Abstract
Background:
A restricted response against citrullinated peptides/proteins, with less isotype usage, has been found in palindromic rheumatism (PR) in comparison with rheumatoid arthritis (RA). We hypothesized that this different antibody response may be observed for other post-translational modified proteins. We compared the prevalence and isotype usage of two specificities of anti-carbamylated peptide/protein antibodies (Anti-CarP) in patients with PR and RA.
Methods:
Cross-sectional study including 54 patients with pure PR and 53 patients with RA, matched by sex, age, disease duration and ACPA. Anti-CarP specificities were determined by home-made enzyme-linked immunosorbent assay tests using a synthetic chimeric fibrin/filaggrin homocitrullinated peptide (CFFHP) and fetal calf serum (FCS) homocitrullinated protein as antigens. IgG, IgA and IgM isotypes were measured.
Results:
Anti-CarP were positive (CFFHP or FCS) in 24% and 64% of patients with PR and RA, respectively (p < 0.005). All Anti-CarP isotype proportions were significantly lower in PR than in RA: Anti-CarP-IgG (24% versus 51%), Anti-CarP-IgA (7% versus 34%) and Anti-CarP-IgM (7% versus 36%). Mean titers of Anti-CarP isotypes were also lower in PR. In Anti-CarP positive patients, the isotype distribution differed between PR and RA: IgG Anti-CarP was used in all PR patients and in 79% of RA patients. By contrast, a significantly lower isotype usage of both IgA (31% versus 53%) and IgM (31% versus 56%) was observed in PR patients. No significant differences in clinical or demographic characteristics were observed according to Anti-CarP status in PR patients, except for a higher prevalence of ACPA and higher mean titers of ACPA and rheumatoid factor in Anti-CarP positive patients.
Conclusion:
Anti-CarP are found in patients with PR but in a lower proportion and with a different isotype usage from in RA, suggesting a distinct B cell response to homocitrullinated antigens in PR.
Keywords: ACPA, Anti-CarP, autoantibodies, B cells, palindromic rheumatism, rheumatoid arthritis
Introduction
Palindromic rheumatism (PR) is an intermittent form of arthritis/periarthritis which may progress to persistent polyarthritis, mainly rheumatoid arthritis (RA).1,2 PR patients may have the characteristic autoantibody profile seen in RA: positive rheumatoid factor (RF) and/or positive anticitrullinated peptide/protein antibodies (ACPA).3–6 ACPA are a biomarker for progression to RA in patients with PR,4 although a subset of ACPA-positive PR patients do not evolve to RA in the long term.7 The ACPA repertoire of PR patients differs from that observed in RA, with fewer fine ACPA specificities and less isotype usage, suggesting that some PR patients have a distinct B cell response to citrullinated antigens that may preclude evolution to persistent RA.8
To our knowledge, ACPA are the only anti-modified protein antibody (AMPA) type that has been analyzed in PR. Anti-carbamylated protein/peptide antibodies (Anti-CarP) are a different AMPA that recognize homocitrullinated antigens and have emerged as a new antibody family frequently found in the sera of RA patients, with a specificity close to that of ACPA but with a lower sensitivity.9 Anti-CarP have been associated with radiographic damage in RA, especially in ACPA-negative patients,9,10 may predict RA progression in patients with inflammatory arthralgia11 and have recently been associated with RA-associated interstitial lung disease.12
The aim of this study was to analyze the prevalence of two Anti-CarP specificities in PR patients and evaluate their isotype usage antibodies with that of patients with established RA. We hypothesized that the Anti-CarP response in PR may be more restricted than in RA, as occurs with the ACPA response.
Patients and methods
Study design and population
We made a cross-sectional study in patients with pure PR (not associated with any rheumatic disease at serum measurement) fulfilling the PR criteria described by Guerne and Weissman13 attending our outpatient clinic. All PR patients were treated according to the criteria of the treating physician. Patients fulfilling diagnostic criteria for other forms of inflammatory arthritis were excluded. Information on the current clinical, serological and treatment features at study entry were used for the analysis. Further details of this cohort are described elsewhere.8,14 Patients with established RA (1987 American College of Rheumatology criteria) matched by age, sex, ACPA positivity and disease duration were included as a control group.
Autoantibody assessment
Serum samples were collected at inclusion for autoantibody assessment. Two types of Anti-CarP were analyzed in sera from PR and RA patients: anti-chimeric fibrin/filaggrin homocitrullinated peptide (Anti-CFFHP) and anti-carbamylated fetal calf serum (Anti-FCS) fine specificities were determined by home-made enzyme-linked immunosorbent assay (ELISA) tests using a synthetic homocitrullinated peptide or carbamylated fetal calf serum as antigens and the non-homocitrullinated versions as the control peptide/protein for the homocitrulline specificity of Anti-CFFHP and Anti-FCS.
Chimeric fibrin/filaggrin homocitrullinated peptide (CFFHP): [HCit620,625] α-fibrin(617–631)-S306, S319 cyclo [Cys306,319, HCit312]filaggrin (304–324) and its non-homocitrullinated version were synthesized by solid-phase peptide synthesis as C-terminal carboxamides on a Novasyn TGR resin (Novabiochem Merck, Germany) following a 9-fluorenylmethoxycarbonyl strategy with subsequent cyclization in solution by forming a disulfide bridge as previously described for other chimeric citrullinated peptides.15,16
To determine Anti-CFFHP, home-made ELISA assays were used. First, CFFHP and non-homocitrullinated peptide as a control for homocitrulline specificity were coupled covalently to microplates (Nunc Immobilizer) diluted to 10 µg/mL in 0.05 M carbonate/bicarbonate (pH 9.6) buffer. 100 μL of peptide solution was added to each microplate well and incubated overnight at 4°C. Each plate contained control wells that included all reagents except the serum sample and the peptide to estimate the background reading. After incubation, the plates were blocked with 2% BSA in 0.05 M carbonate/bicarbonate (pH 9.6) buffer for 1 h at room temperature. Sera were diluted 50-fold in RIA buffer (1% BSA, 350 mM NaCl, 10 mM Tris-HCl, pH 7.6, 1% vol/vol Triton X-100, 0.5% wt/vol Na-deoxycholate, 0.1% SDS) supplemented with 10% fetal bovine serum; 100 μL/well was added and incubated for 1.5 h at room temperature. IgG, IgA and IgM were detected using peroxidase-conjugated rabbit anti-human IgG, rabbit anti-human serum IgA and rabbit anti-human IgM (Fc5µ fragment specific) (Jackson Immunoresearch Europe, UK), respectively, and SIGMAFAST with o-phenylenediamine dihydrochloride as substrate.
To detect Anti-FCS, an ELISA assay using both carbamylated and non-modified FCS as antigens were developed. FCS was carbamylated by incubating a 4 mg/mL concentration with 1M of KCNO (or with 1M of KCl for the controls) for 15 h at 37°C. After incubation, the samples were desalted by centrifugation (Amicon Ultra-0.5 centrifugal filter units, Merck). Carbamylation efficiency was assessed by amino acid analysis of the hydrolyzed samples in a Biochrom 30 amino acid analyzer (Biokrom, UK) using L-Norleucine as the internal standard. The conversion of Lys to homocitrulline was determined as the fraction of the total amount of amino acids.
Anti-FCS was determined by ELISA. All samples were assayed in separate plates (Nunc MaxiSorp, Thermo Fisher Scientific, Denmark) coated with FCS carbamylated and non-modified as antigens overnight at a concentration of 10 µg/mL of carbonate-bicarbonate buffer (0.1 M pH 9.6). The plates were blocked with 1% BSA in PBS-0.05% Tween for 6 h at 4°C and, after washing the plates, diluted serum samples (1:50 in PBS-1% BSA-0.05% Tween) were incubated overnight at 4°C. IgG, IgA and IgM antibodies were detected using alkaline phosphatase-conjugated goat anti-human IgG or rabbit anti-human serum IgA (α chain specific) or rabbit anti-human IgM (Fc5µ fragment specific) (Jackson Immunoresearch Europe, UK), respectively and SIGMAFAST p-nitrophenyl phosphate as substrate.
Reactivity to non-homocitrullinated FCS and CFFHP peptide was subtracted from the reactivity to homocitrullinated FCS and CFFHP. Successive dilutions of a pool of sera from four positive patients were used as a reference standard in all plates and to convert optical density (OD) values to arbitrary units (AUs).
A positive cut-off value was defined as >342.5 AU/mL, >354.0 AU/mL, and >210.5 AU/ml for Anti-FCS-IgG, Anti-FCS-IgA, and Anti-FCS-IgM, respectively, and >115.5 AU/mL, >218.0 AU/mL, >354.0 AU/mL for Anti-CFFHP-IgG, Anti-CFFHP-IgA, and Anti-CFFHP-IgM, respectively. A test was only considered positive and specific for homocitrulline when UA/mL values were higher than the respective cut-off and the difference in OD values between carbamylated (homocitrullinated) and native (non-homocitrullinated) antigens was ⩾0.1.
Serum levels of ACPA were measured using a CCP2 commercial test [ELISA; Immunoscan, Eurodiagnostica; cut-off >50 international units (IUs)] and RF by nephelometry (BNII, Siemens; cut-off >20 IU).
Ethics approval and consent to participate
The study was conducted in accordance with the Declaration of Helsinki and was approved by the Hospital Clinic of Barcelona Research Ethics Committee (approval number 2017/0679). Signed informed consent was obtained from all patients before study enrollment.
Statistical analysis
Between-group differences were analyzed using descriptive statistics as appropriate. Proportions were compared using the χ² or Fisher’s exact test. Continuous variables were analyzed using the Wilcoxon signed rank test or the Mann–Whitney U test and presented as mean and standard deviation (±) or median and interquartile range. Statistical significance was established as two-tailed p-values < 0.05 in all analyses, which were performed using IBM® SPSS® for Windows version 23.0. Missing data was handled with listwise deletion.
Results
Fifty-four PR patients and 54 RA patients were originally included in the study. Serum from one RA patient was not available for Anti-CarP measurement. Therefore, this patient was excluded from the final analysis. Baseline information on sex, age, disease duration, ACPA and RF positivity of PR and RA patients is presented in Table 1. No between-group differences were found for any of these variables.
Table 1.
Study population baseline clinical and serological features.
| PR n = 54 |
RA n = 53 |
p value | |
|---|---|---|---|
| Female, n (%) | 34 (63%) | 34 (64%) | NS |
| Age, mean years (±SD) | 51.2 (±9.3) | 54.5 (±11.2) | NS |
| Disease duration, mean years (±SD) | 11.6 (±10.7) | 8.4 (±6.1) | NS |
| Positive rheumatoid factor, n (%) | 31 (57) | (57) | NS |
| Rheumatoid factor, mean titer (95% CI) | 96 (42–151) | 221 (66–377) | NSa,b |
| Positive ACPA, n (%) | 36 (67%) | 36 (68%) | NS |
| ACPA, mean titer (95% CI) | 354 (219–489) | 496 (335–658) | NSa,b |
Including negative and positive patients.
No difference when analyzing only positive patients (data not shown).
ACPA, anti-citrullinated protein/peptide autoantibodies; CI, confidence interval; n, number; NS, not significant; PR, palindromic rheumatism; RA, rheumatoid arthritis; SD, standard deviation.
Anti-CarP in PR
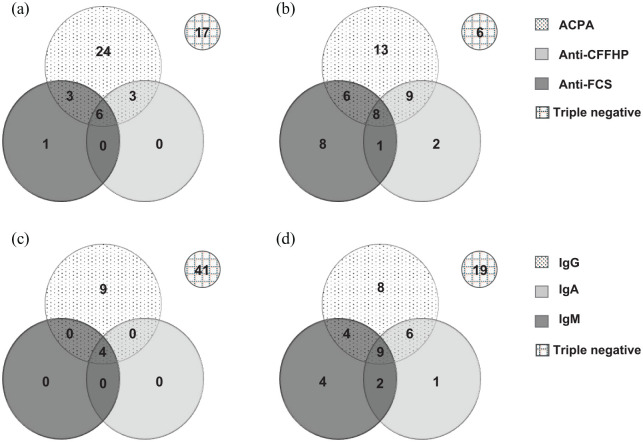
Anti-CarP (FCS or CFFHP) were observed in 13 PR patients (24%). ACPA and Anti-CarP overlap was observed in 12 patients (92% in Anti-CarP positive patients) Figure 1(a). No significant differences were observed in demographic data and clinical or therapeutic findings according to Anti-CarP status in PR patients. However, a significantly higher proportion of ACPA positivity and titers was observed in Anti-CarP PR positive patients. RF titers were higher in these patients (Table 2).
Figure 1.
Antibodies overlap in palindromic rheumatism (PR) and rheumatoid arthritis (RA). (a) PR patients, overlap between Anti-FCS, Anti-CFFHP and ACPA; (b) RA patients, overlap between Anti-FCS, Anti-CFFHP and ACPA; (c) PR patients, Anti-CarP (Anti-FCS and Anti-CFFHP) isotypes overlap; and (d) RA patients, Anti-CarP (Anti-FCS and Anti-CFFHP) isotypes overlap.
ACPA, anti-citrullinated protein/peptide autoantibodies; Anti-CFFHP, anti-chimeric fibrin/filaggrin homocitrullinated peptide antibodies; Anti-FCS, anti-carbamylated fetal calf serum antibodies; Ig, immunoglobulin.
Table 2.
Demographic, clinical, serological and therapeutic features in patients with palindromic rheumatism according to Anti-CarP status.
|
Anti-CarP positive
n = 13 |
Anti-CarP negative
n = 41 |
p value | |
|---|---|---|---|
| Female, n (%) | 7 (54%) | 27 (66%) | NS |
| Age, mean years (±SD) | 50.0 (±9.3) | 52.2 (±11.9) | NS |
| PR onset age, mean years (±SD) | 40.9 (±8.9) | 39.5 (±11.7) | NS |
| Disease duration since PR symptom onset, mean years (±SD) | 9.0 (±6.9.4) | 12.8 (±11.2) | NS |
| Ever smokers, n (%) | 8 (62%) | 26 (63%) | NS |
| Current smokers, n (%) | 6 (46%) | 11 (27%) | NS |
| Smoking cumulative dose ±SD | 15.8 ± 7.8 | 19.0 ± 17.9 | NS |
| Positive rheumatoid factor, n (%) | 10 (77%) | 21 (5%) | NS |
| Rheumatoid factor, mean titer (95% CI) | 239 (76–443) | 51 (34–74) | 0.012 |
| Positive ACPA, n (%) | 12 (92%) | 24 (59%) | 0.04 |
| ACPA, mean titer (95% CI) | 781 (418–1183) | 219 (124–339) | <0.005 |
| Frequency (periodicity) of PR flares | |||
| 1 month | 3 (23%) | 3 (7%) | NS |
| ⩾1 week | 3 (23%) | 8 (20%) | NS |
| PR flares duration | |||
| ⩽72 h, n (%) | 12 (92%) | 35 (85%) | NS |
| 72 h ⩽168 h, n (%) | 1 (8%) | 6 (15%) | NS |
| DMARDs | |||
| HCQ, n (%) | 5 (39%) | 15 (37%) | NS |
| MTX, n (%) | 1 (8%) | 5 (12%) | NS |
| DMARDs, not including HCQ, n (%) | 4 (31%) | 9 (22%) | NS |
| GC, n (%) | 2 (15%) | 6 (15%) | NS |
ACPA, anti-citrullinated protein/peptide autoantibodies; Anti-CarP, anti-carbamylated protein/peptide antibodies; CI, confidence interval; DMARD, disease-modifying anti-rheumatic drug; GC, glucocorticoid; HCQ, hydroxychloroquine; h, hours; MTX, methotrexate; n, number; NS, not significant; PR, palindromic rheumatism; SD, standard deviation.
In PR patients, IgG was the predominant isotype: all 13 Anti-CarP positive patients used the IgG isotype. Anti-FCS-IgG and Anti-CFFHP-IgG were observed in 19% and 17%, respectively, of PR patients [six patients (11%) yielded positive results for both tests]. Anti-CarP-IgA and Anti-CarP-IgM were each found in only four patients (7%) [Table 3 and Figure 1(c)].
Table 3.
Anti-CarP specificities in palindromic rheumatism and rheumatoid arthritis patients.
| Anti-FCS |
Anti-CFFHP |
Any specificity |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PR n (%) |
RA n (%) |
p value | PR n (%) |
RA n (%) |
p value | PR n (%) |
RA n (%) |
p value | ||
| Positive | IgG | 10 (19) | 19 (36) | 0.044 | 9 (17) | 11 (21) | 0.588 | 13 (24) | 27 (51) | 0.004 |
| IgA | 4 (7) | 10 (19) | 0.093 | 1 (2) | 10 (19) | 0.004 | 4 (7) | 18 (34) | 0.001 | |
| IgM | 4 (7) | 13 (25) | 0.018 | 0 (0) | 8 (15) | 0.003 | 4 (7) | 19 (36) | 0.000 | |
| Any isotype | 10 (19) | 23 (43) | 0.005 | 9 (17) | 20 (38) | 0.014 | 13 (24) | 34 (64) | 0.001 | |
Anti-CarP, anti-carbamylated protein/peptide antibodies; Anti-CFFHP, anti-chimeric fibrin/filaggrin homocitrullinated peptide antibodies; Anti-FCS, anti-carbamylated fetal calf serum antibodies; Ig, immunoglobulin; n, number; PR, palindromic rheumatism; RA, rheumatoid arthritis.
Thirteen PR patients were triple negative (negative Anti-CarP, ACPA and RF). No significant differences in clinical and demographic data were observed when comparing triple negative versus Anti-CarP positive patients, or when comparing triple negative versus ACPA positive patients (with or without Anti-CarP; n = 36) except for a shorter flare duration (<72 h) (69% versus 94%; p = 0.036) in the latter group (data not shown).
Anti-CarP in RA
In the RA group (n = 53), Anti-CarP specificities were found in 34 patients (64%). ACPA and Anti-CarP overlap was observed in 23 patients (68% of Anti-CarP positive RA patients) [Figure 1(b)]. IgG was the predominant isotype in the RA group, found in 79% of patients positive for Anti-CarP. Anti-FCS-IgG and Anti-CFFHP-IgG were found in 36% and 21%, respectively, of RA patients [three patients (6%) were positive for both tests]. IgA and IgM Anti-CarP isotypes were found in 34% and 36%, respectively [Table 3 and Figure 1(d)].
Anti-CarP between-group comparison
Positivity for Anti-CarP was significantly lower in PR patients (24% versus 64%; p < 0.005). All isotype proportions were numerically higher in RA patients. Significant differences were found for Anti-FCS-IgG, Anti-FCS-IgM, Anti-CFFHP-IgA and Anti-CFFHP-IgM (Table 3). In addition, PR patients had a lower mean number of positive Anti-CarP specificities (0.52 versus 1.34, p < 0.005). Triple positivity (Anti-CarP, ACPA and RF) was more frequent in RA patients (36% versus 19%; p = 0.044).
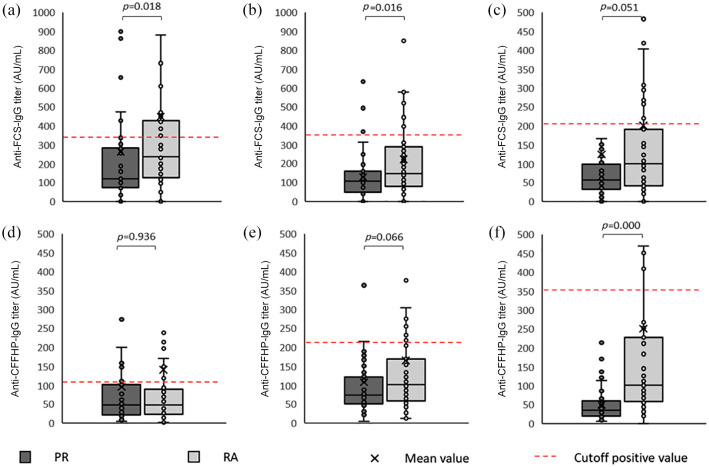
All Anti-CarP isotype mean titers were higher in RA, with mean levels ranging from 1.3- to 5-fold-higher, although the differences were significant only for the Anti-FCS-IgG, Anti-FCS-IgA and Anti-CFFHP-IgM isotypes (p < 0.05 for all comparisons). A trend to significance was observed for Anti-FCS-IgM and Anti-CFFHP-IgA (Figure 2). However, when analyzing only Anti-CarP positive patients, there was no significant between-group (PR Anti-CarP positive versus RA Anti-CarP positive) difference for either specificity titer.
Figure 2.
Anti-CarP titers in palindromic rheumatism and rheumatoid arthritis.
Anti-CarP, anti-carbamylated protein/peptide antibodies; Anti-FCS, anti-carbamylated fetal calf serum antibodies; Anti-CFFHP, anti-chimeric fibrin/filaggrin homocitrullinated peptide antibodies; AU, arbitrary unit; Ig, immunoglobulin; mL, milliliter.
Discussion
Our results show, for the first time, the presence of Anti-CarP, an AMPA different from ACPA, in patients with pure PR. Anti-CarP isotype usage pattern in PR differed from that observed in RA, with a smaller proportion and lower titers in all specificities and isotypes in PR.
Two antigens were chosen to ensure the veracity of the results. First, FCS are by far the most widely-described antigen to test Anti-CarP.9,10,17 Second, CFFHP12 is the homocitrullinated version of a chimeric citrullinated peptide bearing fibrin and filaggrin domains developed by our group. This antigen has been studied in a large series of patients with various rheumatic conditions, together with healthy controls, and has demonstrated the presence of different peptide sequences within the same molecule rendered synergistic effects compared with monomeric peptides.15,18,19 As in a previous study,12 Anti-FCS and Anti-CFFHP behaved similarly.
We found Anti-CarP in over half of RA patients. IgG was the most prevalent isotype (range 21–36%, according to the specificity), in agreement with that reported in patients with established RA.9,20 Anti-CarP were found in only 24% of PR patients. As in RA,21 Anti-CarP were clearly associated with ACPA positivity, even though there was a greater overlap between the two antibodies in PR patients than in RA. Inhibition studies have demonstrated that the overlap between antibodies is not simply due to the remarkably similar chemical structure in the antigenic targets of Anti-CarP and ACPA.22 However, even a highly specific ACPA monoclonal antibody can cross-react toward various post translational modifications (citrulline, homocitrulline and acetylide).23,24 Likewise, immunization with a specific antigen can generate different AMPA.25 An individual patient may have various AMPA clones with distinct reactivity profiles capable of recognizing multiple amino acid motifs, rather than specific proteins.23–25 It might be speculated that PR AMPA clones have a narrowed amino acid motif recognition profile, ensuing a lower cross reactivity.
Besides the association with other autoantibodies, no differences in demographic, clinical or therapeutic characteristics were observed according to Anti-CarP status in PR. The relapsing–remitting nature of palindromic flares resembles the clinical picture of an autoinflammatory disorder,2 and a subgroup of PR patients (mostly ACPA negative) have MEFV mutations, suggesting innate immune activation in PR.26 Nonetheless, the presence of autoantibodies supports the hypothesis that the adaptative immune system is activated in most PR patients.
In established RA, a broad spectrum of Anti-CarP isotypes (IgG, IgM and IgA) have been documented, as in our cohort.21 The isotype distribution is quite different in PR, where IgG clearly predominates, and few patients used the IgA or IgM isotype. IgA is the major Ig isoform produced at mucosal surfaces and plays a major role in the tissue immune function. ACPA-IgA have been found within inflamed lungs and gastrointestinal tissues of RA patients.27 The greatest differences were observed with the IgM isotype. Given the short half-life of IgM, the small proportion of IgM Anti-CarP seen in PR patients may be interpreted as a sign of discontinuous activation of the B cell immune response to modified proteins.28 ACPA followed a similar isotype pattern in PR when analyzed in a previous study.8
The differing AMPA profile in PR patients, with a more restricted pattern, has also been described in the preclinical phases of RA10 and in unaffected relatives of RA patients29 and may account for a less pathogenic role of these autoantibodies.30 ACPA and Anti-CarP may be present several years before RA onset,10,31 and the specificities and titers of both antibodies seem to increase close to RA onset.10,32 Although all ACPA isotypes may be present in the pre-RA stage,31 IgG is the predominant isotype and appears earlier than IgM and IgA.33 It is likely that Anti-CarP isotypes behave similarly in individuals at risk of RA, such as PR patients. Whether this more isotype-restricted AMPA repertoire pattern, with less IgA and IgM isotype use in PR patients, is associated with less propensity to RA progression is unclear. Other factors, such as Fc-glycosylation, have been suggested to play a critical role.28
Our study has the limitations of a small sample size and a selection bias towards treated PR patients with a longstanding disease course, who are probably less prone to evolving to RA, and the results probably cannot be extrapolated to patients with recent onset PR. In addition, the cross-sectional design limited our ability to draw conclusions on the clinical significance or the predictive value for RA progression of our findings.
Conclusion
In conclusion, as with RF and ACPA, Anti-CarP are found in patients with pure PR. Although it is unclear whether PR is a separate entity or a preclinical or abortive form of RA,2,3,34 the similar serological profile observed in PR, even without evolution to persistent arthritis fulfilling RA criteria, strongly suggests that PR may form part of the clinical spectrum of RA. A restricted pattern, with a lower proportion and less isotype usage of Anti-CarP in PR than in RA, suggests a distinct B-cell response against post-translational antigens, which may explain the non-evolution to RA in some patients. However, further studies including recent-onset PR patients are needed to validate our findings, as are long-term prospective evaluations of the clinical significance of the restricted AMPA pattern in PR patients.
Acknowledgments
The authors thank Nuria Sapena and Cristina González Delaurens for sera sampling support and assistance.
Footnotes
Author contributions: RC, SR, SC, IH and RS contributed to the conception and study design. MG, CG and IH conceived, designed and performed the ELISA tests. JI, JR, RM, GS and JG collected samples. RC, SC, VR, JG and AC contributed to data collection. RC, SR, RS and JC analyzed and interpreted the data. RC and RS wrote the first version of the manuscript and JR, JG, JC and IH revised it critically. All authors read and approved the final manuscript.
Availability of data and materials: The datasets used and/or analyzed during the study are available from the corresponding author upon reasonable request.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the Hospital Clinic of Barcelona, Research, Innovation and Education Department (Grant # 37933 to RC) and the Spanish Ministry of Economy, Industry and Competitiveness and the European Regional Development Fund (Grant # RTI2018-094120-B-I00 to IH).
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iDs: Raul Castellanos-Moreira  https://orcid.org/0000-0002-4104-4101
https://orcid.org/0000-0002-4104-4101
Julio Ramirez  https://orcid.org/0000-0002-7047-8056
https://orcid.org/0000-0002-7047-8056
Raimon Sanmarti  https://orcid.org/0000-0002-8864-3806
https://orcid.org/0000-0002-8864-3806
Contributor Information
Raul Castellanos-Moreira, Arthritis Unit, Rheumatology Department, Hospital Clinic of Barcelona, Barcelona, Spain.
Sebastian C. Rodriguez-Garcia, Arthritis Unit, Rheumatology Department, Hospital Clinic of Barcelona, Barcelona, Spain
Sonia Cabrera-Villalba, Arthritis Unit, Rheumatology Department, Hospital Clinic of Barcelona, Barcelona, Spain.
María José Gomara, Unit of Synthesis and Biomedical Applications of Peptides, Institute of Advanced Chemistry of Catalonia, Consejo Superior de Investigaciones Científicas (IQAC-CSIC), Barcelona, Spain.
Georgina Salvador, Rheumatology Department, University Hospital Mutua Tarrasa, Barcelona, Spain.
Virginia Ruiz-Esquide, Arthritis Unit, Rheumatology Department, Hospital Clinic of Barcelona, Barcelona, Spain.
Julio Ramirez, Arthritis Unit, Rheumatology Department, Hospital Clinic of Barcelona, Barcelona, Spain.
Jose Inciarte-Mundo, Arthritis Unit, Rheumatology Department, Hospital Clinic of Barcelona, Barcelona, Spain.
Rosa Morla, Arthritis Unit, Rheumatology Department, Hospital Clinic of Barcelona, Barcelona, Spain.
Cristina Garcia-Moreno, Unit of Synthesis and Biomedical Applications of Peptides, Institute of Advanced Chemistry of Catalonia, Consejo Superior de Investigaciones Científicas (IQAC-CSIC), Barcelona, Spain.
Andrea Cuervo, Arthritis Unit, Rheumatology Department, Hospital Clinic of Barcelona, Barcelona, Spain.
Jose A. Gómez-Puerta, Arthritis Unit, Rheumatology Department, Hospital Clinic of Barcelona, Barcelona, Spain
Juan D. Cañete, Arthritis Unit, Rheumatology Department, Hospital Clinic of Barcelona, Barcelona, Spain
Isabel Haro, Unit of Synthesis and Biomedical Applications of Peptides, Institute of Advanced Chemistry of Catalonia, Consejo Superior de Investigaciones Científicas (IQAC-CSIC), Barcelona, Spain.
Raimon Sanmarti, Arthritis Unit, Rheumatology Department, Hospital Clinic of Barcelona, Villarroel 170, Barcelona, 08036, Spain.
References
- 1. Sanmarti R, Canete JD, Salvador G. Palindromic rheumatism and other relapsing arthritis. Best Pract Res Clin Rheumatol 2004; 18: 647–661. [DOI] [PubMed] [Google Scholar]
- 2. Mankia K, Emery P. Palindromic rheumatism as part of the rheumatoid arthritis continuum. Nat Rev Rheumatol 2019; 15: 687–695. [DOI] [PubMed] [Google Scholar]
- 3. Salvador G, Gomez A, Vinas O, et al. Prevalence and clinical significance of anti-cyclic citrullinated peptide and antikeratin antibodies in palindromic rheumatism. An abortive form of rheumatoid arthritis? Rheumatology (Oxford) 2003; 42: 972–975. [DOI] [PubMed] [Google Scholar]
- 4. Russell AS, Devani A, Maksymowych WP. The role of anti-cyclic citrullinated peptide antibodies in predicting progression of palindromic rheumatism to rheumatoid arthritis. J Rheumatol 2006; 33: 1240–1242. [PubMed] [Google Scholar]
- 5. Khabbazi A, Hajialiloo M, Kolahi S, et al. A multicenter study of clinical and laboratory findings of palindromic rheumatism in Iran. Int J Rheum Dis 2012; 15: 427–430. [DOI] [PubMed] [Google Scholar]
- 6. Mankia K, D’Agostino MA, Wakefield RJ, et al. Identification of a distinct imaging phenotype may improve the management of palindromic rheumatism. Ann Rheum Dis 2019; 78: 43–50. [DOI] [PubMed] [Google Scholar]
- 7. Sanmarti R, Cabrera-Villalba S, Gomez-Puerta JA, et al. Palindromic rheumatism with positive anticitrullinated peptide/protein antibodies is not synonymous with rheumatoid arthritis. A longterm followup study. J Rheumatol 2012; 39: 1929–1933. [DOI] [PubMed] [Google Scholar]
- 8. Cabrera-Villalba S, Gomara MJ, Canete JD, et al. Differing specificities and isotypes of anti-citrullinated peptide/protein antibodies in palindromic rheumatism and rheumatoid arthritis. Arthritis Res Ther 2017; 19: 141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shi J, Knevel R, Suwannalai P, et al. Autoantibodies recognizing carbamylated proteins are present in sera of patients with rheumatoid arthritis and predict joint damage. Proc Natl Acad Sci U S A 2011; 108: 17372–17377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brink M, Verheul MK, Ronnelid J, et al. Anti-carbamylated protein antibodies in the pre-symptomatic phase of rheumatoid arthritis, their relationship with multiple anti-citrulline peptide antibodies and association with radiological damage. Arthritis Res Ther 2015; 17: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ten Brinck RM, van Steenbergen HW, van Delft MAM, et al. The risk of individual autoantibodies, autoantibody combinations and levels for arthritis development in clinically suspect arthralgia. Rheumatology (Oxford) 2017; 56: 2145–2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Castellanos-Moreira R, Rodriguez-Garcia SC, Gomara MJ, et al. Anti-carbamylated proteins antibody repertoire in rheumatoid arthritis: evidence of a new autoantibody linked to interstitial lung disease. Ann Rheum Dis 2020; 79: 587–594. [DOI] [PubMed] [Google Scholar]
- 13. Guerne PA, Weisman MH. Palindromic rheumatism: part of or apart from the spectrum of rheumatoid arthritis. Am J Med 1992; 93: 451–460. [DOI] [PubMed] [Google Scholar]
- 14. Cabrera-Villalba S, Ramirez J, Salvador G, et al. Is there subclinical synovitis in patients with palindromic rheumatism in the intercritical period? A clinical and ultrasonographic study according to anticitrullinated protein antibody status. J Rheumatol 2014; 41: 1650–1655. [DOI] [PubMed] [Google Scholar]
- 15. Perez ML, Gomara MJ, Ercilla G, et al. Antibodies to citrullinated human fibrinogen synthetic peptides in diagnosing rheumatoid arthritis. J Med Chem 2007; 50: 3573–3584. [DOI] [PubMed] [Google Scholar]
- 16. Malakoutikhah M, Gomara MJ, Gomez-Puerta JA, et al. The use of chimeric vimentin citrullinated peptides for the diagnosis of rheumatoid arthritis. J Med Chem 2011; 54: 7486–7492. [DOI] [PubMed] [Google Scholar]
- 17. Truchetet ME, Dublanc S, Barnetche T, et al. Association of the presence of anti-carbamylated protein antibodies in early arthritis with a poorer clinical and radiologic outcome: data from the French ESPOIR cohort. Arthritis Rheumatol 2017; 69: 2292–2302. [DOI] [PubMed] [Google Scholar]
- 18. Sanmarti R, Graell E, Perez ML, et al. Diagnostic and prognostic value of antibodies against chimeric fibrin/filaggrin citrullinated synthetic peptides in rheumatoid arthritis. Arthritis Res Ther 2009; 11: R135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gomara MJ, Rodriguez J, Bleda MJ, et al. Comparative study of the diagnostic and prognostic value of antibodies against chimeric citrullinated synthetic peptides and CCP3/CCP3.1 assays. Clin Chem Lab Med 2018; 56: 285–293. [DOI] [PubMed] [Google Scholar]
- 20. Pecani A, Alessandri C, Spinelli FR, et al. Prevalence, sensitivity and specificity of antibodies against carbamylated proteins in a monocentric cohort of patients with rheumatoid arthritis and other autoimmune rheumatic diseases. Arthritis Res Ther 2016; 18: 276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. van Delft MAM, Verheul MK, Burgers LE, et al. The isotype and IgG subclass distribution of anti-carbamylated protein antibodies in rheumatoid arthritis patients. Arthritis Res Ther 2017; 19: 190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shi J, Willemze A, Janssen GM, et al. Recognition of citrullinated and carbamylated proteins by human antibodies: specificity, cross-reactivity and the ‘AMC-Senshu’ method. Ann Rheum Dis 2013; 72: 148–150. [DOI] [PubMed] [Google Scholar]
- 23. Kissel T, Reijm S, Slot LM, et al. Antibodies and B cells recognising citrullinated proteins display a broad cross-reactivity towards other post-translational modifications. Ann Rheum Dis 2020; 79: 472–480. [DOI] [PubMed] [Google Scholar]
- 24. Sahlstrom P, Hansson M, Steen J, et al. Different hierarchies of anti-modified protein autoantibody reactivities in rheumatoid arthritis. Arthritis Rheumatol. Epub ahead of print 5 June 2020. DOI: 10.1002/art.41385. [DOI] [PubMed] [Google Scholar]
- 25. Kampstra ASB, Dekkers JS, Volkov M, et al. Different classes of anti-modified protein antibodies are induced on exposure to antigens expressing only one type of modification. Ann Rheum Dis 2019; 78: 908–916. [DOI] [PubMed] [Google Scholar]
- 26. Canete JD, Arostegui JI, Queiro R, et al. An unexpectedly high frequency of MEFV mutations in patients with anti-citrullinated protein antibody-negative palindromic rheumatism. Arthritis Rheum 2007; 56: 2784–2788. [DOI] [PubMed] [Google Scholar]
- 27. Holers VM, Demoruelle MK, Kuhn KA, et al. Rheumatoid arthritis and the mucosal origins hypothesis: protection turns to destruction. Nat Rev Rheumatol 2018; 14: 542–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Scherer HU, Huizinga TWJ, Kronke G, et al. The B cell response to citrullinated antigens in the development of rheumatoid arthritis. Nat Rev Rheumatol 2018; 14: 157–169. [DOI] [PubMed] [Google Scholar]
- 29. Koppejan H, Trouw LA, Sokolove J, et al. Role of anti-carbamylated protein antibodies compared to anti-citrullinated protein antibodies in indigenous North Americans with rheumatoid arthritis, their first-degree relatives, and healthy controls. Arthritis Rheumatol 2016; 68: 2090–2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ioan-Facsinay A, Willemze A, Robinson DB, et al. Marked differences in fine specificity and isotype usage of the anti-citrullinated protein antibody in health and disease. Arthritis Rheum 2008; 58: 3000–3008. [DOI] [PubMed] [Google Scholar]
- 31. Kokkonen H, Mullazehi M, Berglin E, et al. Antibodies of IgG, IgA and IgM isotypes against cyclic citrullinated peptide precede the development of rheumatoid arthritis. Arthritis Res Ther 2011; 13: R13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Brink M, Hansson M, Mathsson L, et al. Multiplex analyses of antibodies against citrullinated peptides in individuals prior to development of rheumatoid arthritis. Arthritis Rheum 2013; 65: 899–910. [DOI] [PubMed] [Google Scholar]
- 33. Kelmenson LB, Wagner BD, McNair BK, et al. Timing of elevations of autoantibody isotypes prior to diagnosis of rheumatoid arthritis. Arthritis Rheumatol 2020; 72: 251–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Katz SJ, Russell AS. Palindromic rheumatism: a pre-rheumatoid arthritis state? J Rheumatol 2012; 39: 1912–1913. [DOI] [PubMed] [Google Scholar]