Abstract
Cytomegalovirus (CMV) retinitis (CMVR) has been reported rarely in patients undergoing allogeneic hematopoietic stem cell transplantation (HSCT). In addition, little is known about strategies for ophthalmic surveillance and adequate antiviral treatment of CMVR. A case of CMVR in an allogeneic HSCT recipient is described, including clinical signs and therapy. An adult patient received HSCT from a matched unrelated donor for treatment of a Burkitt lymphoma. Donor and recipients were both CMV positive. Starting on day +40, the patient presented multiple CMV reactivation, treated with valganciclovir, foscarnet and a combination of both. On day +160, the patient started complaining of conjunctival hyperaemia and a decrease in visual acuity. Fundoscopy revealed retinal lesions consistent with CMVR, although whole blood CMV DNAemia was negative. Aqueous humor biopsy showed the presence of CMV infection (CMV DNA 230400 UI/ml). CMVR was treated with foscarnet (180 mg i.v. and 1.2 mg intravitreal injection) combined with anti CMV immunoglobulin at 0.5 ml/kg every 2 weeks. After 4 weeks of systemic therapy, 20 weekly doses of intravitreal foscarnet and six cycles of immunoglobulins, a significant improvement of visual acuity was observed. The treatment was well tolerated with no side effect. In conclusion, our case suggests that systemic and local antiviral treatment combined with CMV-specific-IVIG, may reduce CMV load in the eye of patients with CMVR, leading to a consistent improvement of visual acuity. Systematic ophthalmologic examination should be recommended in HSCT recipients with multiple CMV reactivations and high peak CMV DNA levels.
Keywords: CMV, foscarnet, retinitis
Introduction
Impaired cellular immunity after allogeneic hematopoietic stem cell transplantation (HSCT) may lead to cytomegalovirus (CMV) reactivation, which is associated with increased risk of CMV disease and ultimately to higher morbidity and mortality of HSCT recipients.1 Clinical manifestations of CMV disease usually include colitis, hepatitis and pneumonitis, while CMV retinitis (CMVR) is reported by far more occasionally in hematopoietic stem cell transplantation (HSCT) recipients than in acquired immunodeficiency syndrome (AIDS) patients.2 CMVR, if underestimated or misdiagnosed, frequently leads to visual impairment or blindness,3,4 and therefore needs timely treatment usually combining systemic and intraocular administration.5,6 Ganciclovir represents the treatment of choice for patients with CMVR, although toxicity, in particular myelosuppression, limits its use in a consistent number of cases.2,7–10 Foscarnet is an alternative approach, although clinical experience in this setting is rather limited.7,10,11 Here, we present the case of an HSCT recipient who developed CMVR that was treated successfully by combining systemic and intravitreal administration of foscarnet.
Case presentation
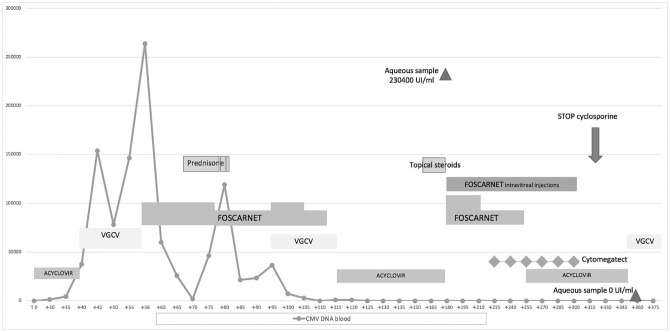
A 32-year-old man was diagnosed with diffuse large B cell lymphoma infiltrating the thorax wall with involvement of costal cartilages in August 2017. He was first treated with six cycles of R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone) followed by four cycles of R-DHAP (rituximab, prednisone, cisplatin and cytarabine) without achieving remission. Surprisingly, disease restaging showed Burkitt lymphoma on chest biopsy, for which the patient was treated with an R Magrath regimen (R-IVAC: rituximab, ifosfamide, etoposide and cytarabine alternated with R-CODOX-M: cyclophosphamide, vincristine, doxorubicin and methotrexate) attaining complete remission. In September 2018, the patient underwent a stem cell transplant from a 10/10 human leukocyte antigen (HLA)-matched unrelated donor (MUD). Donor and recipient were both CMV immunoglobulin G (IgG) positive. The conditioning regimen consisted of thiotepa, busulfan and fludarabine. Graft-versus-host disease (GVHD) prophylaxis included cyclosporine, short course methotrexate and anti thymocyte globulin (2.5 mg/kg for 2 consecutive days). Fluconazole and acyclovir were given as antimicrobial prophylaxis. Neutrophil engraftment occurred on day +27 after transplant. During the first month after transplant, CMV DNAemia, tested two times per week, was around 1300–2000, below the threshold used to start therapy (3000 copies). On day +40, whole blood CMV DNA reached 37,000 UI/ml, and the patient was started on valganciclovir (VGCV) at 900 mg bid. However, after 9 days of therapy, DNAemia increased up to 1,46,800 UI/ml. Second line treatment with foscarnet 120 mg/kg was started promptly, leading to a slow decrease of viral load up to 25,800 UI/ml after 2 weeks of treatment (Figure 1). Following administration of steroids for the treatment of possible gut GVHD, a new flare of CMV DNAemia occurred on day +95. Genotypic resistance testing for the UL54 and UL97 genes was negative. The patient was treated with a combination of foscarnet 120mg/kg and VGCV 450 mg bid, resulting in the clearance of CMV DNAemia after 2 weeks of treatment (Figure 1). On day +160, the patient started complaining of conjunctival hyperaemia and a moderate decrease in visual acuity. Fundoscopy revealed papillary hyperaemia, retinal necrosis, haemorrhage and vasculitis consistent with CMVR (Figure 2), although whole blood CMV DNAemia was negative. Lymphocyte recovery was impaired significantly, with 69 CD4+ cells/μl, 221 CD8+ cells/μl and 82 CD19+ cells/μl. Aqueous humor biopsy showed presence of CMV infection (CMV DNA 230,400 UI/ml) while the presence of other viruses (herpes simplex virus, virus varicella zoster or Epstein Barr virus) was excluded; at the same time CMV serum DNAemia was negative. CMVR, once written informed consent had been obtained, was treated with foscarnet (180 mg i.v. and 1.2 mg intravitreal injection) combined with anti CMV immunoglobulin (cytomegatect) at 0.5 ml/kg every 2 weeks. After 4 weeks of systemic therapy, 20 weekly doses of intravitreal foscarnet and six cycles of immunoglobulins, a significant improvement in visual acuity was observed. The treatment was well tolerated with no side effects. Cyclosporine was tapered and then stopped on day + 315 (Figure 1). At 3 months after discontinuation of treatment, the patient started complaining of blurry vision. Diffuse atrophic chorioretinitis and posterior haemorrhage were observed. Retinal fluoroscopy demonstrated diffuse retinal vasculitis with loss of capillary, bilateral macular oedema, diffuse damage, vitreitis and steadily atrophic CMV lesions consistent with vasculitis requiring the administration of steroids at 1 mg/kg daily. Fundoscopic evaluation 2 weeks later was suggestive of reactivation of the viral infection given the presence of periangioitis and hyalinosis of the temporal area of the right retina. However, whole blood and humor aqueous CMV DNA were both negative. Maintenance therapy with VGCV at 450 mg bid was started during steroid treatment. After 4–6 weeks, visual acuity showed a moderate improvement, allowing a slow steroid taper. At 7 months after the diagnosis of CMV retinitis, the patient is in good general conditions and in continuous haematological remission (Figure 3).
Figure 1.
Clinical course of patient with CMV retinitis following allogeneic HSCT. Day 0 indicates the day of transplantation. The solid line indicates the CMV viral load in whole blood.
CMV, cytomegalovirus; CMVR, CMV retinitis; HSCT, hematopoietic stem cell transplantation; VGCV, valgancyclovir.
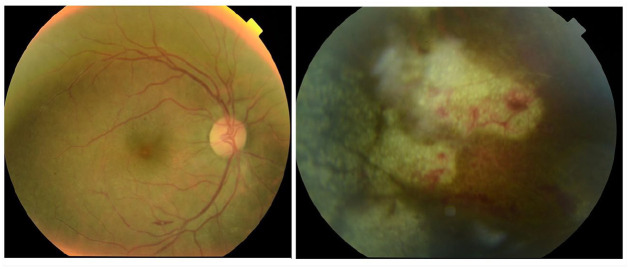
Figure 2.
Fundoscopic finding at the time of initial diagnosis (day+160 after transplantation). Evolutionary CMVR with initial vitreitis, optic papilla hyperaemia, peripheral/equatorial retinal necrosis, retinal haemorrhages, retinal oedema, retinal vasculitis.
CMV, cytomegalovirus; CMVR, CMV retinitis.
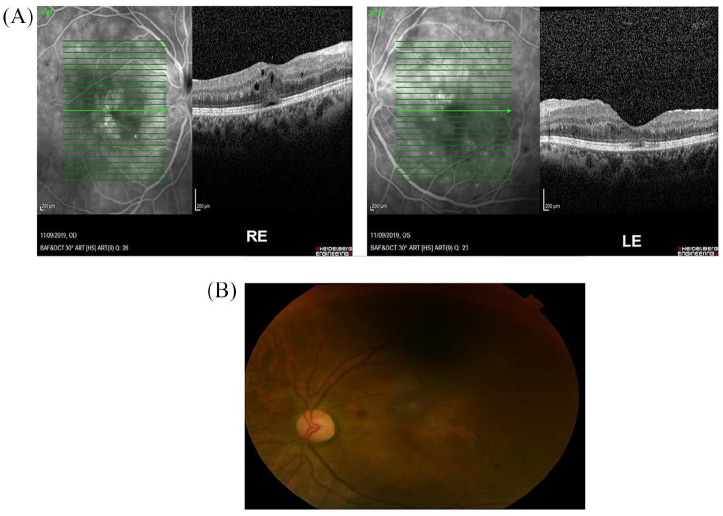
Figure 3.
(A) OCT 3 months after the end of systemic and local antiviral treatment. Regression of CMVR with persistence only of increased central retinal thickness. (B) Retinal fluoroscopy 3 months after the end of systemic and local antiviral treatment.
CMV, cytomegalovirus; CMVR, CMV retinitis; LE, left eye; OCT, optical coherence tomography; RE, right eye.
Discussion
This paper describes a patient who presented with severe CMV retinitis after allogeneic HSCT and highlights the aggressive nature of this complication. CMVR is reported infrequently in adult patients undergoing allogeneic HSCT, with rates ranging between 0.19 and 5.6%, while it appears more frequent in children.2,7,8,12–14 CMVR diagnosis is challenging. Our patient showed characteristics lesions in the fundus and both fluorescein angiography and CMV DNA load in the aqueous humor were consistent with CMVR. Nevertheless, a few cases of asymptomatic CMVR have been described,9,15 underlining the importance of ruling out retinal involvement in immunocompromised hosts with CMV infection. Thus, it is important to stress that CMVR management requires the close cooperation of haematologists with ophthalmologists.
As reported elsewhere, it is not surprising that, in our patient, CMV DNA was not detectable in whole blood at the time of CMVR diagnosis.8,13,16 In fact, it is possible to speculate that the eyes may represent a sanctuary site where immunocompetent cells as well as antiviral therapies penetrate with extreme difficulty.17 According to these observations, our therapeutic approach included the association of both intravenous and intravitreal injections of foscarnet. This antiviral agent was chosen in the light of the poor response to the initial treatment with VGCV and, more importantly, of the presence of a significant peripheral cytopenia, potentially related, at least partly, to VGCV toxicity. Indeed, foscarnet showed efficacy in clearing the virus and clinically resolving CMVR, without any side effect associated with both systemic and local injections. Overall, GCV or Foscarnet represent the most widely used systemic therapies for CMVR.2,7–10,12,18 Only a few patients have been treated with intravitreal injection of GCV (Table 1). Larsson et al. described two patients who received combined systemic and local treatment with intravitreal injections of GCV,12 while Zhang et al. described 12 patients who received combined systemic and intravitreal injection16; 11 eyes showed improvements or stabilization whereas 8 eyes deteriorated. Transplant from a HLA-mismatched donor and CMV DNA copies higher than 1 × 104 were adverse factors for visual outcomes. Miao et al. reported the largest experience on 14 haematological patients with CMVR who were treated with four intravitreal injections of GCV.19 The procedure was well-tolerated and was capable of reducing the level of CMV DNA in the eye. Our patient required a total of 20 intravitreal injections, suggesting that this procedure may be considered rather safe and effective. Moreover, to our knowledge, this is the first case of a patient with CMVR where CMV-specific-IVIG were combined with antiviral treatment. Studies evaluating the efficacy of IVIG in HSCT recipients with CMV infection are lacking.20–22 Since foscarnet is virostatic,23 we can speculate that IVIG might have contributed to control viral replication once systemic treatment with foscarnet has been discontinued.
Table 1.
Summary of published cases of adult patients with CMVR following allogeneic HSCT treated with local antiviral therapy.
| Reference | No. patients | HSCT | Median onset of CMVR (days from HSCT) | Systemic antiviral therapy | Local antiviral therapy | Outcome |
|---|---|---|---|---|---|---|
| Zhang et al.16 | 12 | MUD (7) MMUD(5) |
126 | No | GCV, 4 injections | 4 improvement 4 stabilization 4 deterioration |
| Miao et al.19 | 14 | 156 | No | GCV 4 injections | improvement | |
| Jeon and Lee13 | 10 | MUD (4) MMUD (6) |
3 GCV 3GCV + FOS 4 other |
GCV 4 injections | 4 improvement 5 stabilization |
|
| Zöllner et al.2 | 11 | MUD (2) MMUD(3) |
147 | 2 GCV | GCV 4 injections | 2 improvement 1 deterioration |
CMV, cytomegalovirus; CMVR, CMV retinitis; FOS, foscarnet; GCV, ganciclovir; HSCT, hematopoietic stem cell transplantation; MMUD, mismatched unrelated donor; MUD, matched unrelated donor.
Several risk factors for CMVR have been reported in different studies. Jeon et al. found that HSCT from MUD or mismatched donors, delayed engraftment, peak CMV levels and duration of viremia were factors associated with the development of CMVR in univariate analysis, although only CMV DNA level remained statistically significant in multivariate analysis.13 A study from the Seattle group showed that CMV seropositive recipients, CMV reactivation before day 100, delayed post-transplant lymphocyte recovery and presence of chronic GVHD were factors potentially associated with CMVR.7 All these risk factors were observed in our patient with the exception of GVHD, although a short course of steroids was administered for suspected diagnosis GVHD-related diarrhoea, promptly discontinued after a gut biopsy ruled out histological signs of GVHD. Even in the absence of clinically evident GVHD, our patient showed a remarkably slow lymphocyte recovery, with 69/μl CD4+ T cells at day +160. We can hypothesize that GVHD prophylaxis including antithymocyte globulin (ATG) and the prolonged administration of VGCV (total duration 38 days) may have negatively influenced the immune recovery. Regarding the prolonged use of antiviral drugs, those currently available are toxic and poorly tolerated and must be used wisely. The availability of less toxic compounds might potentially change the paradigm of CMV treatment with long-lasting treatment in selected patients.
In conclusion, our case suggests that systemic and local antiviral treatments with foscarnet, combined with CMV-specific-IVIG, may significantly and safely reduce CMV load in the eye of patients with CMVR, eventually leading to a consistent improvement of visual acuity. Routine ophthalmologic examination should be recommended in HSCT recipients with multiple CMV reactivations and high peak CMV DNA every 2 months until negative viral load, irrespective of visual symptoms or the presence of GVHD.
Footnotes
Authors’ Note: Lucia Brunello is now affiliated with Hematology, AO SS Antonio e Biagio e Cesare Arrigo Alessandria, Italy.
Author contributions: FV: concept/design, drafting manuscript, critical revision of article; AB: concept/design, drafting manuscript, critical revision of article; RN: critical revision of article; IC: critical revision of article; CDC: concept/design, drafting manuscript, critical revision of article; LG: concept/design, drafting manuscript, critical revision of article; FGDR: critical revision of article; GI: concept/design, drafting manuscript, critical revision of article; RC: critical revision of article; LB: concept/design, drafting manuscript, critical revision of article; BB: concept/design, drafting manuscript, critical revision of article.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical approval: Our study did not require an ethical board approval because the drugs used were in label.
ORCID iD: Alessandro Busca  https://orcid.org/0000-0001-5361-5613
https://orcid.org/0000-0001-5361-5613
Contributor Information
Francesco Vassallo, Department of Oncology and Hematology, AOU Citta’ della Salute e della Scienza, Turin, Italy.
Raffaele Nuzzi, Eye Clinic Section, Department of Surgical Sciences, University of Turin, Italy.
Ilaria Cattani, Eye Clinic Section, Department of Surgical Sciences, University of Turin, Italy.
Chiara Dellacasa, Stem Cell Transplant Center, AOU Citta’ della Salute e della Scienza, Turin, Italy.
Luisa Giaccone, Stem Cell Transplant Center, AOU Citta’ della Salute e della Scienza, Turin, Italy.
Francesco Giuseppe De Rosa, Department of Medical Sciences, University of Turin, Italy.
Rossana Cavallo, SC Microbiology and Virology, A.O.U. Città della Salute e della Scienza di Torino, Torino, Italy; Department of Public Health and Pediatrics, University of Torino, Torino, Italy.
Giorgia Iovino, Department of Oncology and Hematology, AOU Citta’ della Salute e della Scienza, Turin, Italy.
Lucia Brunello, Stem Cell Transplant Center, AOU Citta’ della Salute e della Scienza, Turin, Italy.
Benedetto Bruno, Stem Cell Transplant Center, AOU Citta’ della Salute e della Scienza, Turin, Italy.
Alessandro Busca, Stem Cell Transplant Center, AOU Citta’ della Salute e della Scienza, Corso Bramante 88, Turin, 10126, Italy.
References
- 1. Boeckh M, Nichols WG, Papanicolaou G, et al. Cytomegalovirus in hematopoietic stem cell transplant recipients: current status, known challenges, and future strategies. Biol Blood Marrow Transplant 2003; 9: 543–558. [DOI] [PubMed] [Google Scholar]
- 2. Zöllner SK, Herbrüggen H, Kolve H, et al. Cytomegalovirus retinitis in children and adolescents with acute leukemia following allogeneic hematopoietic stem cell transplantation. Transpl Infect Dis 2019; 21: e13089. [DOI] [PubMed] [Google Scholar]
- 3. Lewis RA, Clogston P, Fainstein V, et al. Studies of ocular complications of AIDS Foscarnet-Ganciclovir Cytomegalovirus Retinitis Trial: 1. Rationale, design, and methods. Control Clin Trials 1992; 13: 22–39. [DOI] [PubMed] [Google Scholar]
- 4. Thorne JE, Jabs DA, Kempen JH, et al. Causes of visual acuity loss among patients with AIDS and cytomegalovirus retinitis in the era of highly active antiretroviral therapy. Ophthalmology 2006; 113: 1441–1445. [DOI] [PubMed] [Google Scholar]
- 5. Jabs DA, Ahuja A, Van Natta M, et al. Comparison of treatment regimens for cytomegalovirus retinitis in patients with AIDS in the era of highly active antiretroviral therapy. Ophthalmology 2013; 120: 1262–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Port AD, Orlin A, Kiss S, et al. Cytomegalovirus retinitis: a review. J Ocul Pharmacol Ther 2017; 33: 224–234. [DOI] [PubMed] [Google Scholar]
- 7. Crippa F, Corey L, Chuang EL, et al. Virological, clinical, and ophthalmologic features of cytomegalovirus retinitis after hematopoietic stem cell transplantation. Clin Infect Dis 2001; 32: 214–219. [DOI] [PubMed] [Google Scholar]
- 8. Xhaard A, Robin M, Scieux C, et al. Increased incidence of cytomegalovirus retinitis after allogeneic hematopoietic stem cell transplantation. Transplantation 2007; 83: 80–83. [DOI] [PubMed] [Google Scholar]
- 9. Eid AJ, Bakri SJ, Kijpittayarit S, et al. Clinical features and outcomes of cytomegalovirus retinitis after transplantation. Transpl Infect Dis 2008; 10: 13–18. [DOI] [PubMed] [Google Scholar]
- 10. Ohta H, Matsuda Y, Tokimasa S, et al. Foscarnet therapy for ganciclovir-resistant cytomegalovirus retinitis after stem cell transplantation: effective monitoring of CMV infection by quantitative analysis of CMV mRNA. Bone Marrow Transplant 2001; 27: 1141–1145. [DOI] [PubMed] [Google Scholar]
- 11. Ganly PS, Arthur C, Goldman JM, et al. Foscarnet as treatment for cytomegalovirus retinitis following bone marrow transplantation. Postgrad Med J 1988; 64: 389–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Larsson K, Lönnqvist B, Ringdén O, et al. CMV retinitis is after allogeneic bone marrow transplantation: a report of five cases. Transpl Infect Dis 2002; 4: 75–79. [DOI] [PubMed] [Google Scholar]
- 13. Jeon S, Lee WK. Cytomegalovirus retinitis in a human immunodeficiency virus-negative cohort: long-term management and complications. Ocul Immunol Inflamm 2015; 23: 392–399. [DOI] [PubMed] [Google Scholar]
- 14. Tan PL, Lim LM, Khanlian C, et al. A single-center experience of cytomegalovirus infections in asian pediatric patients undergoing allogeneic hematopoietic stem cell transplant for leukemia in Singapore. Transpl Infect Dis 2014; 16: 556–560. [DOI] [PubMed] [Google Scholar]
- 15. Friedberg DN. Cytomegalovirus retinitis: diagnosis and status of systemic therapy. Journal of Acquired Immune Deficiency Syndromes and Human Retrovirology 1997; 14: S1–S6. [DOI] [PubMed] [Google Scholar]
- 16. Zhang Y, Ruan X, Yang W, et al. High ocular CMV copies and mismatched receipts may predict poor visual prognosis in CMV retinitis patients following allogeneic haematopoietic stem cell transplantation. BMC Ophthalmol 2017; 17: 224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Arevalo JF, et al. Intravitreous and plasma concentrations of ganciclovir and foscarnet after intravenous therapy in patients with aids and cytomegalovirus retinitis. J Infect Dis 1995; 172: 951–956. [DOI] [PubMed] [Google Scholar]
- 18. Zhao N, Liu L, Xu J. Cytomegalovirus retinitis in a patient with secondary acute lymphosarcoma leukemia undergoing allogeneic hematopoietic stem-cell transplantation. Med 2017; 96: 1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Miao H, Tao Y, Jiang YR, et al. Multiple intravitreal injections of ganciclovir for cytomegalovirus retinitis after stem-cell transplantation. Graefe’s Arch Clin Exp Ophthalmol 2013; 251: 1829–1833. [DOI] [PubMed] [Google Scholar]
- 20. Ljungman P, Cordonnier C, Einsele H, et al. Use of intravenous immune globulin in addition to antiviral therapy in the treatment of CMV gastrointestinal disease in allogeneic bone marrow transplant patients: a report from the European Group for Blood and Marrow Transplantation (EBMT). Bone Marrow Transplant 1998; 21: 473–476. [DOI] [PubMed] [Google Scholar]
- 21. Vigil KJ, Adachi JA, Chemaly RF. Analytic review: viral pneumonias in immunocompromised adult hosts. J Intensive Care Med 2010; 25: 307–326. [DOI] [PubMed] [Google Scholar]
- 22. Malagola M, Greco R, Santarone S, et al. CMV management with specific immunoglobulins: a multicentric retrospective analysis on 92 allotransplanted patients. Mediterr J Hematol Infect Dis 2019; 11: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Aschan J, Ringdén O, Ljungman P, et al. Foscarnet for treatment of cytomegalovirus infections in bone marrow transplant recipients. Scand J Infect Dis 1992; 24: 143–150. [DOI] [PubMed] [Google Scholar]