Abstract
Introduction:
The CDC and Illinois Department of Public Health disseminated risk factor criteria for COVID-19 testing early in the pandemic. The objective of this study is to assess the effectiveness of risk stratifying patients for COVID-19 testing and to identify which risk factors and which other clinical variables were associated with SARS-CoV-2 PCR test positivity.
Methods:
We conducted an observational cohort study on a sample of symptomatic patients evaluated at an immediate care setting. A risk assessment questionnaire was administered to every patient before clinician evaluation. High-risk patients received SARS-CoV-2 test and low-risk patients were evaluated by a clinician and selectively tested based on clinician judgment. Multivariate analyses tested whether risk factors and additional variables were associated with test positivity.
Results:
The adjusted odds ratio of testing positive was associated with COVID-19-positive or suspect close contact (aOR 1.56, 95% CI 1.15-2.10), large gathering attendance with a COVID-19-positive individual (aOR 1.92, 95% CI 1.10-3.34), and, with the largest effect size, decreased taste/smell (aOR 2.83, 95% CI 2.01-3.99). Testing positive was associated with ages 45-64 and ≥65 (aOR 1.75, 95% CI 1.25-2.44, and aOR 2.78, 95% CI 1.49-5.16), systolic blood pressures ≤120 (aOR 1.64, 95% CI 1.20-2.24), and, with the largest effect size, temperatures ≥99.0°F (aOR 3.06, 95% CI 2.23-4.20). The rate of positive SARS-CoV-2 test was similar between high-risk and low risk patients (225 [22.2%] vs 50 [19.8%]; P = .41).
Discussion:
The risk assessment questionnaire was not effective at stratifying patients for testing. Although individual risk factors were associated with SARS-CoV-2 test positivity, the low-risk group had similar positivity rates to the high-risk group. Our observations underscore the need for clinicians to develop clinical experience and share best practices and for systems and payors to support policies, funding, and resources to test all symptomatic patients.
Keywords: COVID-19, infectious disease, primary care, quality improvement, diagnostic testing
Introduction
Early in the COVID-19 pandemic, primary care clinicians were challenged to assess patients presenting to clinics and immediate care centers with a novel infectious disease in the context of limited information about signs and symptoms, restricted access to COVID-19 testing, and shortages of personal protective equipment (PPE). The Centers for Disease Control (CDC) and Illinois Department of Public Health (IDPH) disseminated early guidance prioritizing COVID-19 tests to patients with fever and/or signs/symptoms of a lower respiratory illness, as well as known risk factors such as travel to a high risk area or attendance at a large gathering.1 Initial testing was limited to processing at off-site centralized laboratories and often took more than a week for results to return.2 NorthShore University HealthSystem was one of the first hospital systems to initiate an in-house rapid SARS-CoV-2 PCR test and began testing in the ambulatory setting on March 13, 2020.3 With this expanded access to testing, clinicians sought prediction tools to streamline clinical assessments and guide their clinical decisions regarding whom to test for COVID-19. Subsequent systematic reviews identified fever, myalgia or arthralgia, fatigue, and headache as specific for COVID-19, but studies in ambulatory, primary care settings were limited and no prediction model was accurate enough to recommend for general use.4,5 To meet this need, NorthShore adapted the CDC and IDPH criteria into a telephonic pre-rooming risk assessment questionnaire to risk stratify patients for COVID-19 testing prior to the clinician visit. The objective of this study is to assess the effectiveness of risk stratifying patients for COVID-19 testing and identify which risk factors and which other clinical variables were associated with SARS-CoV-2 PCR test positivity.
Methods
We conducted a retrospective cohort study on a sample of patients evaluated for COVID-19 symptoms at 4 immediate care COVID-19 clinics in the northern suburbs of Chicago, Illinois, between 3/22/2020 and 3/28/2020. These clinics were existing immediate care sites that were reserved for patients with fever, upper respiratory, or other COVID-19 symptoms with appropriate staff personal protective equipment and isolation procedures. The clinics did not have easy access to laboratory or imaging studies and patients requiring a further level of evaluation or care were transferred to the closest emergency department. At the time of the study, the positivity rate of the SARS-CoV-2 test was 25% for patients with symptoms. Inclusion criteria were patients with symptoms of possible COVID-19 infection aged 16 and older.
Patients were pre-screened for COVID-19 symptoms during the scheduling process via telephone or mobile app and only symptomatic patients were scheduled for evaluation. Asymptomatic patients with possible exposure to COVID-19 were not included. After patients arrived and checked in for an appointment, a clinician (nurse practitioner or physician) called them and administered a five-question risk assessment questionnaire via telephone. The questions asked (1) presence of any high-risk medical issue such as chronic heart, lung (including asthma on continuous therapy), or kidney disease, age over 65, insulin dependent diabetes mellitus or immunocompromising condition; (2) travel in the past 21 days (Europe, UK, China, Iran, South Korea or Japan); (3) contact with a person who tested positive for COVID-19 or is currently being tested; (4) presence at a large gathering in the past 14 days where a person was known to have COVID-19; and (5) decrease in ability to taste or smell. The question regarding decrease in taste or smell was added on 3/24/2020. Patients who answered yes to any questions were stratified as high-risk and tested for SARS-CoV-2 by the medical assistant as part of the rooming process prior to the clinician evaluation. The clinician could cancel the COVID-19 test after evaluation if they deemed it not necessary. Patients who answered no to all questions were stratified as low-risk and evaluated by the clinician first, who used their clinical judgment to decide whether a SARS-CoV-2 test should be performed (see Figure 1 for risk assessment workflow).
Figure 1.
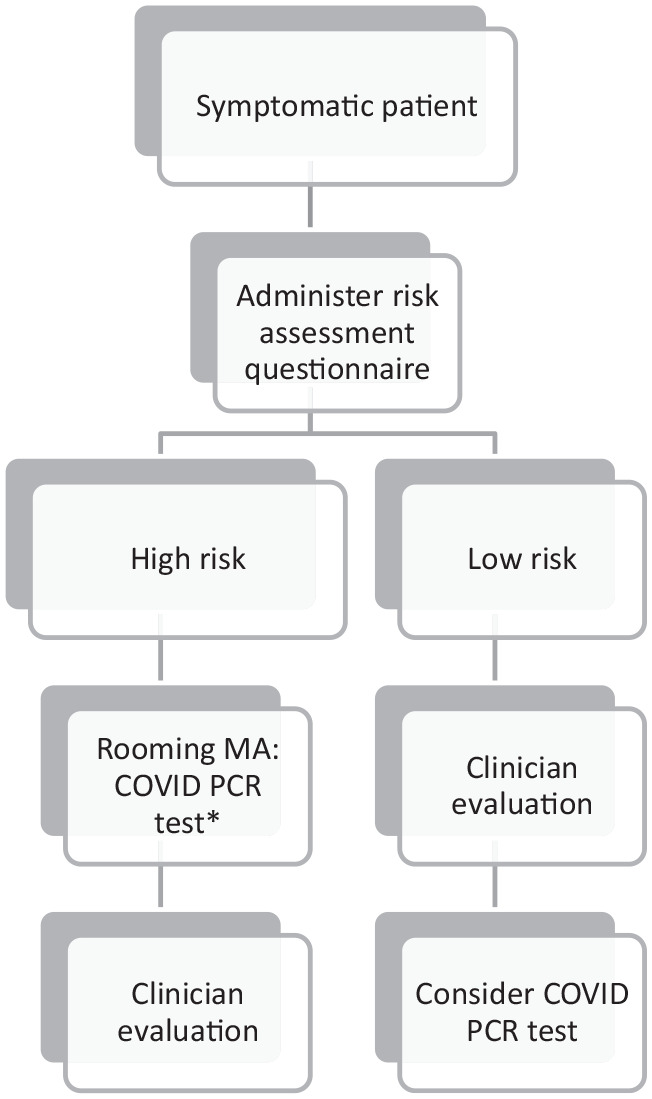
COVID-19 risk assessment questionnaire workflow administered 3/22/2020-3/28/2020.
*After performing an evaluation, clinician could cancel COVID PCR test if not indicated in their medical judgment.
Covid RT-PCR was performed using 1 of 3 assays: an in-house version of the published CDC assay that was validated in conjunction with the Illinois Department of Public Health, or the FDA EUA assays commercially available from Abbott for the automated M2000 and Alinity analyzers. The performance of these 3 assays (sensitivity, specificity, reproducibility, and accuracy) was assessed at launch and at intervals during the past months and is identical. Limit of detection for each is 100 viral copies/ml. Tests were processed at NorthShore University HealthSystem with test results routinely available within 1 to 2 days. All patients, whether high- or low-risk, were otherwise evaluated in standard fashion by the clinicians, including a history, physical exam, ordering of any appropriate point-of-care laboratory tests, and development of an assessment and plan. When appropriate, patients were given clear instructions for quarantine based on CDC recommendations at the time.
We collected patient variables including age, sex, vital signs, and chronic disease diagnoses from the EMR problem list, as well as outcome data including positive SARS-CoV-2 test, COVID-19-specific ER visits, hospitalizations, ICU admissions, intubation, and death within a 2 week follow-up period. Continuous variables were analyzed with t-tests or Wilcoxon rank sum tests and categorical variables with Chi-square tests or Fisher’s exact tests, as appropriate. Univariate and multivariable logistic regression analyses were conducted on the sub-sample of patients who were tested for SARS-CoV-2 to identify additional variables associated with test positivity, adjusting for other covariates of interest including age, gender, vital signs (pulse oximetry, temperature, pulse, respiratory rate, systolic blood pressure), and comorbidities. All analyses were conducted in SAS 9.4 (P < .05 was considered statistically significant). This study was deemed exempt research as a quality improvement project by the NorthShore University HealthSystem Institutional Review Board.
Results
A total of 1542 patients were included in the analysis with descriptive statistics listed in Table 1. High-risk patients were older than low-risk patients (median age 43 vs 39, P < .0001) with slightly higher average respiratory rate (median 18 vs 16, P < .0001) and lower diastolic blood pressure (median 74 vs 78, P = .002) in comparison to low-risk patients. High-risk patients were more likely than low-risk patients to have the presence of heart disease (10.2% vs 5.5%, P = .002), diabetes mellitus (8.6% vs 3.9%, P = .001), asthma (14.4% vs 7.1%, P < .0001), chronic kidney disease (2.1% vs 0.6%, P = .032) and immunosuppression (2% vs 0.2%, P = .006) documented in the electronic health record problem list.
Table 1.
Descriptive Statistics for Patients Aged ≥16-years (N = 1542).
| Overall 1542 (100) N (%a) or Median (IQR) | Risk assessment | P-value | ||
|---|---|---|---|---|
| Low 492 (31.9) N (%a) or Median (IQR) | High 1050 (68.1) N (%a) or Median (IQR) | |||
| Demographics | ||||
| Age | 41 (30 – 53) | 39 (28 – 50) | 43 (31 – 55) | <.0001 |
| Age Group | <.0001 | |||
| 16-44 years old | 873 (56.6) | 307 (62.4) | 566 (53.9) | |
| 45-65 years old | 574 (37.2) | 178 (36.2) | 396 (37.7) | |
| 66+ years old | 95 (6.2) | 7 (1.4) | 88 (8.4) | |
| Gender | .164 | |||
| Female | 910 (59) | 276 (56.1) | 634 (60.4) | |
| Male | 630 (40.9) | 216 (43.9) | 414 (39.4) | |
| Unknown | 2 (0.1) | 0 (0) | 2 (0.2) | |
| Vital Signs | ||||
| Pulse Oximetry (%) | 98 (97-99) | 98 (97-99) | 98 (97-99) | .109 |
| Temperature (° F) | 98.6 (98.2-99.0) | 98.6 (98.3-99.0) | 98.6 (98.2-98.9) | .474 |
| Pulse | 82 (72-93) | 82 (74-92.5) | 82 (72-93) | .112 |
| Respiratory Rate | 17 (16-18) | 16 (16-18) | 18 (16-18) | <.0001 |
| Systolic Blood Pressure | 120 (110-130) | 120 (110-130) | 120 (110-130) | .609 |
| Diastolic Blood Pressure | 76 (68-82) | 78 (70-82) | 74 (68-80) | .002 |
| Comorbidities b | ||||
| Heart Disease c | .002 | |||
| No | 1407 (91.3) | 464 (94.5) | 943 (89.8) | |
| Yes | 134 (8.7) | 27 (5.5) | 107 (10.2) | |
| Diabetes mellitus d | .001 | |||
| No | 1432 (92.9) | 472 (96.1) | 960 (91.4) | |
| Yes | 109 (7.1) | 19 (3.9) | 90 (8.6) | |
| Chronic Lung Disease e | .372 | |||
| No | 1404 (91.1) | 452 (92.1) | 952 (90.7) | |
| Yes | 137 (8.9) | 39 (7.9) | 98 (9.3) | |
| Asthma f | <.0001 | |||
| No | 1355 (87.9) | 456 (92.9) | 899 (85.6) | |
| Yes | 186 (12.1) | 35 (7.1) | 151 (14.4) | |
| Chronic Kidney Disease g | .032 | |||
| No | 1516 (98.4) | 488 (99.4) | 1028 (97.9) | |
| Yes | 25 (1.6) | 3 (0.6) | 22 (2.1) | |
| Immunosupression h | .006 | |||
| No | 1519 (98.6) | 490 (99.8) | 1029 (98) | |
| Yes | 22 (1.4) | 1 (0.2) | 21 (2) | |
Abbreviations: IQR, Interquartile Range.
Descriptive data are reported as median [25th quartile, 75th quartile] for continuous variable of nonnormal distribution, and percentages for categorical data.
Bolded text in column signifies statistically significant P-values (P < .05).
Column Percentages.
Comorbidities were derived from patient problem list. Low-risk patients who did not report comorbidities in the risk assessment questionnaire may have had comorbidities documented in the problem list.
Heart Disease ICD-10 codes I20-I25 or I30-I52 in problem list.
Diabetes ICD-10 codes E10-E14 in problem list.
Chronic Lung Disease ICD-10 codes J40-J44 or J46-J47 in problem list.
Asthma ICD-10 codes J45 in problem list.
Chronic Kidney Disease, codes N18 in problem list.
Immunosuppression, D80-89 in problem list.
Patients who were stratified as high-risk using the risk assessment questionnaire were almost universally tested for COVID-19 while more than half of low-risk patients were tested (1013 [96.5%] vs 252 [51.2%]; P < .0001). A total of 37 high-risk patients (3.5%) were not tested based on clinician judgment. The test positivity rate was not statistically significantly different between high-risk patients and low-risk patients who received a test (225 [22.2%] vs 50 [19.8%]; P = 0.41). COVID-19-related emergency room visits were not statistically significantly different between high- and low-risk groups (38 [3.6%] vs 16 [3.3%]; P = .71). Hospitalizations, ICU admissions, intubation and death were rare and no different between groups.
A total of 1265 patients were tested for SARS-CoV-2 and included in the multivariate logistic regression (results in Table 2). Amongst the variables in the risk assessment questionnaire, the adjusted odds ratio (aOR) of positive SARS-CoV-2 test was higher for close contact with a person who tested positive for COVID-19 or was currently being tested for COVID-19 (aOR 1.56, 95% CI 1.15-2.10), presence at a large gathering in the past 14 days where a person was known to have COVID-19 (1.92, 95% CI 1.10-3.34), or decreased sense of taste/smell (aOR 2.83, 95% CI 2.01-3.99) (Table 2). Examining other variables not included in the risk assessment questionnaire, the aOR of a positive SARS-CoV-2 test was higher for ages 45-64 and ages ≥65 compared to ages 16-44 (aOR 1.75, 95% CI 1.25-2.44, and aOR 2.78, 95% CI 1.49-5.16, respectively), higher for temperatures ≥99.0°F compared to ≤98.9°F (aOR 3.06, 95% CI 2.23-4.20), and higher for systolic blood pressures ≤120 compared to >120 (aOR 1.64, 95% CI 1.20-2.24).
Table 2.
Univariate and Multivariable Logistic Regression Analyses of Variables Associated with SARS-CoV-2 Test Positivity.
| Odds of testing positive uOR (95% CI) | P-value | Odds of testing positive aORa (95% CI) | P-value | |
|---|---|---|---|---|
| Demographics | ||||
| Age group | ||||
| 16-44 | Reference | Reference | ||
| 45-64 | 1.33 (1.00-1.76) | .050 | 1.75 (1.25-2.44) | .001 |
| ≥65 | 1.37 (0.86-2.21) | .189 | 2.78 (1.49-5.16) | .001 |
| Sex | ||||
| Female | Reference | Reference | ||
| Male | 1.70 (1.30-2.22) | .0001 | 1.60 (1.19-2.16) | .002 |
| Vital Signs | ||||
| Pulse Oximetry | ||||
| > 97 % | Reference | Reference | ||
| ≤ 97 % | 1.53 (1.15-2.03) | .004 | 1.39 (1.00-1.92) | .048 |
| Temperature | ||||
| ≤ 98.9° F | Reference | Reference | ||
| ≥ 99.0° F | 2.92 (2.20-3.88) | <.0001 | 3.06 (2.23-4.20) | <.0001 |
| Pulse | ||||
| ≥ 100 | Reference | Reference | ||
| < 100 | 0.79 (0.55-1.13) | .197 | 0.90 (0.60-1.36) | .625 |
| Respiratory Rate | ||||
| < 17 | Reference | Reference | ||
| ≥ 17 | 1.14 (0.87-1.49) | .352 | 0.99 (0.73 - 1.32) | .926 |
| Systolic Blood Pressure | ||||
| > 120 | Reference | Reference | ||
| ≤ 120 | 1.24 (0.95-1.63) | .116 | 1.64 (1.20-2.24) | .002 |
| COMORBIDITIES b | ||||
| Heart Disease c | ||||
| Yes | 0.64 (0.38-1.06) | .083 | 0.56 (0.31-1.02) | .058 |
| Diabetes mellitus d | ||||
| No | Reference | Reference | ||
| Yes | 0.86 (0.51-1.45) | .578 | 0.88 (0.47-1.62) | .676 |
| Chronic Lung Disease e | ||||
| No | Reference | Reference | ||
| Yes | 0.57 (0.33 - 0.96) | .036 | 0.55 (0.30-1.02) | .057 |
| Asthma f | ||||
| No | Reference | Reference | ||
| Yes | 0.64 (0.41-0.98) | .040 | 0.91 (0.55-1.48) | .698 |
| RISK ASSESSMENT QUESTIONNAIRE g | ||||
| Q1. High-risk Medical Issues | ||||
| No | Reference | Reference | ||
| Yes | 0.74 (0.55-0.99) | .040 | 0.81 (0.56-1.18) | .267 |
| Q2. Recent High-risk Travel | ||||
| No | Reference | Reference | ||
| Yes | 1.22 (0.67-2.22) | .519 | 1.34 (0.70-2.56) | .374 |
| Q3. COVID Close Contact | ||||
| No | Reference | Reference | ||
| Yes | 1.28 (0.98-1.67) | .074 | 1.56 (1.15-2.10) | .004 |
| Q4. COVID at Large Gathering | ||||
| No | Reference | Reference | ||
| Yes | 1.97 (1.19-3.26) | .008 | 1.92 (1.10-3.34) | .022 |
| Q5. Decrease in Taste/Smell | ||||
| No | Reference | Reference | ||
| Yes | 2.70 (1.97-3.71) | <.0001 | 2.83 (2.01-3.99) | <.0001 |
| Risk Stratification | ||||
| Low risk | Reference | − | − | |
| High risk | 1.15 (0.82-1.63) | .415 | − | |
Abbreviations: uOR, Unadjusted Odds Ratio; aOR, Adjusted Odds Ratio; 95% CI, 95% Confidence Interval.
Bolded text signifies statistically significant P-values (P < .05).
Adjusted for all covariates of interest: age, gender, vital signs (pulse oximetry, temperature, pulse, respiratory rate, systolic blood pressure), comorbidities (heart disease, diabetes, chronic lung disease, asthma), and other risk factors (question 1-5).
Comorbidities were derived from patient problem list. Low-risk patients who did not report comorbidities in the risk assessment questionnaire may have had comorbidities documented in the problem list.
Heart disease ICD-10 codes I20-I25 or I30-I52 in problem list.
Diabetes ICD-10 codes E10-E14 in problem list.
Chronic lung disease ICD-10 codes J40-J44 or J46-J47 in problem list.
Asthma ICD-10 codes J45 in problem list.
Full text of questions 1-5 listed in methods.
Discussion
We implemented a risk assessment questionnaire and stratification in 4 immediate care COVID-19 clinics to determine if this strategy could improve accuracy and efficiency of diagnosing patients with symptoms of COVID-19. Our results indicate that close contact, attendance at a large gathering with a COVID-19-positive individual, and decreased ability to taste/smell were the only factors in the risk assessment questionnaire that were associated with a positive SARS-CoV-2 test. Among these, decreased ability to taste/smell had the greatest effect size. While almost all high-risk patients received a test per our protocol, clinicians decided to test more than half (51.2%) of low-risk patients after performing a careful history, physical, and assessment. Notably, patients in the high-risk group were no more likely to have a positive test than the low-risk group (22.2% vs 19.8%), indicating that the clinicians observed additional risk factors, symptoms, or concerns meriting a test that were not captured in the questionnaire. A small percent of patients in the low-risk group had high-risk comorbidities in the problem list that they may not have verbalized in the risk questionnaire but were identified by clinicians during a medical history and chart review. Patients in the high-risk group with a negative test may have had an alternative diagnosis—not COVID-19—that also required careful clinical decision-making and judgment by the clinician. Therefore, high-risk versus low-risk stratification was unreliable in predicting positive test results and the risk stratification questionnaire was not effective at stratifying patients for testing.
This study is unique in evaluating a pragmatic risk assessment questionnaire and stratification in an ambulatory, primary care setting. Primary care clinicians use risk assessment tools to determine whom to test for other conditions, including the Centor criteria to assess the risk and need to test for streptococcal pharyngitis.6 An effective risk assessment tool could help primary care clinicians make more accurate COVID-19 diagnoses with a more efficient use of clinical time, resources, PPE, and testing. Prior studies examining signs and symptoms to determine if a patient has COVID-19 were performed in hospital outpatient clinics, emergency rooms or hospital settings. They incorporated laboratory and radiological results into their prediction models which would be unavailable in most primary care and immediate care offices, making it difficult to generalize study results to primary care settings.4,7 A study of prediction models identified age, temperature, and flu-like signs and symptoms, lymphocyte count, neutrophil count, and lung imaging features as common predictors of COVID-19, but none of the models were sufficiently validated to recommend in general practice.5 Our study examined the effect of variables not included in our risk assessment questionnaire and found that age ≥45, temperatures ≥99.0°F, and systolic blood pressures ≤120 were associated with a positive SARS-CoV-2 test. Among these, temperatures ≥99.0°F had the greatest effect size. While we were able to identify these additional associations, we were unable to validate a prediction model that performed well in an immediate care practice setting.
This study has several limitations. Because our study is limited to outcomes from a single hospital system, our results may have underestimated the prevalence of ER visits, hospitalization, and death. Our sample is limited to a time period in late March when the test positivity rate was ~25% for symptomatic patients and influenza prevalence was low. We were unable to capture some important variables that may predict test positivity, including BMI, ethnicity, and workplace or other social or structural determinants of health. We were unable to test all low-risk patients, limiting our ability to comment on the effectiveness of clinician decision-making about COVID-19 testing for low-risk patients compared to universal testing.
The clinical implications of our findings are that primary care clinicians should not rely completely on published risk factors to prioritize patients for SARS-CoV-2 testing. However, recent decrease in sense of smell or taste and temperatures ≥99.0°F were associated with an almost three-fold increased odds of COVID-19 in our study and should prompt a clinician to more highly suspect COVID-19. We have used the results of this study to adapt our approach to risk factor assessment and testing prioritization. Until such time that a reliable prediction model is identified, our best practice is to test all patients presenting with symptoms. As testing has become more available, our current best practice is to offer SARS-CoV-2 testing to all symptomatic patients with suspicion for COVID-19 in order to guide clinical management, quarantine and isolation precautions, and return to work or school guidance.
When testing is limited, the Infectious Disease Society of America, CDC, and local health departments all provide guidance for test prioritization.8,9 More importantly, clinicians evaluating patients in the ambulatory setting have an important role in assessing risk factors for severe COVID-19 to guide the intensity and frequency of follow-up and monitoring for clinical decompensation. The CDC has identified increasing age, cancer, chronic kidney disease, chronic obstructive pulmonary disease, heart conditions, obesity, type 2 diabetes mellitus, smoking, sickle cell disease, pregnancy, and factors related to race and ethnicity as risk factors for severe illness from COVID-19.8–11 Shifts in school attendance and prevalence of other respiratory conditions including influenza and streptococcal pharyngitis as well as prevalence of preventive measures including mask wearing, social distancing, and local policies regulating high-risk activities such as indoor dining will all affect community spread and need to be accounted for in the clinical evaluation of patients with symptoms suspicious for COVID-19 in the coming months. Our observations support other studies that failed to find effective prediction models for SARS-CoV-2 test positivity, underscoring the need for clinicians to develop clinical experience and share best practices and for systems and payors to support policies, funding, and resources to test all symptomatic patients.
Footnotes
Author Contributions: Dr. Oshman had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and Design: Oshman, Caplan, Ali, David
Acquisition, analysis, or interpretation of data: Oshman, Singh, Ali, Khalid, David
Drafting of the manuscript: Oshman, Caplan, Ali, Singh
Revision of the manuscript: Oshman, Caplan, Ali, Singh, Khalid, Jameel, David
Statistical analysis: Oshman, Singh
Supervision: Oshman
Additional Contributions: We wish to acknowledge Nirav Shah, MD (NorthShore University HealthSystem, Evanston, IL) for his contribution to the data extraction plan; Urmila Ravichandran, MS (NorthShore University HealthSystem, Evanston, IL) for performing the data extraction; Bernard Ewigman, MD, MSPH (NorthShore University HealthSystem, Evanston, IL) for providing mentorship in the study design and analysis; Elena Genova, PhD (NorthShore University HealthSystem, Evanston, IL) for performing chart review; Jana Wichelecki, MPH (NorthShore University HealthSystem, Evanston, IL) for providing administrative support; Brigham Temple, MD and Cindy Carpo, MD (NorthShore University HealthSystem, Evanston, IL) for developing the risk assessment questionnaire; Tyler Bauer, MBA (NorthShore University HealthSystem, Evanston, IL) for assisting with administrative implementation.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Dr. Oshman reports stock holdings from Abbott, outside the submitted work. Dr. David reports stock holdings from Genalyte, outside the submitted work. No other disclosures were reported.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical Considerations: This study was deemed exempt research as a quality improvement project by the NorthShore Institutional Review Board.
ORCID iD: Lauren Oshman  https://orcid.org/0000-0001-7134-970X
https://orcid.org/0000-0001-7134-970X
References
- 1. Illinois Department of Public Health. IDPH Interim COVID-19 Guidance. Accessed March 19, 2020 https://www.dph.illinois.gov/sites/default/files/COVID19/20200319_COVID19_Testing%20Authorizati.pdf.
- 2. Appleby J. Why it takes so long to get most COVID-19 test results. Accessed October 5, 2020 https://www.npr.org/sections/health-shots/2020/03/28/822869504/why-it-takes-so-long-to-get-most-covid-19-test-results.
- 3. NorthShore becomes first local hospital network to conduct its own COVID-19 testing [Transcript]. WGN TV. March 17, 2020. [Google Scholar]
- 4. Struyf T, Deeks JJ, Dinnes J, et al. Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19 disease. Cochrane Database Syst Rev. 2020;7:CD013665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wynants L, Van Calster B, Collins GS, et al. Prediction models for diagnosis and prognosis of covid-19 infection: systematic review and critical appraisal. BMJ. 2020;369:1-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Centor RM, Witherspoon JM, Dalton HP, Brody CE, Link K. The diagnosis of strep throat in adults in the emergency room. Med Decis Making. 1981;1:239-246. [DOI] [PubMed] [Google Scholar]
- 7. Sperrin M, Grant SW, Peek N. Prediction models for diagnosis and prognosis in Covid-19 BMJ. 2020;369:m1464. [DOI] [PubMed] [Google Scholar]
- 8. Infectious Diseases Society of America. COVID-19 prioritization of diagnostic testing. Accessed October 8, 2020 http://www.idsociety.org/globalassets/idsa/public-health/covid-19-prioritization-of-dx-testing.pdf.
- 9. Center for Disease Control. Coronavirus Disease 2019 (COVID-19): Evidence used to update the list of underlying medical conditions that increase a person’s risk of severe illness from COVID-19. Accessed November 4, 2020 https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/evidence-table.html.
- 10. Center for Disease Control. Older Adults. Accessed November 4, 2020 https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/older-adults.html.
- 11. Center for Disease Control. COVID-19 Hospitalization and death by race/ethnicity. Accessed November 4, 2020 https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/older-adults.html.


