Abstract
Purpose
Breast cancer is the most commonly diagnosed cancer in women, with many efforts aimed at reducing acute and late toxicity given the generally favorable clinical outcomes with the current standard of care. Carbon ion radiation therapy is an emerging technique that may reduce dose to adjacent organs at risk while allowing dose escalation to the target. Given the efficacy of the standard treatments for breast cancer, there have been few prospective studies to date investigating carbon ion radiation therapy in breast cancer.
Methods
PubMed/Medline, Ebsco, Cochrane, and Scopus were systematically reviewed using the search terms “carbon ion” and “breast” in November 2019. Out of the 76 articles screened, 26 articles were included.
Results
This comprehensive review describes the physical and biological properties of carbon ion radiation therapy, with an emphasis on how these properties can be applied in the setting of breast cancer. Studies investigating the role of carbon ion radiation therapy in early stage breast cancers are reviewed. Additionally, the use of carbon ion radiation therapy in locally advanced disease, recurrent disease, and radiation-induced angiosarcoma are discussed.
Conclusion
Although the data is limited, the early clinical results are promising. Further clinical trials are needed, especially in the setting of locally advanced and recurrent disease, to fully define the potential role of carbon ion radiation therapy in the treatment of breast cancer.
Keywords: Heavy, Particle, Breast, Tumor, Hadron
Introduction
Breast cancer is the most commonly diagnosed cancer in women and the leading cause of cancer-related mortality. In the USA, approximately one in eight women will be diagnosed with the disease, with an estimated 268,600 newly diagnosed invasive cancers and 41,760 deaths due to breast cancer in 2019. Additionally, the incidence of breast cancer is increasing approximately 0.3% per year since 2012 [1]. Radiation therapy, traditionally with photons, is a standard component of curative treatment following lumpectomy in most cases of early stage breast cancer. In addition, radiation therapy is commonly administered following mastectomy in many locally advanced cancers [2–4]. Historically, particle therapy has not been widely used in the treatment of breast cancer. In 2017, an estimated 18.0% of proton and carbon ion radiotherapy (CIRT) facilities in Europe were treating breast cancers [5]. However, given the generally favorable long-term disease control outcomes with modern breast cancer therapy, there has been considerable focus on investigating new radiotherapeutic techniques that minimize exposure to normal tissues with the goal of reducing acute and late adverse effects of treatment [6–9]. Technological advances have enabled improved and less cost delivery of particle therapy. Therefore, interest in particle radiation for breast cancer, including protons and CIRT, has risen in recent years.
The National Institute of Radiologic Sciences (NIRS) opened the first heavy ion accelerator for clinical use in Chiba, Japan in 1994, and currently, there are 13 centers in 5 countries (Austria, China, Germany, Italy, and Japan) treating with CIRT [10–12]. Although few studies have been reported to date, CIRT has distinct physical and biological properties that may benefit a substantial number of patients with breast cancers in the future [13–16].
Discussion
Physics and radiobiology
Irradiation with heavy ions, specifically carbon ions, takes advantage of several unique physical and radiobiological principles to help increase the therapeutic index compared to conventional photon irradiation (Fig. 1). Charged particles, including proton and carbon ions, exhibit a “Bragg peak,” a characteristic energy deposition curve where there is very little energy deposited proximal or distal to a target. Instead, the majority of energy is released at a specific depth. Moreover, a potential advantage of CIRT over proton and photon therapy is that there is a steeper dose fall-off laterally with CIRT. This tighter “penumbra” may further improve radiation precision over these other modalities [11, 17]. Together, these physical properties theoretically reduce dose to organs at risk (OARs), such as the heart and lungs, while also allowing comprehensive treatment of the targeted volume.
Fig. 1.
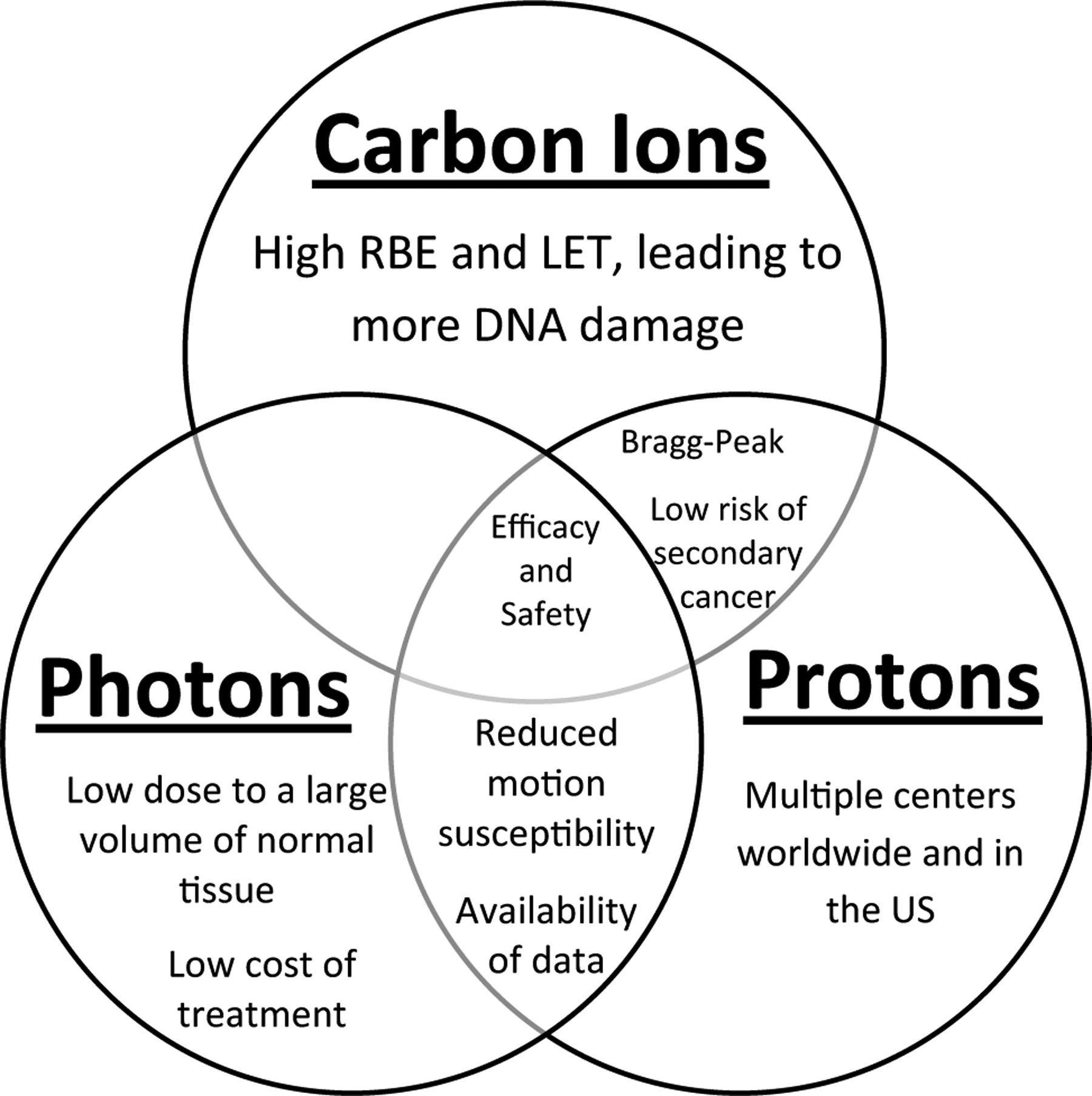
Comparison of the benefits of carbon ion radiotherapy, proton radiotherapy, and conventional photon radiotherapy for the treatment of breast cancers. RBE relative biological effectiveness, LET linear energy transfer
CIRT is considered a high LET (linear energy transfer) radiation, meaning CIRT releases a higher degree of energy per unit of material (i.e., cellular DNA) as compared to low LET irradiation such as photons and protons. This greater ionizing density within the target leads to a higher degree of irreparable DNA damage, which overwhelms the DNA repair mechanisms of cancer cells. Thus, a higher degree of cell kill is observed with CIRT compared to low LET irradiation [17]. Carbon ions are thus considered to have a high RBE (relative biologic effectiveness), which is a ratio of how effective a type of radiation is compared to photon irradiation. For example, the RBE for carbon ranges anywhere from 1.1 to 4 depending on the cell line [18–20], meaning it has a higher effectiveness (1.1–4 times) in a given tissue at a given dose compared to a low-energy photon.
The exact biological mechanisms of cell kill in breast cancer cells following CIRT is still being elicited. The DNA double strand break is the most lethal lesion caused by radiotherapy that must be repaired to prevent cell death. With CIRT, there is appears to be greater clustering of multiple forms of DNA damage in close proximity to double strand breaks rendering these lesions more difficult to repair. Additionally, it has been suggested that clustered DNA damage affects the ataxia telangiectasia mutated (ATM) pathway [17]. Other biological differences between CIRT and photons have been reported. In the MDA-MB-231 human breast cancer line, delivery of sublethal doses of photon irradiation has been shown to promote migration and invasion of the cancer cells. Notably, CIRT suppresses this malignant phenotype in a dose-dependent matter [21]. Interestingly, irradiation with CIRT in mammary tissues have shown upregulated induction of COX2 and DNA damage in tissues outside of the treatment field, which may lead to increased toxicities out of the treatment field [22]. The clinical implication of this is not yet fully realized, although care must be taken to avoid unnecessary out-of-field toxicities.
Furthermore, the addition of CIRT to chemotherapeutic regimens has shown promise in preclinical studies. For example, CIRT has been shown to have superior potential to kill triple-negative breast cancer stem cells in vitro in combination with cisplatin compared to photon irradiation, likely due to enhanced apoptosis and irreparable DNA damage [23].Researchers in China and Japan investigated combining 5-fluorouracil, paclitaxel, and methotrexate, which all substrates of the organic anion transporting polypeptides, an important family of transporter proteins for antineoplastic agents, in combination with CIRT. Combination therapy resulted in greater cytotoxicity involving the MCF7 breast cancer cell line, and show promise in facilitating delivery of these antineoplastic agents [24]. Interestingly, CIRT in combination with caffeine has been shown to inhibit the expression of BRCA1 and its downstream enzymes [25].
Typically, particle radiotherapy is delivered using either passive scatter or active scanning techniques. In passive scatter, the beam is broadened and shaped by compensators to allow for a more conformal dose. With active scanning, a narrow beam is used to deliver the dose as a series of beam-lets that are shaped by magnets. By its nature of dose-delivery, active scanning is often felt to be a superior technique in conforming dose. Matsubara et al. evaluated 11 patients treated in Japan using passive scattering and active scanning, and found that, in the setting of breast cancer, active scanning is not more effective than passive scattering [26]. This is important, as many of the CIRT currently in use do not have active scanning capability.
Clinical applications
Much of the early clinical experience treating breast cancer has been at NIRS. At NIRS, candidates for CIRT include those with T1N0M0, ER positive, and HER2 negative disease without extensive intraductal component, lymphovascular invasion, or located within 5 mm of the skin. Patients usually have two fiducial markers implanted prior to treatment, and are positioned in the supine position with a fixed body shell consisting of neck, breast, and abdominal sections. Doses are typically given between 48 and 60 GyE in four fractions as described in the dose escalation trial in the following section [27].
The first published case report of a patient with stage I breast cancer treated at NIRS was in 2014 [28]. As a follow up on this report, Karasawa et al. reported on seven patients with stage I breast cancer treated with CIRT enrolled in the first prospective phase I trial investigating early stage breast cancer at NIRS. Patients were over 60 years of age, had a life expectancy of over 6 months, were stage I, had a solitary tumor with a diameter of under 2 cm, and were ER positive and HER2 negative. In this dose escalation trial, three patients were treated with 48 GyE, three were treated with 52.8 GyE, and one was treated in 60 GyE. All patients were treated in four fractions over a 1-week period. Patients were immobilized with a thermoplastic cast and treated supine. Following irradiation, all patients underwent tumor excision for pathologic evaluation. Patients tolerated treatment well. Four patients experiencing acute grade 1 skin toxicity, but no other toxicities reported. At 3 months, one patient had a complete response, five patients had a pathologic response, and one patient had stable disease. Six patients were treated with postoperative endocrine therapy (four aromatase inhibitors and two tamoxifen). With follow up of 37–48 months, there were no patients with recurrent disease [29].
In the metastatic setting, there is a case report of a 54-year-old woman with metastatic breast cancer who was treated with a single fraction of CIRT to a dose of 36 GyE to a chemoresistant liver metastasis. She survived for more than 8 years without recurrence [30]. Of note, CIRT may have activating effects outside of the treatment field [22], and may be superior to activating the immune system [31], which may eventually be leveraged in treating oligometastatic disease. In this setting, CIRT can allow for dose escalation to the site of metastasis, such as the liver, while sparing other tissues. Local therapy to the breast could then be performed with standard of care as appropriate. Further trials are needed to investigate the role of CIRT in the setting of metastatic breast cancers.
Early clinical studies, such as those described in the preceding sections, treated patients primarily in the preoperative setting, as the radiobiological and physical advantages of CIRT are minimized in the postoperative setting in the absence of positive margins or other high risk features. There is concern regarding normal tissue toxicity in the absence of a targetable tumor mass in which to target the Bragg Peak. Perhaps the greatest benefit of CIRT can be seen in patients with traditionally difficult to treat and radioresistant tumors [20], such as those with locally advanced breast cancer, positive margins, or unresected nodal disease. For example, patients with locally advanced or inflammatory breast cancer with significant residual disease after preoperative chemotherapy (i.e., chemoresistant breast cancer) have high rates of locoregional recurrence despite surgical resection and standard photon post-mastectomy radiotherapy. These results suggest that residual disease following preoperative chemotherapy may also be a more radioresistant disease biology that may benefit from CIRT. CIRT may also be attractive to treat patients with unresected nodal disease in the supraclavicular fossa or internal mammary nodes. One area of active research using CIRT is for reirradiation [32–34]. CIRT may potentially be a viable treatment option for patients developing recurrent breast cancer of the chest wall following previous irradiation.
CIRT may also be preferential for treating angiosarcoma in the breast, as these are traditionally radioresistant tumors and often present in a previously irradiated area. Angiosarcoma has been previously treated safely with proton beam radiotherapy, and the incidence of angiosarcoma is likely to continue to increase given the good clinical outcomes following current standards of care [35].
Reduction in the risk of secondary malignancy is an important goal of radiation oncologists, as many patients treated for breast cancer have decades-long life expectancies. Previous studies have suggested an approximately 3.4% risk of radiation induced secondary malignancies following radiotherapy [36]. Although there have been no direct comparisons of the risk of secondary malignancy following CIRT or photon irradiation for breast cancer, a study investigating the risk of secondary malignancies in prostate cancer patients found a lower risk with CIRT compared to photon irradiation, possibly due to reduced exposure of normal tissues with CIRT outside of the target volume [37]. Eley et al. estimated the risk of secondary malignancies with plans using proton therapy and CIRT, estimated that the risks are comparable between the two modalities [38].
To our knowledge, there are no currently accruing clinical trials evaluating the use of CIRT for breast cancer. As many of the current clinical trials in Europe and Asia focus on traditionally radioresistant or recurrent diseases, breast cancers have been an under-studied disease site. As previously discussed, unresectable or resistant breast cancers remains a valid option for further clinical trials with CIRT. Additionally, trials using CIRT can be designed similar to already accruing proton trials. Loma Linda is currently investigating proton beam therapy for partial breast irradiation (NCT01310530). Additionally, the University of Florida is investigating the use of proton therapy for lymph node irradiation in breast cancer (NCT01365845). Given the advantages of particle therapy in reducing normal tissue does for lymph node irradiation or partial breast irradiation, these are reasonable areas to design a small phase II trial investigating CIRT. Future trials can also investigate the use of CIRT compared to proton or photon therapy, similar to the current RADCOMP trial (NCT02603341), which is comparing proton and photon irradiation for locally advanced breast cancers.
In summary, there remains much to be learned about the potential role of CIRT for early and locally advanced breast cancers. Preliminary results from the first prospective dose escalation trial are promising. Given the higher RBE of CIRT and favorable pathologic outcomes, it is tempting to speculate that some patients may be treated non-surgically in the future with CIRT alone. Further clinical trials will need to be performed to fully investigate the role of CIRT in the setting of early stage breast cancer, including comparisons with prone positioning, accelerated partial breast irradiation, and intraoperative radiotherapy where normal tissue sparing is already generally favorable. CIRT may potentially be used as a boost in the supine setting, or in patients who have tumors close to the chest wall or in other difficult to treat locations. In the locally advanced and post-mastectomy settings, further dosimetric and clinical trials will be needed to investigate the dose delivered to the chest wall and underlying OARs compared to both proton and photon treatments. Given the higher RBE of CIRT, care will need to be taken in planning to minimize high dose deposition at end of the carbon ion range on the ribs, heart, and lungs.
Conclusion
In conclusion, CIRT is an exciting new treatment modality that has several physical and biological advantages that may help increase the therapeutic index for patients with early stage and locally advanced breast cancer. Further trials are needed to define the role of CIRT in the treatment of breast cancer.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Conflict of interest DMT has received clinical trial funding from Novocure and publishing fees from Springer. The remaining authors declare that they have no conflict of interest.
Research involving human participants and/or animals This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent No informed consent was required as part of this manuscript.
References
- 1.DeSantis CE, Ma J, Gaudet MM, Newman LA, Miller KD, Goding Sauer A, Jemal A, Siegel RL (2019) Breast cancer statistics, 2019. CA Cancer J Clin. 10.3322/caac.21583 [DOI] [PubMed] [Google Scholar]
- 2.Fisher B, Anderson S, Bryant J, Margolese RG, Deutsch M, Fisher ER, Jeong JH, Wolmark N (2002) Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med 347(16):1233–1241. 10.1056/NEJMoa022152 [DOI] [PubMed] [Google Scholar]
- 3.Overgaard M, Hansen PS, Overgaard J, Rose C, Andersson M, Bach F, Kjaer M, Gadeberg CC, Mouridsen HT, Jensen MB, Zedeler K (1997) Postoperative radiotherapy in high-risk premenopausal women with breast cancer who receive adjuvant chemotherapy. Danish Breast Cancer Cooperative Group 82b Trial. N Engl J Med 337(14):949–955. 10.1056/NEJM199710023371401 [DOI] [PubMed] [Google Scholar]
- 4.Overgaard M, Jensen MB, Overgaard J, Hansen PS, Rose C, Andersson M, Kamby C, Kjaer M, Gadeberg CC, Rasmussen BB, Blichert-Toft M, Mouridsen HT (1999) Postoperative radiotherapy in high-risk postmenopausal breast-cancer patients given adjuvant tamoxifen: Danish Breast Cancer Cooperative Group DBCG 82c randomised trial. Lancet 353(9165):1641–1648. 10.1016/S0140-6736(98)09201-0 [DOI] [PubMed] [Google Scholar]
- 5.Weber DC, Abrunhosa-Branquinho A, Bolsi A, Kacperek A, Dendale R, Geismar D, Bachtiary B, Hall A, Heufelder J, Herfarth K, Debus J, Amichetti M, Krause M, Orecchia R, Vondracek V, Thariat J, Kajdrowicz T, Nilsson K, Grau C (2017) Profile of European proton and carbon ion therapy centers assessed by the EORTC facility questionnaire. Radiother Oncol 124(2):185–189. 10.1016/j.radonc.2017.07.012 [DOI] [PubMed] [Google Scholar]
- 6.Vaidya JS, Wenz F, Bulsara M, Tobias JS, Joseph DJ, Keshtgar M, Flyger HL, Massarut S, Alvarado M, Saunders C, Eiermann W, Metaxas M, Sperk E, Sutterlin M, Brown D, Esserman L, Roncadin M, Thompson A, Dewar JA, Holtveg HM, Pigorsch S, Falzon M, Harris E, Matthews A, Brew-Graves C, Potyka I, Corica T, Williams NR, Baum M, Group TT (2014) Risk-adapted targeted intraoperative radiotherapy versus whole-breast radiotherapy for breast cancer: 5-year results for local control and overall survival from the TARGIT-A randomised trial. Lancet 383(9917):603–613. 10.1016/S0140-6736(13)61950-9 [DOI] [PubMed] [Google Scholar]
- 7.Veronesi U, Orecchia R, Maisonneuve P, Viale G, Rotmensz N, Sangalli C, Luini A, Veronesi P, Galimberti V, Zurrida S, Leonardi MC, Lazzari R, Cattani F, Gentilini O, Intra M, Caldarella P, Ballardini B (2013) Intraoperative radiotherapy versus external radiotherapy for early breast cancer (ELIOT): a randomised controlled equivalence trial. Lancet Oncol 14(13):1269–1277. 10.1016/S1470-2045(13)70497-2 [DOI] [PubMed] [Google Scholar]
- 8.Whelan TJ, Pignol JP, Levine MN, Julian JA, MacKenzie R, Parpia S, Shelley W, Grimard L, Bowen J, Lukka H, Perera F, Fyles A, Schneider K, Gulavita S, Freeman C (2010) Long-term results of hypofractionated radiation therapy for breast cancer. N Engl J Med 362(6):513–520. 10.1056/NEJMoa0906260 [DOI] [PubMed] [Google Scholar]
- 9.Haviland JS, Owen JR, Dewar JA, Agrawal RK, Barrett J, Barrett-Lee PJ, Dobbs HJ, Hopwood P, Lawton PA, Magee BJ, Mills J, Simmons S, Sydenham MA, Venables K, Bliss JM, Yarnold JR, Group ST (2013) The UK Standardisation of Breast Radiotherapy (START) trials of radiotherapy hypofractionation for treatment of early breast cancer: 10-year follow-up results of two randomised controlled trials. Lancet Oncol 14(11):1086–1094. 10.1016/S1470-2045(13)70386-3 [DOI] [PubMed] [Google Scholar]
- 10.Kamada T (2015) Twenty years of carbon ion radiation therapy at the national institute of radiological sciences: accomplishments and prospects. Int J Part Ther. 10.14338/IJPT-15-00030.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rackwitz T, Debus J (2019) Clinical applications of proton and carbon ion therapy. Semin Oncol. 10.1053/j.seminoncol.2019.07.005 [DOI] [PubMed] [Google Scholar]
- 12.Particle therapy facilities in clinical operation (2019) Particle therapy co-operative group https://www.ptcog.ch/index.php/facilities-in-operation. Accessed 1 Dec 2019
- 13.Cho I, Seo YS, Jung W, Kim MS (2018) Estimation of the medical need for carbon-ion radiotherapy in Korea. J Radiat Res 59(5):588–592. 10.1093/jrr/rry046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hosseini MAP, Mohamadianpanah MM, Zare-Bandeamiri MM, Mosleh-Shirazi MAP (2018) A preliminary study on the estimation of the number of cancer patients eligible for hadron therapy in Iran and Fars Province. Iran J Med Sci 43(3):313–317 [PMC free article] [PubMed] [Google Scholar]
- 15.Ohno T, Nakano T, Kanai T, Yamada S (2011) Carbon ion radiotherapy at Gunma university: currently indicated cancer and estimation of need. AIP Conf Proc 1336(1):391–396. 10.1063/1.3586127 [DOI] [Google Scholar]
- 16.Krengli M, Orecchia R (2004) Medical aspects of the National Centre For Oncological Hadrontherapy (CNAO-Centro Nazionale Adroterapia Oncologica) in Italy. Radiother Oncol 73(Suppl 2):S21–23. 10.1016/s0167-8140(04)80007-0 [DOI] [PubMed] [Google Scholar]
- 17.Mohamad O, Sishc BJ, Saha J, Pompos A, Rahimi A, Story MD, Davis AJ, Kim DWN (2017) Carbon ion radiotherapy: a review of clinical experiences and preclinical research with an emphasis on DNA damage/repair. Cancers (Basel). 10.3390/cancers9060066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chiblak S, Campos B, Gal Z, Tang Z, Unterberg A, Debus J, Herold-Mende C, Abdollahi A (2012) Photon vs. proton vs. Carbon irradiation of glioma initiating cells. In: 18. Jahreskongress der Deutschen Gesellschaft fur Radioonkologie, DEGRO 2012, Wiesbaden Germany, June 7–10, 2012 [Google Scholar]
- 19.Suzuki M, Kase Y, Kanai T, Ando K (1998) Correlation between cell death and induction of non-rejoining PCC breaks by carbon-ion beams. Adv Space Res 22(4):561–568 [DOI] [PubMed] [Google Scholar]
- 20.Schlaff CD, Krauze A, Belard A, O’Connell JJ, Camphausen KA (2014) Bringing the heavy: carbon ion therapy in the radiobiological and clinical context. Radiat Oncol 9(1):88 10.1186/1748-717X-9-88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Imaizumi H, Sato K, Nishihara A, Minami K, Koizumi M, Matsuura N, Hieda M (2018) X-ray-enhanced cancer cell migration requires the linker of nucleoskeleton and cytoskeleton complex. Cancer Sci 109(4):1158–1165. 10.1111/cas.13545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang TJ, Wu CC, Chai Y, Lam RK, Hamada N, Kakinuma S, Uchihori Y, Yu PK, Hei TK (2015) Induction of non-targeted stress responses in mammary tissues by heavy ions. PLoS ONE 10(8):e0136307 10.1371/journal.pone.0136307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sai S, Vares G, Kim EH, Karasawa K, Wang B, Nenoi M, Horimoto Y, Hayashi M (2015) Carbon ion beam combined with cisplatin effectively disrupts triple negative breast cancer stem-like cells in vitro. Mol Cancer 14:166 10.1186/s12943-015-0429-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou C, Rong Y, Konishi T, Xiang Z, Zihui F, Hong M (2017) Effect of carbon-ion radiation on drug transporters organic anion transporting polypeptides in breast cancer cells. Radiat Res 187(6):689–700. 10.1667/RR14603.1 [DOI] [PubMed] [Google Scholar]
- 25.Li N, Zhang H, Wang Y-L, Zhou X, Hao J-F (2011) Effects of caffeine cotreatment with radiation on breast cancer susceptibility gene BRCA1. Yuanzineng Kexue Jishu/Atom Energy Sci Technol 45(1):124–128 [Google Scholar]
- 26.Matsubara H, Karasawa K, Furuichi W, Wakaisami M, Shiba S, Wakatsuki M, Omatsu T, Inaniwa T, Fukuda S, Kamada T (2018) Comparison of passive and scanning irradiation methods for carbon-ion radiotherapy for breast cancer. J Radiat Res 59(5):625–631. 10.1093/jrr/rry052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsujii H, Kamada T, Kj N, Tsuji H, Karasawa K (2014) Carbonion radiotherapy : principles, practices, and treatment planning. Springer, Japan [Google Scholar]
- 28.Akamatsu H, Karasawa K, Omatsu T, Isobe Y, Ogata R, Koba Y (2014) First experience of carbon-ion radiotherapy for early breast cancer. Jpn J Radiol 32(5):288–295. 10.1007/s11604-014-0300-6 [DOI] [PubMed] [Google Scholar]
- 29.Karasawa K, Omatsu T, Arakawa A, Yamamoto N, Ishikawa T, Saito M, Fukuda S, Kamada T, Working Group for Breast C (2019) A Phase I clinical trial of carbon ion radiotherapy for Stage I breast cancer: clinical and pathological evaluation. J Radiat Res 60(3):342–347. 10.1093/jrr/rry113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harada M, Karasawa K, Yasuda S, Kamada T, Nemoto K (2015) One shot of carbon-ion radiotherapy cured a 6-cm chemo-resistant metastatic liver tumor: a case of breast cancer. Jpn J Radiol 33(9):598–602. 10.1007/s11604-015-0462-x [DOI] [PubMed] [Google Scholar]
- 31.Shimokawa T, Ma L, Ando K, Sato K, Imai T (2016) The future of combining carbon-ion radiotherapy with immunotherapy: evidence and progress in mouse models. Int J Part Ther 3(1):61–70. 10.14338/ijpt-15-00023.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hayashi K, Koto M, Ikawa H, Hagiwara Y, Tsuji H, Ogawa K, Kamada T (2019) Feasibility of Re-irradiation using carbon ions for recurrent head and neck malignancies after carbon-ion radiotherapy. Radiother Oncol 136:148–153. 10.1016/j.radonc.2019.04.007 [DOI] [PubMed] [Google Scholar]
- 33.Hayashi K, Yamamoto N, Karube M, Nakajima M, Tsuji H, Ogawa K, Kamada T (2018) Feasibility of carbon-ion radiotherapy for re-irradiation of locoregionally recurrent, metastatic, or secondary lung tumors. Cancer Sci 109(5):1562–1569. 10.1111/cas.13555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Habermehl D, Wagner M, Ellerbrock M, Buchler MW, Jakel O, Debus J, Combs SE (2015) Reirradiation using carbon ions in patients with locally recurrent rectal cancer at HIT: first results. Ann Surg Oncol 22(6):2068–2074. 10.1245/s10434-014-4219-z [DOI] [PubMed] [Google Scholar]
- 35.Thorpe CS, Niska JR, Brunnhoelzl DC, McGee LA, Kesslering CM, Hartsell WF, Vargas CE (2018) First report of proton beam therapy for breast angiosarcoma from the prospective PCG registry. Acta Oncol 57(7):992–994. 10.1080/0284186X.2017.1423179 [DOI] [PubMed] [Google Scholar]
- 36.Burt LM, Ying J, Poppe MM, Suneja G, Gaffney DK (2017) Risk of secondary malignancies after radiation therapy for breast cancer: comprehensive results. Breast 35:122–129. 10.1016/j.breast.2017.07.004 [DOI] [PubMed] [Google Scholar]
- 37.Mohamad O, Tabuchi T, Nitta Y, Nomoto A, Sato A, Kasuya G, Makishima H, Choy H, Yamada S, Morishima T, Tsuji H, Miyashiro I, Kamada T (2019) Risk of subsequent primary cancers after carbon ion radiotherapy, photon radiotherapy, or surgery for localised prostate cancer: a propensity score-weighted, retrospective, cohort study. Lancet Oncol 20(5):674–685. 10.1016/S1470-2045(18)30931-8 [DOI] [PubMed] [Google Scholar]
- 38.Eley JG, Friedrich T, Homann KL, Howell RM, Scholz M, Durante M, Newhauser WD (2016) Comparative risk predictions of second cancers after carbon-ion therapy versus proton therapy. Int J Radiat Oncol Biol Phys 95(1):279–286. 10.1016/j.ijrobp.2016.02.032 [DOI] [PMC free article] [PubMed] [Google Scholar]


