Abstract
Objective
To empirically test whether apathy and impulse control disorders (ICDs) represent independent, opposite ends of a motivational spectrum.
Methods
In this single-center, cross-sectional study, we obtained retrospective demographics and clinical data for 887 patients with idiopathic Parkinson disease (PD) seen at a tertiary care center. Mood and motivation disturbances were classified using recommended cutoff scores from self-reported measures of apathy, ICD, anxiety, and depression.
Results
Prevalence rates included 29.0% of patients with PD with depression, 40.7% with anxiety, 41.3% with apathy, 27.6% with ICDs, and 17.0% with both apathy and ICD. The majority (61.6%) of people reporting clinically significant ICDs also reported clinically significant apathy, and more than a third of patients with apathy (41.3%) also reported elevated ICD. Anxiety and depression were highest in patients with both apathy and ≥1 ICDs. Dopamine agonist use was higher in people with only ICD compared to people with only apathy. Mood significantly interacted with demographic variables to predict motivational disturbances.
Conclusions
Motivational disturbances are common comorbid conditions in patients with PD. In addition, these complex behavioral syndromes interact with mood in clinically important ways that may influence the design of future clinical trials and the development of novel therapies. This study challenges the concept of apathy and ICD in PD as opposite ends of a spectrum.
Parkinson disease (PD) is recognized as a complex neuropsychiatric condition that includes pervasive motivational disturbances.1 Apathy and impulse control disorders (ICDs) are 2 common debilitating behavioral syndromes frequently observed in patients with PD. ICDs are repetitive reward-seeking behaviors such as pathologic gambling, hypersexuality, binge eating, and compulsive shopping. On the other hand, apathy refers to a reward-deficiency syndrome that can manifest as reduced interest or pleasure in activities and difficulty initiating desired actions. These behaviors are thought to represent opposite extremes of the same spectrum of motivated behavior, although there is scant empirical evidence to support this.2,3
Reported frequencies of apathy and ICD in PD vary considerably on the basis of sample characteristics and diagnostic approach. The prevalence of apathy appears related to changes in dopaminergic function; the estimated frequency of 39.8% in patients with PD drops with the introduction of dopamine replacement therapy and increases as the disease progresses.4 Depending on sample characteristics such as age and cultural background, the reported prevalence of ICDs in PD can range widely.5–7 More recently, it has been reported that the 5-year cumulative incidence of ICDs nears 50%,8 although the true prevalence is likely higher due to underreporting of these often embarrassing behaviors.
Accumulating research suggests that the occurrence of apathy and ICDs cannot be accounted for by dopaminergic changes alone and that they may involve diverse but related etiologic pathways.2,9 However, it is currently difficult to draw meaningful conclusions about the connection between apathy and ICD due to a lack of studies investigating both syndromes in the same patient cohort and because apathy and ICDs have previously been considered mutually exclusive categories. Instead, studying apathy and ICD together would further our understanding of the relationship between these complex behavioral syndromes and influence the design of future clinical trials and novel therapies for these debilitating conditions.
The purpose of this study is to evaluate the hypothesis that apathy and ICDs are independent and opposite (i.e., motivational spectrum hypothesis). First, we establish the prevalence of mood and motivational disturbances in a large cohort of patients with PD. Next, we compare the clinical and demographic variables among 4 subgroups of patients classified by motivational status (i.e., apathetic, ICD, both, and neither). Finally, we investigate the dependency of mood symptoms on motivational disturbances.
Methods
Standard protocol approvals, registrations, and patient consents
This single-center, cross-sectional study was approved by the Institutional Review Board (No. 201901807). All patients provided informed consent before data collection in accordance with the Institutional Review Board under the INFORM database protocol (No. 201501166).
Participants
This study included a convenience sample of 887 patients with PD from the INFORM database followed up by the Movement Disorders Program at the University of Florida Norman Fixel Institute for Neurological Disease, which is designated as a National Parkinson Disease Center of Excellence. At the University of Florida, most patients with movement disorders are approached about whether they would be interested in participating in this research database, and about two-thirds of all patients seen in the clinic are included in the database. Clinical data are collected from these patients at each visit and stored in the database. We included participants >35 years of age meeting a diagnosis of idiopathic PD who completed self-report motivation questionnaires (see below). All patients with PD in the INFORM database are provided these questionnaires before each visit (see below), and participation is optional. Exclusion criteria were concurrent essential tremor diagnoses or any evidence of secondary or atypical parkinsonism, as well as deep brain stimulation therapy.
Procedures
Demographics, disease characteristics (e.g., age at onset, disease severity), and self-reported measures of mood and motivational symptoms were extracted from the INFORM database. Medication data were retrieved from notes in the electronic health record. Medication data were used to compute a total levodopa equivalency daily dose (LEDD) using standard conversion multipliers and an LEDD score comprising only dopaminergic agonists (DADDs). Disease duration was defined relative to each patient's estimated date of symptom onset. Mood symptoms were assessed using self-reported measures of anxiety (Beck Anxiety Inventory [BAI]10) and depressive symptoms (Beck Depression Inventory-II [BDI-II]11). The recommended cutoff score of 14 for the BAI and BDI-II was used to classify clinically significant symptoms of anxiety and depression, respectively. We assessed general cognitive status using the Mini-Mental State Examination (MMSE).12 We found that 86.7% of the sample had MMSE scores >24, a cutoff used to screen for normal cognition.
Motivational disturbances were assessed with validated measures of apathy (Apathy Scale [AS]13) and ICD (Questionnaire for Impulsive-Compulsive Disorders–Rating Scale [QUIP-RS]14) symptoms as recommended by the Movement Disorders Task Force. According to the recommended guidelines, AS scores of ≥14 were classified as clinically significant apathy, while the individual ICD cutoff scores established by Weintraub et al.14 were used to determine ICD status. Throughout, we use the term ICDs to refer to the classic ICDs (hypersexuality, binge eating, compulsive shopping, pathologic gambling), as well as hobbyism/punding and dopamine dysregulation syndrome, which are part of the QUIP-RS assessment. Patients completed these measures on a computer before clinic appointments. Patients could receive help from an informant when filling out self-reported measures. If patients had completed assessments at multiple follow-up visits, only the first time point was considered for this cross-sectional study.
Statistical analyses
Statistical analyses were conducted with IBM SPSS version 22.0 for Windows (Armonk, NY) and R 3.5.2 (R Foundation for Statistical Computing, Vienna, Austria). Missing data analyses were not conducted because patients were included in the present study only if they completed each of the motivational outcome measures. Multidimensional χ2 tests and univariate analyses of variance with post hoc Tukey multiple comparisons (reported p values are adjusted) were used to compare demographic (e.g., age, education, sex) and disease (e.g., disease duration, motor symptom severity, medication use) variables between groups defined by mood and motivational status. Bivariate correlations were used to examine relationships between mood and motivation variables. Hierarchical linear regression was used to identify predictors of AS and QUIP-RS scores. Using the Simple Enter method, all models included age, disease duration, and sex in block 1 and BAI and BDI-II total scores in block 2 to assess the amount of variation accounted for beyond demographic and disease characteristics. We present all comparisons using confidence intervals (CIs) of the difference between 2 groups. However, the mean and SD for all metrics and p values are also provided in the tables.
Data availability
Raw data were collected at the University of Florida's Norman Fixel Institute for Neurological Disease. Derived data supporting the findings of this study are available from the corresponding author on request.
Results
Prevalence of mood and motivational disorders
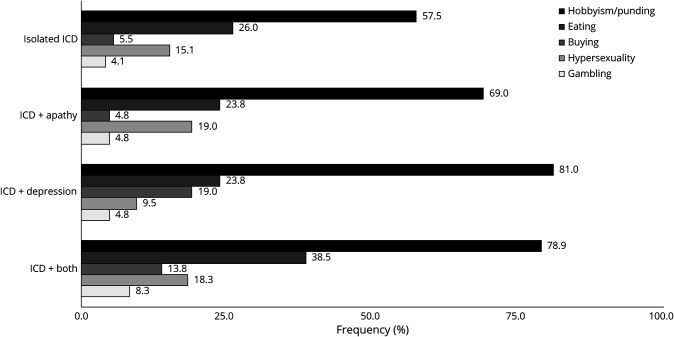
Overall, 41.3% of patients were classified as apathetic. Of these, 43.4% (17.9% of total sample) had an isolated apathy syndrome (i.e., without depression); 27.6% of patients were classified as having ≥1 ICDs; and 17.0% of the sample endorsed clinically significant symptoms of both apathy and ≥1 ICDs. With regard to mood symptoms, 29.0% of patients reported clinically significant depressive symptoms, and 40.7% reported clinically significant symptoms of anxiety, with 22.9% reporting both. The overlapping prevalence of mood and motivational disturbances is illustrated in figure 1.
Figure 1. Overlapping prevalence of mood and motivation.
Percentage of patients with clinically significant scores on mood and motivation inventories using pre-established cutoff scores is shown here. For visualization purposes, darker shades correspond to larger percentages. The total number of patients within each group (N) is shown below that category. ICD = impulse control disorder
Characteristics of motivation subgroups
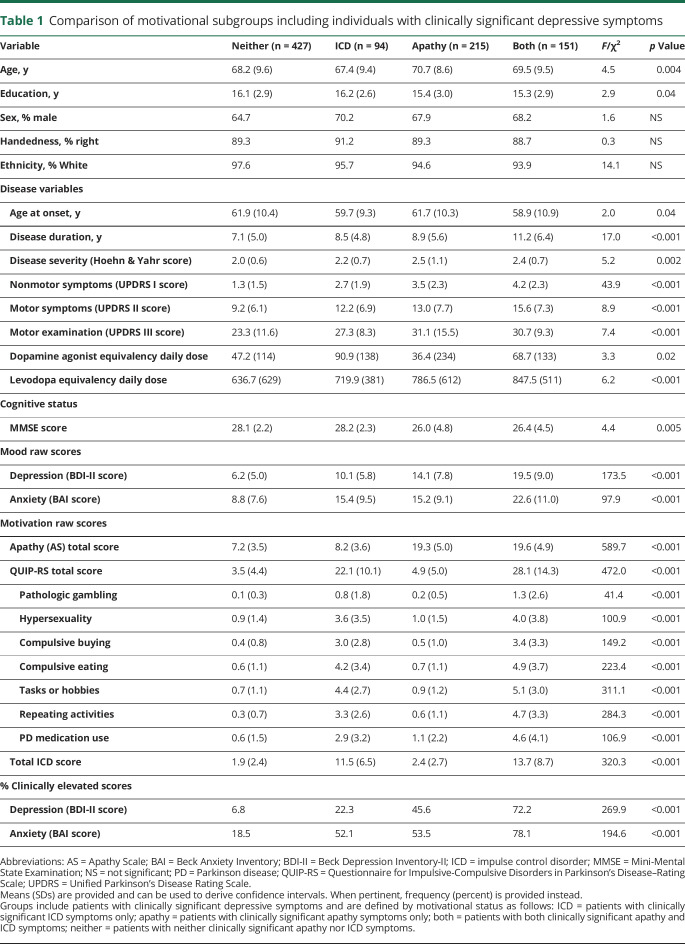
We compared clinical characteristics across motivation subgroups (table 1). Patients in the combined neither or ICD group were significantly younger than patients in the combined apathy or both group (95% CI −3.4 to −0.9 years difference). Patients in the both group had a significantly longer disease duration than patients in the apathy (0.6–4.0 years difference), ICD ( 0.6–4.9 years difference), and neither groups ( 0.6–4.9 years difference). The combined apathy and both groups had a lower general cognitive status than the neither (0.6–3.4 MMSE score difference) group. Patients in the neither group endorsed fewer Unified Parkinson's Disease Rating Scale (UPDRS) I (2.1–3.6 difference) and UPDRS II (3.0–9.9 difference) symptoms and had lower UPDRS III motor severity scores (1.6–13.3 difference) than patients in the both group.
Table 1.
Comparison of motivational subgroups including individuals with clinically significant depressive symptoms
The ICD-only group had higher daily dopamine agonist medication use than patients in the apathy group (95% CI 4.3–104.6 DADD difference) (table 1). There was no significant difference in dopamine agonist use between the ICD and both groups (−31.2 to 75.5 difference). There was also no significant difference in dopamine agonist use between the neither group and the apathy group (−23.8 to 45.4 DADD difference). Total LEDD, which includes dopamine agonist doses, followed trends for disease duration and motor and nonmotor symptom severity (table 1).
Both BDI-II and BAI scores were lowest in the neither group (e.g., compared to the apathy group, 95% CI −9.3 to −6.5 and −8.3 to −4.5 difference, respectively) (table 1). AS scores in the apathy and both groups were significantly higher than in the ICD and neither groups (specifically, apathy vs ICD, apathy vs neither, both vs ICD, and both vs neither; table 1). QUIP-RS total and all subscale scores were highest in patients with both apathy and ICD.
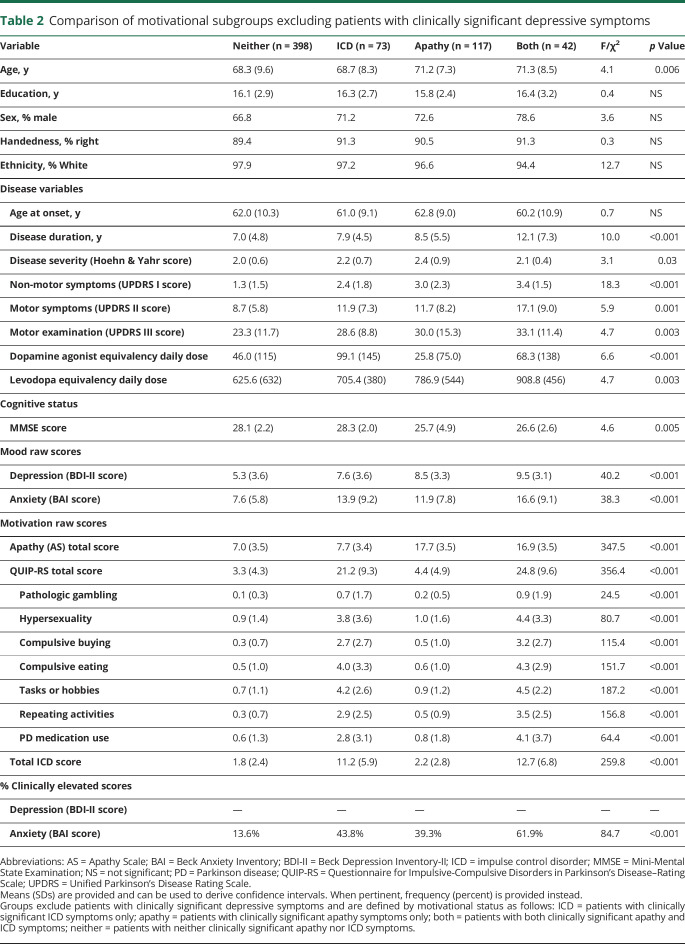
To reduce the influence of mood dysfunction, we additionally compared clinical characteristics across motivation subgroups after excluding patients with clinically significant depression (table 2). These results were largely similar to what we found before excluding patients with depression (table 1). However, after the exclusion of patients with clinically significant depression, education and age at onset differences across groups were no longer noted (tables 1 and 2).
Table 2.
Comparison of motivational subgroups excluding patients with clinically significant depressive symptoms
Influence of mood on motivation subgroups
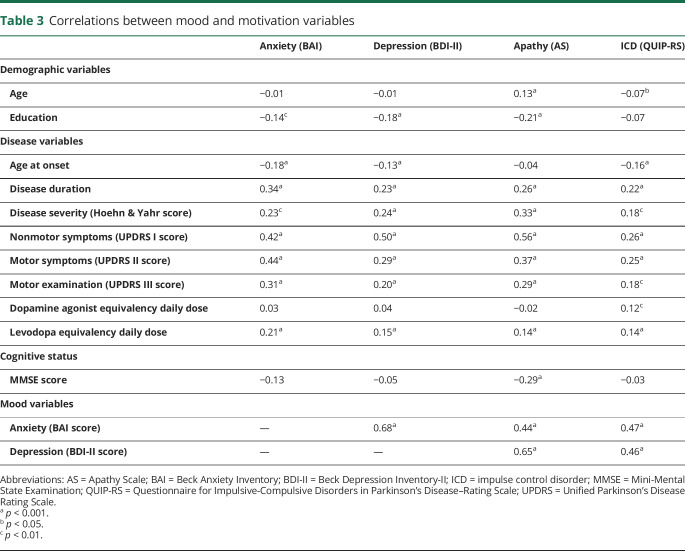
Anxiety and depression each positively correlated with both apathy and ICD symptoms separately (table 3). Specifically, mood symptoms accounted for 14.6% to 23.9% (anxiety R2 95% CI) and 36.9% to 46.8% (depression) of the variance in AS scores and 17.4% to 27.1% (anxiety) and 16.6% to 26.1% (depression) of the variance in QUIP-RS scores. Apathy was positively associated with QUIP-RS scores, accounting for 5.7% to 12.8% of the variance in these scores. Apathy was weakly corelated with age and education, while ICD scores were unrelated to education. Disease variables were generally positively correlated with mood and motivation variables, with the exception of age at onset, which was negatively associated with these variables. However, age at onset was associated with both mood disorders and ICDs but not apathy. MMSE scores correlated with apathy scores but not anxiety, depression, or QUIP-RS score. DADD was correlated only with ICD symptoms, whereas LEDD was correlated with anxiety, depression, apathy, and ICD symptoms.
Table 3.
Correlations between mood and motivation variables
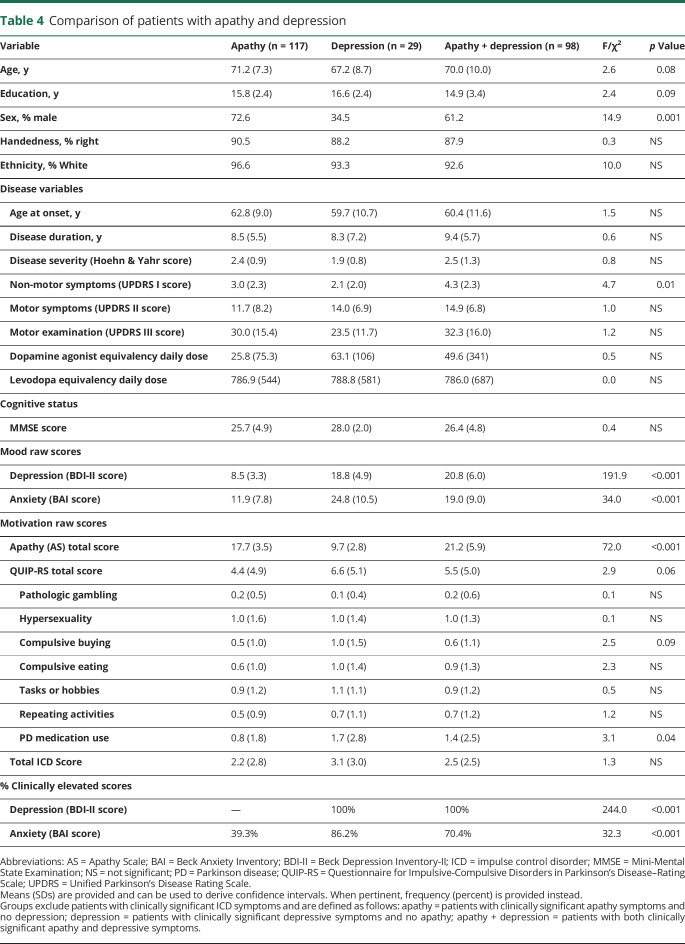
Approximately 27.5% of patients overall reported clinically significant symptoms of apathy and/or depression after we excluded patients with ≥1 ICDs (table 4). The both group endorsed significantly greater UPDRS I nonmotor symptoms than the apathy (95% CI, 0.08–2.4 difference) and depression (0.05–4.3 difference) groups. The apathy group had significantly lower symptoms of depression and anxiety than the depression (−12.6 to −7.9 difference and −17.1 to −8.6 difference, respectively) and both (−13.9 to −10.8 difference and −9.9 to −4.3 difference, respectively) groups. The depression and both groups did not differ in the severity of depressive symptoms. All 3 groups differed significantly in apathy symptoms (table 4), but in post hoc analyses a trend was found only for greater QUIP-RS total scores in the depression vs apathy group (−0.2 to 4.6 difference).
Table 4.
Comparison of patients with apathy and depression
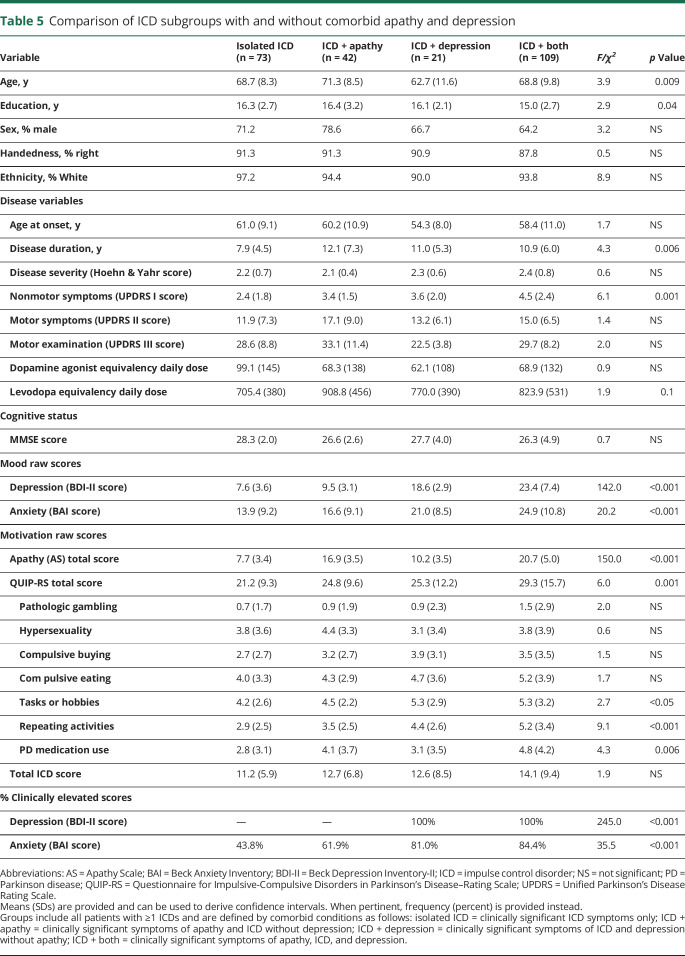
Of the 245 participants meeting criteria for ≥1 ICD, 29.8% had an isolated ICD, 17.1% had an ICD + apathy, 8.6% had an ICD + depression, and 44.5% had an ICD + both apathy and depression (table 5). The ICD + depression group was significantly younger than the ICD + apathy group (95% CI, −15.0 to −2.1 years difference) and the ICD + both group (−11.9 to −0.3 years difference). The isolated ICD group had a trending higher level of education than the ICD + both group (−0.1 to 2.8 years difference), a shorter disease duration than the ICD + apathy (−7.5 to −0.7 years difference) and the ICD + both (−5.7 to −0.4 years difference) groups, and less severe UPDRS I nonmotor symptoms than the ICD + both group (−3.4 to −0.8 difference). The ICD + both group had the greatest symptoms of anxiety and depression, followed by the ICD + depression, ICD + apathy, and isolated ICD groups (table 5). That group also had significantly greater apathy symptoms than all other groups and significantly greater ICD symptoms than the isolated ICD group (3.1–13.2 difference). The frequency of specific ICD subtypes for each group is illustrated in figure 2. The only significant between-group difference in the prevalence of specific ICDs was in rates of hobbyism/punding, which was lowest in the isolated ICD group and highest in the ICD + depression and ICD + both groups.
Table 5.
Comparison of ICD subgroups with and without comorbid apathy and depression
Figure 2. Prevalence of ICD subtypes across mood and motivation subgroups.
Table 4 gives the number of participants in each group. ICD = impulse control disorder
Finally, hierarchical linear regression was used to identify predictors of apathy (AS total) and ICD (QUIP-RS total) symptoms. In the first model predicting AS total scores (adjusted R2 95% CI 0.04–0.11), age (coefficient 95% CI, 0.01–0.13) and disease duration (coefficient 0.24–0.44) emerged as significant predictors. With the addition of block 2 in the second model (adjusted R2 95% CI 0.41–0.52), age (coefficient 0.04–0.13), sex (coefficient −2.14 to −0.28), disease duration (coefficient 0.06–0.23), and BDI-II scores (coefficient 0.50–0.63) were all significant predictors of AS total scores. This combination of variables accounted for a significantly greater amount of variance in AS total scores than demographic and disease variables alone.
In the first model predicting QUIP-RS total scores (adjusted R2 95% CI 0.02–0.09), only disease duration (coefficient 95% CI 0.35–0.72) was identified as a significant predictor while a trend was observed for age (coefficient −0.21 to 0.01). With the addition of block 2 in the second model (adjusted R2 95% CI 0.25–0.37), disease duration lost significance and age remained a trend, while sex (coefficient −5.20 to −1.44), BAI total score (coefficient 0.32–0.56), and BDI-II total score (coefficient 0.25–0.52) were significant predictors of QUIP-RS total scores. This combination of variables accounted for a significantly greater amount of variance in QUIP-RS total scores than demographic and disease variables alone.
Discussion
We found the overall prevalence of apathy to be 41.3%, with nearly half of these individuals (≈18% of the total sample) considered to have an isolated apathy syndrome without clinically significant depressive symptoms. Both values fall within the range previously reported from a large meta-analysis showing an overall prevalence of 39.8% for apathy, with more than half (56.5%) of these patients having concomitant depression.4 This explains in part the substantial variability in apathy rates across studies and highlights the importance of distinguishing symptoms of apathy in the context of depression from an isolated apathy syndrome.
In addition to apathy, ICDs were not uncommon in our cohort, with an overall prevalence of 27.6%. The true prevalence of ICDs in PD is highly contested, with prior reports ranging from 3.5% to 39%, depending on a number of demographic, disease, and treatment characteristics.9 In contrast to previous studies, our participants completed the QUIP-RS on a computer at their convenience before their clinic appointment rather than in a clinic environment where admitting these problems first-hand may be considered embarrassing.6
We identified a sizable group of patients overlooked in previous research: those with comorbid apathy and ICD. The majority (61.6%) of people reporting clinically significant ICD symptoms also reported clinically significant apathy symptoms, and more than a third of patients with elevated apathy (41.3%) also reported elevated ICD symptoms. Even after removal of patients with clinically significant depressive symptoms, these values only dropped to 36.5% and 26.4%, respectively. Several prior studies have examined patients with either ICD or apathy, but not both, under the assumption that that these problems distinctly represent either extreme of a behavioral spectrum of reward and motivation.3,15
Overall, the prevalences of ICD subtypes we report here are generally comparable to other studies.16–22 However, to the best of our knowledge, no prior research has examined the rates of ICD subtypes within distinct groups of individuals with comorbid apathy and mood disturbances in the same patient cohort. We found that the prevalence of hobbyism/punding was higher in patients with comorbid depression.
As expected, there was substantial overlap between mood and motivational disturbances in the current study. Patients with both apathy and ICD had the longest disease duration and most severe symptoms of anxiety and depression. While patients with isolated apathy had lower DADD than those with an isolated ICD, patients with both apathy and an ICD had the highest LEDD use overall and nearly double the DADD of patients with isolated apathy.
The relationship between dopaminergic medication and motivation disorders has been extensively discussed,9 although studies have not considered isolated motivational disorders. As we have shown, the previous finding that people with ICDs use more dopamine agonists applies specifically to the isolated ICD group. Similarly, the finding that people with apathy use fewer dopamine agonists applies specifically to the isolated apathy group. It should be noted that while there is an extensive literature linking dopamine and motivation, the association between dopamine and motivation disorders remains unclear.9 For instance, although dopamine is thought to be a strong risk factor for ICDs, some studies have concluded that other factors are even stronger21,23 or that dopamine is not associated at all.24–27
The largest single category across all groups was the complete overlap of ICD, apathy, anxiety, and depression (figure 1). This group of patients with both mood and motivational disorders tended to comprise patients with more advanced PD with greater nonmotor symptoms. Although much has been learned about the neurobiological mechanisms underlying disturbances in mood and motivated behavior, the developmental interplay of these psychiatric symptoms over the course of the disease remains unclear.
The combination of age, sex, disease duration, and depression significantly predicted the severity of apathy symptoms, with an older age, male sex, longer disease duration, and more severe depressive symptoms associated with more severe symptoms of apathy. This association for age has been widely noted3,13,28–32; however, fewer studies have shown a significant dependency of disease duration or sex on apathy symptoms.3,14,29,33,34 For ICDs, there was a tendency for younger age and more severe anxiety and depression to be associated with more severe symptoms of ICDs. While our results add to a body of literature suggesting that patients with younger-onset PD have increased risk of ICD,16,35–38 it is important to recognize that although patients with an isolated ICD tend to be younger, patients presenting with both apathy and ≥1 ICDs tend to be older. Thus, clinicians could consider screening for both mood and motivational disturbances in routine clinical practice, regardless of the patient's age.
The motivational spectrum is a traditional framework conceptualizing apathy and impulsivity as either a deficiency or surplus, respectively, of motivation and reward sensitivity.39 Overall, the present findings are inconsistent with the current spectrum view of motivational disturbances and may be suggestive of shared underlying pathology. These symptom dimensions interact in clinically meaningful ways both with each other and with comorbid mood symptoms. Conceptualizing disorders of motivation on a single axis of dysfunction (i.e., as opposite extremes of a dopamine-dependent spectrum of motivated behavior) is too simplistic to account for the heterogeneous clinical manifestations and overlapping elements of these conditions.39
Although our data are across participants, ICD appears to occur at a younger age than apathy, and people who have both ICD and apathy are similar in age to people with apathy alone. This suggests that these behaviors may represent compensatory processes. By analogy, people with addiction cycle through stages of reward anticipation, leading to binging and intoxication, followed by a withdrawal or negative affect.40 It may be the case that isolated ICDs are more common in younger patients early in the disease course due to a dopaminergic overdose effect in the relatively well-preserved ventral striatum with the introduction of medications, specifically dopamine agonists. Overactive mesolimbic projections may then begin to normalize as the disease progresses to involve the ventral tegmental area. Reduced dopaminergic signaling associated with normal aging could be compounded by disease progression and possibly addiction-related neurobiological changes, thereby producing a profound apathy syndrome despite a potentially ongoing impulsivity syndrome.
In support of this model, total dopaminergic medication use did not fully stratify individuals with ICD or apathy. Rather, total dopaminergic medication use appeared to increase alongside disease progression, as would be expected, and was highest in people with both ICD and apathy. With regard to dopamine agonists, people with isolated apathy and neither apathy nor ICD had lower use whereas people with either ICD or both ICD and apathy had higher use, without significant differences within those groups. Apathy has been previously linked to an underdosing of dopamine,41 although this result is clearly affected by the presence of ICDs. That is, we found no difference in dopamine agonist use between people with apathy and people with neither apathy nor ICD. These results therefore do not strictly support a relationship between dopamine agonist use and isolated apathy, which is also reflected in our correlation results. In addition, people with both ICD and apathy showed total dopamine agonist use similar to that of people with isolated ICD. Apathy can persist even in the context of high dopamine use. Thus, with regard to both DADD medication use and total dopaminergic medication use, ICD and apathy also cannot be strictly considered opposites on a spectrum of dopamine use.
Although more work is needed to determine whether these conditions overlap from a neurophysiologic standpoint, clinicians should recognize that the occurrence of apathy does not preclude the co-occurrence of ICDs and vice versa. Future studies investigating the mechanism of these neuropsychiatric conditions should include people with both isolated and comorbid motivational disturbances.
We note that this study has several important limitations. First, this is a single-center, cross-sectional study; however, our sample size is quite large. Second, we did not exclude patients with MMSE scores below the recommended cutoff for possible cognitive dysfunction. The majority of our sample obtained MMSE scores within normal limits, which can be atypical for moderate or severe PD patient populations. In addition, despite the wide range of MMSE scores (i.e., 8–30) in the current study, we were unable to ascertain the prevalence of dementia due to the nature of the MMSE as a cognitive screening measure and the lack of more comprehensive neuropsychological assessment data necessary to diagnose dementia. We also did not assess fatigue, which is another common nonmotor symptom of PD often confused with apathy and also closely linked to both depression and cognitive impairment. Further research is needed to clarify the relationship and pathophysiologic underpinnings of these neuropsychiatric symptoms, which are multifactorial and may have important implications for treatment.
It is also important to note that although we used well-validated self-report questionnaires routinely administered in clinical practice, these measures are not diagnostic per se, and mood or motivational disturbances were not confirmed with clinical interview. Lastly, the mode in which self-report questionnaires were administered in the current study may have influenced patient responding. More specifically, our online mode of administration may have provided patients with a sense of anonymity or could have otherwise been perceived as a safer environment, thereby enhancing the willingness of patients to endorse potentially embarrassing symptoms of mood and motivational disorders. Conversely, this method of administration also may have led to underreporting of symptom, because patients may be in denial or simply lack insight into their condition and thereby fail to endorse symptoms that could have potentially been unearthed with follow-up questioning by a clinician or open discussion in the presence of family members. Despite these limitations, our work is highly applicable to the numerous institutions that use these standardized screening instruments in routine clinical practice.
Acknowledgment
The authors thank their funding sources, the University of Florida INFORM database team, and the Norman Fixel Institute for Neurological Diseases, as well as all the participants in this study.
Glossary
- AS
Apathy Scale
- BAI
Beck Anxiety Inventory
- BDI-II
Beck Depression Inventory-II
- CI
confidence interval
- DADD
dopamine agonist daily dose
- ICD
impulse control disorder
- LEDD
levodopa equivalency daily dose
- MMSE
Mini-Mental State Examination
- PD
Parkinson disease
- QUIP-RS
Questionnaire for Impulsive-Compulsive Disorders–Rating Scale
- UPDRS
Unified Parkinson's Disease Rating Scale
Appendix. Authors
Footnotes
Editorial, page 893
Podcast: NPub.org/jffn80
Study funding
This work is supported by the Norman Fixel Institute for Neurological Diseases, the University of Florida Pruitt Family Endowed Faculty Fellowship, the University of Florida Parkinson's Foundation Center of Excellence, the NIH/National Center for Advancing Translational Sciences Clinical and Translational Science Awards to the University of Florida (awards UL1TR001427, KL2TR001429, and TL1TR001428), the National Institute of Neurological Disorders and Stroke (NINDS) award F30NS111841, and the NINDS T32-NS082168 Pre-Doctoral Interdisciplinary Training Program in Movement Disorders and Neurorestoration. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Mazzoni P, Hristova A, Krakauer JW. Why don't we move faster? Parkinson's disease, movement vigor, and implicit motivation. J Neurosci 2007;27:7105–7116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Houeto JL, Magnard R, Dalley JW, Belin D, Carnicella S. Trait impulsivity and anhedonia: two gateways for the development of impulse control disorders in Parkinson's disease? Front Psychiatry 2016;7:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leroi I, Andrews M, McDonald K, et al. Apathy and impulse control disorders in Parkinson's disease: a direct comparison. Parkinsonism Relat Disord 2012;18:198–203. [DOI] [PubMed] [Google Scholar]
- 4.den Brok MG, van Dalen JW, van Gool WA, Moll van Charante EP, de Bie RM, Richard E. Apathy in Parkinson's disease: a systematic review and meta-analysis. Mov Disord 2015;30:759–769. [DOI] [PubMed] [Google Scholar]
- 5.Alvarado-Bolaños A, Cervantes-Arriaga A, Rodríguez-Violante M, et al. Impact of neuropsychiatric symptoms on the quality of life of subjects with Parkinson's disease. J Parkinsons Dis 2015;5:541–548. [DOI] [PubMed] [Google Scholar]
- 6.Papay K, Mamikonyan E, Siderowf AD, et al. Patient versus informant reporting of ICD symptoms in Parkinson's disease using the QUIP: validity and variability. Parkinsonism Relat Disord 2011;17:153–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maréchal E, Denoiseux B, Thys E, Crosiers D, Pickut B, Cras P. Impulse control disorders in Parkinson's disease: an overview from neurobiology to treatment. J Neurol 2015;262:7–20. [DOI] [PubMed] [Google Scholar]
- 8.Corvol JC, Artaud F, Cormier-Dequaire F, et al. Longitudinal analysis of impulse control disorders in Parkinson disease. Neurology 2018;91:e189–e201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eisinger RS, Ramirez-Zamora A, Carbunaru S, et al. Medications, deep brain stimulation, and other factors influencing impulse control disorders in Parkinson's disease. Front Neurol 2019;10:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol 1988;56:893–897. [DOI] [PubMed] [Google Scholar]
- 11.Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- 12.Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–198. [DOI] [PubMed] [Google Scholar]
- 13.Starkstein SE, Mayberg HS, Preziosi TJ, Andrewzejewski P, Leiguarda R, Robinson RG. Reliability, validity, and clinical correlates of apathy in Parkinson's disease. J Neuropsychiatry Clin Neurosci 1992;4:134–139. [DOI] [PubMed] [Google Scholar]
- 14.Weintraub D, Mamikonyan E, Papay K, Shea JA, Xie SX, Siderowf A. Questionnaire for impulsive-compulsive disorders in Parkinson's disease-rating scale. Mov Disord 2011;27:242–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thobois S, Ardouin C, Lhommée E, et al. Non-motor dopamine withdrawal syndrome after surgery for Parkinson's disease: predictors and underlying mesolimbic denervation. Brain 2010;133:1111–1127. [DOI] [PubMed] [Google Scholar]
- 16.Antonini A, Barone P, Bonuccelli U, Annoni K, Asgharnejad M, Stanzione P. ICARUS study: prevalence and clinical features of impulse control disorders in Parkinson's disease. J Neurol Neurosurg Psychiatry 2017;88:317–324. [DOI] [PubMed] [Google Scholar]
- 17.Erga AH, Alves G, Larsen JP, Tysnes OBR, Pedersen KF. Impulsive and compulsive behaviors in Parkinson's disease: the Norwegian ParkWest Study. J Parkinsons Dis 2017;7:183–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bastiaens J, Dorfman BJ, Christos PJ, Nirenberg MJ. Prospective cohort study of impulse control disorders in Parkinson's disease. Mov Disord 2013;28:327–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miyasaki JM, Al Hassan K, Lang AE, Voon V. Punding prevalence in Parkinson's disease. Mov Disord 2007;22:1179–1181. [DOI] [PubMed] [Google Scholar]
- 20.Evans AH, Katzenschlager R, Paviour D, et al. Punding in Parkinson's disease: its relation to the dopamine dysregulation syndrome. Mov Disord 2004;19:397–405. [DOI] [PubMed] [Google Scholar]
- 21.Callesen MB, Weintraub D, Damholdt MF, Møller A. Impulsive and compulsive behaviors among Danish patients with Parkinson's disease: prevalence, depression, and personality. Parkinsonism Relat Disord 2014;20:22–26. [DOI] [PubMed] [Google Scholar]
- 22.Biundo R, Weis L, Abbruzzese G, et al. Impulse control disorders in advanced Parkinson's disease with dyskinesia: the ALTHEA study. Mov Disord 2017;2:1557–1565. [DOI] [PubMed] [Google Scholar]
- 23.Joutsa J, Martikainen K, Vahlberg T, Voon V, Kaasinen V. Impulse control disorders and depression in Finnish patients with Parkinson's disease. Parkinsonism Relat Disord 2012;18:155–160. [DOI] [PubMed] [Google Scholar]
- 24.Phu AL, Xu Z, Brakoulias V, et al. Effect of impulse control disorders on disability and quality of life in Parkinson's disease patients. J Clin Neurosci 2014;21:63–66. [DOI] [PubMed] [Google Scholar]
- 25.Tanaka K, Wada-Isoe K, Nakashita S, Yamamoto M, Nakashima K. Impulsive compulsive behaviors in Japanese Parkinson's disease patients and utility of the Japanese version of the Questionnaire for Impulsive-Compulsive Disorders in Parkinson's Disease. J Neurol Sci 2013;331:76–80. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Y, He AQ, Li L, Chen W, Liu ZG. Clinical characteristics of impulse control and related disorders in Chinese Parkinson's disease patients. BMC Neurol 2017;17:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Riley M, Bakeberg M, Byrnes M, et al. Demographic and clinical predictors of trait impulsivity in Parkinson's disease patients. Park Dis 2018;2018:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oguru M, Tachibana H, Toda K, Okuda B, Oka N. Apathy and depression in Parkinson disease: J Geriatr Psychiatry Neurol 2009;23:35–41. [DOI] [PubMed] [Google Scholar]
- 29.Ziropadja Lj, Stefanova E, Petrovic M, Stojkovic T, Kostic VS. Apathy and depression in Parkinson's disease: the Belgrade PD study report. Parkinsonism Relat Disord 2012;18:339–342. [DOI] [PubMed] [Google Scholar]
- 30.Benito-León J, Cubo E, Coronell C, Group AS. Impact of apathy on health-related quality of life in recently diagnosed Parkinson's disease: the ANIMO study. Mov Disord 2012;27:211–218. [DOI] [PubMed] [Google Scholar]
- 31.Starkstein SE, Merello M, Jorge R, Brockman S, Bruce D, Power B. The syndromal validity and nosological position of apathy in Parkinson's disease. Mov Disord 2009;24:1211–1216. [DOI] [PubMed] [Google Scholar]
- 32.Rodríguez-Violante M, González-Latapi P, Cervantes-Arriaga A, Martinez-Ramirez D, Velázquez-Osuna S, Camacho-Ordoñez A. Apathy and associated factors in Mexican patients with Parkinson's disease. Neurol Sci 2014;35:729–734. [DOI] [PubMed] [Google Scholar]
- 33.Laatu S, Karrasch M, Martikainen K, Marttila R. Apathy is associated with activities of daily living ability in Parkinson's disease. Dement Geriatr Cogn Disord 2013;35:249–255. [DOI] [PubMed] [Google Scholar]
- 34.Pedersen KF, Alves G, Aarsland D, Larsen JP. Occurrence and risk factors for apathy in Parkinson disease: a 4-year prospective longitudinal study. J Neurol Neurosurg Psychiatry 2009;80:1279–1282. [DOI] [PubMed] [Google Scholar]
- 35.Poletti M, Logi C, Lucetti C, et al. A single-center, cross-sectional prevalence study of impulse control disorders in Parkinson disease: association with dopaminergic drugs. J Clin Psychopharmacol 2013;33:691–694. [DOI] [PubMed] [Google Scholar]
- 36.Valença GT, Glass PG, Negreiros NN, et al. Past smoking and current dopamine agonist use show an independent and dose-dependent association with impulse control disorders in Parkinson's disease. Parkinsonism Relat Disord 2013;19:698–700. [DOI] [PubMed] [Google Scholar]
- 37.Djamshidian A, O'Sullivan SS, Wittmann BC, Lees AJ, Averbeck BB. Novelty seeking behaviour in Parkinson's disease. Neuropsychologia 2011;49:2483–2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rossi M, Gerschcovich ER, Achaval DDe, et al. Decision‐making in Parkinson's disease patients with and without pathological gambling. Eur J Neurol 2010;17:97–102. [DOI] [PubMed] [Google Scholar]
- 39.Sinha N, Manohar S, Husain M. Impulsivity and apathy in Parkinson's disease. J Neuropsychol 2013;7:255–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology 2001;24:97–129. [DOI] [PubMed] [Google Scholar]
- 41.Czernecki V, Schüpbach M, Yaici S, et al. Apathy following subthalamic stimulation in Parkinson disease: a dopamine responsive symptom. Mov Disord 2008;23:964–969. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Raw data were collected at the University of Florida's Norman Fixel Institute for Neurological Disease. Derived data supporting the findings of this study are available from the corresponding author on request.