Abstract
Objective
To present standardized diagnostic criteria for idiopathic distal sensory polyneuropathy (iDSP) and its subtypes: idiopathic mixed fiber sensory neuropathy (iMFN), idiopathic small fiber sensory neuropathy (iSFN), and idiopathic large fiber sensory neuropathy (iLFN) for use in research.
Methods
The Analgesic, Anesthetic, and Addiction Clinical Trial Translations, Innovations, Opportunities and Networks (ACTTION) public-private partnership with the Food and Drug Administration convened a meeting to develop consensus diagnostic criteria for iMFN, iSFN, and iLFN. After background presentations, a collaborative, iterative approach was used to develop expert consensus for new criteria.
Results
An iDSP diagnosis requires at least 1 small fiber (SF) or large fiber (LF) symptom, at least 1 SF or LF sign, abnormalities in sensory nerve conduction studies (NCS) or distal intraepidermal nerve fiber density (IENFD), and exclusion of known etiologies. An iMFN diagnosis requires that at least 1 of the above clinical features is SF and 1 clinical feature is LF with abnormalities in sensory NCS or IENFD. Diagnostic criteria for iSFN require at least 1 SF symptom and at least 1 SF sign with abnormal IENFD, normal sensory NCS, and the absence of LF symptoms and signs. Diagnostic criteria for iLFN require at least 1 LF symptom and at least 1 LF sign with normal IENFD, abnormal sensory NCS, and absence of SF symptoms and signs.
Conclusion
Adoption of these standardized diagnostic criteria will advance research and clinical trials and spur development of novel therapies for iDSPs.
Peripheral neuropathies, most of which are chronic distal sensory polyneuropathies (DSPs), are among the most prevalent of neurologic disorders. In 20%–50% of DSP cases, laboratory investigations fail to find a cause, leading to a diagnosis of idiopathic DSP (iDSP, also known as cryptogenic polyneuropathy).1,2 No drugs are approved for the treatment of iDSP and its subtypes, idiopathic mixed fiber sensory neuropathy (iMFN), idiopathic small fiber sensory neuropathy (iSFN), and idiopathic large fiber sensory neuropathy (iLFN). Thus, clinical trials of iDSPs are urgently needed to develop evidence-based therapies.
One potential barrier to developing therapies for iDSP is that eligibility criteria for iDSP research studies do not exist or, depending on the subtype, have considerable variability.3–7 Although some diagnostic criteria are available for DSPs of specific etiology,8,9 and more recently for SFN,3,10,11 no generally accepted criteria are available for iMFN, iSFN, and iLFN. The goal of this article is to present comprehensive and contrasting standardized diagnostic criteria for iMFN, iSFN, and iLFN for clinical research and clinical trials. Clinical trials for novel treatments for iDSP could be designed to examine efficacy and safety in all patients who meet the diagnostic criteria for iDSP, for example, any one of the 3 subtypes or only in 1 or 2 of the subtypes, depending on the mechanism of action of the treatment.
Methods
The Consortium on Clinical Endpoints and Procedures for Peripheral Neuropathy Trials (CONCEPPT) of the Analgesic, Anesthetic, and Addiction Clinical Trial Translations, Innovations, Opportunities and Networks (ACTTION) public-private partnership with the FDA convened in April of 2018 to develop consensus diagnostic criteria for iDSP and its subtypes. An international group of neurologists, clinical trialists, and regulatory experts from academia, government, and the pharmaceutical industry attended. Participants were selected based on their research, clinical, or regulatory expertise relevant to peripheral neuropathy and clinical trial design to represent broadly the relevant disciplines and perspectives while limiting the meeting size to promote productive and efficient discussion. To facilitate discussion, participants presented a set of background lectures, including the results of a systematic review of current diagnostic criteria and research study entry criteria used for iSFN.12 Lectures and the meeting transcript are available at on the ACTTION-CONCEPPT webpage.13 Dr. Freeman served as the facilitator to obtain an initial consensus at the meeting. Based on this initial consensus, an initial draft of the manuscript was developed by Drs. Freeman and Gewandter (i.e., lead authors). The manuscript was sent to all authors for feedback. Subsequently, the lead authors synthesized the input and redistributed the manuscript for further feedback. This process occurred 4 times until no new major themes emerged, and all authors approved the final manuscript.
Background
The sensory neuropathy nomenclature (MFN, SFN, and LFN) is derived from the population of nerve fibers affected in these conditions. These fibers are classified as Aβ, Aδ, and C fibers based on their cell body size, axon diameter, amount of myelination, and conduction velocity. The Aβ fibers have large cell bodies, are heavily myelinated, and have conduction velocities that range from 16 to 100 m/second; the Aδ fibers have medium-sized cell bodies, are lightly myelinated, and have conduction velocities between 5 and 30 m/second; and the C fibers have small cell bodies and are unmyelinated with conduction velocities less than 5 m/second. Aβ fibers are considered large fibers, whereas the Aδ and C fibers are considered small fibers.14–16 Because of their anatomic and consequent neurophysiologic characteristics, Aδ and C fibers, which are predominantly or exclusively injured in SFN, cannot be assessed using standard clinical neurophysiologic techniques. The clinical phenomenology associated with these neuropathies is a function of the sensory modalities subserved by the damaged nerve fibers.
Small fiber sensory modalities include pain, temperature, and poorly localized light touch; consequently, symptoms associated with SFN include spontaneous and evoked pain, numbness, pruritic sensation, and paresthesias.14–21 Large fiber sensory modalities encompass touch, proprioception, pressure, and vibration perception. The deep tendon reflexes are also mediated by the large fibers. Thus, symptoms associated with LFN include numbness, balance impairment, paresthesias, and sensory distortion.14–21 Hence, the symptoms of numbness and paresthesias and impaired light touch perception may be present in both large and small fiber polyneuropathies.14–21
In this article, we provide diagnostic criteria for iMFN (i.e., a polyneuropathy with both small and large fiber features), pure (or isolated) iSFN, and pure (or isolated) iLFN. In doing so, we recognize that pure SFN and LFN may have subclinical large and small fiber pathology or pathophysiology, respectively, for example, detectable with sural nerve biopsy or microneurographic techniques, and that pure SFN and pure LFN may, in some patients, evolve over time into an MFN. The diagnostic criteria for iDSP are fulfilled when the specific diagnostic criteria for any one of the 3 iDSP subtypes are met or in those patients who meet all criteria for SFN and have abnormal nerve conduction studies (termed clinical SFN with abnormal large fiber neurophysiology) or in those patients who meet all criteria for LFN and have abnormal IENFD (termed clinical LFN with abnormal nerve fiber density).
Diagnostic criteria for idiopathic distal sensory polyneuropathy (iDSP)
Symptoms
Polyneuropathy symptoms should be present in a symmetrical, length-dependent distribution for clinical trial inclusion. In some patients, iDSP presents in an asymmetric or non–length-dependent manner. We did not define criteria for that disorder in this article.
To maximize sensitivity and ensure that patients are not excluded based on unique sensory symptom descriptions, a specific list of painful and nonpainful sensory symptom descriptors was deliberately excluded and only 1 sensory symptom was required (table 1). This potentially nonrigorous symptom definition is unlikely to adversely affect diagnosis because the requirements related to signs (see section Signs) and neurophysiologic and neuropathologic tests (see section Neurophysiologic and neuropathologic tests) would be expected to increase the specificity of the criteria. However, to further increase the specificity for entry into clinical trials, investigators may consider requiring at least 1 symptom from at least 2 of the symptom items listed in table 1.
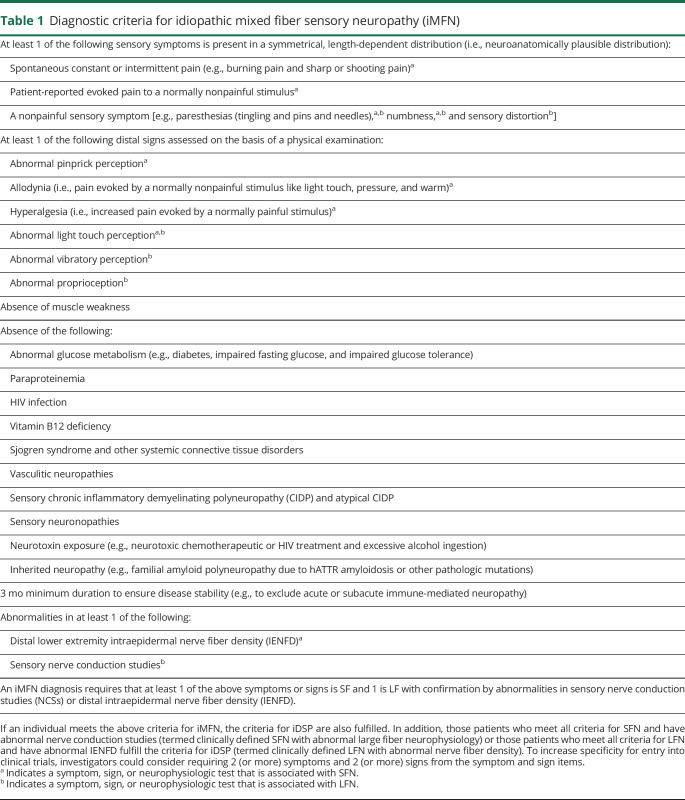
Table 1.
Diagnostic criteria for idiopathic mixed fiber sensory neuropathy (iMFN)
Signs
The diagnosis of iDSP requires abnormality in at least 1 of the clinical signs assessed by sensory neurologic examination (table 1). The following 4 signs: abnormal pinprick perception, abnormal light touch perception, abnormal vibratory perception, and abnormal proprioception were selected because they constitute the core elements of the neurologic clinical examination. In addition, in a recent systematic review, they were found to be the most common signs of sensory dysfunction evaluated in clinician-rated outcome measures for peripheral neuropathy related to chemotherapy, diabetes, HIV, and hATTR.22 Hyperalgesia and allodynia were also included because they are positive signs that indicate a pathologic increase in nerve excitability and are commonly evaluated in patients with neuropathic pain.23 Deep tendon reflexes have been excluded from the diagnostic criteria because of age-related changes in the healthy elderly.24,25 To further increase the specificity for entry into clinical trials, investigators may consider requiring at least 1 sign from at least 2 of the sign items listed in table 1.
Standardized assessment procedures and normative values should be used to identify abnormality in clinical signs (e.g., Rydel-Seiffer tuning fork for vibration26). However, many clinical examination components do not have evidence-based cutoffs and require judgment by a neurologist or a clinician trained in neurologic examination (see Gewandter et al.22 for a description of the available standardized examination assessments and normative values). For clinical trials, the method used to assess each of the clinical signs should be standardized across sites and investigators should be trained to maximize consistency as much as possible. In addition, publication of the standardization methods used in clinical trials would help future investigators and standardize the manner in which these signs are evaluated across studies.
Motor involvement
Muscle weakness (i.e., the main indicator of a motor neuropathy) should be excluded by a neurologist or a clinician trained in neurologic examination. However, depending on the research question, the study goals, or a drug's mechanism of action, it may not be necessary to eliminate motor involvement in all studies. Such an exception should be noted in the entry criteria.
Etiology
Evaluating the cause of all DSPs is important for guiding treatment and entry into clinical trials. Although associations do not guarantee causality, DSP is more prevalent in individuals with certain conditions, exposures to drugs or toxins, and genetic variants, than those in the general population. Thus, these potential causes are likely to contribute to the development of all DSP subtypes and must be excluded as potential etiologies (table 1) when making a diagnosis of idiopathic for all DSP subtypes. The etiologies were selected based on consensus among the authors and from research studies demonstrating associations between these potential etiologies and DSPs.2,25,27–35 These possible etiologies should be considered first-line exclusions and further exclusions36 might be considered under certain circumstances, for example, in phase 2 or 3 clinical trials or in patients with specific medical histories or abnormalities on examination or routine testing. Because some of these excluded disorders (e.g., prediabetes and monoclonal gammopathy) have a high prevalence and may not necessarily be causative in particular patients, depending on the nature of the therapeutic agent, clinical trial or study, one might include patients with these features. If doing so, it should be explicitly stated in the entry criteria.
Furthermore, some, but not all, recent studies37,38 have identified low-frequency gain-of-function mutations in genes encoding sodium channels NaV1.7, NaV1.8, and NaV1.9 (SCN9A, SCN10A, and SCN11A, respectively) in some patients with SFN.31–35 Some studies have observed similar findings in patients with diabetic peripheral neuropathy.34,38,39 These observations have underscored the need for SFN research and a broadly accepted approach for classifying pure or isolated iSFN. Investigators may wish to include patients with specific genetic variants in clinical research studies of DSP subtypes. If so, this should be explicitly stated in the entry criteria.
The role of genetic screening in observational and interventional studies in peripheral neuropathy is evolving as genetic sequencing costs decrease. Depending on the mechanism of action of a putative disease-modifying agent, sequencing may be helpful, but it is not required in all iSFN trials. However, when possible, blood should be drawn and banked for later analysis. Excluding patients with 1 or more first-degree relatives with a DSP may help exclude patients with a hereditary peripheral neuropathy, but given the high population prevalence of DSP, not all studies may require this exclusion.
Duration
Sensory polyneuropathy symptoms should be present and stable for at least 3 months to increase the likelihood that the condition is not acute and reversible. The 3-month minimum is consistent with the requirement of pain chronicity defined by the International Association for the Study of Pain (IASP)40 and the ACTTION-American Pain Society Pain Taxonomy (AAPT).41 Certain forms of peripheral neuropathy can spontaneously improve; thus, for clinical trials, a longer minimum duration of 6 months could be considered. These criteria only apply to patients with chronic iDSPs and not acute or subacute DSPs.
Neurophysiologic and neuropathologic tests
A confimed iDSP diagnosis requires iDSP diagnosis requires either abnormal sensory nerve conduction studies, for example, sural sensory nerve action potential amplitudes (SNAPs) and/or nerve conduction velocity (NCV), or decreased intraepidermal nerve fiber density (IENFD). Sensory nerve conduction and IENFD were chosen as the 2 diagnostic tests for confirming DSP because they detect abnormalities in large and small sensory neurons, respectively, and they have the most extensive age-based normative values for identifying abnormal cases.42,43 However, multiple technical details should be considered when performing these tests. Nerve conduction study values can be affected by electrode setup, accuracy of distance measurements, limb temperature, and testing parameters.44 It is important to standardize nerve conduction analyses and use similar techniques as those used to generate the normative reference values for identifying abnormal cases. The Normative Data Task Force of the American Association of Neuromuscular & Electrodiagnostic Medicine identified high-quality studies reporting nerve conduction normative values with sufficiently detailed technical methods to reproduce the technique in a test sample.44
For IENFD, 2 large, multicenter, multinational studies have established age- and sex-based normative data for identifying abnormality42,43 Importantly, skin biopsy with IENFD analysis is technically challenging and susceptible to false positives of low IENFDs resulting from suboptimal handling including, for example, tissue crush or stretch or thermal damage from inadequate cryoprotection. Strict adherence to handling and processing guidelines is therefore essential (e.g., 50-μm-thick sections, fixed using paraformaldehyde solution, cryoprotected and frozen, sectioned.45 To maximize reliability, it is highly recommended to send skin biopsies for processing and evaluation to experienced laboratories with documented expertise in quantifying IENFD.
Performing nerve conduction studies and assessing IENFDs may be challenging in a multicenter clinical trial. In cases in which these tests are not feasible, and in other specific research situations, a diagnosis of clinically defined iDSP based solely on symptoms, signs, and exclusion of known potential etiologies may be appropriate (see section Etiology); however, this should be explicitly noted in the inclusion criteria. Under this circumstance, to increase specificity for entry into clinical trials, investigators could consider requiring 2 (or more) symptoms and 2 (or more) signs from the symptom and sign items listed in table 1.
Quantitative sensory testing (QST) is an extension of the clinical sensory examination using standardized sensory stimuli and response paradigms. The utility of QST for diagnosing iDSP is still exploratory. The Mayo Clinic Peripheral Nerve Group46 and German Research Network on Neuropathic Pain (DFNS)47 used different equipment and test paradigms to standardized the testing of multiple QST modalities for diagnosing sensory neuropathies and characterizing neuropathic pain. To improve the feasibility of QST in the clinical setting and for large, multisite clinical trials, bedside QST testing methods are currently being developed by several groups.48–50 Limitations of QST include that it is a psychophysical test with inherent subjective variability, a lack of equipment availability in many centers, variability in equipment and test paradigms, limited normative data for nonpainful neuropathies, and that it has no localizing value. The diagnostic criteria for all sensory neuropathies could be supplemented by including QST if performed in centers with technical experience, as in a recent study.10
Corneal confocal microscopy (CCM) has also been used to diagnose DSP. The evidence base supporting this technique is growing, and it may become a promising tool in the future. Using CCM to distinguish patients with and without diabetic peripheral neuropathy (diagnosed via the Toronto Criteria8) has acceptable sensitivity and specificity.51,52 Normative values from a multinational study of healthy volunteers are published.53 However, CCM is currently available at few centers, studies have only been published by a small number of investigators, and its specificity for different nerve fiber populations is not established. More definitive conclusions on its use as a diagnostic tool await larger studies.
The quantitative sudomotor axon reflex test (QSART) quantifies postganglionic C-fiber–mediated sudomotor function (i.e., sweating) via an axon reflex, which is triggered by iontophoresis of a cholinergic agonist. Studies suggest this is a sensitive diagnostic test for iSFN.54 QSART abnormalities may also add diagnostic value for identifying a subpopulation of patients with iSFN with clinical or subclinical autonomic involvement. QSART results are not highly correlated with QST or IENFD55; thus, adding QSART to the diagnostic panel for iSFN increases sensitivity without reducing specificity.56 Normative QSART values are available from a sample of 357 healthy volunteers.57
Laboratory-based QST, CCM, and QSART require specialized equipment and standardized protocols for multisite studies. They are not recommended as core diagnostic criteria or confirmatory tests for multisite iDSP trials at present due to their limited availability, need for trained personnel, and an insufficient evidence base of normative values.
Specific diagnostic criteria for iDSP subtypes
Diagnostic criteria for idiopathic mixed fiber sensory neuropathy (iMFN)
iMFN has both small and large fiber features without specific fiber selectivity. An iMFN diagnosis requires that at least 1 clinical feature is SF and 1 clinical feature is LF (table 1). To increase specificity for entry into clinical trials, investigators could consider requiring 2 (or more) symptoms and 2 (or more) signs from the symptom and sign items listed in table 1.
A confirmed iMFN diagnosis requires abnormal nerve conduction or abnormal IENFD (table 1). In research situations, where nerve conduction or IENFD analyses are not feasible or are deemed unnecessary based on the specific research question, a diagnosis of clinically defined iMFN could be made based on symptoms and signs alone. Under this circumstance, more rigorous clinical criteria are proposed, such as 2 (or more) symptoms and 2 (or more) signs from the symptom and sign items listed in table 1.
Diagnostic criteria for idiopathic small fiber neuropathy (iSFN)
Pure SFN is more frequently diagnosed in the clinical setting because of the development, validation, and widespread availability of IENFD assessment from skin punch biopsy.3,10 An iSFN diagnosis requires the presence of 1 characteristic painful or nonpainful small fiber symptom (e.g., spontaneous or intermittent pain and paresthesias) present in a symmetrical, length-dependent distribution (table 2) and at least 1 small fiber sign. To increase specificity for entry into clinical trials, investigators could consider requiring 2 (or more) symptoms and 2 (or more) signs from the symptom and sign items listed in table 2. The presence of pure LFN symptoms is an exclusion criterion. A pure iSFN diagnosis requires demonstrating normal distal vibration perception and proprioception.3,10 Patients with pure iSFN should have preserved deep tendon reflexes; however, this requirement has been excluded from the diagnostic criteria based on considerations outlined in section Signs.24,25
Table 2.
Diagnostic criteria for idiopathic small fiber sensory neuropathy (iSFN)
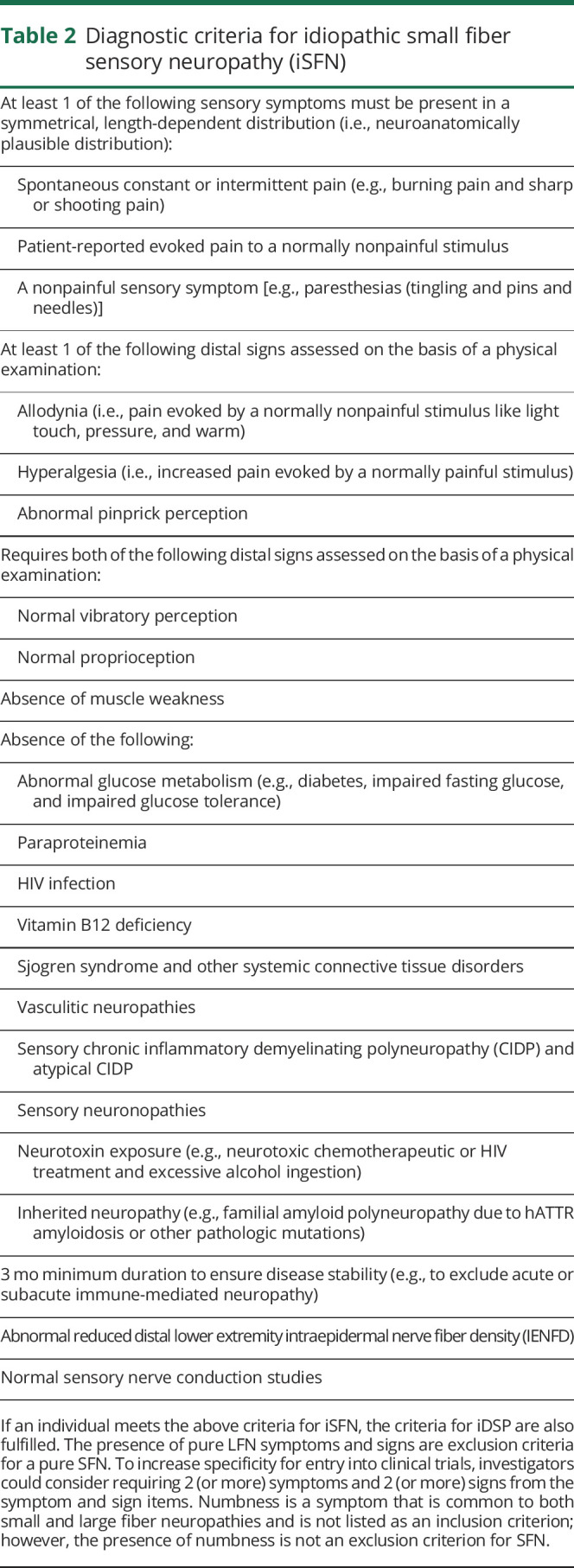
Some patients, otherwise fulfilling all iSFN criteria may report nonpainful sensory symptoms only (e.g., paresthesias). Such patients can be classified as having painless iSFN. Autonomic features may be a clinical accompaniment of iSFN. Patients with iSFN may be classified as iSFN with or without autonomic involvement.55
A confirmed pure iSFN diagnosis requires normal nerve conduction studies and abnormal IENFDs. In some research situations, where nerve conduction or IENFD analyses are not feasible, confirmation of normal vibration perception and proprioception could be used to a make a diagnosis of clinically defined iSFN. Under this circumstance, more rigorous clinical criteria are proposed, such as 2 (or more) symptoms and 2 (or more) signs from the symptom and sign items listed in table 2.
Diagnostic criteria for idiopathic large fiber neuropathy (iLFN)
LFN is well recognized because of the widespread availability of nerve conduction studies, the diagnostic gold standard for large nerve fiber dysfunction. A diagnosis of pure iLFN requires the presence of at least 1 nonpainful sensory symptom (e.g., paresthesias) and 1 large fiber–associated sign, for example, abnormal vibration perception or abnormal proprioception). The presence of pure SFN symptoms is an exclusion criterion. Pinprick perception must be normal, and allodynia and hyperalgesia must be absent. To increase specificity for entry into clinical trials beyond the core criteria, investigators could consider requiring at least 2 (or more) symptoms and 2 (or more) signs from the symptom and sign items listed in table 3.
Table 3.
Diagnostic criteria for idiopathic large fiber sensory neuropathy (iLFN)
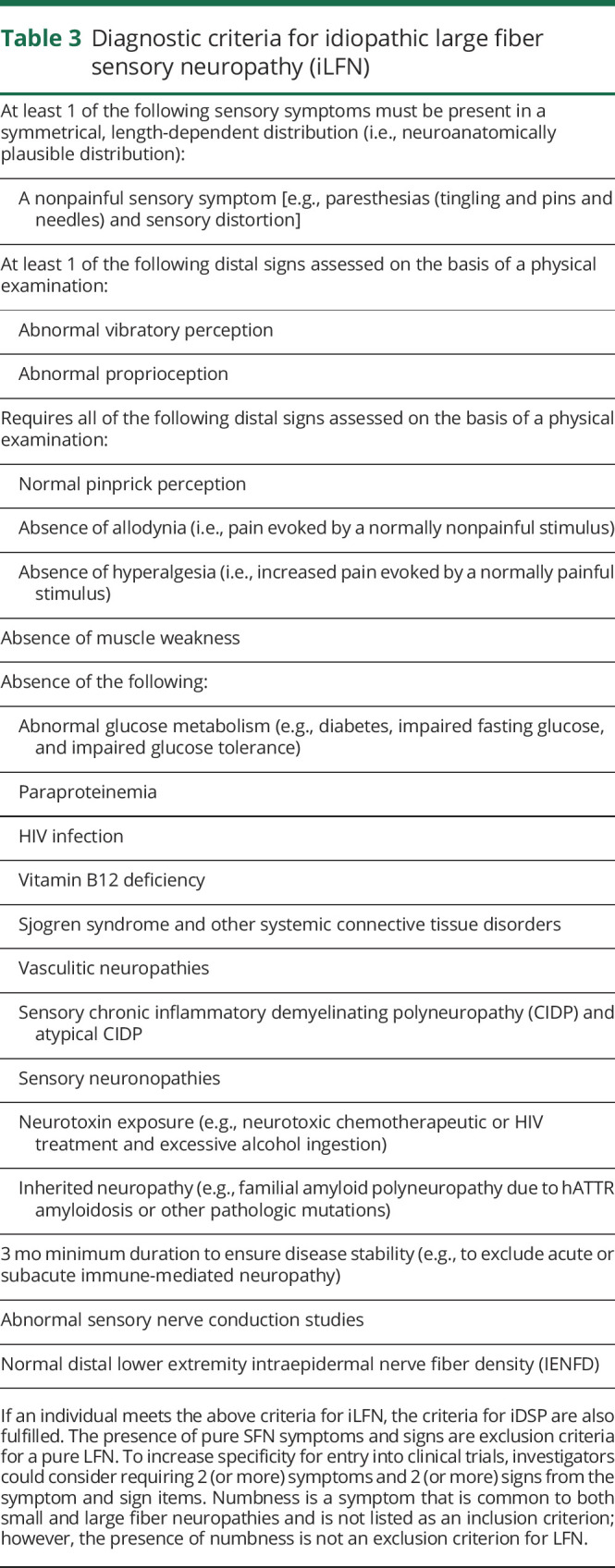
A confirmed iLFN diagnosis requires abnormal nerve conduction with normal IENFD. In some research situations, where nerve conduction or IENFD analyses are not feasible, confirmation of abnormal proprioception and vibration perception with normal pinprick perception and absence of allodynia and hyperalgesia could be used to make a diagnosis of clinically defined iLFN. Under this circumstance, more rigorous clinical criteria are proposed, such as 2 (or more) symptoms and 2 (or more) signs from the symptom and sign items listed in table 3.
Limitations
These diagnostic criteria are based, in part, on systematic literature reviews (one completed before the meeting,22 and a second presented at the meeting). Subsequently, the criteria were developed using a collaborative, iterative expert consensus approach during and after the face-to-face meeting. Others have drawn attention to the methodological limitations of the existing literature that analyzes the sensitivity and specificity of the symptoms, signs, and investigations used to make a diagnosis of DSP and the challenges inherent to using this literature to develop neuropathy diagnostic criteria.58,59 In particular, the role played by bias in potentially inflating the sensitivity and specificity of signs, symptoms, and investigations in these studies.58,59 These challenges lend support to an expert consensus approach to diagnostic criteria that could provide the basis for future methodologically sound studies to determine the sensitivity and specificity of the peripheral neuropathy signs and symptoms.
In these diagnostic criteria, we have attempted to balance sensitivity, specificity, and accuracy, while simultaneously providing sufficient flexibility so that the specific criteria could be tailored to the particular needs of the trial by adding or omitting diagnostic features. This flexible approach will allow these criteria to be used, for example, in community-based epidemiologic studies and clinical trials conducted at centers lacking some diagnostic technology. We have not provided specific guidelines on interviewing patients to best identify symptoms or to perform physical examinations or neurophysiologic tests. Optimizing and standardizing diagnostic methods and analyses should be a research priority. Finally, although these criteria were developed for clinical research, they may, with modifications for individual practice capabilities, guide clinical practice and facilitate research translation into the clinic.
Conclusions
iDSPs are common and often debilitating and currently have no proven efficacious disease-modifying therapies. Because of advances in understanding the pathophysiologic and mechanistic basis of SFN, targeted therapies may be on the horizon. No broadly accepted, comprehensive standardized diagnostic criteria are currently available that segregate iMFN, iSFN, and iLFN. Widespread use of the criteria presented in this article could accelerate progress in developing therapies for these prevalent and often refractory conditions.
Acknowledgment
This article was reviewed and approved by the Executive Committee of the Analgesic, Anesthetic, and Addiction Clinical Trial Translations, Innovations, Opportunities, and Networks (ACTTION) public-private partnership with the US Food and Drug Administration (FDA). Financial support for this project was provided by the ACTTION public-private partnership, which has received research contracts, grants, or other revenue from the FDA, multiple pharmaceutical and device companies, philanthropy, and other sources. The views expressed in this article are those of the authors, and no official endorsement by the FDA or the pharmaceutical and device companies that provided unrestricted grants to support the activities of the ACTTION public-private partnership should be inferred.
Glossary
- CCM
corneal confocal microscopy
- iDSP
idiopathic distal sensory polyneuropathy
- IENFD
intraepidermal nerve fiber density
- iLFN
idiopathic large fiber sensory neuropathy
- iSFN
idiopathic small fiber sensory neuropathy
- LF
large fiber
- NCS
nerve conduction studies
- QST
quantitative sensory testing
- SF
small fiber
Appendix. Authors
Footnotes
Podcast: NPub.org/3cv4m2
Study funding
This article was funded by the ACTTION public-private partnership.
Disclosure
Several authors lead academic or commercial laboratories that process skin biopsies to assess IENFD. Robert H. Dworkin has received in the past 5 years research grants and contracts from the US Food and Drug Administration and the US National Institutes of Health and compensation for serving on advisory boards or consulting on clinical trial methods from Abide, Acadia, Adynxx, Analgesic Solutions, Aptinyx, Aquinox, Asahi Kasei, Astellas, AstraZeneca, Biogen, Biohaven, Boston Scientific, Braeburn, Celgene, Centrexion, Chromocell, Clexio, Concert, Coronado, Daiichi Sankyo, Decibel, Dong-A, Editas, Eli Lilly, Eupraxia, Glenmark, Grace, Hope, Hydra, Immune, Johnson & Johnson, Lotus Clinical Research, Mainstay, Medavante, Merck, Neumentum, Neurana, NeuroBo, Novaremed, Novartis, NSGene, Olatec, Periphagen, Pfizer, Phosphagenics, Quark, Reckitt Benckiser, Regenacy (also equity), Relmada, Sanifit, Scilex, Semnur, Sollis, Spinifex, Syntrix, Teva, Thar, Theranexus, Trevena, Vertex, and Vizuri. Catharina Faber has received grants from the European Commission, the Prinses Beatrix Spierfonds, Grifols, and Lamepro and consulting income from Biogen, Chromocell, Astellas, and Vertex. Eva L. Feldman has received funding from the NIH (R21NS102924, R24DK082841, R01DK107956, and R01DK108173) and the Novo Nordisk Foundation (NNF14SA0006). In the past 36 months, Roy Freeman has received funding from the NIH (U54NS065736, 1R01NS10584401A1, and R01HL111465-01A1) and personal compensation and/or stock options for serving on scientific advisory boards of Abide, Averitas, Applied Therapeutics, Aptinyx, Biogen, Clexio, Ceracor, Cutaneous NeuroDiagnostics, GW Pharma, Lundbeck, NeuroBo, Pfizer, Regenacy, Spinifex, Toray, Theravance, and Vertex. In the past 36 months, Jennifer Gewandter has received research grants from the NIH and consulting income from MundiPharma, Disarm Therapeutics, Asahi Kasei Pharma, Magnolia Neurosciences, Orthogonal Neurosciences, Science Branding Communications, and SK Life Science. In the past 36 months, Simon Haroutounian has received research grants from Pfizer Inc and Disarm Therapeutics and consulting fees from Medoc Ltd and Rafa Laboratories. Ahmet Hoke received grants from the NIH, DOD, and AMRF. In the past 36 months, Dr. Hoke served as a consultant to Pfizer and currently serves on the Scientific Advisory Board of Disarm Therapeutics. Giuseppe Lauria has received grants from the European Commission, Italian Ministry of Health, Lombardy Foundation for Biomedical Research (FRRB), Lombardy Hub for Research and Innovation, and Italian Foundation for the Research in ALS (AriSLA) and consulting income from CSL Behring, Biogen, Vertex, Chromocell, and Astellas. In the past 36 months, Rayaz Malik has received research grants from QNRF and consulting income from Pfizer, Novo Nordisk, Merck Pharma, and Biogen. Anne Louise Oaklander has received research funding from the NIH (R01NS093653 and R01NS095640), Department of Defense (GW140169 and GW130109), and private foundations. Amanda Peltier has received consulting compensation from Akcea, Alnylam, Catalyst and CSL Behring and has grant funding from the NINDS. Elissa Ritt was employed by NuFactor Specialty Pharmacy at the time of the meeting James W. Russell has received funding from the NIH (1R01DK107007-01A1) and Office of Research Development, Department of Veterans Affairs (101RX001030). Dr. Gordon Smith has received research grants from the NIH (NINDS and NIDDK) and consulting income from Disarm Therapeutics, Regenesis, Alexion, and Argenx. In the past 36 months, Roi Treister has received research grants from the ISF (Israel Science Foundation) and EFIC (European pain federation) and consulting income from Medasense, Medoc, Syqe Medical, and Analgesic Solutions. Nurcan Üçeyler has received research grants from Shire Takeda, Idorsia, Sanofi Genzyme, and Biogen. Go to Neurology.org/N for full disclosures.
References
- 1.Hanewinckel R, van Oijen M, Ikram MA, van Doorn PA. The epidemiology and risk factors of chronic polyneuropathy. Eur J Epidemiol 2016;31:5–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Greef BTA, Hoeijmakers JGJ, Gorissen-Brouwers CML, Geerts M, Faber CG, Merkies ISJ. Associated conditions in small fiber neuropathy - a large cohort study and review of the literature. Eur J Neurol 2018;25:348–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Devigili G, Tugnoli V, Penza P, et al. The diagnostic criteria for small fibre neuropathy: from symptoms to neuropathology. Brain 2008;131:1912–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Samuelsson K, Kostulas K, Vrethem M, Rolfs A, Press R. Idiopathic small fiber neuropathy: phenotype, etiologies, and the search for Fabry disease. J Clin Neurol 2014;10:108–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peters MJ, Bakkers M, Merkies IS, Hoeijmakers JG, van Raak EP, Faber CG. Incidence and prevalence of small-fiber neuropathy: a survey in The Netherlands. Neurology 2013;81:1356–1360. [DOI] [PubMed] [Google Scholar]
- 6.Bednarik J, Vlckova-Moravcova E, Bursova S, Belobradkova J, Dusek L, Sommer C. Etiology of small-fiber neuropathy. J Peripher nervous Syst : JPNS 2009;14:177–183. [DOI] [PubMed] [Google Scholar]
- 7.Lacomis D. Small-fiber neuropathy. Muscle & nerve 2002;26:173–188. [DOI] [PubMed] [Google Scholar]
- 8.Tesfaye S, Boulton AJ, Dyck PJ, et al. Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes care 2010;33:2285–2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.England JD, Gronseth GS, Franklin G, et al. Distal symmetric polyneuropathy: a definition for clinical research: report of the American academy of neurology, the American association of electrodiagnostic medicine, and the American academy of physical medicine and rehabilitation. Neurology 2005;64:199–207. [DOI] [PubMed] [Google Scholar]
- 10.Devigili G, Rinaldo S, Lombardi R, et al. Diagnostic criteria for small fibre neuropathy in clinical practice and research. Brain 2019;142:3728–3736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oaklander AL, Nolano M. Scientific advances in and clinical approaches to small-fiber polyneuropathy: a review. JAMA Neurol 2019;76:1240–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haroutounian S, Todorovic MS, Leinders M, Campagnalo M, Gewandter JS, Dworkin RH, Freeman R. Diagnostic criteria for idiopathic small fiber neuropathy: a systematic review. Muscle and Nerve. Epub 2020 September 29. [DOI] [PubMed] [Google Scholar]
- 13.ACTTION CONCEPPT Website. Available at: acttion.org/conceppt. Accessed August 21, 2020. [Google Scholar]
- 14.Abraira VE, Ginty DD. The sensory neurons of touch. Neuron 2013;79:618–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell 2009;139:267–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bourane S, Grossmann KS, Britz O, et al. Identification of a spinal circuit for light touch and fine motor control. Cell 2015;160:503–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ochoa JL, Torebjork HE. Paraesthesiae from ectopic impulse generation in human sensory nerves. Brain 1980;103:835–853. [DOI] [PubMed] [Google Scholar]
- 18.Lennertz RC, Tsunozaki M, Bautista DM, Stucky CL. Physiological basis of tingling paresthesia evoked by hydroxy-alpha-sanshool. J Neurosci 2010;30:4353–4361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Todd AJ. Neuronal circuitry for pain processing in the dorsal horn. Nat Rev Neurosci 2010;11:823–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zimmerman A, Bai L, Ginty DD. The gentle touch receptors of mammalian skin. Science 2014;346:950–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lallemend F, Ernfors P. Molecular interactions underlying the specification of sensory neurons. Trends Neurosci 2012;35:373–381. [DOI] [PubMed] [Google Scholar]
- 22.Gewandter JS, Gibbons CH, Campagnolo M, et al. Clinician-rated measures for distal symmetrical axonal polyneuropathy: ACTTION systematic review. Neurology 2019;93:346–360. [DOI] [PubMed] [Google Scholar]
- 23.Finnerup NB, Haroutounian S, Kamerman P, et al. Neuropathic pain: an updated grading system for research and clinical practice. Pain 2016;157:1599–1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dyck PJ, Davies JL, Litchy WJ, O'Brien PC. Longitudinal assessment of diabetic polyneuropathy using a composite score in the Rochester Diabetic Neuropathy Study cohort. Neurology 1997;49:229–239. [DOI] [PubMed] [Google Scholar]
- 25.England JD, Gronseth GS, Franklin G, et al. Practice Parameter: evaluation of distal symmetric polyneuropathy: role of laboratory and genetic testing (an evidence-based review). Report of the AAN, AANEM, and AAPMR. Neurology 2009;72:185–192. [DOI] [PubMed] [Google Scholar]
- 26.Martina ISJ, van Koningsveld R, Schmitz PIM, et al. Measuring vibration threshold with a graduated tuning fork in normal aging and in patients with polyneuropathy. J Neurol Neurosur Ps 1998;65:743–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Farhad K, Traub R, Ruzhansky KM, Brannagan TH III. Causes of neuropathy in patients referred as "idiopathic neuropathy. Muscle Nerve 2016;53:856–861. [DOI] [PubMed] [Google Scholar]
- 28.Lang M, Treister R, Oaklander AL. Diagnostic value of blood tests for occult causes of initially idiopathic small-fiber polyneuropathy. J Neurol 2016;263:2515–2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith AG, Ramachandran P, Tripp S, Singleton JR. Epidermal nerve innervation in impaired glucose tolerance and diabetes-associated neuropathy. Neurology 2001;57:1701–1704. [DOI] [PubMed] [Google Scholar]
- 30.Plante-Bordeneuve V. Transthyretin familial amyloid polyneuropathy: an update. J Neurol 2018;265:976–983. [DOI] [PubMed] [Google Scholar]
- 31.Faber CG, Hoeijmakers JG, Ahn HS, et al. Gain of function Nanu1.7 mutations in idiopathic small fiber neuropathy. Ann Neurol 2012;71:26–39. [DOI] [PubMed] [Google Scholar]
- 32.Han C, Hoeijmakers JG, Liu S, et al. Functional profiles of SCN9A variants in dorsal root ganglion neurons and superior cervical ganglion neurons correlate with autonomic symptoms in small fibre neuropathy. Brain 2012;135:2613–2628. [DOI] [PubMed] [Google Scholar]
- 33.Huang J, Yang Y, Zhao P, et al. Small-fiber neuropathy Nav1.8 mutation shifts activation to hyperpolarized potentials and increases excitability of dorsal root ganglion neurons. J Neurosci 2013;33:14087–14097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Faber CG, Lauria G, Merkies IS, et al. Gain-of-function Nav1.8 mutations in painful neuropathy. Proc Natl Acad Sci U S A 2012;109:19444–19449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang J, Han C, Estacion M, et al. Gain-of-function mutations in sodium channel Na(v)1.9 in painful neuropathy. Brain 2014;137:1627–1642. [DOI] [PubMed] [Google Scholar]
- 36.Cazzato D, Lauria G. Small fibre neuropathy. Curr Opin Neurol 2017;30:490–499. [DOI] [PubMed] [Google Scholar]
- 37.Wadhawan S, Pant S, Golhar R, et al. NaV channel variants in patients with painful and nonpainful peripheral neuropathy. Neurol Genet 2017;3:e207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blesneac I, Themistocleous AC, Fratter C, et al. Rare NaV1.7 variants associated with painful diabetic peripheral neuropathy. Pain 2018;159:469–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alsaloum M, Estacion M, Almomani R, et al. A gain-of-function sodium channel beta2-subunit mutation in painful diabetic neuropathy. Mol Pain 2019;15:1744806919849802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Treede RD, Rief W, Barke A, et al. Chronic pain as a symptom or a disease: the IASP classification of chronic pain for the international classification of diseases (ICD-11). Pain 2019;160:19–27. [DOI] [PubMed] [Google Scholar]
- 41.Fillingim RB, Bruehl S, Dworkin RH, et al. The ACTTION-American Pain Society Pain Taxonomy (AAPT): an evidence-based and multidimensional approach to classifying chronic pain conditions. J pain : official J Am Pain Soc 2014;15:241–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lauria G, Bakkers M, Schmitz C, et al. Intraepidermal nerve fiber density at the distal leg: a worldwide normative reference study. J Peripher Nervous Syst 2010;15:202–207. [DOI] [PubMed] [Google Scholar]
- 43.Provitera V, Gibbons CH, Wendelschafer-Crabb G, et al. A multi-center, multinational age- and gender-adjusted normative dataset for immunofluorescent intraepidermal nerve fiber density at the distal leg. Eur J Neurol 2016;23:333–338. [DOI] [PubMed] [Google Scholar]
- 44.Dillingham T, Chen S, Andary M, et al. . Establishing high-quality reference values for nerve conduction studies: a report from the normative data task force of the American Association of Neuromuscular & Electrodiagnostic Medicine. Muscle & nerve 2016;54:366–370. [DOI] [PubMed] [Google Scholar]
- 45.Lauria G, Cornblath DR, Johansson O, et al. EFNS guidelines on the use of skin biopsy in the diagnosis of peripheral neuropathy. Eur J Neurol 2005;12:747–758. [DOI] [PubMed] [Google Scholar]
- 46.Dyck PJ, O'Brien PC. Quantitative sensation testing in epidemiological and therapeutic studies of peripheral neuropathy. Muscle & nerve 1999;22:659–662. [DOI] [PubMed] [Google Scholar]
- 47.Rolke R, Baron R, Maier C, et al. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): standardized protocol and reference values. Pain 2006;123:231–243. [DOI] [PubMed] [Google Scholar]
- 48.Osgood E, Trudeau JJ, Eaton TA, et al. Development of a bedside pain assessment kit for the classification of patients with osteoarthritis. Rheumatol Int 2015;35:1005–1013. [DOI] [PubMed] [Google Scholar]
- 49.Freeman R, Baron R, Bouhassira D, Cabrera J, Emir B. Sensory profiles of patients with neuropathic pain based on the neuropathic pain symptoms and signs. Pain 2014;155:367–376. [DOI] [PubMed] [Google Scholar]
- 50.Koulouris AE, Edwards RR, Dorado K, et al. Reliability and validity of the Boston Bedside Quantitative Sensory Testing Battery for Neuropathic Pain. Pain Med 2020; Sep 8:pnaa192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen X, Graham J, Dabbah MA, et al. Small nerve fiber quantification in the diagnosis of diabetic sensorimotor polyneuropathy: comparing corneal confocal microscopy with intraepidermal nerve fiber density. Diabetes care 2015;38:1138–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Perkins BA, Lovblom LE, Bril V, et al. Corneal confocal microscopy for identification of diabetic sensorimotor polyneuropathy: a pooled multinational consortium study. Diabetologia 2018;61:1856–1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tavakoli M, Ferdousi M, Petropoulos IN, et al. Normative values for corneal nerve morphology assessed using corneal confocal microscopy: a multinational normative data set. Diabetes care 2015;38:838–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Low VA, Sandroni P, Fealey RD, Low PA. Detection of small-fiber neuropathy by sudomotor testing. Muscle & nerve 2006;34:57–61. [DOI] [PubMed] [Google Scholar]
- 55.Thaisetthawatkul P, Fernandes Filho JA, Herrmann DN. Autonomic evaluation is independent of somatic evaluation for small fiber neuropathy. J Neurol Sci 2014;344:51–54. [DOI] [PubMed] [Google Scholar]
- 56.Thaisetthawatkul P, Fernandes Filho JA, Herrmann DN. Contribution of QSART to the diagnosis of small fiber neuropathy. Muscle Nerve 2013;48:883–888. [DOI] [PubMed] [Google Scholar]
- 57.Low PA, Denq JC, Opfer-Gehrking TL, Dyck PJ, O'Brien PC, Slezak JM. Effect of age and gender on sudomotor and cardiovagal function and blood pressure response to tilt in normal subjects. Muscle Nerve 1997;20:1561–1568. [DOI] [PubMed] [Google Scholar]
- 58.Benatar M Distal symmetric polyneuropathy: limitations of the proposed case definition. Muscle Nerve 2006;34:131–134. [DOI] [PubMed] [Google Scholar]
- 59.Botez SA, Herrmann DN. Pitfalls of diagnostic criteria for small fiber neuropathy. Nat Clin Pract Neurol 2008;4:586–587. [DOI] [PubMed] [Google Scholar]