Abstract
Objective
to develop a model to predict one- and three-year mortality in patients with dementia attending a hospital, through hospital admission or day/memory clinic.
Design
we constructed a cohort of dementia patients through data linkage of three Dutch national registers: the hospital discharge register (HDR), the population register and the national cause of death register.
Subjects
patients with dementia in the HDR aged between 60 and 100 years registered between 1 January 2000 and 31 December 2010.
Methods
logistic regression analysis techniques were used to predict one- and three-year mortality after a first hospitalisation with dementia. The performance was assessed using the c-statistic and the Hosmer–Lemeshow test. Internal validation was performed using bootstrap resampling.
Results
50,993 patients were included in the cohort. Two models were constructed, which included age, sex, setting of care (hospitalised versus day clinic) and the presence of comorbidity using the Charlson comorbidity index. One model predicted one-year mortality and the other three-year mortality. Model discrimination according to the c-statistic for the models was 0.71 (95% CI 0.71–0.72) and 0.72 (95% CI 0.72–0.73), respectively.
Conclusion
both models display acceptable ability to predict mortality. An important advantage is that they are easy to apply in daily practise and thus are helpful for individual decision-making regarding diagnostic/therapeutic interventions and advance care planning.
Keywords: dementia, prediction, mortality, hospital admission, memory/day clinic, older people
Key points
We developed models to predict 1- and 3-year mortality of dementia patients after their first hospitalisation or day clinic visit.
The models are of acceptable performance and easy to apply in daily practise.
The four risk factors included in the models were age, sex, setting of care (hospital admission or day clinic) and comorbidity.
The models constitute a very useful source of information to identify patients with dementia at differential risk of death.
Introduction
The incidence and prevalence of dementia are increasing worldwide. Currently, 35.6 million people are suffering from the disease and this number is expected to triple in the coming decades [1]. Prognosis is known to be poor, but differs considerably between individuals and depends on underlying factors such as age, sex and comorbidity [2–4].
Management in daily practise, particularly advance care planning (ACP), is inevitably based on the estimated prognosis. However, prognosis is rarely based on a single predictor. A prognostic measure that integrates several risk factors enables stratification of patients into groups at a differential risk of death, and yields a more individualised, accurate estimate of prognosis. In recent years, several models to predict prognosis in dementia have been developed. However, prognosis was not always defined as mortality but for example as progression of disease [5]. Some of these models aimed to predict mortality in a specific patient group as nursing home residents [6, 7]. And other models used specific symptoms (e.g. gait apraxia without using a validated instrument to assess apraxia) [8] or many factors making the use of the model in daily practise complicated [9].
Therefore, the aim of the current study is to develop an easy-to-apply model to predict mortality in patients with dementia attending hospital, through admission or day/memory clinic visit, to support management in daily practise.
Methods
Databases
A cohort was constructed by linking three databases: the Dutch Hospital Discharge Register (HDR), the Dutch Population Register (PR) and the National Cause of Death Register. Since the 1960s, medical and administrative data for admitted and memory/day clinic patients visiting a Dutch hospital are recorded in the HDR. Patients in the Netherlands are referred to the day/memory clinic in case of either with memory-related disorders (memory clinic) or with multi-morbidity, which also might include memory-related disorders (day clinic). Around 100 hospitals participate in the register, which covers 90% of Dutch hospitals. The HDR contains information on patients’ demographics (date of birth, sex), type of hospital, admission data and principle and secondary diagnoses at admission. The principle and secondary diagnoses are determined at discharge and coded using the ninth revision of the International Classification of Diseases (ICD-9-CM) [10]. The PR contains information on all legally residing citizens in the Netherlands, including date of birth, sex, current address, postal code, nationality and native country. In the National Cause of Death register, date of death and all primary and any underlying causes of death are reported. In the Netherlands, it is mandatory to complete a death declaration form after the death of any person. Death reports are coded according to the International statistical Classification of Diseases and Related Health Problems, 10th version [11]. The overall validity of these registries has been shown to be high [12].
Cohort identification
All patients who were admitted in hospital or referred to the day/memory clinic with either a principal or a secondary diagnosis of dementia aged between 60- and 100-years old were selected from the HDR between 1 January 2000 and 31 December 2010. The collected cases were linked with the PR by using the record identification number assigned to each resident in the Netherlands with a unique combination of date of birth, sex and the numeric part of the postal code. The index date was the date patients with dementia were admitted or visited the day/memory clinic for the first time in the study period. Through linkage with the National Cause of Death registry, follow-up information on date of death and principal and underlying causes of death could be obtained. The noted somatic comorbidities, including cardiovascular disease (CVD), were based on discharge diagnoses of previous hospital admissions up to 5 years prior to the index date and obtained from the HDR using the unique record identification number. The validity of ICD codes for CVD has also been shown to be high [13, 14]. Information on severity of disease, presence of risk factors (e.g. hypertension, hypercholesterolemia) or medication use was not available in the registry.
Outcome
One- and three-year mortality risks were calculated. One-year follow up was defined for all included patients as 1 year from the index data of their hospital visit between 2000 and 2009 (n = 50,993). Similarly, three-year follow up was available for all patients included between 2000 and 2007 (n = 38,521).
Statistics
A logistic regression analysis was performed to construct two models, one to predict one- and another to predict three-year mortality among dementia patients admitted to a hospital or visiting a day clinic. Variables considered for the model were: age, sex, setting of care (i.e. day clinic or hospitalisation), type of dementia and comorbidity.
Factors were included in the multivariable analysis if P < 0.10 based on the Wald test. Next, stepwise backward selection was performed leaving a set of variables with the most predictive value for mortality. Age was subdivided into 10 year age-groups. Comorbidity was defined using a modified Charlson comorbidity index (CCI), which proved to be a valid and reliable method to measure comorbidity in clinical research [15]. The updated CCI ranges from 0 to 28 points. Total scores per individual were subdivided into three different groups: 0, 1–2 and >3. Dementia was excluded from the CCI, because all included patients had dementia. Type of dementia was divided into Alzheimer’s disease, vascular dementia and an unknown type of dementia group.
A multivariate mortality risk model was constructed with all variables significantly influencing 1- or 3-year mortality in the univariate analysis. Absolute 1- and 3-year mortality risks were calculated with crosstabs analysis. Absolute mortality risks are presented with 95% confidence intervals. The area under the receiver operating characteristic curve, also known as the c-statistic, and the Hosmer–Lemeshow goodness of fit statistic were used to assess the discrimination and calibration of the models, respectively. In the absence of an external validation cohort, internal validation of the models was performed with the bootstrap method to assess the optimism of the clinical prediction model by randomly drawing 1000 samples from the original data set. All statistical analyses were performed with the SPSS software, version 20.0 (SPSS Inc., Chicago, Illinois, USA) and the R statistics program, version 2.15.1 (R Foundation for Statistical Computing, Vienna, Austria).
Transparency
This report was written using the transparent reporting of multivariable prediction model for individual prognosis or diagnosis statement, a checklist specifically designed for reporting multivariable prediction models [16].
Ethics
Linkage of data from the different registries was performed in agreement with the privacy legislation in the Netherlands [17]. Only anonymised records and data sets are involved. The study did not have to be assessed according to the regulations of the Research complying with the Dutch law on Medical Research in Humans. All linkages and analyses were performed in a secure environment of Statistics Netherlands.
Results
Baseline characteristics
In total, 50,993 patients with dementia (38.7% men) were identified for the one-year model of whom 17,923 died within 1 year (35.1%) after the index hospital visit/admission. For the three-year model, 38,521 patients were included and 23,975 (62.2%) died within 3 years. Baseline characteristics are presented in Table 1.
Table 1.
Factors associated with one- (n = 50,933) and three-year (n = 38,521) mortality among patients with a first hospital admission or day clinic visit with dementia in the Netherlands
| Factor | Model for one-year mortality | Model for three-year mortality | ||||
|---|---|---|---|---|---|---|
| Overall (n = 50,993) N (%) | Univariate analysis OR (95% CI) | Multivariate analysis OR (95% CI) | Overall (n = 38,521) N (%) | Univariate analysis OR (95% CI) | Multivariate analysis OR (95% CI) | |
| Age, years | ||||||
| 60–69 | 2999 (5.9) | Ref. | Ref. | 2243 (5.8) | Ref. | Ref. |
| 70–79 | 15,770 (30.9) | 1.65 (1.50–1.82)* | 1.60 (1.44–1.77)* | 12,046 (31.3) | 1.93 (1.76–2.12)* | 2.01 (1.82–2.22)* |
| 80–89 | 26,593 (52.2) | 2.74 (2.49–3.01)* | 2.59 (2.34–2.87)* | 19,917 (51.7) | 3.54 (3.23–3.87)* | 3.84 (3.48–4.23)* |
| 90–99 | 5631 (11.0) | 5.02 (4.51–5.58)* | 4.76 (4.26–5.33)* | 4315 (11.2) | 8.13 (7.24–9.14)* | 9.29 (8.21–10.52)* |
| Sex | ||||||
| Women | 31,318 (61.4) | Ref. | Ref. | 23,749 (61.7) | Ref. | Ref. |
| Men | 19,675 (38.6) | 1.44 (1.39–1.50)* | 1.68 (1.62–1.75)* | 14,772 (38.3) | 1.59 (1.52–1.66)* | 1.95 (1.86–2.05)* |
| Type of care | ||||||
| Day clinic | 15,688 (30.8) | Ref. | Ref. | 10,598 (27.5) | Ref. | Ref. |
| Inpatient | 35,305 (69.2) | 4.81 (4.58–5.05)* | 4.34 (4.12–4.56)* | 27,923 (72.5) | 3.39 (3.24–3.55)* | 3.04 (2.89–3.19)* |
| Comorbiditya | ||||||
| 0 | 34,561 (67.8) | Ref. | Ref. | 26,503 (68.8) | Ref. | Ref. |
| 1–2 | 13,961 (27.4) | 1.31 (1.26–1.37)* | 1.48 (1.41–1.56)* | 10,270 (26.7) | 1.72 (1.61–1.83)* | 1.33 (1.26–1.40)* |
| > 3 | 2741 (4.8) | 2.08 (1.91–2.25)* | 2.28 (2.02–2.57)* | 1748 (4.5) | 3.32 (2.79–3.96)* | 2.61 (2.31–2.96)* |
| Type of dementiab | ||||||
| Alzheimer | 31,799 (62.4) | Ref. | Ref. | 24,225 (62.9) | Ref. | |
| Vascular dementia | 6555 (12.9) | 0.88 (0.83–0.93)* | 0.94 (0.89–1.00)** | 5078 (13.2) | 0.95 (0.89–1.01) | 1.02 (0.96–1.10) |
| Unknown type | 12,639 (24.8) | 0.58 (0.56–0.61)* | 0.79 (0.76–0.83)* | 9218 (23.9) | 058 (0.55–0.60)* | 0.80 (0.76–0.85)* |
N = number of patients, OR = odds ratio, 95% CI = 95% confidence interval and Ref = reference.
aMeasured with Charlson comorbidity index.
bSome patients had an unspecified type of dementia.
* P-value < 0.05.
** P-value < 0.1.
Derivation of the prediction model
Table 1 shows the results of the univariate and multivariable analysis for one- and three-year mortality. Age, sex, type of care, type of dementia and comorbidity were taken into the models. Figures 1 and 2 show the absolute mortality risks stratified by age, sex, type of care and comorbidity. It made no difference on the other variables in the multivariate analysis whether or not type of dementia was taken into the analysis.
Figure 1.
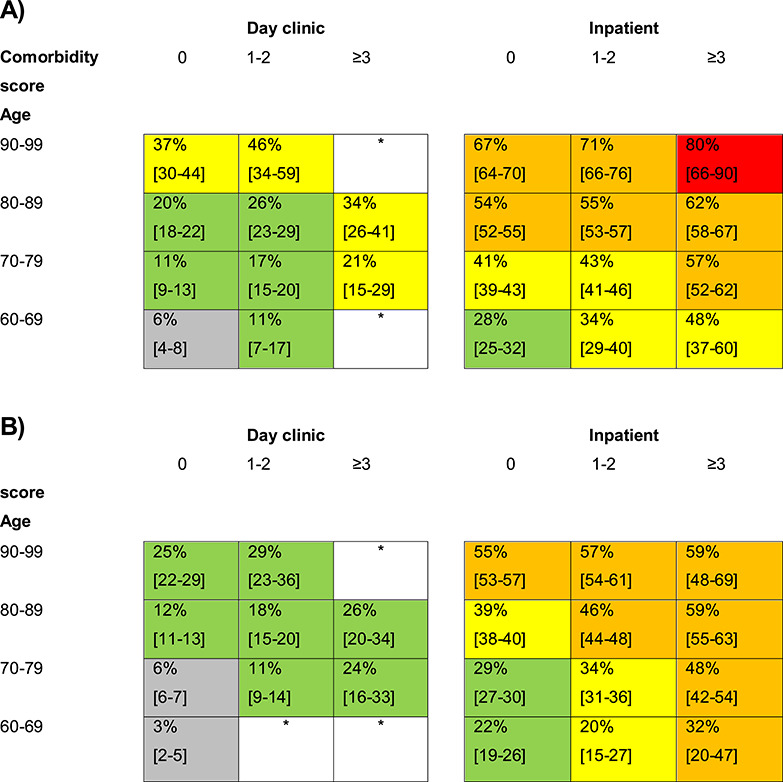
One-year mortality risk for patients with a first hospitalisation or day clinic visit with dementia in the Netherlands, stratified by age, setting of care and comorbidity for men (A) and for women (B). Numbers within individual cells reflect the risk of death within 1 year after the index visit with dementia (%). Between the brackets are the 95% confidence intervals of the percentages. White boxes have not enough data. Grey boxes comprise risks ≤10%, green boxes risks of 11–29%, yellow boxes risks of 30–49%, orange boxes risks 50–79% and red boxes risks ≥80%.
Figure 2.
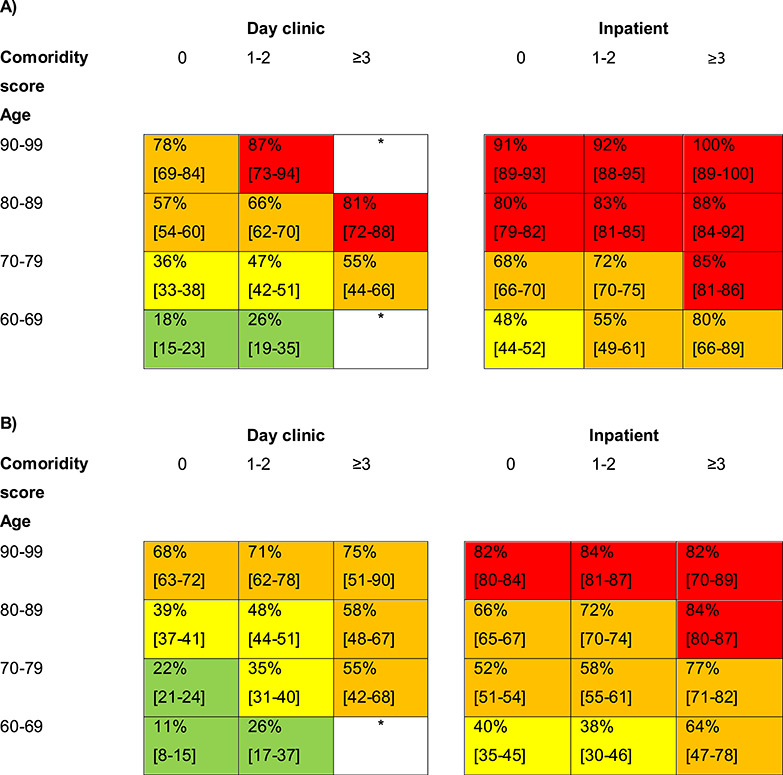
Three-year mortality risk for patients with a first hospitalisation or day clinic visit with dementia in the Netherlands, stratified by age, setting of care and comorbidity for men (A) and for women (B). Numbers within individual cells reflect the risk of death within 3 year after the index visit with dementia (%). Between the brackets are the 95% confidence intervals of the percentages. White boxes have not enough data. Green boxes comprise risks of 11–29%, yellow boxes risks of 30–49%, orange boxes risks 50–79% and red boxes risks ≥80%.
Validation of the model
To examine the discriminative ability of the model, the area under the receiver-operating curve was calculated. The area under the curve was 0.71 (95% CI 0.71–0.72) for the one-year model and 0.72 (95% CI 0.72–0.73) for the three-year model, indicating a fair ability to discriminate between patients who survived and those who deceased. Subsequently, observed outcomes were compared against those predicted by the models using the Hosmer–Lemeshow goodness of fit statistic (Supplementary Table S1). The goodness of fit statistic showed a P-value < 0.0001, generally indicating a poor model fit. However, here, the small P-value is a result of the very large sample size of our cohort as the differences between the predicted and observed frequencies actually were small. Internal validation of the models was performed by randomly drawing 1,000 samples from the original data set. The average c-statistic for the prediction models developed in the bootstrap sample was identical to the c-statistic when the full data set was used (estimate of optimism was 0.0002 for the one-year model and 0.0003 for the three-year model). The slope shrinkage factor for both models was 0.999. These results indicate that the models expected to show fair performance also in other comparable settings (data not shown).
Discussion
We developed a model to predict one- and three-year mortality risk among dementia patients after their first hospitalisation or a day clinic visit. The four factors included in the models were age, sex, setting of care (hospital admission or day clinic) and comorbidity. The models display acceptable discrimination and calibration.
Comparison with other models
Several models have been developed to predict mortality in dementia and some of these models also included other variables. Paradise et al. [8] showed that age and constructional or gait apraxia were independently associated with increased mortality among community-dwelling older adults with dementia. Stern et al. [18] showed in Alzheimer patients that besides sex and age, extrapyramidal signs, psychotic symptoms, duration of illness and cognitive performance influenced mortality. Delva et al. showed in a general population-based cohort that besides sex and age, the number of activities of daily living restrictions was associated with increased mortality and so did Newcomer et al. who included also many other variables making the use of the model complicated in daily care [9, 21]. Delva et al. [19] did not find a contribution of comorbidity to the model; however, the number of missing values for comorbidity was very large in this study. Mitchell et al. [6] developed a tool to predict mortality in patients living in nursing homes with advanced stages of dementia. Only the latter gave an overview of expected mortality risks per risk score making the model easily applicable in daily practise.
Strengths and implications of the model
The prediction model we describe here is based on a large hospital-based cohort of patients with dementia with an almost nationwide coverage and complete follow up. This is unique especially in the field of dementia research, where the participation rate is often low and loss to follow up is high due to accelerated cognitive decline [20].
Furthermore, the performance of the model was acceptable. With the inclusion of four strong variables in the models, we showed that the discriminative ability was fair. The Hosmer–Lemeshow test detected a significant degree of miscalibration, but it is known that this test is sensitive to sample size [21]. Therefore, we provided an overview of the observed versus predicted values. The differences between these values were small. At last, internal validation of the model showed a fair performance of the model.
It is well established that prognosis of dementia is poor in general. However, to identify individuals at differential risk of death is often complicated as patients with dementia represent a heterogeneous group. The models presented in this study provide a more accurate estimate of an individual patient’s mortality risk in daily practise. An important advantage of the models is that they are easy to apply in clinical care. This is important knowledge for the timing of ACP. Notwithstanding that ACP is important for all patients with dementia, the models show which patients have the highest risk and for whom ACP is more urgent.
Limitations of the model
A few limitations need to be addressed. Firstly, the models are not yet externally validated. Secondly, generalizability is restricted to secondary and tertiary care. If someone would want to use the models in other care settings, like the general practitioners office or nursing homes, it would be recommended to examine the performance of the models in that specific setting before use. Thirdly, although the performance of the models was acceptable, efforts should be made to improve the performance, possibly by extension of the models with other factors, including cardiovascular risk factors [22–24], severity of dementia [24, 25], level of education [26] activities of daily living [9, 19] or modifiable risk factors (as hypertension or diabetes mellitus). The HDR lacks information on these determinants. However, given that the performance of the models is already fair and as mortality risks are very high the effect of extension of the model is questionable. Finally, the presence and extent of comorbidity did not include comorbidity that did not lead to a hospital admission. Therefore, comorbidity may have been underestimated. We expect that the effect of underestimation of comorbidity is comparable in all subcategories of our prediction model. Therefore, a significant differential effect on the observed mortality risks is not likely. In an earlier Dutch study, the negative influence of comorbidity on mortality in patients with dementia was also found [27].
We did not include type of dementia in our models with absolute mortality risks, as there was no difference in mortality between Alzheimer’s disease and vascular dementia. We also did not include the unknown type of dementia, because this is a heterogeneous group which is not applicable on a particular patient group in clinical practise. In a large Swedish study, patients with vascular dementia had a higher mortality risk than patients with Alzheimer’s disease [28]. However, the accuracy of diagnosis of these two subtypes (Alzheimer’s disease and vascular dementia) was proven to be high in our database [29]. The difference in our results might derive from the difference in index date. The Swedish study included patients form the moment of dementia diagnosis, while we included patients from the moment they visited the hospital in our study period. Maybe, patients with vascular dementia attended hospital in an earlier stage than patients with Alzheimer’s disease, as the latter disease is a more gradual disease. This could have resulted in a lower than expected mortality risk for the patients with vascular dementia. As our databases do not include severity or stage of dementia, and also the stage of dementia at the index date was unknown, we could not include this information in the models. Our database also does not include any other types of dementia than Alzheimer’s disease and vascular dementia. Therefore, we could not specify other types of dementia and there is a heterogeneous group of patients with unknown type of dementia.
Conclusion
In the present study, we developed two models to predict one- and three-year mortality among patients hospitalised or visiting a day clinic with dementia. We showed that the performance of the models was acceptable. The models constitute a very useful source of information to identify patients with dementia at differential risk of death. An important advantage is that they are easy to apply in daily practise to support individual decision making with respect to diagnostic and therapeutic interventions and ACP.
Supplementary Material
Declaration of Conflicts of Interest
None.
Declaration of Sources of Funding
This work was financially supported by Alzheimer Nederland [project no WE.03-2012-38]. IV was supported by a grant from the Dutch Heart Foundation [grant DHF project “Facts and Figures’”; grant number: 31653251.
References
- 1. World Health Organization (WHO) and Alzheimer's Disease International (ADI) Dementia cases set to triple by 2050 but are still largely ignored. 2012. www.who.int/mediacentre/news/releases/2012/dementia_20120411.
- 2. Jagger C, Andersen K, Breteler MM et al. Prognosis with dementia in Europe: a collaborative study of population-based cohorts. Neurologic diseases in the elderly research group. Neurology 2000; 54: S16–20. [PubMed] [Google Scholar]
- 3. Xie J, Brayne C, Matthews FE. Medical Research Council cognitive function and ageing study collaborators. Survival times in people with dementia: analysis from population based cohort study with 14 year follow-up. BMJ 2008; 336: 258–62. doi: 10.1136/bmj.39433.616678.25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wolfson C, Wolfson DB, Asgharian M et al. A reevaluation of the duration of survival after the onset of dementia. N Engl J Med 2001; 344: 1111–6. doi: 10.1056/NEJM200104123441501. [DOI] [PubMed] [Google Scholar]
- 5. Green C, Zhang S. Predicting the progression of alzheimer's disease dementia: a multidomain health policy model. Alzheimers Dement 2016. 10.1016/j.jalz.2016.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mitchell SL, Miller SC, Teno JM, Davis RB, Shaffer ML. The advanced dementia prognostic tool: a risk score to estimate survival in nursing home residents with advanced dementia. J Pain Symptom Manage 2010; 40: 639–51. doi: 10.1016/j.jpainsymman.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. van der Steen JT, Mitchell SL, Frijters DH, Kruse RL, Ribbe MW. Prediction of 6-month mortality in nursing home residents with advanced dementia: validity of a risk score. J Am Med Dir Assoc 2007; 8: 464–8. doi: 10.1016/j.jamda.2007.05.004 [DOI] [PubMed] [Google Scholar]
- 8. Paradise M, Walker Z, Cooper C et al. Prediction of survival in alzheimer's disease-the LASER-AD longitudinal study. Int J Geriatr Psychiatry 2009; 24: 739–47. doi: 10.1002/gps.2190;10.1002/gps.2190 [DOI] [PubMed] [Google Scholar]
- 9. Newcomer R, Covinsky KE, Clay T, Yaffe K. Predicting 12-month mortality for persons with dementia. J Gerontol B Psychol Sci Soc Sci 2003; 58: S187–98. [DOI] [PubMed] [Google Scholar]
- 10. Dutch Hospital Data http://www.dutchhospitaldata.nl/registraties/lmrlazr/Paginas/default.aspx (January 2014, date last accessed)
- 11. The international statistical classification of diseases, injuries and related health problems. Tenth revision 1992.
- 12. Harteloh P, de Bruin K, Kardaun J. The reliability of cause-of-death coding in the Netherlands. Eur J Epidemiol 2010; 25: 531–8. doi: 10.1007/s10654-010-9445-5 [DOI] [PubMed] [Google Scholar]
- 13. Merry AH, Boer JM, Schouten LJ et al. Validity of coronary heart diseases and heart failure based on hospital discharge and mortality data in the Netherlands using the cardiovascular registry Maastricht cohort study. Eur J Epidemiol 2009; 24: 237–47. doi: 10.1007/s10654-009-9335-x [DOI] [PubMed] [Google Scholar]
- 14. Nieuwkamp DJ, Vaartjes I, Algra A, Rinkel GJ, Bots ML. Risk of cardiovascular events and death in the life after aneurysmal subarachnoid haemorrhage: a nationwide study. Int J Stroke 2012. doi: 10.1111/j.1747-4949.2012.00875.x [DOI] [PubMed] [Google Scholar]
- 15. Quan H, Li B, Couris CM et al. Updating and validating the charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol 2011; 173: 676–82. doi: 10.1093/aje/kwq433 [DOI] [PubMed] [Google Scholar]
- 16. Collins GS, Reitsma JB, Altman DG, Moons KG. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. BMJ 2015; 350: g7594. doi: 10.1136/bmj.g7594. [DOI] [PubMed] [Google Scholar]
- 17. Reitsma JB, Kardaun JW, Gevers E, de Bruin A, van der J, Bonsel GJ. Possibilities for anonymous follow-up studies of patients in dutch national medical registrations using the municipal population register: a pilot study. Ned Tijdschr Geneeskd 2003; 147: 2286–90. [PubMed] [Google Scholar]
- 18. Stern Y, Tang MX, Albert MS et al. Predicting time to nursing home care and death in individuals with Alzheimer disease. JAMA 1997; 277: 806–12. [PubMed] [Google Scholar]
- 19. Delva F, Pimouguet C, Helmer C et al. A simple score to predict survival with dementia in the general population. Neuroepidemiology 2013; 41: 20–8. doi: 10.1159/000346497 [DOI] [PubMed] [Google Scholar]
- 20. Chatfield MD, Brayne CE, Matthews FE. A systematic literature review of attrition between waves in longitudinal studies in the elderly shows a consistent pattern of dropout between differing studies. J Clin Epidemiol 2005; 58: 13–9. doi: 10.1016/j.jclinepi.2004.05.006 [DOI] [PubMed] [Google Scholar]
- 21. Kramer AA, Zimmerman JE. Assessing the calibration of mortality benchmarks in critical care: the Hosmer-Lemeshow test revisited. Crit Care Med 2007; 35: 2052–6. doi: 10.1097/01.CCM.0000275267.64078.B0 [DOI] [PubMed] [Google Scholar]
- 22. Helzner EP, Scarmeas N, Cosentino S, Tang MX, Schupf N, Stern Y. Survival in alzheimer disease: a multiethnic, population-based study of incident cases. Neurology 2008; 71: 1489–95. doi: 10.1212/01.wnl.0000334278.11022.42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zilkens RR, Davis WA, Spilsbury K, Semmens JB, Bruce DG. Earlier age of dementia onset and shorter survival times in dementia patients with diabetes. Am J Epidemiol 2013; 177: 1246–54. doi: 10.1093/aje/kws387 [DOI] [PubMed] [Google Scholar]
- 24. Larson EB, Shadlen MF, Wang L et al. Survival after initial diagnosis of alzheimer disease. Ann Intern Med 2004; 140: 501–9. [DOI] [PubMed] [Google Scholar]
- 25. Zhao Q, Zhou B, Ding D, Guo Q, Hong Z. Prevalence, mortality, and predictive factors on survival of dementia in shanghai, China. Alzheimer Dis Assoc Disord 2010; 24: 151–8. [DOI] [PubMed] [Google Scholar]
- 26. Russ TC, Stamatakis E, Hamer M, Starr JM, Kivimaki M, Batty GD. Socioeconomic status as a risk factor for dementia death: individual participant meta-analysis of 86 508 men and women from the UK. Br J Psychiatry 2013; 203: 10–7. doi: 10.1192/bjp.bp.112.119479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Haaksma ML, Rizzuto D, Ramakers IHGB et al. The impact of frailty and comorbidity on institutionalization and mortality in persons with dementia: a prospective cohort study. J Am Med Dir Assoc 2019; 20: 165–179e2. doi: 10.1016/j.jamda.2018.06.020. [DOI] [PubMed] [Google Scholar]
- 28. Garcia-Ptacek S, Farahmand B, Kareholt I, Religa D, Cudadrado ML, Eriksdotter M. Mortality risk after dementia diagnosis of dementia type and underlying f92actors: a cohort of 15,209 patients based on the Swedisch dementia registry. J Alzheimers Dis 2014; 41: 467–77. doi: 10.3233/JAD-131856 [DOI] [PubMed] [Google Scholar]
- 29. van de Vorst IE, Vaartjes I, Sinneceker L, Bots ML, Koek HL. The validity of a national hospital discharge register data on dementia; a comparative analysis using data from an university medical center. Neth J Med 2015; 73: 69–75. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


