Abstract
This study aimed to explore the influences of microRNA-195 (miRNA-195)/Rap2C/MAPK in the proliferation and apoptosis of small cell lung cancer (SCLC) cells. QRT-PCR analysis were executed to evaluate miRNA-195 expression in lung cancer tissues and SCLC cells, and the western blot was implemented to monitor Rap2C protein level and uncovered whether the MAPK signaling pathway in lung cancer tissues and SCLC cells was activated. The CCK-8 experiment was performed to detect cell proliferation ability, and the flow cytometry was utilized to examine cell apoptosis level. Luciferase reporter gene system was executed to disclose the interaction between miRNA-195 and Rap2C. Subcutaneous implantation mouse models of SCLC cells were constructed to detect cell proliferation in vivo, and Kaplan-Meier method calculated patient survival. The expression of Rap2C was higher in lung cancer tissues and SCLC cells than in normal tissues and cells, while the expression of miRNA-195 was lower in lung cancer tissues and SCLC cells than in normal tissues and cells. miRNA-195 lower expression predicted showed reduced overall survival in lung cancer patients. Further loss of function and enhancement experiments revealed that miRNA-195 overexpression could significantly inhibit SCLC cell proliferation and promote cell apoptosis by upregulation of Bax and down-regulation of bcl-2; Luciferase reporter assay demonstrated that miRNA-195 could bind to Rap2C mRNA and inhibit its expression, Rap2C overexpression also related to the poorer prognosis of lung patients. Knockdown of Rap2C suppressed cell proliferation and expedited apoptosis. In addition, overexpression of Rap2C reversed miRNA-195-induced apoptosis and proliferation inhibition. Furthermore, miRNA195 prohibited the activation of MAPK signaling pathway by down-regulating Rap2C. These consequences indicated that miRNA-195 promotes the apoptosis and inhibits the proliferation of small cell lung cancer (SCLC) cells via inhibiting Rap2C protein-dependent MAPK signal transduction
Keywords: microRNA-195, Rap2C, MAPK pathway, small cell lung cancer, apoptosis and proliferation
Introduction
Lung cancer is a malignant tumor with the highest morbidity and mortality in the world at present,1,2 and it is increasing year by year.3 Lung cancer can be divided into small cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC), of which SCLC accounts for 15% to 20% of the toal lung cancers.4 SCLC is a a kind of malignant epithelial tumor composed of small cells, with high invasiveness, early metastasis, high recurrence rate and drug resistance and other clinicopathological characteristics.5 The current therapeutic effect of small cell lung cancer is poor, with an overall survival rate of less than 6 months.6 Therefore, the treatment and prognosis of lung cancer are still important medical problems and challenges. To explore the pathogenesis of lung cancer is important in promoting the treatment and prevention of lung cancer.
MicroRNA (miRNA) is a large number of non-coding small RNAs, which could inhibit the expression of nearly one-third of human genes at the transcription and translation levels by combining with the 3’-UTR region of the target gene mRNA.7 An increasing number of studies indicate that miRNA mediates many biological processes, including tumorigenesis. Studies have pointed out that miRNA-195 has guiding significance for the diagnosis and prognosis of NSCLC, and participates in regulating the development of NSCLC,8,9 however, the role of miRNA-195 in SCLC has not been unreported.
Rap2 is a Ras-related protein, which belongs to the GTP-binding protein family. Studies have pointed out that Rap2A, 2B and 2C all play a crucial role in the development and progression of tumors including osteosarcoma,10 colorectal cancer11 and breast cancer.12 Nevertheless, the regulation of Rap2C on SCLC has not been reported yet. Ras-MAPK signaling pathway plays an important role in NSCLC,13 but there are few researches of Ras-MAPK on SCLC. Rap2C is a Ras-related protein, which may affect the development of SCLC by modulating MAPK pathway. This study explores the effects of miRNA-195 and Rap2C-MAPK signaling pathway on SCLC.
Materials and Methods
Clinical Sample Collection
Tumor tissues and paired non-cancerous tissues were collected from 40 SCLC patients who underwent surgery in our hospital from march 1, 2015 to November 30, 2019 . Clinicopathological data, including age, gender, tumor size, and TNM stage, were collected and tumor stage was assessed according to international TNM classification (Table 1). All these patients were diagnosed for the first time, without obvious distal metastatic lesions and preoperative radiotherapy or chemotherapy, moreover pathologically identified as SCLC, and the malignancy degree was evaluated in accordance with international TNM clinical staging criteria. The experiment was consented by the ethics committee of our hospital(NO.ERC-20233). All patients signed the informed consent to participate in this study according to the declaration of helsinki.
Table 1.
The Relationship Between Expression Level of miRNA-195 and Clinical Characteristics in Lung Cancer (n = 40).
| Variables | All cases | miRNA-195 expression | P-value | |
|---|---|---|---|---|
| Low | High | |||
| Age at surgery (years) | ||||
| <60 | 22 | 12 | 10 | 0.5688 |
| ≥60 | 18 | 13 | 15 | |
| 8Gender | ||||
| Male | 19 | 10 | 9 | 0.3561 |
| Female | 21 | 8 | 13 | |
| Tumor size (cm) | ||||
| <5 | 17 | 9 | 8 | 0.0430* |
| ≥5 | 23 | 19 | 4 | |
| TNM | ||||
| I + II | 15 | 8 | 7 | 0.0046** |
| III + IV | 25 | 23 | 2 | |
Cell Culture and Transfection
Human respiratory tract epithelial cell line (BEAS-2B) and SCLC cell lines (NCI-H446 and NCI-H526) were purchased from ATCC, which were placed in a humidified cell incubator (37°C) with 5% CO2, using a DMEM medium with 10% FBS, 100 U/ml penicillin and 100 lg /ml streptomycin. miRNA-195 mimic and inhibitor, and plasmid were transfected using lipo2000 transfection reagent according to the instructions.
In Vivo Tumorigenesis Assay
6-8 weeks, male BALB/c nude mice was purchased from Viton Lihua and the mice were prepared to undergo tumor-bearing experiments 3-5 days after resting in the animal room. After digestion and resuspension, A total 1 × 106 cells were subcutaneously injected under the armpit of the mouse. After 30 days, the mice were sacrificed . in the meantime, the tumors growing under the skin were taken out, photographed and weighed. This animal experiment was agreed by the Biomedical Research Ethics Committee of our hospital. All experimental procedures was supported by the Animal Protection and Use Committee of Zhejiang University and conformed the NIH guidelines on laboratory animal protection and safety. Under the standard environmental conditions (22-25°C temperature, 45-50% humidness and 12-hour light-dark cycle), all animals were raised separately with unrestricted access to food and water.
Quantitative Real-Time PCR
Using Trizol reagent (15596026, Invitrogen, Car, Cal, USA) to extract total RNA from lung cancer tissues and SCLC cells, and the reversed transcription was implemented according to the specification of PrimeScript RT reagent Kit (RR047A, Takara, Japan). The DNA templates were quantified using Fast SYBR Green PCR reagent, and the synthesized cDNA Kit (Applied biosystems) was applyed to prepare the reaction system. Relying on ABI PRISM 7300 RT-PCR system (Applied biosystems), qRT-PCR detection was carried out, and each sample was set to 3 replicates. GAPDH served as an internal reference. miRNA expression level was determined using PrimeScript miRNA RT-PCR kit (Takara), which was normalized by U6. The relevant primer sequences utilized in qRT-PCR experiment were exhibited as follows: miR-195, forward 5′-CCT AGC AGC ACA GAA A-3′ and reverse 5′-GAG CAG GCT GGA GAA-3′; U6, forward 5′-CTC GCT TCG GCA GCA CAT A-3′ and reverse 5′-CGC TTC ACG AAT TTG CGT G-3′.14
Western Blot
The cells were collected after digestion and centrifugation, and an appropriate amount of RIPA lysis buffer together with protease inhibitor (Roche, Meylan, France) was added according to the number of cells. The lysis was disposed for 30 minutes on the ice. After centrifugation at 13000 rpm for 20 minutes, the supernatant of the lysate was obtained. The protein concentration was assessed using BCA Protein Assay kit (Beyotime Institute of Biotechnology). The samples were boiled for 10 minutes, then SDS-PAG was performed. After electrophoresis is finished, the protein was transferred to to a PVDF membrane (Millipore, Billerica, MA, USA) through a semi-dry transfer instrument. The membranes were incubated in 5% skim milk-TBST at room temperature for 1 h, and then rinsed 3 times with TBST buffer for 5 minutes. The corresponding first antibody was added and incubated at 4°c shaking table. overnight. Next day, these primary antibodies were recovered, and TBST was utilized to rinse the membrane for 5 minutes (3 times). The secondary antibody was added and incubated for 1 hour at a room temperature, and rinsed 3 times with TBST for 5 minutes. Next, the enhanced chemiluminescence (ThermoFisher Scientific) was added and the obtained images were analyzed by chemiluminescence imager Chemi-Scope mini imagingsystem (Clinx Science). ImageJ software (National Institutes of Health) was applied for quantificational analysis . Antibodies of Rap2C (Abcam, Cambridge, MA, USA; 1: 1000), Bax, Bcl-2, p-ERK, ERK, p-JNK, JNK, p-p38, p38 and GAPDH (Cell signaling Technology, Danvers, MA, USA; 1: 1000) were used in this experiment.
Dual Luciferase Reporter Gene System
The synthetic Rap2C 3′UTR gene fragment was imported into pMIR-reporter gene (Huayueyang Biotechnology, Beijing, China). In consonance with the Rap2C-WT binding site, the mutation of Rap2C-MUT binding site was designed. These composite vectors of Rap2C-WT and MUT were co-transfected into HEK293 T cells (Beinuo Biotechnology, Shanghai, China). After 48 hours transfection, the fluorescence intensity was monitored using a correlative luciferase kit (K801-200; Biovision, Mountain View, CA, USA).
CCK-8 Assay
Cell proliferation was determined using CCK-8 (Dojindo Laboratories, Japan). The cells with logarithmic growth were digested with 0.25% trypsin and gently blew into a single cell. 1 × 104 cells were collected and cell viability was detected by CCK-8 method. After culture, 10 µl CCK-8 reagent was added to each well and incubated at 37 °C for another 4 h. Optical density (OD) values were measured at 450 nm.
Apoptosis Detection
After 48 hours transfection and digestion, cells were collected and adjusted to 1 × 106 cells/mL. These cells (1 ml) were centrifuged at 1500 rpm for 10 minutes. After discarding the supernatant 2 mL PBS was added to the cells. After this, pre-cooled 70% ethanol was utilized to fix these cells at 4°C overnight. After twice washed with PBS, cells were resuspended utilizing 200 µL binding buffer, and then the mixture of Annexin V-FITC (10 μl, ab14085, Abcam, Inc, USA) and PI (5 μl) were added into above cells. After keeping out of the light 15 min at circumambient temperature, the 300 µl binding buffer was supplemented in above reaction system, and the ratio of SCLC cell apoptosis was evaluated through flow cytometry.
Statistical Analysis
The statistical data from the research was analyzed employing SPSS 21.0 (IBM, USA) software, which was exhibited as the mean ± SD. First, the normality and variance homogeneity tests were carried out. The test conforms to the normal distribution and the variance was uniform. The unpaired t test was utilized to analyze the difference between 2 groups. and 1-way ANOVA was used for comparison between groups. Kaplan-Meier method was manipulated to figure up the survival rate of lung cancer patients, and the log-rank test was wielded for univariate analysis. Pearson correlation analysis was implemented to observe the dependency of indicators. ** p < 0.01 represented as the significant different consequences.
Result
Low Expression of miRNA-195 in SCLC Cells
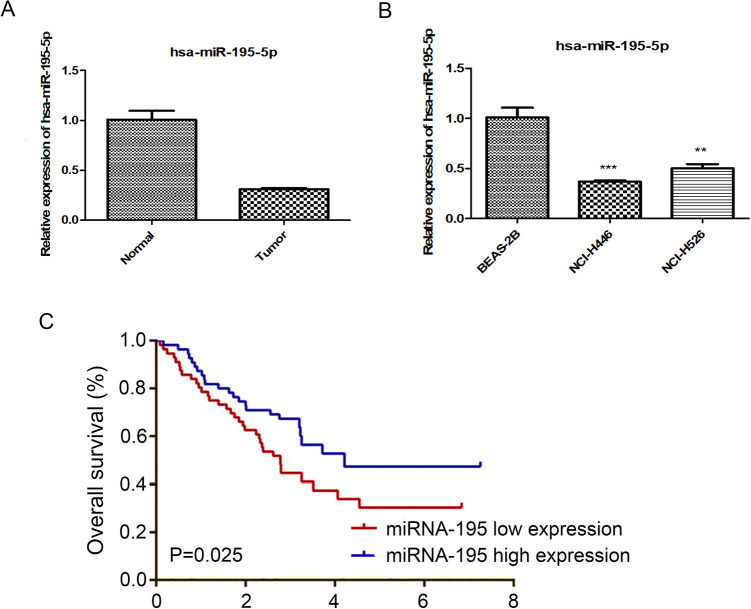
The mRNA expression levels of miRNA-195 were compared between lung cancer tissues and adjacent non-cancerous tissues using RT-PCR. The results indicated that miRNA-195 is down-regulated in lung cancer tissues (Figure 1A). The present study also examined the mRNA levels of miRNA-195 in human lung cancer cell lines. The results demonstrated the mRNA levels of miRNA-195 were significantly lower in NCI-H526 and NCI-H446 human lung cancer cell lines, compared with the BEAS-2B cells (Figure 1B).In 40 clinical SCLC samples, high levels of miRNA-195 mean that patients have longer poorer survival (Figure 1C).
Figure 1.
Low expression of miRNA-195 in SCLC. A: qRT-PCR analysis of miRNA-195 expression in Human lung cancer tissues and paired adjacent normal lung tissues. B: qRT-PCR analysis of miRNA-195 expression in Human lung cancer cells and lung epithelial cells, C: The survival rate for 40 patients with SCLC was analyzed and shown as a Kaplan-Meier graph.
Overexpression of miRNA-195 Inhibits the Proliferation of SCLC Cells and Promotes Apoptosis
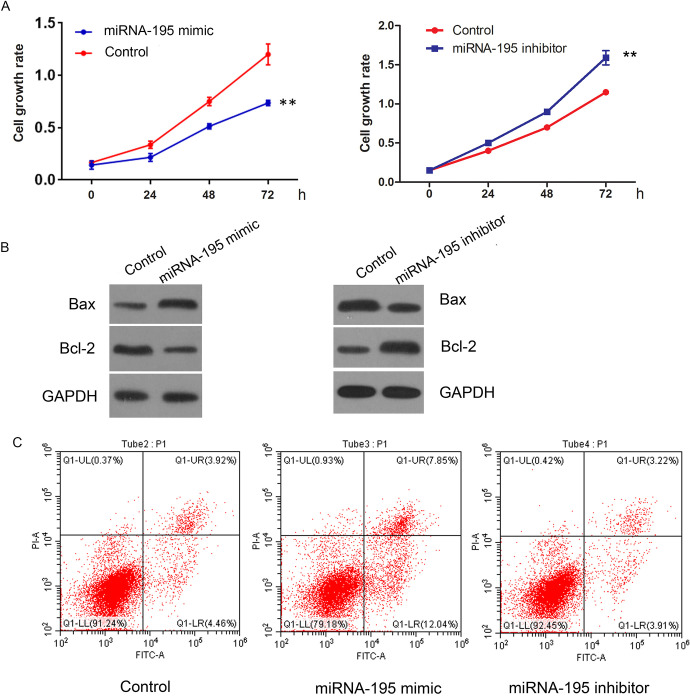
SCLC cells were transfected with miRNA-195 mimic. CCK-8 and flow cytometry result showed that the proliferation ability of SCLC cells decreased significantly after transfection of miRNA-195 mimic (Figure 2A). The apoptosis rate of SCLC cells increased significantly by miRNA-195 overexpression after 24 hours after transfection (Figure 2B). Western blot was further conducted to investigate the importance of miRNA-195 in protein expression of Bcl-2, Bax in SCLC cells. Western blotting test found that 24 h after transfection of miRNA-195 mimic. Compared with control group, the relative protein expression of Bcl-2 protein in the transfected group was significantly decreased (p < 0.05), and the relative protein expression of Bax was significantly increased (p < 0.05) (Figure 2B).
Figure 2.
miRNA-195 overexpression inhibits SCLC cell proliferation and promotes apoptosis. miRNA-195 mimic and miRNA-195 inhibitor were transfected into SCLC cells. A: CCK-8 assay was used to detect cell viability. B: Western Blot analysis of Bax, Bcl-2 expression C: Apoptotic rates were analyzed by using flow cytometry.
miRNA-195 Targets Rap2C
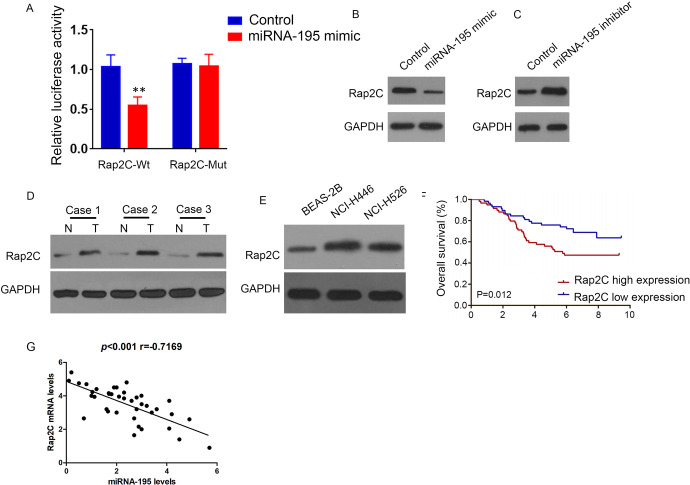
Bioinformatics predicts that Rap2C may be a potential downstream target mRNA of miRNA-195 (TargetScan Release 7.2.; position 62-68 of RAP2C 3’ UTR). The dual luciferase reporter system results indicated that miRNA-195 mimic significantly reduced the activity of Rap2C-wt luciferase, but not Rap2C-mut (Figure 3A). Furthermore, miRNA-195 mimic prohibited Rap2C expression (Figure 3B), while miRNA-195 inhibitor increased Rap2C expression (Figure 3C). Western blot detection of Rap2C protein level in lung cancer tissues and cells, which displayed that Rap2C protein level was enhanced in cancer tissues (Figure 3D) and cells (Figure 3E). Above explorations hinted that Rap2C expression was related to the prognosis of lung cancer patients. The higher the expression level of Rap2C meant the shorter the overall survival time of patients. (Figure 3F), Rap2C mRNA levels was negatively correlated with miRNA-195 expression levels(Figure 3G).
Figure 3.
miRNA-195 targeted regulation of Rap2C. A: Luciferase activity in cells cotransfected with miRNA-195 mimic and luciferase reporters containing Rap2C or mutant transcripts. Data are presented as the relative ratio of firefly luciferase activity of Renilla luciferase activity. B: Western Blot analysis of Rap2C expression in SCLCcells transfected with miRNA-195 mimic. C: Western Blot analysis of Rap2C expression in SCLC cells treatment with miRNA-195 inhibitors. D: Western Blot analysis of Rap2C expression in lung cancer and paired adjacent normal lung tissues. E: Western Blot analysis of Rap2C expression in human lung cancer cells and lung epithelial cells. F: Survival analysis of 40 patients with lung cancer. G: Pearson’s correlation scatter plots of miRNA-195 and Rap2C in lung cancer.
Knockdown of Rap2C Inhibits the Proliferation and Promotes Apoptosis of SCLC Cells
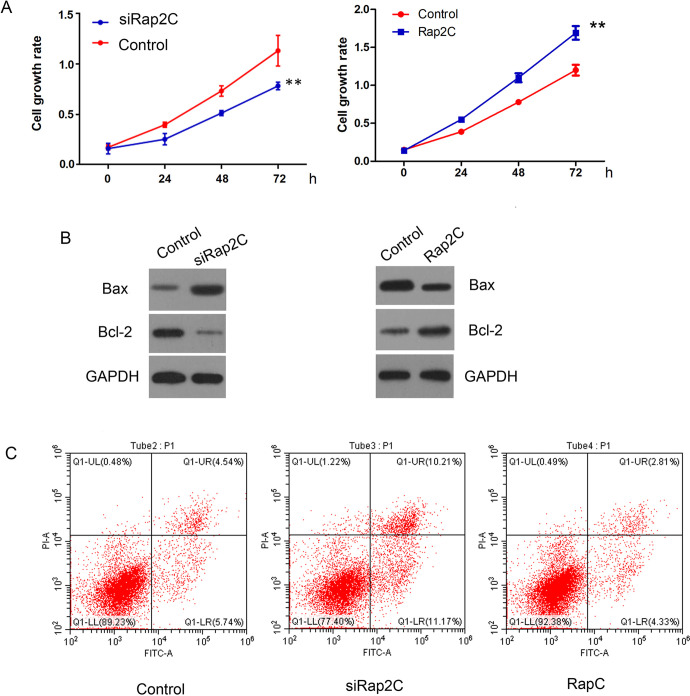
To further confirm Rap2C functions in SCLC cells, we knocked down Rap2C or overexpressed Rap2C expression in SCLC cells. The results presented that knockdown of Rap2C inhibited SCLC cell proliferation (Figure 4A), meanwhile promoted cell apoptosis (Figure 4C) via up-regulation of Bax protein and down-regulation of Bcl-2 as comparison with control group (Figure 4B), while Rap2C overexpression promoted SCLC cell proliferation (Figure 4A), meanwhile inhibited cell apoptosis (Figure 4C) via down-regulation of Bax protein and up-regulation of Bcl-2 as comparison with control group .
Figure 4.
Knockdown of Rap2C restrains SCLC cell proliferation and promotes apoptosis. siRap2C and Rap2C were transfected into SCLC cells. A: CCK-8 assay was used to detect cell viability. B: Western blot analysis of Bax, Bcl-2 in SCLC cells transduced with siRap2C or Rap2C. C: Apoptotic rates of SCLC cells were analyzed by using flow cytometry.
miRNA-195 Regulates SCLC cell Proliferation and Apoptosis Through Rap2C
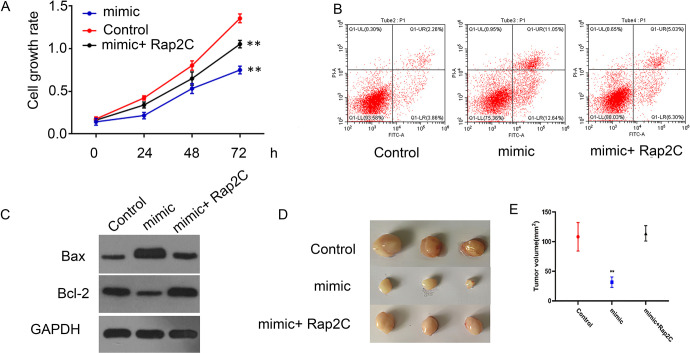
Subsequently, we overexpressed Rap2C expression after transfection with miRNA-195 mimic to examine the effect on SCLC cells. Overexpression of Rap2C significantly reversed the proliferation inhibition (Figure 5A) and apoptosis (Figure 5B) induced by miRNA-195 mimic. In addition, Western blot detection of Bax and Bcl-2 protein level in SCLC cells, which displayed that Rap2C overexpression significantly reversed up-regulation of Bax protein and down-regulation of Bcl-2 induced by miRNA-195 mimic (Figure 5C). To explore whether miRNA-195 affected tumor growth of lung cancer by Rap2C in vivo, the experiment was produced by subcutaneous injection of nude mice with NCI-H520 cells. Compared with the control, miRNA-195 significantly inhibited lung cancer growth, while overexpression of Rap2C overturned the tumor suppression effect caused by miRNA-195 (Figure 5D).
Figure 5.
miRNA-195 regulates the proliferation and apoptosis of SCLC cells through Rap2C. Empty vector and Rap2C were transfected into miRNA-195 overexpressed SCLC cells. A: CCK-8 assay was used to detect cell viability. B: Apoptotic rates were analyzed by using flow cytometry. C: Western blot analysis of Bax, Bcl-2 expression. D: MiRNA-195-overexpressed SCLC cells transfected with Rap2C or control vector were subcutaneously injected in nude mice. Tumor volume was evaluated.
miRNA-195/Rap2C Interferes With SCLC via Regulating MAPK Signaling Pathway
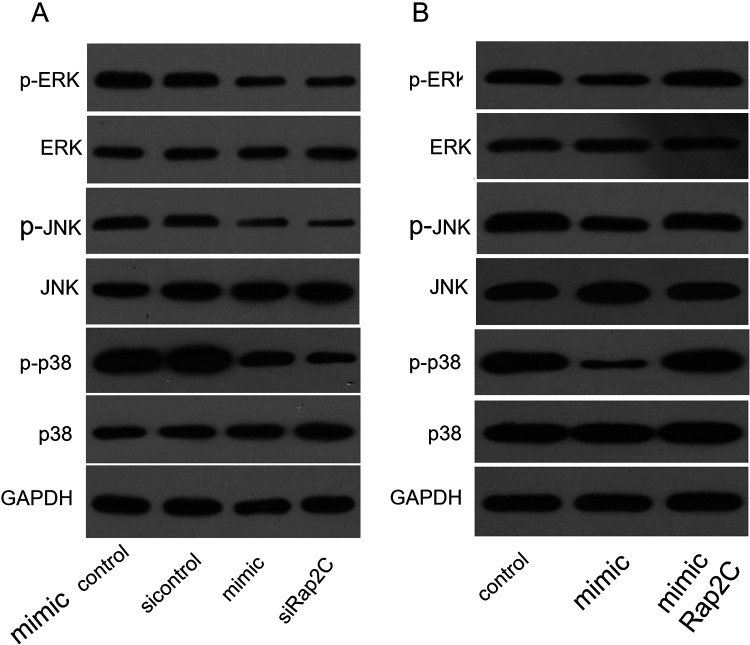
MAPK signaling pathway is very important for cell proliferation and apoptosis. It has been testified that MAPK pathway could affect the biological process of various tumors. Rap2C is a Ras-related protein that could modulate the MAPK signaling pathway. Therefore, we examined the effects of miRNA-195 and Rap2C on MAPK signaling pathway. The results showed that miRNA-195 mimic and siRap2C significantly down-regulated the activation of MAPK signaling pathway (Figure 6A), while that Rap2C overexpression significantly reversed inactivation of MAPK signaling pathway induced by miRNA-195 (Figure 6B).
Figure 6.
miRNA-195 / Rap2C interferes with SCLC via the MAPK signaling pathway. A: Western Blot analysis of p-ERK, ERK, p-JNK, JNK, p-p38, p38 expression in SCLC cells transfected with miRNA-195 mimic control or miRNA-195 mimic or Rap2C siControl or siRap2C. B: Western Blot analysis of p-ERK, ERK, p-JNK, JNK, p-p38, p38 expression in SCLC cells transfected with miRNA-195 mimic and Rap2C.
Discussion
As a malignant tumor with the highest morbidity and mortality in the world, lung cancer severely affects human health. Due to the limitations of current diagnosis and treatment, the prognosis of lung cancer are still poor. Therefore, studying the mechanism of occurrence and development of lung cancer and developing new drug targets has become a major scientific problem to be solved urgently.15,16
A growing amount of research have shown that miRNA play an important role in the occurrence and development of tumors. In this stdy, we found that miRNA-195 expression was decreased in lung cancer, and overexpression of miRNA-195 could inhibit SCLC cell proliferation and accelerated apoptosis. Therefore, miRNA-195 may be a potential target for SCLC tumorigenesis.
Rap2 is a Ras-related protein, which belongs to the GTP-binding protein family. Studies have pointed out that Rap2A, 2B and 2C are all important for the initiation and development of tumors, including osteosarcoma,10 colorectal cancer,11 breast cancer.12 In our study, we discovered that Rap2C was highly expressed in SCLC tissues and cells. Rap2C was predicated as a downstream target gene of miRNA-195. Moreover, miRNA-195 could inhibit the proliferation and promote the apoptosis of small-cell lung cancer cells by directly binding to the upstream region of Rap2C. Furthermore, Knockdown of Rap2C could subdue the SCLC cell proliferation and expedited apoptosis, suggesting that Rap2C may be likely to modulate the pathogenetic process of SCLC.
We further explored the underlying mechanism of miRNA-195/Rap2C in modulating SCLC. Ras-MAPK signaling pathway has been demonstrated to play an important role in NSCLC.13 Rap2C is a Ras-related protein, which may regulate the MAPK signaling pathway. Therefore, MAPK signaling pathway was detected and the results showed that miRNA-195 could inhibit MAPK signaling pathway activation through down-regulatig Rap2C.
This study proposed for the first time the regulatory effect of miRNA-195/Rap2C/MAPK axis on SCLC, which has a guiding effect on the development of drugs to treat lung cancer. At present, there are few types of lung cancer drugs, and the treatment effect is poor. This study suggests that miRNA-195 and Rap2C may be potential drug targets for the treatment of SCLC. MiRNA195 expression can be artificially increased in vivo to inhibit the expression level of Rap2C, and the enzyme activity inhibitor of Rap2C can also be designed to achieve the purpose of treating SCLC.
In addition, because of the difficulty in the diagnosis of SCLC, there is still a lack of relatively simple and convenient diagnostic indicators. This work reminds that the down-regulation of miRNA-195 in serum may serve as a biomarker for diagnosis and prediction of the prognosis of lung cancer patients.17,18 Additionally, Rap2C overexpression could activate the serum response element (SRE),19 which in turn evokes the up-regulation of a series of genes. Based on this, changes in serum level of SRE downstream target genes can be screened to find biomarkers that can be used to guide the diagnosis and prognosis of SCLC.
Abbreviations
- miRNA
MicroRNA
- NSCLC
non-small cell lung cancer
- SCLC
small cell lung cancer
- SRE
serum response element
Footnotes
Authors’ Note: The experiment was often approved by the Ethics Committee of the Changzhou No. 2 People’s Hospital (NO.ERC-20233), and all patients participating in this study provided written informed consent in accordance with the “Helsinki Declaration.”
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Ke Zhang  https://orcid.org/0000-0002-7239-928X
https://orcid.org/0000-0002-7239-928X
References
- 1. Bunn PA., Jr Karnofsky award 2016: a lung cancer journey, 1973 to 2016. J Clin Oncol. 2017;35(2):243–252. [DOI] [PubMed] [Google Scholar]
- 2. Hirsch FR, Scagliotti GV, Mulshine JL, et al. Lung cancer: current therapies and new targeted treatments. Lancet. 2017;389(10066):299–311. [DOI] [PubMed] [Google Scholar]
- 3. Huang C, Liu S, Wang H, et al. LncRNA PVT1 overexpression is a poor prognostic biomarker and regulates migration and invasion in small cell lung cancer. Am J Transl Res. 2016;8(11):5025–5034. [PMC free article] [PubMed] [Google Scholar]
- 4. Howlader N, Noone A, Krapcho M. Seer cancer statistics review (CSR) 1975-2016. Bethesda: MD: National Cancer Institute, 2019. [M].
- 5. Rudin CM, Giaccone G, Ismaila N. Treatment of small-cell lung cancer: American Society of Clinical Oncology endorsement of the American College of Chest Physicians guideline. J Oncol Prac. 2016;12(1): 83–86. [DOI] [PubMed] [Google Scholar]
- 6. Von pawel J, Jotte R, Spigel DR, et al. Randomized phase III trial of amrubicin versus topotecan as second-line treatment for patients with small-cell lung cancer. J Clin Oncol. 2014;32(35):4012–4019. [DOI] [PubMed] [Google Scholar]
- 7. Chi Y, Zhou D. MicroRNAs in colorectal carcinoma—from pathogenesis to therapy. J Exp Clin Cancer Res. 2016;35:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Su K, Zhang T, Wang Y, Hao G. Retraction note: diagnostic and prognostic value of plasma microrna-195 in patients with non-small cell lung cancer. World J Surg Oncol. 2019;17(1):119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yongchun Z, Linwei T, Xicai W, et al. MicroRNA-195 inhibits non-small cell lung cancer cell proliferation, migration and invasion by targeting MYB. Cancer Lett. 2014;347(1):65–74. [DOI] [PubMed] [Google Scholar]
- 10. Wu J, Du W, Wang X, et al. Ras-related protein Rap2c promotes the migration and invasion of human osteosarcoma cells. Oncol Lett. 2018;15(4): 5352–5358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shen Z, Zhou R, Liu C, et al. MicroRNA-105 is involved in TNF-α-related tumor microenvironment enhanced colorectal cancer progression. Cell Death Dis. 2017;8(12):3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhu X, Qiu J, Zhang T, et al. MicroRNA-188-5p promotes apoptosis and inhibits cell proliferation of breast cancer cells via the MAPK signaling pathway by targeting Rap2c. J Cell Physiol. 2020;235(3): 2389–2402. [DOI] [PubMed] [Google Scholar]
- 13. Wang J, Li J, Cao N, Li Z, Han J, Li L. Resveratrol, an activator of SIRT1, induces protective autophagy in non-small-cell lung cancer via inhibiting Akt/mTOR and activating p38-MAPK. Onco Targets Ther. 2018;11:7777–7786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Silver N, Best S, Jiang J, Thein SL. Selection of housekeeping genes for gene expression studies in human reticulocytes using real-time PCR. BMC Mol Biol. 2006;7:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bernhardt EB, Jalal SI. Small cell lung cancer. Cancer Treat Res. 2016;170:301–322. [DOI] [PubMed] [Google Scholar]
- 16. Kalemkerian GP, Schneider BJ. Advances in small cell lung cancer. Hematol Oncol Clin North Am. 2017;31(1):143–156. [DOI] [PubMed] [Google Scholar]
- 17. Fan T, Mao Y, Sun Q, et al. Branched rolling circle amplification method for measuring serum circulating microRNA levels for early breast cancer detection. Cancer Sci. 2018;109(9):2897–2906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chen X, Wang A. Clinical significance of miR-195 in hepatocellular carcinoma and its biological function in tumor progression. Onco Targets Ther. 2019;12:527–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Guo Z, Yuan J, Tang W, et al. Cloning and characterization of the human gene RAP2C, a novel member of Ras family, which activates transcriptional activities of SRE. Mol Biol Rep. 2007;34(3):137–144. [DOI] [PubMed] [Google Scholar]