Abstract
Patients with anti-CV2/collapsin response mediator protein (CRMP)5 antibodies present with more frequent chorea, cerebellar ataxia, uveo/retinal symptoms, and Lambert–Eaton myasthenic syndrome or myasthenia gravis. Chronic intestinal pseudo-obstruction (CIPO) is an intestinal motility dysfunction disease dysmotility that is caused by a neuromuscular disease with recurrent or persistent intestinal obstruction in the absence of mechanical obstruction. We report the case of a patient with CRMP5 antibody-positive paraneoplastic neurological syndrome (PNS) that is associated with autonomic dysfunction (presenting most remarkably as CIPO). CIPO is one of the rarest forms of PNS. Some PNS patients who are positive for anti-CV2/CRMP5 antibodies may have fatal complications such as CIPO. To detect if PNS patients are at risk for CIPO, a timely diagnosis and appropriate treatment are required.
Keywords: Collapsin response mediator protein, CV2/CRMP5 antibodies, chronic intestinal pseudo-obstruction, small-cell lung cancer, paraneoplastic neurological syndrome, intestinal motility dysfunction disease, dysmobility, neuromuscular disease
Introduction
Paraneoplastic neurological syndrome (PNS) is a rare disease that occurs in 0.1% to 10% of cancer patients.1 PNS is an immune-mediated false attack on central, peripheral, or both nervous systems that is caused by the remote effects of a malignant tumor.2 Diagnosing patients with PNS is challenging because tumors causing paraneoplastic neurologic disorders are often asymptomatic and sometimes occult, which makes it difficult to determine the differential diagnosis.3
Chronic intestinal pseudo-obstruction (CIPO) is characterized by the signs and symptoms of mechanical bowel obstruction without mechanical intestinal obstruction, which is often due to derangement of innervation, smooth muscle, and interstitial cells of Cajal. It is a rare, severe, and potentially life-threatening functional digestive disorder that is characterized by a failure of gastrointestinal (GI) propulsion.4 In a preceding report of 121 CIPO patients, most of whom had idiopathic CIPO (70.2%), and the secondary cause was most often systemic sclerosis (16.6%) followed by mitochondrial encephalomyopathy (5.2%), amyloidosis (3.5%), and hypothyroidism (2.6%).5 CIPO can be a paraneoplastic disorder that has been reported in anti-Hu antibody patients,6 but it is rarely found in anti-CV2/CRMP5 patients.7
Case presentation
A 67-year-old Chinese man was admitted to the inpatient department of neurology for bilateral lower limb weakness and bilateral partial ptosis for nearly 20 days. Initially, the patient could still walk slowly by himself and he had associated dysphagia, persistent sweating, dysuria that manifested as frequent urination, less urine per void, prolonged urination time, difficulty with defecation that manifested as laborious, small stool, and abdominal distension with continuous aggravation. The symptoms were progressively aggravated, and within 7 days, he was unable to walk on his own and had accompanying persistent dysphagia, sweating, dysuria, and astriction. About 2 weeks after the first symptom onset, the patient felt bilateral lower limbs stiffness with talipes varus and obvious dorsiflexion of the first toe. Bilateral partial ptosis and diplopia developed 3 days later. The patient had unintended weight loss of 5 kg over the previous 2 months. The patient had a 10-year history of hypertension and a history 3 years previously of cerebral infarction with sequelae of dysarthria. He had a history of smoking for 40 years.
A physical examination revealed bilateral partial ptosis, insufficiency of left eye adduction, right eye abduction with horizontal nystagmus, dysarthria, hoarseness, grade 3 strength for major muscle force in his bilateral lower limbs with hypermyotonia and myoclonia, imprecise bilateral finger–nose test, and obvious Babinski and Chaddock signs on both sides. The muscle strength and muscle tension in his bilateral upper limbs were normal and no other neurological signs were found. The fatigue test and the neostigmine test showed negative results. The abdomen was distended and soft without tenderness, and a vibration water sound was heard as well as weakened bowel sounds.
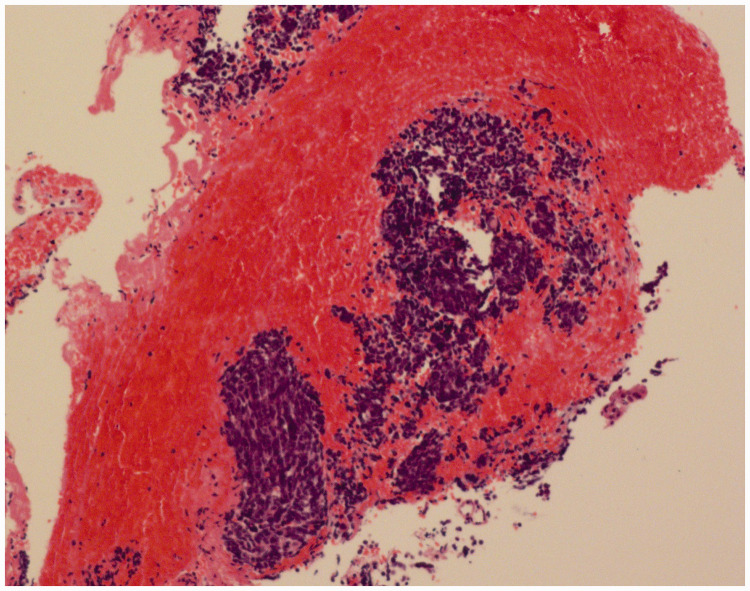
Laboratory tests were performed. Tests for autoimmune diseases, thyroid function test, anti-phospholipid syndrome antibody, three items for rheumatism, and antinuclear antibody (ANA) series were performed and the results were all negative. Blood tumor markers were in the normal range. The patient had intractable low sodium (120.1–132.4 mmol/L, normal range: 137–147 mmol/L) and chlorine (95–96.2 mmol/L, normal range: 99–110 mmol/L) levels. The cerebrospinal fluid (CSF) examination revealed normal intracranial pressure, blood cell count, and glucose levels, as well as elevated protein (560 mg/L, normal range: 150–450 mg/L), slightly reduced chloride (118.0 mmol/L, normal range: 119–129 mmol/L), and increased immunoglobulin (IgG) (76 mg/L, normal range: 0–34 mg/L) levels. Test results for anti-CV2/CRMP5 antibodies in the serum and CSF were positive, while results for anti-AQP4-Ab, NMO-IgG, NMDA-R-Ab, CASPR2-Ab, AMPA2-R-Ab, LGI-1-Ab, GABAB-R-Ab, DPPX-Ab, PNMA2(Ma2/ta), Ri, Yo, Hu, amphiphysin, GM1, and GD1b were negative in serum and CSF. The CSF was also negative for MBP. The above tests were performed by the Peking Union Medical College Hospital (Beijing, China). Test results for anti-acetylcholine receptor (AchR) antibodies, anti-MUSK antibodies, anti-LRP4 antibodies, anti-agrin antibodies, anti-titin antibodies, anti-RyR antibodies, and anti-voltage-gated calcium channel (VGCC) antibodies were negative. Repetitive electrical stimulation showed a normal response. Magnetic resonance imaging (MRI) of the cervical, thoracic, and thoracolumbar vertebrae showed normal results. Head MRI showed multiple old lacunar cerebral infarctions with partial softening focus, which was not related to the current clinical manifestations. Electromyography indicated mild peripheral nerve damage in the upper and lower extremities. Colonoscopy was not arranged due to the patient’s poor physical condition. Abdominal CT showed that part of the small intestine and colon were expanded and contained accumulated gas, and a large amount stool was present in the colon. Positron emission tomography (PET)-CT showed high metabolism in the bronchial opening in the posterior segment of the upper tip of the left lung, which suggested central lung cancer. Bronchoscopy pathology confirmed small cell carcinoma of the upper lobe of the left lung (Figure 1), which showed CGA (+), SYN (weak +), CD56 (+), TTF-1 (+), KI67 (+80%), cytokeratin (CK) (+), and leukocyte common antigen (LCA) (−) by immunohistochemistry.
Figure 1.
Bronchoscopy pathology confirmed small-cell lung cancer in the upper lobe of the left lung.
After intravenous infusion of gamma globulin followed hormone therapy against PNS, radiotherapy, and chemotherapy against small-cell lung cancer, the patient’s central nervous system and CIPO symptoms improved but did not completely disappear. He died of lung cancer 2 years later.
Discussion
Paraneoplastic antibodies can be divided into the following two categories: (1) anti-cytoplasmic antigen antibodies, including anti-Hu, Yo, Ri, PNMA2(Ma2/Ta), CV2/CRMP5, and anti-amphiphysin antibodies, which are significantly correlated with clinical symptoms of the nervous system and potential tumors;8 and (2) antibodies against cell surface membrane proteins such as anti-VGCC, anti-voltage-gated potassium channels (VGKC), and anti-N-methyl-D-aspartate receptor (NMDA) antibody.9 In PNS with anti-cytoplasmic antigen antibodies, T cells are thought to have pathogenic roles, while in PNS with anti-cell surface antigen antibodies, humoral immunity plays a dominant role.
CV2/CRMP5 antibody was initially called as CV2, and it was subsequently identified as a 66 kDa cytoplasmic protein (CRMP5), which is a member of the CRMP family of proteins. Thus, it is also known as anti-CRMP5 antibody. CRMP is a family of five phosphorylated proteins (crmp1–5) that participate in dendrite and axon formation during nerve cell development and regeneration through intracellular signal transduction, which is crucial for neuronal development and repair. CRMP5 can interact with the actin cytoskeleton network in the growth cone and affect growth cone development and neurite outgrowth via this interaction in developing hippocampal neurons.10 However, the detailed mechanisms of how CRMP5 regulates neurite outgrowth remain unclear.
CRMP5 is highly expressed in the developing brain, but it is decreased in the adult brain. In the postnatal stage, CRMP5 expression is restricted to the brain regions with regenerative capacity including the dentate granular layer, olfactory bulb, and rostral migratory stream, which confirms the role of this member in neuronal migration/differentiation.11 CRMP5 mRNA was also detected in neurons of the hypothalamus, thalamus, cortex, amygdala, brainstem, and cerebellum. Recently, Yamashita et al.12 found that CRMP5 is involved in the development, maintenance, and synaptic plasticity in Purkinje cells.
CIPO is characterized by the signs and symptoms of mechanical bowel obstruction without mechanical intestinal obstruction, which often results from derangement of innervation, smooth muscle, and interstitial cells of Cajal. The clinical features of CIPO are pleomorphic and largely depend on the site and extent of the GI tract segment that is involved. The common clinical manifestations of abdominal pain, abdominal distension, nausea, and vomiting may be nonspecific.13 There is no specific diagnostic test or pathological diagnosis for CIPO.13 The diagnostic approach involves exclusion of mechanical GI obstruction, screening for causes of secondary CIPO, and identification of the disease phenotype.14 Abdominal X-ray is the simplest way to confirm intestinal obstruction. In 85% of patients, there is a fluid–gas plane and general dilatation of the intestine. Gas accumulation is common in the colon, while the mechanical ileus is in the distal portion of the intestine. Careful observation of abdominal X-ray film will help to preliminarily distinguish CIPO from mechanical ileus.
Patients with CV2/CRMP5 antibodies presented more frequently with cerebellar ataxia, chorea, uveo-retinal symptoms, and myasthenic syndrome (such as Lambert–Eaton myasthenic syndrome [LEMS] or myasthenia gravis). However, dysautonomia, brainstem encephalitis, and peripheral neuropathy were more frequent in patients who were positive for Hu antibodies.15 CIPO due to PNS associated with anti-CV2/CRMP5 antibodies is rarely reported. Autonomic PNS is typically characterized by chronic GI pseudo-obstruction or obstruction. Postural hypotension usually occurs in conjunction with other PNS rather than alone.16,17 CIPO has been reported to be associated with autonomic neuropathy.7,18 One hypothesis is that disordered extrinsic nerve control of intestinal movement and abnormal autonomic nerve supply of the intestinal tract leads to motor dysfunction of the GI tract.19 Physicians should be reminded to evaluate autonomic nervous function if common features such as orthostatic hypotension, abnormal sweating, bladder dysfunction, and difficulty ejaculation are present.19 The manifestations of intestinal obstruction symptoms and/or bladder movement disorders highly suggest the existence of CIPO.20 This patient had various autonomic dysfunctions such as profuse sweating, dysuria, and difficulty defecating, which are consistent with the symptoms that are mentioned above.
We reviewed the studies on CV2/CRMP5 antibodies that were shown to be associated PNS and summarized the information in Table 1.21–30 These studies demonstrate that CIPO is a rare clinical manifestation of CV2/CRMP5 antibody PNS. Because of a delayed CIPO diagnosis, many patients undergo a variety of surgical procedures.31 Use of rituximab and cyclophosphamide should be considered in patients with paraneoplastic intestinal pseudo-obstruction, which may help to avoid complicated surgical procedures in some patients.32
Table 1.
Anti-CV2/CRMP5 PNS features in recent years.
| References | Gender | Age (years) | Tumor type | Clinical classification/presentation |
|---|---|---|---|---|
| 1. Jarius et al.21 | M | 66 | prostate cancer | NMO |
| 2. Saloustros et al.22 | F | 60 | head and neck cancer | PCD |
| 3. Nakajima et al.23 | F | 41 | SCLC | PON and paraneoplastic opsoclonus–ataxia syndrome |
| 4. Ducray et al.24 | F | 45 | thymoma | NMO |
| 5. Aliprandi et al.25 | M | 80 | prostate adenocarcinoma | PCD |
| 6. Dericioglu et al.26 | F | 54 | SCLC | PSE |
| 7. Verschueren et al.27 | F | 80 | thymoma | rapidly progressive LMNS |
| 8. Rosencher et al.28 | M | 64 | SCLC | visual symptoms with papilledema, cerebellar signs, polyneuropathy |
| 9. Hannawi et al.29 | M | 54 | – | chronic progressive axonal polyradiculoneuropathy |
| 10. Ritzenthaler et al.30 | M | 80 | SCLC | Paraneoplastic chorea and behavioral disorders |
PNS, paraneoplastic neurological syndrome; CRMO, collapsin response mediator protein; M, male; F, female; NMO, neuromyelitis optica; SCLC, small-cell lung cancer; PSE, paraneoplastic striatal encephalitis; PCD, paraneoplastic cerebellar degeneration; LMNS, rapidly progressive lower motor neuron syndrome; PON, paraneoplastic optic neuropathy.
In CIPO patients with anti-Hu antibodies, it is hypothesized that these antibodies interact with extensive lymphocyte infiltration in the intestinal plexus, causing dysfunction and eventually irreversible damage, which results in irreversible intestinal motility disorders.6 The pathological findings in intestinal pseudo-obstruction consist of myenteric plexus infiltration by plasma cells and lymphocytes as well as axonal and neuronal degeneration, and there are significantly fewer neurons in each area of the GI tract.19 However, what is the relationship between CRMP5 and CIPO? There are few relevant studies, and we investigated the CRMP family. More studies have been conducted on CRMP2, which is a novel Ca2+ channel that is mainly expressed in the nervous system and a “neuromodulator” of synaptic strength. It has been speculated that all CRMPs play a role in regulating Ca2+ channel density.33 The role of anti-CRMP5 antibodies is not currently understood. Only one study mentioned that CRMP5 mRNA was also transiently detected in neonatal muscle, which suggests that it may be transiently expressed during onset of innervation at the neuromuscular junction.34 However, there are currently no published studies that report CRMP5 expression in intestinal smooth muscle cells, and further molecular biology research is expected to reveal the advanced mechanism.
Conclusions
We present the case of a patient with CIPO and encephalomyelitis that was associated with anti-CV2/CRMP5 antibodies in a patient with small-cell lung cancer. To the best of our knowledge, this is the first report of paraneoplastic CIPO that is associated with anti-CV2/CRMP5 antibodies in a small-cell lung cancer patient. Some PNS patients with positive anti-CV2/CRMP5 antibody may have fatal complications such as CIPO. CIPO requires more attention during clinical evaluation.
Supplemental Material
Supplemental material, sj-pdf-1-imr-10.1177_0300060520974466 for Anti-CV2/CRMP5 antibody-positive paraneoplastic neurological syndromes with chronic intestinal pseudo-obstruction in a small-cell lung cancer patient: a case report and literature review by Jinhua Yan, Zhongbo Chen, Yumei Liang, Huijia Yang, Lizhi Cao, Yuling Zhou, Yang Zhao and Ying Zhang in Journal of International Medical Research
Footnotes
Ethics approval: Ethics approval was obtained from the Ethics Committee at The First Hospital of Jilin University, China.
Consent for publication: Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor of this journal.
Availability of data and materials: All data generated or analyzed during this study are included in this published article
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported from grants by National Natural Science Foundation of China (NO. 81600924) and the National Key Research and Development Project of China (NO. 2018YFC1312301). The funding supported design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Authors’ contributions: JHY drafted the manuscript; ZBC, YML, HJY, LZC, and YLZ collected the patient information; YZ1 and YZ2 interpreted the data and edited the manuscript.
ORCID iDs: Yumei Liang https://orcid.org/0000-0001-5442-5221
Yang Zhao https://orcid.org/0000-0002-9143-671X
References
- 1.Voltz R. Paraneoplastic neurological syndromes: an update on diagnosis, pathogenesis, and therapy. Lancet Neurol 2002; 1: 294–305. [DOI] [PubMed] [Google Scholar]
- 2.Leypoldt F andWandinger KP.. Paraneoplastic neurological syndromes. Clin Exp Immunol 2014; 175: 336–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grativvol RS, Cavalcante WCP, Castro LHM, et al. Updates in the diagnosis and treatment of paraneoplastic neurologic syndromes. Curr Oncol Rep 2018; 20: 92. [DOI] [PubMed] [Google Scholar]
- 4.Cogliandro RF, De Giorgio R, Barbara G, et al. Chronic intestinal pseudo-obstruction. Best Pract Res Clin Gastroenterol 2007; 21: 657–669. [DOI] [PubMed] [Google Scholar]
- 5.Iida H, Inamori M, Sekino Y, et al. A review of the reported cases of chronic intestinal pseudo-obstruction in Japan and an investigation of proposed new diagnostic criteria. Clin J Gastroenterol 2011; 4: 141–146. [DOI] [PubMed] [Google Scholar]
- 6.Taverna JA, Babiker HM, Yun S, et al. The great masquerader of malignancy: chronic intestinal pseudo-obstruction. Biomark Res 2014; 2: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Izumi Y, Masuda T, Horimasu Y, et al. Chronic intestinal pseudo-obstruction and orthostatic hypotension associated with small cell lung cancer that improved with tumor reduction after chemoradiotherapy. Intern Med 2017; 56: 2627–2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dalmau J andRosenfeld MR.. Paraneoplastic syndromes of the CNS. Lancet Neurol 2008; 7: 327–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Graus F, Delattre JY, Antoine JC, et al. Recommended diagnostic criteria for paraneoplastic neurological syndromes. J Neurol Neurosurg Psychiatry 2004; 75: 1135–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gong X, Tan M, Gao Y, et al. CRMP5 interacts with actin to regulate neurite outgrowth. Mol Med Rep 2016; 13: 1179–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ricard D, Rogemond V, Charrier E, et al. Isolation and expression pattern of human Unc-33-like phosphoprotein 6/collapsin response mediator protein 5 (Ulip6/CRMP5): coexistence with Ulip2/CRMP2 in Sema3a-sensitive oligodendrocytes. J Neurosci 2001; 21: 7203–7214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamashita N, Mosinger B, Roy A, et al. CRMP5 (collapsin response mediator protein 5) regulates dendritic development and synaptic plasticity in the cerebellar Purkinje cells. J Neurosci 2011; 31: 1773–1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Di Nardo G, Di Lorenzo C, Lauro A, et al. Chronic intestinal pseudo-obstruction in children and adults: diagnosis and therapeutic options. Neurogastroenterol Motil 2017; 29. [DOI] [PubMed] [Google Scholar]
- 14.Downes TJ, Cheruvu MS, Karunaratne TB, et al. Pathophysiology, diagnosis, and management of chronic intestinal pseudo-obstruction. J Clin Gastroenterol 2018; 52: 477–489. [DOI] [PubMed] [Google Scholar]
- 15.Honnorat J, Cartalat-Carel S, Ricard D, et al. Onco-neural antibodies and tumour type determine survival and neurological symptoms in paraneoplastic neurological syndromes with Hu or CV2/CRMP5 antibodies. J Neurol Neurosurg Psychiatry 2009; 80: 412–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lorusso L, Hart IK, Ferrari D, et al. Autonomic paraneoplastic neurological syndromes. Autoimmun Rev 2007; 6: 162–168. [DOI] [PubMed] [Google Scholar]
- 17.Ueno T, Hasegawa Y, Hagiwara R, et al. Integrated treatment for autonomic paraneoplastic syndrome improves performance status in a patient with small lung cell carcinoma: a case report. BMC Neurol 2018; 18: 189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maier A, Mannartz V, Wasmuth H, et al. GAD antibodies as key link between chronic intestinal pseudoobstruction, autonomic neuropathy, and limb stiffness in a nondiabetic patient: a CARE-compliant case report and review of the literature. Medicine (Baltimore) 2015; 94: e1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chinn JS andSchuffler MD.. Paraneoplastic visceral neuropathy as a cause of severe gastrointestinal motor dysfunction. Gastroenterology 1988; 95: 1279–1286. [DOI] [PubMed] [Google Scholar]
- 20.Zenzeri L, Tambucci R, Quitadamo P, et al. Update on chronic intestinal pseudo-obstruction. Curr Opin Gastroenterol 2020; 36: 230–237. [DOI] [PubMed] [Google Scholar]
- 21.Jarius S, Wandinger KP, Borowski K, et al. Antibodies to CV2/CRMP5 in neuromyelitis optica-like disease: case report and review of the literature. Clin Neurol Neurosurg 2012; 114: 331–335. [DOI] [PubMed] [Google Scholar]
- 22.Saloustros E, Zaganas I, Mavridis M, et al. Anti-CV2 associated cerebellar degeneration after complete response to chemoradiation of head and neck carcinoma. J Neurooncol 2010; 97: 291–294. [DOI] [PubMed] [Google Scholar]
- 23.Nakajima M, Uchibori A, Ogawa Y, et al. CV2/CRMP5-antibody-related paraneoplastic optic neuropathy associated with small-cell lung cancer. Intern Med 2018; 57: 1645–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ducray F, Roos-Weil R, Garcia PY, et al. Devic’s syndrome-like phenotype associated with thymoma and anti-CV2/CRMP5 antibodies J Neurol Neurosurg Psychiatry 2007; 78: 325–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aliprandi A, Terruzzi A, Rigamonti A, et al. Paraneoplastic cerebellar degeneration with anti-CV2/CRMP5 antibodies and prostate adenocarcinoma. Neurol Sci 2015; 36: 1501–1503. [DOI] [PubMed] [Google Scholar]
- 26.Dericioglu N Gocmen R andTan E.. Paraneoplastic striatal encephalitis and myelitis associated with anti-CV2/CRMP-5 antibodies in a patient with small cell lung cancer. Clin Neurol Neurosurg 2018; 170: 117–119. [DOI] [PubMed] [Google Scholar]
- 27.Verschueren A, Gallard J, Boucraut J, et al. Paraneoplastic subacute lower motor neuron syndrome associated with solid cancer. J Neurol Sci 2015; 358: 413–416. [DOI] [PubMed] [Google Scholar]
- 28.Rosencher L, Maisonobe T, Lavole A, et al. [Neurologic paraneoplastic syndrome with anti-CV2/CRMP5 antibodies revealing a small cell lung cancer. Effectiveness of the lung cancer treatment]. Rev Neurol (Paris) 2012; 168: 371–374. [DOI] [PubMed] [Google Scholar]
- 29.Hannawi Y, Goldsmith CE, Kass JS, et al. A case of severe chronic progressive axonal polyradiculoneuropathy temporally associated with anti-CV2/CRMP5 antibodies. J Clin Neuromuscul Dis 2013; 15: 13–18. [DOI] [PubMed] [Google Scholar]
- 30.Ritzenthaler T Verret JM andHonnorat J.. [Paraneoplastic chorea and behavioral disorders in a patient with anti-CV2/CRMP5 antibodies and two different tumors]. Rev Neurol (Paris) 2010; 166: 90–95. [DOI] [PubMed] [Google Scholar]
- 31.Lu W, Xiao Y, Huang J, et al. Causes and prognosis of chronic intestinal pseudo-obstruction in 48 subjects: a 10-year retrospective case series. Medicine (Baltimore) 2018; 97: e12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Badari A, Farolino D, Nasser E, et al. A novel approach to paraneoplastic intestinal pseudo-obstruction. Support Care Cancer 2012; 20: 425–428. [DOI] [PubMed] [Google Scholar]
- 33.Wang Y, Brittain JM, Wilson SM, et al. Emerging roles of collapsin response mediator proteins (CRMPs) as regulators of voltage-gated calcium channels and synaptic transmission. Commun Integr Biol 2010; 3: 172–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Byk T, Dobransky T, Cifuentes-Diaz C, et al. Identification and molecular characterization of Unc-33-like phosphoprotein (Ulip), a putative mammalian homolog of the axonal guidance-associated unc-33 gene product. J Neurosci 1996; 16: 688–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-imr-10.1177_0300060520974466 for Anti-CV2/CRMP5 antibody-positive paraneoplastic neurological syndromes with chronic intestinal pseudo-obstruction in a small-cell lung cancer patient: a case report and literature review by Jinhua Yan, Zhongbo Chen, Yumei Liang, Huijia Yang, Lizhi Cao, Yuling Zhou, Yang Zhao and Ying Zhang in Journal of International Medical Research