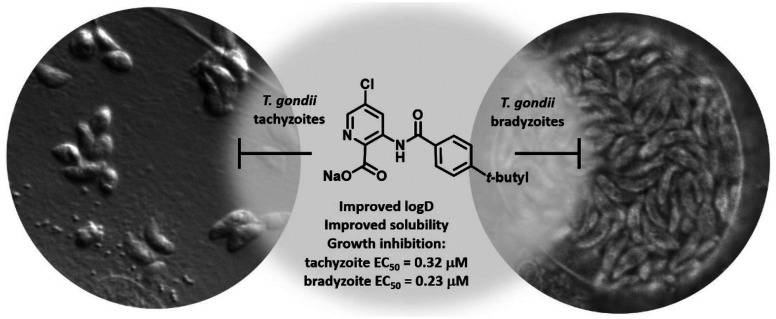
Abstract
Toxoplasma gondii causes a prevalent human infection for which only the acute stage has an FDA-approved therapy. To find inhibitors of both the acute stage parasites and the persistent cyst stage that causes a chronic infection, we repurposed a compound library containing known inhibitors of parasitic hexokinase, the first step in the glycolysis pathway, along with a larger collection of new structural derivatives. The focused screen of 22 compounds showed a 77% hit rate (>50% multistage inhibition) and revealed a series of aminobenzamide-linked picolinic acids with submicromolar potency against both T. gondii parasite forms. Picolinic acid 23, designed from an antiparasitic benzamidobenzoic acid class with challenging ADME properties, showed 60-fold-enhanced solubility, a moderate LogD7.4, and a 30% improvement in microsomal stability. Furthermore, isotopically labeled glucose tracing revealed that picolinic acid 23 does not function by hexokinase inhibition. Thus, we report a new probe scaffold to interrogate dual-stage inhibition of T. gondii.
Keywords: Toxoplasma gondii, picolinic acid, parasite hopping, inhibitor, multistage
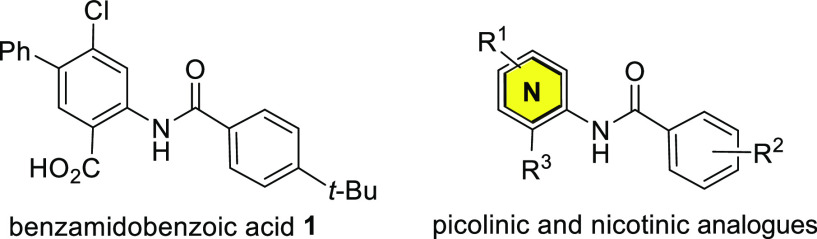
Toxoplasma gondii is responsible for a commonly acquired parasitic infection that is estimated to affect 30–50% of the human population.1 Mainly contracted through consumption of contaminated food or water,2T. gondii parasites rapidly proliferate in the tachyzoite stage, causing an acute infection that recruits a robust host immune response. Chemotherapeutic or immune pressure induces tachyzoite parasites to differentiate into metabolically quiescent bradyzoite cysts that evade the host immune system and are impenetrable to current first-line therapeutics.2,3 Failure to clear parasites from the host results in a lifelong opportunistic chronic infection with significant risk of fatal complications by reactivation in immunocompromised patients.4 While immunocompetent individuals who become infected are typically asymptomatic or may suffer self-limiting flulike illness, ocular lesions have been reported.5 Maternal infection during pregnancy can lead to miscarriage or visual or motor disabilities in newborns.6−8 The acute infection, caused by T. gondii tachyzoites, is frequently treated with a synergistic combination of the antifolate drugs pyrimethamine and sulfadiazine.9,10 Unfortunately, significant side effects11−13 and resistance14 have been reported with use of these drugs, and persistent bradyzoite parasites evade this therapy.15,16 Despite new development17−24 and drug repurposing efforts,25−28 effective treatments for T. gondii, especially those capable of clearing the bradyzoite stage of the parasite, remain an important unmet medical need.15,29−31 We previously described a series of benzamidobenzoic acids, exemplified by 1 (Figure 1), that inhibited the glycolytic hexokinase enzyme of the kinetoplastid parasites Trypanosoma brucei and Leishmania major.(32)
Figure 1.
Probe 1 and next-generation aza analogues.
Through broader screening, we determined that unrelated parasites may be inhibited by these same compounds, though the mechanism may not be shared. For instance, when our compound library was assessed against the glucokinase of the pathogenic free-living protozoa Naegleria fowleri, a neuroinvasive amoeba that causes a rare but highly lethal brain infection, hits were identified, and distinct structure–activity relationships (SARs) emerged from testing these compounds against various parasites.33,34 This parasite-hopping approach26,35−37 reveals opportunities for therapeutic intervention and divergent scaffold optimization due to differences in SARs across a spectrum of familial and distantly related parasites. With this in mind, we curated a small library of 22 compounds from our collection that included compound 1, several related analogues, and a collection of new picolinic and nicotinic acid derivatives that evolved from the benzamidobenzoic acids (Table 1S).
Compounds were evaluated against both tachyzoite38 and bradyzoite39 forms of the T. gondii parasite in separate in vitro screens. Briefly, human foreskin fibroblast (HFF) host cells harboring either T. gondii tachyzoites or in vitro-differentiated bradyzoites were treated with each of the compounds at a single compound concentration of 10 μM.40 Pyrimethamine, which is known to be active against the tachyzoite stage but not the bradyzoite stage, was included as a control (Figure 2).41 Compound 1 displayed >98% growth inhibition for both T. gondii parasite stages at 10 μM, and several of the related 1-arylbenzamidobenzoic acids (compounds 18–22) also resulted in high levels of inhibition (see Figure 2 and Table 1S). These compounds provided some initial SAR insights; however, their high lipophilicity (cLogD7.4 = 5.0–7.4) limited their solubility and development potential. We were encouraged to see that many of the picolinic acid derivatives were also active, as these compounds comparatively had improved physiochemical properties.
Figure 2.
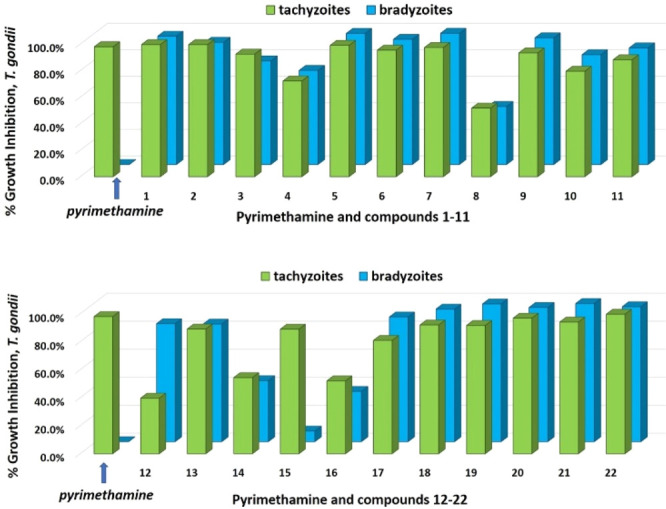
Percent growth inhibition of T. gondii tachyzoites and bradyzoites with compounds 1–22 at 10 μM.
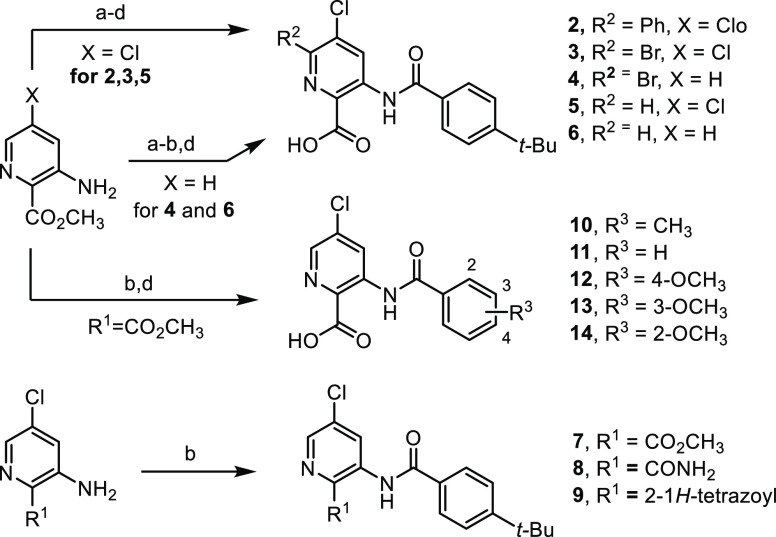
The synthesis of several of the benzamidobenzoic acids32 has been previously disclosed, and new analogues in that series followed similar protocols (see the Supporting Information). The sequence of steps was modified, but the picolinic acid derivatives were generally prepared by benzoylation of 3-amino-5-chloropicolinic acid esters followed by Suzuki coupling when a bromine atom was installed at C6 (Scheme 1). Hydrolysis of the ester functionality revealed the desired acids (compounds 2–6 and 10–14). In some cases, replacement of the carboxylic acid with a bioisostere was surveyed by using the appropriately substituted picolinic derivative and employing the same benzoylation procedure (compounds 7–9). Nicotinic acid analogues 15 and 16 were generated by similar methods (see the Supporting Information).
Scheme 1. Synthesis of Picolinic Acid Derivatives.
Reagents and conditions: (a) 2.6 M Br2 in AcOH, 2 M H2SO4, rt, 4–72 h, 51–55%; (b) ArCOCl, Et3N or i-Pr2EtN, THF or CH3CN, rt to 150 °C, 30–89%; (c) PhB(OH)2, Na2CO3, Pd(PPh3)2Cl2, 10:1 CH3CN/H2O, 100 °C, 0.5 h, 67%; (d) 1.3 M aq LiOH, 3:2 THF/H2O, rt, 12–48 h, 31–93%.
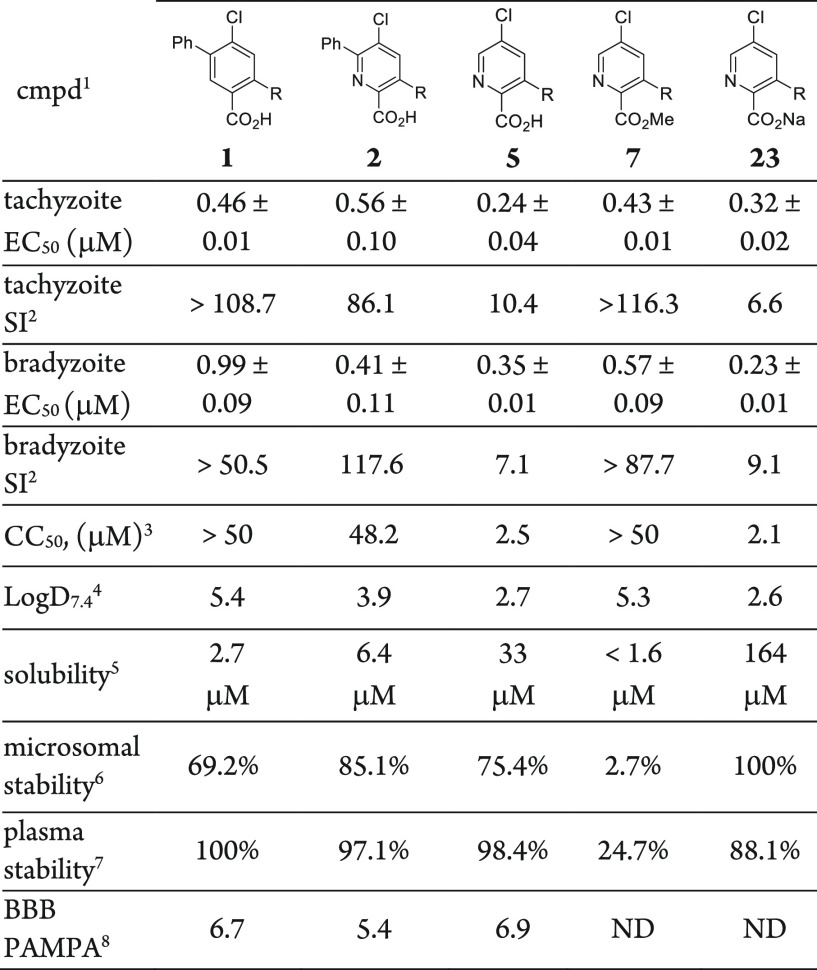
In parallel with compound 1, these second-generation aza analogues 2–16 were subsequently evaluated in dose–response-formatted growth inhibition assays for each stage of the T. gondii parasite.38,39 General cytotoxicity was assessed in uninfected HFF cells over 72 h for comparison with the parasitic assays performed in the same cell line (Table 1). Our benchmark, compound 1, inhibited both tachyzoites and bradyzoites with similar submicromolar potency. Integration of the nitrogen atom into the core to generate the analogous picolinic acid 2 resulted in comparable tachyzoite and bradyzoite inhibition (EC50 = 0.56 and 0.41 μM, respectively). Across the aza series, inhibition was generally congruent between tachyzoites and bradyzoites for a given compound, except in four cases in which inhibition for one stage was significantly favored. The effort revealed that the C6-phenyl ring appended to the picolinic acid core was not necessary to achieve submicromolar inhibition, and removing this moiety helped to reduce the lipophilicity. Conversely, the C5-chloro substituent of the aza analogue set was important for retaining activity (compounds 4–6). Compound 5 showed the best tachyzoite inhibition (EC50 = 0.24 μM) with commensurate bradyzoite activity; however, some apparent toxicity in HFF cells was also noted, affording selectivity indices of 10.4 to 7.1, respectively. The methyl ester derivative 7 showed activity against both parasite forms, though hydrolysis to reveal an active acid metabolite (compound 5) is likely, especially for the bradyzoite assay, which involves base-mediated maturation of parasites. Notably, though, this analogue did not manifest the same levels of toxicity toward HFF cells as observed with compound 5. Other isosteric replacements of the carboxylic acid were also surveyed, revealing that a primary amide isosteric replacement of the acid was inactive; the toxicity of tetrazole 9 confounded the interpretation of any inhibitory effect.
Table 1. T. gondii Tachyzoite and Bradyzoite Inhibition and Cytotoxicity Studies with Compounds 1–16.
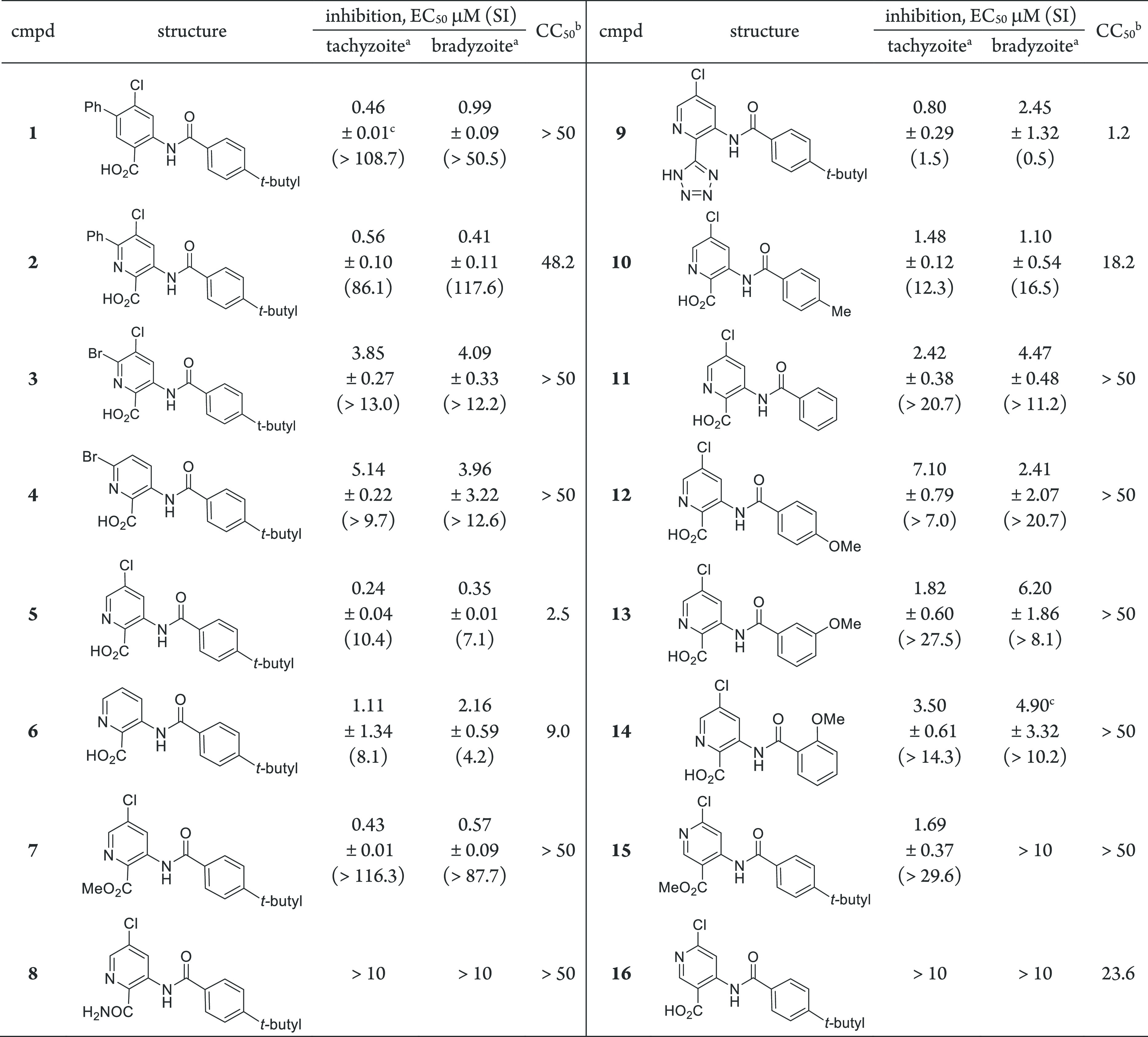
Compounds were minimally tested in triplicate (n ≥ 3) in HFF cells unless otherwise indicated. SI is the selectivity index, given by SI = CC50/EC50.
HFF cell line, 72 h exposure.
Tested in duplicate (n = 2).
The necessity of the 4-tert-butyl appendage of the benzamide moiety was also evaluated. Exchange of the tert-butyl group of 5 with a methyl group (compound 10) or a hydrogen atom (compound 11) eroded the potency against both parasitic forms in a trend that suggests that hydrophobic interactions may be important in this region of the scaffold. This hypothesis was further supported by the more pronounced loss of potency when a more polar 4-methoxyphenyl group was installed in place of the 4-tert-butylphenyl moiety (compound 12). Migration of the methoxy group to other positions on the phenyl ring was not advantageous for potency over other structural modifications already discussed (compounds 13 and 14). Nicotinic acid derivatives 15 and 16 were devoid of inhibitory activity against the bradyzoite form. The benzamidobenzoic acid series, represented by compound 1, possessed challenging physiochemical properties. For instance, lipophilic probe 1 had an experimental LogD7.4 of >5, along with modest solubility and microsomal stability (Table 2). The aza analogues were designed to address these liabilities. Selected picolinic analogues and compound 1 were evaluated for LogD7.4, solubility, microsomal and plasma stability, and brain permeability (PAMPA). Compound 5 had a desirable LogD7.4 value of 2.7 and the most improved solubility and microsomal stability relative to compound 1. Despite the presence of the carboxylic acid, the BBB PAMPA assay predicted that the compound should achieve brain exposure. A pharmacokinetic mouse study was planned; however, we found that compound 5 was difficult to formulate into a solution for proper administration. To enhance the solubility of 5, the sodium salt of the carboxylic acid was generated to afford analogue 23 (Table 2). Parasite growth inhibition was verified, revealing EC50 values for salt 23 comparable to those for the parent compound 5 against both tachyzoites and bradyzoites. Notably, the solubility of salt 23 was improved 5-fold over that of 5, which enabled us to find a particle-free formulation for pharmacokinetic evaluation.
Table 2. ADME Results for Selected Compounds.
R = NHCO-4-tert-butylphenyl.
Selectivity index, given by SI = CC50/EC50.
CC50 in HFF cells after 72 h = cytotoxic concentration at which cell viability is reduced by 50%.
Experimentally determined.
Kinetic aqueous solubility at pH 7.4.
Mouse microsomes; value reported as percent parent remaining after 1 h of exposure
Mouse plasma; percent parent remaining after 1 h of exposure.
BBB PAMPA, Pe (10–6 cm/s). ND = not determined.
To assess the plasma and brain exposure of compound 23, a snapshot pharmacokinetic experiment in mice was conducted. Compound 23 was administered to male BALB/c mice at 15 mg/kg via ip injection as a clear solution. Plasma concentrations were assessed 30 min and 2 h postadministration, and brain exposure was measured at the 2 h time point. Terminal mean plasma and brain exposure (AUC2h = 7883 ng/mL and AUC2h = 816 ng/g, respectively) were determined, affording a brain-to-plasma (B/P) ratio of 0.1. These values translate to 22 μM in plasma and 2.3 μM in brain 2 h postadministration, though because of high protein binding (99.5%), the free fraction is substantially reduced. Encouragingly, compound 23 demonstrated exposure at a low dose in both plasma and brain; however, additional work will be required to improve the selectivity index and increase the exposure to permit suitable dosing in mice for efficacy assessment. The ester of compound 7 may offer potential insight into addressing these characteristics.
Contrary to the tachyzoites, the in vitro-derived bradyzoites used in the primary screen and SAR assessment were not susceptible to pyrimethamine as a control, indicating that the bradyzoites were differentiated. Nonetheless, we evaluated whether the observed inhibition of in vitro-derived bradyzoites was recapitulated with ex vivo-derived bradyzoite cysts using compound 23. Bradyzoites were harvested from the brains of four T. gondii-infected mice 30+ days postinfection, purified, and transferred to fibroblast cells (HFF). Compound 23 was independently added to the cells at three separate concentrations (1, 3, or 6 × EC50) followed by incubation for 3 days. Monolayers were fixed and incubated with an anti-BAG1 antibody that recognizes a bradyzoite-specific antigen, BAG1, which is absent in tachyzoites.42 Fluorescence imaging (Incucyte) of the infected cells showed that compound 23 reduced the fluorescence signal corresponding to BAG1 in a dose -dependent manner compared with the DMSO control, thus demonstrating that compound 23 inhibits ex vivo bradyzoite cysts (Figure 3).
Figure 3.
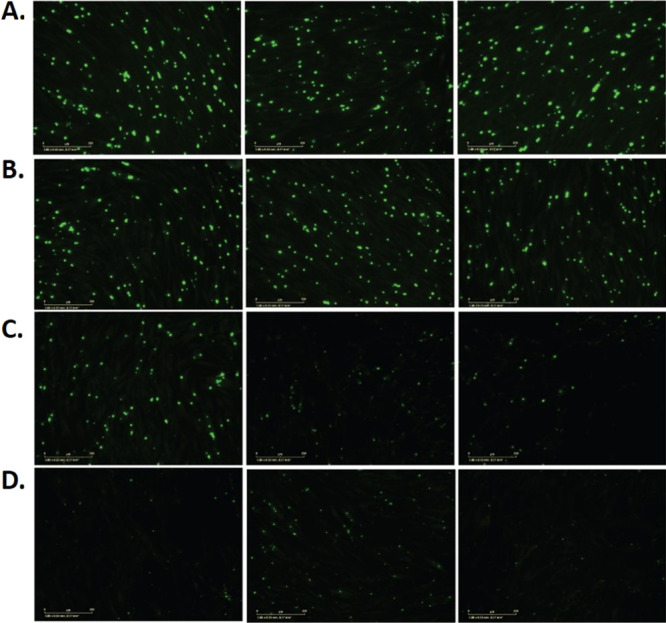
Ex vivo harvested T. gondii cysts from mouse brains in HFF cells incubated with BAG1 antibody and fluorescently imaged after 3 day treatment with (A) DMSO control or (B–D) compound 23 at (B) 1 × EC50, (C) 3 × EC50, or (D) 6 × EC50. Antibody-labeled cysts appear in green. The experiment was performed twice, each time in triplicate, but only one experiment is shown for clarity (see Figure 1S and Table 2S).
A biological target of the picolinic acids is currently unknown. However, the benzamidobenzoic acids from which these compounds were designed, represented by 1, were originally developed as inhibitors of the T. brucei parasite glycolytic enzyme hexokinase 1 (TbHK1).32T. gondii possesses a single copy of its hexokinase (T. gondii hexokinase, TgHK),43,44 and like T. brucei parasites, T. gondii bradyzoites are glycolysis-dependent; however, the tachyzoite stage can use alternative metabolic pathways when necessary. To determine whether compound 23 inhibited TgHK, T. gondii-infected HFF cells were treated with DMSO control or with compound 23 at either 2 or 6 × EC50 4 h postinfection. After 5 h, the medium was changed to glucose-free RPMI medium supplemented with isotopically labeled 13C6-glucose.45 After 10 min of labeling, infections were rapidly quenched, processed, and subjected to broad-spectrum mass spectrometry analysis. Data were evaluated by the Metabolomics Analysis and Visualization Engine (MAVEN)46 software. Analysis of 13C6-labeled metabolite production from the initial glycolysis reactions (Figure 4A) revealed two notable outcomes related to the absence or presence of compound 23.
Figure 4.
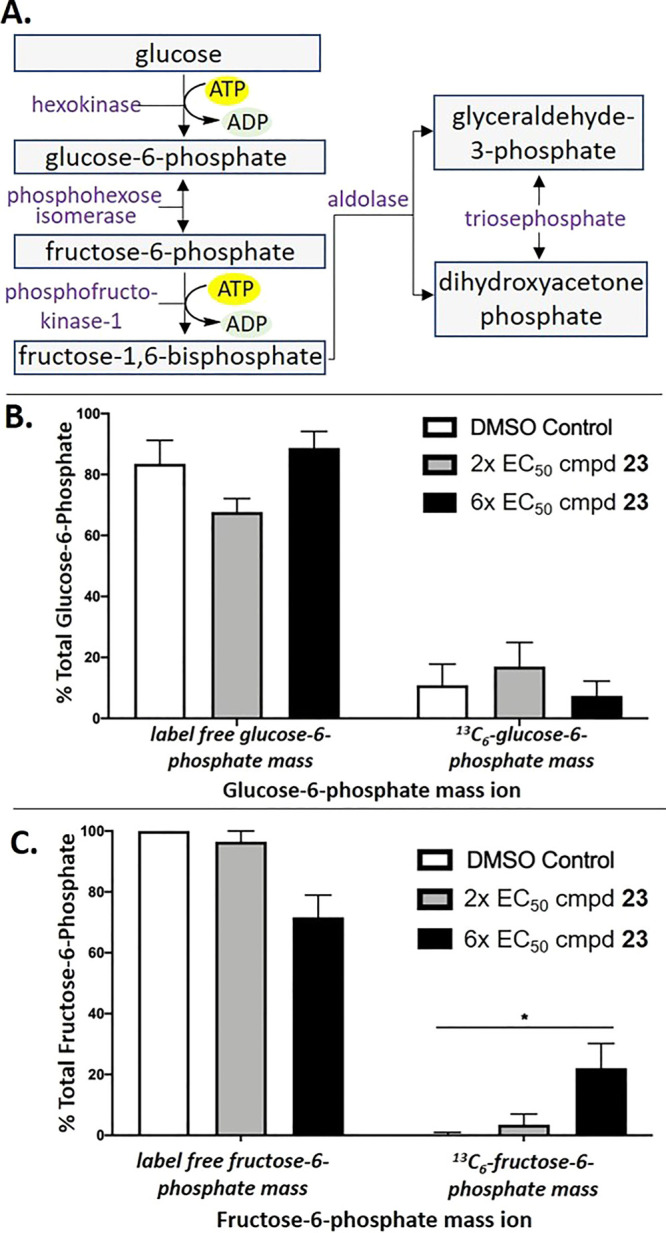
(A) Schematic of initial T. gondii glycolysis reaction pathways. (B, C) Mass spectrometry analyses showing the percentages of the total label-free or 13C6-labeled (B) glucose-6-phosphate and (C) fructose-6-phosphate in T. gondii-infected HFF cells treated with DMSO (white bars) or with compound 23 at 2 × EC50 (gray bars) or 6 × EC50 (black bars). Error bars = 95% confidence interval. Asterisks indicate a significant difference in 13C6-labeled fructose-6-phosphate mass ion (p = 0.02, t test corrected for multiple comparison using the Holm–Sidak method) between the DMSO and 6 × EC50 results. Shown is one representative experiment of two, each performed in triplicate.
In the presence of compound 23, 13C6-glucose-6-phosphate, the product of the hexokinase, did not change significantly in treated parasites compared with the DMSO control, suggesting that the hexokinase was not appreciably inhibited (Figure 4B). In contrast, 13C6-fructose-6-phosphate accumulated in a statistically significant amount compared with DMSO-treated tachyzoites (Figure 4C). An impact of compound 23 on other pathway metabolites was not observed (data not shown). Elucidating the biological target of compound 23 that leads to the observed increase in 13C6-fructose-6-phosphate will require more study; however, these results show that 23 impacts the glycolytic pathway, although inhibition of the hexokinase is not the primary mechanism of action.
Herein we have described the results of a parasite-hopping approach revealing a novel picolinic acid template that inhibits both tachyzoite and bradyzoite T. gondii parasites. Generally, a good correlation between the in vitro potencies toward the two parasite forms was found, and structure–activity relationships were identified for a preliminary collection of 18 compounds, several of which showed submicromolar inhibition. Compared with the first-generation benzamidobenzoic acids, the second-generation picolinic acid derivative 23 was more balanced in terms of its lipophilic character and demonstrated a >30% improvement in mouse microsomal stability and a 60-fold improvement in solubility. Some cellular toxicity in HFF cells was observed, and additional work will be required to enhance the exposure, especially in brain tissue, where the bradyzoite burden is significant. As current FDA-approved therapies are ineffective against T. gondii bradyzoites, these compounds represent an opportunity to study the acute and chronic stages of T. gondii infection with a new probe scaffold.
Acknowledgments
The authors acknowledge the assistance of Dr. Gang Yan and Claire Caschetta in obtaining PAMPA assay data as well as Dr. Gene Ananiev in the UW-Madison Screening Facility for determining HFF cytotoxicity data. The authors thank Dr. Daniel Amador-Noguez for use of the Thermo Fisher Vanquish Horizon UHPLC joined to a Thermo Scientific Q Exactive Orbitrap high-resolution mass spectrometer. This work made use of the instrumentation at the UW-Madison Medicinal Chemistry Center and Analytical Instrumentation Center funded by the UW School of Pharmacy. M.M.K. gratefully acknowledges support from the National Science Foundation Graduate Research Fellowship Program (DGE-1256259) and the American Foundation for Pharmaceutical Education Pre-Doctoral Fellowship in Pharmaceutical Sciences Program. Work from the laboratory of J.C.M. was supported in part by the NIH Center for Biomedical Excellence (COBRE) Grant P20GM109094. L.K.J. acknowledges support from the University of Wisconsin Food Research Institute. B.M.D.G. and G.M.G.-L. were supported by Morgridge Metabolism Interdisciplinary Fellowships from the Morgridge Institute for Research. J.E.G. acknowledges institutional support from the Office of the Vice Chancellor for Research and Graduate Education at the University of Wisconsin-Madison.
Glossary
Abbreviations
- AUC
area under the curve
- BAG1
T. gondii bradyzoite specific heat shock protein
- BBB
blood–brain barrier
- CC50
cytotoxic concentration that results in death of 50% of the cells
- cLogD
calculated partition coefficient for an ionizable compound at a given pH
- EC50
effective concentration of compound necessary to inhibit 50% of the parasites
- HFF
human foreskin fibroblasts
- PAMPA
parallel artificial membrane permeability assay
- SAR
structure–activity relationships
- SI
selectivity index
- TbHK1
Trypanosoma brucei hexokinase 1
- TgHK
T. gondii hexokinase.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsmedchemlett.0c00267.
Assay, experimental details, synthetic procedures, and NMR spectra for all new compounds reported in this work (2–23) (PDF)
Author Contributions
# M.M.K. and B.M.D.G. contributed equally. The manuscript was written through contributions of all authors. All of the authors approved the final version of the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Scallan E.; Hoekstra R. M.; Angulo F. J.; Tauxe R. V.; Widdowson M. A.; Roy S. L.; Jones J. L.; Griffin P. M. Foodborne illness acquired in the United States—major pathogens. Emerging Infect. Dis. 2011, 17 (1), 7–15. 10.3201/eid1701.P11101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittman K. J.; Knoll L. J. Long-Term Relationships: The Complicated Interplay between the Host and the Developmental Stages of Toxoplasma gondii during Acute and Chronic Infections. Microbiol. Mol. Biol. Rev. 2015, 79 (4), 387–401. 10.1128/MMBR.00027-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denkers E. Y.; Gazzinelli R. T. Regulation and Function of T-Cell-Mediated Immunity during Toxoplasma gondii Infection. Clin. Microbiol. Rev. 1998, 11 (4), 569–588. 10.1128/CMR.11.4.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meireles L. R.; Ekman C. C. J.; Franco de Andrade H. Jr.; Luna E. J. d. A. Human Toxoplasmosis Outbreaks and the Agent Infecting Form. Findings From a Systematic Review. Rev. Inst. Med. Trop. Sao Paulo 2015, 57, 369–376. 10.1590/S0036-46652015000500001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park Y.-H.; Nam H.-W. Clinical Features and Treatment of Ocular Toxoplasmosis. Korean J. Parasitol. 2013, 51 (4), 393–399. 10.3347/kjp.2013.51.4.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAuley J. B. Congenital Toxoplasmosis. J. Pediat. Inf. Dis. Soc. 2014, 3 (suppl_1), S30–S35. 10.1093/jpids/piu077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu N.; Duchon J.; Zachariah P. TORCH Infections. Clin. Perinatol. 2015, 42 (1), 77–103. 10.1016/j.clp.2014.11.001. [DOI] [PubMed] [Google Scholar]
- Tenter A. M.; Heckeroth A. R.; Weiss L. M. Toxoplasma gondii: from Animals to Humans. Int. J. Parasitol. 2000, 30 (12), 1217–1258. 10.1016/S0020-7519(00)00124-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters P. J.; Thigpen M. C.; Parise M. E.; Newman R. D. Safety and Toxicity of Sulfadoxine/Pyrimethamine. Drug Saf. 2007, 30 (6), 481–501. 10.2165/00002018-200730060-00003. [DOI] [PubMed] [Google Scholar]
- Pissinate K.; dos Santos Martins-Duarte É.; Schaffazick S. R.; de Oliveira C. P.; Vommaro R. C.; Guterres S. S.; Pohlmann A. R.; de Souza W. Pyrimethamine-loaded Lipid-core Nanocapsules to Improve Drug Efficacy for the Treatment of Toxoplasmosis. Parasitol. Res. 2014, 113 (2), 555–564. 10.1007/s00436-013-3715-6. [DOI] [PubMed] [Google Scholar]
- Macy E.; Poon K-Y T. Self-reported Antibiotic Allergy Incidence and Prevalence: Age and Sex Effects. Am. J. Med. 2009, 122 (8), 778.e1–778.e7. 10.1016/j.amjmed.2009.01.034. [DOI] [PubMed] [Google Scholar]
- Wulf N. R.; Matuszewski K. A. Sulfonamide Cross-Reactivity: Is There Evidence to Support Broad Cross-Allergenicity?. Am. J. Health-Syst. Pharm. 2013, 70 (17), 1483–1494. 10.2146/ajhp120291. [DOI] [PubMed] [Google Scholar]
- Fung H. B.; Kirschenbaum H. L. Treatment Regimens for Patients with Toxoplasmic Encephalitis. Clin. Ther. 1996, 18 (6), 1037–1056. 10.1016/S0149-2918(96)80059-2. [DOI] [PubMed] [Google Scholar]
- Montazeri M.; Mehrzadi S.; Sharif M.; Sarvi S.; Tanzifi A.; Aghayan S. A.; Daryani A. Drug Resistance in Toxoplasma gondii. Front. Microbiol. 2018, 9, 2587. 10.3389/fmicb.2018.02587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neville A. J.; Zach S. J.; Wang X.; Larson J. J.; Judge A. K.; Davis L. A.; Vennerstrom J. L.; Davis P. H. Clinically Available Medicines Demonstrating Anti-Toxoplasma Activity. Antimicrob. Agents Chemother. 2015, 59 (12), 7161–7169. 10.1128/AAC.02009-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konstantinovic N.; Guegan H.; Stäjner T.; Belaz S.; Robert-Gangneux F. Treatment of toxoplasmosis: Current options and future perspectives. Food Waterborne Parasitol. 2019, 15, e00036 10.1016/j.fawpar.2019.e00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidadala R. S.; Rivas K. L.; Ojo K. K.; Hulverson M. A.; Zambriski J. A.; Bruzual I.; Schultz T. L.; Huang W.; Zhang Z.; Scheele S.; DeRocher A. E.; Choi R.; Barrett L. K.; Siddaramaiah L. K.; Hol W. G.; Fan E.; Merritt E. A.; Parsons M.; Freiberg G.; Marsh K.; Kempf D. J.; Carruthers V. B.; Isoherranen N.; Doggett J. S.; Van Voorhis W. C.; Maly D. J. Development of an Orally Available and Central Nervous System (CNS) Penetrant Toxoplasma gondii Calcium-Dependent Protein Kinase 1 (TgCDPK1) Inhibitor with Minimal Human Ether-a-go-go-Related Gene (hERG) Activity for the Treatment of Toxoplasmosis. J. Med. Chem. 2016, 59 (13), 6531–6546. 10.1021/acs.jmedchem.6b00760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsch M. E.; Zhou J.; Gao Y.; Yan Y.; Porter G.; Agnihotri G.; Li Y.; Lu H.; Chen Z.; Thomas S. B. Discovery of Potent and Selective Leads against Toxoplasma gondii Dihydrofolate Reductase via Structure-Based Design. ACS Med. Chem. Lett. 2016, 7 (12), 1124–1129. 10.1021/acsmedchemlett.6b00328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asai T.; Takeuchi T.; Diffenderfer J.; Sibley L. D. Identification of Small-Molecule Inhibitors of Nucleoside Triphosphate Hydrolase in Toxoplasma gondii. Antimicrob. Agents Chemother. 2002, 46 (8), 2393–2399. 10.1128/AAC.46.8.2393-2399.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson C.; Jones N. G.; Hull-Ryde E. A.; Kireev D.; Stashko M.; Tang K.; Janetka J.; Wildman S. A.; Zuercher W. J.; Schapira M.; Hui R.; Janzen W.; Sibley L. D. Identification of small molecule inhibitors that block the Toxoplasma gondii rhoptry kinase ROP18. ACS Infect. Dis. 2016, 2 (3), 194–206. 10.1021/acsinfecdis.5b00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaslip A. T.; Leung J. M.; Carey K. L.; Catti F.; Warshaw D. M.; Westwood N. J.; Ballif B. A.; Ward G. E. A Small-Molecule Inhibitor of T. gondii Motility Induces the Posttranslational Modification of Myosin Light Chain-1 and Inhibits Myosin Motor Activity. PLoS Pathog. 2010, 6 (1), e1000720–e1000721. 10.1371/journal.ppat.1000720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lourido S.; Zhang C.; Lopez M. S.; Tang K.; Barks J.; Wang Q.; Wildman S. A.; Shokat K. M.; Sibley L. D. Optimizing Small Molecule Inhibitors Of Calcium-Dependent Protein Kinase 1 to Prevent Infection by Toxoplasma gondii. J. Med. Chem. 2013, 56 (7), 3068–3077. 10.1021/jm4001314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown K. M.; Suvorova E.; Farrell A.; McLain A.; Dittmar A.; Wiley G. B.; Marth G.; Gaffney P. M.; Gubbels M. J.; White M.; Blader I. J. Forward Genetic Screening Identifies a Small Molecule that Blocks Toxoplasma gondii Growth by Inhibiting Both Host- and Parasite-Encoded Kinases. PLoS Pathog. 2014, 10 (6), e1004180 10.1371/journal.ppat.1004180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leesombun A.; Iijima M.; Umeda K.; Kondoh D.; Pagmadulam B.; Abdou A. M.; Suzuki Y.; Ohba S.-I.; Isshiki K.; Kimura T.; Kubota Y.; Sawa R.; Nihei C.-I.; Nishikawa Y. Metacytofilin Is a Potent Therapeutic Drug Candidate for Toxoplasmosis. J. Infect. Dis. 2020, 221 (5), 766–774. 10.1093/infdis/jiz501. [DOI] [PubMed] [Google Scholar]
- Alday P. H.; Doggett J. S. Drugs in Development for Toxoplasmosis: Advances, Challenges, and Current Status. Drug Des., Dev. Ther. 2017, 11, 273–293. 10.2147/DDDT.S60973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radke J. B.; Burrows J. N.; Goldberg D. E.; Sibley L. D. Evaluation of Current and Emerging Antimalarial Medicines for Inhibition of Toxoplasma gondii Growth in Vitro. ACS Infect. Dis. 2018, 4 (8), 1264–1274. 10.1021/acsinfecdis.8b00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittmar A. J.; Drozda A. A.; Blader I. J. Drug repurposing screening identifies novel compounds that effectively inhibit Toxoplasma gondii growth. mSphere 2016, 1 (2), e00042-15. 10.1128/mSphere.00042-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J. L.; Si H. F.; Shang X. F.; Zhang X. K.; Li B.; Zhou X. Z.; Zhang J. Y. New Life for an Old Drug: In Vitro and In Vivo Effects of the Anthelmintic Drug Niclosamide against Toxoplasma gondii RH strain. Int. J. Parasitol.: Drugs Drug Resist. 2019, 9, 27–34. 10.1016/j.ijpddr.2018.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland M. M.; Zach S. J.; Wang X.; Potluri L. P.; Neville A. J.; Vennerstrom J. L.; Davis P. H. Review of Experimental Compounds Demonstrating Anti-Toxoplasma Activity. Antimicrob. Agents Chemother. 2016, 60 (12), 7017–7034. 10.1128/AAC.01176-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunay I. R.; Gajurel K.; Dhakal R.; Liesenfeld O.; Montoya J. G. Treatment of Toxoplasmosis: Historical Perspective, Animal Models, and Current Clinical Practice. Clin. Microbiol. Rev. 2018, 31 (4), e00057-17. 10.1128/CMR.00057-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montazeri M.; Sharif M.; Sarvi S.; Mehrzadi S.; Ahmadpour E.; Daryani A. A Systematic Review of In vitro and In vivo Activities of Anti-Toxoplasma Drugs and Compounds (2006–2016). Front. Microbiol. 2017, 8, 25. 10.3389/fmicb.2017.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaherty D. P.; Harris M. T.; Schroeder C. E.; Khan H.; Kahney E. W.; Hackler A. L.; Patrick S. L.; Weiner W. S.; Aubé J.; Sharlow E. R.; Morris J. C.; Golden J. E. Optimization and Evaluation of Antiparasitic Benzamidobenzoic Acids as Inhibitors of Kinetoplastid Hexokinase 1. ChemMedChem 2017, 12 (23), 1994–2005. 10.1002/cmdc.201700592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milanes J. E.; Suryadi J.; Abendroth J.; Van Voorhis W. C.; Barrett K. F.; Dranow D. M.; Phan I. Q.; Patrick S. L.; Rozema S. D.; Khalifa M. M.; Golden J. E.; Morris J. C. Enzymatic and Structural Characterization of the Naegleria fowleri Glucokinase. Antimicrob. Agents Chemother. 2019, 63 (5), e02410-18. 10.1128/AAC.02410-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalifa M. M.; Morris J. C.; Golden J. E.. Development of Inhibitors of Naegleria fowleri. Unpublished work.
- Devine W.; Thomas S. M.; Erath J.; Bachovchin K. A.; Lee P. J.; Leed S. E.; Rodriguez A.; Sciotti R. J.; Mensa-Wilmot K.; Pollastri M. P. Antiparasitic Lead Discovery: Toward Optimization of a Chemotype with Activity Against Multiple Protozoan Parasites. ACS Med. Chem. Lett. 2017, 8 (3), 350–354. 10.1021/acsmedchemlett.7b00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh B.; Bernatchez J. A.; McCall L.-I.; Calvet C. M.; Ackermann J.; Souza J. M.; Thomas D.; Silva E. M.; Bachovchin K. A.; Klug D. M.; Jalani H. B.; Bag S.; Buskes M. J.; Leed S. E.; Roncal N. E.; Penn E. C.; Erath J.; Rodriguez A.; Sciotti R. J.; Campbell R. F.; McKerrow J.; Siqueira-Neto J. L.; Ferrins L.; Pollastri M. P. Scaffold and Parasite Hopping: Discovery of New Protozoal Proliferation Inhibitors. ACS Med. Chem. Lett. 2020, 11 (3), 249–257. 10.1021/acsmedchemlett.9b00453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klug D. M.; Gelb M. H.; Pollastri M. P. Repurposing Strategies for Tropical Disease Drug Discovery. Bioorg. Med. Chem. Lett. 2016, 26 (11), 2569–2576. 10.1016/j.bmcl.2016.03.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferkorn E. R.; Pfefferkorn L. C. Specific Labeling of Intracellular Toxoplasma gondii with Uracil. J. Protozool. 1977, 24 (3), 449–453. 10.1111/j.1550-7408.1977.tb04774.x. [DOI] [PubMed] [Google Scholar]
- Knoll L. J.; Boothroyd J. C. Isolation of Developmentally Regulated Genes from Toxoplasma gondii by a Gene Trap with the Positive and Negative Selectable Marker Hypoxanthine-Xanthine-Guanine Phosphoribosyltransferase. Mol. Cell. Biol. 1998, 18 (2), 807–814. 10.1128/MCB.18.2.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss L. M.; Ma Y. F.; Takvorian P. M.; Tanowitz H. B.; Wittner M. Bradyzoite Development in Toxoplasma gondii and HSP70 Stress Response. Infect. Immun. 1998, 66 (7), 3295–3302. 10.1128/IAI.66.7.3295-3302.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstanley P. Drug Treatment of Toxoplasmic Encephalitis In Acquired Immunodeficiency Syndrome. Postgrad. Med. J. 1995, 71 (837), 404–408. 10.1136/pgmj.71.837.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohne W.; Hunter C. A.; White M. W.; Ferguson D. J. P.; Gross U.; Roos D. S. Targeted Disruption of the Bradyzoite-specific Gene BAG1 Does Not Prevent Tissue Cyst Formation in Toxoplasma gondii. Mol. Biochem. Parasitol. 1998, 92 (2), 291–301. 10.1016/S0166-6851(97)00236-3. [DOI] [PubMed] [Google Scholar]
- Fleige T.; Fischer K.; Ferguson D. J. P.; Gross U.; Bohne W. Carbohydrate Metabolism in the Toxoplasma gondii Apicoplast: Localization of Three Glycolytic Isoenzymes, the Single Pyruvate Dehydrogenase Complex, and a Plastid Phosphate Translocator. Eukaryotic Cell 2007, 6 (6), 984–996. 10.1128/EC.00061-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajria B.; Bahl A.; Brestelli J.; Dommer J.; Fischer S.; Gao X.; Heiges M.; Iodice J.; Kissinger J. C.; Mackey A. J.; Pinney D. F.; Roos D. S.; Stoeckert C. J. Jr.; Wang H.; Brunk B. P. ToxoDB: an Integrated Toxoplasma gondii Database Resource. Nucleic Acids Res. 2008, 36 (Database issue), D553–D556. 10.1093/nar/gkm981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson W.; Martorelli Di Genova B.; Gallego-Lopez G.; Dawson A. R.; Stevenson D. M.; Amador-Noguez D.; Knoll L. J. Dual Metabolomic Profiling Uncovers Toxoplasma Manipulation of the Host Metabolome and the Discovery of a Novel Parasite Metabolic Capability. PLoS Pathog. 2020, 16 (4), e1008432 10.1371/journal.ppat.1008432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melamud E.; Vastag L.; Rabinowitz J. D. Metabolomic Analysis and Visualization Engine for LC–MS Data. Anal. Chem. 2010, 82 (23), 9818–26. 10.1021/ac1021166. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.