Abstract
Multidisciplinary (MD) care is essential in the management of patients with spondyloarthritis (SpA) and is one of the main pillars of disease management and patient care. However, evidence supporting the effectiveness and benefits of this strategy in SpA is scarce. In this review we discuss the three types of MD care models: (i) combined clinics (MD units), including ‘face to face’, ‘parallel’ and ‘circuit approach’ clinics; (ii) MD team meetings; (iii) group consultations. The most frequently used model in SpA studies has been the ‘parallel’ combined clinic and usually encompasses a rheumatologist and another specialist, most commonly a dermatologist or a gastroenterologist, that work in tandem according to predefined referral criteria and treatment algorithms. MD working seems to improve the care of patients with SpA by a better identification and diagnosis of the disease, an earlier and more comprehensive treatment approach, and better outcomes for patients in terms of disease activity, physical function, quality of life and patient satisfaction. Nevertheless, challenges remain. Data on effectiveness and feasibility are scarce and are mostly derived from studies with design issues and often without a unidisciplinary care comparator arm. Although patient centricity is one of the core values of patient care and MD setting in SpA, the patient often does not play an active role in most of the MD settings studied or in common clinical practice. Further efforts should be made so that MD care reflects patients’ expectations and needs. Overcoming these limits will help to implement successfully SpA MD care in daily clinical practice and subsequently to achieve a higher quality of care for our patients.
Keywords: combined clinics, multidisciplinary team meetings, multidisciplinary working, spondyloarthritis
Introduction
A multidisciplinary (MD) approach involves drawing appropriately from multiple disciplines to explore problems outside normal boundaries and reach solutions based on a new understanding of complex situations. An MD team (MDT) can be defined as a group of health and social care professionals with specialised skills and expertise working in a coordinated way.1
Spondyloarthritis (SpA) is a heterogeneous group of interrelated inflammatory conditions that, according to the latest Assessment of SpondyloArthritis Society criteria,2 can be classified in two main entities: axial and peripheral. This group encompasses several disorders, that is, ankylosing spondylitis (AS), reactive arthritis, psoriatic arthritis (PsA), inflammatory bowel disease-related arthritis (IBD-SpA) and undifferentiated SpA, with overlapping clinical manifestations and shared genetic markers. Managing these complex conditions is challenging and often requires a coordinated approach between rheumatologists and other specialists such as dermatologists, gastroenterologists, ophthalmologists, cardiologists, radiologists, physiotherapists, etc. The importance of MD input in SpA has been emphasised by including it as an overarching principle of both international3–6 and national7–10 treatment recommendations. However, despite the increasing recognition and wide endorsement of such approaches, there is a paucity of evidence supporting the effectiveness and benefits of this strategy in SpA studies, and results have been conflicting.11–13 Even less is reported about the feasibility and logistics of implementing MD working in a clinical practice setting.
The objective of this article is to give a comprehensive overview of MD working in the management of SpA through a literature review. More specifically, we aim to: (i) describe the characteristics of MD care models; (ii) describe their effectiveness and feasibility in comparison with the unidisciplinary approach; (iii) provide guidance on how to quantify and audit the benefits of MD working; (iv) identify the limits of the current canon of knowledge and clinical practice challenges in order to identify areas for further research.
Characteristics of MD working in SpA studies
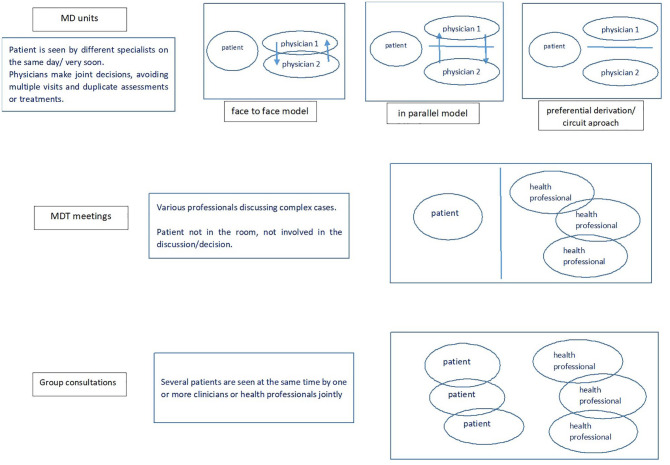
There are several models of MD working currently being used in the management of patients with SpA (Figure 1). The most frequently reported model is the MD care unit, which usually involves a combined clinic where the patient is seen by several team members in a coordinated way. The diagnosis and therapeutic management are usually established according to a predefined algorithm. The team composition and workflow process may vary from unit to unit, but there are essentially three types of combined clinics. They were summarised by Queiro et al.14 in a work based on a comprehensive evaluation of all the PsA MD care models across Spain. However, these models are not specific for PsA or for Spain, since they are widely used in other SpA and other rheumatic disease studies and internationally.13,15–17
Figure 1.
Types of multidisciplinary care models (original).
There are three main MD models currently used in the management of patients with spondyloarthritis: MD care units (combined clinics), MDT meetings and group consultations. This figure depicts the advantages and disadvantages, as well as how each model is organised and how patients and health professionals interact within the model. MD, multidisciplinary; MDT, multidisciplinary team.
The first and most common model of combined clinics used in SpA studies is the ‘face-to-face’ model.18–26 It is designed as a concurrent, synchronous care model: the patient is seen on the same day, usually in the same room by all members of the team, as one clinical experience. In the ‘parallel’ model, team members see the patient separately but in parallel: they stagger their evaluations in that same day visit, and then decide on the optimal management approach. The third model is the ‘preferential derivation’ or ‘circuit approach’, which is more similar to habitual clinical practice in the sense that every team member has their own usual agenda, but there are predefined referral criteria and the possibility of referring the patient to the other team member(s) to be seen in a very early timeframe.27–32
Another type of MD working is represented by the MDT meetings, which can be defined as regular discussions of patients, comprising various professionals including physicians from different specialties, nurse specialists, pharmacists and other health professionals (e.g. occupational therapists, physiotherapists, psychologists, etc). Although data from studies on MDT meetings in SpA are very scarce,33 these meetings are reported to be used in rheumatology in general34,35 and more often in other specialties such as oncology.36 In addition, the use of telemedicine has improved the use of MDT meetings in diverse clinical settings, with benefits for all key healthcare elements, that is, for patients, healthcare professionals, hospital and the state, in terms of quality of care, costs, accessibility, organisation and acceptability.37 Telemedicine is particularly useful to link specialist teams for whom distance is a barrier, or to create closer relationships between primary, secondary and tertiary healthcare and patients. It has been applied in various specialties, especially in oncology and dermatology, but also in cardiology, neurology, nutritional medicine, psychology and psychiatry, pharmacology and orthopaedics.37 In rheumatology, the video-conferencing MDT model has been successfully implemented in rare autoimmune diseases: the Eastern Network for Rare Autoimmune Disease (ENRAD) meetings developed at Addenbrooke’s Hospital in Cambridge, UK has been awarded the Best Practice Award 2018 by the British Society of Rheumatology.35 The ENRAD scheme provides dial-in conferences for health professionals to be able to discuss cases centrally with various relevant specialists whilst still treating patients locally, and subsequently improving patient care (diagnosis, treatment time) and costs (time, money, resources).
By including diverse health professionals, MDT meetings have the advantage of providing a holistic service, which is essential in heterogeneous diseases such as SpA where MD input is needed. The multiperspective view on the patient and disease makes these meetings suitable for discussing complex cases and diagnostic or treatment challenges. However, the patients are most often not present at the meeting, and treatment recommendations may be made without considering patient preferences and personal needs, which is a major limitation of this MD model. Our experience of MDT meetings running in our own institution illustrates well this type of MD working.33 In 2016, a monthly PsA and 2-monthly IBD-SpA MDT and hepatology-SpA MDT were established. They are attended by consultants and fellows (with a special interest in psoriatic disease, SpA and IBD), rheumatology trainees, specialist and research nurses and a biologic pharmacist. The format consists of 1–1.5 h’ of discussions of a list of patients (6 up to 19), and usually they are challenging cases in terms of diagnosis or treatment. History, laboratory results and imaging are reviewed using the electronic patient record and MDT recommendations are recorded and communicated to the patient and general practitioner where relevant.33 Figure 2 depicts a case collection from our departmental MDT meetings where patients with complex SpA have benefited from MD working. The MDT input has contributed to a better management and better outcome of the patient’s disease in each case, illustrating well the advantage of implementing these meetings in clinical practice. MDT meetings were established not only to align SpA management to existing guidelines,3–6 but also for clinical service and safety improvement, service commissioning and research, that is, academic research study inception and execution, and recruitment to clinical trials.
Figure 2.
Collection of cases of patients with spondyloarthritis that have benefited from an MDT input. All cases were discussed in one of the MDT meetings taking place in the rheumatology department at Addenbrooke’s Hospital, Cambridge, UK.
DMARD, disease-modifying antirheumatic drug; HBV, hepatitis B virus; IL, interleukin; MDT, multidisciplinary team; PsA, psoriatic arthritis; TNF, tumour necrosis factor.
Although we did not find any studies reporting results on group consultations on patients with SpA, this type of MD working might be a beneficial MD care option, since it is suitable for both acute and chronic settings, where both patients with chronic stable and active newly diagnosed or flaring SpA can be seen together. ‘Group consultations’ is an overarching term to describe care models in which several patients are seen by one or more clinicians or health professionals jointly.17 Besides the standard aspects of the usual MD care models, group consultations also provide the advantage of peer support, combining features of health professional–patient interaction and patient support groups. Other claimed benefits include better patient outcomes and service access, reduced costs and time, and greater system flexibility in dealing with patients with different care needs in a timely manner.17 Group consultations have been successfully used in chronic diseases like diabetes, where improvements in costs, physiological outcomes, and patient and clinician satisfaction have been demonstrated.38,39 They could be implemented in rheumatological conditions, including SpA.
In terms of team composition, the majority of studies of MD working included MDTs usually consisting of two physicians, one of whom is a rheumatologist and the other a dermatologist,22–26,29,31 gastroenterologist18–21 or ophthalmologist.27 Only a few studies included teams comprising diverse disciplines, such as cardiologists, endocrinologists, orthopaedics, specialist nurses, psychologists, medical assistants, physiotherapists, occupational therapists and volunteer patients.20,28–30,32 The composition of an MDT should differ to suit the needs of the condition being managed. In a systematic literature review of MD healthcare working in rheumatology,12 Crossland et al. summarised the different professionals in rheumatological MDTs in published studies and national health guidelines. The most frequently cited and recommended professionals were physiotherapists, rheumatologists and occupational therapists, followed by social workers, nurses and nurse specialists, and psychologists. However, as the reviewed studies were mainly of patients with rheumatoid arthritis (RA), ‘inflammatory arthritis’ and fibromyalgia, the generalisability of results to SpA services remains unclear, particularly in terms of the need to address extra-articular manifestations and comorbidities.
Outcomes and outcome measures evaluated in studies on MD working in SpA
The outcomes assessed in studies on MD care in SpA varied according to their main objective. Most of these studies were designed to identify new cases or calculate prevalence of different types of SpA amongst the referred patients. Therefore, the most frequently evaluated outcome was early diagnosis of SpA,27 axial SpA,30 enteropathic SpA20–22 or PsA.23,25,26,31 Diagnostic delay,21,30 reclassification of diagnosis24,26,32 and descriptive characteristics of cases21,31 were also reported. Disease-related outcomes were frequently reported, such as disease activity of arthritis, skin psoriasis or IBD,18,19,25,28,29 physical function,18,19,28,29 comorbidities, disease complications and adverse events.24 Some studies assessed therapeutic adjustment, especially when starting a synthetic or biologic disease-modifying antirheumatic drug.23,24,26,32 Patient-reported outcomes (PROs) have frequently been reported in studies of MD working in SpA, in particular quality of life (QoL)18,19,25,29 and patient global assessment,18,19,25 and more rarely, global wellness,19 activity limitations and participation restrictions,29 reinforcing patient centricity.
Although considered by some to be an essential metric of service quality, patient satisfaction with the MD care was very poorly addressed or measured in SpA studies.23 In terms of feasibility, there is also a significant lack of data on health service and human resources utilisation, administrative burden, time and associated economic cost.28 The main outcomes assessed in studies on MD working in SpA, matched with their outcome measures, are depicted in Table 1.
Table 1.
Outcomes and outcome measures evaluated in studies of multispecialty working in spondyloarthritis.
| Outcomes | Outcome measures | |
|---|---|---|
| Diagnosis | Early diagnosis | Assessment of SpondyloArthritis Society criteria; New York criteria The Classification Criteria for Psoriatic Arthritis; Moll and Wright criteria Rheumatologist’s/dermatologist’s (clinical) judgement Not defined (‘standard diagnostic criteria for inflammatory bowel diseases and rheumatic diseases’) |
| Diagnosis delay | The total lag time from joint symptom onset to the first rheumatological assessment Diagnostic delay: the time interval between the onset of symptoms and the correct diagnosis being made Physician-related diagnostic delay: the time interval between the initial visit to a physician and the time of diagnosis |
|
| Reclassification of diagnosis | Number of patients, n (%) | |
| Disease related | Disease activity | Musculoskeletal: • The Bath Ankylosing Spondylitis Disease Activity Index • The Ankylosing Spondylitis Disease Activity Score – C-reactive protein • Disease Activity in Psoriatic Arthritis Gastroenterology: • Crohn’s disease activity index • the partial Mayo Psoriasis: • Psoriasis Area Severity Index |
| Physical function | Bath Ankylosing Spondylitis Metrology Index Bath Ankylosing Spondylitis Functional Index Bath Ankylosing Spondylitis Patient Global Score Health Assessment Questionnaire |
|
| Comorbidities | Prevalence of diabetes, hypertension, hyperlipidaemia and current/past smoking status | |
| Complications during follow up/adverse events | Prevalence of infection and adverse medication effects (i.e. elevated liver function test, headache) | |
| Treatment | Therapeutic adjustment | Number of patients, n (%) having had their treatment changed |
| Patient reported outcomes | Quality of life | Inflammatory Bowel Disease Questionnaire Short Form (SF36) Dermatology Life Quality Index Psoriatic Arthritis Impact of Disease |
| Global wellness | • Health Assessment Questionnaire • Short Form (SF36) • Patient Global Assessment |
|
| Patient global assessment | Patient Global Assessment | |
| Activity limitations and participation restrictions | The Canadian Occupational Performance Measure | |
| Patient satisfaction | Satisfaction questionnaire (developed by the multidisciplinary team) | |
| Feasibility/costs | Health service utilisation | Questionnaire developed by the Stanford University School of Medicine with four indicators (i.e. outpatient visits, emergency visits, hospitalisations and hospitalisation days) |
Effectiveness of MD care compared with unidisciplinary care in the management of SpA
In terms of effectiveness, data seem to show that MD working improves the quality of care in SpA by a better identification and diagnosis,20–27 an earlier and more comprehensive treatment approach23,24,32 and better outcomes for patients in terms of disease activity, physical function or QoL,18,19,25,28,29,31 as well as healthcare service utilization and patient satisfaction.23
Early diagnosis, delayed diagnosis and diagnosis reclassification
In a multicentre study, Juanola et al.27 showed that a large proportion of patients with clinically significant anterior uveitis (n = 798) had an undiagnosed axial (50.2%) and peripheral SpA (17.5%). In patients with IBD, combined gastroenterology–rheumatology clinics helped to identify patients with enteropathic SpA and other musculoskeletal conditions.20,21 In an Italian MD model21 including 1495 patients with IBD seen in the gastroenterology outpatient department, 269 had musculoskeletal pain and were referred to the combined clinic. Of these, 136 patients (50.5%) were diagnosed with enteropathic SpA. In another Italian MD model20 in which patients were identified according to predefined ‘red flag’ issues, a new diagnosis of enteropathic SpA was made in 9/44 (21%) patients with IBD. In dermatology–rheumatology MD care units,23–26,31,32 the percentage of undiagnosed PsA among patients with psoriasis and musculoskeletal symptoms usually referred from dermatology to these combined clinics varied significantly from 20.6%31 up to 71.5%.25 In a study by Zabotti et al.,22 patients with early RA or PsA were assessed in combined dermatology– rheumatology clinics and a quarter of the subjects initially diagnosed as seronegative early RA were reclassified as PsA.
Conigliaro et al.21 found a mean diagnostic delay of 5.2 years of enteropathic SpA in patients with IBD assessed in a gastroenterology–rheumatology combined clinic. When compared with retrospective results from a similar cohort of patients evaluated prior to the establishment of the combined clinic, the authors found that the diagnostic delay was reduced in the group of patients benefiting from an MD assessment.
Moreover, Li et al.30 also demonstrated that diagnostic delay of axial SpA was shorter in patients being evaluated in an MD clinic compared with standard care. Following MD clinic introduction, the median physician diagnostic delay defined as the time interval between the initial visit to a physician and the time of axial SpA diagnosis decreased from 13 months to 1 month (p = 0.026).
The reclassification of diagnosis is reported in some studies, with a frequency of 19%32 to 47%26 for rheumatological conditions.
The results of these studies begin to evidence the virtues of MD working, especially of combined clinics and particularly in terms of identifying undiagnosed cases of SpA. However, only one study30 included a control group as a comparison with standard care.
Disease activity and disability
Disease activity and physical function scores also improved in studies evaluating the role of MD care in patients with SpA.18,19,25,28,29
In studies of patients with IBD and enteropathic SpA managed in combined gastroenterology–rheumatology clinics, musculoskeletal disease activity as assessed by the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) and Ankylosing Spondylitis Disease Activity Score – C-reactive protein, gastrointestinal disease activity as assessed by the partial MAYO and Crohn’s Disease Activity Index,18,19 physical function scores assessed by the Bath Ankylosing Spondylitis Functional Index (BASFI)18,19 and Health Assessment Questionnaire18 significantly improved and were maintained compared with baseline during 6-, 12- and 24-month follow-up visits.18,19 In the DErmo-Rheumatologic combined clinic of 116 patients with PsA,25 both skin activity and articular disease activity significantly improved from baseline to 48 weeks. Whilst these three studies did not include a control group of standard care, two studies of MD working in the management of patients with AS included a control group of patients assigned to usual care.28,29 Liang et al.28 demonstrated that after 6 months, the intervention group receiving intensive transitional care by a nurse-led MDT exhibited significant score improvements in disease activity (BASDAI: p < 0.001) and physical function (BASFI: p = 0.022) compared with the control group receiving routine care. The nurse-led MDT transitional care included disease assessment and treatment adjustment, as well as health education, psychological support, rehabilitation guidance and access to online communication, all coordinated by the nurses.
Kjeken et al.29 also found significant overall improvement in favour of the MD intervention group compared with standard care with regards to disease activity scores [BASDAI: mean difference over the 1-year period 10.0; 95% confidence interval (CI): –3.7; –16.3], well-being scores (–7.3; 95% CI: –1.0; –14.7) and some of the Short Form-36 (SF36) subdomain scores (mean differences ranging from 5.8 for pain to 10.7 for role physical). MD intervention consisted of a 3-week rehabilitation programme conducted by an MDT including a physician, a nurse, a physiotherapist and an occupational therapist.
QoL
QoL has also been shown to improve in patients with enteropathic SpA,18,19 PsA25 and AS28,29 through an MD approach, although none included a standard care comparator group. A significant improvement in global QoL scores (SF-36 questionnaire physical and mental health components) were demonstrated in these studies.18,19,25,28,29 In addition, disease-specific QoL scores, such as the gastrointestinal specific QoL score,18 skin psoriasis-specific Dermatology Life Quality Index and PsA-specific PsA Impact of Disease31 followed the same pattern.
In the two studies that included a control group,28,29 results were relatively similar. Patients with AS assigned to the intervention group of a nurse-led MDT care reported significant improvement in all components of the SF-36, that is, physical functioning, mental health, general health perceptions, vitality, social role functioning, physical role functioning, emotional role functioning and bodily pain.28 Likewise, in the randomised control trial of patients with AS29 there was a significant overall intervention effect in favour of the rehabilitation group in some of the SF-36 components, for example, role physical (overall treatment effect mean 7.0; 95% CI: 0.03, 13.9, p = 0.05), role mental (10.7; 95% CI: 3.2, 18.1, p = 0.006), bodily pain (5.8; 95% CI: 0.5, 11.0, p = 0.03) and social functioning (7.0; 95% CI: 0.03, 13.9, p = 0.05).
Limitations of activity and participation were assessed in one study,29 but only at baseline as part of the therapeutic plan decision and not as an outcome.
Comorbidities and adverse events
Comorbidities are rarely assessed as an outcome in studies of MD care in patients with SpA. However, results from a cohort of patients with psoriasis and/or PsA evaluated in a combined clinic showed that the prevalence of concomitant conditions was high in these patients: 45% had hypertension, 46% hyperlipidaemia, 19% diabetes and 36% past/current smoking.24 The same study showed that during the 6-year follow-up period, the incidence of therapy-related adverse medical events, including adverse drug reactions and infections was 3% in the psoriasis population and 6% in the PsA population.24
Therapeutic adjustment
In terms of treatment change, findings from studies of patients with PsA attending MD care units showed that it leads not only to an earlier diagnosis, but that these patients are also more likely to be initiated on a new drug or to have their medication escalated.23,24,32 In a Spanish dermatology–rheumatology combined clinic,23 out of the 112 patients with psoriasis and PsA assessed, the number of patients with therapeutic adjustment increased, and 55.4% and 42% of patients changed their topic and systemic treatments, respectively. Similar results were reported by another dermatology–rheumatology combined clinic, the Center for Skin and Related Musculoskeletal Diseases (SARM) in Boston, USA, in which 268 patients with psoriasis and PsA were evaluated over a 6-year period.24 Patients were more likely to receive a systemic medication after the evaluation in SARM compared with before (25% versus 15%, respectively; odds ratio of 5.1). Patients were also more likely to be treated with a biologic agent after the evaluation in SARM compared with before (37% versus 16%, respectively).
In the ‘pSORRIDI’ experience, 75 patients with psoriasis (of whom 30 had PsA) were assessed by an MDT consisting of dermatology, rheumatology, cardiology and endocrinology specialists in order to determine the prevalence and appropriateness of therapeutic approach of comorbidities.32 An adjustment in treatment of comorbid conditions was needed in 76.2% patients undergoing rheumatology evaluations, 61.1% and 33.3% for endocrinology and cardiology assessments, respectively.
Patient satisfaction
All MD settings, including those designed for SpA, should be patient centred and aim to improve the quality of care for patients.40 However, patients’ opinion and satisfaction were rarely assessed in these studies. In a combined dermatology–rheumatology clinic in which 112 patients with psoriasis and PsA were evaluated,23 the level of satisfaction was very high (93% reported with MD care compared with their previous care) and 95.4% reported the quality of the information given in the MD care unit as good/very good. Moreover, all patients considered that their disease was better controlled in this MD care unit. More research is needed on patient satisfaction and domains of MD care important to patients.
Feasibility of MD compared with unidisciplinary care in the management of SpA
Although particularly important, data on the feasibility of MD care (i.e. cost, time, administrative burden, utilisation of health care services) are rather scarce. In one study on MD working in patients with AS, Liang et al.28 showed that a nurse-led MD care programme resulted not only in better disease outcomes, but also in a better health service utilisation. After 6 months, the experimental group exhibited significant score improvement in hospitalisations compared with the control group (p = 0.014).
More information on feasibility items is crucial in evaluating MD models, since it may help to identify difficulties in setting up an MD care unit and justify the associated additional economic costs and human resources. Queiro et al.41 recently reported results from the ongoing NEXUS 2.0 project designed to analyse the situation of PsA MD care models already or in the process of being embedded across Spain. The authors have shown that the level of implementation of quality standards was extremely low amongst all 50 PsA MD centres in Spain, ranging from 2% to 28%. Barriers to implementation included: administration and bureaucracy; poor communication between departments; demotivated health professionals; limited training and lack of a culture of involvement of nurses; lack of human resources in general; lack of physical space; computer systems ill-prepared for efficient data collection and analysis; insufficient time; lack of effort to homogenise the plan of action; difficult evaluation of costs, etc. A better understanding of these limitations will further promote a more cost-effective use of MD care.
Audit and standards of care of MD working in SpA
It is important to quantify, through audit, the benefits of MD versus unidisciplinary approaches in terms of effectiveness and feasibility. Being able to justify the additional team members’ time and associated economic cost is important to commission services. Unfortunately, the lack of standard care as a control group in the published studies makes this challenging. There is a paucity of information on how to conduct audits of MDTs in SpA, but some initiatives have been undertaken.42–44 An Italian Delphi consensus identified a set of overarching principles to guide the implementation of an MD approach for the management of SpA-related immune-mediated inflammatory diseases (IMIDs), including SpA, psoriasis, PsA, Crohn’s disease, ulcerative colitis and uveitis.42 The goals of such MD approaches were to increase the diagnosis of concomitant IMIDs, improve decision-making, increase patient satisfaction and increase patient adherence with treatment. Several advantages were described including early referral and diagnosis, early recognition of other IMIDs and optimization of treatment to improve patient quality of care. Finally, the other statements covered aspects such as team composition and tools needed for diagnosis and follow up. These principles may be further used as a foundation for developing more specific recommendations or for establishing MD care models.
There is a growing interest in defining such standards of care in PsA.43,44 A Spanish scientific committee of rheumatology and dermatology experts has developed a consensus statement on considerations when establishing a PsA MD unit.43 Several statements and practices were grouped into 11 domains: justification; objectives; premises; source of patients; referral criteria; procedures; length of follow up; administration; coordination; track-record; satisfaction.
A national project in Spain aiming to promote and standardise MD care in patients with PsA (the NEXUS project) generated a list of 25 standards of care and 24 quality indicators for the MD care.44 Standards of care included: appropriate physical infrastructure and technical equipment; access to nursing care; access to laboratory and imaging; access to other health professionals and to evidence-based treatments; the development of care plans. The structure and process quality indicators reaching a final consensus included: defining MD model objectives and referral criteria; establishing a team composition and responsibilities; clinic workflow scheme and data collection; medical reports.44
Similar initiatives have been conducted for IBD MD care units,45,46 but focused on collaboration between surgeons, endoscopists, radiologists and nutritionists, without considering concomitant rheumatological comorbidities.
Challenges of MD in SpA and research agenda
The importance of MD working in SpA is broadly recognised and the number of related studies reporting advantages over unidisciplinary care is increasing. However, areas of low knowledge remain and need further study. We therefore propose below a future research agenda (Table 2).
Table 2.
Research agenda for multidisciplinary care in spondyloarthritis.
| Theme | Research question |
|---|---|
| MD models | Defining the most adequate type of MD model in SpA: MD care units, MD team meetings or group consultations? Defining MD team composition: how many?; which health professionals? Defining target population: which stage of disease? |
| Outcomes and outcome measures | Identifying outcomes that are important for patients. Identifying outcomes that are important for health professionals. Identifying outcomes that are important for hospitals/departments. Identifying outcome measures and instruments that are adequate for SpA. |
| Effectiveness | Comparing effectiveness of MD care to unidisciplinary care. Comparing effectiveness between MD care models. |
| Feasibility | Comparing feasibility of MD care to unidisciplinary care. Comparing feasibility between MD care models. Identifying barriers in implementing MD models in clinical practice. |
| Audit | Defining items to be included in an audit quantifying effectiveness and feasibility of MD care in comparison with the unidisciplinary working. |
| Patient involvement | Defining the role of the patient in an MD setting. Identifying patient needs and expectations with regards to MD care. Identifying outcomes that are important for patients. |
MD, multidisciplinary; SpA, spondyloarthritis.
One of the most important areas of unmet need is the lack of patient involvement in research or service inception. SpA guidelines and recommendations invariably include patient input these days.3–6,47,48 Although QoL and other PROs are used as outcomes in studies on MD in SpA, direct feedback from patients is rare. Among all the studies on MD care in patients with SpA that we have identified through this review only one study included patient satisfaction as an outcome.23 Likewise, only one of the initiatives of recommendations for MD working in SpA has included patients’ input in the process.44 Whilst all MD care models described above claim to be patient centred, the patient does not play an active role in any of the models. Further work should be undertaken to improve this limitation, as the current studies might not capture patients’ needs and expectations.49–51
In a similar manner, further research should also be performed regarding physicians’ and health professionals’ expectations and satisfaction, which has been identified as a barrier to implementing standards of quality in PsA MD care models.41
Although effectiveness is one of the aspects most frequently addressed in studies of MD working in SpA, the outcomes used to assess it vary largely across studies. They usually cover domains such as disease activity, disability or QoL, and rarely consider aspects important to patients. In addition, their corresponding outcome measures are also different among studies and may not be always adequate in the context of such a heterogeneous disease like SpA. For example, most of the studies on MD in PsA have used disease activity scores such as BASDAI or Disease Activity in PsA, which cover only some of the disease aspects, omitting others of equal importance, for example, skin psoriasis, enthesitis or dactylitis. Another limit of evaluating the effectiveness of the MDT approach in patients with SpA is that most of the studies did not use a comparison group, thus the superiority versus the unidisciplinary care cannot be clearly proven, only assumed. Furthermore, it is important not only to compare MD with standard care, but also to compare between different types and variations of MD models, so that an MD setting would be more cost-, time- and resource-effective.
Another area that needs further research is the feasibility of MD working in SpA, since data on the subject are very scarce. Further information is needed not only regarding costs and time, but also some other practical aspects of implementation, such as: utilisation of human resources (team member availability and training); administrative burden; the impact on healthcare service utilisation. Moreover, the role of the local or national organisation limits should also be of interest, since the applicability of MD models to different healthcare systems may strongly depend on this.
Little is known about the SpA population mostly likely to benefit from MD care, for example, the stage of disease at first diagnosis, those with persistent high disease activity or those in longstanding remission but with complications, etc. Identifying the target population most in need of an MD approach and the factors that might predict better outcomes in these patients align with stratified and precision medicine approaches.
There is growing evidence that the treat-to-target strategy results in better disease outcomes in patients with SpA.52,53 It would be of interest to determine how and if MD working could contribute and improve treat-to-target recommendations for SpA. We should learn from other specialities that have implemented MD care, such as oncology, where MDT meetings are considered essential, not optional, in the delivery of cancer care.54 The current literature provides evidence that MDT meetings lead to significant changes in the way cancer patients are assessed and managed. Again, there are limited publications on the benefits with regards to patient outcomes such as survival, QoL or patient experience.36
Finally, we should also learn from the COVID-19 experience with telemedicine on how we might implement MD working using technology better in the future. The COVID-19 outbreak has reshaped medical care through the need for implementing social distancing measures and reducing non-urgent hospital attendance, in particular for outpatient appointments or elective surgeries. In this context, telemedicine, especially video consultations, have been promoted and widely used since the beginning of the pandemic.55,56 Various types of telehealth encounters were reported to be used, such as telephone or video consultations, e-consult (asynchronous clinician-to-clinician communication based on record review), remote patient monitoring with various devices, sensors, patient-initiated messaging or chat-boxes.57,58
The use of telemedicine since the start of the outbreak has proven to have many benefits including accessibility, reducing time required for diagnosis and initiating treatment, saving costs, facilitating a close follow up and preventing the risk of contagion.58 However, several disadvantages were also reported. Main drawbacks were the hindering of the relationship between health professionals and their patients, as well as between health professionals, issues concerning the quality of health information and organisational and administrative difficulties.58,59 Therefore several challenges need to be addressed in the future, such as: insufficient financial resources; lack of technological infrastructure; digital divide (systematic gap between patients who benefit more and those who benefit less from the digitalisation process); security and liability concerns; overall arrangement of policymakers and lack of a regulatory framework to authorize, integrate and reimburse telemedicine; general scepticism on data use, privacy and security.58,59 Lessons learned from this experience will be useful for the global use and integration of telemedicine into medical care and public health in the future, as well as for improving the use of telehealth in MD working.
Conclusion
Despite the lack of strong and reliable evidence to support its benefits compared with standard care, MD working is an essential part of the care of patients with SpA. Further studies and initiatives should be developed so that the challenges and limits of MD care can be improved.
Acknowledgments
DRJ and TG acknowledge the Cambridge Arthritis Research Endeavour charity for supporting academic and research activities.
Footnotes
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Ethical statement: Our study did not require an ethical board approval because this was a literature review based upon already published studies.
ORCID iD: Tania Gudu  https://orcid.org/0000-0002-8973-323X
https://orcid.org/0000-0002-8973-323X
Contributor Information
Tania Gudu, Department of Rheumatology, Cambridge University Hospitals NHSFT, Cambridge, UK.
Deepak R. Jadon, Rheumatology Research Unit, Addenbrooke’s Hospital, Cambridge, CB2 0QQ, UK; Department of Rheumatology, Cambridge University Hospitals NHSFT, Cambridge, UK; Department of Medicine, University of Cambridge, UK.
References
- 1. NHS England. Service component handbook – MDT development: working toward an effective multidisciplinary/multiagency team, https://www.england.nhs.uk/publication/making-it-happen-multi-disciplinary-team-mdt-working/ (2015, accessed 11 February 2020).
- 2. Rudwaleit M, van der Heijde D, Landewé R, et al. The assessment of spondyloarthritis international society classification criteria for peripheral spondyloarthritis and for spondyloarthritis in general. Ann Rheum Dis 2011; 70: 25–31. [DOI] [PubMed] [Google Scholar]
- 3. Smolen JS, Schöls M, Braun J, et al. Treating axial spondyloarthritis and peripheral spondyloarthritis, especially psoriatic arthritis, to target: 2017 update of recommendations by an international task force. Ann Rheum Dis 2018; 77: 3–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. van der Heijde D, Ramiro S, Landewé R, et al. 2016 update of the ASAS-EULAR management recommendations for axial spondyloarthritis. Ann Rheum Dis 2017; 76: 978–991. [DOI] [PubMed] [Google Scholar]
- 5. Gossec L, Smolen JS, Ramiro S, et al. European League Against Rheumatism (EULAR) recommendations for the management of psoriatic arthritis with pharmacological therapies: 2015 update. Ann Rheum Dis 2016; 75: 499–510. [DOI] [PubMed] [Google Scholar]
- 6. Coates LC, Kavanaugh A, Mease PJ, et al. Group for research and assessment of psoriasis and psoriatic arthritis 2015 treatment recommendations for psoriatic arthritis. Arthritis Rheumatol 2016; 68: 1060–1071. [DOI] [PubMed] [Google Scholar]
- 7. NICE guidelines. Spondyloarthritis in over 16s: diagnosis and management, https://www.nice.org.uk/guidance/ng65/chapter/Recommendations (2017, accessed 23 March 2020). [PubMed]
- 8. Ward MM, Deodhar A, Gensler LS, et al. 2019 update of the American college of rheumatology/spondylitis association of America/spondyloarthritis research and treatment network recommendations for the treatment of ankylosing spondylitis and nonradiographic axial spondyloarthritis. Arthritis Rheumatol 2019; 71: 1599–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wendling D, Lukas C, Prati C, et al. 2018 update of French Society for Rheumatology (SFR) recommendations about the everyday management of patients with spondyloarthritis. Joint Bone Spine 2018; 85: 275–284. [DOI] [PubMed] [Google Scholar]
- 10. Kiltz U, Rudwaleit M, Sieper J, et al. [Evidence-based recommendations on diagnostics and therapy of axial spondyloarthritis: S3 guidelines of the German Society of Rheumatology (DGRh) in cooperation with the Association of the Scientific Medical Societies in Germany (AWMF)]. Z Rheumatol 2017; 76: 111–117. [DOI] [PubMed] [Google Scholar]
- 11. Vliet Vlieland TP, Li LC, MacKay C, et al. Does everybody need a team? J Rheumatol 2006; 33: 1897–1899. [PubMed] [Google Scholar]
- 12. Crossland V, Field R, Ainsworth P, et al. Is there evidence to support multidisciplinary healthcare working in rheumatology? A systematic review of the literature. Musculoskeletal Care 2015; 13: 51–56. [DOI] [PubMed] [Google Scholar]
- 13. Soleymani T, Reddy SM, Cohen JM, et al. Early recognition and treatment heralds optimal outcomes: the benefits of combined rheumatology-dermatology clinics and integrative care of psoriasis and psoriatic arthritis patients. Curr Rheumatol Rep 2017; 20: 1. [DOI] [PubMed] [Google Scholar]
- 14. Queiro R, Coto P, Rodríguez J, et al. Multidisciplinary care models for patients with psoriatic arthritis. Reumatol Clin 2017; 13: 85–90. [DOI] [PubMed] [Google Scholar]
- 15. Queiro R, Coto P. Multidisciplinary care for psoriatic disease: where we are and where we need to go. Rheumatology (Oxford) 2017; 56: 1829–1831. [DOI] [PubMed] [Google Scholar]
- 16. Visalli E, Crispino N, Foti R, et al. Multidisciplinary management of psoriatic arthritis: the benefits of a comprehensive approach. Adv Ther 2019; 36: 806–816. [DOI] [PubMed] [Google Scholar]
- 17. Jones T, Darzi A, Egger G, et al. Process and systems: a systems approach to embedding group consultations in the NHS. Future Healthc J 2019; 6: 8–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Luchetti MM, Benfaremo D, Bendia E, et al. Clinical and patient reported outcomes of the multidisciplinary management in patients with inflammatory bowel disease-associated spondyloarthritis. Eur J Intern Med 2019; 64: 76–84. [DOI] [PubMed] [Google Scholar]
- 19. Luchetti MM, Benfaremo D, Ciccia F, et al. Adalimumab efficacy in enteropathic spondyloarthritis: a 12-mo observational multidisciplinary study. World J Gastroenterol 2017; 23: 7139–7149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lorenzetti R, Scolieri P, Guarini A, et al. Integrated gastroenterology and rheumatology ambulatory: an innovative approach for enteropathic spondyloarthritis early diagnosis. Ann Ist Super Sanita 2019; 55: 246–248. [DOI] [PubMed] [Google Scholar]
- 21. Conigliaro P, Chimenti MS, Ascolani M, et al. Impact of a multidisciplinary approach in enteropathic spondyloarthritis patients. Autoimmun Rev 2016; 15: 184–190. [DOI] [PubMed] [Google Scholar]
- 22. Zabotti A, Errichetti E, Zuliani F, et al. Early psoriatic arthritis versus early seronegative rheumatoid arthritis: role of dermoscopy combined with ultrasonography for differential diagnosis. J Rheumatol 2018; 45: 648–654. [DOI] [PubMed] [Google Scholar]
- 23. Urruticoechea-Arana A, Serra Torres M, Hergueta Diaz M, et al. Experience and satisfaction with a multidisciplinary care unit for patients with psoriasis an psoriatic arthritis. Reumatol Clin 2019; 15: 237–241. [DOI] [PubMed] [Google Scholar]
- 24. Velez NF, Wei-Passanese EX, Husni ME, et al. Management of psoriasis and psoriatic arthritis in a combined dermatology and rheumatology clinic. Arch Dermatol Res 2012; 304: 7–13. [DOI] [PubMed] [Google Scholar]
- 25. Luchetti MM, Benfaremo D, Campanati A, et al. Clinical outcomes and feasibility of the multidisciplinary management of patients with psoriatic arthritis: two-year clinical experience of a dermo-rheumatologic clinic. Clin Rheumatol 2018; 37: 2741–2749. [DOI] [PubMed] [Google Scholar]
- 26. Luelmo J, Gratacós J, Moreno Martínez-Losa M, et al. A report of 4 years of experience of a multidisciplinary unit of psoriasis and psoriatic arthritis. Reumatol Clin 2014; 10: 141–146. [DOI] [PubMed] [Google Scholar]
- 27. Juanola X, Loza Santamaría E, Cordero-Coma M. Description and prevalence of spondyloarthritis in patients with anterior uveitis: the SENTINEL interdisciplinary collaborative project. Ophthalmology 2016; 123: 1632–1636. [DOI] [PubMed] [Google Scholar]
- 28. Liang L, Pan Y, Wu D, et al. Effects of multidisciplinary team-based nurse-led transitional care on clinical outcomes and quality of life in patients with ankylosing spondylitis. Asian Nurs Res (Korean Soc Nurs Sci) 2019; 13: 107–114. [DOI] [PubMed] [Google Scholar]
- 29. Kjeken I, Bø I, Rønningen A, et al. A three-week multidisciplinary in-patient rehabilitation programme had positive long-term effects in patients with ankylosing spondylitis: randomized controlled trial. J Rehabil Med 2013; 45: 260–267. [DOI] [PubMed] [Google Scholar]
- 30. Li J, Xu Y, Chen Y, et al. A multidisciplinary clinic approach to improve physician-related diagnostic delay for patients with axial spondyloarthritis: a retrospective study. J Int Med Res 2019; 47: 2483–2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Reich K, Krüger K, Mössner R, et al. Epidemiology and clinical pattern of psoriatic arthritis in Germany: a prospective interdisciplinary epidemiological study of 1511 patients with plaque-type psoriasis. Br J Dermatol 2009; 160: 1040–1047. [DOI] [PubMed] [Google Scholar]
- 32. Esposito M, Faleri S, Babino G, et al. From patients’ needs to treatment outcomes in psoriasis: results from the ‘pSORRIDI’ experience. J Int Med Res 2016; 44(Suppl. 1): 95–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gladman DD, Coates LC, Jadon DR, et al. The benefits and challenges of setting up a longitudinal psoriatic arthritis database. J Rheumatol Suppl 2018; 94: 26–29. [DOI] [PubMed] [Google Scholar]
- 34. Richeldi L, Launders N, Martinez F, et al. The characterisation of interstitial lung disease multidisciplinary team meetings: a global study. ERJ Open Res 2019; 5: 00209-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. British Society for Rheumatology. The best practice award, https://www.rheumatology.org.uk/practice-quality/best-practice-awards (2018, accessed 20 April 2020).
- 36. Pillay B, Wootten AC, Crowe H, et al. The impact of multidisciplinary team meetings on patient assessment, management and outcomes in oncology settings: a systematic review of the literature. Cancer Treat Rev 2016; 42: 56–72. [DOI] [PubMed] [Google Scholar]
- 37. Aghdam MRF, Vodovnik A, Hameed RA. Role of telemedicine in multidisciplinary team meetings. J Pathol Inform 2019; 10: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sadikot SM, Das AK, Wilding J, et al. Consensus recommendations on exploring effective solutions for the rising cost of diabetes. Diabetes Metab Syndr 2017; 11: 141–147. [DOI] [PubMed] [Google Scholar]
- 39. Riley SB, Marshall ES. Group visits in diabetes care: a systematic review. Diabetes Educ 2010; 36: 936–944. [DOI] [PubMed] [Google Scholar]
- 40. Gossec L, Dougados M, Dixon W. Patient-reported outcomes as end points in clinical trials in rheumatoid arthritis. RMD Open 2015; 1: e000019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Queiro R, Coto P, Joven B, et al. Current status of multidisciplinary care in psoriatic arthritis in Spain: NEXUS 2.0 project. Reumatol Clin 2020; 16: 24–31. [DOI] [PubMed] [Google Scholar]
- 42. Rizzello F, Olivieri I, Armuzzi A, et al. Multidisciplinary management of spondyloarthritis-related immune-mediated inflammatory disease. Adv Ther 2018; 35: 545–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gratacos-Masmitja J, Luelmo-Aguilar J, Zarco-Montejo P, et al. Points to consider in the foundation of multidisciplinary units for psoriatic arthritis: a Delphi study and a systematic review of the literature. Adv Ther 2017; 33: 2150–2159. [DOI] [PubMed] [Google Scholar]
- 44. Gratacós J, Luelmo J, Rodríguez J, et al. Standards of care and quality indicators for multidisciplinary care models for psoriatic arthritis in Spain. Rheumatol Int 2018; 38: 1115–1124. [DOI] [PubMed] [Google Scholar]
- 45. Calvet X, Panés J, Alfaro N, et al. Delphi consensus statement: quality indicators for inflammatory bowel disease comprehensive care units. J Crohns Colitis 2014; 8: 240–251. [DOI] [PubMed] [Google Scholar]
- 46. Louis E, Dotan I, Ghosh S, et al. optimising the inflammatory bowel disease unit to improve quality of care: expert recommendations. J Crohns Colitis 2015; 9: 685–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. de Wit M, Kirwan JR, Tugwell P, et al. Successful stepwise development of patient research partnership: 14 years’ experience of actions and consequences in Outcome Measures in Rheumatology (OMERACT). Patient 2017; 10: 141–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Goel N, O’Sullivan D, Steinkoenig I, et al. Tackling patient centricity: a report from the GRAPPA 2016 annual meeting. J Rheumatol 2017; 44: 703–705. [DOI] [PubMed] [Google Scholar]
- 49. Tillett W, Adebajo A, Brooke M, et al. Patient involvement in outcome measures for psoriatic arthritis. Curr Rheumatol Rep 2014; 16: 418. [DOI] [PubMed] [Google Scholar]
- 50. Stamm TA, Nell V, Mathis M, et al. Concepts important to patients with psoriatic arthritis are not adequately covered by standard measures of functioning. Arthritis Rheum 2007; 57: 487–494. [DOI] [PubMed] [Google Scholar]
- 51. Dures E, Hewlett S, Lord J, et al. important treatment outcomes for patients with psoriatic arthritis: a multisite qualitative study. Patient 2017; 10: 455–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Coates LC, Moverley AR, McParland L, et al. Effect of tight control of inflammation in early psoriatic arthritis (TICOPA): a UK multicentre, open-label, randomised controlled trial. Lancet 2015; 386: 2489–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Moltó A, López-Medina C, van den Bosch F, et al. THU0370 cluster-randomized pragmatic clinical trial evaluating the potential benefit of a tight-control and treat-to-target strategy in axial spondyloarthritis: the results of the TICOSPA trial. Ann Rheum Dis 2020; 79(Suppl.1): abstract 417. [Google Scholar]
- 54. Taylor C, Munro AJ, Glynne-Jones R, et al. Multidisciplinary team working in cancer: what is the evidence? BMJ 2010; 340: c951. [DOI] [PubMed] [Google Scholar]
- 55. Ohannessian R, Duong TA, Odone A. Global telemedicine implementation and integration within health systems to fight the COVID-19 pandemic: a call to action. JMIR Public Health Surveill 2020; 6: e18810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wosik J, Fudim M, Cameron B, et al. Telehealth transformation: COVID-19 and the rise of virtual care. J Am Med Inform Assoc 2020; 27: 957–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Vidal-Alaball J, Acosta-Roja R, Pastor Hernández N, et al. Telemedicine in the face of the COVID-19 pandemic. Version 2. Aten Primaria 2020; 52: 418–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hong Z, Li N, Li D, et al. Telemedicine during the COVID-19 pandemic: experiences from western China. J Med Internet Res 2020; 22: e19577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Nittas V, von Wyl V. COVID-19 and telehealth: a window of opportunity and its challenges. Swiss Med Wkly 2020; 150: w20284. [DOI] [PubMed] [Google Scholar]