Abstract
Citrus leaf and fruit spot is one of the most important biotic constraints of citrus production in Ethiopia. The symptomatic leaf and fruit samples were collected from 29 orchards of 15 major citrus growing districts of Ethiopia. One hundred sixty-seven fungal isolates were recovered and identified to species level through DNA barcoding; and their relationships were established using multigene phylogeny. The internal transcribed spacers, long subunit and actin gene sequences revealed that those 167 isolates belonged to either Collectotrichum gloeosporioides or Collectotrichum boninense species complexes (sensu lato), but no recovery of Pseudocercospora angolensis, the primary causal agent of the citrus leaf and fruit spot disease. Detached leaf assays confirmed pathogenicity of isolates of both C. gloeosporioides and C. boninense species complexes on citrus. They reproduced disease symptoms and the pathogens were re-isolated from symptomatic tissues. This study reports frequent association of C. gloeosporioides and C. boninense species complexes with citrus fruit and leaf spot disease in Ethiopia. This finding suggests the need for in-depth studies to determine the roles of C. gloeosporioides and C. boninense species complexes in citrus fruit and leaf spot disease epidemiology.
Keywords: DNA barcode marker, Internal transcribed spacer, Long subunit, Actin gene, Pathogenicity, Colletotrichum, Citrus
Introduction
Citrus (Citrus spp.) are economically important fruits in Ethiopia [1,2]. The total acreage and the annual production of citrus are estimated at 7040 hectares and 72459 tons, respectively [3,4]. The commercial citrus farms are located mainly in the central rift valley and the eastern parts of Ethiopia. They contribute about 46% to the total citrus production of the country. Whereas, small-scale citrus productions are scattered throughout the country. Most citrus fruits are consumed fresh while some are processed for juice and marmalade [1]. Sweet oranges and lime are exported to Djibouti, Europe, and the Middle East [5]. However, the citrus production in Ethiopia is severely constrained by diseases including Citrus Fruit and Leaf Spot Disease (CFLSD) [2,6]. The CFLSD is caused by a fungus Pseudocercospora angolensis [7]. It can cause yield losses of 20% to 100% [8]. It also affects fruit quality and yields of essential oils [9]. Since the first report in Angola and Mozambique in 1952 [10], the disease has been reported from 22 African countries and Yemen [2,8,11,12]. In Ethiopia, the disease was first reported in 1988 from the southern part of the country [13]. Later, it spread to south, southwest, and northwest parts of Ethiopia and cause heavy crop damage, often total crop loss [2,6].
The CFLSD attacks leaves, fruits, and young twigs [14]. Symptoms on the leaves are characterized by circular, mostly solitary spots with light brown or grayish centers which blacken with sporulation [8]. The lesions are often surrounded by dark brown margins and prominent yellow halos. Infection on fruit produces circular to irregular, discrete or coalescent spots. It also produces yellow halos which is surrounded by tumor-like growth on young fruits [15]. The infection of P. angolensis seems to predispose citrus fruits to secondary infection by Colletotrichum gloeosporioides. Infection on stem usually occurs from infected petiole. Several lesions on stem may cause defoliation and die back symptoms [10,16]. Despite high economic importance of CFLSD in sub-Saharan Africa, our understanding of biology and epidemiology of the causal agent is very limited [12]. Therefore, this study was conducted to isolate the causal agent associated with symptomatic citrus leaf and fruit samples collected from the major citrus growing areas of Ethiopia and examine pathogen diversity based on the multiple gene sequences and phylogeny.
Materials and Methods
Fungal isolates
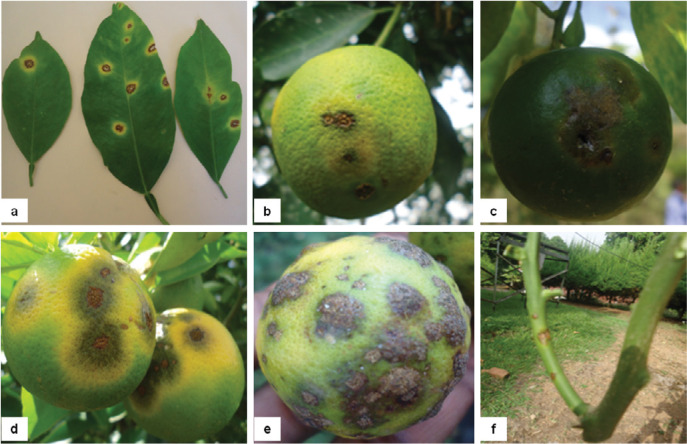
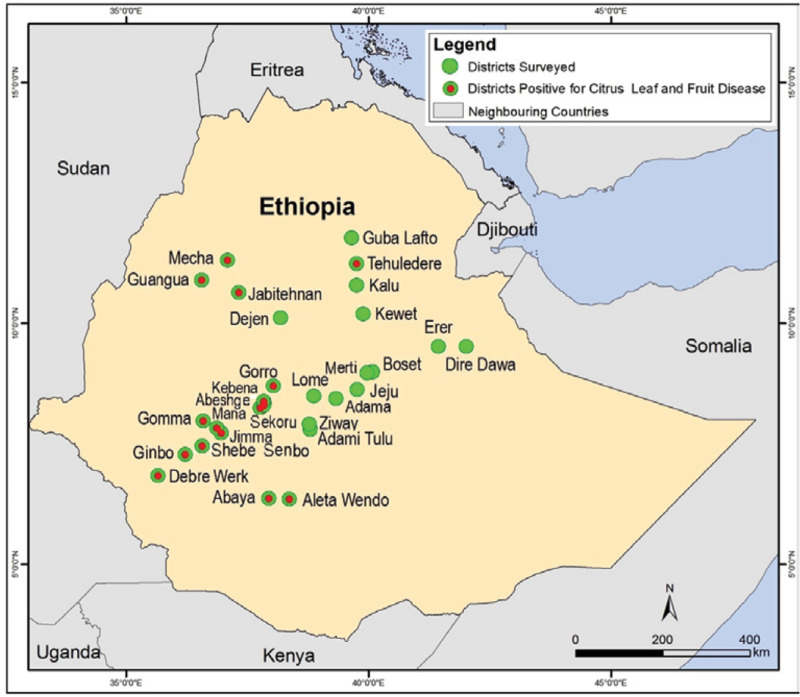
Citrus leaves and fruits with distinct symptoms of CFLSD (Figure 1) were collected from citrus production areas of Ethiopia (Figure 2) in between 2012 and 2014. Among 49 citrus orchards surveyed in 29 districts, the disease was recorded in 29 orchards of 15 districts. The symptomatic leaf and fruit samples collected from these orchards were transported to laboratory in ice chest for isolating causal agent. Leaf and fruit tissues comprising both infected and healthy parts were excised, surface disinfected in 70% ethanol for 1 min followed by 1% sodium hypochlorite solution for 10 min and rinsed three times with sterile distilled water. Disinfected tissues were blot dried and placed on potato dextrose agar (PDA; Oxoid, Basingstoke, Hampshire, UK) plates amended with 50 ppm streptomycin sulfate. Cultures were incubated in dark at room temperature. A total of 167 fungi were isolated (Table 1) and monosporic or single-hyphal tip cultures were established. Stock cultures were preserved on PDA slants supplemented with 10% glycerol at 4°C.
Figure 1.
Symptoms of citrus leaf and fruit spot disease in Ethiopia. (a) Leaves with spots, (b to e) symptomatic fruits, and (f) young twig with lesions.
Figure 2.
Map of Ethiopia showing the districts surveyed, and the districts with fruit and leaf spot disease symptoms. Locations were plotted as per GPS coordinates using ArcGIS (version 10) software.
Table 1.
Isolates of Colletotrichum species and their origin in Ethiopia.
| Isolate | Host plant | Plant part | Region | District | Collection Site |
|---|---|---|---|---|---|
| ETHCTR001 | Citrus sinensis | Leaf | Southwest Ethiopia | Ginbo | Megenagna |
| ETHCTR002 | Citrus sinensis | Fruit | Central Ethiopia | Kebena | Aregita |
| ETHCTR003 | Citrus sinensis | Fruit | Central Ethiopia | Geta | Kebul |
| ETHCTR004 | Citrus sinensis | Fruit | Central Ethiopia | Abeshege | Rumuga |
| ETHCTR006 | Citrus sinensis | Leaf | South Ethiopia | Damot Pulasa | Denba Galie |
| ETHCTR007 | Citrus sinensis | Fruit | Central Ethiopia | Abeshege | Holie |
| ETHCTR008 | Citrus sinensis | Leaf | Southwest Ethiopia | Debre Werk | Bebeka |
| ETHCTR009 | Citrus sinensis | Leaf | Southwest Ethiopia | Mana | Gube Bosoka |
| ETHCTR012 | Citrus sinensis | Leaf | Southwest Ethiopia | Shebe Senbo | Kishe-Kosta |
| ETHCTR013 | Citrus sinensis | Leaf | Central Ethiopia | Abeshege | Jejeba |
| ETHCTR014 | Citrus sinensis | Leaf | Central Ethiopia | Abeshege | Jejeba |
| ETHCTR016 | Citrus aurantium | Leaf | Central Ethiopia | Wolisso | Fodu Gora |
| ETHCTR017 | Citrus aurantium | Fruit | Central Ethiopia | Wolisso | Fodu Gora |
| ETHCTR018 | Citrus sinensis | Leaf | Central Ethiopia | Wolisso | Fodu Gora |
| ETHCTR019 | Citrus aurantium | Leaf | Central Ethiopia | Wolisso | Fodu Gora |
| ETHCTR020 | Citrus sinensis | Leaf | Central Ethiopia | Abeshege | Holie |
| ETHCTR021 | Citrus sinensis | Leaf | South Ethiopia | Damot Pulasa | Denba Galie |
| ETHCTR022 | Citrus sinensis | Leaf | South Ethiopia | Damot Pulasa | Denba Galie |
| ETHCTR023 | Citrus sinensis | Leaf | South Ethiopia | Damot Pulasa | Denba Galie |
| ETHCTR024 | Citrus sinensis | Leaf | Central Ethiopia | Cheha | Chifangira |
| ETHCTR025 | Citrus sinensis | Leaf | Central Ethiopia | Cheha | Chifangira |
| ETHCTR026 | Citrus sinensis | Leaf | Central Ethiopia | Cheha | Sisena Mitia |
| ETHCTR027 | Citrus sinensis | Leaf | Central Ethiopia | Cheha | Sisena Mitia |
| ETHCTR028 | Citrus sinensis | Leaf | Central Ethiopia | Cheha | Sisena Mitia |
| ETHCTR029 | Citrus sinensis | Leaf | Central Ethiopia | Cheha | Sisena Mitia |
| ETHCTR031 | Citrus sinensis | Leaf | South Ethiopia | Damot Pulasa | Denba Galie |
| ETHCTR032 | Citrus sinensis | Leaf | South Ethiopia | Boloso Sore | Areka |
| ETHCTR033 | Citrus sinensis | Fruit | South Ethiopia | Boloso Sore | Areka |
| ETHCTR034 | Citrus sinensis | Fruit | South Ethiopia | Boloso Sore | Areka |
| ETHCTR037 | Citrus sinensis | Fruit | Central Ethiopia | Sekoru | Gibe |
| ETHCTR038 | Citrus reticulate | Leaf | Central Ethiopia | Sekoru | Gibe |
| ETHCTR039 | Citrus sinensis | Leaf | South Ethiopia | Abaya | Guangua |
| ETHCTR041 | Citrus reticulate | Leaf | Northwest Ethiopia | Jabitehnan | Finoteselam |
| ETHCTR042 | Citrus sinensis | Leaf | Northwest Ethiopia | Jabitehnan | Finoteselam |
| ETHCTR043 | Citrus sinensis | Fruit | Northwest Ethiopia | Jabitehnan | Finoteselam |
| ETHCTR044 | Citrus sinensis | Fruit | Northwest Ethiopia | Jabitehnan | Finoteselam |
| ETHCTR045 | Citrus sinensis | Leaf | Southwest Ethiopia | Mana | Gube Bosoka |
| ETHCTR046 | Citrus sinensis | Fruit | Southwest Ethiopia | Mana | Gube Bosoka |
| ETHCTR047 | Citrus sinensis | Leaf | Central Ethiopia | Gorro | Adami Wedessa |
| ETHCTR048 | Citrus aurantium | Leaf | South Ethiopia | Abaya | Guangua |
| ETHCTR049 | Citrus sinensis | Leaf | Southwest Ethiopia | Gomma | Agaro |
| ETHCTR050 | Citrus aurantium | Leaf | South Ethiopia | Abaya | Lado |
| ETHCTR051 | Citrus sinensis | Fruit | South Ethiopia | Abaya | Guangua |
| ETHCTR052 | Citrus sinensis | Leaf | Southwest Ethiopia | Gomma | Agaro |
| ETHCTR053 | Citrus sinensis | Leaf | Central Ethiopia | Abeshege | Gasorie |
| ETHCTR054 | Citrus sinensis | Fruit | Southwest Ethiopia | Gomma | Agaro |
| ETHCTR055 | Citrus sinensis | Leaf | Southwest Ethiopia | Mana | Gube Bosoka |
| ETHCTR056 | Citrus sinensis | Leaf | South Ethiopia | Abaya | Guangua |
| ETHCTR057 | Citrus sinensis | Leaf | South Ethiopia | Abaya | Guangua |
| ETHCTR058 | Citrus sinensis | Fruit | Central Ethiopia | Abeshege | Gasorie |
| ETHCTR059 | Citrus sinensis | Leaf | Northwest Ethiopia | Guangua | Chagni |
| ETHCTR060 | Citrus sinensis | Leaf | Northwest Ethiopia | Guangua | Chagni |
| ETHCTR061 | Citrus sinensis | Fruit | Northwest Ethiopia | Jabitehnan | Finoteselam |
| ETHCTR062 | Citrus sinensis | Fruit | Northwest Ethiopia | Jabitehnan | Finoteselam |
| ETHCTR063 | Citrus sinensis | Leaf | South Ethiopia | Abaya | Guangua |
| ETHCTR064 | Citrus sinensis | Fruit | South Ethiopia | Abaya | Guangua |
| ETHCTR065 | Citrus sinensis | Fruit | Central Ethiopia | Kebena | Aregita |
| ETHCTR066 | Citrus sinensis | Fruit | Central Ethiopia | Kebena | Aregita |
| ETHCTR067 | Citrus sinensis | Fruit | Southwest Ethiopia | Debre Werk | Bebeka |
| ETHCTR068 | Citrus sinensis | Leaf | Southwest Ethiopia | Debre Werk | Bebeka |
| ETHCTR069 | Citrus sinensis | Leaf | Southwest Ethiopia | Debre Werk | Bebeka |
| ETHCTR070 | Citrus sinensis | Leaf | Central Ethiopia | Kebena | Aregita |
| ETHCTR071 | Citrus sinensis | Leaf | Central Ethiopia | Kebena | Aregita |
| ETHCTR072 | Citrus reticulate | Fruit | Northwest Ethiopia | Jabitehnan | Finoteselam |
| ETHCTR073 | Citrus reticulate | Fruit | Northwest Ethiopia | Jabitehnan | Finoteselam |
| ETHCTR074 | Citrus sinensis | Leaf | South Ethiopia | Aleta Wendo | Omacho Chawa |
| ETHCTR075 | Citrus sinensis | Leaf | South Ethiopia | Aleta Wendo | Omacho Chawa |
| ETHCTR077 | Citrus sinensis | Leaf | Northwest Ethiopia | Guangua | Chagni |
| ETHCTR078 | Citrus sinensis | Fruit | Central Ethiopia | Abeshege | Tawela |
| ETHCTR081 | Citrus sinensis | Fruit | Central Ethiopia | Abeshege | Gasorie |
| ETHCTR082 | Citrus sinensis | Fruit | Central Ethiopia | Abeshege | Gasorie |
| ETHCTR084 | Citrus sinensis | Leaf | Southwest Ethiopia | Ginbo | Megenagna |
| ETHCTR085 | Citrus sinensis | Leaf | Southwest Ethiopia | Ginbo | Megenagna |
| ETHCTR086 | Citrus sinensis | Leaf | Southwest Ethiopia | Shebe Senbo | Kishe-Kosta |
| ETHCTR088 | Citrus sinensis | Leaf | Northwest Ethiopia | Jabitehnan | Finoteselam |
| ETHCTR089 | Citrus sinensis | Fruit | Southwest Ethiopia | Mana | Gube Bosoka |
| ETHCTR090 | Citrus sinensis | Leaf | Northwest Ethiopia | Jabitehnan | Finoteselam |
| ETHCTR091 | Citrus sinensis | Leaf | Northwest Ethiopia | Jabitehnan | Finoteselam |
| ETHCTR092 | Citrus sinensis | Fruit | Northwest Ethiopia | Guangua | Chagni |
| ETHCTR093 | Citrus sinensis | Fruit | Northwest Ethiopia | Guangua | Chagni |
| ETHCTR094 | Citrus sinensis | Leaf | South Ethiopia | Abaya | Guangua |
| ETHCTR095 | Citrus sinensis | Leaf | Northwest Ethiopia | Guangua | Chagni |
| ETHCTR096 | Citrus sinensis | Leaf | Southwest Ethiopia | Shebe Senbo | Kishe-Kosta |
| ETHCTR097 | Citrus sinensis | Fruit | South Ethiopia | Abaya | Guangua |
| ETHCTR098 | Citrus sinensis | Leaf | South Ethiopia | Boloso Sore | Areka |
| ETHCTR101 | Citrus sinensis | Leaf | Central Ethiopia | Abeshege | Layignaw Tatesa |
| ETHCTR103 | Citrus aurantium | Leaf | South Ethiopia | Abaya | Lado |
| ETHCTR104 | Citrus sinensis | Leaf | Southwest Ethiopia | Gomma | Agaro |
| ETHCTR105 | Citrus sinensis | Leaf | Southwest Ethiopia | Gomma | Agaro |
| ETHCTR106 | Citrus sinensis | Leaf | Southwest Ethiopia | Gomma | Agaro |
| ETHCTR107 | Citrus sinensis | Fruit | Southwest Ethiopia | Gomma | Agaro |
| ETHCTR108 | Citrus sinensis | Leaf | Northwest Ethiopia | Jabitehnan | Finoteselam |
| ETHCTR109 | Citrus sinensis | Leaf | Northwest Ethiopia | Jabitehnan | Finoteselam |
| ETHCTR110 | Citrus sinensis | Leaf | Northwest Ethiopia | Jabitehnan | Finoteselam |
| ETHCTR111 | Citrus sinensis | Fruit | Northwest Ethiopia | Jabitehnan | Finoteselam |
| ETHCTR112 | Citrus sinensis | Leaf | Central Ethiopia | Abeshege | Layignaw Tatesa |
| ETHCTR113 | Citrus sinensis | Leaf | Central Ethiopia | Abeshege | Layignaw Tatesa |
| ETHCTR114 | Citrus sinensis | Leaf | North Central | Tehuledere | Hayk/Jarre |
| ETHCTR115 | Citrus sinensis | Leaf | South Ethiopia | Aleta Wendo | Omacho Chawa |
| ETHCTR116 | Citrus sinensis | Leaf | South Ethiopia | Aleta Wendo | Omacho Chawa |
| ETHCTR117 | Citrus sinensis | Leaf | Southwest Ethiopia | Mana | Gube Bosoka |
| ETHCTR118 | Citrus reticulate | Fruit | Northwest Ethiopia | Jabitehnan | Finoteselam |
| ETHCTR119 | Citrus reticulate | Fruit | Northwest Ethiopia | Jabitehnan | Finoteselam |
| ETHCTR120 | Citrus reticulate | Fruit | Northwest Ethiopia | Jabitehnan | Finoteselam |
| ETHCTR121 | Citrus reticulate | Fruit | Northwest Ethiopia | Jabitehnan | Finoteselam |
| ETHCTR122 | Citrus sinensis | Leaf | Southwest Ethiopia | Ginbo | Balewold |
| ETHCTR123 | Citrus sinensis | Leaf | Southwest Ethiopia | Ginbo | Balewold |
| ETHCTR124 | Citrus sinensis | Leaf | Southwest Ethiopia | Ginbo | Balewold |
| ETHCTR127 | Citrus sinensis | Leaf | Southwest Ethiopia | Shebe Senbo | Kishe-Kosta |
| ETHCTR128 | Citrus sinensis | Leaf | Southwest Ethiopia | Shebe Senbo | Kishe-Kosta |
| ETHCTR129 | Citrus sinensis | Leaf | Southwest Ethiopia | Debre Werk | Bebeka |
| ETHCTR130 | Citrus sinensis | Leaf | Southwest Ethiopia | Debre Werk | Bebeka |
| ETHCTR131 | Citrus sinensis | Leaf | Southwest Ethiopia | Debre Werk | Bebeka |
| ETHCTR132 | Citrus sinensis | Fruit | Central Ethiopia | Abeshege | Tawela |
| ETHCTR133 | Citrus sinensis | Fruit | Central Ethiopia | Abeshege | Tawela |
| ETHCTR134 | Citrus sinensis | Leaf | Central Ethiopia | Abeshege | Gasorie |
| ETHCTR136 | Citrus sinensis | Leaf | Central Ethiopia | Abeshege | Gasorie |
| ETHCTR137 | Citrus sinensis | Fruit | Central Ethiopia | Abeshege | Gasorie |
| ETHCTR138 | Citrus sinensis | Fruit | Central Ethiopia | Abeshege | Gasorie |
| ETHCTR139 | Citrus sinensis | Leaf | Southwest Ethiopia | Debre Werk | Bebeka |
| ETHCTR140 | Citrus sinensis | Leaf | Southwest Ethiopia | Debre Werk | Bebeka |
| ETHCTR141 | Citrus sinensis | Leaf | Southwest Ethiopia | Debre Werk | Bebeka |
| ETHCTR142 | Citrus sinensis | Leaf | Southwest Ethiopia | Debre Werk | Bebeka |
| ETHCTR143 | Citrus sinensis | Leaf | Southwest Ethiopia | Debre Werk | Bebeka |
| ETHCTR146 | Citrus sinensis | Leaf | Southwest Ethiopia | Gomma | Agaro |
| ETHCTR148 | Citrus sinensis | Leaf | Central Ethiopia | Abeshege | Gasorie |
| ETHCTR151 | Citrus sinensis | Fruit | Southwest Ethiopia | Debre Werk | Bebeka |
| ETHCTR152 | Citrus sinensis | Fruit | Southwest Ethiopia | Debre Werk | Bebeka |
| ETHCTR153 | Citrus sinensis | Fruit | Southwest Ethiopia | Debre Werk | Bebeka |
| ETHCTR154 | Citrus sinensis | Fruit | Northwest Ethiopia | Jabitehnan | Finoteselam |
| ETHCTR156 | Citrus sinensis | Fruit | South Ethiopia | Abaya | Guangua |
| ETHCTR157 | Citrus sinensis | Leaf | Southwest Ethiopia | Debre Werk | Bebeka |
| ETHCTR158 | Citrus sinensis | Leaf | Southwest Ethiopia | Debre Werk | Bebeka |
| ETHCTR159 | Citrus sinensis | Leaf | Southwest Ethiopia | Debre Werk | Bebeka |
| ETHCTR160 | Citrus sinensis | Leaf | Southwest Ethiopia | Gomma | Agaro |
| ETHCTR161 | Citrus sinensis | Leaf | Northwest Ethiopia | Guangua | Chagni |
| ETHCTR162 | Citrus sinensis | Fruit | Northwest Ethiopia | Jabitehnan | Finoteselam |
| ETHCTR163 | Citrus sinensis | Leaf | Northwest Ethiopia | Jabitehnan | Finoteselam |
| ETHCTR164 | Citrus sinensis | Leaf | Northwest Ethiopia | Jabitehnan | Finoteselam |
| ETHCTR165 | Citrus sinensis | Leaf | Northwest Ethiopia | Jabitehnan | Finoteselam |
| ETHCTR166 | Citrus sinensis | Fruit | South Ethiopia | Boloso Sore | Areka |
| ETHCTR167 | Citrus sinensis | Fruit | South Ethiopia | Boloso Sore | Areka |
| ETHCTR169 | Citrus sinensis | Fruit | South Ethiopia | Boloso Sore | Areka |
| ETHCTR170 | Citrus sinensis | Leaf | Southwest Ethiopia | Ginbo | Megenagna |
| ETHCTR172 | Citrus sinensis | Leaf | Southwest Ethiopia | Ginbo | Megenagna |
| ETHCTR173 | Citrus sinensis | Leaf | Southwest Ethiopia | Mana | Gube Bosoka |
| ETHCTR174 | Citrus sinensis | Leaf | Southwest Ethiopia | Mana | Gube Bosoka |
| ETHCTR175 | Citrus reticulata | Leaf | Northwest Ethiopia | Jabitehnan | Finoteselam |
| ETHCTR176 | Citrus reticulata | Leaf | Northwest Ethiopia | Jabitehnan | Finoteselam |
| ETHCTR178 | Citrus reticulata | Leaf | Northwest Ethiopia | Jabitehnan | Finoteselam |
| ETHCTR179 | Citrus reticulata | Leaf | Northwest Ethiopia | Jabitehnan | Finoteselam |
| ETHCTR180 | Citrus reticulata | Leaf | Northwest Ethiopia | Jabitehnan | Finoteselam |
| ETHCTR181 | Citrus sinensis | Fruit | Northwest Ethiopia | Jabitehnan | Finoteselam |
| ETHCTR182 | Citrus sinensis | Fruit | Northwest Ethiopia | Jabitehnan | Finoteselam |
| ETHCTR183 | Citrus sinensis | Fruit | Northwest Ethiopia | Jabitehnan | Finoteselam |
| ETHCTR184 | Citrus sinensis | Fruit | Northwest Ethiopia | Jabitehnan | Finoteselam |
| ETHCTR185 | Citrus sinensis | Fruit | Northwest Ethiopia | Jabitehnan | Finoteselam |
| ETHCTR186 | Citrus sinensis | Fruit | Northwest Ethiopia | Jabitehnan | Finoteselam |
| ETHCTR187 | Citrus sinensis | Leaf | Central Ethiopia | Cheha | Sisena Mitia |
| ETHCTR188 | Citrus sinensis | Fruit | Central Ethiopia | Geta | Kebul |
| ETHCTR189 | Citrus sinensis | Leaf | South Ethiopia | Damot Pulasa | Denba Galie |
| ETHCTR190 | Citrus sinensis | Leaf | South Ethiopia | Damot Pulasa | Denba Galie |
| ETHCTR192 | Citrus sinensis | Leaf | Northwest Ethiopia | Jabitehnan | Finoteselam |
| ETHCTR193 | Citrus sinensis | Fruit | Northwest Ethiopia | Jabitehnan | Finoteselam |
| ETHCTR194 | Citrus sinensis | Leaf | Central Ethiopia | Abeshege | Holie |
| ETHCTR197 | Citrus aurantium | Leaf | Central Ethiopia | Wolisso | Fodu Gora |
| ETHCTR198 | Citrus aurantium | Fruit | Central Ethiopia | Wolisso | Fodu Gora |
DNA extraction
Fungal genomic DNA was extracted using the procedures described earlier [17,18] with some modifications. Mycelium was scraped from the surface of a week-old cultures, added with fine sand, and crushed in a mortar and pestle. The finely ground mycelium was transferred into 1.5 ml Eppendorf tubes with sterile glass beads, and were pulverized with GenoGrinder at 25 rpm for 3 min in 0.5 ml extraction buffer (3% CTAB, 200 mM Tris-HCl (pH 8.0), 0.5 M NaCl, 10 mM EDTA (pH 8.0), and 2% SDS (preheated at 65°C)). To each sample, 150 μl of sodium acetate (3 M, pH 5.2) was added and mixed by inverting the tube. The mixtures were incubated at -20°C for 10 min and centrifuged at 14800 rpm for 10 min at room temperature. The supernatant was transferred into a new Eppendorf tube and 300 μl of ice-cold isopropanol was added and mixed by gentle inverting. The mixture was kept at room temperature for 5 min. Precipitated DNA was pelleted by centrifugation. The supernatant was poured off and the DNA pellet was washed with 0.5 ml of 70% ethanol and centrifuged for 5 min. The supernatant was decanted, and the pellet was dried at room temperature by placing the tubes face down on paper towels for 20 min. The pellet was re-suspended in 50 μl low-salt TE buffer and stored at -20°C. The concentration and quality of DNA was checked by NanoDrop 2000c Spectrophotometer (Thermo Fisher Scientific, Walthum, MA, USA), and visualized on a 1% agarose gel (Sigma-Aldrich, Saint Louis, MO, USA) stained with SYBR Safe DNA gel stain under ultra-violet light (UVP BioImaging Systems, Upland, CA, USA).
PCR amplification
Three loci including the 5.8 S nuclear ribosomal gene with the two flanking ITS regions, the portion of Large Sub Unit (LSU), and partial sequences of the ACT gene were amplified and sequenced using universal primer pairs ITS-1F/ITS-4 [19,20], LROR/LR5 [21,22], and ACT-512F/ACT-783R [23], respectively. Genomic DNA from Acremonium species isolate 133 was used as positive control whereas reaction with no DNA template was used for negative control.
All PCRs were performed in 20 µl reaction volumes containing AccuPower PCR PreMix (Bioneer, Daejeon, Republic of Korea), 0.8 μl of 10 µM of each forward and reverse primers, and 2 µl template DNA (20 ng/μl). PCR reactions were performed in a GeneAmp PCR System 9700 thermal cycler (Applied Biosystems, Foster City, CA, USA). The PCR conditions for the three loci were optimized using gradient PCR. The PCR cycling conditions for the ITS regions constituted an initial denaturation step at 94°C for 5 min, followed by 35 cycles of denaturation at 94°C for 45 secs, primer annealing at 48°C for 45 sec, and primer extension at 72°C for 1 min; and a final extension step at 72°C for 10 min. The PCR programs for LSU were an initial denaturation step at 95°C for 5 min, followed by 35 cycles at 94°C for 30 sec, at 43°C for 30 sec, and at 72°C for 1 min; and a final extension step at 72°C for 10 min. PCR reaction profiles for partial ACT gene comprised an initial denaturation at 96°C for 2 min, followed by 35 cycles at 94°C for 30 sec, at 61°C for 45 sec, and at 72°C for 45 sec; and a final extension step at 72°C for 10 min.
PCR products were subjected to electrophoresis in 1.5% agarose gels stained with SYBR Safe DNA gel stain at 70 V for 45 min and visualized under UV light (UVP BioImaging Systems). The sizes of amplicons were determined against a 100 bp molecular weight marker (Invitrogen, Carlsbad, CA, USA). The PCR products with the expected sizes were cleaned using the GeneJET (Thermo Fisher Scientific) for ITS and QIAquick (Qiagen, Venlo, The Netherlands) PCR purification kit for LSU and ACT as instructed by the manufacturers. The concentration and quality of the purified PCR products were determined by NanoDrop 2000c spectrophotometer (Thermo Fisher Scientific) and visualized on 1.5% agarose gel electrophoresis.
DNA sequencing and alignment
Purified PCR products were sequenced using the same forward and reverse primers used for PCR amplifications with BigDye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems) and were run on an ABI 3130x l DNA analyzer (Applied Biosystems) at BecA-ILRI Hub, Nairobi, Kenya.
The nucleotide sequence datasets obtained from forward and reverse primers were inspected, edited, and assembled into consensus contigs using CLC Main Workbench v7.5.1 (CLC bio, Prismet, Denmark). The sequences were analyzed using BLASTN v2.2.30 (http://blast.ncbi.nlm.nih.gov/Blast.cgi) program [24] against the GenBank database based on the best hits of the query sequences that were used to assign identities to the test isolates. Multiple sequence alignments were performed with MAFFT v7.221 [25] using the auto alignment strategy with the 200 PAM/ K=2 scoring matrix and a gap opening penalty of 1.53 with an offset value of 0.0. The ambiguous regions of each gene sequences were removed with Gblocks v0.91b [26]. Resulting sequence alignments were evaluated and manually edited where necessary using MEGA v6.06 [27] software.
Phylogenetic analyses
Phylogenetic analyses were performed for each multiple sequence alignment of the ITS, LSU, and ACT as well as for the combined dataset of the three loci using different statistical methods to differentiate the isolates by species complex. jModelTest v2.1.7 [28] as well as the Modeltest [29] implemented in the MEGA were used to estimate the best-fit models of nucleotide substitution and the corresponding general time-reversible (GTR) substitution rate parameters, shape of the four-category gamma distribution and fraction of invariable sites for each gene using corrected Akaike Information Criterion (AICc) and the Bayesian Information Criterion (BIC) scores. For each locus, 167 sequence datasets were used to reconstruct phylogenetic trees. Published ITS, LSU and ACT nucleotide sequences of 13 isolates of Colletotrichum spp. from the GenBank database were included as reference species (Table 2). Colletotrichum acutatum J. H. Simmonds (GenBank accession numbers: DQ286124, DQ286125, and JQ949687) [30,31] was designated as outgroup in all analyses for the reconstruction of the phylogenetic trees.
Table 2.
Details of reference isolates used in this study.
| Species | Accession numbera | Host | Country | GenBank numberb | Reference | ||
|---|---|---|---|---|---|---|---|
| ITS | LSU | ACT | |||||
| C. aenigma | ICMP 18608, LC0038, C1253.4 | Persea americana | Israel | NR_120140 | JN940409 | JX009443 | Cai and Weir [41] |
| C. aotearoa | C1252.9, AR2802, ICMP 18532 | Kunzea ericoides, Pueraria, Vitexlucens | New Zealand, USA | JX010198 | DQ286187 | JX009544 | Farr et al. [31]; Weir et al. [41] |
| C. asianum | C1187, LC0036, CPC 20981 | Mangifera indica, unknown, Fruits | Australia, Thailand, Brazil | JX010192 | JN940407 | KC566879 | Cai 2011; Weir et al. [41]; Braganca (unpublished data) |
| C. boninense | CBS 128547, ICMP 10338 | Camellia sp. | New Zeealand | JQ005159 | DQ286169 | JQ005507 | Farr et al. [31]; Damm et al. |
| C. fructicola | ICMP 12568, LC0032, CMM3811 | Persea americana, unknown, Mangiferaindica | Australia, Thailand, Brazil | JX010166 | JN940418 | KC702919 | Cai 2011; Weir et al. [41]; Chowdappa and Chethana2014 (unpublished data) |
| C. gloeosporioides | OCAC24, LC0553, C1254.3 | Elettariacardamomum, unknown, Citrus sp. | India, China, USA | KJ813602 | JN940414 | JX009494 | Cai 2011; Weir et al. [41]; Chowdappa and Chethana2014 (unpublished data) |
| C. gloeosporioides | GM62-L03, PP143, CPC 20904 | Annona muricata; Fruits | Colombia, Brazil | KC512137 | FJ890371 | KC566853 | Gazis and Chaverri (unpublished data); Braganca (unpublished data); Alvarezet al. |
| C. gloeosporioides | Strain 8, GJS01-199, CBS 953.97 | Olive, Citrus sinensis, Theobroma | Italy, Cameroon | JN121209 | DQ286177 | GQ856782 | Farr et al. [31]; Yang et al. (unpublished data); Faeddaet al. |
| C. gloeosporioides | CK13b7, AR4031, CBS 131329 | Citrus limon, Fruits | Cameroon, Brazil | JX436791 | AY539807 | KC566856 | Berner et al. (unpublished data); Braganca (unpublished data); Douanla-Meli et al. (unpublished data) |
| C. gloeosporioides | M2P3D7, CBS 122687, C1014.6 | Soybean, Leucospermum sp., Citrus sp., | Brazil, SouthAfrica, NewZealand | JX258787 | EU552111 | JX009462 | Marincowitz et al. unpublished data); Cnossen-Fassoni et al. (unpublisheddata); Weir et al. |
| C. karstii | OCAC4, CBS 102667, BRIP:28443a | Elettariacardamomum; Passiflora; Mangifera indica | India, NewZealand, Australia | KJ813595 | DQ286173 | JQ005551 | Farr et al., Damm et al.; Chowdappa and Chethana (unpublished data) |
| C. siamense | ICMP 12567, FAU 553, CBS 114054/BPI 747978 C1315.2 | Persea americana, Fragaria, Coffeaarabica | Australia, USA, Thailand | JX010250 | AF543786 | JX009518 | Farr et al., Damm et al.; Chowdappa and Chethana (unpublished data) |
| Glomerella cingulata | ICMP 10646, AR 2799 | Camellia sasanqua, Pueraria lobata | USA | JX010225 | DQ286193 | JX009563 | Farra et al.; Weir et al. 2012 |
| C. acutatum (outgroup) | MEP1323, CBS:126521 | Vaccinium, Anemone F1 hybrid | New Zealand, Netherlands | DQ286124 | DQ286125 | JQ949687 | Farr et al. and Damm et al. |
a BPI: The U.S. National Fungus Collections; BRIP: Plant Pathology Herbarium, Department of Primary Industries, Queensland, Australia; CBS: Culture collection of the Centraalbureau voor Schimmelcultures, Fungal Biodiversity Centre, Utrecht, The Netherlands; CMM: Culture Collection of Phytopathogenic Fungi Prof. Maria Menezes (Colecao de Culturas de Fungos Fitopatogenicos Prof. Maria Menezes), Brazil; CPC: Culture collection of Pedro Crous, housed at CBS; FAU: Florida Atlantic University, Harbor Branch Marine Microbial Database, Boca Raton, Florida; ICMP: International Collection of Microorganisms from Plants; GJS: Gary J. Samuels searchable database;
b ITS: internal transcribed spacers and intervening 5.8S nrDNA; LSU: partial long subunit of nrDNA gene; ACT: partial actin gene.
To determine whether the three sequence datasets were congruent and combinable, tree topologies of 70% reciprocal Neighbor-joining (NJ) bootstrap with Maximum Likelihood (ML) distances (1000 replicates) with substitution models determined separately for each partition using Model test were compared visually [32]. The analyses showed that individual genes were broadly congruent, thus nucleotide alignments of the three genes were concatenated using scripts in Microsoft Office Excel 2007 program. Phylogeny reconstruction was performed by NJ method [33] using the MEGA software. The percentage of replicate trees in which the associated taxa clustered together was evaluated with a bootstrap analysis with 1000 replicates [34]. The tree was drawn to scale, with branch lengths in the same units as those of the evolutionary distances used to infer the phylogenetic tree. The evolutionary distances were computed using the Kimura 2- parameter substitution model [35]. All alignment positions containing gaps and missing data were removed and the rate variation among sites was modeled with a gamma distribution (shape parameter=5). All branches with bootstrap values of less than 50 were collapsed.
The evolutionary history was inferred using the Maximum Parsimony (MP) method on the combined multilocus alignments using Tree-Bisection-Reconstruction (TBR) algorithm with search level 3 in which the initial trees were obtained by 10 random sequence additions. Alignment gaps and missing data were eliminated and the rate of variation among sites was modeled with gamma distribution. The confidence values for clades within the resulting tree were determined using a bootstrap analysis with 1000 replicates [34]. Tree length (TL), consistency index (CI), retention index (RI), rescaled consistency index (RC) and homoplasy index (HI) were calculated for one of the most parsimony trees.
A Markov Chain Monte Carlo (MCMC) algorithm was used to generate phylogenetic trees with Bayesian probabilities using MrBayes v3.2.1 [36] for the combined multilocus sequence datasets. Based on the results of the jModelTest, the Bayesian analysis for all loci was performed using the Dirichlet (1,1,1,1) nucleotide frequency distribution, and GTR model with gamma-distributed rate variation across sites and a proportion of invariable sites. The analyses of two MCMC chains on the full data set were run from random trees for 4 × 106 generations and sampled from the posterior every 1000 generations until the split frequency reached below 0.02. The first 25% of the trees were discarded as burn-in phase of the analysis and posterior probabilities were determined from the remaining trees. The effective sample size and traces of all parameters and convergence of the two runs were checked using the internal diagnostics of the standard deviation of split frequencies and performance scale reduction factors, and then checked externally with Tracer v1.6 [37]. A summary maximum clade credibility species tree was built with TreeAnnotator v1.7.1 [38] using a 25% burn-in and a posterior probability limit of 0.5. The resulting phylogenetic trees were drawn and edited using TreeGraph v2.4.0-456 beta [39]. All tree branches with values of less than 0.50 were collapsed.
Sequences derived in the present study were deposited in the GenBank with accession numbers from KT282463 to KT282629 (ACT), from KT282630 to KT282796 (ITS), and from KT282797 to KT282963 (LSU), whereas alignments and trees were deposited into TreeBASE (http://purl.org/phylo/treebase/phylows/study/TB2:S17920).
Pathogenicity tests
Representative isolates of C. gloeosporioides and C. boninense species complexes recovered from symptomatic plant tissues were tested for pathogenicity in different citrus species (two cultivars each of sweet orange, mandarin, lemon, lime, and grapefruit). Pathogenicity test was carried out following the standard techniques [40] on healthy detached leaves. Young leaves from two-year old plants were artificially inoculated with each isolate by placing three drops of aqueous suspension of 106 spores per ml, or mycelia suspension. Spores were obtained from a week-old culture grown on PDA, suspended in sterile water, and filtered through two layers of sterile cheesecloth. In all tests, inoculation with sterile water was used as control. Each test isolate was inoculated on four leaves. Inoculated leaves were incubated on agar media for up to three weeks at 26°C. Inoculated leaves were assessed daily for the development of disease symptoms. At the end of each test, symptomatic tissues were surface disinfested and placed on water agar to confirm recovery of the inoculated isolates. Re-isolated cultures were examined for growth and morphological characteristics with the parent cultures. The experiment was conducted twice.
Results
PCR amplification
Three universal primer pairs (ITS-1F/ITS4, LROR/LR5 and ACT-512F/ACT-783R) were used to amplify the target ITS, LSU and ACT loci. All 167 fungal DNA samples were successfully amplified and sequenced. The amplified ITS, LSU and ACT loci were of approximately 600 bp, 900 bp and 300 bp in size, respectively. The average sizes of assembled sequences of the test isolates used in the present study were 570 bp for ITS, 855 bp for LSU, and 250 bp for ACT gene. Among isolates, there were only slight variations in amplicon size with few inconsistencies due to variable length nucleotide repeats.
Identification of the test isolates
The multilocus sequences identified the associated fungi isolates to the species level. Among the 167 fungal isolates, 163 were identified as C. gloeosporioides (or its teleomorph G. cingulata) species complex (sensu lato; s.l.) using BLASTN search tool. Four isolates were recognized as C. boninense species complex.
The ITS sequence analysis delineated 163 isolates as C. gloeosporioides species complex (C. aenigma=2, C. aotearoa=2, and C. gloeosporioides sensu stricto [s.s.]=159). One isolate was resolved as C. karstii. Three isolates were identified as C. truncatum. The ITS sequence data resolved all the isolates to species level.
The analysis of portion of LSU revealed that 93 isolates were recognized as C. gloeosporioides s.l. (C. asianum=3, C. fructicola=4, and C. gloeosporioides s.s.=86), while four isolates were identified as C. boninense species complex. The rest 70 isolates were belonged to G. cingulata. The partial LSU data identified all the isolates to species level.
The partial ACT nucleotide sequence data resolved 163 isolates as C. gloeosporioides s.l. (C. aenigma=2, C. asianum=1, C. crassipes=1, C. fructicola=4, C. siamense=1, and C. gloeosporioides s.s.=154), one isolate as C. karstii, and three isolates as C. magnisporum. The partial ACT sequences discriminated all the isolates to species level.
The sequences of the ITS, LSU and ACT barcode markers discriminated all the Colletotrichum isolates studied to the species level. The results further indicated that CFLSD in Ethiopia have frequent association of C. gloeosporioides species complex.
Phylogenetic analyses
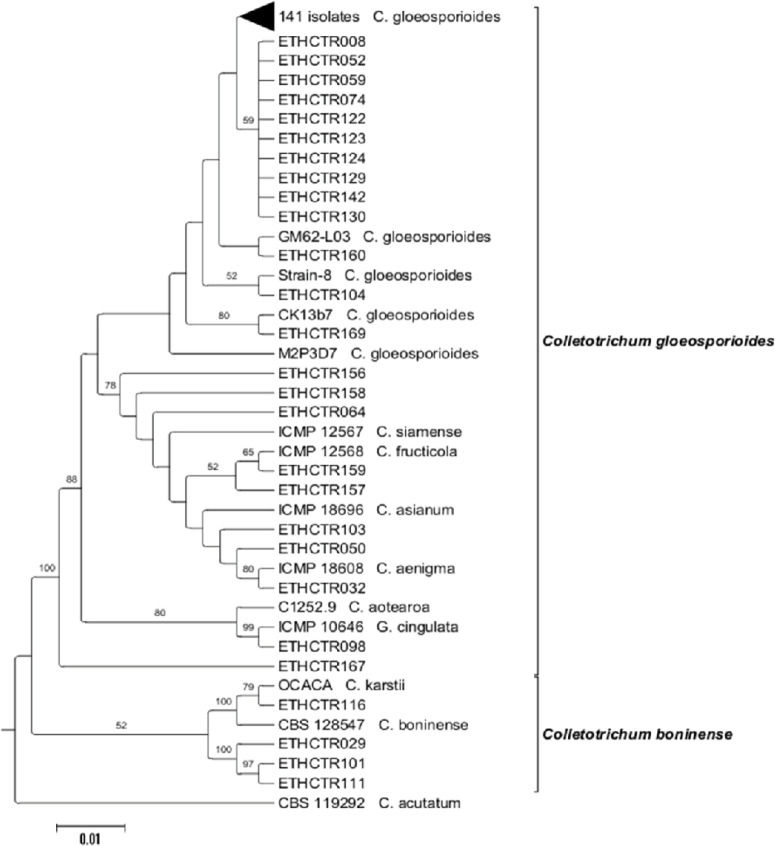
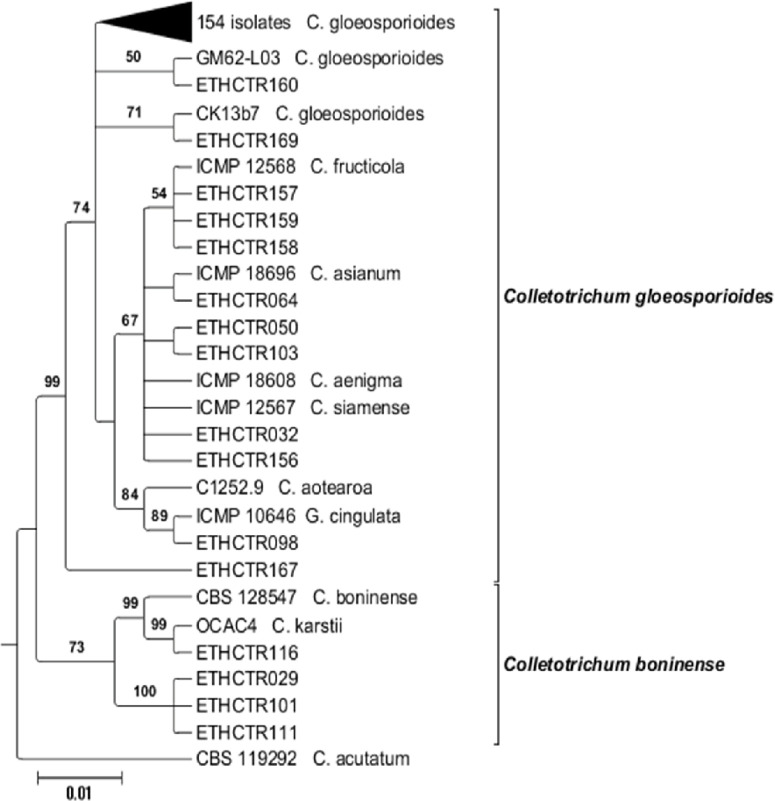
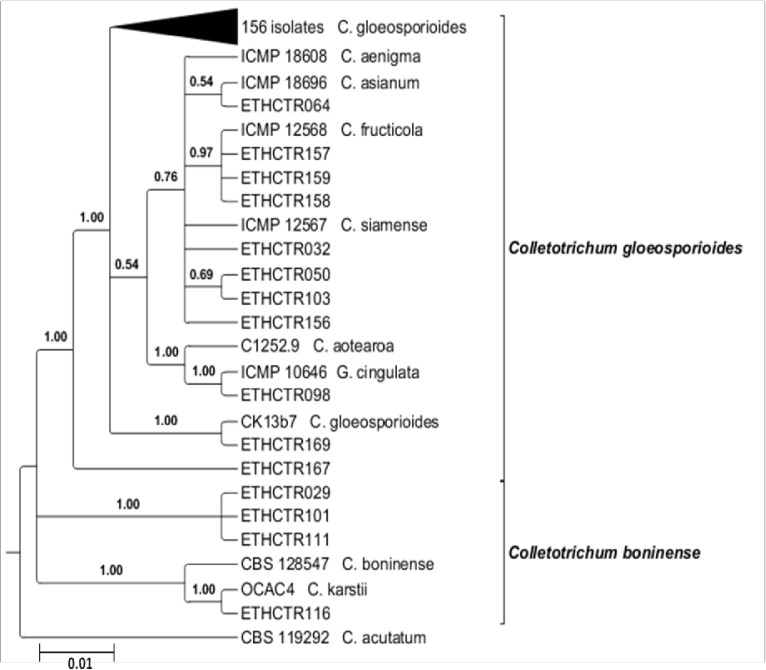
The relatedness among fungal isolates was established through multi-gene phylogeny. The trees drawn from each individual dataset (ITS, LSU, and ACT loci) using NJ and MP had similar topology (data not shown) for the 70% reciprocal NJ bootstrap trees, which allowed us to combine them. The phylogenetic analysis of the combined sequences from the three loci using NJ, MP and Bayesian methods resulted similar grouping of isolates (Figures 3 to 5) into C. gloeosporioides [41] and C. boninense [42] species complexes.
Figure 3.
Neighbor-joining phylogenetic tree of concatenated ITS, LSU and ACT sequences of 167 Colletotrichum isolates generated in this study and 13 isolates from other studies, retrieved from the GenBank. Bootstrap support values (1000 replicates) of ≥ 50 are shown above the nodes. The tree was rooted with Colletotrichum acutatum (CBS 119292) as outgroup. The scale bar indicates the number of expected nucleotide changes per site.
In the combined multilocus analyses (gene boundaries of ITS: 1–527, LSU: 528-1385, ACT: 1386-1623) of 181 isolates including the 13 reference isolates and the outgroup (C. acutatum CBS 119292), 1913 characters including the alignment gaps were processed. Of these characters, 143 were parsimony informative; 236 were parsimony uninformative; and 1364 were constant. Parsimony analysis resulted in three most parsimonious trees. One of the most parsimonious trees (tree length=341, CI=0.584615, RI=0.829921, RC=0.485185, and HI=0.415385) obtained with the combined multiple sequence alignment of the three loci using MP method is presented in Figure 4.
Figure 4.
One of the most parsimonious trees obtained from a heuristic search of combined ITS, LSU and ACT sequences of 167 isolates generated in this study and 13 published reference isolates from the GenBank in the Colletotrichum gloeosporioides and Colletotrichum boninense species complexes. Bootstrap support values (1000 replicates) of ≥ 50 are shown at the nodes. Colletotrichum acutatum (CBS 119292) is used as outgroup. The scale bar indicates the number of expected changes per site.
The overall topology for all equally most parsimonious trees was similar; but they differed in the position of isolates within the clades. Out of the 8002 trees, 3001 trees were used to calculate the consensus tree and posterior probabilities. The analysis resulted in the delineation of four main clades within the isolates studied in this paper (Figure 5). Most of the isolates clustered in the first clade (C. gloeosporioides s.l.) with a bootstrap support and Bayesian posterior probability values of 74/1.0. The first main clade consists of several closely related species including C. aenigma, C. asianum, C. fructicola and C. siamense (67/0.76), C. aotearoa and G. cingulata (84/1.0), and C. gloeosporioides s.s. The second clade contained only one isolate, representing C. gloeosporioides s.l. (99/1.0). Colletotrichum karstii (99/1.0) and C. boninense (99/1.0) belonged to the third main clade. The fourth main clade consists of the C. acutatum isolate used as outgroup for the phylogenetic analysis [43-61].
Figure 5.
A Bayesian inference phylogenetic tree which illustrates the relationships of 167 isolates generated in this study and 13 published reference isolates from the GenBank in the Colletotrichum gloeosporioides and Colletotrichum boninense species complexes. The tree was built using concatenated sequences of the ITS, LSU, and LSU genes, each with a separate models of DNA evolution. Bayesian posterior probability values of ≥ 0.5 are shown above the nodes. Colletotrichum acutatum (CBS 119292) is used as outgroup. The scale bar indicates the number of expected changes per site.
Pathogenicity tests
Pathogenicity tests on detached leaves confirmed the ability of both Colletotrichum species complexes to produce the disease symptoms. Leaf spots symptom observed on citrus leaves under the field conditions are shown in Figure 6a. All test isolates in the C. gloeosporioides and C. boninense species complexes caused foliar disease symptoms on inoculated citrus leaves (Figure 6b), and the test isolates were consistently recovered from inoculated symptomatic leaf tissues. Some isolates caused necrosis of entire leaf area. Water inoculated controls remained healthy (Figure 6c).
Figure 6.
Pathogenic behaviour of the tested isolates of Colletotrichum on sweet orange leaves. (a) Naturally observed spots, (b) symptoms developed on artificially inoculated leaf, and (c) no symptom on water inoculated leaves.
Discussion
Citrus fruit and leaf spot is a devastating disease of citrus in sub-Saharan Africa and Yemen [12]. A total of 167 fungal isolates were recovered from leaf and fruit samples with prominent symptoms of CFLSD. The multiple gene sequences distinguished all 167 isolates as members of genus Colletotrichum or teleomorph Glomerella which suggests that fruit and leaf spot disease of citrus in Ethiopia have complete association of C. gloeosporioides s.l. and C. boninense s.l. species complexes. The genus Colletotrichum is classified within the Fungi imperfecti. It belongs to the morphological classification of the phylum Ascomycota [51,62]. The fungus comprises Colletotrichum as anamorph or asexual state while Glomerella as sexual or teleomorph state [42,62]. Phylogenetic analysis of Colletotrichum reveals that the genus comprises nine major clades [62]. However, the taxonomy and phylogeny of Colletotrichum remains in a state of flux because many uncertainties exist with regard to the systematics of fungal pathogens from this genus [51]. The names C. gloeosporioides and C. boninense are commonly used in both broad (sensu lato) and strict (sensu stricto) senses. When used in a broad sense, they refer to the C. gloeosporioides [41] and C. boninense [42] species complexes.
Pseudocercospora angolensis has been reported as the causal agent of CFLSD [7]. In Ethiopia, Derso [6] and Yesuf [2] reported P. angolensis as the causal agent of CFLSD. Both authors tried to characterize the causal pathogen based on cultural and morphological characteristics. However, the pathogen description stated in both papers do not match with the typical characteristics of the P. angolensis reported by other scientists. Yesuf [2] indicated that apart from P. angolensis, C. gloeosporioides was found one of the important pathogens associated with citrus in the areas he surveyed. The infection of P. angolensis appears to predispose citrus fruits to secondary infection by C. gloeosporioides [10,16]. However, the isolations of C. gloeosporioides s.l. and C. boninense s.l. species complexes and no revival of P. angolensis from symptomatic citrus leaf and fruit samples in this study were highly surprising results. Such observations might have been attributed to the infection and colonization of host tissues by Colletotrichum species prior to sampling and/or the differences in the growth behavior between Colletotrichum and Pseudocercospora isolates. The former situation is more likely as all leaf and fruit samples had well developed disease symptoms at the time of collection. The latter situation may also hold true because of the relatively faster growth rate of Colletotrichum (6.3 mm/day) to Pseudocercospora (1.2 mm/day) [43,44] on general isolation medium which might have favored the frequent recovery of fast growing Colletotrichum species. Our efforts to isolate P. angolensis from leaves with early symptom of CFLSD also completed with isolation of Colletotrichum species at high frequency (Moges, Admassu, Belew, Yesuf, Maina and Ghimire unpublished results). The pathogenicity test of C. gloeosporioides s.l. and C. boninense s.l. isolates in detached leaf assay produced foliar symptoms and the test isolates were consistently recovered. These observations indicate important roles of Colletotrichum species on CFLSD. Therefore, inoculation study with P. angolensis and Colletotrichum species individually and in combination is important to determine the exact roles of these fungi in CFLSD initiation, development, and epidemics.
The Colletotrichum species complexes isolated in this study have been reported to show highly variable cultural and morphological characteristics [41,45], making these attributes less reliable to determine species complex [46]. Therefore, multiple gene based molecular phylogeny is preferred [47]. The multigene phylogeny adopted in this study discriminated 167 Colletotrichum isolates in two major groups: C. gloeosporioides and C. boninense species complexes with high species diversity (C. aenigma, C. aotearoa, C. asianum, C. boninense s.s., C. crassipes, C. fructicola, C. gloeosporioides s.s., C. karstii, C. magnisporum, C. siamense, C. truncatum and Glomerella cingulata). Although C. acutatum has been reported as the main pathogen of citrus anthracnose disease complexes worldwide [48], no isolate of C. acutatum was recorded from our study. Majority of taxa identified in this study have worldwide distribution, and many isolates cause diseases in agriculturally important crops [30,41,49,50]. Moreover, Colletotrichum is among ten most important plant pathogenic fungi [51].
Colletotrichum gloeosporioides species complex causes both preharvest diseases such as wither tip on twigs, tear stain [52] and fruit stem-end rot [53], and postharvest anthracnose [54] in various plant species. It is commonly associated with Key Lime Anthracnose and other postharvest diseases on citrus species [41]. It also causes post-bloom fruit drop on sweet orange in Brazil [48]. The ability of C. fructicola and C. gloeosporioides strains to cause anthracnose on citrus fruits has been demonstrated in China [55]. In the tropical Asia, Colletotrichum siamense causes anthracnose disease on a wide range of tropical fruits e.g. Ficus racemosa, Azadirachta indica and Mangifera indica [50]. Recently, C. gloeosporioides and C. karstii have been reported to cause severe lesions on sweet orange fruits (Citrus sinensis) in Italy, and C. gloeosporioides was more aggressive than C. karstii [56]. The virulence and pathogenicity of C. asianum, C. fructicola, and C. karstii have been demonstrated on various plant species including mango, papaya, banana, guava, and bell pepper [49].
Colletotrichum boninense s.l., once considered to belong to the C. gloeosporioides complex, was first described from Crinum asiaticum var. sinicum and Cucumis melo from Bonin Islands, Japan, where the species was associated with a variety of host plants [57]. Since then, this species has been reported as a pathogen causing leaf and fruit anthracnose on different host plants [42,45]. For instances, C. boninense has been found to be associated with diseases of Proteaceae in Australia and Zimbabwe, and with Eucalyptus in South Africa [58], Dracaena and Pachira in China, Passiflora in New Zealand and Hippeastrum in Brazil and the Netherlands [31], berries and twigs of Coffea in Vietnam [59], and avocado in Mexico [60]. Colletotrichum boninense and C. gloeosporioides have been shown to infect Protea leaves and stems in South Africa [61].
Conclusion
The results of this study indicated a wider distribution of citrus fruit and leaf spot disease across citrus growing regions of Ethiopia. The multigene sequences (ITS, LSU, and ACT) analyses of all 167 fungal isolates recovered from CFLSD samples revealed them of genus Colletotrichum and surprisingly, none of the isolates were Pseudocercospora angolensis. Multigene phylogeny differentiated the 167 isolates into C. gloeosporioides s.l. or C. boninense s.l. species complexes. As of our knowledge, most of the taxa identified in this study are the first report on their association with citrus fruit and leaf spot symptoms in Ethiopia. The present findings suggest the absolute/ frequent association of C. gloeosporioides species complex with fruit and leaf spot symptoms of citrus in Ethiopia. We suggest the need for in-depth studies to examine roles of P. angolensis, C. gloeosporioides and C. boninense species complexes in citrus fruit and leaf spot disease epidemiology. This information would be helpful in developing effective disease management strategies.
Acknowledgements
This project was supported by the BecA-ILRI Hub through the Africa Biosciences Challenge Fund (ABCF) program. The ABCF Program is funded by the Australian Department for Foreign Affairs and Trade (DFAT) through the BecA-CSIRO partnership; the Syngenta Foundation for Sustainable Agriculture (SFSA); the Bill & Melinda Gates Foundation (BMGF); the UK Department for International Development (DFID) and; the Swedish International Development Cooperation Agency (SIDA). The authors acknowledge Leah Kago, Collins Mutai, Moses Njahira and Martina Kyalo for providing general support during the laboratory work. We are thankful to Joyce Njuguna and Trushar Shah for bioinformatics support and help in evolutionary phylogenetic analyses. The first author acknowledges the Ethiopian Institute of Agricultural Research and Jimma University for the permission to carry this research at BecA-ILRI Hub.
References
- 1.Gebre-Mariam S. (2003) Status of commercial fruit production in Ethiopia. Ethiopian Agricultural Research Organization, Addis Ababa, Ethiopia. [Google Scholar]
- 2.Yesuf M. (2007) Distribution and management of Phaeoramularia leaf and fruit spot disease of citrus in Ethiopia. Fruits 62: 99-106. [Google Scholar]
- 3.Central Statistical Agency of Ethiopia (2015) Large and medium scale commercial farms sample survey 2014/25. Statistical Bulletin, CSA, Addis Ababa, Ethiopia. [Google Scholar]
- 4.Central Statistical Agency of Ethiopia (2015) Agricultural sample survey 2014/2015: Report on area and production of major crops for private peasant holding. Statistical Bulletin 578, CSA, Addis Ababa, Ethiopia. [Google Scholar]
- 5.Joosten F, Boselie D, Wolde B, Dessalegn L (2011) Exporting fruit and vegetables from Ethiopia: Assessment of development potentials and investment options in the export-oriented fruit and vegetable sector. Ethiopia-Netherlands Horticulture Partnership Programme, Addis Ababa, Ethiopia. [Google Scholar]
- 6.Derso E. (1999) Occurrence, prevalence and control methods of Phaeoramularia leaf and fruit spot disease of citrus in Ethiopia. Fruits 54: 225-232. [Google Scholar]
- 7.Pretorius MC, Crous PW, Groenewald JZ, Braun U (2003) Phylogeny of some cercosporoid fungi from Citrus. Sydowia 55: 286-305. [Google Scholar]
- 8.Seif AA, Hillocks RJ (1993) Phaeoramularia fruit and leaf spot of citrus with special reference to Kenya. Int J Pest Manage 39: 44-50. [Google Scholar]
- 9.Kuate J, Jazet Dongmo PM, Ducelier D, Damesse F, Menut C, et al. (2003) Effect of citrus leaf spot disease (Phaeoramularia angolensis) on the content and chemical composition of essential oils from orange peel. Fruits 58: 143-149. [Google Scholar]
- 10.De Carvalho T, Mendes O (1952) A cercosporiosis is Citrus. Motambique 72:1-8. [Google Scholar]
- 11.Derso E. (1997) Leaf and fruit spot: A new disease of citrus in Ethiopia. Proceedings of the Seventh Crop Science Society of Ethiopia Annual Conference, Ethiopia. [Google Scholar]
- 12.Yesuf M. (2013) Pseudocercospora leaf and fruit spot disease of citrus: Achievements and challenges in the citrus industry: A review. Agric Sci 4: 324-328. [Google Scholar]
- 13.Yimenu J. (1993) Preliminary survey report on leaf spot disease of citrus in Sidamo. Coffee Development Authority, Addis Ababa, Ethiopia. [Google Scholar]
- 14.Timmer LW, Garnsey SM, Broadbent P (2003) Diseases of citrus. In: Ploetz RC. (ed). Diseases of Tropical Fruit Crops. Wallingford, CAB International; pp. 163-195. [Google Scholar]
- 15.Kuate J. (1998) Citrus leaf and fruit spot disease caused by Phaeoramularia angolensis. Cah Agric 7: 121-129. [Google Scholar]
- 16.Seif AA, Kungu JN (1990) Association of Colletotrichum species with the fruit and leaf spot of citrus caused by Phaeoramularia angolensis. National Agricultural Research Laboratories, Annual Report of Plant Pathology Department for 1990, Nairobi, Kenya. [Google Scholar]
- 17.Cenis JL. (1992) Rapid extraction of fungal DNA for PCR amplification. Nucleic Acids Res 20: 2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murray MG, Thompson WF (1980) Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res 8: 4321-4325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gardes M, Bruns TD (1993) ITS primers with enhanced specificity for basidiomycetes - application to the identification of mycorrhizae and rusts. Mol Ecol 2: 113-118. [DOI] [PubMed] [Google Scholar]
- 20.White TJ, Bruns T, Lee S, Taylor JW (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ, (eds). PCR Protocols: A guide to methods and applications. Academic Press, New York, USA: pp. 315-322. [Google Scholar]
- 21.Moncalvo JM, Wang HH, Hseu RS (1995) Phylogenetic relationships in Ganoderma inferred from the internal transcribed spacers and 25S ribosomal DNA sequences. Mycologia 87: 223-238. [Google Scholar]
- 22.Vilgalys R, Hester M (1990) Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J Bacteriol 172: 4238-4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carbone I, Kohn LM (1999) A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 91: 553-556. [Google Scholar]
- 24.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215: 403-410. [DOI] [PubMed] [Google Scholar]
- 25.Katoh K, Standley DM (2013) MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol Biol Evol 30: 772-780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Talavera G, Castresana J (2007) Improvement of phylogenies after removing divergent and ambiguously aligned blocks from protein sequence alignments. Syst Biol 56: 564-577. [DOI] [PubMed] [Google Scholar]
- 27.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30: 2725-2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Darriba D, Taboada GL, Doallo R, Posada D (2012) jModelTest 2: More models, new heuristics and parallel computing. Nat Methods 9: 772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Posada D, Crandall KA (1998) Model test: Testing the model of DNA substitution. Bioinformatics 14: 817-818. [DOI] [PubMed] [Google Scholar]
- 30.Damm U, Cannon PF, Woudenberg JHC, Crous PW (2012) The Colletotrichum acutatum species complex. Stud Mycol 73: 37-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Farr DF, Aime MC, Rossman AY, Palm ME (2006) Species of Colletotrichum on Agavaceae. Mycol Res 110: 1395-1408. [DOI] [PubMed] [Google Scholar]
- 32.Mason-Gamer RJ, Kellogg EA (1996) Testing for phylogenetic conflict among molecular data sets in the tribe Triticeae (Gramineae). Syst Biol 45: 524-545. [Google Scholar]
- 33.Saitou N, Nei M (1987) The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol Biol Evol 4: 406-425. [DOI] [PubMed] [Google Scholar]
- 34.Felsenstein J. (1985) Confidence limits on phylogenies: An approach using the bootstrap. Evolution 39: 783-791. [DOI] [PubMed] [Google Scholar]
- 35.Kimura M. (1980) A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol 16: 111-120. [DOI] [PubMed] [Google Scholar]
- 36.Ronquist F, Teslenko M, Van Der Mark P, Ayres DL, Darling A, et al. (2012) MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol 61: 539-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rambaut A, Suchard MA, Xie D, Drummond AJ (2014) Tracer v1.6. [Google Scholar]
- 38.Drummond AJ, Suchard MA, Xie D, Rambaut A (2012) Bayesian phylogenetics with BEAUti and the BEAST 1.7. Mol Biol Evol 29: 1969-1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stover BC, Muller KF (2010) TreeGraph 2: Combining and visualizing evidence from different phylogenetic analyses. BMC Bioinformatics 11: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Agrios GN. (2005) Plant pathology. Academic Press, San Diego, California, USA. [Google Scholar]
- 41.Weir BS, Johnston PR, Damm U (2012) The Colletotrichum gloeosporioides species complex. Stud Mycol 73: 115-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Damm U, Cannon PF, Woudenberg JHC, Johnston PR, Weir BS, et al. (2012). The Colletotrichum boninense species complex. Stud Mycol 73: 1-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Elmsly TA, Dixon J (2008) Growth rates of ripe rot fungi at different temperatures. New Zealand Avocado Growers’ Association Annual Research Report 8: 77-84. [Google Scholar]
- 44.Lee MH, Lee HK, Cho PH, Kim YS, Cho SK, et al. (2015) Survey and screening of fungicide for the control of tomato black leaf mold Pseudocercospora fuligena. Res Plant Dis 21: 94-98. [Google Scholar]
- 45.Johnston PR, Pennycook SR, Manning MA (2005) Taxonomy of fruit-rotting fungal pathogens: What’s really out there? N Z Plant Prot 58: 42-46. [Google Scholar]
- 46.Phoulivong S, Cai L, Chen H, McKenzie EHC, Abdelsalam K, et al. (2010) Colletotrichum gloeosporioides is not a common pathogen on tropical fruits. Fungal Divers 44: 33-43. [Google Scholar]
- 47.Crouch JA, Clarke BB, Hillman BI (2009) What is the value of ITS sequence data in Colletotrichum systematics and species diagnosis? A case study using the falcatespored graminicolous Colletotrichum group. Mycologia 101: 648-656. [DOI] [PubMed] [Google Scholar]
- 48.Lima WG, Sposito MB, Amorim L, Golcalves FP, deFilho PAM (2011) Colletotrichum gloeosporioides, A new causal agent of citrus post-bloom fruit drop. Eur J Plant Pathol 131: 157-165. [Google Scholar]
- 49.Lima NB, Lima WG, Tovar-Pedraza JM, Michereff SJ, Camara MPS (2015) Comparative epidemiology of Colletotrichum species from mango in northeastern Brazil. Eur J Plant Pathol 141: 679-688. [Google Scholar]
- 50.Udayanga D, Manamgoda DS, Liu X, Chukeatirote E, Hyde KD (2013) What are the common anthracnose pathogens of tropical fruits? Fungal Divers 61: 165-179. [Google Scholar]
- 51.Dean R, Van Kan JAL, Pretorius ZA, Hammond-Kosack KE, Di Pietro A, et al. (2012) The top 10 fungal pathogens in molecular plant pathology. Mol Plant Pathol 13: 414-430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Benyahia H, Jrifi A, Smaili C, Afellah M, Timmer LW (2003) First report of Colletotrichum gloeosporioides causing wither tip on twigs and tear stain on fruit of citrus in Morocco. Plant Pathol 52: 798. [Google Scholar]
- 53.Kaur R, Rewal HS, Sethi A (2007) Pre-harvest stem-end rot in citrus cultivars due to Colletotrichum gloeosporioides. Eur J Hortic Sci 72: 20-25. [Google Scholar]
- 54.Freeman S, Shabi E (1996) Cross-infection of subtropical and temperate fruits by Colletotrichum species from various hosts. Physiol Mol Plant Pathol 49: 395-404. [Google Scholar]
- 55.Huang F, Chen GQ, Hou X, Fu YS, Cai L, et al. (2013) Colletotrichum species associated with cultivated citrus in China. Fungal Divers 61: 61-74. [Google Scholar]
- 56.Aiello D, Carrieri R, Guarnaccia V, Vitale A, Lahoz E, et al. (2015) Characterization and pathogenicity of Colletotrichum gloeosporioides and C. karstii causing preharvest disease on Citrus sinensis in Italy. J Phytopathol 163: 168-177. [Google Scholar]
- 57.Moriwaki J, Sato T, Tsukiboshi T (2003) Morphological and molecular characterization of Colletotrichum boninense species nov. from Japan. Mycoscience 44: 47-53. [Google Scholar]
- 58.Lubbe CM, Denman S, Cannon PF, Groenewald JZ, Lamprecht SC, et al. (2004) Characterization of Colletotrichum species associated with diseases of Proteaceae. Mycologia 96: 1268-1279. [PubMed] [Google Scholar]
- 59.Nguyen THP, Sall T, Bryngelsson T, Liljeroth E (2009) Variation among Colletotrichum gloeosporioides isolates from infected coffee berries at different locations in Vietnam. Plant Pathol 58: 898-909. [Google Scholar]
- 60.Silva-Rojas HV, Avila-Quezada GD (2011) Phylogenetic and morphological identification of Colletotrichum boninense: A novel causal agent of anthracnose in avocado. Plant Pathol 60: 899-908. [Google Scholar]
- 61.Lubbe CM, Denman S, Lamprecht SC, Crous PW (2006). Pathogenicity of Colletotrichum species to Protea cultivars. Australas Plant Pathol 35: 37-41. [Google Scholar]
- 62.Cannon PF, Damm U, Johnston PR, Weir BS (2012). Colletotrichum– Current status and future directions. Stud Mycol 73: 181-213. [DOI] [PMC free article] [PubMed] [Google Scholar]