Abstract
Introduction
Clinoptilolite has antiviral, antibacterial, anti-inflammatory, antidiabetic, and anticancer properties due to its biological activities. In various cancer cell culture studies, it has been reported effective against tumour cells and gave positive results in treatment of various tumours in dogs. No study was found on the effects of the nanoparticulate form, nanoclinoptilolite, on cancer cells. The aim of this study was to determine its cytotoxic and apoptotic effects in canine osteosarcoma (OSA) cell culture.
Material and Methods
Doses at 50% inhibitory concentration were determined by measuring the dose- and duration-dependent cytotoxicity of nanoclinoptilolite on canine D-17 osteosarcoma cells by methylthiazol tetrazolium (MTT) test for 24 h, 48 h, and 72 h. Murine caspase-3 and -7 activity and expression levels of the BAX and BCL2 genes were measured using RT-PCR to investigate the apoptotic effect.
Results
Nanoclinoptilolite decreased cell viability and induced caspase-3- and -7-mediated apoptosis in treated canine OSA cells. Furthermore, its application to canine OSA cells downregulated the expression of BCL2 and upregulated the expression of proapoptotic BAX.
Conclusion
Clinoptilolite, which was previously demonstrated to have anticancer properties, decreased cell viability effectively and rapidly and increased the apoptotic cell ratio in a novel use in nanoparticle form, exhibiting this effect by increasing the BAX/BCL2 ratio.
Keywords: nanoclinoptilolite, canine osteosarcoma cell line, apoptosis, caspase-3 and -7, BAX and BCL2 expression
Introduction
Diversity in the aetiology of cancer poses a challenge in determining a definitive treatment method and achieving results. Surgical intervention, administration of chemotherapeutic drugs, radiotherapy, immunotherapy, and alternative medicine to strengthen the immune system and support the treatment administered are all used in cancer regimens. Administration of immunostimulatory agents such as plant extracts or zeolites containing different antioxidants and investigation of their anti-cancer effects have been the major topics studied in recent years.
Osteosarcoma (OSA), common in dogs and especially in large breeds, accounts for 85% of all skeletal tumours (12, 30). OSA develops in the metaphyseal region of canine long bones and sometimes in the ribs and skull. The most common locations are the distal radius, proximal humerus, proximal tibia, distal tibia, and distal femur (21). No long-term survival has been achieved by standard chemotherapy for OSA in dogs due to the metastatic nature of this type of cancer (20, 26, 30). Osteosarcoma is very aggressive and frequently causes secondary tumours. At the time of diagnosis, there is usually one more tumour other than the primary location. Therefore, the standard treatment for bone cancer is the surgical removal of the primary tumour, followed by destruction of the remaining tumour cells with chemotherapy (26, 30).
Although the incidence of the disease is about ten times higher in the dog population than in humans, OSA is also the most common form of malignant bone cancer in children (13). Both clinical and molecular evidence has shown that OSA shares many common characteristics in humans and dogs such as anatomical location, presence of histopathologically diagnosed metastatic disease, development of chemotherapy-resistant metastases and altered expression/activation (5). In addition, overlapping transcriptional profiles and DNA copy-number variations in dogs and children have suggested that these diseases may also have significant similarity at the molecular level (5). Therefore, the studies conducted in the field of canine OSA have been considered to have possible value for research into human OSA in terms of study model and drug development.
Zeolites have unique and extraordinary physical and chemical properties. Clinoptilolite is a natural, nontoxic zeolite with ion exchange and adsorbent properties (23). Recently, studies on the use of clinoptilolite in veterinary and human medicine have been conducted. The antidiarrhoeic property is the best-known biological activity of natural clinoptilolite (27). It has been reported that it absorbs glucose very effectively (10) and has an immunostimulatory effect (2, 23, 24, 32). Also, some results show that silicates and aluminosilicates cause alterations in the expression of genes of proteins involved in cell signalling (23). Silicate particles ingested by cells have been reported to stimulate mitogen-activated protein kinase (MAPK), protein kinase C, and stress-activated protein kinases (SAPKs) (18). Moreover, they also activate important transcription factors such as activator protein-1 (AP-1) and NF-κB (nuclear factor kappa-light-chain-enhancer of activated B cells), and proinflammatory cytokines such as IL-lα, IL-6, and TNF (31). The anti-cancer effects of clinoptilolite have been demonstrated on in vitro animal models and on different tumour types in in vivo dog studies. These results demonstrate the anti-tumour effect of clinoptilolite and its potential role as an adjuvant in anti-cancer treatment (23, 24).
In the field of healthcare, nanodrug formulations can easily move across the blood–brain barrier and be used for therapeutic purposes due to their very small size (3, 4, 8, 15, 29). Preliminary studies on the use of nanoproducts have been performed in cell cultures and then performed on experimental animals. In this study, clinoptilolite nanoparticulated using chitosan biopolymer was added to canine OSA cell culture to investigate its effects on cells. This study aimed to determine the cytotoxic and apoptotic effects of nanoclinoptilolite administered to a canine OSA cell line and to evaluate its effects on apoptosis.
Material and Methods
Preparation of nanoparticle-containing clinoptilolite
In this study, clinoptilolite was encapsulated into nanoparticles prepared using chitosan biopolymer. For this purpose, nanoparticle synthesis was carried out in a spray drier using 1% chitosan, clinoptilolite solution, and thiaminpyrophosphate acetic acid. During the optimisation and characterisation of the nanoparticles obtained, size measurements were made with a Zetasizer Nano ZS90 particle size analyser (Malvern Panalytical, Malvern, UK). Nanoparticle synthesis was performed by Nanovet (Izmir, Turkey).
Cell line. The cells were supplied from the American Type Culture Collection (ATCC, USA). Frozen canine OSA cells of the D-17 line (CCL-183) were thawed and propagated in a sterile medium including Eagle’s minimum essential medium with 10% foetal bovine serum (Sigma-Aldrich, USA), 100 units/mL of penicillin and 100 mg/mL of streptomycin (Gibco, USA), and 1% MEM non-essential amino acid solution (Sigma-Aldrich) at 37°C in a 5% CO2 atmosphere of 80% humidified air. Subsequently, when the number of cells in the flask had increased by 80%– 90%, the cells were removed using trypsin-EDTA solution and passaged. Following the addition of trypsin-EDTA, the solution was kept in the incubator and as soon as the cells were observed to separate from the flask they had been adhering to, pipetting was performed by adding fresh medium in a double volume of trypsin-EDTA and the pipette content was transferred to the flask with fresh medium in the cells solution or to the plate at the desired density for analyses.
Methylthiazol tetrazolium (MTT) assay. After the canine D-17 OSA cells were removed from the stock when they reached the required number, the MTT method was used to determine the cytotoxic effect of clinoptilolite on these cells and the dose range. The main stock was prepared to obtain a 1,000 μg/mL concentration of clinoptilolite converted into nanoparticles. Diluting the prepared stock solution with fresh medium, clinoptilolite concentrations of 10, 20, 30, 40, 50, 75, 100, 150, and 200 μg/mL were prepared. Canine D-17 OSA cells in these solutions were incubated at 37°C in 95% humidified air and 5% CO2 for 24, 48, and 72 h. At the end of the incubation, 100 μL of the prepared MTT solution was added to each sample and incubated again for 4 h. After the incubation, the liquid above the cells was removed and 100 μL of dimethyl sulphoxide was added to each sample, after which the samples were read at 570 nm. Each experiment was repeated four times. The wells containing only cells and medium with no clinoptilolite were considered positive controls. The data obtained were used to calculate the IC50 value in Graphpad Prism 5.0 software (GraphPad, San Diego, CA, USA). For each incubation time, the absorbance values of the clinoptilolite-treated cells were normalised to the control value and the IC50 values were calculated.
Caspase-3 and -7 activity analyses. To examine caspase-3 and -7 activity, the cells were seeded in 12-well plates at a density of 250,000 cells/well. After exposure to 10, 20, and 30 μg/mL nanoclinoptilolite concentrations for 24 and 48 h, caspase-3 and -7 activity was analysed using the Muse Caspase-3/7 Assay Kit (Millipore Sigma, St Louis, MO, USA) according to the user’s guide. Assay results were measured using the Muse Cell Analyzer (Millipore Sigma).
Determination of BAX and BCL2 gene expression
RNA isolation and cDNA synthesis. The D-17 OSA cells into which nanoclinoptilolite was introduced were obtained in the form of pellets. For this purpose, cells were removed with trypsin-EDTA at the end of the incubation period and centrifugation was performed. RNA was isolated from the obtained cells using a RiboEx commercial RNA isolation kit (GeneAll Biotechnology, Seoul, Korea) and the concentration of the RNA samples was measured at 260 nm wavelength in a Multiskan FC Photometer microplate reader (Thermo Fisher Scientific, Vantaa, Finland). To obtain more reliable results at the qRT-PCR stage, the A260/280 ratio determining the RNA quality was designated, and cDNA synthesis was carried out with a Transcriptor High Fidelity cDNA Synthesis kit (Roche, Penzberg, Germany) with appropriate samples using a LightCycler Nano real-time PCR system (Roche). The cDNA obtained was completed to 100 μL, divided into aliquots and stored at 4°C for immediate use or at −20°C for later qRT-PCR study.
Preparation of primers. For RT-PCR analysis, primers were supplied from Atlas Biyoteknoloji (Ankara, Turkey) in lyophilised form. They were centrifuged and diluted using H2O with diethyl pyrocarbonate (DEPC) and a primary stock with a concentration of 100 μM was obtained. In order to increase the solubility of the primers, the solutions were heated in a thermal block at 37°C for 5 min. An intermediate primer stock in a concentration of 10 μM was prepared from the 100 μM concentration stock to be used in the PCR reaction. For this purpose, 10 μL was taken from the primer stock and diluted with 90 μL of H2O containing DEPC.
The expression levels of BCL2 and BAX genes involved in apoptotic pathways were determined using the qRT-PCR method on a LightCycler 480 (Roche). In the implementation of the method, the primer sets specific to the relevant genes were used and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) served as the reference gene. Cross referencing to the RNA data of canine BCL2, BAX and GAPDH genes from GenBank, base sequences were evaluated and checked using the specificities of the primers created using online BLAST software (NCBI). Each cDNA sample was tested three times, and the cycle threshold (Ct) value was determined. In each replication, the PCR applications not containing cDNA or reverse transcriptase were used as negative controls. The Ct value was also determined for GAPDH to be used as the reference gene and the expression of the tested genes was normalised to this gene. Next, the ΔΔCt method developed by Livak and Schmittgen (19) was used to calculate the differences between the groups.
Statistical analysis. All the data were expressed as mean values ± standard deviations. A P value of <0.05 was considered significant. A Shapiro–Wilk test was used for evaluation of the normality of distributions, and a one-way ANOVA test was used for normal distribution. SPSS for Windows version 22 (IBM SPSS, Armonk, NY, USA) was used to perform statistical analysis.
Table 1.
Primer sequences used for qRT-PCR
| NCBI reference sequence | Gene | Forward primer (5´→3´) | Reverse primer (5´→3´) |
|---|---|---|---|
| NM_001003142.2 | GAPDH | AGTCAAGGCTGAGAACGGGAAA | TCCACAACATACTCAGCACCAGC |
| NM_001002949.1 | BCL2 | CATGCCAAGAGGGAAACACCAGAA | GTGCTTTGCATTCTTGGATGAGGG |
| NM_001003011.1 | BAX | TTCCGAGTGGCAGCTGAGATGTTT | TGCTGGCAAAGTAGAAGAGGGCAA |
Results
The effect of nanoclinoptilolite on canine OSA cell viability was evaluated by the MTT method. Exposure of canine D-17 OSA cells to nanoclinoptilolite resulted in a statistically significant decrease in viable cells in a time- and dose-dependent manner (Fig. 1 and Table 2). IC50 values of the cells were 35.64 μg/mL, 26.29μg/mL, and 7.99 μg/mL at 24, 48, and 72 h, respectively.
Fig. 1.
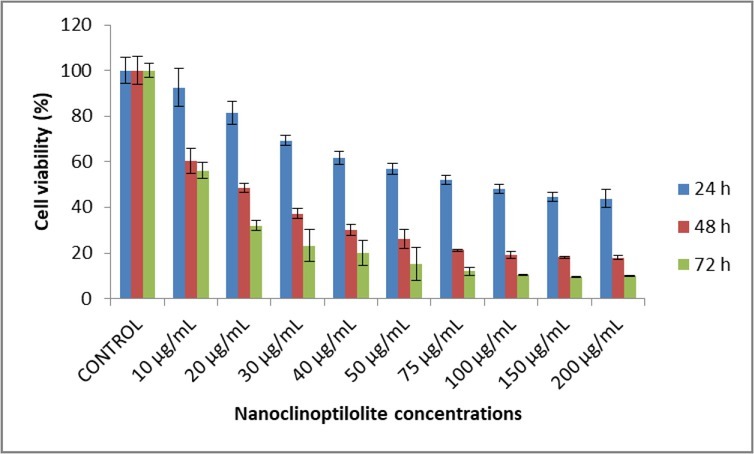
Effect of nanoclinoptilolite on cell viability of canine OSA D-17
Table 2.
Effect of nanoclinoptilolite on cell viability of canine D-17 OSA. The results represent the mean ± standard deviation
| Nanoclinoptilolite concentration (μg/mL) | 24 h | 48 h | 72 h | ||||||
|---|---|---|---|---|---|---|---|---|---|
| % Viability | X | P | % Viability | X | P | % Viability | X | P | |
| Control | 100 ± 5.66 | a | 100 ± 6.09 | a | 100 ± 3.15 | a | |||
| 10 | 92.62 ± 8.29 | a | >0.05 | 60.24 ± 5.50 | b | *** | 56.08 ± 3.57 | b | *** |
| 20 | 81.45 ± 5.11 | b | *** | 48.50 ± 1.83 | c | *** | 31.95 ± 2.24 | c | *** |
| 30 | 69.35 ± 2.24 | c | *** | 37.27 ± 2.25 | d | *** | 23.23 ± 6.89 | cd | *** |
| 40 | 61.66 ± 3.01 | cd | *** | 30.12 ± 2.23 | de | *** | 19.87 ± 5.45 | de | *** |
| 50 | 56.88 ± 2.45 | de | *** | 26.16 ± 4.14 | ef | *** | 15.14 ± 7.29 | def | *** |
| 75 | 51.92 ± 2.02 | def | *** | 21.19 ± 0.42 | fg | *** | 12.02 ± 1.70 | ef | *** |
| 100 | 48.08 ± 1.87 | ef | *** | 19.14 ± 1.55 | fg | *** | 10.31 ± 0.26 | ef | *** |
| 150 | 44.60 ± 1.80 | f | *** | 18.19 ± 0.53 | g | *** | 9.41 ± 0.15 | f | *** |
| 200 | 43.80 ± 3.91 | f | *** | 18.00 ± 0.74 | g | *** | 9.92 ± 0.28 | f | *** |
* P < 0.05; ** P < 0.01; *** P < 0.001 compared to control
Statistical difference between groups with different letters in the same column is significant
Statistical difference between groups with different letters in the same column is significant
Statistical difference between groups with different letters in the same column is significant
Statistical difference between groups with different letters in the same column is significant
Statistical difference between groups with different letters in the same column is significant
Statistical difference between groups with different letters in the same column is significant
Statistical difference between groups with different letters in the same column is significant
Statistical difference between groups with different letters in the same column is significant
Statistical difference between groups with different letters in the same column is significant
Statistical difference between groups with different letters in the same column is significant
Statistical difference between groups with different letters in the same column is significant
There was no statistically significant difference in caspase-3 and -7 activity in cells inoculated with 10 μg/mL and 20 μg/mL concentrations of clinoptilolite for 24 h, compared to the control. However, there was a statistically significant increase in this activity in cells to which a 30 μg/mL concentration of nanoclinoptilolite was added. The percentage of early apoptotic cells induced by this concentration increased to 6.20% and for late apoptotic cells it increased to 6.57%. The total apoptotic cell content was determined at 12.77% and that of dead cells at 0.52% (Table 3, Fig. 2 A–D). Canine D-17 OSA cells treated with a 10 μg/mL concentration of nanoclinoptilolite for 48 h showed no statistically significant difference when compared to the control. However, the percentage of early apoptotic cells induced by 20 μg/mL and 30 μg/mL concentrations of nanoclinoptilolite increased to 4.48% and 8.95%, and the percentage of late apoptotic cells increased to 9.73% and 14.85%, respectively (Table 4, Fig. 2 E–G).
Table 3.
Live/apoptotic/necrotic cell ratios in canine D-17 OSA cells treated for 24 h with 10, 20, and 30 μg/mL of nanoclinoptilolite and in control group cells
| % live | % apoptotic | % dead | |
|---|---|---|---|
| Control | 92.3 ± 2.36a | 7.62 ± 1.83a | 0.12 ± 0.017a |
| 10 μg/mL | 93.65 ± 1.61a | 5.78 ± 0.87a | 0.57 ± 0.18b |
| 20 μg/mL | 91.57 ± 0.43a | 7.60 ± 0.32a | 0.95 ± 0.004 c |
| 30 μg/mL | 86.65 ± 0.41b | 12.77 ± 0.51b | 0.52 ± 0.07b |
| P | 0.020 | 0.031 | 0.05 |
Statistical difference between groups with different letters in the same column is significant
Statistical difference between groups with different letters in the same column is significant
Statistical difference between groups with different letters in the same column is significant
Fig. 2.
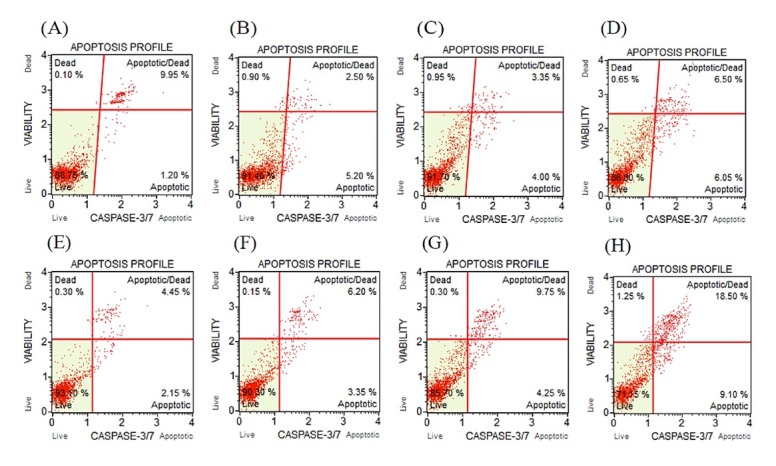
Caspase-3 and -7 activity after treatment with different concentrations of nanoclinoptilolite in canine D-17 OSA cells. Treatment over 24 h: A – control; B – 10 μg/mL; C– 20 μg/mL; D – 30 μg/mL. Treatment over 48 h: E – control; F – 10 μg/mL; G – 20 μg/mL; H– 30 μg/mL
Table 4.
Live/apoptotic/necrotic cell ratios in canine D-17 OSA cells treated for 48 h with 10, 20, and 30 μg/mL of nanoclinoptilolite and in control group cells
| % live | % apoptotic | % dead | |
|---|---|---|---|
| Control | 92.63 ± 0.63a | 7.25 ± 0.73a | 0.22 ± 0.04a |
| 10 g/mL | 91.3 ± 0.41a | 8.53 ± 0.93a | 0.17 ± 0.02a |
| 20 μg/mL | 85.35 ± 0.30b | 14.2 ± 0.5b | 0.45 ± 0.09a |
| 30 μg/mL | 23.8 ± 1.96c | 23.8 ± 1.93c | 1.2 ± 0.03b |
| P | 0.020 | 0.031 | 0.05 |
Statistical difference between groups with different letters in the same column is significant
Statistical difference between groups with different letters in the same column is significant
Statistical difference between groups with different letters in the same column is significant
Expression of BAX (pro-apoptotic) and BCL-2 (anti-apoptotic) proteins was determined by RT-PCR to investigate the apoptotic effect, and BAX/BCL-2 ratios were calculated. There was no significant change in BAX/BCL-2 ratios compared to the control (0 μM) over the 24-h incubation period (data not shown). At the end of 48-h incubation with nanoclinoptilolite, the BAX/BCL-2 ratio was found to be 3.00-, 8.88-, and 14.24-fold higher compared with the control cells for 10, 20, and 30 μg/mL of nanoclinoptilolite doses, respectively (Table 5, Fig. 3).
Table 5.
Effect of nanoclinoptilolite on the Bax/Bcl-2 ratio in canine D-17 OSA cells for 24 and 48 h (* P<0.05)
| Nanoclinoptilolite concentration | 24 h | 48 h |
|---|---|---|
| 10 μg/mL | 0.44 | 3.00 |
| 20 μg/mL | 0.44 | 8.88 |
| 30 μg/mL | 0.75 | 14.24 |
Fig. 3.
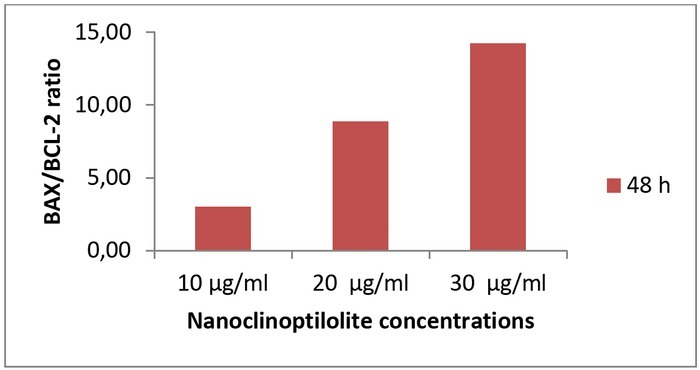
Effect of nanoclinoptilolite on the BAX/BCL-2 ratio in canine D-17 OSA cells
Discussion
The biological activities of clinoptilolite include glucose absorption, diarrhoea symptom relief, and immune response stimulation (16). It was shown that chitosan-coated clinoptilolite nanoparticles statistically significantly decreased canine D-17 OSA cell viability at 24, 48, and 72 h. There are various studies administering clinoptilolite to cancer cells in pure form (6, 16, 23, 24). In these studies, the researchers challenged cancer cells with clinoptilolite and investigated various pathways in cancer cell lines. Pavlevic et al. (23) used small clinoptilolite particles to investigate the mechanical therapeutic effect of natural clinoptilolite and its toxic and anti-cancer effects. They administered clinoptilolite at doses of 0.5, 5, and 50 mg/mL to diploid fibroblast (Hef522), cervical carcinoma (HeLa), colon carcinoma (CaCo-2, HT-29, and SW-620), breast carcinoma (MCF-7 and SkBr-3), and mouse fibrosarcoma cell lines and carried out a viability assay by MTT at the end of a 72 h incubation. They found that cell proliferation was significantly inhibited by a dose of 50 mg/mL in all cell lines except Hef522 and SW620. In this study, the strongest inhibition (50%) was observed in mouse fibrosarcoma cells. One of the action mechanisms of zeolites is reported to be the absorption of serum components (7). Adsorption of molecules involved in signal conduction steps such as inositol phosphatides and calcium may also contribute to their therapeutic efficacy. They have been reported to be strong performers in lipid absorption studies. Similar results have been observed in the adsorption of proteins. Alterations in membrane sequence and interactions of membrane proteins with other proteins may also take place since membrane translocation is required for activation of protein kinase B/Akt (25). Researchers have reported that zeolite administration leads to inhibition of protein kinase B/Akt pathways in the cell and then results in apoptosis (22). Akt has been reported to inactivate p27KIP1, an important cyclin inhibitor and tumour suppressor molecule, which has been shown to be activated by antioxidants (9). Moreover, researchers have shown that zeolites absorb and deactivate nitric oxide and other oxidants. The study by Katic et al. (16) showed that proliferation was inhibited in clinoptilolite-challenged mouse fibrosarcoma (FSAR), small-cell lung carcinoma (SCCVII), human pancreatic carcinoma (MiaPaCa2), and normal diploid fibroblast (V138) cell lines and demonstrated by MTT test that apoptosis was also activated. Ashana Gazhi et al. (6) aimed to investigate the anti-cancer effect of zeolites other than clinoptilolites in cancer cell lines in vitro. For this purpose, they carried out viability tests with MTT in cervical carcinoma (HeLa), human pancreatic tumour (AsPc-1), and human embryonic retinoblast (HER) cells to which were applied zeolite X and zeolite Y at doses of 5mg/mL and 50 mg/mL, respectively. They reported that both zeolites inhibited proliferation and reduced cell viability. They also suggested the use of zeolites as an alternative and complementary method in the treatment of cancer.
Chitosan is a molecule used in the fields of biotechnology and pharmaceutics. Coating drug nanoparticles with it improves drug delivery systems (3, 15). A chitosan-coated nanoparticular form increases the specificity and bioavailability of drugs and reduces the toxicity of drugs used in the treatment of cancer (15). This polysaccharide has many important biological properties, including bioactivity, biodegradability, and biocompatibility with reactive chemical groups containing NH2 and OH (3). Previous studies have confirmed that some types of nanoparticles can prevent the proliferation of cancer cells and that apoptosis of cancer cells is associated with reactive oxygen species– mediated (ROS-mediated) pathways (15). In this study, clinoptilolite, which is a natural product itself, was coated with a natural polymer, chitosan, and made to interact with cancer cells. Ali et al.(3) in their study added chitosan-coated methotrexate to human MCF-7 breast cancer cell lines at doses of 10, 20, 30, 40 and 50 μg/mL and the cell viability was measured by MTT analysis after 24, 48, and 72 h. It was shown that chitosan-coated drug nanoparticles statistically significantly decreased cell viability at 24, 48, and 72 h. In addition, the same researchers reported that chitosan-coated methotrexate decreased cell viability much more effectively than pure methotrexate and chitosan applied separately and its anticancer effect was more evident. Cengiz (8) also applied chitosan-loaded nanoparticles to MCF-7 human breast cancer cells and found that chitosan had no toxic effect on them. It was also shown that the necrotic effect was more dominant in the cells exposed to small interfering RNA (siRNA) and the apoptotic effect was more dominant in the siRNA-loaded chitosan nanoparticle–administered cells. Almutairi et al. (4) showed that chitosan-coated raloxifene reduced cell proliferation more effectively than the free drug and induced apoptosis in human lung and liver cancer cells. Saravanabhavan et al. (29) reported that the chitosan carrier system did not cause any toxicity to normal osteoblast cells or degrade their viability. Chitosan nanoparticles were demonstrated to directly cause cell apoptosis by inducing intracellular ROS-mediated mitochondrial damage and ER stress in a study by Jiang et al. (15) evaluating their effects on hepatocellular carcinoma cells based on endoplasmic stress and ROS-mediated mitochondrial damage. Chitosan nanoparticles with a small particle size and positive surface charge were reported to exhibit high anti-tumour activity in hepatocellular carcinoma cells and might be a promising agent for further evaluation in cancer therapy.
Caspases play an important role in the apoptotic process. During apoptosis, caspase activity causes damage in DNA, leading to further destruction of cellular components by changing cell morphology. In this study, it was found that 24 h incubation of a D-17 cell culture in which nanoclinoptilolite was administered at different doses increased the apoptotic cell rate at a dose of 30 μg/mL, while 48 h incubation effectively induced apoptosis at both 20 μg/mL and 30 μg/mL doses. This effect of nanoclinoptilolite on D-17 cells was observed to occur through the caspase-3 and -7 activity. Nanoclinoptilolite demonstrates this effect by interacting with multiple molecular targets, and this occurs by activation of biochemical pathways that induce apoptosis. The BCL-2 family are proteins involved in the regulation of apoptosis and act by suppressing or stimulating cell death (11). BAX protein is a member of the BCL-2 family that induces apoptosis, whereas BCL-2 blocks programmed cell death without affecting cellular proliferation (28). The balance between pro-apoptotic and anti-apoptotic proteins can determine the occurrence of apoptosis. Downregulation of BCL-2 expression may result in an increase in the BAX/BCL-2 ratio and an increase in free BAX. In such case, BAX activates apoptotic cascades by undergoing translocation in the mitochondria (1). In this study, it was found that 24 h incubation of different doses of nanoclinoptilolite did not cause a significant change in the BAX/BCL-2 ratio. However, following 48 h of incubation, it induced apoptosis by causing a 3-, 8.8-, and 14.4-fold increase in the BAX/BCL-2 ratio at doses of 10, 20, and 30 μg/mL, respectively. Consequently, it was determined that nanoclinoptilolite changed the ratio between BAX and BCL-2 in favour of apoptosis after 48 h of incubation. This rate is an important predictor demonstrating the activation of the mitochondrial apoptotic pathway and is used for this purpose in studies (14, 17, 33).
In conclusion, the polymer chitosan as a drug delivery system is currently widely favoured as an effective and useful nanoparticular material. Clinoptilolite, a natural zeolite, was converted into a nanoparticle in this study by encapsulation with chitosan. To determine its effect on cancer cells, it was administered to canine OSA cells at different doses and durations, and its influence on cell proliferation and apoptosis was determined for each dose and duration. It was found that the nanoparticular form of clinoptilolite, which in ordinary particular form was previously demonstrated to have anticancer properties by decreasing cell viability, effectively and rapidly increased apoptotic cell ratio and exhibited this effect by causing an increase in BAX/BCL-2 ratio. In addition, it was concluded that nanoclinoptilolite showed this effect at much lower doses compared to the levels at which it was used in its pure form in the previous studies.
Footnotes
Conflict of Interest
Conflict of Interests Statement: The authors declare that there is no conflict of interests regarding the publication of this article.
Financial Disclosure Statement: This study was supported by the Adnan Menderes University (Project number VTF-17025).
Animal Rights Statement: None required.
References
- 1.Adams J.M., Corry S.. The Bcl-2 protein family: arbiters of cell survival. Science. 1998;281:1322–1326. doi: 10.1126/science.281.5381.1322. [DOI] [PubMed] [Google Scholar]
- 2.Aikoh T., Tomokuni A., Matsukii T., Hydoh F., Ueki H., Otsuki T., Ueki A.. Activation-induced cell death in human peripheral blood lymphocytes after stimulation with silicate in vitro. Int J Oncol. 1998;12:1355–1359. doi: 10.3892/ijo.12.6.1355. [DOI] [PubMed] [Google Scholar]
- 3.Ali E.M.M., Elashkar A.A., El-Kassas H.Y., Salim E.I.. Methotrexate loaded on magnetide iron nanoparticles coated with chitosan: biosynthesis, characterization and impact on human breast cancer MCF-7 cell line. Int J Biol Macromol. 2018;120:1170–1180. doi: 10.1016/j.ijbiomac.2018.08.118. [DOI] [PubMed] [Google Scholar]
- 4.Almutairi F.M., Abd-Raboub A.A., Mervat S., Mohamed M.S.. Raloxifene-encapsulated hyaluronic acid-decorated chitosan nanoparticles selectively induce apoptosis in lung cancer cells. Bioorg Med Chem. 2019;27:1629–1638. doi: 10.1016/j.bmc.2019.03.004. [DOI] [PubMed] [Google Scholar]
- 5.Angstadt A.Y., Motsinger-Reif A., Thomas R., Kisseberth W.C., Couto C.G., Duval D.L., Nielsen D.M., Modiano J.F., Breen M.. Characterization of canine osteosarcoma by array comparative genomic hybridization and RT-qPCR: signatures of genomic ımbalance in canine osteosarcoma parallel the human counterpart. Genes Chromosom Cancer. 2011;50:859–874. doi: 10.1002/gcc.20908. [DOI] [PubMed] [Google Scholar]
- 6.Azhana Ghazi N., Khairina İ.A.H., Nizam N.A., Malek N., Hamdan S.. The effects of zeolite X and Y on cancer cell lines. J Sci Technol. 2012;4:33–40. [Google Scholar]
- 7.Bedioui F.. Zeolite-encapsulated and clay-intercalated metal porphyrin phthalocyanine and Schiff-base complexes as models for biomimetic oxidation catalysts: an overview. Coord Chem Rev. 1995;144:39–68. [Google Scholar]
- 8.Cengiz B.B. Inhibition of ABCE1 and Erf3 proteıns ın breast cancer cell lines with siRNA loaded nanoparticles. 2013. Hacettepe University Department of Nanotechnology and Nanomedicine Master of Science Thesis.
- 9.Chinery R., Brockman J.A., Peeler M.O., Shyr Y., Beauchamp R.D., Coffey R.J.. Antioxidants enhance the cytotoxicity of chemotherapeutic agents in colorectal cancer: a p53-independent induction of p21WAF1/CIP1 via C/EBP. Natl Med. 1997;3:1233–1241. doi: 10.1038/nm1197-1233. [DOI] [PubMed] [Google Scholar]
- 10.Concepcion-Rosabal B., Rodriguez-Fuentes G., Simon-Carballo R.. Development and featuring of the zeolitic active principle FZ: a glucose adsorbent. Zeolites. 1997;19:47–50. [Google Scholar]
- 11.Deveraux Q.L., Reed J.C.. IAP family proteins – suppressors of apoptosis. Genes Dev1999. 13:239–252. doi: 10.1101/gad.13.3.239. [DOI] [PubMed] [Google Scholar]
- 12.Ehrhart N.P., Ryan S.D., Fan T.M. Withrow S.J., Vail D.N., Page R.L. Withrow and MacEwen’s Small Animal Clinical Oncology. Saunders, St Louis, Missouri: 2013. Tumors of the skeletal system; pp. 463–503. edited by. [Google Scholar]
- 13.Fenger J.M., London C.A., Kisseberth W.C.. Canine osteosarcoma: a naturally occurring disease to ınform pediatric oncology. ILAR J. 2014;55:69–85. doi: 10.1093/ilar/ilu009. [DOI] [PubMed] [Google Scholar]
- 14.Huang Z., Chen G., Shi P.. Emodin induced apoptosis in human breast cancer BCap-37 cells throught the mitocondrial signaling pathway. Arch Pharm Res. 2008;31:742–748. doi: 10.1007/s12272-001-1221-6. [DOI] [PubMed] [Google Scholar]
- 15.Jiang Y., Yu X., Su C., Zhao L., Shi Y.. Chitosan nanoparticles induced the antitumor effect in hepatocellular carcinoma cells by regulating ROS-mediated mitochondrial damage and endoplasmic reticulum stress. Artif Cells Nanomed Biotechnol. 2019;47:747–756. doi: 10.1080/21691401.2019.1577876. [DOI] [PubMed] [Google Scholar]
- 16.Katic M., Bosnjak B., Gall-Troselj K., Dikic I., Pavelic K.A.. Clinoptilolite effect on cell media and the consequent effects on tumor cells in vitro. Front Biosci. 2006;1:1722–1732. doi: 10.2741/1918. [DOI] [PubMed] [Google Scholar]
- 17.Kim S.J., Kim H.J., Kim H.R., Lee S.H., Cho S.D., Choi C.S., Nam J.S., Jung J.Y.. Antitumor actions of baicalein and wogonin in HT29 human colorectal cancer cells. Mol Med Rep. 2012;6:1443–1449. doi: 10.3892/mmr.2012.1085. [DOI] [PubMed] [Google Scholar]
- 18.Lim Y., Kim S.H., Kim K.A., Oh M.W., Lee K.H.. Involvement of protein kinase C, phospholipase C, and protein kinase pathways in oxygen radical generation by asbestos-stimulated alveolar macrophages. Environ Health Perspect. 1997;105:1325–1327. doi: 10.1289/ehp.97105s51325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Livak K.J., Schmittgen T.D.. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 20.Mealey K., Bentjen S.A., Gay J.M., Hosick H.L.. Dexamethasone treatment of a canine, but not human, tumour cell line increases chemoresistance independent of P-glycoprotein and multidrug resistance-related protein expression. Vet Comp Oncol. 2003;1:67–75. doi: 10.1046/j.1476-5829.2003.00009.x. [DOI] [PubMed] [Google Scholar]
- 21.Messerschmitt P.J., Garcia R.M., Abul-Karim F.W., Greenfield E.M., Getty P.J.. Osteosarcoma. J Am Acad Orthop Surg. 2009;17:515–527. doi: 10.5435/00124635-200908000-00005. [DOI] [PubMed] [Google Scholar]
- 22.Moore S.M., Rintoul R.C., Walker R., Chilvers E.R., Haslett C., Sethi T.. The presence of a constitutively active phosphoinositide 3-kinase in small cell lung cancer cells mediates anchorage-independent proliferation via a protein kinase B and p70 s6k-dependent pathway. Cancer Res. 1998;58:5239–5247. [PubMed] [Google Scholar]
- 23.Pavelic K., Hadzija M., Bedrica L.J., Pavelic J., Dikic I., Katic M.K., Bosnar M.H., Kapitanovic S., Poljak Blazi M., Krizanac S., Stojkovic R., Jurin M., Subotic B., Colic M.. Natural zeolite clinoptilolite: new adjuvant in anticancer therapy. J Mol Med. 2001;78:708–720. doi: 10.1007/s001090000176. [DOI] [PubMed] [Google Scholar]
- 24.Pavelic K., Katic M., Sverko V., Marotti T., Bosnjak B., Balog T., Stojkovic R., Radacic M., Colic M., Poljak-Blazi M.. Immunostimulatory effect of natural clinoptilolite as a possible mechanism of its antimetastatic activity. J Cancer Res Clin Oncol. 2002;128:37–44. doi: 10.1007/s00432-001-0301-6. [DOI] [PubMed] [Google Scholar]
- 25.Peterson M.W., Kirschbaum J.. Asbestos-induced lung epithelial permeability: potential role of nonoxidant pathways. Am J Physiol. 1998;19:262–268. doi: 10.1152/ajplung.1998.275.2.L262. [DOI] [PubMed] [Google Scholar]
- 26.Poirier V.J., Hershey A.E., Burgess K.E., Phillips B., Turek M.M., Forrest L.J., Beaver L., Vail D.M.. Efficacy and toxicity of paclitaxel (Taxol) for the treatment of canine malignant tumors. J Vet Intern Med. 2004;18:219–222. doi: 10.1892/0891-6640(2004)18<219:eatopt>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 27.Rodriguez-Fuentes G., Barrios M.A., Iraizoz A., Perdomo I., Cedre B.. Entrex-anti-diarrheic drug based on purified natural clinoptilolite. Zeolites. 1997;19:441–448. [Google Scholar]
- 28.Salakou S., Kardamakıs D., Tsamandas A.C., Zolota V., Apostolakis E., Tzelepi V., Papathanasopoulos P., Bonikos D.S., Papapetropoulos T., Petsas T., Dougenis D.. Increased Bax/Bcl-2 ratio up-regulates caspase-3 and ıncreases apoptosis in the thymus of patients with myasthenia gravis. In vivo. 2007;21:123–132. [PubMed] [Google Scholar]
- 29.Saravanabhavana S.S., Rethinasabapathy M., Zsoltb S., Kalambettud A.B., Elumalaie S., Janakiramana M., Huh Y.S., Natesan B.. Graphene oxide functionalized with chitosan based nanoparticles as a carrier of siRNA in regulating Bcl-2 expression on Saos-2 & MG-63 cancer cells and its inflammatory response on bone marrow derived cells from mice. Mater Sci Eng C Mater Biol Appl. 2019;99:1459–1468. doi: 10.1016/j.msec.2019.02.047. [DOI] [PubMed] [Google Scholar]
- 30.Selvarajah G.T., Kirpensteijn J.. Prognostic and predictive biomarkers of canine osteosarcoma. Vet J. 2010;185:28–35. doi: 10.1016/j.tvjl.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 31.Simeonova P., Torium W., Kommineni C., Erkan M., Muson A.E., Rom W.N., Luster M.I.. Molecular regulation of IL-6 activation by asbestos in lung epithelial cells role of reactive oxygen species. J Immunol. 1997;59:3921–3928. [PubMed] [Google Scholar]
- 32.Ueki A., Yamaguchi M., Ueki H.. Polyclonal human T-cell activation by silicate in vitro. Immunology. 1994;82:332–335. [PMC free article] [PubMed] [Google Scholar]
- 33.Yu J.Q., Bao W., Lei J.C.. Emodin regulates apoptotic pathway in human liver cancer cells. Phytother Res. 2013;27:251–257. doi: 10.1002/ptr.4703. [DOI] [PubMed] [Google Scholar]


