Abstract
Hepatitis C virus (HCV) infections represent a global health challenge; however, developing a vaccine for treatment of HCV infection has remained difficult as heterogeneous HCV contains distinct genotypes, and each genotype contains various subtypes and different envelope glycoproteins. Currently, there is no effective preventive vaccine for achieving global control over HCV. In our efforts to improve upon current HCV vaccines we designed a synthetically accessible adjuvant platform, wherein we synthesized 11 novel lipidated tucaresol analogues to assess their immunological potential. Using a tucaresol-based adjuvant approach, truncated lipid-variants together with an engineered E1E2 antigen construct, namely E2ΔTM3, elicited antibody (Ab) responses that were significantly higher than tucaresol. In sum, antibody end-point titer values largely corroborated HCV neutralization data with a simplified lipidated tucaresol variant affording the highest end point titer and % neutralization. This study lays the groundwork for additional permutations in tucaresol adjuvant design, including the examination of other proteins in vaccine development.
Keywords: Vaccines, adjuvant, hepatitis C virus, phenol
Hepatitis C virus (HCV) infection is a global health problem that threatens to escalate amid the ongoing opioid pandemic. Specifically, the rise in injectable drug use in the United States has grown in concert with HCV cases over the past decade.1,2 HCV infection surveillance data from 2010 to 2014 reported a 2-fold increase that mirrored opioid substance use disorder admissions.1 This suggests a common thread between acute HCV infections and the opioid pandemic.3 Moreover, HCV is among the major contributors to serious liver cirrhosis and fibrosis that often lead to hepatocellular carcinoma and permanent liver damage.4,5 Despite progress toward an HCV vaccine, current regimens are complex, with immunization lasting over multiple months and dosages. Furthermore, distinct HCV antigen genotypes are required to elicit broadly neutralizing antibodies (bnAbs) for an effective immune response.6 Therefore, there is a continued need to develop simpler, safer, and effective vaccines to combat the risks caused by HCV.
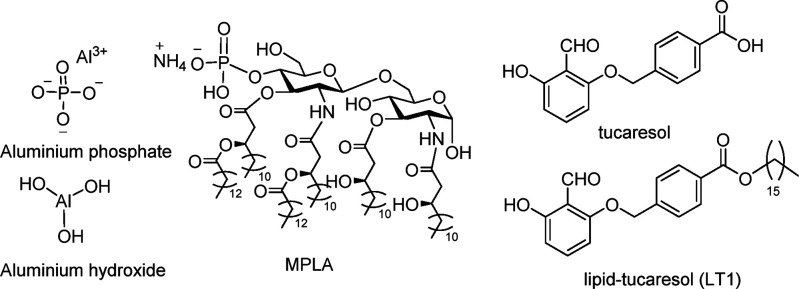
One area for vaccine advancement is the design of suitable adjuvants that function to enhance the immune response to a coadministered antigen. Notwithstanding FDA-approved adjuvants available including the delivery systems alum and AddaVax, Oil Emulsion Adjuvant MF59 (not listed), and the immune potentiator Monophosphoryl Lipid A (MPLA) (Figure 1).7−9 The simplest of the current armamentarium of FDA approved adjuvants is alum, which is a heterogeneous mixture of inorganic salts of aluminum phosphate and hydroxide and remains the most important adjuvant because of its low cost and ease of manufacture. Indeed, alum is used in over 80% of FDA approved vaccines.10
Figure 1.
Structures of alum, MPLA, tucaresol, and LT1.
Yet, despite the widespread use of alum, a clear understanding that governs alum’s physiochemical interaction with an antigen is lacking. Furthermore, alum has little capacity to stimulate a broad cellular immune response.11,12 Moreover, some developmental vaccines that are formulated with alum either cannot sustain an Ab response or have adverse side effects and fail toxicological requirements.
The challenge of developing improved vaccines has been accelerated by using combinations of adjuvant systems. One notable example is the GlaxoSmithKline (GSK) adjuvants ASO1, ASO2, ASO4, and AS15 that are used in single and multicomponent vaccine formulations to achieve complementary or synergistic bnAb enhancement of the immune response.13 MPLA is also an example of a complex-glycoside lipid adjuvant that is approved for use with alum facilitating a more potent immune response.7,8 In the context of HCV, MPLA has been used with an engineered E2 subunit of the HCV envelope to enhance immunogenicity.14 However, this truncated E1E2 with MPLA formulation was found to be a weak immunogen that elicited low levels of bnAbs. To circumvent these issues, both flag-tagged and purified Fc untagged rE1E2 have been investigated (without MPLA) but to no avail, eliciting only comparable Ab levels to wild type (WT) E1E2.15,16
Tucaresol (Figure 1) is an example of a synthetic adjuvant that has been shown to elicit both humoral and cellular responses, that are thought to be mediated through its aldehyde functionality.17 The aldehyde moiety is hypothesized to substitute/compliment with antigen-presenting cells through Schiff base formation, thus mimicking innate chemical interactions between immune cells (Figure 1).18,19 As a Schiff-base forming molecule, tucaresol would take part in highly dynamic, reversible, and rapid reaction processes. These interactions play a role in the overall immune costimulatory mechanism originally postulated by Rhodes.18 Tucaresol’s effect on the immune response is known to be concentration-dependent, with increasing immunopotentiation at lower doses; however, immunosuppression is observed at higher doses.17 With these known constraints, a series of modifications to the tucaresol core have been undertaken with a 16-carbon alkyl chain modification being the most successful (LT1, Figure 1), which has been examined in liposomal and nonliposomal formulation delivery systems.20,21 However, liposomal LT1 vs liposomal MPLA formulations only gave comparable titers and immune response in a methamphetamine vaccine mouse model.20
To further enhance tucaresol’s adjuvant platform, we detail the synthesis of a series of tucaresol concentric adjuvants through lipidation of the tucaresol framework and evaluating the structure activity relationship (SAR) of the tucaresol scaffold. Complementary to our previous research, these efforts would also establish the importance of the benzaldehyde and phenol moieties of these lipidated variants.21
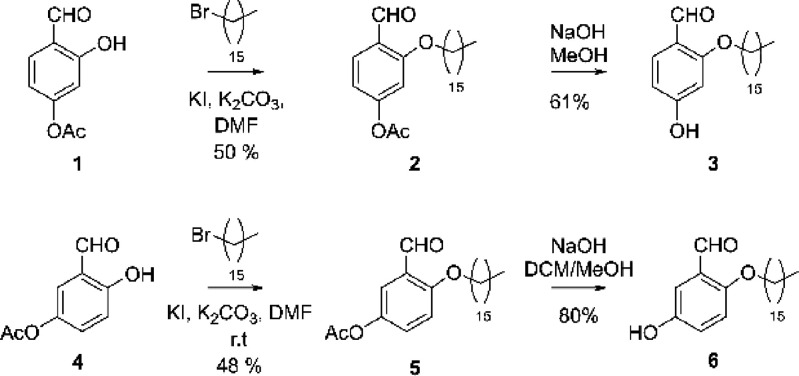
Our overall synthetic tactic to the abridged lipid-tucaresol analogues was to (1) eliminate phenoxymethyl benzoic acid embedded within tucaresol, which would allow for enhanced solubility in PBS; (2) probe the importance of the aldehyde moiety and phenol copy number/regioplacement; and (3) test the role for the alkyl lipid attachment in an HCV vaccine model while keeping consistent with the adjuvanting properties of previously developed tucaresol-lipids, which are being examined in a methamphetamine vaccine (unpublished). Based upon this research agenda efforts were initiated by accessing the selectively protected phenols 1 and 4, wherein each was alkylated providing 2 and 5 in moderate yields. Deprotection under basic conditions afforded the target lipid-phenols 3 and 6 (Scheme 1).
Scheme 1. Synthesis of Monophenols (3 and 6).
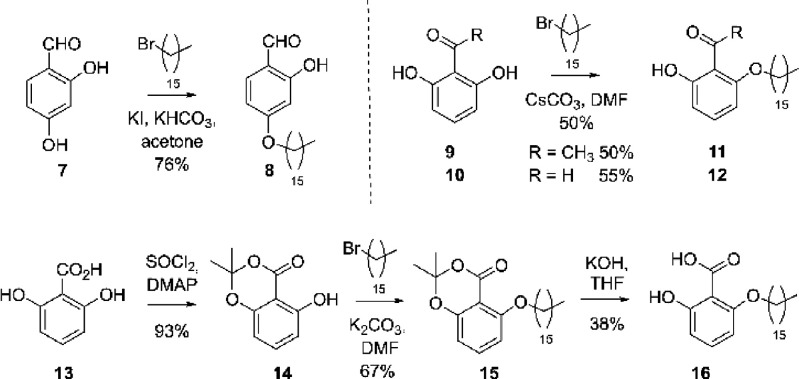
A third hydroxybenzaldehyde in this series was prepared via alkylation at the less sterically encumbered phenol of 7, which awarded 8 (Scheme 2). To evaluate the role of the aldehyde, we elected to synthesize benzoic and acetophenone variants. The latter was introduced through selective alkylation of 9 and 10, providing 11 and 12. To access the acid, 2,6-dihydroxybenzoic acid 13 was protected granting phenol, 14. Alkylation of 14 furnished 15 in a workable yield, and subsequent deprotection gave the targeted benzoic acid analogue 16 (Scheme 2).
Scheme 2. Synthesis of Monophenols (8, 11, 12, and 16).
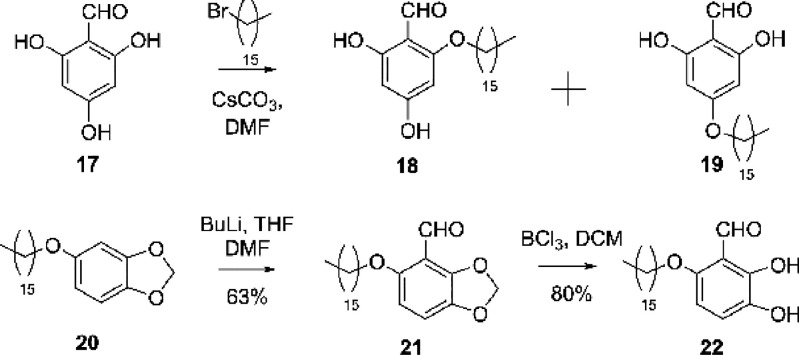
Direct alkylation of 2,4,6-trihydroxybenzaldehyde 17 provided easy access to 18 and 19, which were separated by chromatography without the need for protecting group manipulation. The 2,3-dihydroxybenzaldehyde 22 was obtained from 20 via alkylation of commercially available seasmol. Thus, addition of butyl lithium to 20 in dimethylformide allowed access to aldehyde 21,22 which conferred 22 upon treatment with borontrichloride (Scheme 3).
Scheme 3. Synthesis of Diphenols (18, 19, and 22).
With the series of putative adjuvants in hand, we evaluated our HCV vaccine. We envisioned that improvement in vaccine design could be accomplished by optimizing protein solubility to include a recombinant envelope glycoprotein E2 antigen without the transmembrane domain, namely E2ΔTM323. For the purposes of this study, we elected to use a single antigen E2ΔTM3, which has previously been effective in producing broadly neutralizing antibodies against HCV1 (Genotype 1a strain) in both rodents and humans.23−27 The E2ΔTM3 protein comprises the complete ectodomain; it also ensures that all the antigenic sites are accessible on the E2 core and that the protein can be readily taken up by cells. This technique has been widely applied to many E2 constructs.14,28,29
A well-established tucaresol mouse vaccination schedule initially pioneered by Rhodes was used in our HCV testing.17 Thus, each priming HCV vaccine was formulated with 25 μg of E2ΔTM3 antigen and 200 μg of experimental tucaresol adjuvant administered on day one. Mice then received 200 μg doses of adjuvant only on days 2–5 thereafter to give 1 mg of adjuvant per mouse per vaccination period evenly split over five consecutive days. Booster injections were performed under the same schedule at 4-week intervals post prime injections, except with 5 μg of E2ΔTM3 antigen given on the first day. E2ΔTM3 and adjuvant were injected subcutaneously into BALB/C mice at weeks 0, 4, 8, and 12 (see SI). Adjuvant positive controls included tucaresol, and the negative control included E2ΔTM3 without adjuvant. Bleeds were performed before the priming injections and 2 days after the booster injections. Mouse weight was monitored on a weekly basis, and infection at the site of injection was noted (SI)
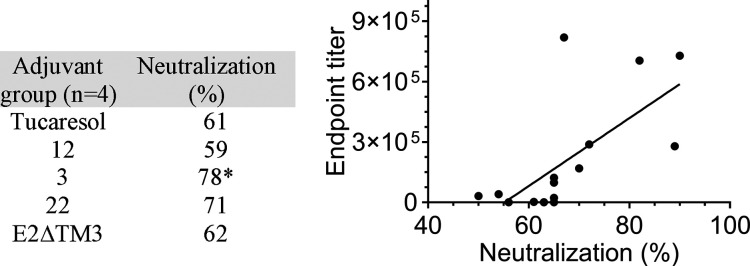
Antibody end point titers in response to vaccination were elucidated by an ELISA-based measurement of sera IgG specific to the native E1E2 antigen. Adjuvant 3 (Table 1) generated the highest E2-specific serum IgG titers, thus appearing to be the superior adjuvant based upon this ELISA data. Compound 3 also produced the least injection site infection. While adjuvants 22 and 12 revealed highly comparable end point IgG values to adjuvant 3, infection at the site of injection was markedly greater.
Table 1. Average Serum IgG End Point Titers (Left) and Error Measurement (Right) Calculated at 3× Background Absorbance for Individual Mice (n = 4) for Each Group.
| End
point titer values |
||
|---|---|---|
| Adjuvant | Bleed 2 | Bleed 3 |
| Tucaresol | 4471 ± 1489 | 74493 ± 39166 |
| 12 | 17295 ± 10253 | 253271 ± 189742 |
| 3 | 18777 ± 8992 | 290929 ± 149783 |
| 6 | 5740 ± 5470 | 8900 ± 7851 |
| 8 | 1883 ± 1578 | 3051 ± 2202 |
| 11 | 3967 ± 1127 | 15869 ± 4507 |
| 16 | 8021 ± 3288 | 32083 ± 13151 |
| 22 | 17623 ± 9902 | 281958 ± 158437 |
| 19 | 8404 ± 2286 | 33616 ± 9145 |
| 18 | 5862 ± 2464 | 23449 ± 9854 |
| E2ΔTM3 | 185 ± 50 | 21 ± 8 |
Based upon ELISA data, the best performing adjuvants were then tested for their ability to neutralize HCV pseudotype virus infectivity in vitro. E1E2 glycoproteins were expressed from an autologous genotype 1a strain HCV (H77) and an unrelated envelope glycoprotein from lymphocytic choriomeningitis virus (LCMV), the latter being used as a negative control (SI). Monoclonal antibody (mAb)19B3 was used as a positive control for all neutralization experiments following literature protocols.30 Positive neutralization, defined as >50%, was highest for the purified polyclonal Ab pooled serum sample of 3 with 78% neutralization at a concentration of 1 μg/mL. This was statistically significant relative to the tucaresol control at the same concentration. For each of the samples tested, high end point titers also correlated with high % neutralization data (P < 0.0016) (Table 2).
Table 2. Left: In Vitro Pseudotype Virus Particle Neutralization with Pooled Immune Sera.a Right: Correlation of Individual (End Point Titer Values EPT) with Neutralization % Values for Each Mouse.
A one-way ANOVA was performed for each immunization group, followed by a Dunnett’s post hoc comparison test, respectively. *P < 0.05. Significance is denoted by an asterisk (*). Neutralization values were averaged for each group (n = 4).
Taken together, these data suggest that introducing a simplified lipid-adjuvant based system positively affects autologous neutralization and presents itself as an alternative approach for rational HCV vaccine design, potentially overcoming the need for elaborate vaccine delivery systems and complex adjuvants. From a tucaresol scaffolding stance, the aldehyde appears to be essential for a robust immune response. Moreover, regiochemistry of the phenol in relationship to the aldehyde was also critical with the para position most favorable, followed by ortho relative to the aldehyde.
For this structural class, the change in physiochemical properties by alteration of pKa (increase in acidity) as seen for ortho hydroxyl analogues 12, 18, 19, and 22 may be responsible for the toxic effects. In addition, the possibility of intramolecular H-bond formation by interaction of the ortho phenol with the aldehyde carbonyl may present additional effects such as assistance with Schiff-base formation, catalytic effects, and subsequent increase in lipophilicity. Strengthening this conclusion, meta substitution of a phenol as seen for 6 was detrimental toward the immune response and no toxicity was observed. Although toxic effects were not observed for both the benzoic (16) and acetophenone (11) structures; with these derivatives a downward trend was seen with the immune response, further strengthening the argument of Schiff-base formation. Moreover, steric effects, i.e. positioning of the lipid chain, are best seen by comparison of adjuvants 3 and 8. Here a significant difference in immune response is observed for 3, but both showed no toxicity despite the ortho phenol displayed in 8. Notably, the end point titer values were 3-fold greater relative to parent tucaresol, which demonstrates that 3 is the best adjuvant in this structural class.
In summary, we have developed a new adjuvant platform and designed a vaccine formulation for neutralizing Ab responses against HCV in vivo. In particular, adjuvants containing E2ΔTM3 antigen elicited promising E2-specific IgG titers with superior neutralizing capacities compared to their respective antigen and tucaresol controls. A positive correlation of % neutralization and end point titers was achieved for lipidated variants of tucaresol for the first time, in a simplified administration procedure without the use of liposomes. While there is scope for further optimization of the vaccine protocol, the direct comparison of truncated tucaresol variants, including removal of the phenoxymethyl benzoic acid and alteration of phenol number and regiochemistry has provided us with solid grounding for enhancing opportunities for new adjuvant development.
Acknowledgments
This work was supported by funding from AI137868.
Glossary
Abbreviations
- HCV
hepatitis C virus.
- COVID-19
coronavirus disease 2019
- MPLA
monophosphoryl lipid A.
- Ab
antibody
- mAb
monoclonal antibody
- SAR
structure activity relationship
- (LCMV)
lymphocytic choriomeningitis virus
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsmedchemlett.0c00413.
Experimental details of the synthetic adjuvants prepared, vaccine administration protocol, and immunological assay methods (PDF)
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Zibbell J. E.; Asher A. K.; Patel R. C.; Kupronis B.; Iqbal K.; Ward J. W.; Holtzman D. Increases in Acute Hepatitis C Virus Infection Related to a Growing Opioid Epidemic and Associated Injection Drug Use, United States, 2004 to 2014. Am. J. Public Health 2018, 108, 175. 10.2105/AJPH.2017.304132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharareh N.; Hess R.; White S.; Dunn A.; Singer P. M.; Cochran J. A vulnerability assessment for the HCV infections associated with injection drug use. Prev. Med. 2020, 134, 106040. 10.1016/j.ypmed.2020.106040. [DOI] [PubMed] [Google Scholar]
- Liang T. J.; Ward J. W. Hepatitis C in Injection-Drug Users - A Hidden Danger of the Opioid Epidemic. N. Engl. J. Med. 2018, 378, 1169. 10.1056/NEJMp1716871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Sierra C.; Arizcorreta A.; Diaz F.; Roldan R.; Martin-Herrera L.; Perez-Guzman E.; Giron-Gonzalez J. A. Progression of Chronic Hepatitis C to Liver Fibrosis and Cirrhosis in Patients Coinfected with Hepatitis C Virus and Human Immunodeficiency Virus. Clin. Infect. Dis. 2003, 36, 491. 10.1086/367643. [DOI] [PubMed] [Google Scholar]
- Ghouri Y. A.; Mian I.; Rowe J. H. Review of hepatocellular carcinoma: Epidemiology, etiology, and carcinogenesis. J. Carcinog. 2017, 16, 1. 10.4103/jcar.JCar_9_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health O.Guidelines for the screening, care and treatment of persons with chronic hepatitis C infection; Updated version, April 2016 ed.; World Health Organization: Geneva, 2016. [PubMed] [Google Scholar]
- Thoelen S.; De Clercq N.; Tornieporth N. A prophylactic hepatitis B vaccine with a novel adjuvant system. Vaccine 2001, 19, 2400. 10.1016/S0264-410X(00)00462-X. [DOI] [PubMed] [Google Scholar]
- Harper D. M.; Franco E. L.; Wheeler C.; Ferris D. G.; Jenkins D.; Schuind A.; Zahaf T.; Innis B.; Naud P.; De Carvalho N. S.; Roteli-Martins C. M.; Teixeira J.; Blatter M. M.; Korn A. P.; Quint W.; Dubin G. Efficacy of a bivalent L1 virus-like particle vaccine in prevention of infection with human papillomavirus types 16 and 18 in young women: a randomised controlled trial. Lancet 2004, 364, 1757. 10.1016/S0140-6736(04)17398-4. [DOI] [PubMed] [Google Scholar]
- Calabro S.; Tritto E.; Pezzotti A.; Taccone M.; Muzzi A.; Bertholet S.; De Gregorio E.; O’Hagan D. T.; Baudner B.; Seubert A. The adjuvant effect of MF59 is due to the oil-in-water emulsion formulation, none of the individual components induce a comparable adjuvant effect. Vaccine 2013, 31, 3363. 10.1016/j.vaccine.2013.05.007. [DOI] [PubMed] [Google Scholar]
- Petrovsky N.; Aguilar J. C. Vaccine adjuvants: Current state and future trends. Immunol. Cell Biol. 2004, 82, 488. 10.1111/j.0818-9641.2004.01272.x. [DOI] [PubMed] [Google Scholar]
- Lindblad E. B. Aluminium compounds for use in vaccines. Immunol. Cell Biol. 2004, 82, 497. 10.1111/j.0818-9641.2004.01286.x. [DOI] [PubMed] [Google Scholar]
- Marrack P.; McKee A. S.; Munks M. W. Towards an understanding of the adjuvant action of aluminium. Nat. Rev. Immunol. 2009, 9, 287. 10.1038/nri2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garçon N.; Chomez P.; Van Mechelen M. GlaxoSmithKline Adjuvant Systems in vaccines: concepts, achievements and perspectives. Expert Rev. Vaccines 2007, 6, 723. 10.1586/14760584.6.5.723. [DOI] [PubMed] [Google Scholar]
- Bazzill J. D.; Ochyl L. J.; Giang E.; Castillo S.; Law M.; Moon J. Interrogation of Antigen Display on Individual Vaccine Nanoparticles for Achieving Neutralizing Antibody Responses against Hepatitis C Virus. Nano Lett. 2018, 18, 7832. 10.1021/acs.nanolett.8b03601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krapchev V. B.; Rychłowska M.; Chmielewska A.; Zimmer K.; Patel A. H.; Bieńkowska-Szewczyk K. Recombinant Flag-tagged E1E2 glycoproteins from three hepatitis C virus genotypes are biologically functional and elicit cross-reactive neutralizing antibodies in mice. Virology 2018, 519, 33. 10.1016/j.virol.2018.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan M.; Law J.; Wong J. A. J.-X.; Hockman D.; Landi A.; Chen C.; Crawford K.; Kundu J.; Baldwin L.; Johnson J.; Dahiya A.; LaChance G.; Marcotrigiano J.; Law M.; Foung S.; Tyrrell L.; Houghton M. Native Folding of a Recombinant gpE1/gpE2 Heterodimer Vaccine Antigen from a Precursor Protein Fused with Fc IgG. J. Virol. 2017, 91, e01552 10.1128/JVI.01552-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes J.; Chen H.; Hall S. R.; Beesley J. E.; Jenkins D. C.; Collins P.; Zheng B. Therapeutic potentiation of the immune system by costimulatory Schiff-base-forming drugs. Nature 1995, 377, 71. 10.1038/377071a0. [DOI] [PubMed] [Google Scholar]
- Rhodes J. Evidence for an intercellular covalent reaction essential in antigen-specific T cell activation. J. Immunol. 1989, 143, 1482. [PubMed] [Google Scholar]
- Rhodes J. Erythrocyte rosettes provide an analogue for Schiff base formation in specific T cell activation. J. Immunol. 1990, 145, 463. [PubMed] [Google Scholar]
- Collins K. C.; Schlosburg J. E.; Lockner J. W.; Bremer P. T.; Ellis B. A.; Janda K. D. Lipid tucaresol as an adjuvant for methamphetamine vaccine development. Chem. Commun. (Cambridge, U. K.) 2014, 50, 4079. 10.1039/C4CC00682H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang C. S.; Ellis B.; Zhou B.; Janda K. D. Heat shock proteins: A dual carrier-adjuvant for an anti-drug vaccine against heroin. Bioorg. Med. Chem. 2019, 27, 125. 10.1016/j.bmc.2018.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Schaewen M.; Ploss A. Murine models of hepatitis C: what can we look forward to?. Antiviral Res. 2014, 104, 15. 10.1016/j.antiviral.2014.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choo Q. L.; Kuo G.; Ralston R.; Weiner A.; Chien D.; Van Nest G.; Han J.; Berger K.; Thudium K.; Kuo C. Vaccination of chimpanzees against infection by the hepatitis C virus. Proc. Natl. Acad. Sci. U. S. A. 1994, 91, 1294. 10.1073/pnas.91.4.1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey S. E.; Houghton M.; Coates S.; Abrignani S.; Chien D.; Rosa D.; Pileri P.; Ray R.; Di Bisceglie A. M.; Rinella P.; Hill H.; Wolff M. C.; Schultze V.; Han J. H.; Scharschmidt B.; Belshe R. B. Safety and immunogenicity of HCV E1E2 vaccine adjuvanted with MF59 administered to healthy adults. Vaccine 2010, 28, 6367. 10.1016/j.vaccine.2010.06.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralston R.; Thudium K.; Berger K.; Kuo C.; Gervase B.; Hall J.; Selby M.; Kuo G.; Houghton M.; Choo Q. L. Characterization of hepatitis C virus envelope glycoprotein complexes expressed by recombinant vac cinia viruses. J. Virol. 1993, 67, 6753. 10.1128/JVI.67.11.6753-6761.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majerus S. L.; Alibhai N.; Tripathy S.; Durst T. New syntheses of dillapiol [4,5-dimethoxy-6-(2-propenyl)-1,3-benzodioxole], its 4-methylthio and other analogs. Can. J. Chem. 2000, 78, 1345. 10.1139/v00-138. [DOI] [Google Scholar]
- Ruwona T. B.; Giang E.; Nieusma T.; Law M. Fine Mapping of Murine Antibody Responses to Immunization with a Novel Soluble Form of Hepatitis C Virus Envelope Glycoprotein Complex. J. Virol. 2014, 88, 10459. 10.1128/JVI.01584-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopal R.; Jackson K.; Tzarum N.; Kong L.; Ettenger A.; Guest J.; Pfaff J. M.; Barnes T.; Honda A.; Giang E.; Davidson E.; Wilson I. A.; Doranz B. J.; Law M. Probing the antigenicity of hepatitis C virus envelope glycoprotein complex by high-throughput mutagenesis. PLoS Pathog. 2017, 13, e1006735 10.1371/journal.ppat.1006735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L.; Tzarum N.; Lin X.; Shapero B.; Sou C.; Mann C. J.; Stano A.; Zhang L.; Nagy K.; Giang E.; Law M.; Wilson I. A.; Zhu Proof of concept for rational design of hepatitis C virus E2 core nanoparticle vaccines. J. Sci. Adv. 2020, 6, 6225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aleman F.; Tzarum N.; Kong L.; Nagy K.; Zhu J.; Wilson I. A.; Law M. Immunogenetic and structural analysis of a class of HCV broadly neu-tralizing antibodies and their precursors. Proc. Natl. Acad. Sci. U. S. A. 2018, 115, 7569. 10.1073/pnas.1802378115. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.