Abstract
Objective
To determine the leading causes of death in intracerebral hemorrhage (ICH) survivors, we used administrative data from 3 large US states to identify adult survivors of a first-time spontaneous ICH and track all hospital readmissions resulting in death.
Methods
We performed a longitudinal analysis of prospectively collected claims data from hospitalizations in California (2005–2011), New York (2005–2014), and Florida (2005–2014). Adult residents admitted with a nontraumatic ICH who survived to discharge were included. Patients were followed for a primary outcome of any readmission resulting in death. The cause of death was defined as the primary diagnosis assigned at discharge. Multivariable Cox proportional hazards and multinomial logistic regression were used to determine factors associated with the risk for and cause of death.
Results
Of 72,432 ICH survivors (mean age 68 years [SD 16], 48% female), 12,753 (18%) died during a median follow-up period of 4.0 years (interquartile range 2.3–6.3). The leading causes of death were infection (34%), recurrent intracranial hemorrhage (14%), cardiac disease (8%), respiratory failure (8%), and ischemic stroke (5%). Death in patients with atrial fibrillation (AF) was more likely to be caused by ischemic stroke (odds ratio [OR] 2.4, 95% confidence interval [CI] 1.9–2.9, p < 0.001) and less likely to be caused by recurrent intracranial hemorrhage (OR 0.7, 95% CI 0.6–0.8, p < 0.001) compared to patients without AF.
Conclusions
Infection is the leading cause of death in all ICH survivors. Survivors with AF were at increased risk for death from ischemic stroke. These findings will help prioritize interventions aimed to improve long-term survival and recovery in ICH survivors.
Spontaneous, nontraumatic intracerebral hemorrhage (ICH) remains the most devastating form of stroke. Within 1 month of injury, 40% of patients with ICH die, and by 1 year, this number increases to 54%.1 However, stroke case fatality, particularly in patients aged 75 and younger, has significantly decreased during the past 2 decades.2–4 Use of anticoagulation reversal agents, early access to critical care, and development of dedicated inpatient stroke units have all contributed to lower rates of in-hospital mortality following spontaneous ICH.5–9 With increased survival rates achieved by some hospitals, more patients are surviving a first-time ICH, leading to a growing population of ICH survivors.
Although short-term fatality after ICH has decreased due to improved care, long-term mortality remains high. ICH survivors experience an elevated risk for death long after survival of the first-time ICH.10,11
The long-term causes of death in the ICH survivor population are unclear. Survivors of ICH are at risk for subsequent ischemic stroke, as those with atrial fibrillation (AF) are often not restarted on anticoagulation therapy.12 Survivors also remain at risk for recurrent hemorrhage, a complication that is estimated to occur in about 2%–7% of patients.13–15 But it is not yet clear whether increased mortality in ICH survivors is driven by recurrent stroke or by other causes of illness and death. It is also unknown whether the leading causes of death differ in ICH survivors with AF.
To date, no comprehensive analysis of causes of death in ICH survivors has been performed. We therefore aimed to characterize long-term mortality in survivors of first-time ICH in a diverse, US-based population. We used administrative claims data from the Healthcare Cost and Utilization Project State Inpatient Databases to assess the risk of mortality and leading causes of death in ICH survivors to inform interventions to improve survival in this population.
Methods
Study design
We performed a retrospective, longitudinal analysis of prospectively collected administrative claims data from the State Inpatient Databases and State Emergency Department Databases as part of the Healthcare Cost and Utilization Project. All data were de-identified, standardized, and checked for quality. Data from the State Inpatient Databases were available from California between 2005 and 2011, New York between 2005 and 2014, and Florida between 2005 and 2014 and the State Emergency Department Database was available from California for 2005–2011. These states were chosen because of their large, diverse populations and availability of desired data elements. Given that de-identified participant data from publicly available databases were used, this study was exempt from review from our institutional review board.
Subjects
Hospitalizations with a primary diagnosis of spontaneous ICH were identified using a previously validated ICD-9-CM code (431) in the DX1 position.16 Adult patients (age ≥18) who were admitted from 2005 to 2010 in California and 2005 to 2013 in New York and Florida were included in the analysis to allow for at least 1 year of potential follow-up in 2011 and 2014, respectively. Patients who were admitted with concurrent trauma were excluded. Patients who died prior to discharge or were discharged to hospice were considered nonsurvivors and were excluded from the longitudinal analysis. Patients discharged to hospice were considered nonsurvivors because up to 93% of patients with stroke die within 6 months after admission to hospice.17 It is also unlikely that deaths occurring after immediate discharge to hospice following the index hemorrhage were caused by a disease other than the index hemorrhage itself. Each patient in the State Inpatient Databases is assigned a unique identifier, which allows subsequent readmissions to be linked to the index event. Patients who lacked this longitudinal identifier and patients who were not California, New York, or Florida residents were excluded.
Outcome
Patients were followed from the time of their index ICH event to their date of death or last available follow-up point. The primary outcome was all-cause mortality after hospital discharge for index ICH. We tracked all subsequent inpatient hospital admissions in the 3 states and emergency department admissions (available in California only) in included participants for admissions that resulted in death. Death was identified by a discharge disposition of dead or discharge to hospice. In the infrequent case that a patient was discharged to hospice and then readmitted, the first admission that resulted in a discharge to hospice was considered the event resulting in death. Cause of death was determined using the ICD-9-CM code assigned as the primary diagnosis at discharge. Any hospital readmission that resulted in death within 30 days of the index admission that had a primary diagnosis of ICH (ICD-9-CM code 431) was considered death caused by late effects of the index ICH.
Covariates
Demographic characteristics (age, sex, race/ethnicity) and baseline comorbidities (hypertension, hyperlipidemia, smoking, congestive heart failure [CHF], myocardial infarction [MI], chronic kidney disease, prior malignancy, diabetes, and coagulopathy) were identified using validated ICD-9-CM codes and used as covariates in multivariable models. In the administrative data used here, race and ethnicity are included in one data element. Race is categorized into White, Black, Hispanic, Asian or Pacific Islander, Native American, and Other, and ethnicity takes precedence over race. Our study identified very few Native American patients, and Native Americans were therefore grouped into the “Other” category. The Elixhauser comorbidity index, which is intended to predict risk for in-hospital mortality based on predefined comorbidities, was also extracted for each patient using ICD-9-CM codes and was included as a covariate.18 The Agency for Healthcare Research and Quality weighting system for calculating the Elixhauser comorbidity index was used.18 Length of index hospital stay, prior do not resuscitate order, and insurance payer were also included. Insurance payer lists the expected primary payer for the hospital admission and includes Medicare, Medicaid, private, self-pay, or other. Dual eligible Medicare/Medicaid patients are included in the “Medicare” category. Our analysis was restricted to Medicare, Medicaid, and private insurance patients.
Statistical analysis
Descriptive statistics are reported using means (SD) and medians (interquartile range [IQR]) for normally and non-normally distributed continuous variables, respectively. Discrete variables are reported using counts (%). Unadjusted comparisons involving categorical and continuous variables were performed using χ2 and unpaired t tests, respectively. Kaplan-Meier survival statistics were used to assess cumulative mortality risk during each year after index ICH. Time in years from index ICH was used as the time index. We used the log-rank test and Cox proportional hazards regression to evaluate the association between covariates and risk of death in unadjusted and adjusted analyses, respectively. Visual inspection confirmed the proportional hazard assumption. Multinomial logistic regression was used to identify factors associated with causes of mortality. The category “other” was used as the reference level cause of death in the multinomial model. Multivariable models were built by selecting covariates with p < 0.1 in univariable analyses, forcing universal confounders age and sex into the model, backwards eliminating covariates with p > 0.1, and removing collinear variables identified by a variance inflation factor >5. R version 3.4.1 was used for all analyses.
Standard protocol approvals, registrations, and patient consents
Given that only publicly available, deidentified, administrative claims data were used, this study was exempt from review by the institutional review board and individual patient consent.
Data availability
The study was completed using publicly available data provided by the Healthcare Cost and Utilization Project, as described above. The data can be found at hcup-us.ahrq.gov/.
Results
Study population
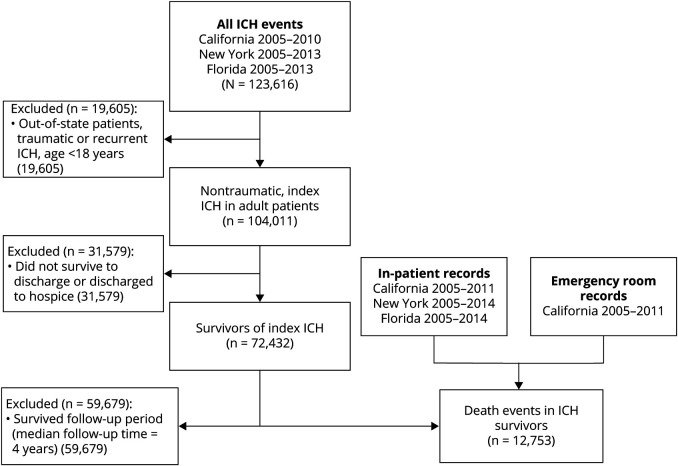
We identified 104,011 patients with a first-ever spontaneous ICH. Of these patients, 72,432 (70%) survived to discharge and were included in our analysis (figure 1). The mean age of included patients was 68 years (SD 15) and 48% were female (table 1). The median follow-up time was 4.0 years (IQR 2.3–6.3).
Figure 1. Study inclusion criteria.
Flowchart summarizing the number of patients meeting inclusion criteria for the study. ICH = intracerebral hemorrhage.
Table 1.
Baseline characteristics
Mortality
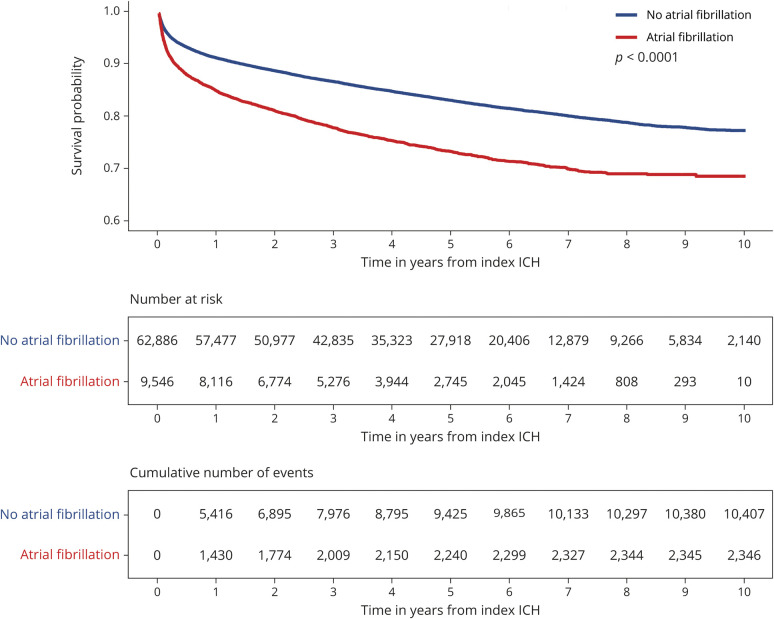
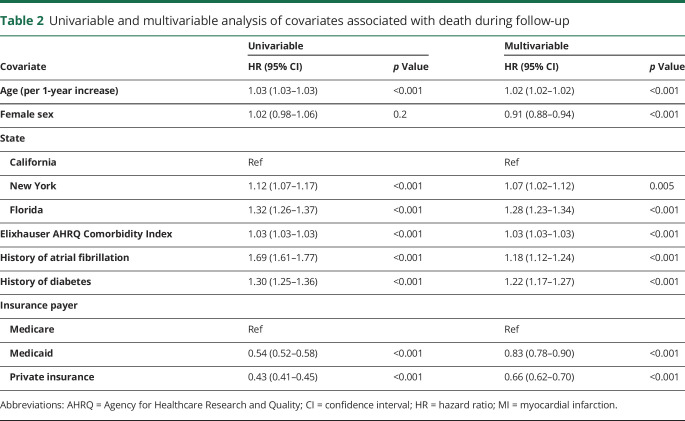
During the follow-up period, 12,753 (18%) ICH survivors were readmitted for events that resulted in death. At 1 year, the cumulative risk for death was 9.5% (95% confidence interval [CI] 9.2–9.7) and the median time to death was 10 months (IQR 2–31) for all patients and 7 months (IQR 1–24) in patients with AF (figure 2). We identified both statistically and clinically significant differences between patients who survived vs died during follow-up. Compared to patients who survived, patients who died were older (73 vs 67 years, p < 0.001) and more likely to have preexisting comorbidities, including history of AF (18% vs 12%, p < 0.001), chronic kidney disease (13% vs 8%, p < 0.001), congestive heart failure (11% vs 6%, p < 0.001), and diabetes (25% vs 21%, p < 0.001) (table 1). In adjusted analysis, history of AF (hazard ratio [HR] 1.18 [95% CI 1.12–1.24]) and diabetes (HR 1.22 [95% CI 1.17–1.27) were associated with an increased risk of death (table 2). Patients with higher Elixhauser comorbidity indices were also at increased risk for death (HR 1.03 [95% CI 1.03–1.03]). Patients with private insurance (HR 0.66 [95% CI 0.62–0.70]) or Medicaid (HR 0.83 [95% CI 0.78–0.90]) were significantly less likely to die during follow-up compared to patients with Medicare.
Figure 2. Cumulative risk for death in patients with and without atrial fibrillation.
Kaplan-Meier survival curve summarizing the risk of death during follow-up separated by history of atrial fibrillation. ICH = intracerebral hemorrhage.
Table 2.
Univariable and multivariable analysis of covariates associated with death during follow-up
Causes of death
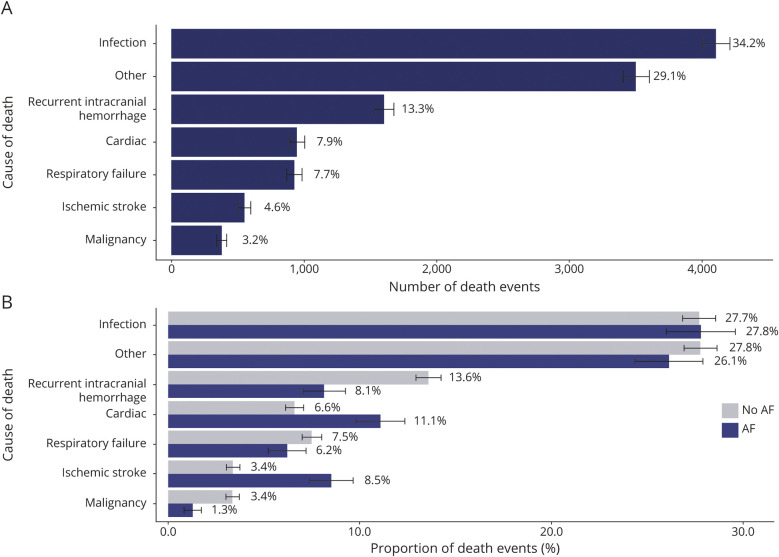
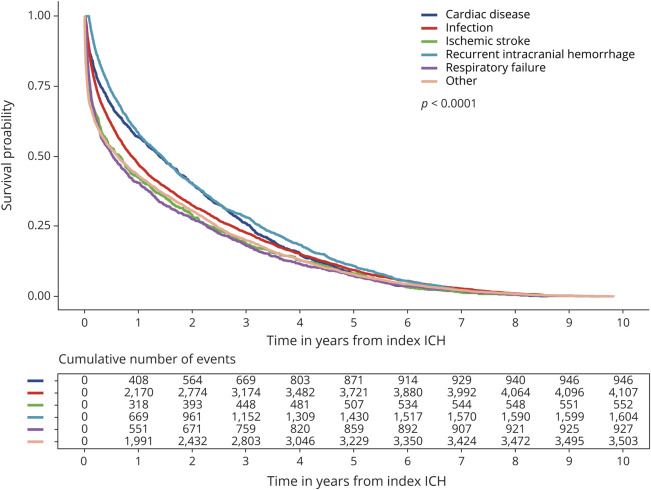
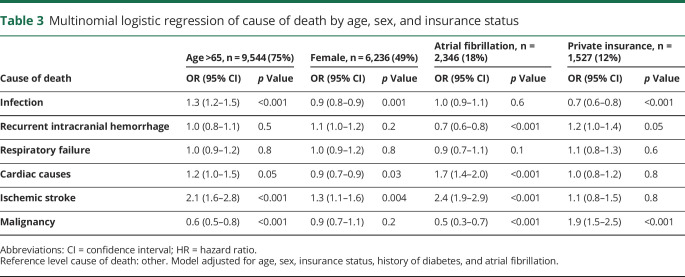
The leading causes of death in all patients were infection (34%), recurrent intracranial hemorrhage (including ICH, subarachnoid hemorrhage, and subdural hemorrhage) (13%), cardiac disease (including MI, cardiac arrhythmias, cardiac arrest, and heart failure) (8%), respiratory failure (8%), ischemic stroke (5%), and malignancy (3%) (figure 3). Of 4,107 infection-related deaths, 66% were caused by sepsis, 14% by aspiration pneumonia, 11% by viral and bacterial pneumonia, 6% by other types of infection, including those caused by implants or grafts (ICD-9-CM code 996.6X), and 3% by urinary tract infections. The median time to death was 10 months when caused by infection, 17 months when caused by recurrent intracranial hemorrhage or cardiac disease, 6 months when caused by respiratory failure or malignancy, and 8 months when caused by ischemic stroke (figure 4). In patients with AF, death was more likely to be caused by ischemic stroke (9% vs 3%, p < 0.001) or cardiac disease (11% vs 7%, p < 0.001), and less likely to be caused by recurrent intracranial hemorrhage (8% vs 14%, p < 0.001), than in patients without AF (figure 3). Patients who were older than 65 years were more likely to die from infection (OR 1.3, 95% CI 1.2–1.5, p < 0.001) or ischemic stroke (OR 2.1, 95% CI 1.6–2.8, p < 0.001) than younger patients (table 3). Female survivors were slightly less likely to die from infection (OR 0.9, 95% CI 0.8–0.9, p = 0.001) but were more likely to experience mortality caused by ischemic stroke (OR 1.3, 95% CI 1.1–1.6, p = 0.004) than male patients. Those with private insurance, compared to Medicare/Medicaid or no insurance, were at decreased risk for death caused by infection (OR 0.7, 95% CI 0.6–0.8, p < 0.001). Other causes of death accounted for 30% of deaths in all patients (figure 3). Each individual diagnosis within the “other” group accounted for <2% of deaths independently.
Figure 3. Leading causes of death in intracerebral hemorrhage survivors.
Bar graph summarizing the leading causes of death (A) in all patients and (B) separated by history of atrial fibrillation (AF).
Figure 4. Kaplan-Meier survival curves by cause of death.
Kaplan-Meier survival curves separated by the leading causes of death in intracerebral hemorrhage (ICH) survivors.
Table 3.
Multinomial logistic regression of cause of death by age, sex, and insurance status
Discussion
Using administrative claims data from 3 large and diverse US states, we identified a 1-year cumulative risk of in-hospital death of 9.5% in ICH survivors. Older patients with more comorbidities, including AF, CHF, and prior MI, were more likely to die during follow-up. The leading causes of death in ICH survivors were infection, recurrent intracranial hemorrhage, respiratory failure, cardiac disease, and ischemic stroke. Death in patients with AF was significantly more likely to be due to ischemic stroke or cardiac disease, and less likely to be caused by recurrent intracranial hemorrhage.
Across all patients, the leading cause of death following survival of a spontaneous ICH was infection, with 34% of all deaths that occurred at hospital readmissions due to infectious causes. Our findings are consistent with previous studies that found that infection accounts for the majority of readmissions occurring within 30 days of the incident bleed, and that readmissions due to infection are associated with high rates of mortality.19 Poststroke infection is a well-known risk. Our study adds evidence that the risk for infection may not be isolated to the in-hospital stay, and that infection confers a significant risk for death during long-term follow-up, particularly in patients older than 65 years. This aligns closely with what is observed in clinical practice as patients frequently return to the hospital with infections after acute brain injuries. Development of infection during follow-up may be driven by prolonged use of antibiotics leading to infections like Clostridium difficile, pressure ulcers in disabled and immobile patients, and urinary tract infections caused by repeated catheterization. Further, infection-related deaths in ICH survivors with neurologic deficits resulting in dysfunctional swallowing may be likely to be due to aspiration pneumonia.20,21 Additional research is needed to determine the underlying cause of high numbers of death due to infection, but special attention should be paid to prevention, early detection, and treatment of infection not only during the acute phase, but also during long-term follow-up to promote survival after ICH.
Second to infection, recurrent intracranial bleeding was the next most common cause for mortality. The number of deaths due to recurrent intracranial hemorrhage found here is in line with prior studies of ICH recurrence that have reported recurrence rates between 2% and 7%.13–15 However, very few studies have examined mortality due to recurrent stroke, and here we provide evidence that recurrent hemorrhage is a significant source for mortality in ICH survivors throughout the long-term follow-up period. Previous research has highlighted clinical and demographic factors associated with the risk for ICH recurrence. First, lobar location of the incident bleed may confer an elevated risk for rebleeding.13,15,22,23 This increase in risk is likely driven by incident hemorrhages caused by underlying cerebral amyloid angiopathy, a known predictor of lobar hemorrhage that also provokes a 7-fold increase in the risk for recurrent ICH.24,25 Further, inadequate blood pressure control and severity of hypertension during follow-up has been associated with ICH recurrence in both lobar and nonlobar hemorrhages.26 Blood pressure management is likely a crucial component of secondary stroke prevention, not only for patients with deep, hypertension-mediated hemorrhages, but in those with lobar hemorrhages as well. Overall, minimizing recurrent bleeding through effective secondary prevention strategies will extend the gains we have made thus far for patients with spontaneous ICH.
We report that patients with AF were significantly more likely to die during the follow-up period, and death in patients with AF was disproportionately due to ischemic stroke or cardiac causes and less likely to be caused by recurrent intracranial hemorrhage. These findings align with a host of previous studies demonstrating that history of AF significantly increases one's risk for death due to ischemic stroke and cardiac illness.27–30 ICH survivors with AF may not be restarted on their previous anticoagulation therapy, which may or may not decrease their risk for a recurrent hemorrhage while placing them at elevated risk for subsequent ischemic stroke.31 Optimal secondary prevention strategies in ICH survivors with AF are critical for the long-term survival of these patients and clinical trials are underway to address this question.
In addition to differences in risk for in-hospital death based on presence of preexisting comorbidities, we also found that patients with private insurance or Medicare, compared to Medicaid, were at significantly lower risk for death during follow-up. Prior studies have reported disparities in stroke-related health outcomes based on insurance payer that support this result, but it is not currently clear what drives these differences.32,33 It is possible that patients with Medicaid experience inadequate control of preexisting comorbidities, such as hypertension, which may contribute to increased risk for significant health events, such as recurrent intracranial hemorrhage, leading to death.26,34 Further research is needed to determine the underlying causes of differences in outcome based on insurance status after stroke.
Our study has several important limitations to consider. First, administrative claims data only include information on hospitalizations. We were therefore unable to fully assess the number of deaths and causes for death in patients who died at home or outside of the hospital. The frequency of in-hospital deaths following stroke is decreasing, while at-home deaths and death occurring at hospice facilities are becoming more common.35 Consequently, our study underestimates the number of deaths that occurred during follow-up and misrepresents the prevalence of causes of death more likely to occur outside of the hospital. Still, as of 2017, the plurality of poststroke deaths occurred in a hospital.35 Also, a small portion of patients was likely readmitted in other states and was missed by our analysis. We took several steps to include as many patients and death events in our analysis as possible. Readmissions that resulted in discharge to hospice, either an inpatient facility or home with hospice services, were included in the analysis and considered equivalent to readmissions resulting in death. Further, we used emergency department data, available in California, to identify deaths that occurred prior to an inpatient admission. Therefore, some deaths occurring at hospice facilities or at home or in an emergency department setting could be studied.
A second limitation is the use of ICD-9-CM codes to determine the cause of death. It is possible that the primary diagnosis was miscoded, or that the primary diagnosis does not accurately reflect the true cause of death. Despite this limitation, it is likely that ICD-9-CM codes are more reliable than other sources of diagnosis data, such as self-report data provided by families, for determining cause of death. Also, it is possible that readmissions with a primary diagnosis of ICH did not represent a true ICH recurrence, and instead represented readmissions for sequelae of the index hemorrhage where the ICD-9-CM code for ICH was carried forward. To mitigate this source of error, readmissions within 30 days of the index ICH that carried a primary diagnosis of ICH were not considered a recurrent hemorrhage, but rather death due to late effects of the index ICH. In addition, the administrative data used here lack disease-specific details and neither the severity nor anatomical location of the index hemorrhage could be studied. Finally, the data lack information on prescription medications, rendering us unable to assess the impact of antithrombotic therapy or other medication therapy on long-term risk of death in the ICH survivor population. Future studies using well-reported prescription data should evaluate the influence of medication use on long-term survival following ICH.
We found that the leading cause of long-term death after ICH is infection, followed by recurrent intracranial hemorrhage and respiratory failure. Our findings highlight the opportunity to extend improved outcomes in ICH survivors through the prevention of long-term causes of mortality.
Glossary
- AF
atrial fibrillation
- CHF
congestive heart failure
- CI
confidence interval
- HR
hazard ratio
- ICD-9-CM
International Classification of Diseases, 9th revision, Clinical Modification
- ICH
intracerebral hemorrhage
- IQR
interquartile range
- MI
myocardial infarction
Appendix. Authors
Footnotes
CME Course: NPub.org/cmelist
Study funding
A.C.L. is supported by the NIH (R03NS112859). S.B.M. is supported by the NIH (K23NS105948) and the Leon Levy Foundation. H.K. is supported by the NIH (R01NS097443, U01NS095869, R01HL144541, U01NS106513). L.H.S. is supported by the NIH (R01NS095993, R01NS097728, U01NS113445). G.J.F. is supported by the NIH (K76AG059992, R03NS112859), the American Heart Association (18IDDG34280056), the Yale Pepper Scholar Award (P30AG021342), and the Neurocritical Care Society Research Fellowship. K.N.S. is supported by the NIH (U24NS107136, U24NS107215, R01NR018335, R01NS107215, U01NS106513, R03NS112859) and the American Heart Association (18TPA34170180, 17CSA33550004). The funding entities had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Disclosure
L. Kuohn, A. Leasure, Dr. Acosta, K. Vanent, and Dr. Murthy report no disclosures. Dr. Kamel serves as co-PI for the NIH-funded ARCADIA trial, which receives in-kind study drug from the BMS-Pfizer Alliance and in-kind study assays from Roche Diagnostics, serves as Deputy Editor for JAMA Neurology, serves as a steering committee member of Medtronic's Stroke AF trial (uncompensated), serves on an endpoint adjudication committee for a trial of empagliflozin for Boehringer-Ingelheim, and has served on an advisory board for Roivant Sciences related to Factor XI inhibition. Dr. Matouk, Dr. Sansing, Dr. Falcone, and Dr. Sheth report no disclosures. Go to Neurology.org/N for full disclosures.
References
- 1.van Asch CJ, Luitse MJ, Rinkel GJ, van der Tweel I, Algra A, Klijn CJ. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. Lancet Neurol 2010;9:167–176. [DOI] [PubMed] [Google Scholar]
- 2.Jolink WM, Klijn CJ, Brouwers PJ, Kappelle LJ, Vaartjes I. Time trends in incidence, case fatality, and mortality of intracerebral hemorrhage. Neurology 2015;85:1318. [DOI] [PubMed] [Google Scholar]
- 3.Koton S, Schneider AL, Rosamond WD, et al. Stroke incidence and mortality trends in US communities, 1987 to 2011. JAMA 2014;312:259–268. [DOI] [PubMed] [Google Scholar]
- 4.Schmidt M, Jacobsen JB, Johnsen SP, Bøtker HE, Sørensen HT. Eighteen-year trends in stroke mortality and the prognostic influence of comorbidity. Neurology 2014;82:340. [DOI] [PubMed] [Google Scholar]
- 5.Parry‐Jones AR, Sammut‐Powell C, Paroutoglou K, et al. An intracerebral hemorrhage care bundle is associated with lower case fatality. Ann Neurol 2019;86:495–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuramatsu JB, Gerner ST, Schellinger PD, et al. Anticoagulant reversal, blood pressure levels, and anticoagulant resumption in patients with anticoagulation-related intracerebral hemorrhage. JAMA 2015;313:824–836. [DOI] [PubMed] [Google Scholar]
- 7.Peter L, Patricia F, Ronning Ole M, et al. Stroke unit care benefits patients with intracerebral hemorrhage. Stroke 2013;44:3044–3049. [DOI] [PubMed] [Google Scholar]
- 8.Diringer MNM, Edwards DF. Admission to a neurologic/neurosurgical intensive care unit is associated with reduced mortality rate after intracerebral hemorrhage. Crit Care Med 2001;29:635–640. [DOI] [PubMed] [Google Scholar]
- 9.McKinney JS, Cheng JQ, Rybinnik I, Kostis JB; the Myocardial Infarction Data Acquisition System (MIDAS 22) Study Group. Comprehensive stroke centers may be associated with improved survival in hemorrhagic stroke. J Am Heart Assoc 2015;4:e001448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ekker MS, Verhoeven JI, Vaartjes I, Jolink WMT, Klijn CJM, de Leeuw FE. Association of stroke among adults aged 18 to 49 Years with long-term mortality. JAMA 2019;321:2113–2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rutten-Jacobs LCA, Arntz RM, Maaijwee NAM, et al. Long-term mortality after stroke among adults aged 18 to 50 years. JAMA 2013;309:1136–1144. [DOI] [PubMed] [Google Scholar]
- 12.Claude HJ, Greenberg Steven M, Anderson Craig S, et al. Guidelines for the management of spontaneous intracerebral hemorrhage. Stroke 2015;46:2032–2060. [DOI] [PubMed] [Google Scholar]
- 13.Bailey RD, Hart RG, Benavente O, Pearce LA. Recurrent brain hemorrhage is more frequent than ischemic stroke after intracranial hemorrhage. Neurology 2001;56:773–777. [DOI] [PubMed] [Google Scholar]
- 14.Hanger HC, Wilkinson TJ, Fayez-Iskander N, Sainsbury R. The risk of recurrent stroke after intracerebral haemorrhage. J Neurol Neurosurg Psychiatry 2007;78:836–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Poon MTC, Fonville AF, Al-Shahi Salman R. Long-term prognosis after intracerebral haemorrhage: systematic review and meta-analysis. J Neurol Neurosurg Psychiatry 2014;85:660. [DOI] [PubMed] [Google Scholar]
- 16.McCormick N, Bhole V, Lacaille D, Avina-Zubieta JA. Validity of diagnostic codes for acute stroke in administrative databases: a systematic review. PLoS One 2015;10:e0135834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harris PS, Stalam T, Ache KA, et al. Can hospices predict which patients will die within six months? J Palliat Med 2014;17:894–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moore BJ, White S, Washington R, Coenen N, Elixhauser A. Identifying increased risk of readmission and in-hospital mortality using hospital administrative data: the AHRQ elixhauser comorbidity index. Med Care 2017;55:698. [DOI] [PubMed] [Google Scholar]
- 19.Lord AS, Lewis A, Czeisler B, et al. Majority of 30-day readmissions after intracerebral hemorrhage are related to infections. Stroke 2016;47:1768–1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Armstrong JR, Mosher BD. Aspiration pneumonia after stroke. Neurohospitalist 2011;1:85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martino R, Foley N, Bhogal S, Diamant N, Speechley M, Teasell R. Dysphagia after stroke: incidence, diagnosis, and pulmonary complications. Stroke 2005;36:2756–2763. [DOI] [PubMed] [Google Scholar]
- 22.Passero S, Burgalassi L, D'Andrea P, Battistini N. Recurrence of bleeding in patients with primary intracerebral hemorrhage. Stroke 1995;26:1189–1192. [DOI] [PubMed] [Google Scholar]
- 23.Hill MD, Silver FL, Austin PC, Tu JV. Rate of stroke recurrence in patients with primary intracerebral hemorrhage. Stroke 1970;2000;31. [DOI] [PubMed] [Google Scholar]
- 24.Charidimou A, Imaizumi T, Moulin S, et al. Brain hemorrhage recurrence, small vessel disease type, and cerebral microbleeds. Neurology 2017;89:820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Biffi A, Halpin A, Towfighi A, et al. Aspirin and recurrent intracerebral hemorrhage in cerebral amyloid angiopathy. Neurology 2010;75:693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Biffi A, Anderson CD, Battey TW, et al. Association between blood pressure control and risk of recurrent intracerebral hemorrhage. JAMA 2015;314:904–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wolf PA, Dawber TR, Thomas HE, Kannel WB. Epidemiologic assessment of chronic atrial fibrillation and risk of stroke: the Framingham Study. Neurology 1978;28:973. [DOI] [PubMed] [Google Scholar]
- 28.Marini C, De Santis F, Sacco S, et al. Contribution of atrial fibrillation to incidence and outcome of ischemic stroke: results from a population-based study. Stroke 2005;36:1115–1119. [DOI] [PubMed] [Google Scholar]
- 29.Lee E, Choi EK, Han KD, et al. Mortality and causes of death in patients with atrial fibrillation: a nationwide population-based study. PLos One 2018;13:e0209687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stewart S, Hart CL, Hole DJ, McMurray JJ. A population-based study of the long-term risks associated with atrial fibrillation: 20-year follow-up of the Renfrew/Paisley study. Am J Med 2002;113:359–364. [DOI] [PubMed] [Google Scholar]
- 31.Murthy SB, Gupta A, Merkler AE, et al. Restarting anticoagulant therapy after intracranial hemorrhage: a systematic review and meta-analysis. Stroke 2017;48:1594–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.James ML, Grau-Sepulveda MV, Olson DM, et al. Insurance status and outcome after intracerebral hemorrhage: findings from get with the guidelines-stroke. J Stroke Cerebrovasc Dis 2014;23:283–292. [DOI] [PubMed] [Google Scholar]
- 33.Medford‐Davis LN, Fonarow GC, Bhatt DL, et al. Impact of insurance status on outcomes and use of rehabilitation services in acute ischemic stroke: findings from get with the guidelines‐stroke. J Am Heart Assoc 2016;5:e004282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Walsh KB, Woo D, Sekar P, et al. Untreated hypertension: a powerful risk factor for lobar and nonlobar intracerebral hemorrhage in whites, blacks, and Hispanics. Circulation 2016;134:1444–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cross SH, Kaufman BG, Warraich HJ. Trends in location of death for individuals with cerebrovascular disease in the United States. JAMA Neurol 2019;76:1399–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The study was completed using publicly available data provided by the Healthcare Cost and Utilization Project, as described above. The data can be found at hcup-us.ahrq.gov/.