Abstract

We utilized synthetic photochemistry to generate novel sp3-rich scaffolds and report the design, synthesis, and biological testing of a diverse series of amides based on the 1-(amino-methyl)-2-benzyl-2-aza-bicyclo[2.1.1]hexane scaffold. Preliminary antimalarial screening of the library provided promising compounds with activity in the 1–5 μM range with an enhanced hit rate. Further evaluation (solubility, drug metabolism and pharmacokinetics (DMPK), and toxicity) of a selected compound (9) suggested that this series represents an excellent opportunity for further optimization with the framework offering multiple opportunities for the addition of uniquely vectorally positioned extra functionality.
Keywords: Drug discovery, antimalarial, photochemistry, sp3-rich substituted bridged pyrrolidines, 3D character
Malaria is an infectious disease transmitted by mosquitoes infected with five species of single-celled eukaryotic Plasmodium parasites (most commonly Plasmodium falciparum and Plasmodium vivax). In 2018, there were an estimated 228 million cases of malaria globally, with half of the world’s population at risk. Malaria transmission continues to affect more than 90 countries and territories around the world, inflicting a tremendous burden in sub-Saharan Africa with more than 90% of cases on the African continent. In 2018, malaria was estimated to cause 405,000 deaths, 99% of which are due to P. falciparum in Africa. Parasite resistance against currently recommended artemisinin-based combination therapies (ACTs) is a major concern, and new antimalarial drugs with novel modes of action are urgently needed as well as drugs that interrupt the parasite life cycle by blocking transmission to the vectors, prevent infection, and target malaria species that transiently remain dormant in the liver.1,2
Finding new medicines for malaria has proven to be challenging; part of this stems from the difficulty of identifying molecules from screening that are tractable and novel starting points. The hit rate found when screening conventional chemical libraries, which also contain compounds with known antimicrobial motifs, has been reported to be 0.4–1%;3 however two recent screens carried out by Medicines for Malaria on libraries with compounds with known antimicrobial motifs excluded showed much lower hit rates of 0.07% and 0.09% respectively (hit defined as compound with IC50 > 2 μM). Clemons et al. examined compounds from different sources (commercial, research, natural) for their protein-binding behaviors and found that these correlate with general trends in stereochemical and shape descriptors for these compound collections. Increasing the content of sp3-hybridized and stereogenic centers in novel compounds improved binding selectivity and most importantly hit frequency.4 This was in contrast to those from commercial sources, which generally comprise the majority of current screening collections.
We were prompted to look at synthetic methodologies that could be used to efficiently generate novel, complex, sp3-rich frameworks that could be utilized in the generation of a number of novel libraries to undergo screening for antimalarial activity. For the past five years, we have been investigating the use of photochemistry, in particular [2 + 2] cycloadditions, to produce novel chemical entities as desirable starting points for drug discovery.5,6
Apart from the apparent positive impact on hit finding, another key importance of sp3-enrichment was highlighted by Lovering et al.,7 who utilized fraction sp3 (Fsp3) where Fsp3 = (number of sp3 hybridized carbons/total carbon count) as a simple and interpretable measurement of the complexity of molecules. They were able to show that complexity (as measured by Fsp3) appears to correlate with the chance of success of compounds transitioning from discovery through clinical testing to marketed drugs. They also demonstrated that increased saturation generally correlated with improved solubility, an often a desirable property for a drug.7 In later work, Lovering described how increasing complexity reduces promiscuity and CYP450 enzyme inhibition, with increased promiscuity being linked to toxicity and ultimately to failure.8
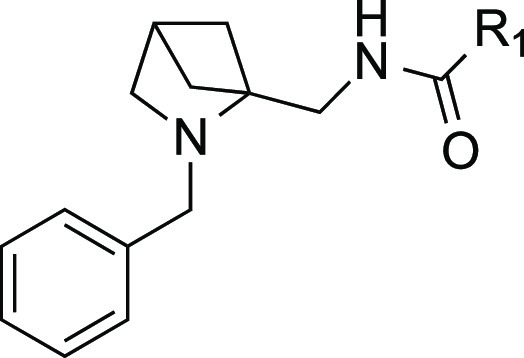
With these influences in mind, we set out to design a novel sp3-rich screening library and demonstrate its utility by identifying novel antimalarial leads. As previously reported, we have focused some of our efforts on analogues and derivatives of 2,4-methanoproline 1 (Figure 1).6 For this effort, we selected 1-(amino-methyl)-2-benzyl-2-aza-bicyclo[2.1.1]hexane 2a as an ideal key scaffold for the preparation of the library. During the completion of the work in this publication, 2a was reported by Levterov et al. as a precursor to the two differentially protected N-Boc derivatives 2b and 2c.9 Although there have been numerous reports of the “aminoproline” 3, its analogues, and their biological activities, there are relatively few reports of biological activity associated with the 2,4-methano-bridged variant. Two examples are the insect repellent/antifeedant activity of compounds such as 2d(10) and the potential therapeutic application of compounds such as 4, found to be potent inhibitors of the glycine transporter GlyT1.11
Figure 1.
Proline analogues.
The N-benzoyl ethyl ester of 2,4-methanoproline 5 was prepared by the method of Malpass et al. in a two step process from ethyl pyruvate (Scheme 1) via an intramolecular [2 + 2] olefin photocycloaddition reaction.12 The original syntheses of the N-benzoyl methyl ester of 2,4-methanoproline were published in back-to-back articles, the first utilizing the methyl pyruvate13 and the second starting from serine.14
Scheme 1. Synthesis of Ethyl 2-Benzoyl-2-azabicyclo[2.1.1]hexane-1-carboxylate 5.
Reagents and conditions: (a) allylamine then PhCOCl, Et3N, toluene (47%); (b) hν, acetone (40%).
The N-benzoyl ethyl ester of 2,4-methanoproline 5 was used to prepare 2a in a three stage process (Scheme 2); first reduction with lithium aluminum hydride to produce the N-benzyl alcohol 6, followed by a Mitsunobu reaction with phthalimide to generate the phthalimide derivative 7, and finally hydrazinolysis to yield the desired material. Levterov et al. used an alternative approach via the tosylate of 6, followed by conversion to the azide and then Staudinger reduction.9
Scheme 2. Synthesis of 1-(Amino-methyl)-2-benzyl-2-aza-bicyclo[2.1.1]hexane 2a.
Reagents and conditions: (a) LiAlH4, THF (78%); (b) phthalimide, polymer-supported PPh3, DIAD, THF (83%); (c) H2NNH2, EtOH (72%); (d) DIPEA, HATU, DCM, R1 carboxylic acid or DIPEA, COMU, DCM, R1 carboxylic acid (See Table 1 for yields).
A diverse 234-member array of amide derivatives of 2a was then prepared using a parallel synthesis/purification method employing standard amide forming reactions with HATU or COMU as coupling agents. (See Supporting Information for a full list of the compounds synthesized).
The compounds were assessed for their antimalarial activity using a 3D7 SYBR Green I in vitro assay.15,16 All compounds were screened in a single point assay at a concentration of 10 μM to identify those with activity >50% inhibition. Actives and a representative selection of less active compounds were screened in potency mode with a top concentration of 25 μM following a 10-point 1 in 3 dilution series (see Table 1; see Supporting Information for a full list of the screening data for the compounds synthesized).
Table 1. Antimalarial Activities of Amide Derivatives of 1-(Amino-methyl)-2-benzyl-2-aza-bicyclo[2.1.1]hexane 2a in the 3D7 SYBR Green I In Vitro Assay.


The 68 compounds titrated displayed IC50 values in the range 1 to 25 μM with a reasonable correlation between the activity in the primary assay and the titrated IC50 assay (Figure 2). Three compounds have activity <2 μM providing a hit rate of 1.3% based on this cut off, which compares extremely favorably with the hit rates of the two recent screens described earlier.
Figure 2.

Plot of % inhibition in the primary assay at 10 μM and titrated IC50 value (μM).
The unsubstituted benzamide (203) was found to be inactive (−20% @ 10 μM); however, positioning a chloro at the 3-position of the phenyl ring gave useful activity (51, IC50 13 μM). The bioisosteric 3-bromo (89, 35% @ 10 μM) and 3-methyl (109, 7.6% @ 10 μM) analogues showed no advantage. However, the 3-trifluoromethoxy (38, IC50 9.3 μM) showed a possible improvement. The 2-chloro (143, −7.9% @ 10 μM) and 4-chloro (81, 38% @ 10 μM) proved to be less active.
Using the 3-chloro as the starting point for further analysis, additional substitution at the 2-position (e.g., the 2,3-dichloro compound 147, −8.2% @ 10 μM) and the 6-position (e.g., the 3-chloro-6-methyl analogue 62, IC50 19 μM) proved detrimental. In contrast, 3,4-disubstitution offered a modest improvement in activity (21, IC50 5.1 μM). Additional substitution at the 5-position proved to be the most advantageous (e.g., the 3-chloro-5-trifluoromethyl analogue 9, IC50 1.3 μM). A range of derivatives with lipophilic substituents in the 3,5-positions were also active. In general, the analogous benzylamides were less active (see 43 vs 21, 67 vs 42, 80, vs 46, and 146 vs 72); however substitution at the benzylic position dramatically improved activity with the spirocyclobutyl compound 26 (IC50 6.2 μM) proving to be active, and impressively the spirocyclopropyl compound 8 (IC50 1.1 μM) proved to be the most active in the library. In a further trend, the fluorostyryl compounds 44, 65, and 52 all proved to be more active than their benzyl or phenyl counterparts (where prepared). In general, heterocyclic amides are not well tolerated, the exception being the isoxazole (14, IC50 3.4 μM).
A range of physicochemical properties were calculated using a Jupyter notebook17 and ChemAxon tools. In general, the compounds conform to the “rule of five” definition of drug-likeness with log P < 5 and molecular weight <500. In terms of ionization, the majority are predicted to be basic and protonated at physiological pH, albeit with a range of pKa from 7.2 to 8.4. Early profiling data received for 9 confirmed this with a log D = 3.2, and kinetic solubility >200 μM. Early DMPK and toxicity profiling further indicated its value as an early lead candidate (HLM CLint = 27 μL·min–1·mg–1 and RHeps CLint = 8 μL·min–1·(106 cells)−1) and an IC50 in a Hep G2 cell assay of >17 μM (the maximum concentration of the assay). The compound was also assessed against the multiple drug resistant strain Dd2 in an LDH format assay alongside the nonresistant 3D7 strain. Excitingly, the compound was found to have similar activities against both strains (Dd2 IC50 5.4 μM and 3D7 IC50 4.3 μM).
The ChEMBL Neglected and Tropical Disease Archive18 contains a number of data sets of screening and medicinal chemistry campaigns directed toward NTD targets including malaria. These data sets were imported into Vortex, InChiKeys were generated and a script was run comparing InChiKeys for all compounds. While there is considerable overlap between some of the ChEMBL data sets, none of the photodiversity compounds were present in any of the previously tested data sets.
Comparison of the “bridged” azabicyclic framework with that of the pyrrole and pyrrolidine (one enantiomer selected) analogues shows that the framework offers many more divergent vectors for further elaboration and thus exploration of 3D space (Figure 3).19
Figure 3.
Spacial comparison of pyrrole, pyrrolidine, and azabicycle cores.
As in our previous publication,6 further analysis was carried out to assess relative shape across these three frameworks using principle moments of inertia (PMI)20 and plane of best fit (PBF).21 These data are tabulated below (Table 2) and are consistent with the suggestion that the azabicycle occupies an area of chemical space described as more “three-dimensional” (note that compounds with ∑NPR (sum of NPR1 and NPR2) ≥ 1.07 and PBF scores ≥ 0.6 are deemed by Firth et al. to reside in 3D space).20
Table 2. Comparison of the Normalized Principle Moments of Inertia (NPR) and Plane of Best Fit (PBF) of Pyrrole, Pyrrolidine, and Azabicycle Cores.
| structure | NPR1 | NPR2 | ∑NPR | PBF |
|---|---|---|---|---|
| pyrrole | 0.22 | 0.84 | 1.06 | 0.58 |
| pyrrolidine | 0.32 | 0.86 | 1.18 | 0.70 |
| azabicycle | 0.36 | 0.91 | 1.27 | 0.83 |
In conclusion, as researchers seek greater novelty and sp3 content in their screening sets, photocycloaddition reactions ought to provide highly desirable templates. Here we have shown that the 2,4-methanoproline derived template provided a unique library with an enhanced hit rate, good drug-like properties, and a viable starting point for further optimization in an antimalarial program.
Acknowledgments
The authors thank the following colleagues for providing small molecule analysis data: Dr. Alaa Abdul-Sada (mass spectrometry), Dr. Iain Day (NMR), and Dr. Mark Roe (X-ray crystallography) at the University of Sussex. We also thank Dr. Susanta Kumar of Mondal TCG Lifesciences Pvt Ltd, India, for log D, kinetic solubility, LDH, and DMPK assays. We are also grateful to the Universities of Sussex and Bristol.
Glossary
Abbreviations
- COMU
1-cyano-2-ethoxy-2-oxoethylidenaminooxy)dimethylamino-morpholino-carbenium hexafluorophosphate
- CYP450
cytochrome P450
- DCM
dichloromethane
- DIAD
diisopropyl azodicarboxylate
- DIPEA
diisopropylethylamine
- DMPK
drug metabolism and pharmacokinetics
- HATU
1-[bis(dimethylamino)methylene]-1H-1,2,3-triazolo[4,5-b]pyridinium 3-oxide hexafluorophosphate
- HLM
human liver microsomes
- THF
tetrahydrofuran
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsmedchemlett.0c00486.
Synthetic methods, characterization, biological assay methods and results, and crystallographic and computational modeling data (PDF)
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
We gratefully acknowledge support for Luke Elliott from the Engineering and Physical Sciences Research Council (EP/P013341/1).
The authors declare the following competing financial interest(s): B.C. and K.I.B.M. are employees of their respective universities and cofounders and owners of Photodiversity Ltd. J.D. and B.L. are employees of Medicines for Malaria Venture (MMV).
Supplementary Material
References
- For a comprehensive source of materials relating to malaria, its current treatment, and global research and development efforts, please see: World malaria report 2019, https://apps.who.int/iris/handle/10665/330011.
- Tse E. G.; Korsik M.; Todd M. H. The past, present and future of anti-malarial medicines. Malar. J. 2019, 18, 93. 10.1186/s12936-019-2724-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flannery E. L.; Chatterjee A. K.; Winzeler E. A. Antimalarial drug discovery - approaches and progress towards new medicines. Nat. Rev. Microbiol. 2013, 11, 849–862. 10.1038/nrmicro3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemons P. A.; Bodycombe N. A.; Carrinski H. A.; Wilson J. A.; Shamji A. F.; Wagner B. K.; Koehler A. N.; Schreiber S. L. Small molecules of different origins have distinct distributions of structural complexity that correlate with protein-binding profiles. Proc. Natl. Acad. Sci. U. S. A. 2010, 107, 18787–18792. 10.1073/pnas.1012741107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox B.; Booker-Milburn K. I.; Elliott L. D.; Robertson-Ralph M.; Zdorichenko V. Escaping from flatland: [2 + 2] photocycloaddition; conformationally constrained sp3-rich scaffolds for lead generation. ACS Med. Chem. Lett. 2019, 10, 1512–1517. 10.1021/acsmedchemlett.9b00409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox B.; Zdorichenko V.; Cox P. B.; Booker-Milburn K. I.; Paumier R.; Elliott L. D.; Robertson-Ralph M.; Bloomfield G. Escaping from flatland: substituted bridged pyrrolidine fragments with inherent three-dimensional character. ACS Med. Chem. Lett. 2020, 11, 1185–1190. 10.1021/acsmedchemlett.0c00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovering F.; Bikker J.; Humblet C. Escape from flatland: increasing saturation as an approach to improving clinical success. J. Med. Chem. 2009, 52, 6752–6756. 10.1021/jm901241e. [DOI] [PubMed] [Google Scholar]
- Lovering F. Escape from Flatland 2: complexity and promiscuity. MedChemComm 2013, 4, 515–519. 10.1039/c2md20347b. [DOI] [Google Scholar]
- Levterov V. V.; Michurin O.; Borysko P. O.; Zozulya S.; Sadkova I. V.; Tolmachev A. A.; Mykhailiuk P. K. Photochemical in-flow synthesis of 2,4-methanopyrrolidines: pyrrolidine analogues with improved water solubility and reduced lipophilicity. J. Org. Chem. 2018, 83, 14350–14361. 10.1021/acs.joc.8b02071. [DOI] [PubMed] [Google Scholar]
- Stevens C. V.; Smagghe G.; Rammeloo T.; De Kimpe N. Insect repellent/antifeedant activity of 2,4-methanoproline and derivatives against a leaf- and seed-feeding pest insect. J. Agric. Food Chem. 2005, 53, 1945–1948. 10.1021/jf048222u. [DOI] [PubMed] [Google Scholar]
- Dargazanli G.; Estenne-Bouhtou G.; Mafroud A.-K.. N-[(2-azabicyclo[2.1.1]hex-1-yl)-aryl-methyl]-benzamide derivatives, preparation thereof, and therapeutic use thereof. WO2010092286, August 19, 2010.
- Malpass J. R.; Patel A. B.; Davies J. W.; Fulford S. Y. Modification of 1-substituents in the 2-azabicyclo[2.1.1]hexane ring system; approaches to potential nicotinic acetylcholine receptor ligands from 2,4-methanoproline derivatives. J. Org. Chem. 2003, 68, 9348–9355. 10.1021/jo035199n. [DOI] [PubMed] [Google Scholar]
- Pirrung M. C. Total synthesis of 2,4-methanoproline. Tetrahedron Lett. 1980, 21, 4577–4578. 10.1016/0040-4039(80)80077-3. [DOI] [Google Scholar]
- Hughes P.; Martin M.; Clardy J. Synthesis of 2,4-methanoproline. Tetrahedron Lett. 1980, 21, 4579–4580. 10.1016/0040-4039(80)80078-5. [DOI] [Google Scholar]
- Baragaña B.; Hallyburton I.; Lee M. C. S.; Norcross N. R.; Grimaldi R.; Otto T. D.; Proto W. R.; Blagborough A. M.; Meister S.; Wirjanata G.; Ruecker A.; Upton L. M.; Abraham T. S.; Almeida M. J.; Pradhan A.; Porzelle A.; Martínez M. S.; Bolscher J. M.; Woodland A.; Norval S.; Zuccotto F.; Thomas J.; Simeons F.; Stojanovski L.; Osuna-Cabello M.; Brock P. M.; Churcher T. S.; Sala K. A.; Zakutansky S. E.; Jiménez-Díaz M. B.; Sanz L. M.; Riley J.; Basak R.; Campbell M.; Avery V. M.; Sauerwein R. W.; Dechering K. J.; Noviyanti R.; Campo B.; Frearson J. A.; Angulo-Barturen I.; Ferrer-Bazaga S.; Gamo F. J.; Wyatt P. G.; Leroy D.; Siegl P.; Delves M. J.; Kyle D. E.; Wittlin S.; Marfurt J.; Price R. N.; Sinden R. E.; Winzeler E.; Charman S. A.; Bebrevska L.; Gray D. W.; Campbell S.; Fairlamb A. H.; Willis P.; Rayner J. C.; Fidock D. A.; Read K. D.; Gilbert I. H.; Luksch T. A novel multiple-stage antimalarial agent that inhibits protein synthesis. Nature 2015, 522 (7556), 315–320. 10.1038/nature14451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smilkstein M.; Sriwilaijaroen N.; Kelly J. X.; Wilairat P.; Riscoe M. Simple and inexpensive fluorescence-based technique for high-throughput antimalarial drug screening. Antimicrob. Agents Chemother. 2004, 48 (5), 1803–1806. 10.1128/AAC.48.5.1803-1806.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain C. J.; Macs in chemistry, physicochemical property profile iPython notebook, https://www.macinchem.org/reviews/ipython/calcproperties2.php (accessed Oct 13, 2020).
- ChEMBL, malaria data, https://chembl.gitbook.io/chembl-ntd/ (accessed Oct 13, 2020).
- Molecular operating environment (MOE), https://www.chemcomp.com/Products.htm. (accessed Oct 13, 2020).
- Firth N. C.; Brown N.; Blagg J. Plane of best fit: a novel method to characterize the three-dimensionality of molecules J. J. Chem. Inf. Model. 2012, 52, 2516–2525. 10.1021/ci300293f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer W. H. B.; Schwarz M. K. Molecular shape diversity of combinatorial libraries: a prerequisite for broad bioactivity. J. Chem. Inf. Comput. Sci. 2003, 43, 987–1003. 10.1021/ci025599w. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.






