Abstract
Objectives
To assess the frequency of transient orthostatic hypotension (tOH) and its clinical impact in Parkinson disease (PD), we retrospectively studied 173 patients with PD and 173 age- and sex-matched controls with orthostatic intolerance, who underwent cardiovascular autonomic function testing under continuous noninvasive blood pressure (BP) monitoring.
Methods
We screened for tOH (systolic BP fall ≥20 mm Hg or diastolic ≥10 mm Hg resolving within the first minute upon standing) and classic OH (cOH, sustained systolic BP fall ≥20 mm Hg or diastolic ≥10 mm Hg within 3 minutes upon standing). In patients with PD, we reviewed the medical records of the 6 months preceding and following autonomic testing for history of falls, syncope, and orthostatic intolerance.
Results
tOH occurred in 24% of patients with PD and 21% of controls, cOH in 19% of patients with PD and in none of the controls, independently of any clinical–demographic or PD-specific characteristic. Forty percent of patients with PD had a history of falls, in 29% of cases due to syncope. Patients with PD with history of orthostatic intolerance and syncope had a more severe systolic BP fall and lower diastolic BP rise upon standing, most pronounced in the first 30–60 seconds.
Conclusions
tOH is an age-dependent phenomenon, which is at least as common as cOH in PD. Transient BP falls when changing to the upright position may be overlooked with bedside BP measurements, but contribute to orthostatic intolerance and syncope in PD. Continuous noninvasive BP monitoring upon standing may help identify a modifiable risk factor for syncope-related falls in parkinsonian patients.
Cardiovascular autonomic failure is a common nonmotor feature of Parkinson disease (PD).1,2 Orthostatic hypotension (OH) is its hallmark sign and is characterized by a sustained blood pressure (BP) fall within 3 minutes upon standing.3,4 OH typically manifests with recurrent syncope and orthostatic intolerance (lightheadedness, blurred vision, or tremulousness upon standing) and has a significant impact on daily life in patients with PD.5–7
Different groups reported that OH increases the risk of falls in PD,8–19 while others did not.20–23 This suggests that additional factors may contribute to the symptomatic burden of cardiovascular autonomic failure in PD.
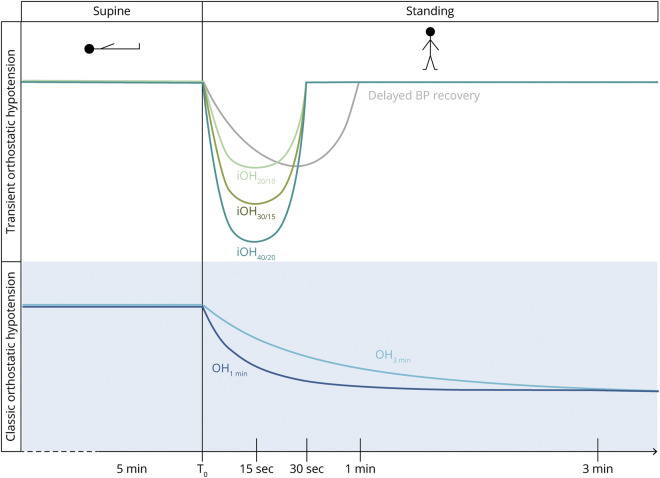
Besides classic OH (cOH), transient forms of OH (tOH) have been described (figure 1).4,24 Initial OH consists of a profound BP fall right after standing up, which resolves within 30 seconds.4 It manifests with transient, severe lightheadedness and blurred vision and represents an underrecognized cause of syncope in younger adults.24 Failure to stabilize the BP back to baseline values within the first 30 seconds upon standing, a phenomenon called delayed BP recovery, occurs in up to 30% of elderly individuals and has been associated with falls, orthostatic intolerance, and frailty.25–27
Figure 1. Overview of abnormal blood pressure (BP) changes upon standing.
Different types of transient forms of orthostatic hypotension (tOH) (upper panel) and classic orthostatic hypotension (cOH) (lower panel). iOH40/20, iOH30/15, iOH20/10: initial orthostatic hypotension (iOH) with systolic BP fall ≥40/30/20 mm Hg or diastolic >20/15/10 mm Hg within 15 seconds upon standing, recovering at 30 seconds; delayed BP recovery: systolic BP fall ≥20 mm Hg at 30 seconds upon standing, but not meeting the criteria for cOH; OH1min, OH3min: sustained orthostatic BP fall ≥20 mm Hg systolic or ≥10 mm Hg diastolic within 1 or 3 minutes upon standing.
We determined the frequency of tOH in patients with PD vs age- and sex-matched controls with orthostatic intolerance. In patients with PD, we assessed the relationship of tOH with history of falls, syncope, and orthostatic intolerance and retrospectively reviewed the therapeutic measures adopted for tOH.
Methods
Recruitment
We retrospectively screened all patients who underwent cardiovascular autonomic function testing at the Medical University of Innsbruck between March 2007 and March 2020.
Inclusion criteria were diagnosis of PD28,29 or PD with dementia30 at last available neurologic follow-up, completed standing test during autonomic function testing, and detailed medical records available, including at least 1 visit in the 6 months preceding or following the autonomic tests.
Exclusion criteria were diabetes mellitus, other major neurologic diseases (polyneuropathies, stroke, epilepsy), treatment with neuroleptics in a dose and time course consistent with drug-induced parkinsonism, low quality of biosignals at autonomic function testing, missing or incomplete medical records, less than 5 years disease duration at the time of cardiovascular autonomic function testing, and less than 12 months follow-up available.
Patients with PD were matched 1:1 for age and sex with patients referred in the same time frame for orthostatic intolerance to the autonomic function laboratory, who fulfilled the same inclusion and none of the exclusion criteria, but had a negative neurologic history.
Standard protocol approvals, registrations, and patient consents
Due to the retrospective and noninterventional nature of the study, the protocol was not required by Austrian law to receive approval by the local ethical committee and no written informed consent was needed. The study was performed in accordance with the Declaration of Helsinki and followed the current European regulation for data protection.
Clinical–demographic data set
For each patient, the following information was collected from the medical records contemporary to autonomic function testing:
Demographic characteristics: age, sex
PD-related characteristics: disease duration, Hoehn & Yahr (H&Y) stage, presence of postural instability (>3 steps backwards on pull test, tendency to fall on pull test, spontaneous falls or near falls unrelated to syncope or presyncope),31,32 akinetic-rigid parkinsonian phenotype, abnormal postures (including antecollis, camptocormia, and Pisa syndrome), l-dopa fluctuations (“off” phenomena and dyskinesias), freezing of gait, use of assistive devices (rollator, walkers, stocks, or crutches), number of follow-up months between cardiovascular autonomic function testing and last-available follow-up
Comorbidities: cardiovascular disease (including heart failure, cardiac arrhythmias, coronary artery disease, valvular hearth disease) and hypertension
PD treatments: dopaminergic medication intake, l-dopa equivalent daily dose (LEDD) calculated according to Tomlinson et al.,33 deep brain stimulation
Other medications: use of other CNS-acting drugs (including benzodiazepines, antidepressants, neuroleptics, amantadine and anticholinergic drugs), antihypertensive or antihypotensive agents, total number of drugs
In patients with PD, we reviewed the medical records of the 6 months preceding and following autonomic function testing for history of falls, syncope, and orthostatic intolerance. We defined the following:
Fall: an event that resulted in the patient coming to rest inadvertently on the ground or floor or other lower level (bit.ly/2XkvsPa).
Syncope: transient loss of consciousness, characterized by rapid onset, short duration, and spontaneous complete recovery4; additional requirements were amnesia for the event by the patient or loss of responsivity during the event ascertained by the caregiver; we also filed the position in which syncope had occurred (standing, sitting, or supine).
Orthostatic intolerance: symptoms of end-organ hypoperfusion, including weakness, nausea, tremulousness, blurred vision, headache, or “coat-hanger pain” (pain in the neck and shoulder region) developing upon standing34 and ameliorated by lying down, but not fulfilling the criteria for syncope.
Cardiovascular autonomic function tests
All cardiovascular autonomic function tests were performed in a quiet room, with a mean temperature of 22°C, mostly between 8:30 am and 12:00 pm and on regular medication. ECG was recorded with 4 peripheral electrodes. We monitored BP with noninvasive beat-to-beat finger-cuff plethysmography, with the left hand fixed at heart level and calibrated with oscillometric arm cuff BP measurements at the end of the supine phase and at regular intervals during the orthostatic challenge (Task Force Monitor, CNSystems, Graz, Austria).
The standing test is part of the standard autonomic test battery in our center and consists of 5 minutes of rest in the supine position, followed by 5 minutes of active standing. For the purposes of the present study, we manually controlled all examinations for artifacts and missing signals prior to the analysis. A locally developed software sampled the lowest systolic and diastolic BP values and the highest heart rate reached within 15 seconds upon standing and calculated the heart rate and systolic and diastolic BP values by averaging 15 consecutive values at the 5th minute supine, 1st and 3rd minute upon standing, and by averaging 5 consecutive values at the 30th second upon standing.
Where available, heart rate and BP counter-regulation during the Valsalva maneuver and the deep breathing ratio were calculated following the methodology described elsewhere.35,36
Definitions
We applied a 4-tier definition of tOH (figure 1, upper panel):
Initial OH (iOH40/20): systolic BP fall ≥40 mm Hg or diastolic ≥20 mm Hg within 15 seconds upon standing with recovery within 30 seconds4
Initial OH 30/15 (iOH30/15): systolic BP fall ≥30 mm Hg or diastolic ≥15 mm Hg within 15 seconds of standing with recovery within 30 seconds
Initial OH 20/10 (iOH20/10): systolic BP fall ≥20 mm Hg or diastolic ≥10 mm Hg within 15 seconds of standing with recovery within 30 seconds
Delayed BP recovery: systolic BP values ≥20 mm Hg below baseline 30 seconds after standing with recovery within 1 minute24
Sustained orthostatic BP falls were defined as follows (figure 1, lower panel):
Classic OH (cOH3min): sustained systolic BP fall ≥20 mm Hg or diastolic ≥10 mm Hg within 3 minutes upon standing3
Classic OH 1 minute (cOH1min): sustained systolic BP fall ≥20 mm Hg or diastolic ≥10 mm Hg developing already 1 minute after standing up
Therapeutic measures for tOH in PD
In patients with PD diagnosed with tOH at cardiovascular autonomic function testing, we reviewed the medical record following autonomic testing for the therapeutic approach adopted and the subsequent follow-up visit for the patient's reported outcome.
Statistical analysis
We reported qualitative variables by frequency (percentage) and compared them with the Pearson χ2 test (or Fisher exact test, where appropriate). We summarized quantitative variables by median (1st quartile; 3rd quartile), tested for normal distribution with the Kolmogorov-Smirnov test (or the Shapiro-Wilk test, if n <50), and investigated for differences between groups by using the t test (or analysis of variance [ANOVA], if >2 groups) and the Mann-Whitney U test (or the Kruskal-Wallis test, if >2 groups), according to their distribution. We applied the Benjamini-Hochberg correction to multiple comparisons.37
We assessed the overall frequency of orthostatic BP dysregulation, tOH, and cOH in patients with PD vs controls with a logistic regression analysis adjusted for possible confounding factors highlighted by the univariate analysis. In patients with PD, we investigated the clinical demographic factors associated with history of falls, syncope, and orthostatic intolerance in a univariate fashion, followed by binary logistic regression analysis and calculation of the receiver operating characteristic (ROC) curve. Finally, we applied an ANOVA for repeated measurements adjusted for the LEDD to investigate the orthostatic heart rate and BP behavior of patients with PD with and without history of falls, syncope, and orthostatic intolerance. We performed the statistical analysis with IBM (Armonk, NY) SPSS Statistics version 24.0 and considered 2-sided p values <0.05 statistically significant.
Data availability
The first and the last authors take full responsibility for the integrity of data and agree to share any data not published within this article upon reasonable request from any qualified investigator.
Results
Study population
We screened 291 patients with PD (55 with dementia [PDD]) and excluded 118 of them because of matching one or more exclusion criteria. We included 173 patients with PD (144 PD, 29 PDD) and 173 age- and sex-matched controls with orthostatic intolerance in the final analysis. In patients with PD, the median follow-up time between autonomic function testing and the last available visit was 46 (21; 78) months and confirmed the neurologic diagnosis in all of them. Table 1 shows the clinical–demographic characteristics of the study population. Patients with PD had on average a higher number of regular medications (p < 0.001), were more frequently on CNS-acting drugs other than dopaminergic medications (p < 0.001), and on antihypotensive agents (p = 0.003) than controls. Hypertension (p = 0.003) and antihypertensive regimens were more frequent in the control group (p = 0.010).
Table 1.
Clinical–demographic characteristics of the patients with Parkinson disease (PD) and of the age- and sex-matched controls with orthostatic intolerance
Frequency of orthostatic BP dysregulation in PD vs controls
Seventy-one (41%) patients with PD and 37 (21%) controls showed a pathologic BP regulation upon standing. After adjusting for the total number of drugs and use of antihypotensive, antihypertensive, and CNS-acting drugs other than dopaminergic medications, patients with PD showed a 2.0 odds ratio (OR; 95% confidence interval [CI] 1.2–3.5; p = 0.013) for any form of orthostatic BP dysregulation.
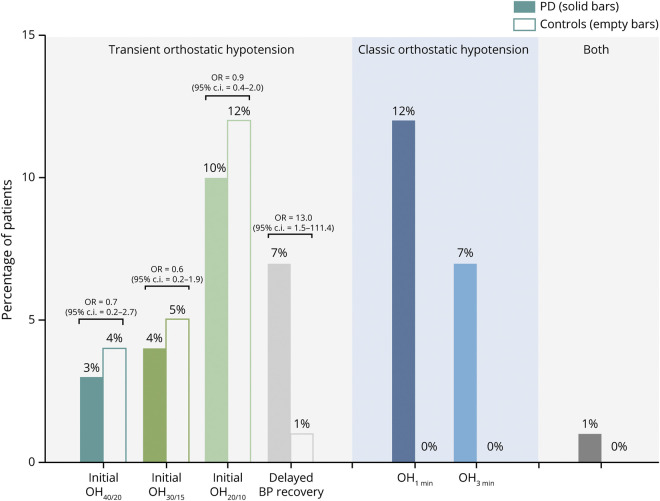
tOH occurred in 41 (24%) patients with PD and 37 (21%) controls (OR 1.1 [95% CI 0.6–2.0], p = 0.759). The frequency of iOH40/20, iOH30/15, and iOH20/10 did not differ between patients with PD and controls, but patients with PD showed a significantly higher frequency of delayed orthostatic BP recovery (p = 0.019; figure 2). cOH was present in 32 (19%) patients with PD, but in none of the controls: 20 patients with PD (12%) fulfilled the OH criteria already after the first minute upon standing (OH1min). The combination of both tOH and cOH occurred in 2 patients with PD (1%) and no controls.
Figure 2. Frequency of abnormal orthostatic blood pressure (BP) changes in patients with Parkinson disease (PD) vs controls.
Odds ratios (ORs) were calculated after adjusting for potential confounding factors highlighted by the univariate analysis reported in table 1. CI = confidence interval; iOH = initial orthostatic hypotension; OH = orthostatic hypotension.
Patients with PD with tOH showed no difference in the clinical–demographic or PD-specific characteristics vs those with cOH or with a normal BP counterregulation upon standing. Also among the controls, we observed no difference in the clinical–demographic characteristics of patients with tOH vs those without.
Cardiovascular autonomic function indices in PD vs controls
A Valsalva maneuver was available in 117 patients with PD, deep breathing in 140. The frequency of pathologic age-adjusted Valsalva ratio, deep breathing ratio, or missing BP overshoot in phase IV of the Valsalva maneuver did not differ between patients with PD with or without orthostatic BP dysregulation or between patients with PD with tOH vs cOH.
Among the control patients, 120 had had a Valsalva maneuver and 118 a deep breathing. The frequency of altered cardiovascular autonomic function indices did not differ between patients with tOH vs those without.
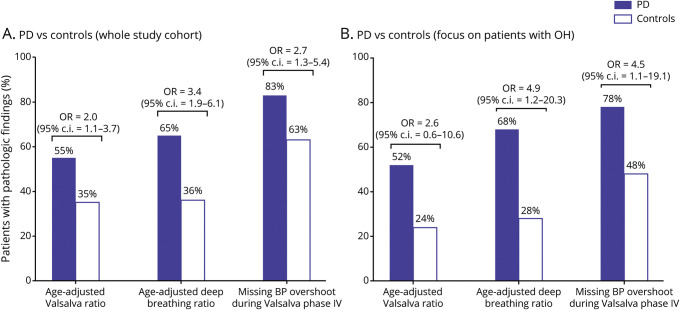
After adjusting for possible confounding factors (table 1), the PD group more often had altered cardiovascular autonomic function indices than the controls (figure 3A). Also, when focusing on tOH-positive cases only, patients with PD showed a significantly higher frequency of pathologic deep breathing ratio and missing BP overshoot at the end the Valsalva maneuver than controls (figure 3B).
Figure 3. Cardiovascular autonomic function tests in patients with Parkinson disease (PD) vs controls.
Frequency of pathologic cardiovascular autonomic function indices in the overall PD and control population (A) and in the transient orthostatic hypotension (OH)–positive PD vs control cases (B). Odds ratios (ORs) were calculated after adjusting for potential confounding factors highlighted by the univariate analysis reported in table 1. BP = blood pressure; CI = confidence interval.
History of falls in PD
Sixty-nine patients with PD (40%) experienced falls in the 6 months preceding or following cardiovascular autonomic function testing. At univariate analysis (table 2), history of falls was associated with longer disease duration (p < 0.001), more advanced H&Y stage (p < 0.001), presence of postural instability (p < 0.001), akinetic-rigid phenotype (p = 0.008), l-dopa fluctuations (p = 0.002), freezing of gait (p < 0.001), use of assistive devices (p < 0.001), higher LEDD (p = 0.002), and history of syncope (p < 0.001). The multivariate analysis confirmed an association of falls with postural instability (OR 2.1 [95% CI 1.0–4.2], p = 0.041), freezing of gait (OR 3.1 [95% CI 1.3–7.5], p = 0.012), and history of syncope (OR 43.9 [95% CI 8.7–220.3], p < 0.001). The area under the ROC curve was 0.863 (95% CI 0.8–0.9).
Table 2.
Univariate analysis of clinical-demographic characteristics associated with falls, syncope, and orthostatic intolerance in Parkinson disease (PD)
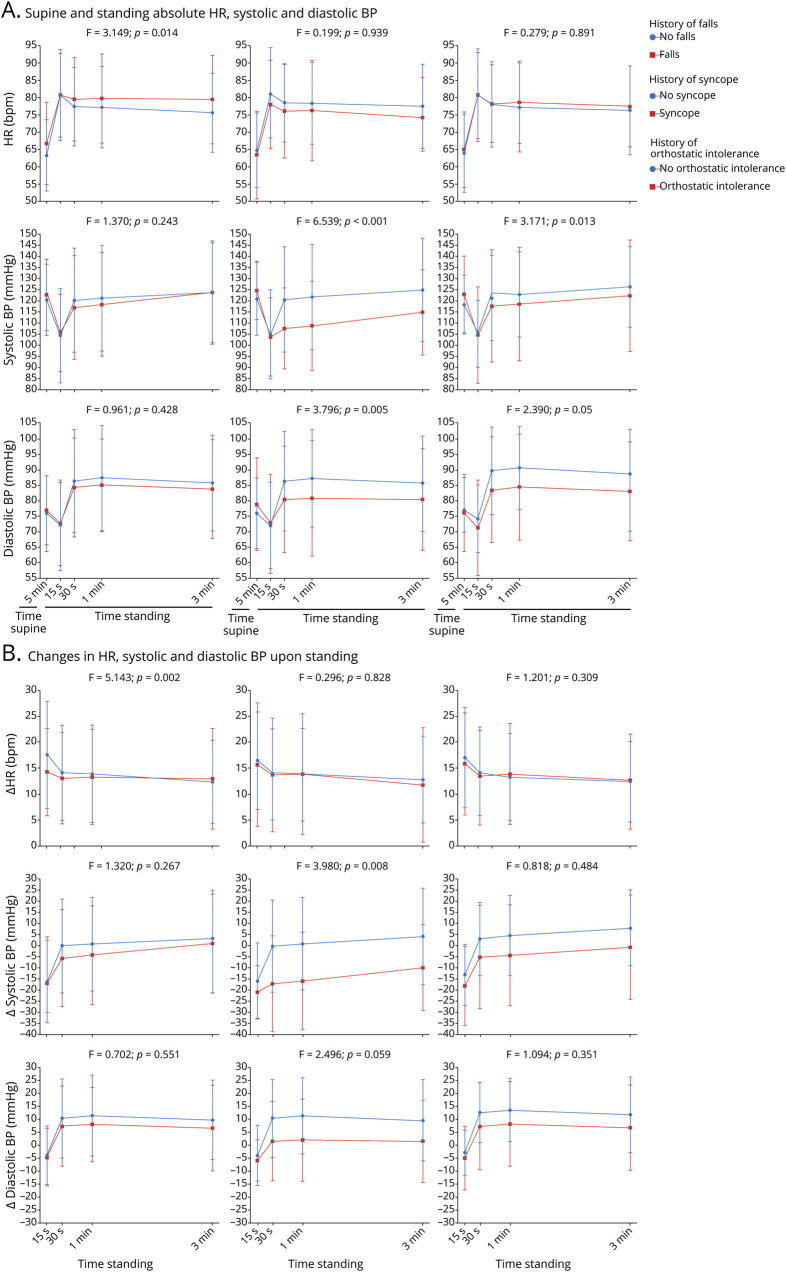
The frequency of falls did not differ between patients with PD with or without pathologic BP regulation upon standing (42% vs 38%, figure 4), nor between patients with PD with tOH vs cOH (41% vs 43%, figure 4). One of the 2 patients with both tOH and cOH had a positive history of falls.
Figure 4. Clinical milestones in patients with Parkinson disease (PD) with transient orthostatic hypotension, classic orthostatic hypotension, or no orthostatic blood pressure (BP) dysregulation.
CI = confidence interval; OR = odds ratio.
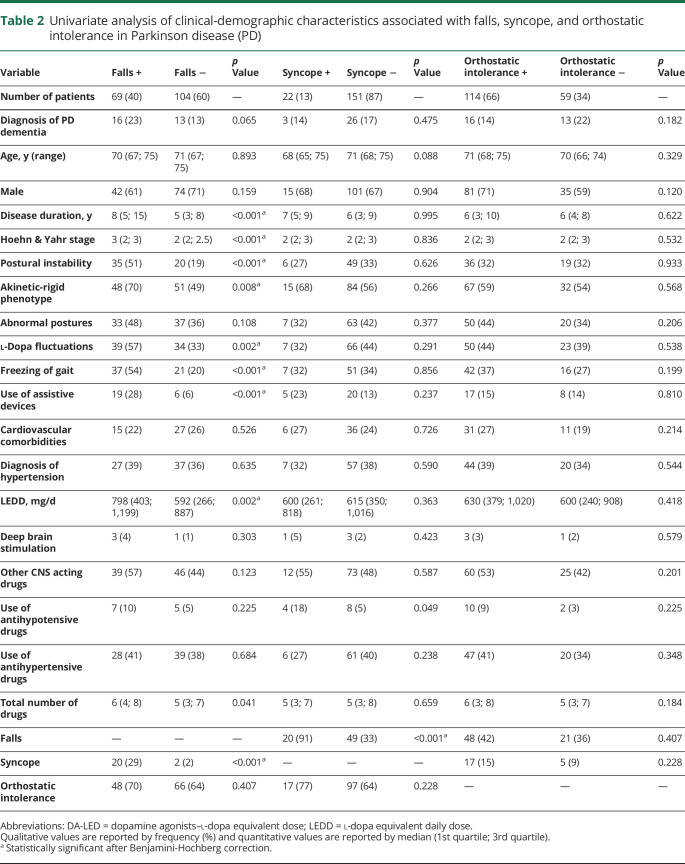
After adjusting for the LEDD, continuous heart rate and BP monitoring showed a milder heart rate increase within the first 15 seconds upon standing in patients with PD with history of falls vs those without (p = 0.033; figure 5).
Figure 5. Analysis of variance of supine and standing heart rate (HR) and systolic and diastolic blood pressure (BP).
(A) Analysis of variance of supine and standing absolute heart rate and systolic and diastolic orthostatic blood pressure values in parkinsonian patients with and without history of falls (left), syncope (middle), and orthostatic intolerance (right). (B) analysis of variance of heart rate and systolic and diastolic blood pressure changes upon standing in parkinsonian patients with and without history of falls (left), syncope (middle), and orthostatic intolerance (right).
History of syncope in PD
Syncope was documented in 22 patients with PD (13%) in the 6 months preceding or following cardiovascular autonomic function testing. Syncope occurred upon standing in all but 2 patients and was associated with falls (p < 0.001), but no other clinical, demographic, or PD-specific characteristics (table 2).
History of syncope was present in 17% of patients with PD with an altered BP regulation upon standing and in 10% of those without, not reaching statistical significance (figure 4). The frequency of syncope did not differ between patients with PD diagnosed with tOH vs cOH at cardiovascular autonomic function testing (10% vs 23%, figure 4). One of the 2 patients with both tOH and cOH had experienced syncope in the 6 months preceding autonomic testing.
Upon standing, patients with PD with history of syncope showed lower absolute systolic BP values after 30 seconds (p = 0.015) and 1 minute upon standing (p = 0.016; figure 5, left panel), and more severe systolic BP falls, most pronounced after 30 seconds (p < 0.001) and 1 minute (p = 0.001) in the upright position (figure 5, right panel). Orthostatic diastolic BP increase was missing or reduced in patients with PD with history of syncope vs those without, also most pronounced after 30 seconds (p = 0.011) and 1 minute upon standing (p = 0.006; figure 5, right panel).
History of orthostatic intolerance in PD
Orthostatic intolerance was documented in 114 patients with PD (66%), without any significant association with clinical, demographic, or PD-specific features (table 2).
The frequency of orthostatic complaints did not differ between patients with PD diagnosed with any form of orthostatic BP dysregulation vs those without (69% vs 64%, figure 4), nor between those diagnosed with tOH vs cOH (64% vs 73%, figure 4). Both patients with combined tOH and cOH had a history of orthostatic intolerance.
At continuous heart rate and BP monitoring, patients with PD with orthostatic intolerance had a greater systolic BP fall 30 seconds (p = 0.008), 1 minute (p = 0.010), and 3 minutes (p = 0.007) upon standing (figure 5, right panel). Patients with orthostatic intolerance also showed lower diastolic BP values between the first 30 seconds (p = 0.017) and 3rd minute upon standing (p = 0.021; figure 5, left panel) and a less pronounced orthostatic diastolic BP increase, most pronounced within the first minute upon standing (p = 0.014 at 30 seconds; p = 0.024 at 1 minute; figure 5, right panel).
Therapeutic approaches in patients with PD with tOH
After autonomic function testing, 18 out of 39 patients with tOH received a specific treatment for orthostatic intolerance. Nine patients received nonpharmacologic measures (elastic abdominal binder, increased water and salt intake, and head-up tilt overnight): 6 of them reported an improvement of orthostatic symptoms at the follow-up visit. In 6 patients, previously scheduled antihypertensive drugs were removed (ACE-inhibitors, diuretics or β-blockers), with symptomatic benefit in 3 of them. Three patients received adrenergic agents (midodrine or droxidopa), with subjective improvement in one.
Discussion
We found that at least one form of orthostatic BP dysregulation was present in 41% of patients with PD referred for cardiovascular autonomic function testing, independently of disease stage, medications, or presence of cardiovascular comorbidities.
To our knowledge, this is the first study specifically investigating tOH in PD. iOH40/20, as defined by the 2018 Guidelines for the Diagnosis and Management of Syncope of the European Society of Cardiology,4 occurred rarely. The observed frequency of delayed orthostatic BP recovery was also lower than previously reported for the general aging population.26,27 Patients with PD mostly showed milder transient orthostatic BP changes, that is, iOH20/10, but overall, tOH was at least as common as cOH in the present study.
When comparing our PD cohort with age- and sex-matched patients with orthostatic intolerance but negative neurologic history, we found similar frequencies of iOH, but delayed orthostatic BP recovery was significantly more frequent in patients with PD than in controls. This suggests that while iOH may represent an age-related phenomenon in PD, delayed BP recovery might be more closely related to the underlying neurodegenerative disorder.
In young adults, tOH develops after a transient mismatch between cardiac output and vascular tone when changing to the upright position due to vasodilation in the postural muscles.38 By contrast, the pathophysiology of milder forms of tOH, including delayed orthostatic BP recovery in the elderly population, is not fully understood. We found that tOH-positive patients with PD showed a higher frequency of altered cardiovascular autonomic indices with respect to tOH-positive controls. Incipient cardiovascular autonomic failure might therefore contribute to the development of tOH in PD.
In order to clarify the clinical relevance of tOH in the setting of PD, we assessed its impact on major outcomes like falls, syncope, and symptoms of orthostatic intolerance.
Beyond established PD-specific risk factors for falls, such as the presence of postural instability and freezing of gait,39 we found a strong association between falls and history of syncope, which almost invariably occurred upon standing in our cohort. The orthostatic character suggests that orthostatic BP dysregulation may have indeed represented the underlying cause of syncope and, indirectly, of fall in these patients.
Patients with orthostatic BP dysregulation showed higher frequencies of falls, syncope, and orthostatic intolerance vs those without, but neither a diagnosis of tOH nor of cOH was associated with a significantly higher risk for any of the abovementioned clinical outcome measures. In fact, OH remains asymptomatic in a substantial proportion of patients40 and additional hemodynamic and individual factors might determine the degree of cerebral hypoperfusion and functional impairment in single patients.41,42 To this end, we observed a less pronounced heart rate increase upon standing in patients with history of falls vs those without, indicating that an insufficient chronotropic response to orthostatic challenges might increase the risk of falls in PD.
In patients with history of syncope, we observed a more severe systolic BP fall, lower absolute systolic BP values, and a milder diastolic BP rise upon standing, most pronounced between 30 seconds and 1 minute after changing to the upright position. Beyond the amplitude of orthostatic BP fall, the lowest BP value reached upon standing may determine the degree of cerebral hypoperfusion in PD41 and systolic, rather than diastolic, BP changes may be more relevant in determining the onset of syncope.43 Like in the aging population, also in PD the first 30–60 seconds upon standing appear to represent a crucial time point for an appropriate stabilization of orthostatic hemodynamic responses.25
Similar to syncope, history of orthostatic intolerance was associated with a more severe systolic BP fall, lower diastolic BP values, and reduced diastolic BP rise upon standing, most pronounced during the first minute. This confirms that transient orthostatic BP falls represent an underrecognized, yet disabling, phenomenon in patients with PD.
When reviewing the therapeutic measures adopted in patients with PD with tOH, we found Class IV evidence for patient-reported symptomatic improvement after ceasing previously scheduled antihypertensive medications and introducing nonpharmacologic measures. Although very preliminary, these observations suggest that first-line therapeutic approaches used to treat cOH may prove effective for tOH as well.44–47
This study has some limitations. Due to its retrospective nature, collection of clinical information was not fully standardized, although relying on a well-structured electronic archive. Since we screened patients who underwent autonomic function tests, our study may have been biased towards an autonomic PD phenotype: the observed prevalence of OH was lower than previously reported by other groups, however.2 The fact that cOH occurred in none of the control patients also suggests that the stringent exclusion criteria applied (e.g., presence of diabetes mellitus, polyneuropathy) effectively excluded secondary causes of autonomic neuropathy in the study cohort.
Performing a supine to standing BP test with a standard arm cuff, referred to as Schellong test in German-speaking countries, is a cost- and time-effective bedside screening tool for OH in parkinsonian patients with orthostatic complaints.4,48 This does not capture transient orthostatic BP falls, which are as frequent as cOH in PD and may cause orthostatic intolerance and syncope. Performing a standing test under continuous heart rate and BP monitoring may thus help identify a modifiable cause of syncope-related falls in patients with suggestive medical history. Given its frequency and potential symptomatic impact, we suggest including tOH as an outcome measure in future interventional trials targeting OH.
Glossary
- ANOVA
analysis of variance
- BP
blood pressure
- CI
confidence interval
- cOH
classic orthostatic hypotension
- H&Y
Hoehn & Yahr
- iOH
initial orthostatic hypotension
- LEDD
l-dopa equivalent daily dose
- OH
orthostatic hypotension
- OR
odds ratio
- PD
Parkinson disease
- PDD
Parkinson disease with dementia
- ROC
receiver operating characteristic
- tOH
transient forms of orthostatic hypotension
Appendix. Authors
Footnotes
CME Course: NPub.org/cmelist
Study funding
This was an academic study without external financial support. Dr Campese received a travel bursary from the Sant’Anna School of Advanced Studies of Pisa.
Disclosure
Dr. Fanciulli reports royalties from Springer Nature Publishing Group and Thieme Verlag, speaker fees from the Austrian Autonomic Society, Austrian Neurology Society, Austrian Parkinson Society, Ordensklinikum Linz, International Parkinson Disease, Movement Disorders Society, and Theravance Biopharma, and research grants from the Stichting ParkinsonFond, the US MSA Coalition, and the Österreichischer Austausch Dienst, outside of the submitted work. N. Campese, Georg Goebel, J.P. Ndayisaba, S. Eschlboeck, C. Kaindlstorfer, C. Raccagni, and R. Granata report no disclosures relevant to the manuscript. U. Bonuccelli reports speaker fees from Lundbeck, Lusofarmaco, and UCB Pharma, outside of the submitted work. R. Ceravolo reports speaker fees from UCB Pharma, Zambon, General Electric, Lusofarmaco, AbbVie, and Bial, outside of the submitted work. Dr. Seppi reports personal fees from Teva, UCB, Lundbeck, AOP Orphan Pharmaceuticals AG, Roche, Grünenthal, and AbbVie, honoraria from the International Parkinson and Movement Disorders Society, and research grants from the FWF Austrian Science Fund, Michael J. Fox Foundation, and International Parkinson and Movement Disorder Society, outside the submitted work. Dr. Poewe reports consultancy and lecture fees in relation to clinical drug programs for PD from AbbVie, Allergan, AstraZeneca, BIAL, Boehringer-Ingelheim, Boston Scientific, Britannia, Cynapsus, Grünenthal, Intec, Ipsen, Lundbeck, Medtronic, Merz Pharmaceuticals, Novartis, Neuroderm, Orion Pharma, Prexton, Teva, UCB, and Zambon and royalties from Thieme, Wiley Blackwell, Oxford University Press, and Cambridge University Press, outside of the submitted work. Dr. Wenning reports consultancy and lecture fees from AbbVie, Affiris, AstraZeneca, Bial, Biogen, Biohaven, Lilly, Lundbeck, Merz, Mynorix, Modag, Novartis, Roche, Ono, Orion Pharma, Teva, Theravance, UCB, and Zambon, research grants from the FWF Austrian Science Fund, the Austrian National Bank, Cure PSP, NINDS, the US MSA-Coalition, Parkinson Fonds Austria, and International Parkinson and Movement Disorder Society, and royalties from Cambridge University Press, Elsevier, Lippincott Williams & Wilkins, McGraw-Hill, Oxford University Press, Springer, Thieme, and Wiley Blackwell, outside the submitted work. Go to Neurology.org/N for full disclosures.
References
- 1.Barone P, Antonini A, Colosimo C, et al. The PRIAMO study: a multicenter assessment of nonmotor symptoms and their impact on quality of life in Parkinson's disease. Mov Disord 2009;24:1641–1649. [DOI] [PubMed] [Google Scholar]
- 2.Velseboer DC, de Haan RJ, Wieling W, Goldstein DS, de Bie RM. Prevalence of orthostatic hypotension in Parkinson's disease: a systematic review and meta-analysis. Parkinsonism Relat Disord 2011;17:724–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Freeman R, Wieling W, Axelrod FB, et al. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin Auton Res 2011;21:69–72. [DOI] [PubMed] [Google Scholar]
- 4.Brignole M, Moya A, de Lange FJ, et al. 2018 ESC Guidelines for the diagnosis and management of syncope. Eur Heart J 2018;39:1883–1948. [DOI] [PubMed] [Google Scholar]
- 5.Merola A, Romagnolo A, Rosso M, et al. Orthostatic hypotension in Parkinson's disease: does it matter if asymptomatic? Parkinsonism Relat Disord 2016;33:65–71. [DOI] [PubMed] [Google Scholar]
- 6.Hauser RA, Biaggioni I, Hewitt LA, Vernino S. Integrated analysis of droxidopa for the treatment of neurogenic orthostatic hypotension in patients with Parkinson disease. Mov Disord Clin Pract 2018;5:627–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Freeman R. Clinical practice. Neurogenic orthostatic hypotension. N Engl J Med 2008;358:615–624. [DOI] [PubMed] [Google Scholar]
- 8.Pilotto A, Romagnolo A, Tuazon JA, et al. Orthostatic hypotension and REM sleep behaviour disorder: impact on clinical outcomes in alpha-synucleinopathies. J Neurol Neurosurg Psychiatry 2019;90:1257–1263. [DOI] [PubMed] [Google Scholar]
- 9.Czarkowska H, Tutaj M, Rudzińska M, et al. Cardiac responses to orthostatic stress deteriorate in Parkinson disease patients who begin to fall. Neurol Neurochir Pol 2010;44:339–349. [DOI] [PubMed] [Google Scholar]
- 10.Romagnolo A, Zibetti M, Merola A, et al. Cardiovascular autonomic neuropathy and falls in Parkinson disease: a prospective cohort study. J Neurol 2019;266:85–91. [DOI] [PubMed] [Google Scholar]
- 11.Gray P, Hildebrand K. Fall risk factors in Parkinson's disease. J Neurosci Nurs 2000;32:222–228. [DOI] [PubMed] [Google Scholar]
- 12.Rudzinska M, Bukowczan S, Stozek J, et al. Causes and consequences of falls in Parkinson disease patients in a prospective study. Neurol Neurochir Pol 2013;47:423–430. [DOI] [PubMed] [Google Scholar]
- 13.Merola A, Sawyer RP, Artusi CA, et al. Orthostatic hypotension in Parkinson disease: impact on health care utilization. Parkinsonism Relat Disord 2018;47:45–49. [DOI] [PubMed] [Google Scholar]
- 14.Merola A, Romagnolo A, Rosso M, et al. Autonomic dysfunction in Parkinson's disease: a prospective cohort study. Mov Disord 2018;33:391–397. [DOI] [PubMed] [Google Scholar]
- 15.Matinolli M, Korpelainen JT, Korpelainen R, Sotaniemi KA, Myllylä VV. Orthostatic hypotension, balance and falls in Parkinson's disease. Mov Disord 2009;24:745–751. [DOI] [PubMed] [Google Scholar]
- 16.Rascol O, Perez-Lloret S, Damier P, et al. Falls in ambulatory non-demented patients with Parkinson's disease. J Neural Transm 2015;122:1447–1455. [DOI] [PubMed] [Google Scholar]
- 17.De Pablo-Fernandez E, Tur C, Revesz T, Lees AJ, Holton JL, Warner TT. Association of autonomic dysfunction with disease progression and survival in Parkinson disease. JAMA Neurol 2017;74:970–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Williams DR, Watt HC, Lees AJ. Predictors of falls and fractures in bradykinetic rigid syndromes: a retrospective study. J Neurol Neurosurg Psychiatry 2006;77:468–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Allan LM, Ballard CG, Rowan EN, Kenny RA. Incidence and prediction of falls in dementia: a prospective study in older people. PLoS One 2009;4:e5521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wood BH, Bilclough JA, Bowron A, Walker RW. Incidence and prediction of falls in Parkinson's disease: a prospective multidisciplinary study. J Neurol Neurosurg Psychiatry 2002;72:721–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koller WC, Glatt S, Vetere-Overfield B, Hassanein R. Falls and Parkinson's disease. Clin Neuropharmacol 1989;12:98–105. [DOI] [PubMed] [Google Scholar]
- 22.Michalowska M, Fiszer U, Krygowska-Wajs A, Owczarek K. Falls in Parkinson's disease: causes and impact on patients' quality of life. Funct Neurol 2005;20:163–168. [PubMed] [Google Scholar]
- 23.O'Sullivan SS, Massey LA, Williams DR, et al. Clinical outcomes of progressive supranuclear palsy and multiple system atrophy. Brain 2008;131:1362–1372. [DOI] [PubMed] [Google Scholar]
- 24.van Wijnen VK, Finucane C, Harms MPM, et al. Noninvasive beat-to-beat finger arterial pressure monitoring during orthostasis: a comprehensive review of normal and abnormal responses at different ages. J Intern Med 2017;282:468–483. [DOI] [PubMed] [Google Scholar]
- 25.Finucane C, O'Connell MD, Fan CW, et al. Age-related normative changes in phasic orthostatic blood pressure in a large population study: findings from the Irish Longitudinal Study on Ageing (TILDA). Circulation 2014;130:1780–1789. [DOI] [PubMed] [Google Scholar]
- 26.Romero-Ortuno R, Cogan L, Foran T, Kenny RA, Fan CW. Continuous noninvasive orthostatic blood pressure measurements and their relationship with orthostatic intolerance, falls, and frailty in older people. J Am Geriatr Soc 2011;59:655–665. [DOI] [PubMed] [Google Scholar]
- 27.Finucane C, O'Connell MD, Donoghue O, Richardson K, Savva GM, Kenny RA. Impaired orthostatic blood pressure recovery is associated with unexplained and injurious falls. J Am Geriatr Soc 2017;65:474–482. [DOI] [PubMed] [Google Scholar]
- 28.Hughes AJ, Ben-Shlomo Y, Daniel SE, Lees AJ. What features improve the accuracy of clinical diagnosis in Parkinson's disease: a clinicopathologic study. Neurology 1992;42:1142–1146. [DOI] [PubMed] [Google Scholar]
- 29.Postuma RB, Berg D, Stern M, et al. MDS clinical diagnostic criteria for Parkinson's disease. Mov Disord 2015;30:1591–1601. [DOI] [PubMed] [Google Scholar]
- 30.Emre M, Aarsland D, Brown R, et al. Clinical diagnostic criteria for dementia associated with Parkinson's disease. Mov Disord 2007;22:1689–1707; quiz 1837. [DOI] [PubMed] [Google Scholar]
- 31.Goetz CG, Tanner CM, Klawans HL. The pharmacology of olivopontocerebellar atrophy. Adv Neurol 1984;41:143–148. [PubMed] [Google Scholar]
- 32.Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology 1967;17:427–442. [DOI] [PubMed] [Google Scholar]
- 33.Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE. Systematic review of levodopa dose equivalency reporting in Parkinson's disease. Mov Disord 2010;25:2649–2653. [DOI] [PubMed] [Google Scholar]
- 34.Fanciulli A, Wenning GK. Multiple-system atrophy. N Engl J Med 2015;372:249–263. [DOI] [PubMed] [Google Scholar]
- 35.Fanciulli A, Strano S, Ndayisaba JP, et al. Detecting nocturnal hypertension in Parkinson's disease and multiple system atrophy: proposal of a decision-support algorithm. J Neurol 2014;261:1291–1299. [DOI] [PubMed] [Google Scholar]
- 36.Low PA, Denq JC, Opfer-Gehrking TL, Dyck PJ, O'Brien PC, Slezak JM. Effect of age and gender on sudomotor and cardiovagal function and blood pressure response to tilt in normal subjects. Muscle Nerve 1997;20:1561–1568. [DOI] [PubMed] [Google Scholar]
- 37.Benjamini Y, Drai D, Elmer G, Kafkafi N, Golani I. Controlling the false discovery rate in behavior genetics research. Behav Brain Res 2001;125:279–284. [DOI] [PubMed] [Google Scholar]
- 38.Wieling W, Krediet CT, van Dijk N, Linzer M, Tschakovsky ME. Initial orthostatic hypotension: review of a forgotten condition. Clin Sci 2007;112:157–165. [DOI] [PubMed] [Google Scholar]
- 39.van der Marck MA, Klok MP, Okun MS, et al. Consensus-based clinical practice recommendations for the examination and management of falls in patients with Parkinson's disease. Parkinsonism Relat Disord 2014;20:360–369. [DOI] [PubMed] [Google Scholar]
- 40.Freeman R, Illigens BMW, Lapusca R, et al. Symptom recognition is impaired in patients with orthostatic hypotension. Hypertension 2020;75:1325–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Palma JA, Gomez-Esteban JC, Norcliffe-Kaufmann L, et al. Orthostatic hypotension in Parkinson disease: how much you fall or how low you go? Mov Disord 2015;30:639–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Indelicato E, Fanciulli A, Poewe W, Antonini A, Pontieri FE, Wenning GK. Cerebral autoregulation and white matter lesions in Parkinson's disease and multiple system atrophy. Parkinsonism Relat Disord 2015;21:1393–1397. [DOI] [PubMed] [Google Scholar]
- 43.Fedorowski A, Hamrefors V, Sutton R, et al. Do we need to evaluate diastolic blood pressure in patients with suspected orthostatic hypotension? Clin Auton Res 2017;27:167–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Biaggioni I, Arthur Hewitt L, Rowse GJ, Kaufmann H. Integrated analysis of droxidopa trials for neurogenic orthostatic hypotension. BMC Neurol 2017;17:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gibbons CH, Schmidt P, Biaggioni I, et al. The recommendations of a consensus panel for the screening, diagnosis, and treatment of neurogenic orthostatic hypotension and associated supine hypertension. J Neurol 2017;264:1567–1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eschlböck S, Wenning G, Fanciulli A. Evidence-based treatment of neurogenic orthostatic hypotension and related symptoms. J Neural Transm 2017;124:1567–1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kaufmann H, Norcliffe-Kaufmann L, Palma JA. Baroreflex dysfunction. N Engl J Med 2020;382:163–178. [DOI] [PubMed] [Google Scholar]
- 48.Fanciulli A, Campese N, Wenning GK. The Schellong test: detecting orthostatic blood pressure and heart rate changes in German-speaking countries. Clin Auton Res 2019;29:363–366. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The first and the last authors take full responsibility for the integrity of data and agree to share any data not published within this article upon reasonable request from any qualified investigator.