Abstract
Objective
To find determinants of the occurrence of repetitive compound muscle action potential (R-CMAP) and to assess the efficacy of channel blocker therapy in slow-channel congenital myasthenic syndrome (SCCMS).
Methods
Neurologic examination, EMG study, laboratory test, muscle biopsy, and next-generation and Sanger sequencing; literature review of reported patients with SCCMS, including EMG, kinetics of mutant acetylcholine receptors (AChRs), and response to therapy; and simulation of the decay phase of endplate potential (EPP) were performed.
Results
Three newly characterized and 57 reported patients with SCCMS with mutations of AChR subunits were included. In patients with R-CMAP, the length of channel opening bursts of mutant AChR was increased 8.68 ± 2.82 (mean ± SD)-fold compared to wild-type; in patients without R-CMAP, the length was increased 3.84 ± 0.65-fold (95% confidence interval 3.18–6.50, p = 0.000014). The EPP amplitude after refractory period of action potential in muscle fiber is above the threshold in patients with R-CMAP but below the threshold in patients without R-CMAP. In patients with good results from channel blocker therapy, treatment was initiated 11.60 ± 5.17 years after onset of symptoms; in patients with no to moderate benefit from channel blocker therapy, treatment was initiated 30.70 ± 12.72 years after onset (95% confidence interval −28.57 to −9.63, p = 0.00089).
Conclusions
In SCCMS, the R-CMAP occurrence is related to the extent of prolongation of the opening episodes of mutant AChR channel. Channel blocker treatment is more effective the sooner it is started after the onset of symptoms.
Classification of evidence
This study provides Class IV evidence that channel blocker therapy in patients with SCCMS improves symptoms.
The congenital myasthenic syndromes (CMS) are diverse disorders characterized by fatigable muscle weakness due to abnormal neuromuscular transmission caused by mutations in no fewer than 33 genes.1–3 To date, about a half of CMS stem from mutations in genes encoding subunits of the muscle nicotinic acetylcholine receptor (AChR), a pentameric ligand-gated cationic ion channel composed of homologous subunits with stoichiometry (α1)2β1δε. Recessive mutations causing loss of function and dominant mutations causing gain of function in AChR subunits result in fast-channel and slow-channel CMS (SCCMS), respectively. The SCCMS is characterized by slow decay of miniature endplate potential (EPP) caused by prolonged opening episodes of AChR ion channel4 due to increased affinity of AChR for acetylcholine, increased gating efficiency of the channel, or both.1,4–6 Slow-channel mutations of AChR can also result in spontaneous channel openings of the unliganded receptor and desensitize the receptor at physiologic rates of stimulation.1 The progressive depolarization can result in a postsynaptic depolarization block, and the prolonged and spontaneous channel openings produce calcium overloading of the postsynaptic region. Calcium overloading of the postsynaptic region produces an endplate myopathy manifested by loss of AChR from degenerating junctional folds that promotes diffusion of acetylcholine molecules from the widened synaptic space and compromised safety margin of neuromuscular transmission.7
EMG study is crucial for the diagnosis of an SCCMS. The prolonged opening events of the AChR channel exceed the refractory period of the sodium channel in muscle so that a single nerve stimulus can result in a repetitive (R-) compound muscle action potential (CMAP).8 Patients with SCCMS usually respond well to long-lived channel blockers of the AChR channel9–12 but do not respond to or are even worsened by acetylcholinesterase (AChE) inhibitor.1 Thus, R-CMAPs and negative response to AChE inhibitor are important criteria for the diagnosis of the SCCMS. However, absent R-CMAP6,13,14 and initial positive response to an AChE inhibitor were also reported in SCCMS.5,6,15–18 The factors causing these atypical electrophysiologic and pharmacologic features and the importance of understanding the mechanisms underlying these phenomena for better diagnosis and treatment of the SCCMS have not been addressed.
Here, we describe the clinical, genetic, and electrophysiologic findings and the response to the channel blocker in 3 patients with SCCMS. Furthermore, by analyzing the EMG findings, channel kinetics of mutant AChRs, and treatment data in patients with SCCMS reported in the literature, we explore the determinants of the occurrence of R-CMAP and of therapeutic efficacy of channel blockers.
Methods
Standard protocol approvals, registrations, and patient consents
The index patient had generalized muscle weakness for 40 years. Her 2 daughters had similar symptoms. They were referred to the Department of Neurology, Xuanwu Hospital, Capital Medical University in May 2017 for further investigation of their myasthenia. The study was approved by Medical Ethics Committee of Xuanwu Hospital (No. 2017/084). Each patient gave informed consent to allow us to perform genetic tests, to include them in this study, and to present any findings from their participation.
Laboratory tests and EMG studies of new patients
Laboratory tests were performed on the index patient and her 2 daughters, including a complete blood count with differential, liver function tests, fasting glucose, blood urea, nitrogen, creatinine, electrolytes, creatine kinase, lactate, thyroxine, thyroid-stimulating hormone, and anti-AChR and anti-muscle‐specific kinase (MuSK) antibodies. Needle EMG was performed in the index patient. Repetitive nerve stimulation and nerve conduction studies were performed in all 3 patients.
Muscle biopsy of a new patient
The left biceps in the proband's second daughter was biopsied, and frozen sections stained with hematoxylin & eosin, trichrome, NADH dehydrogenase, succinate dehydrogenase, pH 4.3, 4.6, and 9.4, preincubated ATPase, periodic acid–Schiff, Oil-Red O, and nonspecific esterase were examined.
Mutation analysis of new patients
Genomic DNAs were extracted from peripheral blood by standard methods. Genetic analysis was performed in the proband with next-generation sequencing of 434 genes associated with neuromuscular diseases. We focused on the genes that have been reported in association with CMS. Sanger sequencing was performed to confirm variants of clinical interest in the proband and to identify mutation in the proband's 2 daughters.
Primary research questions and classification of level of evidence
We aim to answer 2 questions. (1) Is the occurrence of R-CMAP associated with the kinetic defect of a corresponding mutation harbored in patients with SCCMS? (2) Is the efficiency of therapy related to the age at onset, the age at channel blocker treatment initiation, or the delay of treatment after the onset of disease? This study provides Class IV evidence that for people with SCCMS channel blocker therapy improves symptoms because of the absence of a non–channel blocker–treated control group and the open-label design.
Literature search to identify reported patients with SCCMS
We searched SCCMS articles published in English from January 1, 1977, to November 1, 2019, in PubMed. The key words included slow channel, slow-channel, slow channel myasthenia, slow-channel myasthenia, slow channel congenital myasthenic syndrome, slow-channel CMS, SCCMS, CMS1A, CMS1B, CMS1C, CMS1D, slow decay of EPP, slow-decay of EPP, and prolonged burst duration of AChR. Two authors (L.D. and X.-M.S.) evaluated the eligible reports independently. The patients with SCCMS were selected by 2 criteria: the causative mutation is identified, and clinical features are adequately documented.
Collection of electrophysiologic and therapeutic effectiveness data in reported patients with SCCMS
To correlate the occurrence of R-CMAP and the kinetic defects of corresponding mutations, we reviewed EMG data and the opening burst durations of mutant AChRs. To identify the possible factors associated with therapeutic efficacy, we collected data on age at disease onset, age at initiation of channel blocker treatment, and efficacy of treatment. To assess the treatment efficacy, we categorized patients into 2 groups: a group with description of “significant benefit” or “clear benefit, dramatic improvement, significant response, or significant benefit” and a second group with “no to moderate benefit” with terms of “no improvement, some or partial response, mild or modest benefit without changes in neurologic presentation.”
Simulation of the declining phase of the EPP
To explore the relationship between the length of opening burst of mutant AChR identified in patients and the occurrence of R-CMAP in the EMG study, we needed to examine whether the amplitude of EPP after the refractory period of muscle sodium channel (Na1.4) was above the threshold for the second action potential (AP). To this end, we apply the following equation to simulate the decline phase of EPP:
Here, A is the amplitude of EPP at time t; A0 is the peak amplitude at time 0; τ1 and τ2 are decay times of EPP from wild-type and mutant AChR, respectively, because both wild-type and mutant AChRs are expressed in postsynaptic regions of all patients with SCCMS; and E1 and E2 indicate surface expression levels of wild-type and mutant AChRs, respectively, with the assumption that normal expression of the wild-type receptor originates from 1 allele in SCCMS. Because E1 is set to 1 for wild-type, E2 is set to a relative value to E1. We set A0 to 42.6 mV, an average of amplitude of control EPPs recorded at the endplates of a patient control harboring 2 causative heterozygous mutations of Na1.4 channel studied at Mayo Clinic.19 In this participant, the endplates expressed wild-type AChR, but the muscle fibers did not contract on nerve stimulation due to dysfunction of the muscle fiber sodium channel. Thus, the amplitude of EPP in this individual can be used as a normal control. The amplitude of EPP was corrected for resting membrane potential of −80 mV and nonlinear summation.20 The decay time of EPP in control human EP (τ1) is 4.55 ± 0.11 milliseconds (mean ± standard error), which was obtained from 190 EPPs in control human muscle specimens at the Mayo Clinic Muscle Research Laboratory.21 τ2 Is the product of τ1 multiplied by the increase fold of the opening burst duration of corresponding mutant AChR.
Statistics
We compared 2 independent groups of data for significant difference with values of p < 0.05 and with no zero contained in the 95% confidence interval (CI) by the Student 2-tailed t test. All statistical analyses were performed with IBM SPSS version 23.0 (Armonk, NY).
Data availability
The dataset analyzed in the study is available on request.
Results
Clinical features of new patients
The proband, a 48-year-old woman, could not keep up with peers in sports since age 7 years. She had mild bilateral ptosis and limited ocular ductions without double vision and limb muscles weakness since age 10 years. She was diagnosed as having myasthenia gravis and treated with prednisone for ∼2 weeks due to respiratory insufficiency at age 28 years. At 46 years of age, she needed assistance in walking, turning over, getting out of bed, and even brushing her teeth, and she noted mild difficulty swallowing and weakening of her voice. Pyridostigmine 60 mg 3 times daily improved her symptoms only partially. At age 48 years, she had mild ptosis with limited ocular movements without diplopia, as well as moderate facial and bulbar weakness. Her muscle strength on Medical Research Council scale was 2/5 for the neck flexor, 3−/5 for the neck and finger extensor muscles, and 3/5 for the upper and lower proximal limb muscles.
The proband's first daughter is 30 years old. She had poor suck at birth and delayed motor milestones. Eyelid ptosis and progressive muscle weakness appeared after age 3 years, with inability to rise from squatting at 5 years of age and increasing difficulty in walking and inability to run at 15 years of age. Physical examination at age 30 years revealed ophthalmoparesis, moderate facial weakness, scoliosis of the spine, and a waddling gait. Her muscle strength was 2/5 for both proximal upper limb and finger extensor muscles and 3/5 for lower legs. Muscle bulk was diffusely reduced.
The proband's second daughter is 24 years old. She was born healthy with normal motor milestones. Fatigable weakness appeared at age 7 years. Her ptosis occurred at the age 13 years without double vision. She had difficulties in climbing stairs and running but was able to complete her daily housework. On examination, she had mild ptosis, restricted ocular movements without diplopia, and mild facial weakness. The muscle strength was 4/5 for neck muscles, 5-/5 for proximal upper limbs, 4/5 for finger extensor muscles, and 5−/5 for the distal leg muscles. Her speech, respiratory, and swallowing functions were normal.
Laboratory tests, EMG studies, and muscle biopsy of the new patients
A complete blood count with differential, liver function tests, fasting glucose, blood urea, nitrogen, creatinine, electrolytes, creatine kinase, lactate, thyroxine, and thyroid-stimulating hormone were normal in the proband and her 2 daughters. Anti-AChR and anti-MuSK antibodies were negative.
Needle EMG in the proband showed low-amplitude short-duration complex motor unit potentials in the tibialis anterior and vastus lateralis muscles. Nerve conductions were normal, and no R-CMAP was detected in the patients with CMAP amplitude >10 mV. Repetitive nerve stimulation at 3 Hz in the proband showed 26% to 47% decrement of the fourth compared with the first CMAP in abductor digiti minimi, trapezius, and orbicularis oculi muscle. The proband's daughters had 29% to 40% decrement of CMAP in abductor digiti minimi.
A muscle biopsy of the younger daughter showed slight denervation atrophy. Necrotic and regenerating fibers were absent, and the connective tissue elements were unremarkable. There was a normal distribution of oxidative enzyme reactivity. The random distribution of histochemical fiber types was preserved. The atrophic fibers were of either histochemical type. The muscle fiber glycogen and lipid contents were normal. Some scattered angular atrophic fibers overreacted to nonspecific esterase.
Mutation analysis of the new patients
The proband harbored a heterozygous c.835G>T variant in the exon 8 of the AChR ε-subunit, indicating replacement of a valine by a phenylalanine at codon 279 (p.εV279F) with Human Genome Variation Society nomenclature or at codon 259 of the mature peptide (p.εV259F) with legacy nomenclature. The variant occurs in the middle of the second transmembrane domain (M2) of the ε-subunit that lines the AChR channel pore and has been characterized as a dominant mutation causing an SCCMS. The εV259F mutation reduces the surface expression of the receptor to 45% of wild-type and prolongs the length of channel opening bursts 4.37-fold compared to the wild-type.14 The mutation was also present in both daughters.
Therapeutic response of the new patients
After the proband was diagnosed as having slow-channel syndrome, she was started on fluoxetine with an initial dose of 20 mg/d and gradual increase to 60 mg/d (1.5 mg/kg/d), tapering off pyridostigmine gradually from 180 mg/d. She tolerated fluoxetine well but felt worse when pyridostigmine was reduced to 150 mg/d. With combined administration of fluoxetine (up to 60 mg/d) and pyridostigmine (180 mg/d) for 6 months, her muscle strength improved from grade 2 to 4 in neck flexor and grade 3 to 4 in limb muscles; her endurance and activities of daily living were also improved, but her ptosis remained unchanged. At this point, withdrawal of pyridostigmine was tried. In 24 hours after discontinuing pyridostigmine, the strength of her neck and limb muscles was reduced to grade 3. After she was on pyridostigmine for 24 hours, her muscle strength improved to grade 4 rapidly.
The proband's older daughter began taking fluoxetine at 1.5 mg/kg/d at age 30 years. After 1 month of treatment, she was able to walk longer distances but still could not run. Her upper limb muscle strength has improved to 3/5. The strength of the lower limbs was unchanged. Because her mother had improved with pyridostigmine combined with the channel blocker, she was prescribed pyridostigmine 30 mg 3 times daily. Like her mother, she benefitted from the combined fluoxetine and pyridostigmine, with improvement of proximal limb muscle strength to 4/5.
The proband's younger daughter started on fluoxetine (60 mg/d, 1.5 mg/kg/d) at age 24 years. Her fatigable weakness remarkably improved. She was able to run and go hiking, but her ptosis remained unchanged. Pyridostigmine combined with fluoxetine for a month provided no additional benefit.
Clinical features of patients with SCCMS reported in literature
We reviewed a total of 57 patients in 26 reports (table 1). Thirty-four patients had symptom onset between early infancy and 1 year of age; 7 patients had onset between 1 and 10 years of age; and 16 patients presented after 10 years of age. The common clinical features were ptosis and ophthalmoparesis, generalized weakness, and weakness prominent in distal upper limbs. Twenty-two patients had cervical muscle weakness; 20 patients had facial and bulbar weakness. Fourteen patients had respiratory insufficiency; 11 patients had scoliosis. Of note, the age at onset of symptoms in 1 patient and the clinical features of another patient were not described.
Table 1.
Clinical features of reported patients with SCCMS
Mutations identified in patients with SCCMS
Patients with SCCMS harbored 25 mutations in different domains of all 4 AChR subunits, as shown in tables 1 and 2. Nine mutations occurred in the α-subunit (αG153S,5,13,15,17 αV156M,13 αN217K,18,22,23 αS226Y,24 αS226F,24 αV249F,7 αT254I,13 αS269I,13 αC418W18,25), 5 mutations in the β-subunit (βV229F,23 βL262M,26 βT265S,15 βV266M,15,17,22,23 βV266A6), 3 mutations in the δ-subunit (δS268F,23,27 δS268Y,15 δL273F21), and 8 mutations in the ε-subunit (εL221F,28 εI257F,29 εV259F,14 εV259L,15,16 εT264P,4,8,30 εV265A,6 εL269F,15,18,22,31–33 εS278del34).
Table 2.
R-CMAP in patients and open burst durations in mutant AChRs
In terms of location, most mutations (15 of 25, 60%) are in the second transmembrane domain (M2), 5 mutations are in the first transmembrane domain (M1) (αN217K, αS226Y, αS226F, εL221F, βV229F), 2 mutations are near the agonist binding sites of extracellular domains (αG153S, αV156M), 2 mutations are in the extracellular M2-M3 linker (αS269I, εS278del), and 1 mutation is in the fourth transmembrane domain (αC418W).
Electrophysiologic studies in reported patients with SCCMS
As shown in table 2, EMG studies were described in 46 patients, with 43 patients previously reported and 3 new patients from this study. R-CMAP was detected in 40 patients and was absent in 6 patients.6,13,14 Single-channel patch-clamp studies were performed to measure the channel opening burst durations of 16 mutant AChRs carried in 33 patients with R-CMAP and 3 mutants carried in 6 patients without R-CMAP.
To compare data published by different laboratories, in each report, we calculated how many times the length of channel opening bursts in mutant AChR exceeded that of wild-type AChR (table 2). The average length of opening bursts of mutant AChRs in corresponding patients with SCCMS without R-CMAP (SCCMS without R-CMAP) increased 3.84 ± 0.65-fold (mean ± SD) from wild-type AChR, whereas that of other mutants in patients with R-CMAP (SCCMS with R-CMAP) increased 8.68 ± 2.82-fold. Thus, the mutations in SCCMS without R-CMAP prolonged the burst duration 2.26-fold less than those in SCCMS with R-CMAP (95% CI 3.18–6.50, p = 0.000014) (table 2 and figure 1).
Figure 1. Comparison of the prolongation of the mutant AChR channel openings in SCCMS with and without R-CMAP.

Each dot represents a mutant acetylcholine receptor (AChR); each box indicates mean and SD. Comparison of patients with and without repetitive compound muscle action potential (R-CMAP) shows 95% confidence interval of 3.18 to 6.50 with the p value indicated in the figure. SCCMS = slow-channel congenital myasthenic syndrome.
Simulation of the declining phase of EPP in patients with SCCMS
To explore the mechanism underlining the association of the R-CMAP occurrence in patients with the mutant AChR kinetics, as described in Methods, we used equation 1 to simulate the declining phases of virtual EPPs in control, SCCMS with R-CMAP, and SCCMS without R-CMAP. We set both E1 and E2 to 1 in all patients because SCCMS mutations generally do not affect the receptor expression. The first component decay time of EPP (τ1) in SCCMS is the same as the decay time of EPP in control (4.55 milliseconds).21 We derived the decay times of the second components of EPPs (τ2) in patients with SCCMS from the products of τ1 multiplied by the average fold change of opening burst lengths of corresponding mutant AChRs, 8.68 and 3.84 (table 2) in SCCMS with and without R-CMAP, respectively.
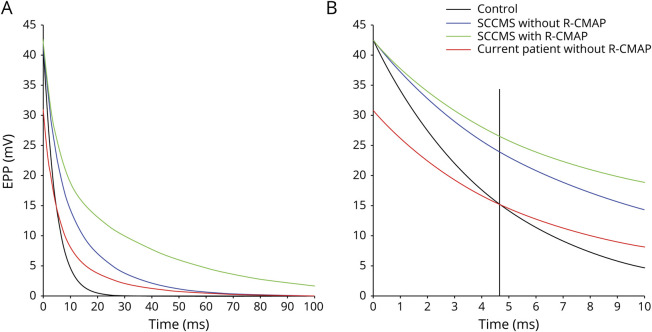
As shown in figure 2A, the EPP peak amplitudes in the control and SCCMS groups with or without R-CMAP are all 42.6 mV.19 EPPs in patients with SCCMS decay much more slowly than the EPP of the control. The absolute refractory period in innervated human muscles varies from 2.2 to 4.6 milliseconds.35 As shown in figure 2B, the EPP amplitudes at 4.6 milliseconds are 16, 27, and 23 mV in control, SCCMS with R-CMAP, and SCCMS without R-CMAP, respectively. An EPP with an amplitude greater than the threshold of 25 mV is able to produce an AP in a human muscle fiber.20,36 Thus, after the refractory period of the muscle sodium channels, the EPP amplitude at the postsynaptic membrane of patients with SCCMS with R-CMAP exceeds the threshold for evoking the second AP, but those in controls and in patients with SCCMS without R-CMAP are below the threshold.
Figure 2. Simulation of the decay phase of EPPs in SCCMS and control.
(A) All simulations are performed on the basis of equation 1 in Methods. (B) Section of panel A within time scale of 10 milliseconds. Vertical line indicates the refractory time of action potential in muscle. EPP = endplate potential; R-CMAP = repetitive compound muscle action potential; SCCMS = slow-channel congenital myasthenic syndrome.
Moreover, we simulated the declining phase of virtual EPP in current patients harboring εV259F mutation with E2 set to 0.45 due to its expression level at 45% of wild-type14 and with τ2 set to the product of τ1 multiplied by 4.37, the fold change of opening burst length compared to wild-type (table 2). Although the EPP in our patients decays more slowly than in the control, the peak amplitude of the EPP is 30.9 mV (figure 2A), lower than control, and the amplitude of decayed EPP after the refractory period of the first AP is only 15 mV, far below the threshold for the second AP (figure 2B).
Therapeutic efficacies of channel blockers in reported SCCMS
We identified 17 previously reported patients who were treated with channel blockers (table 3). Including the 3 new patients, the symptoms and the distribution of weakness were similar in the groups with significant and with no to partial benefit. The age at onset in patients with significant benefit ranged from birth to 43 years with an average of 11.80 ± 16.94 years (mean ± SD, n = 10), and the age at onset in patients with no to partial benefit was from birth to 11 years with an average of 3.40 ± 3.98 years (n = 10). Comparison between the 2 groups shows that the corresponding 95% CI is from −3.86 to 20.66 with p = 0.17 (table 3 and figure 3A). The age at the initial channel blocker therapy in patients with the significant benefit was from 12 to 43 years with an average of 23.40 ± 13.29 years, whereas that in the no to partial benefit group was from 20 to 52 years with an average of 34.10 ± 13.43 years. The statistical analysis shows no difference (95% CI −23.25 to 1.85, p = 0.090) (table 3 and figure 3B). The delay in time of treatment from onset of the disease in the significant benefit group was 11.60 ± 5.17 years with a median of 13 years, whereas that in the no to partial benefit group was 30.70 ± 12.72 years with a median of 33 years. The statistical analysis shows a significant difference between the 2 groups (95% CI −28.57 to −9.63, p = 0.00089) (table 3 and figure 3C). Thus, patients with significant benefit from the channel blocker treatment had significantly less delay in the initiation of therapy than patients with no to partial benefit.
Table 3.
Efficacy of channel blocker therapy
Figure 3. Comparison of the response to channel blockers in SCCMS with age at onset, age at starting treatment, and delay time of treatment.
Each dot represents a patient; each column indicates mean and SD. Comparisons between significant response and partial or no response with (A) age at onset, (B) age at the start of channel blocker treatment, and (C) the delay time of treatment show 95% confidence intervals of −3.86 to 20.66, −23.25 to 1.85, and −28.57 to −9.63, respectively, with p values indicated in the figure. SCCMS = slow-channel congenital myasthenic syndrome.
Discussion
We report 3 patients with SCCMS caused by a V259F variant in the AChR ε-subunit. They had no R-CMAP in EMG studies. Two patients partially responded to channel blocker but improved with additional use of the AChE inhibitor pyridostigmine. Systematic analysis of current and previously reported patients with SCCMS reveals that the occurrence of R-CMAP correlates with the extent of the channel opening prolongation of mutant AChR and that earlier initiation of channel blocker therapy results in better treatment response.
The detection of the R-CMAP has been considered an essential criterion for the diagnosis of a SCCMS since the first patient with slow channel was reported.8 It is assumed that the absence of the R-CMAP in some patients with SCCMS is due to low initial CMAP amplitude6 or to insufficient rest during testing.8 However, current patients had a CAMP amplitude >10 mV, and a Polish patient with SCCMS harboring the same εV259F variant as our patients had no R-CMAP in 2 EMG studies performed with an interval of 20 years.14 This suggests that the lack of R-CMAP may be associated with the specific mutation. The underlying mechanism to evoke R-CMAP is that the amplitude of decayed EPP after refractory period of the sodium channel activated for the first AP is above the threshold able to produce the second AP. The duration of opening burst of AChR at the single channel level reflects the decay time of miniature endplate currents or potential and EPP or endplate current. A longer duration of channel opening bursts of AChR causes slower decay of EPP and a higher amplitude of decayed EPP. To date, it is unknown how many fold increase of opening burst duration of mutant AChR channel is sufficient for the second AP. Our study reveals that <4-fold prolongation of opening burst length of mutant AChR is insufficient to produce the second AP because of the decay EPP amplitude below the AP threshold after the refractory period of the first one. Decreased expression of mutant AChR reduces the EPP peak amplitude and causes the decay amplitude further lower than the threshold for the second AP.
In SCCMS, the gain-of-function mutations of AChR subunits prolong EPPs, resulting in depolarization of the postsynaptic membrane, reduction of AChR available for activation due to desensitization, and an endplate myopathy associated with degeneration of the junctional folds and loss of AChR.1,4,6,7,37 A prolonged delay from symptom onset to the start of appropriate therapy results in more severe endplate myopathy and greater loss of AChR from the neuromuscular junction. Structural damages at the postsynaptic region reduce the effectiveness of channel blocker therapy. That explains the varied efficacy of channel blocker treatment in SCCMS with varied delay time from onset to the start of treatment. The association between treatment efficacy and the delay time is well reflected in our newly identified patients in the same family. The mother and the older daughter with longer delay in starting therapy had less response to therapy than the younger daughter, who had a shorter delay before the start of therapy. The proband and the older daughter, but not the younger daughter, responded favorably to pyridostigmine when combined with fluoxetine, likely due to increased binding of acetylcholine molecules to balance the shortening of the channel open time of reduced receptors. Salbutamol, a β2-adrenergic agonist, also provided additional benefit with channel blocker treatment in 4 patients with SCCMS, as shown in table 3.18,25,34 Hence, the additional AChE inhibitor and salbutamol are beneficial to patients with SCCMS with long delay until the initiation of channel blocker therapy.
This work provides mechanistic explanation of the lack of R-CMAP and the variation of efficacy of channel blocker therapy in patients with SCCMS. However, our study is retrospective, and the treatment efficacy was evaluated on the basis of descriptions from different clinicians without uniform criteria. Moreover, the wide range of disease onset and extent of symptom severity in patients with the same mutation suggest that other factors may also influence the efficacy of treatment.
Acknowledgment
The authors thank the patient and her family for their participation in the study.
Glossary
- AP
action potential
- AChE
acetylcholinesterase
- AChR
acetylcholine receptor
- CI
confidence interval
- CMAP
compound muscle AP
- CMS
congenital myasthenic syndromes
- EPP
endplate potential
- MuSK
muscle‐specific kinase
- R-CMAP
repetitive CMAP
- SCCMS
slow-channel CMS
Appendix. Authors


Footnotes
Class of Evidence: NPub.org/coe
Study funding
Supported by the National Key R&D Program of China, Precision Medicine Project (2017YFC0907700), Chinese National Natural Science Funds (81641049), NIH (R01 NS109491), and Myasthenia Gravis Foundation of America (FP00094864).
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Engel AG, Shen XM, Selcen D, Sine SM. Congenital myasthenic syndromes: pathogenesis, diagnosis, and treatment. Lancet Neurol 2015;14:420–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thompson R, Abicht A, Beeson D, et al. A nomenclature and classification for the congenital myasthenic syndromes: preparing for FAIR data in the genomic era. Orphanet J Rare Dis 2018;13:211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oury J, Liu Y, Topf A, et al. MACF1 links Rapsyn to microtubule- and actin-binding proteins to maintain neuromuscular synapses. J Cell Biol 2019;218:1686–1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ohno K, Hutchinson DO, Milone M, et al. Congenital myasthenic syndrome caused by prolonged acetylcholine receptor channel openings due to a mutation in the M2 domain of the epsilon subunit. Proc Natl Acad Sci USA 1995;92:758–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sine SM, Ohno K, Bouzat C, et al. Mutation of the acetylcholine receptor alpha subunit causes a slow-channel myasthenic syndrome by enhancing agonist binding affinity. Neuron 1995;15:229–239. [DOI] [PubMed] [Google Scholar]
- 6.Shen XM, Okuno T, Milone M, et al. Mutations causing slow-channel myasthenia reveal that a valine ring in the channel pore of muscle AChR is optimized for stabilizing channel gating. Hum Mutat 2016;37:1051–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Milone M, Wang HL, Ohno K, et al. Slow-channel myasthenic syndrome caused by enhanced activation, desensitization, and agonist binding affinity attributable to mutation in the M2 domain of the acetylcholine receptor alpha subunit. J Neurosci 1997;17:5651–5665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Engel AG, Lambert EH, Mulder DM, et al. A newly recognized congenital myasthenic syndrome attributed to a prolonged open time of the acetylcholine-induced ion channel. Ann Neurol 1982;11:553–569. [DOI] [PubMed] [Google Scholar]
- 9.Harper CM, Engel AG. Safety and efficacy of quinidine sulfate in slow-channel congenital myasthenic syndrome. Ann NY Acad Sci 1998;841:203–206. [DOI] [PubMed] [Google Scholar]
- 10.Fukudome T, Ohno K, Brengman JM, Engel AG. AChR channel blockade by quinidine sulfate reduces channel open duration in the slow-channel congenital myasthenic syndrome. Ann NY Acad Sci 1998;841:199–202. [DOI] [PubMed] [Google Scholar]
- 11.Fukudome T, Ohno K, Brengman JM, Engel AG. Quinidine normalizes the open duration of slow-channel mutants of the acetylcholine receptor. Neuroreport 1998;9:1907–1911. [DOI] [PubMed] [Google Scholar]
- 12.Harper CM, Fukodome T, Engel AG. Treatment of slow-channel congenital myasthenic syndrome with fluoxetine. Neurology 2003;60:1710–1713. [DOI] [PubMed] [Google Scholar]
- 13.Croxen R, Newland C, Beeson D, et al. Mutations in different functional domains of the human muscle acetylcholine receptor alpha subunit in patients with the slow-channel congenital myasthenic syndrome. Hum Mol Genet 1997;6:767–774. [DOI] [PubMed] [Google Scholar]
- 14.Fidzianska A, Ryniewicz B, Shen XM, Engel AG. IBM-type inclusions in a patient with slow-channel syndrome caused by a mutation in the AChR epsilon subunit. Neuromuscul Disord 2005;15:753–759. [DOI] [PubMed] [Google Scholar]
- 15.Chaouch A, Muller JS, Guergueltcheva V, et al. A retrospective clinical study of the treatment of slow-channel congenital myasthenic syndrome. J Neurol 2012;259:474–481. [DOI] [PubMed] [Google Scholar]
- 16.Outteryck O, Richard P, Lacour A, et al. Novel epsilon subunit mutation of the muscle acetylcholine receptor causing a slow-channel congenital myasthenic syndrome. J Neurol Neurosurg Psychiatry 2009;80:450–451. [DOI] [PubMed] [Google Scholar]
- 17.Mihaylova V, Scola RH, Gervini B, et al. Molecular characterisation of congenital myasthenic syndromes in southern Brazil. J Neurol Neurosurg Psychiatry 2010;81:973–977. [DOI] [PubMed] [Google Scholar]
- 18.Durmus H, Shen XM, Serdaroglu-Oflazer P, et al. Congenital myasthenic syndromes in Turkey: clinical clues and prognosis with long term follow-up. Neuromuscul Disord 2018;28:315–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsujino A, Maertens C, Ohno K, et al. Myasthenic syndrome caused by mutation of the SCN4A sodium channel. Proc Natl Acad Sci USA 2003;100:7377–7382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elmqvist D, Johns TR, Thesleff S. A study of some electrophysiological properties of human intercostal muscle. J Physiol 1960;154:602–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shen XM, Milone M, Wang HL, et al. Slow-channel myasthenia due to novel mutation in M2 domain of AChR delta subunit. Ann Clin Transl Neurol 2019;6:2066–2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Engel AG, Ohno K, Milone M, et al. New mutations in acetylcholine receptor subunit genes reveal heterogeneity in the slow-channel congenital myasthenic syndrome. Hum Mol Genet 1996;5:1217–1227. [DOI] [PubMed] [Google Scholar]
- 23.Vohra BP, Groshong JS, Maselli RA, Verity MA, Wollmann RL, Gomez CM. Focal caspase activation underlies the endplate myopathy in slow-channel syndrome. Ann Neurol 2004;55:347–352. [DOI] [PubMed] [Google Scholar]
- 24.Ohno K, Wang HL, Shen XM, et al. Slow channel mutations in the center of the M1 transmembrane domain of acetylcholine receptor (AChR) alpha subunit. Neurology 2000;54:A183. [Google Scholar]
- 25.Shen XM, Deymeer F, Sine SM, Engel AG. Slow-channel mutation in acetylcholine receptor alphaM4 domain and its efficient knockdown. Ann Neurol 2006;60:128–136. [DOI] [PubMed] [Google Scholar]
- 26.Gomez CM, Maselli R, Gammack J, et al. A beta-subunit mutation in the acetylcholine receptor channel gate causes severe slow-channel syndrome. Ann Neurol 1996;39:712–723. [DOI] [PubMed] [Google Scholar]
- 27.Gomez CM, Maselli RA, Vohra BP, et al. Novel delta subunit mutation in slow-channel syndrome causes severe weakness by novel mechanisms. Ann Neurol 2002;51:102–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Croxen R, Hatton C, Shelley C, et al. Recessive inheritance and variable penetrance of slow-channel congenital myasthenic syndromes. Neurology 2002;59:162–168. [DOI] [PubMed] [Google Scholar]
- 29.Shen XM, Ohno K, Milone M, Brengman J, Tsujino A, Engel AG. Effect of residue side-chain mass on channel kinetics in second transmembrane domain of muscle AChR. Mol Biol Cell 2003;14:223a. [Google Scholar]
- 30.Azuma Y, Nakata T, Tanaka M, et al. Congenital myasthenic syndrome in Japan: ethnically unique mutations in muscle nicotinic acetylcholine receptor subunits. Neuromuscul Disord 2015;25:60–69. [DOI] [PubMed] [Google Scholar]
- 31.Colomer J, Muller JS, Vernet A, et al. Long-term improvement of slow-channel congenital myasthenic syndrome with fluoxetine. Neuromuscul Disord 2006;16:329–333. [DOI] [PubMed] [Google Scholar]
- 32.Tan JZ, Man Y, Xiao F. A missense mutation in epsilon-subunit of acetylcholine receptor causing autosomal dominant slow-channel congenital myasthenic syndrome in a Chinese family. Chin Med J (Engl) 2016;129:2596–2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gomez CM, Gammack JT. A leucine-to-phenylalanine substitution in the acetylcholine receptor ion channel in a family with the slow-channel syndrome. Neurology 1995;45:982–985. [DOI] [PubMed] [Google Scholar]
- 34.Finlayson S, Spillane J, Kullmann DM, et al. Slow channel congenital myasthenic syndrome responsive to a combination of fluoxetine and salbutamol. Muscle Nerve 2013;47:279–282. [DOI] [PubMed] [Google Scholar]
- 35.Farmer TW, Buchthal F, Rosenfalck P. Refractory period of human muscle after the passage of a propagated action potential. Electroencephalogr Clin Neurophysiol 1960;12:455–466. [DOI] [PubMed] [Google Scholar]
- 36.Seifter J, Austin R, Sloane D. An overview of nerve cell physiology and the autonomic nervous system. In: Seifter J, Austin R, Sloane D, editors. Concepts in Medical Physiology. Philadelphia: Lippincott Williams & Wilkins; 2005: 49–70. [Google Scholar]
- 37.Groshong JS, Spencer MJ, Bhattacharyya BJ, et al. Calpain activation impairs neuromuscular transmission in a mouse model of the slow-channel myasthenic syndrome. J Clin Invest 2007;17:2903–2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Engel AG, Hutchinson DO, Nakano S, et al. Myasthenic syndromes attributed to mutations affecting the epsilon subunit of the acetylcholine receptor. Ann NY Acad Sci 1993;681:496–508. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The dataset analyzed in the study is available on request.