Abstract
The World Health Organization suggests storing human urine for at least 6 months at 20°C prior to application as fertilizer to reduce the potential health risks from pathogenic organisms. Such a storage condition for human urine,however,not only requires along period of time and large space but also ignores the risk of nitrogen losses. In this study, human urine underwent thermal treatment during storage to improve disinfection and to inhibit urea hydrolysis. Microbial indicators such as Escherichia coli and fecal coliforms and the concentration of ammonia/ammonium were investigated in urine samples that were stored at 60°C and 70°C. Both the inactivation of indicators and decomposition of urea improved under storage temperatures of 60°C and 70°C compared with storage under ambient temperature. Therefore, human urine is recommended to be stored at 70°C for 7 days for hygienic and stabilization purposes. Under this storage condition, pH is maintained below 8.0 and ammonia/ammonium content is maintained at approximately 800 mg/L.
Keywords: human urine, hygienization, stabilization, thermal treatment
INTRODUCTION
The concept of source-separated human feces and urine collection to promote sustainable sanitation solutions from the local to the global level has gained increased attention in recent decades (Boh et al. 2013; O'Neal & Boyer 2013). Given that urine contains available nutrients for plant growth, including nitro-gen, phosphorus, and potassium (Lind et al. 2001; Karak & Bhattacharyya 2011), urine is a good source of green (Akpan-Idiok et al. 2012) and multinutrient fertilizer (Karak & Bhattacharyya 20n), as well as providing a range of environmental benefits (Tidaker et al. 2007). Several technologies on the
This is an Open Access article distributed under the terms of the Creative Commons Attribution Licence (CC BY 4.0), which permits copying, adaptation and redistribution, provided the original work is properly cited (http://creativecommons.org/licenses/by/4.0/). doi: 10.2166/washdev.2017.142
laboratory or industriai level have been utilized to recover nutrients from urine for agricultural purposes (Pronk & Koné 2009). These technologies include struvite recovery via precipitation or electricity generation (Sakthivel et al. 2012; You et al. 2014), nitrification and distillation (Udert & Wachter 2012), and stripping and absorption (Basakçilardan-Kabakci et al. 2007; Zhang et al. 2015). The direct application of urine as fertilizer after proper storage is favored in rural and suburban areas.
Urea is biochemically hydrolyzed into ammonium , bicarbonate , and hydroxyl (OH-), thus increasing pH and ammonium concentration (Vinnerâs et al 2006; Jons-son & Vinnerâs 2007) and exerting lethal effects on microorganisms. Therefore, pH, storage duration, and temperature are the major factors that influence the storage process of human urine. Moreover, temperature is a crucial parameter that influences microbial inactivation rates (Maurer et al 2006). High pH, high temperature, and long storage periods are required to produce safe and hygienic liquid fertilizer (Magri et al 2015; Hu et al 2016). Therefore, guidelines from the World Health Organization (WHO 2006) recommend a storage period of 6 months at 20 "Corhigher for the safe application of human urine on unrestricted crops. However, nitrogen loss via ammonia volatilization as a result of urea hydrolysis is another issue that should be addressed for the collection and transport of stored urine (Udert et al 2006). Ammonia volatilization not only decreases the efficiency of nitrogen recovery but also adversely affects environmental and human health (Galloway & Cowling 2002). In addition, the storage time of 6 months requires a large volume of storage tanks and is not cost-effective.
Hence, it is necessary to develop an efficient method that minimizes the required volume of storage tanks for human urine storage, as well as promoting the disinfection and stability of stored human urine. Although freezing concentrates almost 80% of the nutrients in urine to 25% of the original volume (Lind et al 2001), the utilization of frozen urine is problematic. Acidification or chemical oxidation (Hellström et al 1999; Zhang et al 2013) can inhibit urea decomposition; however, adding reagents to the urine is not an ideal solution. Temperature considerably affects urine storage and benefits pathogen elimination. For example, increasing storage temperature from 24 °Cto34 °C considerably decreases the number of enteric pathogens in diluted urine (Höglund et al 1998; Vin-neräs et al 2008). In addition, bacteria, such as Salmonella typhimurium, Streptococcus faecalis,and Escherichia coli,are inactivated at 65 °C within4 min (Fjendbo et al 1998). Mean-while, Enterococcus faecalis and E. coliare eliminated within 20 s under 65 °C (Spinks et al 2006). Moreover, solar pasteuri-zation at 55 °C decreases E. coli counts below the detectable limit (Dobrowsky et al 2015). Thus, utilizing the thermal effect on pathogen inactivation is an interesting alternative for human urine storage. Similar works on the disinfection of gray water, rainwater, and secondary effluent disinfection by solar energy showed that thermal disinfection is efficient (Amin et al 2014; Giannakis et al 2014; Lee et al 2016). Temperatures above 50 "Csignificantly affect the chemical hydrolysis of urea (Frankenberger & Tabatabai 1982). Urea hydrolysis is catalyzed by the urease produced by urease-producing bacteria (UPB); theoptimal temperatureof ureaseactivityisnormally 650C(Hagenkamp-Korth et al 2015). High temperatures, however, can reduce urea hydrolysis by inhibiting UPB growth. Therefore, the thermal storage of urine has potentially high sanitizing efficiency while minimizing urea hydrolysis.
Given that these advantageous properties have not been discussed in the presented literature, this study aims to investigate the thermal storage of human urine at 60 °C and 70 °C. It also aims to determine the effects of storage temperature on pathogen inactivation and urea decomposition. E. coli and fecal coliforms were used as the indicators of bacterial inactivation, whereas pH and ammonia were used as the main indicators of urea hydrolysis.
MATERIAL AND METHODS
Experimental set-up
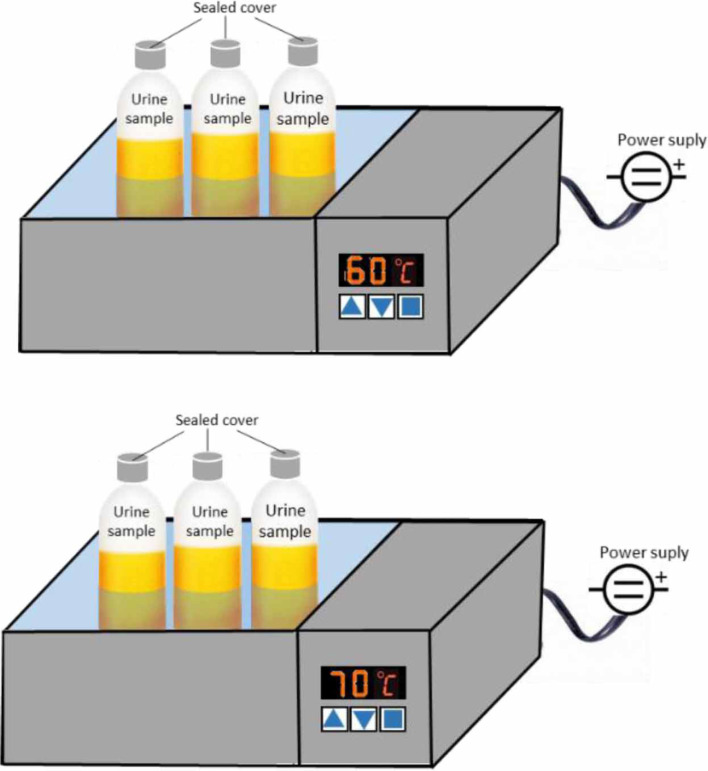
Fresh urine samples were stored in 30 sealed 50-mL glass bot-tles to avoid cross-contamination during sampling. All the bottles were disinfected and dried prior to filling. The storage temperatures of 600C and 70 °C were controlled with a water bath. The urine samples were then cooled down to ambient temperature after a reasonable storage time. Urine that was stored at ambient temperature was used as control. The actual ambient temperature was measured daily. Each experimental scenario was maintained for several days until the end of the experiment. The physical, chemical, and biological parameters of the urine samples were tested. Figure 1 presents the installation set-up of the experiment.
Figure 1.
Experimental installation with water bath to maintain the temperature of urine storage.
Urine samples
Fresh urine was collected from toilets at the School of Civil and Resource Engineering, University of Science and Technology, Beijing, China. To simulate a low-flushing urinal, the collected urine samples were diluted at a urine: distilled water ratio of 2:1 prior to distribution into experimental bottles. Given that urine was randomly collected in a plastic bucket from urinals with-out flushing water, the initial composition of fresh human urine varied from time to time. In this research, three experimental scenarios were investigated; thus, the initial characteristics of urine differed, as presented in Table 1.
Table 1.
Main characteristics of fresh urine collected for the experimental scenarios
| Scenarios | pH | Ammonia/ammonium (mg/L) | Fecal coliforms (CFU/L) | E. coli (CFU/L) |
|---|---|---|---|---|
| 1 | 7.15 | 292.49 | 1.4 x 106 | 2.0 x 104 |
| 2 | 6.84 | 501.52 | 2.4 x 103 | 2.0x 103 |
| 3 | 7.26 | 331.43 | 3.4 x 104 | - |
means not detected.
Sample analysis
Chemical analysis
pH was measured with a hand-held pH meter (HACH HQ30d, USA) and a corresponding electrode (pHC10101). Urine in glass bottles was measured immediately after sampling. Ammonia/ammonium content was analyzed calorimetrically with a DR/600 spectrophotometer (HACH, Co., USA) at 420 nm. All assays were performed in triplicate. Results were presented as averaged values.
Microbial analysis
There is currently no consensus on which organism(s) is the most useful hygiene indicator. Furthermore, there is no national regulation that mandates a single standard for urine reuse. According to the WHO Guidelines for the Safe Use of Wastewater, Excreta, and Greywater (WHO 2006), one or more of the total coliform, fecal coliform, and E. coli bacteria are used as wastewater effluent or reuse standards. Fecal coliforms originate from feces and are indicators of possible disease transmission (Fuhrmeister et al 2015); thus, fecal coliforms are often used as primary bacterial indicators. Moreover, the United States Environmental Protection Agency recommended E. coli and Enterococci as health risk indicators for water. Thus, fecal coliforms and E. coli were used as the core indicators in this study.
Initially, fecal coliform and E. coli in the urine samples were analyzed daily at the same sampling time. The sampling interval was adjusted during the later periods of storage. All the samples were diluted to a suitable concentration for parameter measurement.
The standard membrane filter method was used to quantify fecal coliform and E. coli (APHA 2012). An appropriate volume of urine sample was filtered through a 0.45-|im acetate cellulose filter. The filtered sample was placed in MFC broth and incubated at (44.5 ± 0.2) °C for 24 h. Blue colonies were counted to estimate the population of fecal coliforms. The number of fecal coliforms was presented as colony-forming unit per liter (CFU/L). To quantify E. coli, the filtered membrane was placed on Fuchsin basic sodium sulfite agar at 37 °C for 24 h and then transferred to NA-MUG agar for further incubation at 36 ± 1 °C for 4 h. Then, the bacteria were counted under a 366-nm ultraviolet lamp. Bacteria with blue fluorescence were counted to estimate the population of E. coli.
RESULTS AND DISCUSSION
Inactivation of bacteria
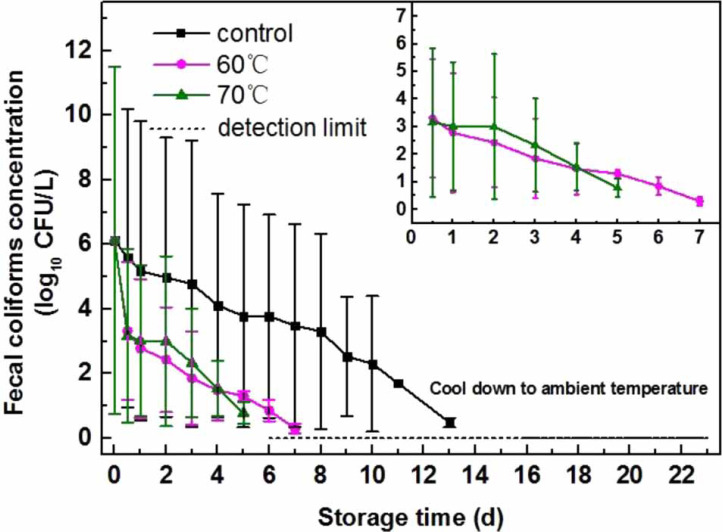
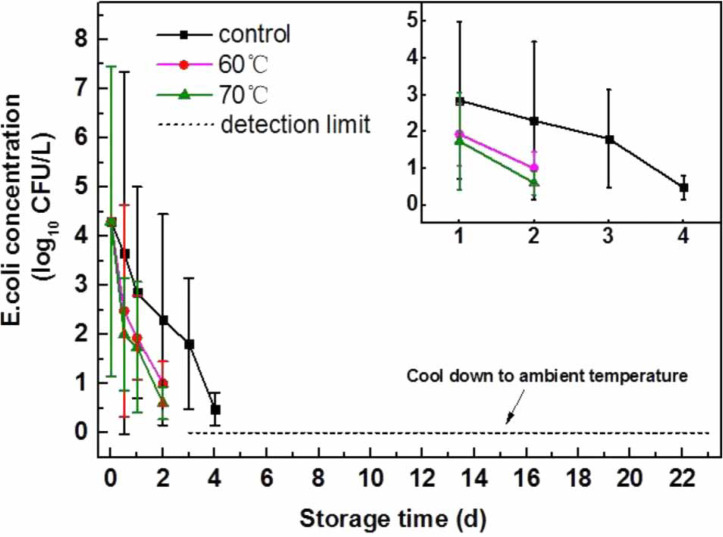
The overall storage time-dependence of the fecal coliform and E. coli concentrations in the three stored diluted urine samples is presented in Figures 2 and 3, respectively. An inset that represents the time-dependent elimination process is shown on the upper right quarter of the figures. Both fecal coKforms and E. coli decreased rapidly within the first week in urine that was stored at 60 °Cor70 °C compared with urine that was stored at ambient temperature.
Figure 2.
Fecal coliform concentration in the three urine samples during storage.
Figure 3.
E. coli concentrations in the three urine samples during storage.
Figure 2 demonstrates that the inactivation of fecal coli-forms in urine that was stored at ambient temperature was considerably slower than that in urine that was stored at high temperatures. In the former, the concentration of fecal coliforms decreased to undetectable levels on the 16th day of storage. The population of fecal coliforms in the urine samples that were stored at 60 °C and 70 °C decreased rapidly from 6 log to approximately 3 log on the 1st day of storage. The rate of population decline gradually stabilized and fecal coliform concentrations decreased to undetectable levels on the 8th and 6th days of storage. Similar results were obtained for E. coli. As shown in Figure 3, E. coli reached undetectable levels on the 3rd day of storage at 60 °C and 70 °C. By contrast, E. coli reached undetectable levels on the 5th day of storage at ambient temperature.
The thermally stored urine was cooled down to ambient temperature. Then, the microbial indicators were continuously tested to evaluate the long-lasting disinfecting effect of high temperature. The three bacterial indicators were not reactivated in the two urine samples, which indi-cated that thermal storage has a stable disinfection effect. The experimental results showed that compared with the ambient temperature, high temperatures accelerate disinfection.
Urea hydrolysis
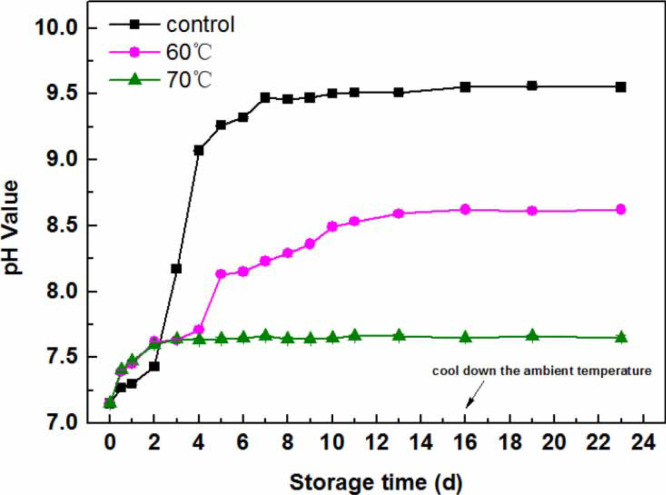
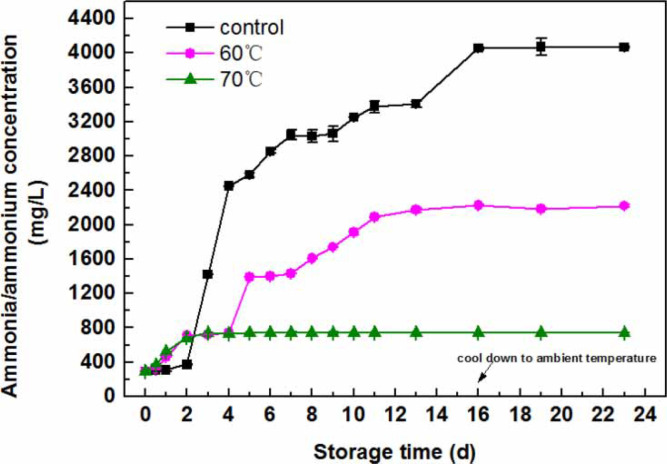
To reflect urea hydrolysis, the storage time-dependence of pH values and the ammonia/ammonium concentrations of the three urine samples were investigated. The results are presented in Figures 4 and 5. The actual ambient temperature during the experimental period varied from 15 °C to 20.5 °C. The urine stored under ambient temperature had high pH (9.07) and high ammonia/ammonium concentration (2,453.32 mg/L) from the 4th day of storage onwards. Although both storage temperatures of 60 °C and 70 °C quickly removed fecal coliforms and E. coli, pH levels and ammonia/ammonium concentration differed at these temperatures. The pH value of the urine that was stored at 60 °C was initially 7.15, which then increased to 8.13 on the 5th day of storage, reached approximately 8.53 after 13 days of storage, and finally stabilized. Meanwhile, ammonia concentration increased from 292.49 mg/L to 1,392.59 mg/L before reaching approximately 2,173.04 mg/L. However, only a slight increase in pH and ammonia concentration was noted in the urine stored at 70 °C. pH increased from 7.15 to 7.60 and the ammonia concentration increased from 292.48 mg/L to 685.01 mg/L on the 2nd day of storage before stabilizing.
Figure 4.
pH value of the diluted urine during the storage.
Figure 5.
Ammonia/ammonium concentration of the diluted urine during storage.
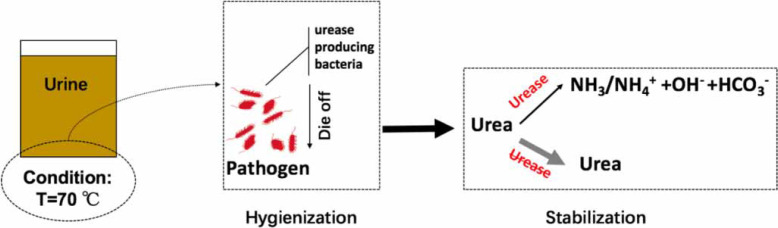
Under ambient storage conditions, the main process for disinfection is urea hydrolysis and high pH together with high ammonia/ammonium concentration, which are lethal to fecal coliforms and E. coli. In this experiment, the variation in pH and ammonia/ammonium concentration was correlated with the removal of fecal coliforms and E. coli. The thermal effect is responsible for pathogen inactivation in the thermally stored urine. Moreover, the high storage temperature caused the rapid removal of fecal coliforms and E. coli (Figures 2 and 3). Thermal storage has been hypothesized not only to enhance disinfection efficiency but also to prevent urea from hydrolyzing into ammonia/ammonium. Urea hydrolysis is catalyzed by urease from UPB. The thermal effect, how-ever, inactivates UPB, thus inhibiting urease production during storage. In this study, the inactivation of bacteria was slower at 60 °C than at 70 °C; thus, the remaining bacterial concentration in the urine that was stored at 60 °C was higher than that of the urine that was stored at 70 °C. As a result, more UPB remained in the urine that was stored at 60 °C during early storage. Therefore, urea hydrolysis occurred. Although temperatures of both 70 °C and 60 °Cinfluenced urease activity, 70 °C had a more significant effect on urease because the optimal temperature for urease activity is 65 °C(Hagenkamp-Korth et al 2015) and UPB is less active. The reduction of urease concentration via UPB inactivation likely contributes more to the inhibition of urea hydrolysis. Therefore, considering disinfection effect and urea hydrolysis, storage at 70 °C for 7 days produces the optimal pathogenic bacteria inacti-vation and urea stabilization. The storage process is as described in Figure 6.
Figure 6.
Process for disinfection and stabilization of human urine under thermal storage of 70 WC.
Replication tests
Based on the above experimental results, thermal storage, i.e., storing urine at 70 °C for 7 days, was applied to human urine in two duplicate tests to test the reproduci-bility of the selected operational conditions. Heated urine was naturally cooled down to ambient temperature for another 7 days to investigate the disinfection and stabilization effect of thermal storage. The experimental results for the thermal storage of the two urine samples are presented in Figure 7.
Figure 7.
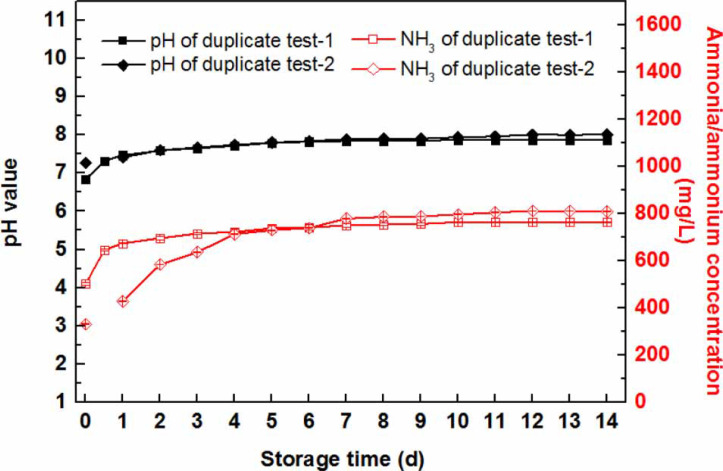
pH values and ammonia/ammonium concentrations in the two urine samples.
Fecal coliforms and E. coli in the two urine samples were completely inactivated within 2 days of storage. Fecal coliforms and E. coli decreased to undetectable levels after the first storage day, particularly in duplicate-1. For urea hydrolysis, little variation was found in the pH and ammo-nia/ammonium concentration of the two urine samples during the 7 days of storage. These results further supported the efficiency and reproducibility of the previous experimental results. The pH values of the two urine samples on the 7th day of storage were 7.84 and 7.89, whereas the ammonia concentrations were 749.39 and 778.46 mg/L, i.e., only a slight increase was observed. Moreover, disinfection and stability were retained even after storing the urine under ambient temperature, as evidenced by the lack of significant changes in the microbial, physical, and chemical character-istics of the samples.
Thermal treatment is a potential alternative technology for community toilets with source-separated systems. In fecal sludge composting, applying high temperatures for a short duration may be as effective as applying low temperatures for a longer duration (Day et al 2001). Given that similar operational parameters are suitable for urine storage, increasing temperature from the ambient to 70 °C during the thermal storage of urine can minimize storage tank volume. Thus, the thermal storage of urine is suitable for commu-nities with limited space and is a potential, inexpensive solution for some solar energy-rich regions. Moreover, this system can be heated with renewable energy, such as biogas and ground source heat. The present study proved that the thermal treatment of human urine at 70 °C for 7 days for disinfection and stabilization can be achieved tech-nically. However, a more comprehensive study on the tradeoff between energy costs, environmental impact/ resource use from heating with traditional energy sources (compared with renewable energies), and efficient nutrient recovery from a waste stream should be discussed before the practical application of thermal storage for human urine.
CONCLUSIONS
In this study, the thermal treatment of urine at 60°C and 70°C was conducted and evaluated in terms of disinfection and stabilization. Storage at 70 °C for 7 days realized optimal pathogenic bacteria inactivation and urea stabilization. High storage temperature had multiple advantages for human urine treatment, including: (1) acceleration of pathogen inactivation; (2) inhibition of urea decomposition; and (3) minimization of storage tank volume. Urea hydrolysis was likely inhibited by the decreased UPB count because urease production became limited.
Further research should be conducted before this storage system can be used for practical applications. Future studies should: (1) use more indicators for sanitation monitoring to broaden the spectrum of cross-sectional pathogenic inacti-vation efficiency to find an optimal storage temperature; (2) investigate the mechanism of microorganism inactivation during high-temperature storage; (3) compare the loss of different gaseous nitrogen forms from thermal storage with that from conventional storage; and (4) analyze energy balance and minimize energy consumption.
ACKNOWLEDGEMENTS
The authors would like to acknowledge the financial support provided by the Bill and Melinda Gates Foundation. The authors would also like to thank the National Environment and Energy International Cooperation Base for their support. The first two authors contributed equally to the work.
REFERENCES
- Akpan-Idiok A. U., Udo I. A. & Braide E I. 2012. The use of human urine as an organic fertilizer in the production of okra (abelmoschus esculentus) in south eastern Nigeria. Resources, Conservation and Recycling 62,14-20. [Google Scholar]
- Amin M. T., Alazba A. A., Amin M. N. & Han M. Y.. 2014. Cost-effective and sustainable solutions to enhance the solar disinfection efficiency improving the microbiological quality of rooftop-harvested rainwater. Desalination and Water Treatment 52 (28-30), 5252-5263. [Google Scholar]
- APHA 2012. Standard Methods for the Examination of Water and Wastewater. American Public Health Association, Washington, DC. [Google Scholar]
- Basakcilardan-Kabakci S., ipekoglu A. N. & Talinli I.. 2007. Recovery of ammonia from human urine by stripping and absorption. Environmental Engineering Science 24 (5), 615-624. [Google Scholar]
- Boh M. Y., Germer J., Müller T. & Sauerborn J.. 2013. Comparative effect of human urine and ammonium nitrate application on maize (Zea mays l.) grown under various salt (nacl) concentrations. Journal of Plant Nutrition & Soil Science 176, 703-711. [Google Scholar]
- Day M., Shaw K., Stofella P. & Kahn B.. 2001. Biological chemical and physical processes of composting compost utilization. In: Compost Utilization In Horticultural Cropping Systems (Stoffella P. J. & Kahn B. A., eds). CRC Press, Boca Raton, FL. [Google Scholar]
- Dobrowsky P. H., Carstens M., De Villiers J., Cloete T. E. & Khan W.. 2015. Efficiency of a closed-coupled solar pasteurization system in treating roof harvested rainwater. Science of the Total Environment 536, 206-214. [DOI] [PubMed] [Google Scholar]
- Fjendbo J. A. J., Nohr K., Sorensen H. & Boisen F.. 1998. Decontamination of drinking water by direct heating in solar panels. Journal of Applied Microbiology 85 (3), 441-447. [DOI] [PubMed] [Google Scholar]
- Frankenberger W. T. Jr & Tabatabai M. A.. 1982. Amidase and urease activities in plants. Plant and Soil 64 (2), 153-166. [Google Scholar]
- Fuhrmeister E. R., Schwab K. J. & Julian T. R.. 2015. Estimates of nitrogen, phosphorus, biochemical oxygen demand, and fecal coliforms entering the environment due to inadequate sanitation treatment technologies in 108 low and middle income countries. Environmental Science & Technology 49 (19), 11604-11611. [DOI] [PubMed] [Google Scholar]
- Galloway J. N. & Cowling E. B.. 2002. Reactive nitrogen and the world: 200 years of change. Ambio A Journal of the Human Environment 31 (31), 64-71. [DOI] [PubMed] [Google Scholar]
- Giannakis S., Darakas E., Escalas-Canellas A. & Pulgarin C.. 2014. The antagonistic and synergistic effects of temperature during solar disinfection of synthetic secondary effluent. Journal of Photochemistry and Photobiology A Chemistry 280,14-26. [Google Scholar]
- Hagenkamp-Korth F., Haeussermann A. & Hartung E.. 2015. Effect of urease inhibitor application on urease activity in three different cubicle housing systems under practical conditions. Agriculture, Ecosystems & Environment 202, 168-177. [Google Scholar]
- Hellström D., Johansson E. & Grennberg K.. 1999. Storage of human urine: acidification as a method to inhibit decomposition of urea. Ecological Engineering 12 (3-4), 253-269. [Google Scholar]
- Höglund C., Stenstrom T., Jonsson H. & Sundin A.. 1998Evaluation of faecal contamination and microbial die-off in urine separating sewage systems. Water Science and Technology 38 (6), 17-25. [Google Scholar]
- Hu, Fan M., Wang B., Qu H., B. & Zhu S.. 2016. Constructing the ecological sanitation: a review on technology and methods. Journal of Cleaner Production 125,1-21. [Google Scholar]
- Jonsson H. & Vinnerâs B.. 2007. Experiences and suggestions for collection systems for source separated urine and faeces. Gewasserschutz Wasser Abwasser 206, 13. [DOI] [PubMed] [Google Scholar]
- Karak T. & Bhattacharyya P.. 2011. Human urine as a source of alternative natural fertilizer in agriculture: a flight of fancy or an achievable reality. Resources, Conservation and Recycling 55 (4), 400-408. [Google Scholar]
- Lee , Song W., Son J. , Gutierrez J. H. , Kang M. P., Km T. , D. & Lee L. P.. 2016. Solar optics-based active panel for solar energy storage and disinfection of greywater. Biomicrofluidics 10, 0541205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind B., Ban Z. & Byden S.. 2001. Volume reduction and concentration of nutrients in human urine. Ecological Engineering 16 (4), 561-566. [Google Scholar]
- Magri M. E., Fidjeland J., Jonsson H., Albihn A. & Vinneras B.. 2015. Inactivation of adenovirus, reovirus and bacteriophages in fecal sludge by Ph and ammonia. Science of the Total Environment 520, 213-221. [DOI] [PubMed] [Google Scholar]
- Maurer M., Pronk W. & Larsen T. A.. 2006. Treatment processes for source-separated urine. Water Research 40 (17), 3151-3166. [DOI] [PubMed] [Google Scholar]
- O'Neal J. A. & Boyer T. H.. 2013. Phosphate recovery using hybrid anion exchange: applications to source-separated urine and combined wastewater streams. Water Research 47 (14), 5003-5017. [DOI] [PubMed] [Google Scholar]
- Pronk W. & Kone D.. 2009. Options for urine treatment in developing countries. Desalination 248 (1-3), 360-368. [Google Scholar]
- Sakthivel, S. R., Tilley E. & Udert K. M.. 2012. Wood ash as a magnesium source for phosphorus recovery from source-separated urine. Science of the Total Environment 419,68-75. [DOI] [PubMed] [Google Scholar]
- Spinks A. T., Dunstan R. H., Harrison T., Coombes P. & Kuczera G.. 2006. Thermal inactivation of water-borne pathogenic and indicator bacteria at sub-boiling temperatures. Water Research 40 (6), 1326-1332. [DOI] [PubMed] [Google Scholar]
- Tidaker P., Mattsson B. & Jonsson H.. 2007. Environmental impact of wheat production using human urine and mineral fertilisers -a scenario study. Journal of Cleaner Production 15 (1), 52-62. [Google Scholar]
- Udert, K. M. & Wachter M.. 2012. Complete nutrient recovery from source-separated urine by nitrification and distillation. Water Research 46 (2), 453-464. [DOI] [PubMed] [Google Scholar]
- Udert K. M., Larsen T. A. & Gujer W.. 2006. Fate of major compounds in source-separated urine. Water Science and Technology 54 (11-12), 413-420. [DOI] [PubMed] [Google Scholar]
- Vinneras B., Palmquist H., Balmer P. & Jonsson H.. 2006. The characteristics of household wastewater and biodegradable solid waste - a proposal for new Swedish design values. Urban Water Journal 3 (1), 3-11. [Google Scholar]
- Vinneras B., Nordin A., Niwagaba C. & Nyberg K.. 2008. Inactivation of bacteria and viruses in human urine depending on temperature and dilution rate. Water Research 42 (15), 4067-4074. [DOI] [PubMed] [Google Scholar]
- WHO 2006. Guidelines for the Safe Use of Wastewater, Excreta and Greywater in Agriculture and Aquaculture. World Health Organization, Geneva. [Google Scholar]
- You J., Greenman J., Melhuish C. & Ieropoulos I.. 2014. Electricity generation and struvite recovery from human urine using microbial fuel cells. Chemical Technology and Biotechnology 91 (3), 647-654. [Google Scholar]
- Zhang Y., Li Z., Zhao Y., Chen S. & Mahmood I. B.. 2013. Stabilization of source-separated human urine by chemical oxidation. Water Science and Technology 67 (9), 1901-1907. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Li Z. & Mahmood I. B.. 2015. Effects of corn cob produced biochars on urea recovery from human urine and their application as soil conditioners. CLEAN - Soil, Air, Water 43 (8), 1167-1173. [Google Scholar]