Abstract
BACKGROUND
Feijoa is widely used in medicine due to their anti-inflammatory, antioxidant, antimicrobial and antitumor properties. The current investigation studied the proliferative and regenerative effect of acetonic extract of Feijoa sellowiana on stem cells.
METHODS
Acetone extract of Feijoa was prepared using percolator and rotary machines. Human bone marrow stem cells (hBMSCs) were used as experimental in vitro model and characterized morphologically, by flowcytometry, and differentiation properties. The toxicity of the extract on hBMSCs was determined by MTT assay. The viability and growth kinetics of hBMSCs treated to Feijoa was determined. Real time PCR was used for changes in expression of proliferative and apoptotic genes on day 7th.
RESULTS
MTT assay demondtrated that Feijoa at doses less than 200 ng/ml did not show any cytotoxic effect on hBMSCs and increased the cell proliferation until day 3rd followed by a non-significant slow decreasing trend until day 7th. Population doubling time (PDT) showed a decline until day 3rd followed by an increase until day 7th. A significant rise in expression of Bax and decline in Bcl-2 expression were noted on day 7th.
CONCLUSION
The modulatory activity of Feijoa may be responsible for its increasing effect on cell proliferation till day 3rd. Therefore, when faster proliferation during a shorter time period is targeted, Feijoa can be safely added to the culture media in the first three days.
Key Words: Feijoa sellowiana, Bone marrow stem cells, Growth kinetics, Apoptosis
INTRODUCTION
Feijoa (Feijoa sellowiana (O. Berg) Burret) is an evergreen tree belonging to Myrtaceae family, with gray branches, elliptical buds, white and red flowers, and sweet-smelling leaves and is indigenous to western highlands of Paraguay, southern Brazil, Uruguay and northern Argentina.1-6 This plant entered the Mediterranean region in the late nineteenth century and also entered into Islamic Republic of Iran in 1973 through the Republic of Azerbaijan. This shrub is just grown on the northern strip of Iran.7 Feijoa has several biological features such as antibacterial,8 analgesic and anti-inflammatory,9-11 antioxidant and anticancer12,13 effects.
The leaves of Feijoa are waste materials without any utilization and little research has been done on the leaves which have phenolic compounds.14 Compounds such as a-tocopherol, flavone, stigmasterol, b-carotene, some unreported longchain esters of tyrosol and a novel galactolipid have been extracted from the leaves of Feijoa.15,16 Moreover, antioxidant activity of the aqueous extract of Feijoa has previously been demonstrated.17
Investigation on human myeloid leukemia cells showed that flavonoids in Feijoa induce apoptosis through caspase activation, overexpression of p16, p21 and tumor necrosis factor-related apoptosis-inducing ligand (TRAIL), increase in histone and non-histone acetylation and inhibition in histone deacetylase (HDAC) pathways.18 The antioxidant activity of methanolic extract of Feijoa was illustrated in mouse liver via 3,4-methylenedioxymethamphetamine (MDMA, or ecstasy) denoting to the medicinal property of Feijoa. MDMA is a ring-substituted amphetamine derivative that was synthesized in 1912 by Merck chemical company and has attracted a great deal of attention in recent years. It has widespread abuse as a recreational drug by the young generation.19 This study was undertaken to evaluate the proliferative and regenerative effect of acetonic extract of Feijoa sellowiana on human bone marrow derived stem cells (hBMSCs).
MATERIALS AND METHODS
Feijoa (Feijoa sellowiana [(O. Berg) Burret] leaves were collected from Ramsar Research Center, northern Iran in spring of 2017. To remove the epiphytic hosts, which are normally present on the surface, the leaves were treated with 0.8% Triton X-100. Distilled water was used for extensive washing and then the leaves were dried on filter papers in a dark and dry place for two weeks and were later changed into powder. Totally, 100 g of the powder was placed in a percolator machine for 72 hours in 1000 mL of acetone (Merck, Germany), while the acetone solvent was rotated at 45°C and 50 rpm in a rotary machine (IKA, Germany) to be completely evaporated for the solvent.20
The study was approved by Islamic Azad University Ethics Committee, Yasooj Branch, Yasooj, Iran (30/99-1111/519). Bone marrow (BM) samples were provided from the Bone Marrow Transplantation Center of Nemazi Hospital, affiliated to Shiraz University of Medical Sciences, Shiraz, Iran. A written signed consent form was provided from the subjects by Hematology Research Center, Shiraz University of Medical Sciences, Shiraz, Iran. BM was diluted with an equal volume of Dulbecco’s Modified Eagle Medium (DMEM; Biovet, Bulgaria) and was carefully layered over an equal volume of Ficoll-Paque product (without intermixing, GE Healthcare Life Sciences, UK) as described before.21
It was centrifuged at 1500 rpm and 20°C for 30 min to separate a second layer. The interface between the plasma and the Ficoll-Paque layer consisted of mononuclear cells (MNCs) were seeded in 75 cm flasks containing 88% alpha minimal essential medium (αMEM; Biovet, Bulgaria), 10% fetal bovine serum (FBS; Biovet, Bulgaria), 1% penicillin and streptomycin (Biovet, Bulgaria), and 1% L-glutamine (Sigma, USA). hBMSCs were transferred in 5% CO2 incubator at 37°C with saturated humidity (Memmert, Germany). The medium was changed every 3 days until 80% confluence and sub-cultured using 0.25% trypsin (Gibco, USA) till passage 3rd.
The adhered cells were assessed morphologically to be spindle shape. In vitro differentiation to osteogenic lineage was assessed by seeding 5×104 hBMSCs in a 12-well plate containing osteogenic medium of DMEM, 10% FBS, 200 μM L-ascorbic acid, 10 mM glycerol phosphate and 100 nM dexamethasone, while the media was changed every three days until 3 weeks. Alizarin red (Sigma-Aldrich, USA) staining that bound to calcium mineralized deposits revealing a red color when osteogenic differentiation was induced.
In vitro differentiation to adipogenic lineage was investigated by seeding 5×104 hBMSCs in a 12-well plate containing adipogenic medium of DMEM, 10% FBS, 1 μmol/L dexamethasone, 200 μg/mL insulin, 0.5 mmol/L isobutylmethylxanthine, and 60 μmol/L indomethacin (all from Sigma-Aldrich, USA) for 21 days, while the medium was replaced every 3 days. Then, the cells were stained with Oil red O (Sigma-Aldrich, USA) to evaluate presence of red color droplets, when adipogenic differentiation was induced. Flowcytometry of hBMSCs was conducted for expression of mesenchymal markers (CD73 and CD90) and hematopoietic markers (CD34, and CD45) (Dako, Denmark) in passage 3rd.
MTT assay was done to determine the probable toxicity of Feijoa for hBMSCs. Totally, 1×104 hBMSCs were seeded in a 96-well plate (Invitrogen, USA). After 24 hours, the medium was changed and different doses of Feijoa (1.25, 2.5, 5, 10, 20, 200 and 2000 ng/mL) were added and transferred to 5% CO2 incubator for one day at 37°C and saturated humidity. Twenty micro-liter of MTT was added for 4 hours for each 200 μL of the culture medium. It was later centrifuged for 5 minutes, the supernatant was removed and 200 μL of DMSO (Sigma-Aldrich, USA) was added to the remaining. Optical absorption at 570 nm was read by an ELISA plate reader (Polarstar Omega-BMG Lab Tech, Germany). Based on the highest percentage of cell viability that 200 μL/mL of Feijoa induced in hBMSCs until 7 days, this concentration was selected as optimum dose in all experiments (Figure 1).
Fig. 1.
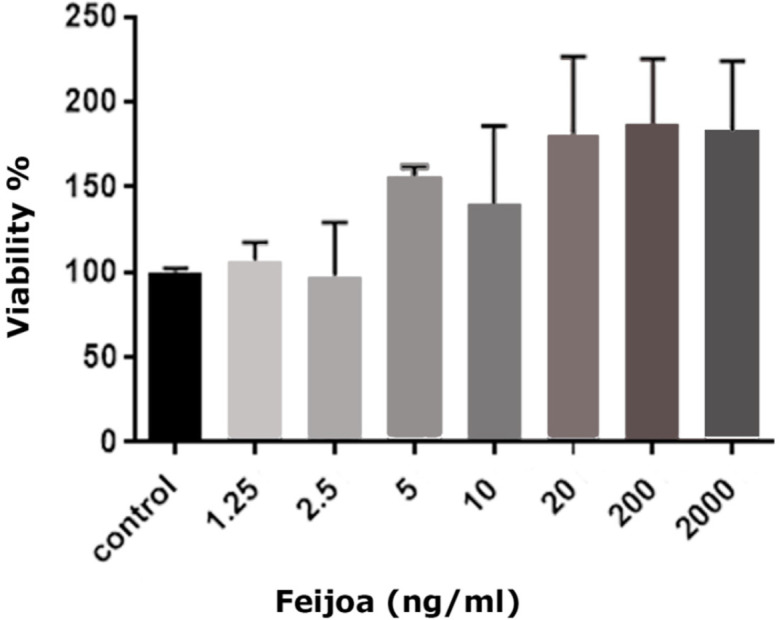
MTT assay of different concentrations of Feijoa after 7 days regarding the cell viability
hBMSCs from passage 3rd were seeded in 24-well culture plates at a density of 4×104 cells per well and transferred in a 5% CO2 incubator at 37°C and saturated humidity. After 3 days, the culture media was changed and Feijoa at different time intervals was added. Acetone was used as a control. To evaluate cell viability, 0.4% trypan blue solution (Biowest, France) was added to the cell suspension and counted each day using a Neubauer hemocytometer slide and a phase contrast microscope until 7 days. The cell viability was calculated at optical density of 570 nm on a microplate reader (Floustar Omega, BMG LabTech, Germany) (n=4) using the following formula, while Ac and Ab were considered as the absorbance in the control and blank wells. %Survival rate=A sample–(Ab×Ac–Ab)×100.22
The population doubling time (PDT) was measured and the growth curve was plotted using the following the formula of PDT=T ln2=ln(Xe/Xb); while T is the incubation time in hours, Xb is the cell number at the beginning of the incubation time, and Xe is considered as the cell number at the end of the incubation time. The mean number of cells at each time point was plotted by GraphPad Prism (GraphPad software Inc., San Diego, CA, USA). Cells were cryopreserved in 10% (V/V) dimethyl sulphoxide (DMSO; MP Bio USA), 50% (V/V) fetal bovine serum and 40% DMEM, at a density of 1×106 cells/mL until use.22
Total cellular RNA was isolated from hBMSCs using RNA extraction kit (CinnaGen, Iran). Applying a nanodrop™ spectrophotometer (Nanodrop; Thermo Fisher Scientific, USA), the quantity and quality of RNA were assessed by determining the ratio of optical density at 260/280 nm. They were stored at 80˚C until cDNA synthesis. The cDNA was synthesized by 1,000 ng total RNA in a first strand cDNA synthesis reaction using RevertAid™ First Strand cDNA Synthesis kit (Thermo Fisher Scientific, USA).22
Quantitative real time PCR (qPCR) was conducted by ABI Biosystems StepOne and the RealQ Plus 2x Master Mix Green (Ampliqon A/S, Odense, Denmark). In each reaction, 200 nM of each primer (Table 1) was added to target the specific sequence. Specific primers targeting Bax and Bcl-2 were prepared (Table 1), while the TBP housekeeping gene was as internal control for qPCR reactions. The qPCR condition was established for 10 minutes at 94˚C followed by 40 cycles of 15 sec at 94˚C, 60 sec at 58˚C and final extension of 7 minutes at 72˚C. The amplification signals of different samples were normalized to TBP cycle threshold (Ct), and then 2 ΔΔCq methods were applied to compare mRNA level of activated vs. the control, which were shown as fold change in data analysis. The data was analyzed by independent samples t test, using GraphPad Prism 6 (GraphPad Software, Inc., La Jolla, CA, USA). The P<0.05 was considered statistically significant.
Table 1.
The specific primer sequences of the targeted genes
| Genes | Primer sequences | Size (bp) |
|---|---|---|
| BAX | Forward: 5’- GCCCTTTTGCTTCAGGGTTTCA -3’ | 108 |
| Reverse: 5’- CAGCTTCTTGGTGGACGCAT -3’ | ||
| Bcl-2 | Forward: 5’- ACGAGTGGGATGCGGGAGATGTG-3’ | 245 |
| Reverse: 5’- GCGGTAGCGGCGGGAGAAGTC-3’ | ||
| TBP | Forward: 5’- GGATAAGAGAGCCACGAACCAC-3’ | 139 |
| Reverse: 5’- TTAGCTGGAAAACCCAACTTCTG-3’ |
bp, base pair
RESULTS
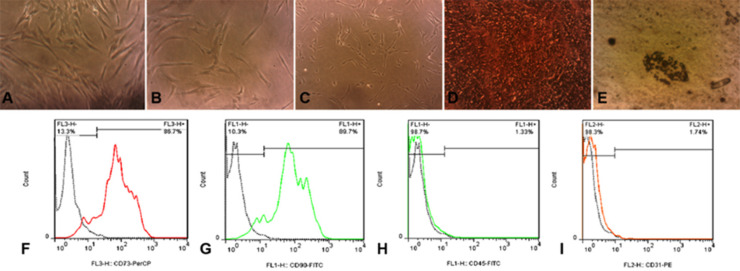
hBMSCs were all adhered to the culture flasks. These cells were spindle shape in different passages (Figure 2). Three weeks later, these cells in osteogenic media showed presence of calcium deposits confirmed by Alizarin red staining in red color (Figure 2). Regarding adipogenic differentiation, hBMSCs were positive for red oil droplets in adipogenic media (Figure 2). The cells were also positive for expression of mesenchymal markers of CD73 and CD90, and negative for hematopoietic markers of CD34 and CD45 (Figure 2).
Fig. 2.
hBMSCs in Passage 1 (A), Passage 2 (B), and Passage 3 (C) (100×). Osteogenic induction (D), and adipogenic differentiation (E). Flowcytometry being positive for mesenchymal markers of CD73 (F) and CD90 (G) and negative for hematopoietic markers of CD34 (H) and CD45 (I).
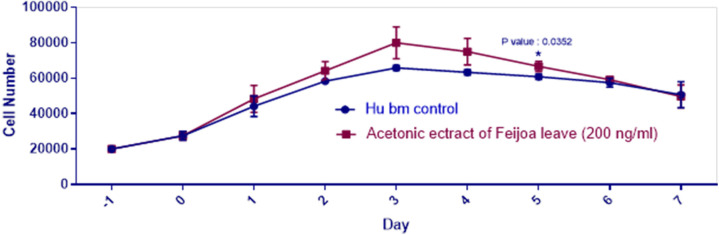
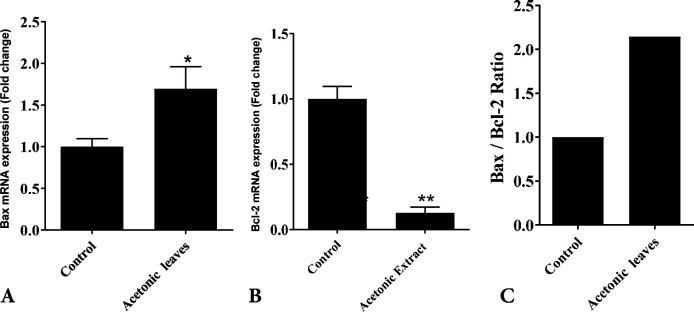
MTT assay showed that acetonic extract of Feijoa leaves had no cytotoxic effects on hBMSCs at doses ≤200 ng/mL (Figure 2). PDT over seven days in control and hBMSCs groups treated with 200 ng/mL of acetonic extract of Feijoa was presented in Table 2. PDT showed a decline until day 3rd followed by an increase until day 7th. The growth curve was plotted based on the average number of cells treated with 200 ng/mL of acetonic extract of Feijoa during a seven days period. An increasing trend in cell proliferation till day 3 was noted, while proliferation demonstrated a further decrease when compared to the control group (Figure 3). Real time PCR for expression of Bax and Bcl-2 genes of the cells treated with 200 ng/mL of acetonic extract of Feijoa exhibited an increase in expression of Bax gene, and a decrease in expression of Bcl-2 gene (P≤0.05) on day 7th (Figure 4).
Table 2.
PDT of control and treated cells to 200 ng/mL of acetonic extract of Feijoa leave
| Variable |
Population doubling time (n)
|
|
|---|---|---|
| Day | Control | Feijoa (200 ng/mL) |
| 0 | 0 | 0 |
| 1 | 21 | 17 |
| 2 | 31 | 29 |
| 3 | 42 | 39 |
| 4 | 58 | 54 |
| 5 | 75 | 71 |
| 6 | 94 | 92 |
| 7 | 125 | 113 |
Fig. 3.
The growth curve of hBMSCs treated with 200 ng/mL of acetonic extract of Feijoa leave during seven days in comparison to the control group (P<0.05). Hu bm: Human bone marrow
Fig. 4.
The expression of Bax (P<0.05) (A) and Bcl-2 genes (P<0.01) (B) and the ratio of Bax\Bcl-2 (C) genes for control and hBMSCs treated with 200 ng/mL of acetonic extract of Feijoa leave on day 7th.
DISCUSSION
In our study identical to other studies, hBMSCs had mesenchymal properties confirmed morphologically, by osteogenic and adipogenic induction and by flowcytometry.23 Regarding growth kinetics of these cells, we noted an increasing trend until day 3rd, and then a decrease until day 7th when exposed to the acetonic extract of Feijoa. It was shown that, when Caco-2 and HT-29 cells were exposed to Feijoa sellowiana for 24 hours, it demonstrated anti-inflammatory effects24 and prevented lipid peroxidation in intestinal epithelial cells.25,26 These findings may explain the increasing trend noted in proliferation of hBMSCs during the first 3 days of our study. Feijoa can also improve the lactase and sucrase-isomaltase activities and inhibit cell proliferation too. It has anticancer activities.27 These activities may be responsible for the decreasing effect of Feijoa on proliferation of hBMSCs and can explain the growth kinetics in our study.
Bax and Bcl-2 apoptotic genes have been of tremendous interest to clinicians who study cancer therapy. There are two classes of Bcl-2 proteins including pro-apoptotic proteins (Bax, Bad, Bid, Bik) and anti-apoptotic proteins (Bcl-2, Bcl-XL, Bcl-W), while anti-apoptotic proteins are responsible for apoptosis through delaying the mitochondrial release of cytochrome-c, and that the proapoptotic proteins activate such releases.28 Bax as a pro-apoptotic protein was shown to trigger apoptosis by increasing the opening of the mitochondrial voltage-dependent anion channels, which induce the loss in its membrane potential. The protein Bcl-2 is a key inhibitor of apoptosis, and its aberrant expression was demonstrated in a wide range of solid tumors.29 The ratio of Bax to Bcl-2 denotes to the susceptibility of a cell to apoptosis too.30 Our findings revealed that Feijoa significantly increased the expression of Bax gene, while decreased Bcl-2 expression, suggesting that apoptotic mechanisms of Feijoa are responsible for up regulation of Bax expression.30
These results are consistent with other studies reporting that Bax and Bcl-2 play an important role in apoptosis, growth kinetics and cell differentiation. Our results resemble the findings of cytotoxic potential effects of E. billardieri on MCF-7, A549, HepG-2 and HT-29 cell lines.31-33
CONCLUSION
The modulatory activity of Feijoa may be responsible for its increasing effect on cell proliferation till day 3rd. Therefore, when faster proliferation during a shorter time period is targeted, Feijoa can be safely added to the culture media in the first three days. This study also revealed that acetonic leave extract of Feijoa significantly induced apoptosis on day 7th by increasing expression of Bax and decreasing of Bcl-2 genes. However, more molecular studies should be undertaken to achieve a definite conclusion to determine the probable apoptotic pathways of the cytotoxic activity of Feijoa on day 7th.
ACKNOWLEDGEMENTS
We appreciate Stem Cell Technology Research Canter of Shiraz University of Medical Sciences for providing the laboratory works. The article is part of PhD thesis of Mr. Hossein Rasekh that was financially supported by Islamic Azad University, Yasooj Branch, Yasooj, Iran (30/99-1111/519) and their kind support is appreciated.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
References
- 1.Pasquariello MS, Mastrobuoni F, Di Patre D, Zampella L, Capuano LR, Scortichini M, Petriccione M. Agronomic, nutraceutical and molecular variability of feijoa (Acca sellowiana (O. Berg) Burret) germplasm. Scientia Horticulturae. 2015;191:1–9. [Google Scholar]
- 2.Sun-Waterhouse D, Wang W, Waterhouse GI, Wadhwa SS. Utilisation potential of feijoa fruit wastes as ingredients for functional foods. Food and Bioprocess Technology. 2013;6:3441–55. [Google Scholar]
- 3.Elmastaş M, Gedikli F. Total phenolic compounds and antioxidant capacity of leaf, dry fruit and fresh fruit of feijoa (Acca sellowiana, Myrtaceae) Journal of Medicinal Plants Research. 2010;4:1065–72. [Google Scholar]
- 4.Weston RJ. Bioactive products from fruit of the feijoa (Feijoa sellowiana, Myrtaceae): A review. Food Chemistry. 2010;121:923–6. [Google Scholar]
- 5.Basile A, Conte B, Rigano D, Senatore F, Sorbo S. Antibacterial and antifungal properties of acetonic extract of Feijoa sellowiana fruits and its effect on Helicobacter pylori growth. J Med Food. 2010;13:189–95. doi: 10.1089/jmf.2008.0301. [DOI] [PubMed] [Google Scholar]
- 6.Aoyama H, Sakagami H, Hatano T. Three new flavonoids, proanthocyanidin, and accompanying phenolic constituents from Feijoa sellowiana. Biosci Biotechnol Biochem. 2018;82:31–41. doi: 10.1080/09168451.2017.1412246. [DOI] [PubMed] [Google Scholar]
- 7.Mousavi M, Bimakr M, Ghoreishi SM, Ganjloo A. Supercritical carbon dioxide extraction of bioactive compounds from Feijoa (Feijoa sellowiana) leaves. Nutrition and Food Sciences Research. 2018;5:23–31. [Google Scholar]
- 8.Tuncel NB, Yılmaz N. Optimizing the extraction of phenolics and antioxidants from feijoa (Feijoa sellowiana, Myrtaceae) Journal of Food Science and Technology. 2015;52:141–50. [Google Scholar]
- 9.Arguilles MC, Watson RR. Feijoa (pineapple guava) fruit: a role in health promotion? In: Watson RR, Preedy RV, Boca Raton, editors. Bioactive foods and extracts cancer treatment and prevention. Florida: CRC Press; 2010. pp. 603–8. [Google Scholar]
- 10.El Dib R, Moharram F, Marzouk M, El-Shenawy S, El-Sayed H. Anti-inflammatory and analgesic activities of Feijoa sellowiana Berg leaves and investigation of their phenolic constituents. Planta Medica. 2007;73:P_051 . [Google Scholar]
- 11.Rossi A, Rigano D, Pergola C, Formisano C, Basile A, Bramanti P, Senatore F, Sautebin L. Inhibition of inducible nitric oxide synthase expression by an acetonic extract from Feijoa sellowiana Berg. fruits. J Agric Food Chem. 2007;55:5053–61. doi: 10.1021/jf070510d. [DOI] [PubMed] [Google Scholar]
- 12.Nakashima H. Biological activity of Feijoa peel extracts. Occasional Papers of the Kagoshima University Research Center for the Pacific Islands. 2001;34:169–75. [Google Scholar]
- 13.Vuotto ML, Basile A, Moscatiello V, De Sole P, Castaldo-Cobianchi R, Laghi E, Ielpo MT. Antimicrobial and antioxidant activities of Feijoa sellowiana fruit. Int J Antimicrob Agents. 2000;13:197–201. doi: 10.1016/s0924-8579(99)00122-3. [DOI] [PubMed] [Google Scholar]
- 14.Zhu F. Chemical and biological properties of feijoa (Acca sellowiana) Trends in Food Science & Technology. 2018;81:121–31. [Google Scholar]
- 15.Weston RJ. Bioactive products from fruit of the feijoa (Feijoa sellowiana, Myrtaceae): A review. Food Chemistry. 2010;121:923–6. [Google Scholar]
- 16.Turco F, Palumbo I, Andreozzi P, Sarnelli G, De Ruberto F, Esposito G, Basile A, Cuomo R. Acetonic Extract from the Feijoa sellowiana Berg Fruit Exerts Antioxidant Properties and Modulates Disaccharidases Activities in Human Intestinal Epithelial Cells. Phytother Res. 2016;30:1308–15. doi: 10.1002/ptr.5629. [DOI] [PubMed] [Google Scholar]
- 17.Bell TJ, Draper SL, Centanni M, Carnachan SM, Tannock GW, Sims IM. Characterization of Polysaccharides from Feijoa Fruits ( Acca sellowiana Berg) and Their Utilization as Growth Substrates by Gut Commensal Bacteroides Species. J Agric Food Chem. 2018;66:13277–84. doi: 10.1021/acs.jafc.8b05080. [DOI] [PubMed] [Google Scholar]
- 18.do Amarante C, Souza A, Beninca T, Steffens C, editors Characterization of dietary attributes and mineral composition of the fruit in Brazilian genotypes of feijoa. Acta Horticulturae. 2018;30:947–954. [Google Scholar]
- 19.Kalant H. The pharmacology and toxicology of “ecstasy” (MDMA) and related drugs. CMAJ. 2001;165:917–28. [PMC free article] [PubMed] [Google Scholar]
- 20.Karami M, Saeidnia S, Nosrati A. Study of the Hepatoprotective Activity of Methanolic Extract of Feijoa sellowiana Fruits Against MDMA using the Isolated Rat Liver Perfusion System. Iran J Pharm Res. 2013;12:85–91. [PMC free article] [PubMed] [Google Scholar]
- 21.Iravani K, Sobhanmanesh A, Ashraf MJ, Hashemi SB, Mehrabani D, Zare S. The Healing Effect of Conditioned Media and Bone Marrow-Derived Stem Cells in Laryngotracheal Stenosis: A Comparison in Experimental Dog Model. World J Plast Surg. 2017;6:190–7. [PMC free article] [PubMed] [Google Scholar]
- 22.Zare S, Mehrabani D, Jalli R, Saeedi Moghadam M, Manafi N, Mehrabani G, Jamhiri I, Ahadian S. MRI-Tracking of Dental Pulp Stem Cells In Vitro and In Vivo Using Dextran-Coated Superparamagnetic Iron Oxide Nanoparticles. J Clin Med. 2019;8 doi: 10.3390/jcm8091418. doi: 10.3390/jcm8091418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mehrabani D, Mojtahed Jaberi F, Zakerinia M, Hadianfard MJ, Jalli R, Tanideh N, Zare S. The Healing Effect of Bone Marrow-Derived Stem Cells in Knee Osteoarthritis: A Case Report. World J Plast Surg. 2016;5:168–74. [PMC free article] [PubMed] [Google Scholar]
- 24.Monforte MT, Fimiani V, Lanuzza F, Naccari C, Restuccia S, Galati EM. Feijoa sellowiana Berg fruit juice: anti-inflammatory effect and activity on superoxide anion generation. J Med Food. 2014;17:455–61. doi: 10.1089/jmf.2012.0262. [DOI] [PubMed] [Google Scholar]
- 25.Turco F, Palumbo I, Andreozzi P, Sarnelli G, De Ruberto F, Esposito G, Basile A, Cuomo R. Acetonic Extract from the Feijoa sellowiana Berg Fruit Exerts Antioxidant Properties and Modulates Disaccharidases Activities in Human Intestinal Epithelial Cells. Phytother Res. 2016;30:1308–15. doi: 10.1002/ptr.5629. [DOI] [PubMed] [Google Scholar]
- 26.Keles H, Ince S, Kucukkurt I, Tatli II, Akkol EK, Kahraman C, Demirel HH. The effects of Feijoa sellowiana fruits on the antioxidant defense system, lipid peroxidation, and tissue morphology in rats. Pharm Biol. 2012;50:318–25. doi: 10.3109/13880209.2011.608074. [DOI] [PubMed] [Google Scholar]
- 27.Motohashi N, Kawase M, Shirataki Y, Tani S, Saito S, Sakagami H, Kurihara T, Nakashima H, Wolfard K, Mucsi I, Varga A, Molnar J. Biological activity of feijoa peel extracts. Anticancer Res. 2000;20:4323–9. [PubMed] [Google Scholar]
- 28.Ebrahimzadeh MA, Enayatifard R, Khalili M, Ghaffarloo M, Saeedi M, Yazdani Charati J. Correlation between Sun Protection Factor and Antioxidant Activity, Phenol and Flavonoid Contents of some Medicinal Plants. Iran J Pharm Res. 2014;13:1041–7. [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu L, Wang J, Wei T, Gao J, He H, Chang X, Yan T. Effects of Naringenin on inflammation in complete freund’s adjuvant-induced arthritis by regulating Bax/Bcl-2 balance. Inflammation. 2015;38:245–51. doi: 10.1007/s10753-014-0027-7. [DOI] [PubMed] [Google Scholar]
- 30.Fang Z, Yang Q, Luo W, Li GH, Xiao J, Li F, Xiong W. Differentiation of GFP-Bcl-2-engineered mesenchymal stem cells towards a nucleus pulposus-like phenotype under hypoxia in vitro. Biochem Biophys Res Commun. 2013;432:444–50. doi: 10.1016/j.bbrc.2013.01.127. [DOI] [PubMed] [Google Scholar]
- 31.Raisova M, Hossini AM, Eberle J, Riebeling C, Wieder T, Sturm I, Daniel PT, Orfanos CE, Geilen CC. The Bax/Bcl-2 ratio determines the susceptibility of human melanoma cells to CD95/Fas-mediated apoptosis. J Invest Dermatol. 2001;117:333–40. doi: 10.1046/j.0022-202x.2001.01409.x. [DOI] [PubMed] [Google Scholar]
- 32.Bai L, Lennon DP, Caplan AI, DeChant A, Hecker J, Kranso J, Zaremba A, Miller RH. Hepatocyte growth factor mediates mesenchymal stem cell-induced recovery in multiple sclerosis models. Nat Neurosci. 2012;15:862–70. doi: 10.1038/nn.3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chatzitolios A, Venizelos I, Tripsiannis G, Anastassopoulos G, Papadopoulos N. Prognostic significance of CD95, P53, and BCL2 expression in extranodal non-Hodgkin’s lymphoma. Ann Hematol. 2010;89:889–96. doi: 10.1007/s00277-010-0945-x. [DOI] [PubMed] [Google Scholar]