SUMMARY
Fibroblast growth factor 21 (FGF21) is an endocrine hormone produced by the liver that regulates nutrient and metabolic homeostasis. FGF21 production is increased in response to macronutrient imbalance and signals to the brain to suppress sugar intake and sweet-taste preference. However, the central targets mediating these effects have been unclear. Here, we identify FGF21 target cells in the hypothalamus and reveal that FGF21 signaling to glutamatergic neurons is both necessary and sufficient to mediate FGF21-induced sugar suppression and sweet-taste preference. Moreover, we show that FGF21 acts directly in the ventromedial hypothalamus (VMH) to specifically regulate sucrose intake but not non-nutritive sweet-taste preference, body weight or energy expenditure. Finally, our data demonstrates that FGF21 affects neuronal activity by increasing activation and excitability of neurons in the VMH. Thus, FGF21 signaling to glutamatergic neurons in the VMH is an important component of the neurocircuitry that functions to regulate sucrose intake.
eTOC
FGF21 is a liver-derived hormone that signals to the brain to regulate macronutrient intake and energy homeostasis. Here, it is shown that FGF21 administration signals to glutamatergic neurons in the ventromedial hypothalamus (VMH) to suppress sugar intake but not increase energy expenditure, while enhancing glucose responsiveness of VMH glucose-sensitive neurons to elevated glucose levels.
Graphical Abstract
INTRODUCTION
The prevalence of obesity worldwide represents an ongoing health-care crisis, likely driven by changes in diet composition and an increasingly sedentary lifestyle. Humans exhibit a wide range of dietary preferences and it is recognized that changes in macronutrient preference can contribute to metabolic dysfunction (Solon-Biet et al., 2014). Lifestyle modifications that target dietary macronutrient composition represent a promising approach for preventing body weight gain and metabolic disease in obesogenic environments. Macronutrient preference is controlled by the central nervous system (CNS) through the integration and processing of complex metabolic information relayed via peripheral hormones and metabolites (Calvo and Egan, 2015). For a differential preference of macronutrient intake to occur, one or more senses (e.g., taste) must distinguish nutrient content as well as potential changes in energy content (Thibault and Booth, 1999). Carbohydrates, or sugars, are highly palatable and promote ingestive behavior through gustatory and post-ingestive pathways (Sclafani, 2001; Zuker, 2015). The rewarding properties of sugars consist of both sweet taste and nutrient caloric value which are sensed and transmitted via distinct neural circuits for higher order processing (Tellez et al., 2016). Afferent signals from taste buds in the mouth are transmitted to hindbrain regions, such as the nucleus tractus solitarius (NTS), which relay taste information through projections to forebrain areas including the hypothalamus (Fernstrom et al., 2012). The hypothalamus is an important satiety center in the CNS which responds to peripheral cues but also possesses regions such as the ventromedial hypothalamus (VMH), which are critical for glucose sensing and the regulation of glucose homeostasis (Andermann and Lowell, 2017; Elmquist et al., 2005; Vianna and Coppari, 2011). However, the identity of metabolic cues and their central targets that regulate macronutrient preference are incompletely understood.
The liver-derived hormone fibroblast growth factor 21 (FGF21) is an important regulator of macronutrient intake. Single nucleotide polymorphisms (SNPs) at the FGF21 locus are associated with changes in macronutrient intake, specifically increased carbohydrate intake (Chu et al., 2013; Frayling et al., 2018; Tanaka et al., 2013) and increased preference for sweets (Soberg et al., 2017). FGF21 expression is induced in the liver in response to high carbohydrate (Fisher et al., 2017; Lundsgaard et al., 2017; von Holstein-Rathlou et al., 2016) and protein restriction (Laeger et al., 2014; Solon-Biet et al., 2016), but is maximally induced by low-protein, high carbohydrate feeding (Solon-Biet et al., 2016). FGF21 then enters the circulation to act on the CNS to suppress simple sugar intake and sweet taste preference (Talukdar et al., 2016a; von Holstein-Rathlou et al., 2016). Notably, FGF21 appears to specifically suppress carbohydrate intake without reducing total caloric intake in mice (von Holstein-Rathlou et al., 2016).
FGF21 is a pleotropic endocrine hormone with multiple metabolic functions (BonDurant and Potthoff, 2018) and in addition to its effects on regulating simple sugar intake, FGF21 also regulates energy homeostasis and acute insulin sensitivity through direct actions on the CNS and adipose tissues, respectively (BonDurant et al., 2017; Lan et al., 2017; Owen et al., 2014). Some FGF21 analogs (LY2405319 and PF-05231023) significantly reduce body weight and markedly lower lipid levels in type 2 diabetic and obese subjects, but the effects on glycemia and insulin sensitivity are less clear (Gaich et al., 2013; Talukdar et al., 2016b).
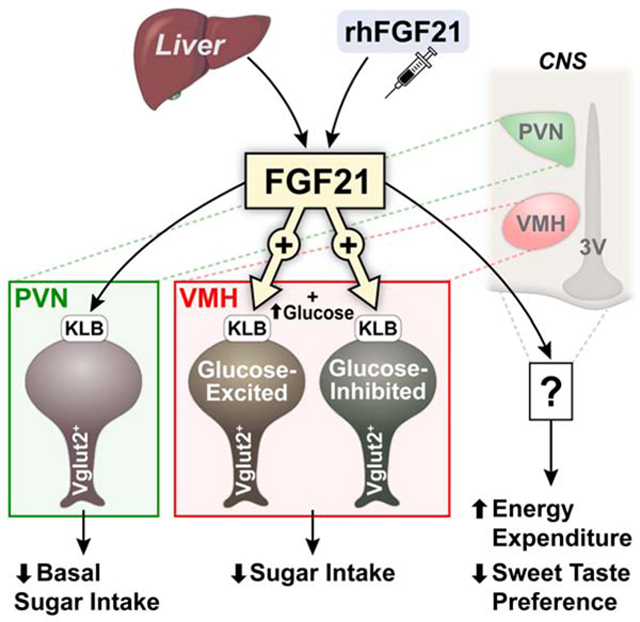
FGF21 acts through a cell-surface receptor complex comprised of the canonical FGF receptor (FGFR1c) and its obligate co-receptor, β-klotho (KLB) (Adams et al., 2012; Ding et al., 2012; Ogawa et al., 2007). While FGFR1c is ubiquitously expressed, KLB is expressed in specific metabolic tissues thereby conferring signaling specificity for FGF21 (Fon Tacer et al., 2010). FGF21 crosses the blood-brain barrier (Hsuchou et al., 2007) and associates with its receptor complex expressed in different regions of the CNS. We demonstrated previously that the hypothalamus is an important target site for FGF21-mediated suppression of simple sugar intake (von Holstein-Rathlou et al., 2016). Using knock-in reporter mice that express Cre recombinase under the control of the endogenous Klb promoter, here, we show that multiple cell types have the capacity to respond to FGF21, but that FGF21 signaling to glutamatergic, but not GABAergic or dopaminergic, neurons is necessary and sufficient for FGF21-mediated suppression of sucrose intake and non-nutritive sweet taste preference. In addition, we show that KLB is expressed in the VMH and FGF21 signaling directly to the VMH is important for FGF21-mediated suppression of simple sugar intake but not its effects on energy expenditure. Finally, we reveal that FGF21 potentiates the response of both glucose-excited and glucose-inhibited neurons to increases, but not decreases, in glucose levels. Together, this work identifies the VMH as an important target of FGF21 in the hypothalamus capable of decreasing sucrose consumption to protect the liver from metabolic stress.
RESULTS
Characterization of β-klotho Expression in the Mouse Brain
Attempts to identify targets of FGF21 in the CNS have been hindered by low β-klotho (KLB) expression in the CNS and the lack of reliable antibodies to detect KLB. To identify cells that express KLB in vivo, we engineered mice that express CRE-recombinase and tdTomato under the control of the endogenous Klb promoter. These Klb-CRE mice were generated by inserting, in frame with the ATG in exon 1 of Klb, a construct containing the CRE-recombinase and the tdTomato reporter coding sequence separated by an IRES sequence (Fig. 1A and Fig. S1A,B). This allows the expression of both proteins (CRE and tdTomato) from one bi-cistronic mRNA. As expected, based on the known expression of KLB in the liver, tdTomato expression can be readily observed in the livers of Klb-CRE mice (Fig. S1C). Notably, however, since endogenous expression of KLB in the CNS is so low compared to other tissues, tdTomato expression was not visible in any brain regions of these mice (Fig. S1D). To circumvent this issue, we crossed Klb-CRE mice to Ai14-tdTomato reporter mice to allow high CRE-dependent expression of tdTomato (Klb-CRE;Ai14 tdTomato). Expression of tdTomato was then readily observed in multiple areas of the hypothalamus of Klb-CRE;Ai14 tdTomato mice including the VMH, suprachiasmatic nucleus (SCN), paraventricular nucleus (PVN), and arcuate nucleus (ARC) (Fig. 1B). KLB- positive cells were also readily observed in hindbrain regions including the area postrema (AP) and NTS (Fig. S1E). In contrast, very few cells were observed in the nucleus accumbens (NAc) (Fig. S1F).
Figure 1. β-Klotho is expressed in multiple cell types in the hypothalamus.
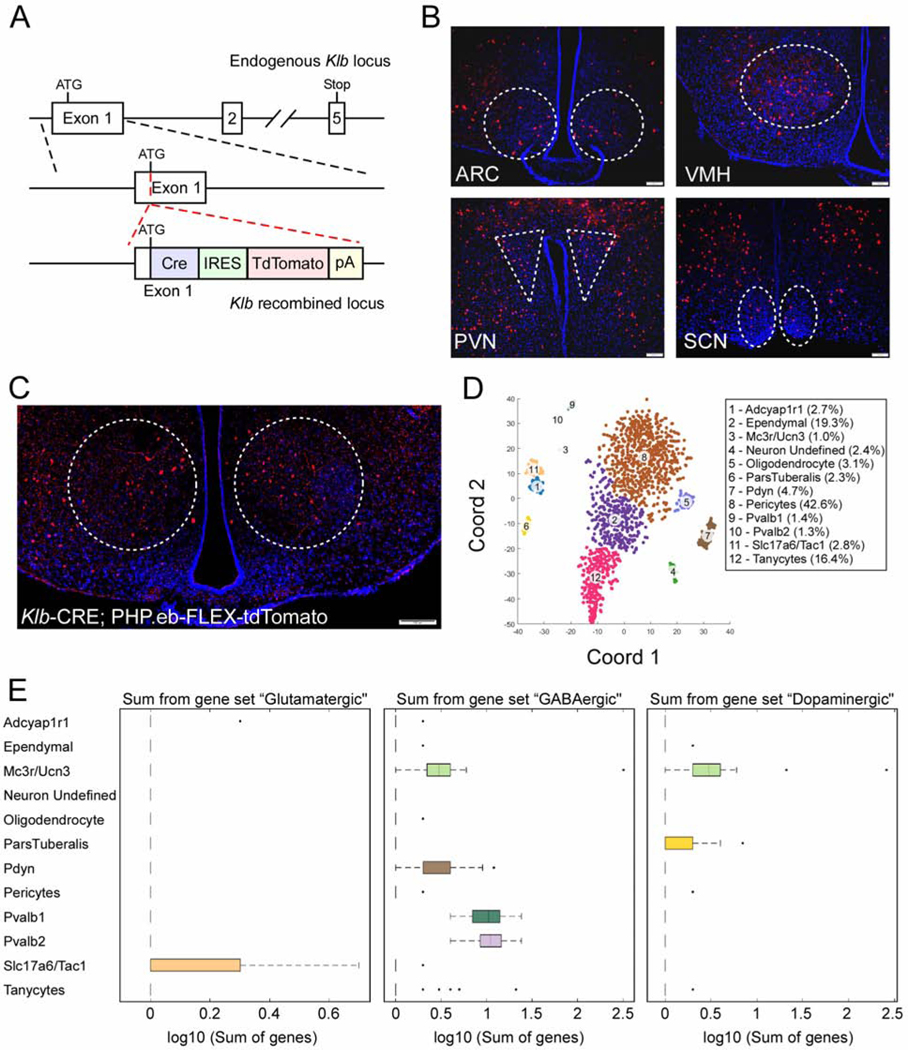
(A) Schematic representation of the targeting strategy of KLB-Cre mice. Solid lines represent chromosome sequences. Cre, Cre recombinase coding sequence; IRES, internal ribosome entry site; tdTomato, fluorescence reporter gene; pA, polyadenylation are all represented by colored rectangles.
(B) Representative fluorescence imaging (n = 20 total images analyzed) for tdTomato positive cells in the arcuate nucleus (ARC), ventromedial hypothalamus (VMH), paraventricular nucleus (PVN), and the superchiasmatic nucleus (SCN) of 12-week-old Klb-CRE;Ai14 tdTomato mice.
(C) Fluorescence imaging for tdTomato positive cells in the VMH of 12-week old Klb-CRE mice following infection with a PHP.eb-FLEX-tdTomato virus.
(D) tSNE analysis from single cell RNA sequencing on FACs sorted tdTomato-expressing cells from hypothalami of 11–13-week-old male Klb-CRE;Ai14 tdTomato mice. Cell types were defined based on differentially expressed genes within the indicated clusters.
(E) Expression of glutamatergic, GABAergic, and dopaminergic markers in identified cell clusters represented in box plots. Scale bars, 100μm.
To determine whether the observed tdTomato expression was a result of lineage tracing or active KLB expression, we administered a PHP.eb-FLEX-tdTomato reporter virus to adult Klb-CRE mice. Administration of a PHP.eb virus results in systemic infection of cells in the CNS (Chan et al., 2017). Consistent with the expression of tdTomato in Klb-CRE;Ai14 tdTomato mice, tdTomato expression was observed in a similar pattern in the hypothalamus of Klb-CRE mice administered PHP.eb-FLEX-tdTomato, although the VMH exhibited particularly robust expression (Fig. 1C).
To assess in an unbiased manner which cells express Klb in the hypothalamus, we performed single-cell RNA sequencing (scRNAseq) on FACS sorted tdTomato-expressing cells from hypothalami of Klb-CRE;Ai14 tdTomato mice. This was accomplished using a new, customized single-cell sequencing platform (BD Rhapsody), to assess cellular signatures identified through previous scRNAseq studies (Campbell et al., 2017; Chen et al., 2017; Romanov et al., 2017). This panel allows efficient analysis of both low- and high-abundant transcripts (Table S1 and S2). Following efficient cell sorting for viable cells (Fig. S1G), quality filtering, and annotation of the sequencing data, we obtained 1,904 sorted putative KLB+ cells. We then performed tSNE analysis and unsupervised clustering and identified 12 clusters of neuronal and non-neuronal cell types (Fig. 1D). Among the neuronal clusters we observed expression of glutamatergic, GABAergic, and dopaminergic markers with some overlapping expression in certain populations (Fig. 1E). We obtained similar results performing scRNAseq using the 10X Chromium platform (Fig. S1H). In addition, these results are in concordance with previous data indicating Klb is expressed in glutamatergic, GABAergic, dopaminergic and non-neuronal cell types within the hypothalamus (Romanov et al., 2017).
FGF21 Signals to Glutamatergic Neurons to Suppress Sugar and Non-nutritive Sweet-Taste Preference
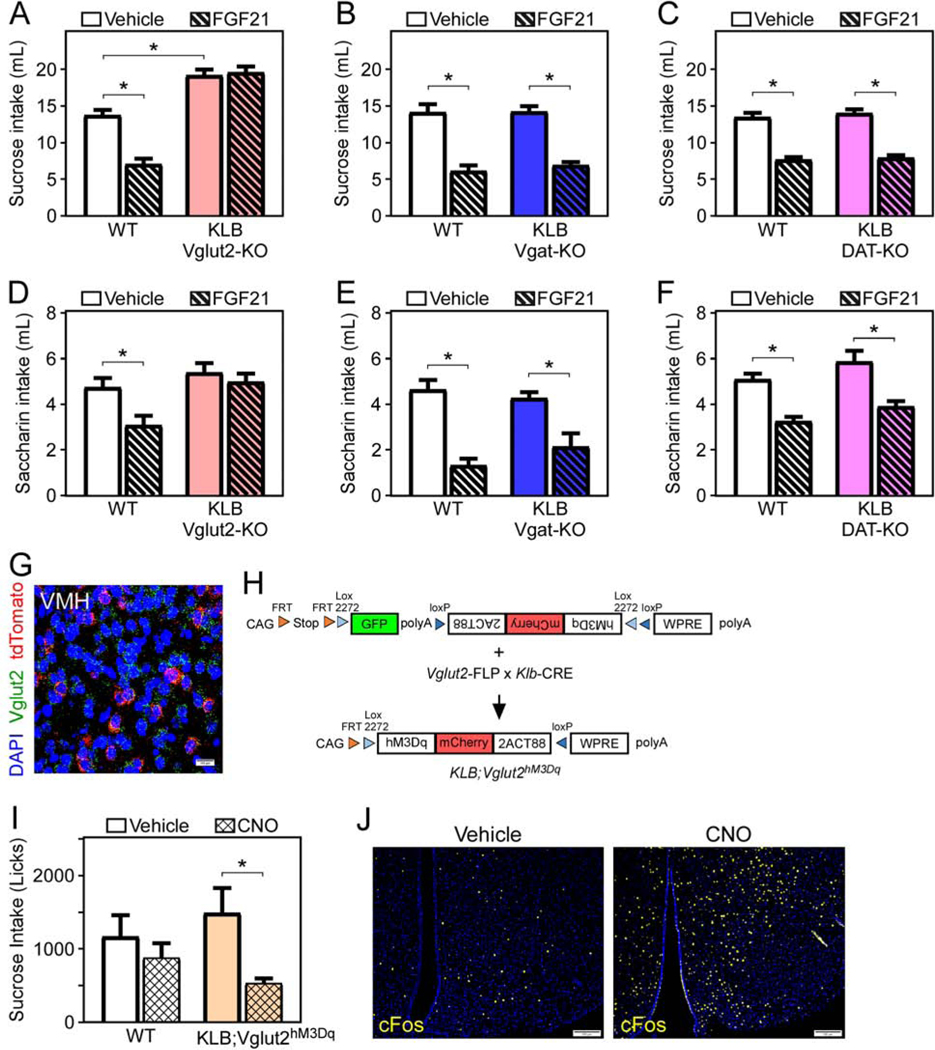
To begin to explore the neuronal target(s) of FGF21 action, we evaluated if FGF21’s effects are mediated primarily by excitatory (i.e., glutamatergic, VGLUT2+), inhibitory (i.e., GABAergic, VGAT+), or dopaminergic (i.e., DAT+) neurons. The purpose of this approach is to provide insight into the molecular identity of the neurons mediating FGF21’s effect on sugar satiety and provide information regarding the function of FGF21-responsive neurons (excitatory versus inhibitory versus modulatory). To accomplish this, we generated mice that lack β-klotho specifically in glutamatergic (KLB Vglut2-KO), GABAergic (KLB Vgat-KO), or dopaminergic (KLB DAT-KO) neurons by crossing KLBfl/fl mice (Ding et al., 2012) with Vglut2-IRES-CRE (Vong et al., 2011), Vgat-IRES-CRE (Vong et al., 2011), or DAT-IRES-CRE mice (Backman et al., 2006), respectively. As expected, the expression pattern of Vglut2-, Vgat-, and DAT-expressing cells in the CNS was as previously reported (Fig. S2A). All wild-type (WT) and littermate knock-out mice were given ad-libitum access to a 10% sucrose solution and water using a two-bottle choice paradigm, and sucrose intake and preference were assessed as described (von Holstein-Rathlou et al., 2016). Briefly, we acclimated mice for 4 days with daily i.p. injections of vehicle, and then offered water and sucrose ad-libitum for 3 days while administering daily vehicle injections. Following 3 days of vehicle injections, the same mice were administered FGF21 for 3 days and 10% sucrose solution and water intake was assessed throughout this time. Similar to mice lacking FGF21 (FGF21 KO) (von Holstein-Rathlou et al., 2016), KLB Vglut2-KO mice exhibit a marked elevation in sucrose intake relative to WT littermate controls (Fig. 2A and S2B). Notably, compared to male and female WT mice, which markedly reduce sugar intake in response to FGF21 (Fig. 2A, S2B and S2C), KLB Vglut2-KO mice are resistant to FGF21-mediated suppression of sucrose intake and preference (Fig. 2A, S2B, and S2C). Importantly, FGF21 administration had no effect on water intake (Fig. S2B). A direct comparison of WT male and female mice revealed no differences in FGF21-mediated suppression of sucrose intake between sexes (Fig. S2D). In contrast to KLB Vglut2-KO mice, KLB Vgat-KO and KLB DAT-KO mice exhibit no change in sucrose preference and are phenotypically similar to WT mice in the ability of FGF21 to suppress sucrose intake (Fig. 2B,C).
Figure 2. FGF21 signaling to glutamatergic neurons is necessary and sufficient for FGF21-mediated suppression of sucrose intake.
(A-C) Fluid intake during two bottle choice of 10% sucrose versus water in 11–13 week old male WT mice or mice lacking KLB in (A) Vglut2- (KLB Vglut2-KO), (B) Vgat- (KLB Vgat-KO), or (C) DAT-expressing cells (KLB DAT-KO) while receiving daily injections of vehicle (3 days) followed by daily injections of FGF21 (1 mg/kg; 3 days) via i.p. injection (n = 11–21/group).
(D-F) Fluid intake during two-bottle choice of 0.2% saccharin versus water in 11–13-week-old male WT and (D) KLB Vglut2-KO, (E) KLB Vgat-KO, and (F) KLB DAT-KO mice while receiving daily injections of vehicle (3 days) followed by daily injections of FGF21 (1 mg/kg; 3 days) via i.p. injection (n = 7–17/group).
(G) Fluorescence in situ hybridization (FISH) demonstrating co-localization of Vglut2 (green) and tdTomato (red) in the ventromedial hypothalamus (VMH) of Klb-CRE;Ai14 tdTomato mice.
(H) Schematic representation of the generation of KLB;Vglut2hM3Dq triple knock-in mice. CAG, CAG promoter coding sequence; FRT, FRT sequence; LoxP, LoxP sequence; GFP; green fluorescent protein; hM3Dq-mCherry; excitatory DREADDs construct; WPRE, gene enhancer sequence; polyA, polyadenylation.
(I) Fluid intake (licks) during two-bottle choice of 10% sucrose intake versus water assessed using a lickometer in 11–13-week-old WT and KLB;Vglut2hM3Dq male mice treated with vehicle or clozapine-n-oxide (CNO) (1 mg/kg) via i.p. injections for 3 days (n = 6–8/group).
(J) Immunofluorescence imaging for cFos in the VMH of WT and KLB;Vglut2hM3Dq following 1-hour treatment with CNO.
Values are mean +/− SEM. (*, P < 0.05 compared to WT). Statistical analyses were conducted using 2-way ANOVA for multiple comparisons. Scale bars, 100μm.
To examine whether the effects of FGF21 on sweet taste preference were also mediated by KLB on Vglut2+ neurons, we next provided WT, KLB Vglut2-KO, KLB Vgat-KO, and KLB DAT-KO mice ad-libitum access to water and the non-caloric sweetener saccharin in the presence and absence of FGF21 using the two-bottle choice paradigm. While WT, KLB Vgat-KO, and KLB DAT-KO mice responded to FGF21 by reducing their saccharin intake, FGF21 had no effect on lowering saccharin intake in KLB Vglut2-KO mice (Fig. 2D-F). Consistent with the scRNAseq data and loss-of-function data, in situ hybridization confirmed that a subset of KLB-expressing neurons in the hypothalamus express Vglut2 (Fig. 2G). Together, these data indicate that FGF21 requires direct signaling to glutamatergic neurons to reduce both sugar intake and sweet-taste preference.
Next, we tested whether activation of glutamatergic KLB neurons (Vglut2+; KLB+) was sufficient to suppress sugar intake by expressing Designer Receptors Exclusively Activated by Designer Drugs (DREADDs) specifically in Vglut2+; KLB+ neurons. To accomplish this, we utilized transgenic mice that express a dual FLP and CRE-dependent human Gq-coupled M3 muscarinic receptor (hM3Dq) C-terminally tagged with mCherry (RC::FL-hM3Dq mice; Fig. 2H) (Sciolino et al., 2016). These mice were crossed to mice which express FLP recombinase under the control of the endogenous Vglut2 promoter (Vglut2-IRES-FLP), and Klb-CRE mice to yield triple knock-in mice (KLB;Vglut2hM3Dq; Fig. 2H). As expected, KLB;Vglut2hM3Dq mice only express mCherry when all three alleles are present (Fig. S2E). KLB;Vglut2hM3Dq and control mice were acclimated to two-bottle choice of sucrose and water for 2 hours daily using a lickometer to analyze drinking behavior. While the DREADDs activating ligand clozapine-N-oxide (CNO) had no effect on WT mice, it significantly reduced sucrose licks and intake in KLB;Vglut2hM3Dq mice (Fig. 2I). To identify hM3Dq-activated neurons, we subsequently treated mice with CNO for 1 hour, collected their brains, and then stained for cFos. Consistent with DREADD-mediated activation of neurons, KLB;Vglut2 hM3Dq mice had higher cFos staining in the hypothalamus compared to their vehicle injected littermate controls (Fig. 2J). These data demonstrate that acute activation of glutamatergic KLB neurons is sufficient to suppress sucrose intake.
FGF21 Signaling to the Paraventricular Nucleus of the Hypothalamus is Not Required to Suppress Sugar Intake
Multiple studies have implicated the PVN as a target site of FGF21 action (Liang et al., 2014; Song et al., 2018; von Holstein-Rathlou et al., 2016). The PVN produces numerous neuropeptides that regulate energy homeostasis and sugar preference, including oxytocin (Klockars et al., 2015; Ott et al., 2013) and arginine-vasopressin (AVP) (Song and Albers, 2018). To determine whether FGF21 signals directly to oxytocin or AVP neurons to mediate sugar satiety, we generated mice that lack KLB specifically in oxytocin neurons or AVP neurons by crossing KLBfl/fl mice with Oxytocin-IRES-CRE mice (Wu et al., 2012) (KLB Oxy-KO) or AVP-IRES-CRE mice (Harris et al., 2014) (KLB AVP-KO), respectively. We analyzed sugar intake in KLB Oxy-KO, KLB AVP-KO, and WT littermate control mice in the absence and presence of FGF21 using a two-bottle choice assay. Both KLB Oxy-KO and KLB AVP-KO mice exhibited significantly elevated basal sucrose intake compared to WT littermate controls (Fig. 3A,B). However, both KLB Oxy-KO and KLB AVP-KO mice significantly decreased sucrose intake in response to FGF21 to a similar extent as WT controls (Fig. 3A,B). Since previous studies have implicated corticotrophin-releasing hormone (CRH) neurons as a potential target for FGF21 (Owen et al., 2014), we next generated KLB CRH-KO mice by crossing KLBfl/fl mice with CRH-IRES-CRE mice (Taniguchi et al., 2011) (KLB CRH-KO) and assessed sugar intake and preference in the presence and absence of FGF21. Similar to KLB Oxy-KO and KLB AVP-KO mice (Fig. 3A,B), both WT and KLB CRH-KO mice responded to FGF21 by decreasing sucrose intake (Fig. S3A). We next explored the importance of direct FGF21 action on the entire PVN by crossing KLBfl/fl mice with Sim1-CRE mice which express Cre-recombinase in the PVN (Balthasar et al., 2005). Consistent with KLB Oxy-KO and KLB AVP-KO mice, KLB Sim1-KO mice had higher basal sucrose intake compared to WT littermate controls but significantly decreased sucrose intake in response to FGF21 (Fig. 3C). In contrast, deletion of KLB from hindbrain regions including the AP and NTS using Phox2b-Cre mice, did not affect basal or FGF21-mediated suppression of sucrose intake (Fig. S3B). Together, these data demonstrate that FGF21 signaling directly to the PVN influences basal sucrose intake but is not required for FGF21-mediated suppression of sucrose intake.
Figure 3. The role of FGF21 signaling to the PVN.
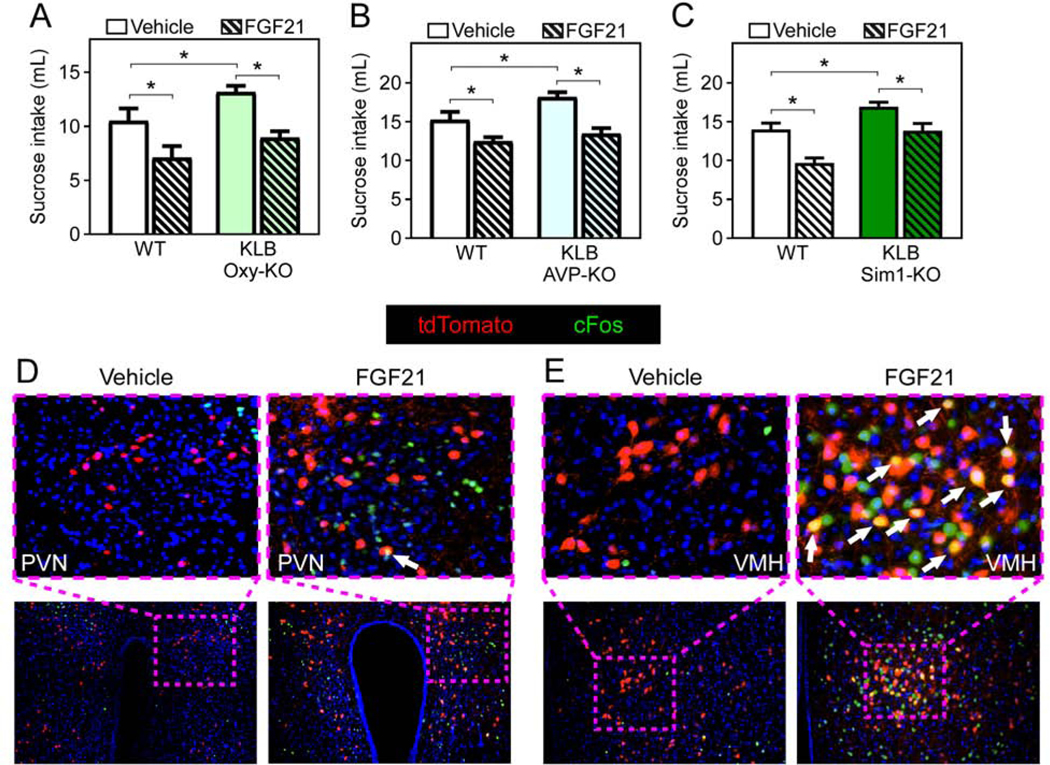
(A-C) Fluid intake during two-bottle choice of 10% sucrose versus water in in 11–13 week old male WT mice or mice lacking KLB in (A) Oxytocin- (KLB Oxy-KO), (B) arginine vasopressin-(KLB AVP-KO), or (C) Sim1-expressing cells (KLB Sim1-KO) treated with vehicle or FGF21 (1 mg/kg) i.p. injection for 3 days (n = 6–15/group).
(D and E) Immunofluorescence imaging for cFos in the (D) paraventricular nucleus of the hypothalamus (PVN) or (E) ventromedial hypothalamus (VMH) from Klb-CRE;Ai14 tdTomato mice treated with vehicle or FGF21 (1 mg/kg) i.p. injection for 1 hour (white arrows, cFos and KLB double-positive cells).
Values are mean +/− SEM. (*, P < 0.05 compared to WT). Statistical analyses were conducted using 2-way ANOVA for multiple comparisons.
FGF21 Signaling to the Ventromedial Hypothalamus is Required to Suppress Sugar Intake but not to Increase Energy Expenditure
To identify direct central targets of FGF21 action, we next evaluated regions of the hypothalamus that are activated in response to FGF21 using cFos staining. While we identified increased cFos expression in multiple regions of the hypothalamus in response to FGF21 administration, including the PVN as we have demonstrated previously (von Holstein-Rathlou et al., 2016), very few cFos positive cells were KLB+ (Fig. 3D). Instead, we observed significant cFos reactivity in KLB+ neurons in the VMH (Fig. 3E). In situ hybridization for steroidogenic factor 1 (SF1), a VMH-specific marker, and tdTomato in Klb-CRE;Ai14 tdTomato mice confirmed the presence of KLB+ neurons in the VMH (Fig. S4A). Additionally, in silico analysis of single-cell sequencing data acquired in VMH neurons (Kim et al., 2019) revealed that all KLB+ neurons in the VMH co-express Vglut2 (Fig. S4B).
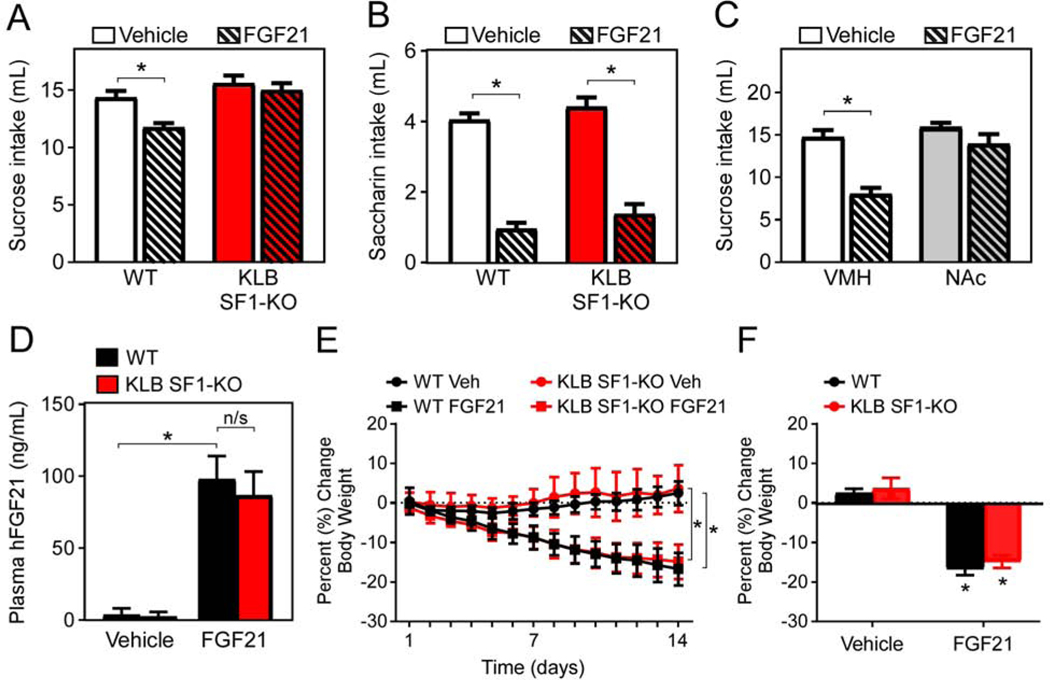
The VMH is a key brain region involved in feeding and metabolism. Studies altering the nutrient- and hormone-sensing pathways in SF1+ neurons have confirmed the role of glutamatergic neurons in the VMH in the regulation of food intake and glucose metabolism (Shimazu and Minokoshi, 2017; Tong et al., 2007). To determine whether FGF21 acts in the VMH to decrease sugar consumption, we generated mice lacking KLB in SF1+ neurons by crossing KLBfl/fl mice with SF1-CRE mice (KLB SF1-KO). We then assessed sucrose intake in WT and KLB SF1-KO mice in the absence and presence of FGF21. Importantly, while sucrose intake was lowered in WT mice in response to FGF21, FGF21-mediated suppression of sugar intake was impaired in KLB SF1-KO mice (Fig. 4A). Surprisingly, however, FGF21’s ability to suppress the intake of the non-caloric sweetener saccharin was maintained (Fig. 4B). Together with the KLB Vglut2-KO data (Fig. 2A), these data suggest that FGF21 signaling to glutamatergic neurons in the VMH is important for maximal suppression of sugar intake, but FGF21 can also act on additional regions of the CNS to reduce non-nutritive sweet taste preference.
Figure 4. FGF21 signaling to the VMH is necessary and sufficient for FGF21 to suppress sucrose intake, but not to lower body weight.
(A) Fluid intake during two-bottle choice of 10% sucrose versus water in 11–13-week-old male WT mice and mice lacking KLB in SF1+ neurons (KLB SF1-KO) while receiving daily injections of vehicle (3 days) followed by daily injections of FGF21 (1 mg/kg; 3 days) via i.p. injection (n = 21–23/group).
(B) Fluid intake during two-bottle choice of 0.2% saccharin versus water in 11–13-week-old male WT and KLB SF1-KO while receiving daily injections of vehicle (3 days) followed by daily injections of FGF21 (1 mg/kg; 3 days) via i.p. injection (n = 21–23/group).
(C) Fluid intake during two-bottle choice of 10% sucrose versus water in WT mice following vehicle or FGF21 (0.4 mg/mL) cannula infusions directly into the ventromedial hypothalamus (VMH) or the nucleus accumbens (NAc) for 3 days (n = 7–9/group).
(D) Plasma levels of human FGF21 in 16–18-week-old male diet-induced obese (DIO) WT and DIO KLB SF1-KO mice continuously treated with vehicle or FGF21 by osmotic minipumps for 2 weeks (1 mg/kg/d) (n = 6–8/group).
(E and F) (E) Daily and (F) final percent change in body weight of mice from (D).
Values are mean +/− SEM. (*, P < 0.05 compared to WT). Statistical analyses were conducted using 2-way ANOVA for multiple comparisons.
To further examine whether FGF21 action directly in the VMH suppresses sugar intake, WT mice were administered either vehicle or FGF21 directly into the VMH. Consistent with effects of i.p. injections of FGF21, unilateral intra-VMH FGF21 administration in WT mice significantly reduced sucrose intake compared to vehicle control mice (Fig. 4C). This FGF21-mediated reduction of sugar intake was not observed in mice receiving intra-NAc FGF21 administration (Fig. 4C). Together, these data indicate that FGF21 signaling to the VMH is critical for its effects to suppress sugar intake.
Extended FGF21 administration increases energy expenditure, promotes weight loss, and improves metabolic profiles through direct signaling to the CNS (BonDurant et al., 2017; Owen et al., 2014). The VMH is also an important hypothalamic site controlling energy homeostasis (Timper and Bruning, 2017). Since FGF21 signaling to the VMH is required for its effects to suppress sucrose intake, we next assessed whether direct signaling to the VMH is also required for the beneficial effects of chronic FGF21 treatment to regulate energy homeostasis. To do so, we administered FGF21 continuously to diet-induced obese (DIO) WT and DIO KLB SF1-KO mice by osmotic minipumps for 2 weeks. Circulating levels of human FGF21 during pharmacological administration via minipump, which are markedly higher than physiological FGF21 levels (von Holstein-Rathlou et al., 2016), were similar in both DIO WT and DIO KLB SF1-KO mice (Fig. 4D). Continuous administration of FGF21 via osmotic minipumps resulted in a significant decrease in body weight in both DIO WT and KLB SF1-KO mice (Fig. 4E,F). Extended administration of FGF21 also increased thermogenic gene expression in brown adipose tissue and lowered circulating triglycerides to a similar extent in both DIO WT and KLB SF1-KO mice (Fig. S4C,D) without decreasing food intake (Fig. S4E), consistent with previous studies (BonDurant et al., 2017; Owen et al., 2014). Finally, FGF21 administration also improved insulin sensitivity in both DIO WT and KLB SF1-KO mice (Fig. S4F,G). Together, these results demonstrate that FGF21 signaling to the VMH is required for its effects on suppression of sucrose intake but is not required to stimulate weight loss and improve insulin sensitivity in DIO mice.
FGF21 Increases Excitability and Activity of KLB-expressing Neurons in the VMH
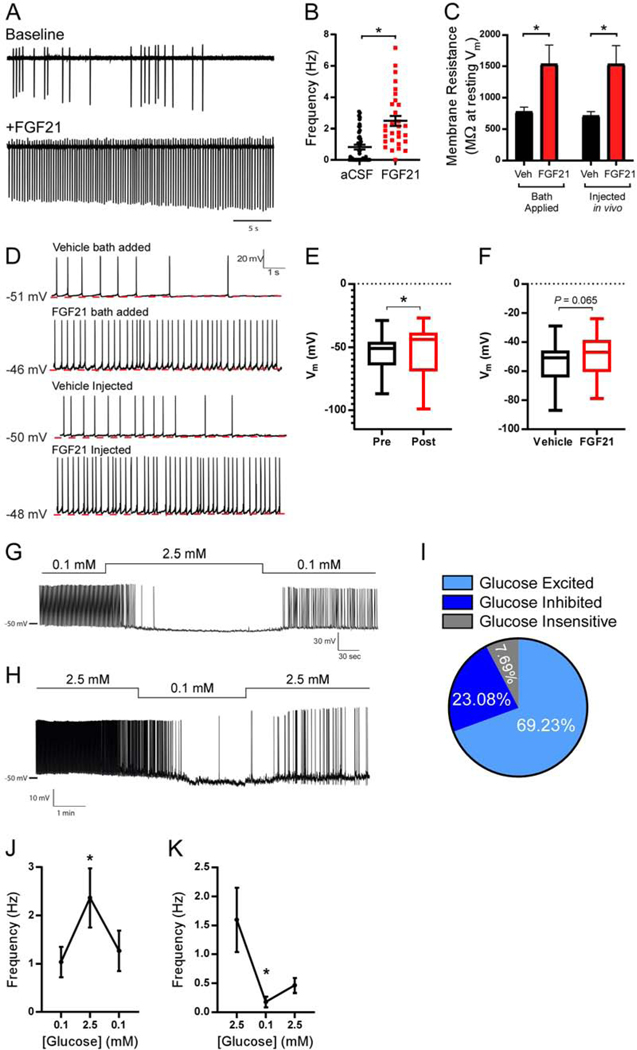
To date, no in-depth analyses on the effects of FGF21 on neuronal activity in KLB-expressing neurons have been conducted in brain slices or in vivo. In order to understand how FGF21 regulates activity of KLB+ neurons in the VMH, we performed cell-attached recordings of action potentials (APs) of KLB+ neurons in Klb-CRE mice infected with PHPeb-FLEX-tdTomato virus. Four weeks following initial infection, acute brain slices were prepared and AP frequencies were recorded at baseline (aCSF) and in response to FGF21 application. In the presence of synaptic blockers (CNQX, AP5, PTX), FGF21 application to the slices promoted a robust increase in the frequency of APs relative to baseline (Fig. 5A,B), suggesting FGF21 is capable of directly increasing the activity of KLB+ VMH neurons. To determine whether FGF21 mediates its effects on firing rates through altering membrane resistance, we investigated the current-voltage (I-V) relationship, which is a metric of neuron excitability, in KLB+ VMH neurons. To do so, we performed whole-cell current clamp recordings from KLB+ VMH neurons to measure changes in membrane voltage in response to increasing steps of hyperpolarizing current injections. In addition to applying FGF21 directly to slices, we also i.p. administered FGF21 or vehicle in vivo 1 hour prior to acute brain slice preparation. No change in overall membrane resistance (Rm) was observed when calculated from the I-V curves (Overall Rm FGF bath added: vehicle: 766.1 ± 44.6 MΩ, n = 37 neurons, FGF21 bath added: 712.7.5 ± 81.8 MΩ, n = 23 neurons P = 0.6, unpaired Student’s t-test; Overall Rm FGF injected: vehicle: 806.1 ± 69.9 MΩ, n = 16 neurons, FGF21 injected: 790.9 ± 50.1 MΩ, n = 37 neurons P = 0.86, unpaired Student’s t-test). However, when measured at resting membrane potential, we observed a significant and dramatic increase in membrane resistance when FGF21 was bath applied to the slices (Fig. 5C). Strikingly, we observed a remarkably similar effect on membrane resistance when FGF21 was delivered in vivo, exhibiting a doubling of the membrane resistance relative to vehicle administration (Fig. 5C). These results show that FGF21 does not affect overall Rm values but significantly increases membrane resistance when measured at resting membrane potential (Vm), possibly due to an FGF21-inhibited conductance specific to near resting Vm values. Collectively these results indicate that FGF21 may increase AP frequency in KLB+ neurons in the VMH through enhancing neuron excitability in a cell-autonomous manner. In support of this notion, when measuring resting membrane potential during whole-cell current clamp recordings in KLB+ VMH neurons we observed that both application of FGF21 directly to slices, and to a smaller extent, in vivo administration of FGF21 promoted depolarization of the resting membrane potential which correlated with increases in AP frequency relative to vehicle-treated mice (Fig. 5D-F). Given the resting membrane potential was measured in the presence of synaptic blockers, these data suggest that FGF21 depolarizes the resting membrane potential of KLB+ neurons in the VMH through a cell autonomous mechanism.
Figure 5. FGF21 increases excitability and activity of KLB-expressing neurons in the VMH.
(A) Representative traces of action current firing using cell-attached recordings in KLB+ VMH neurons in acute brain slices at baseline (top trace) and following FGF21 application (bottom trace, 50 ng/ul).
(B) Average firing frequency at baseline (aCSF) and following FGF21 application in KLB+ VMH neurons in acute brain slices (n = 45 neurons/3 mice for aCSF, n = 30 neurons/3 mice for FGF21). Mean +/− SEM plotted, unpaired two-tailed Student’s t-test.
(C) The average membrane resistance plotted in KLB+ VMH neurons in slices receiving bath application of FGF21 (50 ng/ul, n = 24 neurons/3 mice) or vehicle (n = 30 neurons/3 mice) and in vivo administration of FGF21 (1 mg/kg, i.p., n = 13 neurons/3 mice) or vehicle (n = 15 neurons/3 mice), 2-way ANOVA w/ Sidak’s multiple comparison test.
(D) Average resting membrane potential and traces of whole cell current clamp recordings in KLB+ VMH neurons in vehicle injected (n = 53 neurons/4 mice), FGF21 bath added (50 ng/ul, n = 37 neurons/4 mice), and FGF21 injected mice (1 mg/kg, i.p., n = 37 neurons/4 mice).
(E and F) Membrane potential measured in (E) KLB+ neurons in the VMH in slices before (pre) and following application of FGF21 in bath (post) or (F) KLB+ neurons in the VMH in slices from mice administered vehicle or FGF21 by i.p. injection as described in C. Kolmogorov-Smirnov test was used when comparing pre- and post-FGF21 bath application while an unpaired two-tailed Student’s t-test was used to compare between vehicle and FGF21 i.p. injected mice.
(G) Representative trace of a whole-cell current clamp recording in a glucose inhibited KLB+ VMH neuron in response to the indicated glucose concentrations.
(H) Representative trace of a whole cell current clamp recording in a glucose excited KLB+ VMH neuron in response to the indicated glucose concentrations.
(I) Pie chart illustrating the percentage of KLB+ VMH neurons which are glucose-excited, glucose-inhibited, and glucose insensitive in (n = 26 neurons/3 mice).
(J-K) Average action potential frequency in KLB+ VMH glucose excited neurons in response to indicated glucose concentrations (n = 7 neurons for (J) and 7 neurons for (K) from 4 mice each).
Values are mean +/− SEM. (*, P < 0.05). Comparisons were made using a paired two-tailed Student’s t-test.
The VMH is a critical site regulating glucose homeostasis. Specialized VMH glucose-sensing neurons integrate peripheral cues with changes in blood glucose levels (Shimazu and Minokoshi, 2017). Glucose-excited and glucose-inhibited neurons in the VMH increase and decrease their activity, respectively, in response to increases of interstitial glucose concentrations from 0.1 mM to 2.5 mM (Routh, 2010). Given the ability of VMH neurons to respond differentially to fluctuating glucose concentrations and FGF21’s ability to decrease sucrose consumption via signaling to the VMH, we next performed whole-cell current clamp analysis of KLB+ neurons in the VMH in response to increases (0.1 mM>2.5 mM>0.1 mM) or decreases (2.5 mM>0.1 mM>2.5 mM) in glucose concentration (Fig. 5G-K). These studies revealed a heterogeneous population of KLB+ neurons that were either glucose-excited, glucose-inhibited, or glucose-insensitive, with the majority of cells being glucose-excited (~70%) by exogenous glucose treatment in the absence of FGF21 (Fig. 5I).
FGF21 Alters Calcium Signaling in Glucose-excited and Glucose-inhibited Neurons Following Increases, but not Decreases, in Glucose Levels
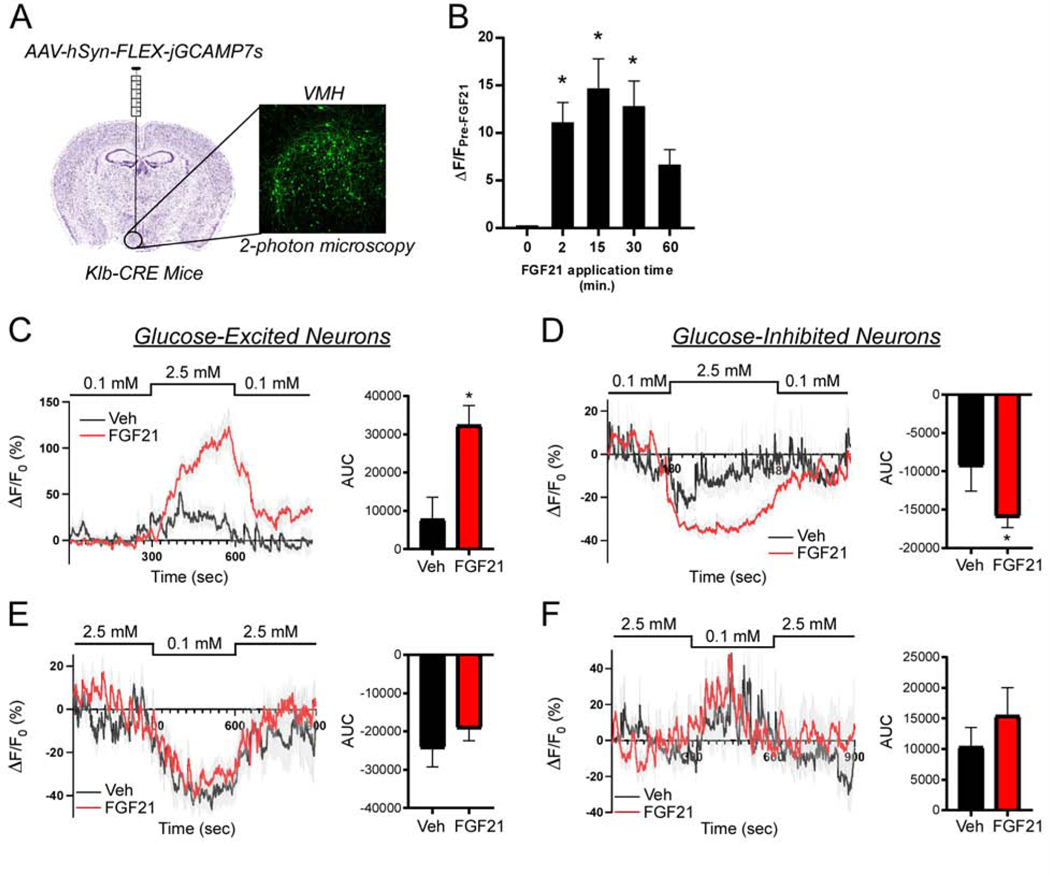
To explore the responsiveness of KLB+ neurons to increasing or decreasing glucose concentrations in the presence or absence of FGF21, we targeted a CRE-dependent GCaMP7s to KLB+ neurons by unilateral stereotactic injections of an AAV9-syn-FLEX-GCaMP7s virus into the VMH of Klb-CRE mice (Fig. 6A). Three to four weeks following injection, acute brain slices were prepared and changes in GCaMP7s fluorescence were assessed using two-photon microscopy. To confirm our electrophysiological results suggesting FGF21 can directly increase activity of KLB+ neurons in the VMH, we applied FGF21 directly to acute slices and measured GCaMP7s fluorescence. A time course of acute FGF21 application to slices revealed a rapid increase in Ca2+ concentration in KLB+ VMH neurons upon FGF21 application (Fig. 6B), confirming our electrophysiologic findings and suggests FGF21 is able to directly increase activity of KLB+ VMH neurons.
Figure 6. FGF21 alters calcium signaling in glucose-responsive neurons during increasing, but not decreasing, glucose levels.
(A) Experimental approach to assess calcium (Ca2+) signaling in KLB+ neurons in the ventromedial hypothalamus (VMH) using 2-photon microscopy and the Ca2+-sensitive indicator GCaMP7s via AAV-hSyn-FLEX-jGCaMP7s injection in the VMH of Klb-CRE male mice and imaging acutely prepared slices.
(B) Change in GCaMP7s fluorescence in neurons from VMH slices following treatment with FGF21 (50 ng/μl) at 0, 2, 15, 30, and 60 minutes (n = 14 neurons from 3 slices); one-way ANOVA (*, P < 0.05 relative to time 0).
(C-F) Average traces and area under the curve (AUC) of the percent change in GCaMP7s fluorescence relative to baseline fluorescence (ΔF/F0) in response to the indicated glucose concentrations in KLB+ VMH glucose excited (C,E) and glucose inhibited neurons (D,F) in acute brain slices prepared from mice i.p. injected with vehicle or FGF21 (1 mg/kg) 1 hour prior to acute brain slice preparation. For (C) n = 22 and 56 glucose excited neurons from 7 vehicle and 7 FGF21 treated mice, respectively. For (D) n = 15 and 61 glucose inhibited neurons from 7 vehicle and 7 FGF21 treated mice, respectively. For (E) n = 15 and 21 glucose excited neurons from 7 vehicle and 7 FGF21 treated mice, respectively. For (F) n = 11 and 20 glucose inhibited neurons from 7 vehicle and 7 FGF21 treated mice, respectively.
Values plotted are mean +/− SEM. (*, P < 0.05 compared to vehicle). Comparisons were made using an unpaired two-tailed Student’s t-test for comparison of AUC.
To explore how in vivo FGF21 administration affects the activity of KLB+ VMH neurons, Klb-CRE mice expressing GCaMP7s in the VMH (Fig. 6A) were administered either FGF21 or vehicle by i.p. injection for 1 hour prior to acute brain slice preparation. We then perfused slices with aCSF containing increasing (0.1 mM>2.5 mM>0.1 mM glucose, successively; Fig. 6C,D) or decreasing (2.5 mM>0.1 mM>2.5 mM glucose, successively; Fig. 6E,F) concentrations of glucose. Consistent with the patch clamp results, distinct subpopulations of KLB+ neurons were identified that are glucose-excited, glucose-inhibited, and glucose-insensitive (Fig. 6C-F). Notably, FGF21 administration markedly potentiates changes in Ca2+ concentration observed in both glucose-excited and glucose-inhibited neurons in response to increasing glucose relative to vehicle treated mice (Fig. 6C,D). Importantly, however, FGF21 administration did not enhance Ca2+ concentration changes in KLB+ neurons in response to decreasing glucose concentrations relative to vehicle treated mice (Fig. 6E,F). Taken together, these data suggest that FGF21 enhances glucose sensitivity in response to increasing, but not decreasing, concentrations of glucose in KLB+ neurons in the VMH.
DISCUSSION
Our work provides important new insights into how the liver-derived hormone FGF21 signals to the CNS to regulate macronutrient intake. Using knock-in reporter (i.e., Klb-CRE) mice, we find that KLB is expressed in many regions of the hypothalamus. These findings are in contrast to previous work reporting KLB restriction to the SCN (Bookout et al., 2013), which may be due to the detection limits of KLB by QPCR (Bookout et al., 2013). Consistent with our results here, more recent work observed expression of KLB in other areas of the hypothalamus, especially the VMH (Hultman et al., 2019). In addition, in contrast to work suggesting FGF21 signals to oxytocin neurons to suppress sugar preference (Matsui et al., 2018), our data with mice lacking KLB in oxytocin neurons (KLB Oxy-KO) demonstrates that FGF21 signaling directly to oxytocin neurons is not required for FGF21-mediated suppression of simple sugar intake but does not exclude a physiological role of FGF21 action on oxytocin neurons and Sim1+ neurons to influence basal sucrose intake. Our findings that FGF21-mediated suppression of sucrose intake is retained in mice lacking KLB in Sim1+ neurons (i.e., in the PVN) is surprising given our previous results that bilateral stereotaxic injections of an AAV-Cre-expressing virus targeted to the PVN of KLBfl/fl mice impaired FGF21-mediated suppression of sucrose intake (von Holstein-Rathlou et al., 2016). Instead, we now report that FGF21 signaling to glutamatergic neurons is absolutely required for its effects on simple sugar intake and sweet-taste preference, both physiologically and pharmacologically. In addition, we find that FGF21 signaling to SF1+ neurons in the VMH is important for simple sugar intake but is dispensable for FGF21’s effects to suppress non-nutritive sweet taste preference, increase energy expenditure and reduce body weight. As VMH neurons project to the PVN (Cheung et al., 2013; Lo et al., 2019), our previous findings may have resulted from KLB+ VMH neurons being infected with Cre through their projection terminals in the PVN when AAV-Cre was administered bilaterally to the PVN. In contrast, our studies here used genetic models to provide more targeted deletion of KLB in specific neurons.
In addition to identifying an important site of FGF21 action in the CNS, this work also reveals how FGF21 alters activity of KLB-expressing neurons. Using our novel Klb-CRE mouse model and the PHP.eb-FLEX-tdTomato virus to label KLB expressing cells, we used electrophysiology and calcium imaging to reveal that FGF21 markedly increases the frequency of action potential firing in KLB-expressing neurons. We propose that this is related to FGF21’s ability to depolarize the resting membrane potential of KLB+ neurons allowing for the generation of APs in response to otherwise sub-threshold depolarizations. One potential explanation for the profound ability of FGF21 to double membrane resistance, both in vivo and when applied to slices, is that FGF21 may close chloride leak channels based on the observed reversal potential in our I-V curves. Future work will focus on the molecular mechanism by which FGF21 enhances membrane resistance and consequently neuron excitability.
Interestingly, many of the KLB+ neurons in the VMH also exhibited sensitivity to changes in glucose concentrations. FGF21 sensitized these KLB+ VMH glucose-excited and glucose-inhibited neurons during periods of hyperglycemia but not hypoglycemia under the recording conditions used in this study. Further, as FGF21 is also induced during prolonged fasting to regulate glucose and lipid homeostasis (BonDurant and Potthoff, 2018), the possibility that glucose-sensitive KLB+ neurons respond to FGF21 under specific glucose concentrations to ensure control of this physiological response to maintain macronutrient balance warrants further investigation. Additionally, these findings suggest that KLB+ VMH glucose-excited neurons represent a novel subset of neurons that do not protect against hyperglycemia through enhancing glucose disposal, but instead through suppressing simple sugar intake. The mechanism for this effect and the circuits affected by FGF21 action on the VMH are important future areas of study.
Physiological levels of FGF21 are induced by either high-carbohydrate or low-protein conditions (BonDurant and Potthoff, 2018; Solon-Biet et al., 2016). We have proposed previously that this liver-brain endocrine axis may function to promote foraging of other macronutrients by suppressing sugar intake during macronutrient imbalance (either high-carbohydrate or low-protein) (von Holstein-Rathlou et al., 2016). Specifically, we showed that FGF21 suppresses simple sugar intake without altering protein intake or preference (von Holstein-Rathlou et al., 2016). In contrast, FGF21 was recently reported to promote increases in protein intake (Hill et al., 2019). However, these latter experiments were conducted using very acute FGF21 administration (a single intracerebroventricular injection) and without comparing the effect of FGF21 on protein intake to simple sugar (i.e., sucrose) intake. Since imbalances in dietary amino acid concentrations are detected by the anterior piriform cortex (APC) (Anthony and Gietzen, 2013; Morrison and Laeger, 2015), we explored whether KLB+ neurons are located in this brain region. Indeed, KLB+ neurons are located in the APC, and thus, additional work is needed to determine whether FGF21 actually affects protein intake and whether this could be mediated through FGF21 signaling to the APC.
An interesting result from these studies is that FGF21’s ability to suppress sucrose intake and sweet-taste preference can be dissociated. Currently, it is unclear how this occurs and how other FGF21 target regions regulating sweet taste preference do not function to suppress sucrose intake in the absence of FGF21 action on the VMH. Perhaps a hierarchical order exists for FGF21 to suppress the distinct pathways regulating the hedonic versus nutritive value of sugar (Tellez et al., 2016; Veldhuizen et al., 2017). Multiple studies have revealed that the nutritive value of sucrose and glucose are sensed post-ingestively to establish a preference for nutritive sugars (de Araujo et al., 2008; Fernstrom et al., 2012; Ren et al., 2010; Sclafani et al., 2011). Recently, this sugar-specific, rather than sweet-taste-specific, pathway mediating sugar preference was shown to be transmitted through a circuit from vagal ganglia to the caudal nucleus of the solitary tract (cNST) (Tan et al., 2020). It is interesting to speculate that FGF21 signaling specifically to the VMH, which alters glucose sensitivity in neurons in this region, may somehow modulate the activity of this gut-to-brain axis regulating sugar preference to decrease sugar intake. This question, along with the other questions raised above, are important future areas of study. Together, our data presented here provide important insights into the central mechanisms of FGF21 action and the pathways controlling macronutrient intake.
LIMITATIONS OF STUDY
While our experimental setup for electrophysiology recording and calcium imaging in slices was suitable to evaluate responses of glucose-excited and glucose-inhibited neurons to increases in glucose, we cannot rule out the possibility that the fraction of observed glucose-inhibited neurons might be an underestimation due to possible damage to glucose sensing mechanisms by exposure to high-glucose levels during slice preparations. Future studies will determine whether the effects of FGF21 on glucose sensitive neurons is specific to increasing, but not decreasing, glucose concentrations. Finally, while Sim1+ and SF1+ neurons are located in the PVN and VMH, respectively, we cannot exclude the possibility that these neurons in other brain regions are involved on FGF21’s effects on basal sugar intake and suppression of sugar intake.
STAR METHODS
LEAD CONTACT AND MATERIALS AVAILABILITY
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Matthew Potthoff (matthew-potthoff@uiowa.edu).
DATA AND CODE AVAILABILITY
RNA sequencing data is available in the NCBI Gene Expression Omnibus (GEO) and Sequence Read Archive (SRA) under accession number GEO: GSE150923. Further requests for data are available upon correspondence with the Lead Contact.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
All experiments presented in this study were conducted according to the animal research guidelines from NIH and were approved by the University of Iowa IACUC.
Animals
The following mice were utilized in these studies (Jackson stock number in parenthesis): KLBfl/fl (Ding et al., 2012), Ai14-tdTomato (007914) (Madisen et al., 2010), Vglut2-IRES-CRE (028863) (Vong et al., 2011), Vgat-IRES-Cre (028862) (Vong et al., 2011), DAT-IRES-CRE (006660) (Backman et al., 2006), Oxytocin-IRES-CRE (024234) (Wu et al., 2012), AVP-IRES-CRE (023530) (Harris et al., 2014), CRH-IRES-CRE (012704) (Taniguchi et al., 2011), SF1-CRE (012462) (Dhillon et al., 2006), Vglut2-IRES-FLP (030212), RC::FL-hM3Dq (026942) (Sciolino et al., 2016), and Sim1-Cre (006451)(Balthasar et al., 2005). Klb-CRE mice were generated by GenOway by inserting, in frame with the β-klotho (gene symbol: Klb) ATG in exon 1, a construct containing the CRE recombinase and the tdTomato reporter coding sequence separated by an IRES sequence via homologous recombination. Proper insertion was confirmed by Southern blot analysis at both the 5’ and 3’ ends. All mice used in experiments were individually housed under a 12 hr light/dark cycle at room temperature (21–23ºC). Littermates of the same sex were randomly assigned to experimental groups. Animal ages are reported in figure and supplemental figure legends. All animals were used in scientific experiments for the first time. This includes no previous exposures to pharmacological substances. All mice were on a C57BL/6J genetic background. Chow diets were provided to all mice given two-bottle tastants (Teklad 2920x). High-fat diets (Research Diets; catalog #D12492) were provided to the indicated mice to induce mice to obesity. Health status was normal for all animals.
METHOD DETAILS
Surgery
Adult mice (6–12 weeks old) were anesthetized with 2%–3% isofluorane and placed on a stereotaxic frame. Heat pads were used through the duration of the surgery to keep the body temperature stable. Eye ointment was applied to keep the eyes from drying. An incision was made to the skin to expose the skull after asepsis with Betadine and medical alcohol was applied. For viral injection, a craniotomy was made and a 1uL Hamilton syringe was slowly inserted into the target region. 0.4–0.6 μL virus was injected with an injection speed of 0.02 μL per minute. For in-vitro calcium imaging, pGP-AAV9-syn-FLEX-jGCaMP7s-WPRE virus was unilaterally injected into the VMH (AP −1.46; ML +/−0.6; DV −5.3) of KLB-CRE mice. For drug infusions, unilateral 26-gauge stainless steel guide cannulae (Plastics One, Roanoke VA) were implanted over the VMH (AP −1.46; ML +/−0.6; DV −5.3) or the NAc (AP +1.34; ML +/−0.75; DV −4.55) of C57BL6/J mice. The cannulae were secured to the skull with stainless steel screws and dental cement. Animals were allowed to recover for at least 2 weeks after surgery. Drug infusions were administered using an internal cannula (33-gauge) that extended 1mm beyond the tip of the cannulae over a period of 5min in a final volume of 1 μL. For PHP.eB injections, mice were anesthetized with 3% isofluorane and retro-orbitally injected with pAAV-FLEX-tdTomato (8×1012 vg/ml). Mice were allowed to recover for 4 weeks post-surgery to allow for proper viral spread, and brains were collected and processed for immunofluorescent imaging.
Administration of Recombinant Human FGF21 by Minipump
16–18 week old WT and KLB SF1-KO male mice on 60% HFD (Research Diets) for 8 weeks were randomized by body weight to receive vehicle or FGF21 via minipump (Alzet). Mice were individually housed for 1 week prior to surgery. DIO mice were than subcutaneously implanted with osmotic minipumps containing recombinant human FGF21 (1 mg/kg/day) or vehicle. All mice were maintained in single housing with continued HFD feeding for 2 weeks. Body weights were recorded daily.
Insulin tolerance tests
For insulin tolerance tests, DIO mice were injected i.p. with vehicle 3 days prior to the baseline ITT. Following two weeks of recovery, mice were then treated once a day with FGF21 (1 mg/kg/day) for 3 weeks and the insulin tolerance test was repeated. Under both conditions, mice were fasted for 5 hours and then injected i.p. with 0.75 U insulin/kg BW and tail blood was collected at 0, 15, 30, 60, 90, and 120 minutes after injection. Plasma glucose levels were subsequently measured with a colorimetric assay (Wako).
Plasma Analysis
Human FGF21 (Biovendor) and mouse insulin (Crystal Chem) were measured using commercially available ELISAs. Blood was collected into 300K2E microvettes (Sarstedt) and spun at 3000 rpm for 30 min at 4C to separate plasma. Plasma triglycerides were measured using a colorimetric assay (Infinity, Thermo Scientific). All measurements were performed according to the manufacturer’s instructions.
Gene expression
Gene expression analyses were performed as described (BonDurant et al., 2017). RNA was isolated from brown adipose tissue following Trizol (Invitrogen) protocol. 2 micograms RNA from each sample was used to generate cDNA (High-Capacity cDNA Reverse Transcription Kit; Life Technologies), and QPCR was conducted using SYBR green (Invitrogen).QPCR primer sequences are as follows: Bmp8b: 5’ -TCAACACAACCCTCCACATCA-3’ , 5’ - AGATCGGAGCGTCTGAAGATC-3’; U36B4: 5’ -CGTCCTCGTTGGAGTGACA-3’ , 5’ - CGGTGCGTCAGGGATTG-3’; UCP1: 5’-AAGCTGTGCGATGTCCATGT-3’, 5’-AAGCCACAAACCCTTTGAAAA-3’; Elovl3: 5’-CTTCGAGACGTTTCAGGACTTAAG-3’, 5’-TCTGGCCAACAACGATGAG-3’.
Single-cell RNA-Sequencing
The method used here for generating single cell suspensions for FACs sorting was modified from a previously published protocol (Chung et. al, 2017, Nature). Brains were dissected from mice immediately following decapitation and placed in ice cold artificial cerebrospinal fluid (ACSF) containing 126 mM NaCl, 20 mM NAHCO3, 20 mM dextrose, 3 mM KCl, 1.25 mM NaH2PO4, 2 mM CaCl2, 2 mM MgCl2 which was bubbled with carbogen gas (95% O2 and 5% CO2) for at least 15 minutes prior to dissection. The hypothalamus was then dissected from each brain, and three hypothalami were pooled for each capture. The tissue was then minced and placed in 2mg/ml Pronase in ACSF and incubated at 37°C for 30 minutes. Following incubation, the supernatant was removed, and tissue was resuspended in HBSS w/ 10% FBS. The tissue was then titrurated using fire polished Pasteur pipets and strained through a 70 μM cell strainer.
Hoechst cell viability dye (Hoechst 33258) was added to the cell suspension at a final dilution of 4ug/ml just prior to sorting to allow for selection of living cells during FACs sorting. Cells were then FACs sorted for tdTomato fluorescence and viability via Hoechst staining. Sorting was performed on a BD FACSAriaII using a 130 μM nozzle, a sheath pressure of 10 p.s.i., and in the single cell sorting mode at the University of Iowa Flow Cytometry Facility. FACS isolated cells were then loaded on the BD Rhapsody where capture of the single cells was performed per manufacturer instructions. Following lysis of the cells and capture of mRNA on oligonucleotide barcoded beads the cDNA synthesis was performed per manufacturer instructions. cDNA libraries were then generated using two custom primer panels (with one primer panel containing lowly expressed genes and the other containing highly expressed genes with overlapping genes incorporated into each panel (Table S1 and S2) designed by BD and generated by Integrated DNA Technologies (IDT) targeted against cell type specific markers for cell types previously identified in the hypothalamus based on previous publications (Romanov et. al, Chen et. al). cDNA libraries were then sequenced on Illumina HiSeq 4000 sequencers at the University of Iowa Genomics Core. Sequencing data was then quality filtered and annotated using the BD Rhapsody analysis pipeline while also correcting for artifacts which arise during library construction. The filtered and annotated data was then plotted using an unsupervised tSNE clustering algorithm and putative cell types were identified based on differential gene expression relative to other clusters using the BD Data View software package. Experiments were replicated using the 10X Chromium and Cell Ranger Pipeline for analyses.
In silico analysis of deposited SMART-seq data.
SMART-seq data from (Kim et al., 2019) was downloaded from https://data.mendeley.com/datasets/ypx3sw2f7c/1 and loaded into the R package Seurat (v3.1) and filtered using default parameters. Gene expression in each cell was normalized using the NormalizeData function (which normalizes individual gene expression/cell by the total number of molecules/cell, multiplies that value by a scale factor of 10,000/cell, and then log-transforms the scaled value). Then the expression of each gene was scaled using the ScaleData function which scales the mean expression of each gene to the variance of its expression across all cells. We then subclustered only neurons which express KLB to determine expression of Slc17a6 in subclusters of KLB+ neurons in the VMH. Subclusters within KLB+ neurons were determined by identifying highly variable genes which was used as an input for dimensionality reduction via principle component analysis (PCA). The identified principle components were then used as an input for clustering analysis using the FindClusters function which identified 5 unique clusters within KLB+ neurons.
Immunohistochemistry and Fluorescence In-Situation Hybridization
For immunohistochemistry, mice were anesthetized and transcardially perfused using 25 mL PBS followed by 25 mL 4% paraformaldehyde (PFA). Brains were collected and post fixed for 24 hours in 4% PFA and transferred to a 10%–30% sucrose gradient solution for 48 hours. Brains were cryoprotected in Tissue-Tek optimal cooling temperature (OCT) compound and stored in a −80 freezer until use. 30 μm coronal sections were cut using a thermo-cryostat (UIOWA; Thermo HM525 Cryostat). For c-Fos staining (1:1000, Cell Signaling), brains were collected without perfusion. Brain slices were washed and permeabilized with PBST (0.4% Triton X-100 in PBS) over 15 minutes and incubated with blocking solution (5% normal goat or donkey serum in PBST) for 1 hour at room temperature (RT). Slices were incubated in primary antibody overnight at 4°C. The following day slices were washed in PBST over 15 minutes and incubated in the proper fluorescently-conjugated secondary antibodies (1:250, Life Technologies) at RT for 1 hour. Finally, slices were mounted on slides with VECTASHIELD Antifade Mounting Media with DAPI (Vector Laboratories). Slices were imaged using an Olympus BX61 Light Microscope.
For Fluorescence In-Situ Hybridization (FISH), mice were euthanized, and brain tissue was collected and frozen in 2-methylbutane and embedded in OCT for storage at −80°C. Next, 10 μm coronal sections were cut and processed using the RNAscope Multiplex FISH protocol for fresh-frozen tissue (320851; Advanced Cell Diagnostics). Briefly, brain sections were fixed using 4% paraformaldehyde, pretreated with protease IV, followed by hybridization with target probes, which contained 20 double Z oligo probe pairs for the specific RNA target of interest (Vglut2, Cat No. 550031; SF1, Cat No. 445731; tdTomato Cat No. 317041-C2; Advanced Cell Diagnostics). All probe concentrations were premade by manufacturer. Subsequent hybridization was completed with RNAscope detection reagents to amplify the fluorescent signal. All Images were captured using a Zeiss LSM 710 confocal microscope at 20X or 40X magnification, maintained by the Central Microscopy Research Facility (CMRF) at the University of Iowa, which was acquired with funding from the NIH shared instrumentation grant 1 S10 RR025439–01.
Two-bottle choice experiments
For two-bottle tastant experiments, drinking tubes were constructed and test fluids were presented following the Monell Mouse Taste Phenotyping Project specifications (http://www.monell.org/MMTPP/), and mice were offered the indicated amount of each test fluid versus water. Animals were individually caged and habituated to regular handling with mock i.p. injections for 4 days before the commencement of experiments. Animals were injected either with vehicle or FGF21 prior to accessing fluid solutions with ad-libitum chow unless otherwise stated. Mice were injected with vehicle for 3 days, followed by FGF21 for 3 days and fluid intake was measured daily. Fluid solution positions were switched daily to prevent learning bias. Solutions were available 23h/day and fluid intake was recorded and tubes were refilled during the remaining hour.
For 2-bottle choice experiments, all solutions were prepared with deionized water and served at RT. The spillage from drinking tubes was estimated daily by recording the change in fluid levels of two drinking tubes that were placed on an empty cage where one tube contained that days test solution, and the other tube contained water.
Recombinant FGF21 was generated and provided by Novo Nordisk. For intake/preference studies, mice received i.p. injections of vehicle or FGF21 at 1 mg/mL and were put back in home-cages with access to fluid solutions. Shorter duration experiments were performed using a lickometer system. This custom system allows both fluid intake and drinking activity (number of licks, time between licks, etc.) to be assessed in real-time (Lafayette Instrument Neuroscience). Mice were placed in a lickometer with two-bottle fluid solutions with no access to food for 2 hours at 7 hours after the initiation of the light phase (zeitgeber time (ZT) 7). For chemogenetic experiments, mice received i.p. CNO (1 mg/kg) injections 15 minutes prior to being placed in lickometer cages with fluid solutions to allow for proper circulation. For drug infusion experiments mice received vehicle infusions for 3 days followed by FGF21 infusions for 3 days into the VMH or NAc. Animals were placed in lickometer cages 15 minutes following drug infusions.
Calcium Imaging
Brains were rapidly dissected from mice following decapitation and placed in ice-cold NMDG-HEPES aCSF cutting solution (in mM): 92 NMDG, 2.5 KCl, 1.25 NaH2PO4, 30 NaHCO3, 20 HEPES, 25 glucose, 2 thiourea, 5 Na-ascorbate, 3 Na-pyruvate, 0.5 CaCl2·2H2O, and 10 MgSO4·7H2O. Brains were then sliced in 300 μM sections on a vibratome before being placed in 37 degree Celsius NMDG-HEPES aCSF cutting solution for at least 10 minutes, but no greater than 15 minutes before being moved to room temperature recovery buffer (92 NaCl, 2.5 KCl, 1.25 NaH2PO4, 30 NaHCO3, 20 HEPES, 25 glucose, 2 thiourea, 5 Na-ascorbate, 3 Na-pyruvate, 0.5 CaCl2·2H2O, and 10 MgSO4·7H2O) for at least 1 hour before recording. For Ca2+ imaging slices were placed in a recording chamber on the stage of an Olympus FVMPE RS Multiphoton Microscope and imaged for changes in GCaMP7s fluorescence (λEx = 935 nm) using a super 20x water immersion lens (Olympus, NA = 1.0) during perfusion with recording solution (92 NaCl, 2.5 KCl, 1.25 NaH2PO4, 30 NaHCO3, 20 HEPES, 2 CaCl2, and 2 MgSO4). Prior to initiation of imaging, slices were incubated for 10 minutes in either 0.1 mM glucose or 2.5 mM glucose depending on the experiment. Imaging of slices was then performed using stimulation paradigms in which slices were exposed to increases in glucose concentration (0.1 mM glucose>2.5 mM glucose>0.1 mM glucose) or decreases in glucose concentration (2.5 mM glucose>0.1 mM glucose>2.5 mM glucose) in separate experiments. At the end of the experiment recording solution w/ 50 μM KCl was applied to slices to confirm and identify all viable cells in the slice. Glucose sensitive neurons were identified based on reversible changes in GCaMP7s fluorescence in response to the changes in glucose concentration described above. Image stacks were stabilized using the TurboReg plugin on FIJI and fluorescence was quantified for the soma of individual cells following creation of a mask using the ImageJ multimeasure analysis function.
Electrophysiology
Slice preparation was performed as described previously (Ting et al., 2018). Briefly, 8 week old Klb-cre mice infected with PHP.eb-FLEX-tdTomato virus were sacrificed and brains were immersed in NMDG-HEPES aCSF cutting solution (in mM): 92 NMDG, 2.5 KCl, 1.25 NaH2PO4, 30 NaHCO3, 20 HEPES, 25 glucose, 2 thiourea, 5 Na-ascorbate, 3 Na-pyruvate, 0.5 CaCl2·2H2O, and 10 MgSO4·7H2O. Brain tissue is kept in 95% O2 / 5% CO2 aerated ice-cold cutting solution and 300 μm thick fresh slices containing the hypothalamus were obtained with vibratome and transferred to 95% O2 / 5% CO2 aerated and HEPES containing artificial cerebrospinal fluid (aCSF) incubation solution containing (in mM): 92 NaCl, 2.5 KCl, 1.25 NaH2PO4, 30 NaHCO3, 20 HEPES, 25 glucose, 2 thiourea, 5 Na-ascorbate, 3 Na-pyruvate, 2 CaCl2·2H2O, and 2 MgSO4·7H2O. The sections were incubated in this solution for at least 30 minutes and placed in the recording chamber which has the recording aCSF (in mM): 124 NaCl, 2.5 KCl, 1.25 NaH2PO4, 24 NaHCO3, 12.5 glucose, 5 HEPES, 2 CaCl2·2H2O, and 2 MgSO4·7H2O.
Loose seal recordings are performed using aCSF in pipette and action currents are monitored in the presence of a cocktail of synaptic blockers CNQX (10 μM) + AP5 (50 μM) + PTX (50 μM). Whole cell recordings were performed in current clamp mode and membrane voltage were recorded from tdTomato-expressing KLB+ VMH neurons using electrodes with 4–5 MΩ tip resistances in the presence of synaptic blockers. Pipette solution contained (in mM): 145 K-gluconate, 1 MgCl2, 10 HEPES, 1.1 EGTA, 2 Mg-ATP, 0.5 Na2-GTP, and 5 Na2-phosphocreatine (pH 7.3 with KOH; 290–295 mOsm). Recordings were corrected for liquid junction potential. Current injection protocol was set for 300 ms pulses of 30 pA (or 50 pA) steps starting from −150 pA to +150 pA (or 300 pA).
MultiClamp 700B Amplifier (Molecular Devices, San Jose, CA) and Axon™ pCLAMP™ 11.3 software (Molecular Devices, San Jose, CA) were used to obtain and analyze data. Vm was monitored before and after administration of FGF21 (50 ng/μl) into the bath through perfusion. Overall Rm was calculated by analyzing the hyperpolarizing voltage deflection in response to current injection protocol of 300 ms pulses of 50 pA steps starting from −150 pA and using steady state portion of the pulses. Rm values at the resting membrane potential was calculated at voltage clamp mode using the steady state current in response to 10 mV of depolarizing voltage pulse for 100 ms.
QUANTIFICATION AND STATISTICAL ANALYSIS
Sample sizes for experiments were determined based on sample sizes used in similar experiments reported previously in the literature. Statistical analysis was conducted using GraphPad Prism software in which distribution of the data was analyzed to determine the appropriate statistical test to be applied. The statistical test used for each comparison is described in the figure legends corresponding to the specific figure. Data are presented as the mean ± SEM unless otherwise noted with P < 0.05 being the cut-off for a result to be considered significant. “n” corresponds to the number of individual mice or neurons analyzed as indicated. Read quality filtering for single-cell RNA sequencing was performed on paired-end reads to exclude sequencing reads with lengths <66 base pairs for R1 and <64 base pairs for R2. Additionally, if the mean base quality score of either R1 or R2 was <20 then the read pair was dropped. Lastly, the single-nucleotide frequency (SNF) of each read was also used to filter sequencing results in which an SNF >0.54 for R1 or >.79 for R2 prompted exclusion of the read pair. Only reads which passed all three quality filters were annotated and used to determine putative cells and quantify unique molecular identifiers for each cell.
For fluid intake studies, mice were randomly assigned to groups and animals that were unwilling to drink were removed from analyses.
Supplementary Material
Table S1, related to STAR methods
List of genes and amplicon sequences for panel 1 of single cell RNAseq for BD Rhapsody.
Table S2, related to STAR methods
List of genes and amplicon sequences for panel 2 of single cell RNAseq for BD Rhapsody.
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| c-FOS (9F6) Rabbit mAb | Cell Signaling Technology | Cat#:2250, RRID:AB_2247211 |
| F(ab’)2-Goat anti-Rabbit IgG (H+L) Secondary Antibody, Alexa Fluor 488 | Life Technologies | A-11070 |
| F(ab’)2-Goat anti-Rabbit IgG (H+L) Secondary Antibody, Alexa Fluor 568 | Life Technologies | A-21069 |
| Vglut2 (RNAScope) | Advanced Cell Diagnostics | Cat#: 550031 |
| Tdtomato (RNAScope) | Advanced Cell Diagnostics | Cat#: 317041-C2 |
| SF1 (RNAScope) | Advanced Cell Diagnostics | Cat#: 445731 |
| Bacterial and Virus Strains | ||
| pGP-AAV-syn-FLEX-jGCaMP7s-WPRE | Douglas Kim & GENIE Project | Cat#:104491-AAV9, RRID:Addgene_104491 |
| pAAV-FLEX-tdTomato (AAV PHP.eB) | Edward Boyden | Cat#:28306-PHPeb, RRID:Addgene_28306 |
| Biological Samples | ||
| Chemicals, Peptides, and Recombinant Proteins | ||
| Recombinant Human FGF21 | Novo Nordisk | N/A |
| Human Insulin | Sigma | I9278 |
| N-Methyl-D-glucamine | Sigma | Cat#:M2004 |
| KCl | Research Products International | Cat#:P41025–500.0 |
| NaCl | Research Products International | Cat#:S23020–500.0 |
| NaH2PO4 | Research Products International | Cat#:S23185–500.0 |
| NaHCO3 | Research Products International | Ca#:S25060–500.0 |
| HEPES | Research Products International | Cat#:H75030–50.0 |
| Glucose | Research Products International | Cat#:G32045–500.0 |
| MgSO4-7H2O | Research Products International | Cat#:M65240–100.0 |
| CaCl2-2H2O | Research Products International | Cat#:C36200–500.0 |
| Sodium Ascorbate | Research Products International | Cat#:S42175–100.0 |
| Thiourea | Sigma | Cat#:T8656 |
| Sodium Pyruvate | Sigma | Cat#:P2256 |
| Pronase | Roche | Cat#: 10165921001 |
| HBSS (w/out CaCl2, MgCl2, MgSO4) | Gibco | Cat#:14175–095 |
| Fetal Bovine Serum | Gibco | Cat#:26140–079 |
| CNQX | Tocris | Cat#:0190 |
| TTX | Tocris | Cat#:1078 |
| D-AP5 | Tocris | Cat#:0106 |
| Pertussis Toxin | Tocris | Cat#:3097 |
| Critical Commercial Assays | ||
| Targeted mRNA Reagent Kit 4 pack | BD | Cat#:633771 |
| Glucose Autokit | Wako Chemicals | 439–90901 |
| Human FGF21 ELISA | Biovendor | RD191108200R |
| Human Insulin ELISA | Chrystal Chem | 90080 |
| Deposited Data | ||
| Raw and processed scRNAseq data | Gene Expression Omnibus | Pending |
| Experimental Models: Cell Lines | ||
| Experimental Models: Organisms/Strains | ||
| Klb-CRE | This paper | |
| Ai14;tdTomato | The Jackson Laboratory | 007914 |
| KLB fl/fl | Dr. Steven Kliewer; UTSW | Ding et al., 2012 |
| Vglut2-IRES-Cre | The Jackson Laboratory | 028863 |
| Vgat-IRES-CRE | The Jackson Laboratory | 028862 |
| DAT-IRES-CRE | The Jackson Laboratory | 006660 |
| Oxytocin-IRES-CRE | The Jackson Laboratory | 024234 |
| AVP-IRES-CRE | The Jackson Laboratory | 023530 |
| CRH-IRES-CRE | The Jackson Laboratory | 012704 |
| SF1-CRE | The Jackson Laboratory | 012462 |
| Vglut2-IRES-FLP | The Jackson Laboratory | 030212 |
| RC::Fl-hM3Dq | The Jackson Laboratory | 026942 |
| Sim1-Cre | The Jackson Laboratory | 006451 |
| C57BL/6J | The Jackson Laboratory | 003548 |
| Oligonucleotides | ||
| BD Rhapsody Custom Primer Panel | BD/IDT | Cat#:633743 |
| Bmp8b: 5' -TCAACACAACCCTCCACATCA-3', 5' -AGATCGGAGCGTCTGAAGATC-3' | This paper | N/A |
| U36B4: 5' -CGTCCTCGTTGGAGTGACA-3', 5' -CGGTGCGTCAGGGATTG-3' | This paper | N/A |
| UCP1: 5'-AAGCTGTGCGATGTCCATGT-3', 5'-AAGCCACAAACCCTTTGAAAA-3' | This paper | N/A |
| Elovl3: 5'-CTTCGAGACGTTTCAGGACTTAAG-3', 5'-TCTGGCCAACAACGATGAG-3' | This paper | N/A |
| Recombinant DNA | ||
| Software and Algorithms | ||
| ImageJ | Schneider et al., 2012 | https://imagej.nih.gov/ij/ |
| BD Genomics Data View | BD | bitbucket.org/CRSwDev/dataview |
| BD Rhapsody Analysis pipeline | BD | sbgenomics.com/bdgenomics |
| Axon pCLAMP 11.3 | Molecular Devices | N/A |
| Scurry mouse activity wheel with dual lickometer | Lafayette Instrument Neuroscience | 80822S |
| Graphpad Prism 7 | Graphpad | http://www.graphpad.com/scientific-software/prism/ |
| Seurat | Stuart, Butler, et al., 2019 | https://satijalab.org/seurat/ |
| Other | ||
| Sucrose | Sigma | Cat#:S0389 |
| Saccharin | Sigma | Cat#:109185 |
| Chow | Teklad | 2920x |
| 60% High fat diet | Research Diets | D12492 |
| Mounting media with DAPI | Vector Laboratories | Cat#: H-1200 |
| Cannula | PlasticsOne | N/A |
| Triton X-100 | Sigma | 9002-93-1 |
| Tissue-Tek Optimal Cutting Temperature (OCT) Compound | Sakura | 4583 |
| Minipump | Alzet | 1002 |
| Microvet | Sarstedt | 90–1091 |
| Trizol | Invitrogen | 15596026 |
| High-Capacity cDNA Reverse Transcription Kit | Life Technologies | 4368814 |
Highlights.
Hypothalamic scRNAseq analyses in Klb-CRE mice identified central targets of FGF21
FGF21 signals to Vglut2+ neurons to reduce sugar intake and sweet taste preference.
FGF21 signals to ventromedial hypothalamus (VMH) neurons to suppress sugar intake.
FGF21 markedly enhances glucose sensitivity of β-klotho neurons in the VMH.
Context and Significance.
The prevalence of obesity continues to increase worldwide due to changes in dietary composition and behaviors. In particular, excessive consumption of sugars has been linked to metabolic disease. Fibroblast growth factor 21 (FGF21) is a liver-derived hormone that signals to the brain to reduce sugar intake, but the mechanism for this effect was unknown. Here, Jensen-Cody et al. show that FGF21 signals to glutamatergic neurons to lower sugar intake and sweet-taste preference. Interestingly, the researchers demonstrate that FGF21 signals to specific neurons in the hypothalamic region of the brain to lower sugar intake by enhancing their sensitivity to glucose. These findings provide important new insights into how FGF21 functions to regulate dietary preferences and provide a step towards a molecular therapy to qualitatively improve dietary choices.
ACKNOWLEDGMENTS
We thank Dr. Birgitte Andersen (Novo Nordisk) for providing FGF21 protein, Dr. Justin Grobe (Univ. of Iowa) for project insights, Dr. Huxing Cui (Univ. of Iowa) for providing Sim1-Cre mice, and Mr. Tate Neff (Univ. of Iowa) for technical assistance. This work was funded by the National Institutes of Health (NIH) R01DK106104 (M.J.P.), R01AA027654 (M.J.P.), T32 DK112751 (K.H.F.), F32 DK117510 (K.E.C.), and F31 DK117515 (S.O.J.), American Heart Association (AHA) 18PRE33960377 (S.A.S.), Veterans Affairs Merit Review Program I01BX004634 (M.J.P.), the University of Iowa Carver College of Medicine (M.J.P.), and the Novo Nordisk Foundation Center for Basic Metabolic Research (NNF18CC003490; M.P.G.). Some of the data presented herein were obtained at the Flow Cytometry Facility supported by the NIH (1 S10 OD016199-01A1). The authors would like to acknowledge use of the University of Iowa Central Microscopy Research Facility and Genomics Division of the Iowa Institute of Human Genetics.
Footnotes
DECLARATION OF INTERESTS
The authors declare no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Adams AC, Cheng CC, Coskun T, and Kharitonenkov A. (2012). FGF21 requires betaklotho to act in vivo. PLoS One 7, e49977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andermann ML, and Lowell BB (2017). Toward a Wiring Diagram Understanding of Appetite Control. Neuron 95, 757–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony TG, and Gietzen DW (2013). Detection of amino acid deprivation in the central nervous system. Curr Opin Clin Nutr Metab Care 16, 96–101. [DOI] [PubMed] [Google Scholar]
- Backman CM, Malik N, Zhang Y, Shan L, Grinberg A, Hoffer BJ, Westphal H, and Tomac AC (2006). Characterization of a mouse strain expressing Cre recombinase from the 3’ untranslated region of the dopamine transporter locus. Genesis 44, 383–390. [DOI] [PubMed] [Google Scholar]
- Balthasar N, Dalgaard LT, Lee CE, Yu J, Funahashi H, Williams T, Ferreira M, Tang V, McGovern RA, Kenny CD, et al. (2005). Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell 123, 493–505. [DOI] [PubMed] [Google Scholar]
- BonDurant LD, Ameka M, Naber MC, Markan KR, Idiga SO, Acevedo MR, Walsh SA, Ornitz DM, and Potthoff MJ (2017). FGF21 Regulates Metabolism Through Adipose-Dependent and -Independent Mechanisms. Cell Metab 25, 935–944 e934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BonDurant LD, and Potthoff MJ (2018). Fibroblast Growth Factor 21: A Versatile Regulator of Metabolic Homeostasis. Annu Rev Nutr 38, 173–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookout AL, de Groot MH, Owen BM, Lee S, Gautron L, Lawrence HL, Ding X, Elmquist JK, Takahashi JS, Mangelsdorf DJ, et al. (2013). FGF21 regulates metabolism and circadian behavior by acting on the nervous system. Nat Med 19, 1147–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo SS, and Egan JM (2015). The endocrinology of taste receptors. Nat Rev Endocrinol 11, 213–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell JN, Macosko EZ, Fenselau H, Pers TH, Lyubetskaya A, Tenen D, Goldman M, Verstegen AM, Resch JM, McCarroll SA, et al. (2017). A molecular census of arcuate hypothalamus and median eminence cell types. Nat Neurosci 20, 484–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan KY, Jang MJ, Yoo BB, Greenbaum A, Ravi N, Wu WL, Sanchez-Guardado L, Lois C, Mazmanian SK, Deverman BE, et al. (2017). Engineered AAVs for efficient noninvasive gene delivery to the central and peripheral nervous systems. Nat Neurosci 20, 1172–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Wu X, Jiang L, and Zhang Y. (2017). Single-Cell RNA-Seq Reveals Hypothalamic Cell Diversity. Cell Rep 18, 3227–3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung CC, Kurrasch DM, Liang JK, and Ingraham HA (2013). Genetic labeling of steroidogenic factor-1 (SF-1) neurons in mice reveals ventromedial nucleus of the hypothalamus (VMH) circuitry beginning at neurogenesis and development of a separate non-SF-1 neuronal cluster in the ventrolateral VMH. J Comp Neurol 521, 1268–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu AY, Workalemahu T, Paynter NP, Rose LM, Giulianini F, Tanaka T, Ngwa JS, Group CNW, Qi Q, Curhan GC, et al. (2013). Novel locus including FGF21 is associated with dietary macronutrient intake. Hum Mol Genet 22, 1895–1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Araujo IE, Oliveira-Maia AJ, Sotnikova TD, Gainetdinov RR, Caron MG, Nicolelis MA, and Simon SA (2008). Food reward in the absence of taste receptor signaling. Neuron 57, 930–941. [DOI] [PubMed] [Google Scholar]
- Dhillon H, Zigman JM, Ye C, Lee CE, McGovern RA, Tang V, Kenny CD, Christiansen LM, White RD, Edelstein EA, et al. (2006). Leptin directly activates SF1 neurons in the VMH, and this action by leptin is required for normal body-weight homeostasis. Neuron 49, 191–203. [DOI] [PubMed] [Google Scholar]
- Ding X, Boney-Montoya J, Owen BM, Bookout AL, Coate KC, Mangelsdorf DJ, and Kliewer SA (2012). betaKlotho is required for fibroblast growth factor 21 effects on growth and metabolism. Cell Metab 16, 387–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmquist JK, Coppari R, Balthasar N, Ichinose M, and Lowell BB (2005). Identifying hypothalamic pathways controlling food intake, body weight, and glucose homeostasis. J Comp Neurol 493, 63–71. [DOI] [PubMed] [Google Scholar]
- Fernstrom JD, Munger SD, Sclafani A, de Araujo IE, Roberts A, and Molinary S. (2012). Mechanisms for sweetness. J Nutr 142, 1134S–1141S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher FM, Kim M, Doridot L, Cunniff JC, Parker TS, Levine DM, Hellerstein MK, Hudgins LC, Maratos-Flier E, and Herman MA (2017). A critical role for ChREBP-mediated FGF21 secretion in hepatic fructose metabolism. Mol Metab 6, 14–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fon Tacer K, Bookout AL, Ding X, Kurosu H, John GB, Wang L, Goetz R, Mohammadi M, Kuro-o M, Mangelsdorf DJ, et al. (2010). Research resource: Comprehensive expression atlas of the fibroblast growth factor system in adult mouse. Mol Endocrinol 24, 2050–2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frayling TM, Beaumont RN, Jones SE, Yaghootkar H, Tuke MA, Ruth KS, Casanova F, West B, Locke J, Sharp S, et al. (2018). A Common Allele in FGF21 Associated with Sugar Intake Is Associated with Body Shape, Lower Total Body-Fat Percentage, and Higher Blood Pressure. Cell Rep 23, 327–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaich G, Chien JY, Fu H, Glass LC, Deeg MA, Holland WL, Kharitonenkov A, Bumol T, Schilske HK, and Moller DE (2013). The effects of LY2405319, an FGF21 analog, in obese human subjects with type 2 diabetes. Cell Metab 18, 333–340. [DOI] [PubMed] [Google Scholar]
- Harris JA, Hirokawa KE, Sorensen SA, Gu H, Mills M, Ng LL, Bohn P, Mortrud M, Ouellette B, Kidney J, et al. (2014). Anatomical characterization of Cre driver mice for neural circuit mapping and manipulation. Front Neural Circuits 8, 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill CM, Laeger T, Dehner M, Albarado DC, Clarke B, Wanders D, Burke SJ, Collier JJ, Qualls-Creekmore E, Solon-Biet SM, et al. (2019). FGF21 Signals Protein Status to the Brain and Adaptively Regulates Food Choice and Metabolism. Cell Rep 27, 2934–2947 e2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsuchou H, Pan W, and Kastin AJ (2007). The fasting polypeptide FGF21 can enter brain from blood. Peptides 28, 2382–2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultman K, Scarlett JM, Baquero AF, Cornea A, Zhang Y, Salinas CBG, Brown J, Morton GJ, Whalen EJ, Grove KL, et al. (2019). The central fibroblast growth factor receptor/beta klotho system: Comprehensive mapping in Mus musculus and comparisons to nonhuman primate and human samples using an automated in situ hybridization platform. J Comp Neurol 527, 2069–2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DW, Yao Z, Graybuck LT, Kim TK, Nguyen TN, Smith KA, Fong O, Yi L, Koulena N, Pierson N, et al. (2019). Multimodal Analysis of Cell Types in a Hypothalamic Node Controlling Social Behavior. Cell 179, 713–728 e717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klockars A, Levine AS, and Olszewski PK (2015). Central oxytocin and food intake: focus on macronutrient-driven reward. Front Endocrinol (Lausanne) 6, 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laeger T, Henagan TM, Albarado DC, Redman LM, Bray GA, Noland RC, Munzberg H, Hutson SM, Gettys TW, Schwartz MW, et al. (2014). FGF21 is an endocrine signal of protein restriction. J Clin Invest 124, 3913–3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan T, Morgan DA, Rahmouni K, Sonoda J, Fu X, Burgess SC, Holland WL, Kliewer SA, and Mangelsdorf DJ (2017). FGF19, FGF21, and an FGFR1/beta-Klotho-Activating Antibody Act on the Nervous System to Regulate Body Weight and Glycemia. Cell Metab 26, 709–718 e703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Q, Zhong L, Zhang J, Wang Y, Bornstein SR, Triggle CR, Ding H, Lam KS, and Xu A. (2014). FGF21 maintains glucose homeostasis by mediating the cross talk between liver and brain during prolonged fasting. Diabetes 63, 4064–4075. [DOI] [PubMed] [Google Scholar]
- Lo L, Yao S, Kim DW, Cetin A, Harris J, Zeng H, Anderson DJ, and Weissbourd B. (2019). Connectional architecture of a mouse hypothalamic circuit node controlling social behavior. Proc Natl Acad Sci U S A 116, 7503–7512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundsgaard AM, Fritzen AM, Sjoberg KA, Myrmel LS, Madsen L, Wojtaszewski JFP, Richter EA, and Kiens B. (2017). Circulating FGF21 in humans is potently induced by short term overfeeding of carbohydrates. Mol Metab 6, 22–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, Ng LL, Palmiter RD, Hawrylycz MJ, Jones AR, et al. (2010). A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci 13, 133–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui S, Sasaki T, Kohno D, Yaku K, Inutsuka A, Yokota-Hashimoto H, Kikuchi O, Suga T, Kobayashi M, Yamanaka A, et al. (2018). Neuronal SIRT1 regulates macronutrient-based diet selection through FGF21 and oxytocin signalling in mice. Nat Commun 9, 4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison CD, and Laeger T. (2015). Protein-dependent regulation of feeding and metabolism. Trends Endocrinol Metab 26, 256–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa Y, Kurosu H, Yamamoto M, Nandi A, Rosenblatt KP, Goetz R, Eliseenkova AV, Mohammadi M, and Kuro-o M. (2007). BetaKlotho is required for metabolic activity of fibroblast growth factor 21. Proc Natl Acad Sci U S A 104, 7432–7437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott V, Finlayson G, Lehnert H, Heitmann B, Heinrichs M, Born J, and Hallschmid M. (2013). Oxytocin reduces reward-driven food intake in humans. Diabetes 62, 3418–3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen BM, Ding X, Morgan DA, Coate KC, Bookout AL, Rahmouni K, Kliewer SA, and Mangelsdorf DJ (2014). FGF21 acts centrally to induce sympathetic nerve activity, energy expenditure, and weight loss. Cell Metab 20, 670–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren X, Ferreira JG, Zhou L, Shammah-Lagnado SJ, Yeckel CW, and de Araujo IE (2010). Nutrient selection in the absence of taste receptor signaling. The Journal of neuroscience : the official journal of the Society for Neuroscience 30, 8012–8023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanov RA, Zeisel A, Bakker J, Girach F, Hellysaz A, Tomer R, Alpar A, Mulder J, Clotman F, Keimpema E, et al. (2017). Molecular interrogation of hypothalamic organization reveals distinct dopamine neuronal subtypes. Nat Neurosci 20, 176–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Routh VH (2010). Glucose sensing neurons in the ventromedial hypothalamus. Sensors (Basel) 10, 9002–9025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciolino NR, Plummer NW, Chen YW, Alexander GM, Robertson SD, Dudek SM, McElligott ZA, and Jensen P. (2016). Recombinase-Dependent Mouse Lines for Chemogenetic Activation of Genetically Defined Cell Types. Cell Rep 15, 2563–2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sclafani A. (2001). Post-ingestive positive controls of ingestive behavior. Appetite 36, 79–83. [DOI] [PubMed] [Google Scholar]
- Sclafani A, Touzani K, and Bodnar RJ (2011). Dopamine and learned food preferences. Physiol Behav 104, 64–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimazu T, and Minokoshi Y. (2017). Systemic Glucoregulation by Glucose-Sensing Neurons in the Ventromedial Hypothalamic Nucleus (VMH). J Endocr Soc 1, 449–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soberg S, Sandholt CH, Jespersen NZ, Toft U, Madsen AL, von Holstein-Rathlou S, Grevengoed TJ, Christensen KB, Bredie WLP, Potthoff MJ, et al. (2017). FGF21 Is a Sugar-Induced Hormone Associated with Sweet Intake and Preference in Humans. Cell Metab 25, 1045–1053 e1046. [DOI] [PubMed] [Google Scholar]
- Solon-Biet SM, Cogger VC, Pulpitel T, Heblinski M, Wahl D, McMahon AC, Warren A, Durrant-Whyte J, Walters KA, Krycer JR, et al. (2016). Defining the Nutritional and Metabolic Context of FGF21 Using the Geometric Framework. Cell Metab 24, 555–565. [DOI] [PubMed] [Google Scholar]
- Solon-Biet SM, McMahon AC, Ballard JW, Ruohonen K, Wu LE, Cogger VC, Warren A, Huang X, Pichaud N, Melvin RG, et al. (2014). The ratio of macronutrients, not caloric intake, dictates cardiometabolic health, aging, and longevity in ad libitum-fed mice. Cell Metab 19, 418–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song P, Zechner C, Hernandez G, Canovas J, Xie Y, Sondhi V, Wagner M, Stadlbauer V, Horvath A, Leber B, et al. (2018). The Hormone FGF21 Stimulates Water Drinking in Response to Ketogenic Diet and Alcohol. Cell Metab 27, 1338–1347 e1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Z, and Albers HE (2018). Cross-talk among oxytocin and arginine-vasopressin receptors: Relevance for basic and clinical studies of the brain and periphery. Front Neuroendocrinol 51, 14–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talukdar S, Owen BM, Song P, Hernandez G, Zhang Y, Zhou Y, Scott WT, Paratala B, Turner T, Smith A, et al. (2016a). FGF21 Regulates Sweet and Alcohol Preference. Cell Metab 23, 344–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talukdar S, Zhou Y, Li D, Rossulek M, Dong J, Somayaji V, Weng Y, Clark R, Lanba A, Owen BM, et al. (2016b). A Long-Acting FGF21 Molecule, PF-05231023, Decreases Body Weight and Improves Lipid Profile in Non-human Primates and Type 2 Diabetic Subjects. Cell Metab 23, 427–440. [DOI] [PubMed] [Google Scholar]
- Tan HE, Sisti AC, Jin H, Vignovich M, Villavicencio M, Tsang KS, Goffer Y, and Zuker CS (2020). The gut-brain axis mediates sugar preference. Nature 580, 511–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T, Ngwa JS, van Rooij FJ, Zillikens MC, Wojczynski MK, Frazier-Wood AC, Houston DK, Kanoni S, Lemaitre RN, Luan J, et al. (2013). Genome-wide meta-analysis of observational studies shows common genetic variants associated with macronutrient intake. Am J Clin Nutr 97, 1395–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi H, He M, Wu P, Kim S, Paik R, Sugino K, Kvitsiani D, Fu Y, Lu J, Lin Y, et al. (2011). A resource of Cre driver lines for genetic targeting of GABAergic neurons in cerebral cortex. Neuron 71, 995–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tellez LA, Han W, Zhang X, Ferreira TL, Perez IO, Shammah-Lagnado SJ, van den Pol AN, and de Araujo IE (2016). Separate circuitries encode the hedonic and nutritional values of sugar. Nat Neurosci 19, 465–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibault L, and Booth DA (1999). Macronutrient-specific dietary selection in rodents and its neural bases. Neurosci Biobehav Rev 23, 457–528. [DOI] [PubMed] [Google Scholar]
- Timper K, and Bruning JC (2017). Hypothalamic circuits regulating appetite and energy homeostasis: pathways to obesity. Dis Model Mech 10, 679–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting JT, Lee BR, Chong P, Soler-Llavina G, Cobbs C, Koch C, Zeng H, and Lein E. (2018). Preparation of Acute Brain Slices Using an Optimized N-Methyl-D-glucamine Protective Recovery Method. J Vis Exp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong Q, Ye C, McCrimmon RJ, Dhillon H, Choi B, Kramer MD, Yu J, Yang Z, Christiansen LM, Lee CE, et al. (2007). Synaptic glutamate release by ventromedial hypothalamic neurons is part of the neurocircuitry that prevents hypoglycemia. Cell Metab 5, 383–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldhuizen MG, Babbs RK, Patel B, Fobbs W, Kroemer NB, Garcia E, Yeomans MR, and Small DM (2017). Integration of Sweet Taste and Metabolism Determines Carbohydrate Reward. Curr Biol 27, 2476–2485 e2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vianna CR, and Coppari R. (2011). A treasure trove of hypothalamic neurocircuitries governing body weight homeostasis. Endocrinology 152, 11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Holstein-Rathlou S, BonDurant LD, Peltekian L, Naber MC, Yin TC, Claflin KE, Urizar AI, Madsen AN, Ratner C, Holst B, et al. (2016). FGF21 Mediates Endocrine Control of Simple Sugar Intake and Sweet Taste Preference by the Liver. Cell Metab 23, 335–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vong L, Ye C, Yang Z, Choi B, Chua S Jr., and Lowell BB (2011). Leptin action on GABAergic neurons prevents obesity and reduces inhibitory tone to POMC neurons. Neuron 71, 142–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Xu Y, Zhu Y, Sutton AK, Zhao R, Lowell BB, Olson DP, and Tong Q. (2012). An obligate role of oxytocin neurons in diet induced energy expenditure. PLoS One 7, e45167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuker CS (2015). Food for the brain. Cell 161, 9–11. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1, related to STAR methods
List of genes and amplicon sequences for panel 1 of single cell RNAseq for BD Rhapsody.
Table S2, related to STAR methods
List of genes and amplicon sequences for panel 2 of single cell RNAseq for BD Rhapsody.
Data Availability Statement
RNA sequencing data is available in the NCBI Gene Expression Omnibus (GEO) and Sequence Read Archive (SRA) under accession number GEO: GSE150923. Further requests for data are available upon correspondence with the Lead Contact.