins by tandem mass spectrometry. Anal Chem (2003) 75:4646–58. 10.1021/ac0341261 [DOI] [PubMed] [Google Scholar]
- 24. Rouhi P, Jensen LD, Cao Z, Hosaka K, Lanne T, Wahlberg E, et al. Hypoxia-induced metastasis model in embryonic zebrafish. Nat Protoc (2010) 5:1911–8. 10.1038/nprot.2010.150 [DOI] [PubMed] [Google Scholar]
- 25. Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, et al. Fiji: an open-source platform for biological-image analysis. Nat Methods (2012) 9:676–82. 10.1038/nmeth.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gyorffy B, Lanczky A, Eklund AC, Denkert C, Budczies J, Li Q, et al. An online survival analysis tool to rapidly assess the effect of 22,277 genes on breast cancer prognosis using microarray data of 1,809 patients. Breast Cancer Res Treat (2010) 123:725–31. 10.1007/s10549-009-0674-9 [DOI] [PubMed] [Google Scholar]
- 27. Szczerba BM, Castro-Giner F, Vetter M, Krol I, Gkountela S, Landin J, et al. Neutrophils escort circulating tumour cells to enable cell cycle progression. Nature (2019) 566:553–7. 10.1038/s41586-019-0915-y [DOI] [PubMed] [Google Scholar]
- 28. Wculek SK, Malanchi I. Neutrophils support lung colonization of metastasis-initiating breast cancer cells. Nature (2015) 528:413–7. 10.1038/nature16140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zaretsky JZ, Barnea I, Aylon Y, Gorivodsky M, Wreschner DH, Keydar I. MUC1 gene overexpressed in breast cancer: structure and transcriptional activity of the MUC1 promoter and role of estrogen receptor alpha (ERalpha) in regulation of the MUC1 gene expression. Mol Cancer (2006) 5:57. 10.1186/1476-4598-5-57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Vazquez Rodriguez G, Abrahamsson A, Jensen LDE, Dabrosin C. Adipocytes Promote Early Steps of Breast Cancer Cell Dissemination via Interleukin-8. Front Immunol (2018) 9:1767. 10.3389/fimmu.2018.01767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kuo JC. Mechanotransduction at focal adhesions: integrating cytoskeletal mechanics in migrating cells. J Cell Mol Med (2013) 17:704–12. 10.1111/jcmm.12054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mc MR, Lund CC, Oncley JL. Unbound amino acid concentrations in human blood plasmas. J Clin Invest (1957) 36:1672–9. 10.1172/JCI103568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ananieva EA, Wilkinson AC. Branched-chain amino acid metabolism in cancer. Curr Opin Clin Nutr Metab Care (2018) 21:64–70. 10.1097/MCO.0000000000000430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rosi A, Ricci-Vitiani L, Biffoni M, Grande S, Luciani AM, Palma A, et al. (1) H NMR spectroscopy of glioblastoma stem-like cells identifies alpha-aminoadipate as a marker of tumor aggressiveness. NMR Biomed (2015) 28:317–26. 10.1002/nbm.3254 [DOI] [PubMed] [Google Scholar]
- 35. Chen L, Miao Y, Liu M, Zeng Y, Gao Z, Peng D, et al. Pan-Cancer Analysis Reveals the Functional Importance of Protein Lysine Modification in Cancer Development. Front Genet (2018) 9:254. 10.3389/fgene.2018.00254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kocher R. Effects of a Low Lysine Diet on the Growth of Spontaneous Mammary Tumors in Mice and on the N2 Balance in Man. Cancer Res (1944) 4:251–6. [Google Scholar]
- 37. Gandhi N, Das GM. Metabolic Reprogramming in Breast Cancer and Its Therapeutic Implications. Cells (2019) 8:1–33. 10.3390/cells8020089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Galdiero MR, Garlanda C, Jaillon S, Marone G, Mantovani A. Tumor associated macrophages and neutrophils in tumor progression. J Cell Physiol (2013) 228:1404–12. 10.1002/jcp.24260 [DOI] [PubMed] [Google Scholar]
- 39. Nath D, Hartnell A, Happerfield L, Miles DW, Burchell J, Taylor-Papadimitriou J, et al. Macrophage-tumour cell interactions: identification of MUC1 on breast cancer cells as a potential counter-receptor for the macrophage-restricted receptor, sialoadhesin. Immunology (1999) 98:213–9. 10.1046/j.1365-2567.1999.00827.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kim DH, Wirtz D. Focal adhesion size uniquely predicts cell migration. FASEB J (2013) 27:1351–61. 10.1096/fj.12-220160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sokeland G, Schumacher U. The functional role of integrins during intra- and extravasation within the metastatic cascade. Mol Cancer (2019) 18:12. 10.1186/s12943-018-0937-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Palangat M, Anastasakis DG, Fei DL, Lindblad KE, Bradley R, Hourigan CS, et al. The splicing factor U2AF1 contributes to cancer progression through a noncanonical role in translation regulation. Genes Dev (2019) 33:482–97. 10.1101/gad.319590.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yoshida K, Sanada M, Shiraishi Y, Nowak D, Nagata Y, Yamamoto R, et al. Frequent pathway mutations of splicing machinery in myelodysplasia. Nature (2011) 478:64–9. 10.1038/nature10496 [DOI] [PubMed] [Google Scholar]
- 44. Honma K, Iwao-Koizumi K, Takeshita F, Yamamoto Y, Yoshida T, Nishio K, et al. RPN2 gene confers docetaxel resistance in breast cancer. Nat Med (2008) 14:939–48. 10.1038/nm.1858 [DOI] [PubMed] [Google Scholar]
- 45. Takahashi RU, Takeshita F, Honma K, Ono M, Kato K, Ochiya T. Ribophorin II regulates breast tumor initiation and metastasis through the functional suppression of GSK3beta. Sci Rep (2013) 3:2474. 10.1038/srep02474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kennecke H, Yerushalmi R, Woods R, Cheang MC, Voduc D, Speers CH, et al. Metastatic behavior of breast cancer subtypes. J Clin Oncol (2010) 28:3271–7. 10.1200/JCO.2009.25.9820 [DOI] [PubMed] [Google Scholar]
- 47. Lindstrom LS, Karlsson E, Wilking UM, Johansson U, Hartman J, Lidbrink EK, et al. Clinically used breast cancer markers such as estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2 are unstable throughout tumor progression. J Clin Oncol (2012) 30:2601–8. 10.1200/JCO.2011.37.2482 [DOI] [PubMed] [Google Scholar]
- 48. Dias AS, Almeida CR, Helguero LA, Duarte IF. Metabolic crosstalk in the breast cancer microenvironment. Eur J Cancer (2019) 121:154–71, 121. 10.1016/j.ejca.2019.09.002 [DOI] [PubMed] [Google Scholar]
- 49. Perez-Riverol Y, Csordas A, Bai J, Bernal-Llinares M, Hewapathirana S, Kundu DJ, et al. The PRIDE database and related tools and resources in 2019: improving support for quantification data. Nucleic Acids Res (2019) 47:D442–50. 10.1093/nar/gky1106 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE (49) partner repository with the dataset identifier PXD022008.