Abstract
Background:
Alopecia areata (AA) is a chronic nonscarring alopecia that involves hair follicles and is characterized by patchy areas of hair loss without any signs of clinical inflammation. Platelet-ri-ch plasma (PRP) has a high platelet concentration. Anti-inflammatory effect of PRP may be of great help in AA.
Aims and Objectives:
Study was conducted to compare the outcome of treatment and side effects of intralesional PRP versus triamcinolone in AA.
Materials and Methods:
40 patients with alopecia areata were allocated into 2 groups and treated with triamcinolone and PRP injections. The response was analyzed by SALT score (severity of alopecia tool score) and hair regrowth grade (HRG) scale. Inferential statistical tools such as t-test, Mann–Whitney U test, and Chi-square test were used.
Results:
16 patients in each group completed the study. While comparing the decrease in SALT score at different intervals of time, there was a significant difference in SALT score reduction during the second review between PRP group and triamcinolone group (P = 0.028). After the first and final review, results did not show any statistically significant difference between the two groups. While comparing the hair regrowth scale between treatments, there was no statistical significance. 12.5% patients in PRP group reported excellent response after final review (HRG scale 4), compared to none in triamcinolone group.
Conclusions:
Platelet-rich plasma is a safe, effective, steroid sparing, and suitable alternative in AA. Only side effect noted was pain during injections in both the groups.
Keywords: Alopecia areata, platelet-rich plasma, triamcinolone acetonide
Introduction
Alopecia areata (AA) is a common nonscarring alopecia characterized by patchy areas of hair loss without any signs of clinical inflammation. Intralesional corticosteroid is considered as the first-line therapy for adults with less than 50% of scalp area involvement. It may be associated with local side effects like skin atrophy, telangiectasia, and burning.[1] Platelet-rich plasma (PRP) is a simple and effective treatment for alopecia areata without major side effects. PRP contains many growth factors and has beneficial effects on wound healing and hair growth.[2,3] Antiinflammatory effect of PRP may be of great help in AA. This study was undertaken to compare the outcome of treatment with intralesional PRP versus triamcinolone in AA.
Materials and Methods
This study was conducted in the Department of Dermatology, in a tertiary care center in north Kerala, over a period of 1 year. Aims and objectives of the study were to compare the outcome of treatment and side effects of intralesional PRP versus triamcinolone in alopecia areata. Informed consent from the patients, and institutional ethical and research committee clearance were obtained. Consecutive sampling method was used.
Inclusion criteria
Patients who were willing for the study
All patients presenting with a circumscribed patch of hair loss without any signs of inflammation or scarring
Patients with AA confined to scalp
Patients who have not taken any treatment for alopecia areata during the last 3 months
Patient age of more than 18 years.
Exclusion Criteria:
Patients who were not willing for the study
Pregnant or lactating patients
Extensive lesions (more than 5 lesions)
Patients with AA in areas other than scalp.
Size of the AA patch was not measured. The patients were allocated into Group A and Group B. All the odd numbers were allocated to Group A and the even numbers to Group B. Each group received 3 injections, one at baseline, one at 4 weeks, and one at 8 weeks. All the baseline investigations including blood sugar, complete blood count, liver and renal function tests, HIV, HBsAg, anti-HCV antibodies, prothrombin time, activated partial thromboplastin time, and INR were done.
Group A received intralesional injection of PRP. Fifteen ml of blood was withdrawn into a vacutainer containing sodium citrate and centrifuged at 1500 rpm for 15 min. Upper layer containing buffy coat and plasma was taken into another vacutainer without anticoagulant and then centrifuged at 2500 rpm for 10 min. Lower one-third containing PRP was injected at 45° angle into deep dermis and subcutis with an insulin syringe, 0.1 ml, 1 cm apart to the lesion. Average PRP concentration obtained was 6-7 lakhs per microliter.
Group B received intralesional injection of triamcinolone acetonide (10 mg/ml). It was given intradermally into lesion and administered using insulin syringe 0.1 ml injection 1 cm apart. The two modalities of treatment were continued for a period of 12 weeks at an interval of 4 weeks apart and were followed up at every visit. A digital photograph of the patches was taken at every visit starting from baseline and evaluated. The response was analyzed by SALT score, i.e., severity of alopecia tool score, and hair regrowth grade (HRG) scale.[4] Scale-1: 0%–25% improvement, scale-2: 26%–50% improvement, scale-3: 51%–75% improvement, and scale-4: 76%–100% improvement.
SALT Score
Scalp is divided into 4 areas namely:
Vertex-40% (0.4) of scalp surface area
Right profile of scalp-18% (0.18) of scalp surface area
Left profile of scalp-18% (0.18) of scalp surface area
Posterior aspect of scalp-24% (0.24) of scalp surface area.
Percentage of hair loss in any of these areas is percentage hair loss multiplied by percent surface area of the scalp in that area. SALT score is the sum of percentage of hair loss in all the above-mentioned areas.
Collected data was analyzed in terms of frequency, percentage, mean, median, standard deviation, and inter-quartile range. Inferential statistical tools such as t-test, Mann–Whitney U test, and Chi-square test were used. P value of less than 0.05 was considered significant.
Results
Of the 40 patients enrolled in the study after informed consent, 20 were enrolled to PRP group and other 20 to triamcinolone group. 16 patients in each group completed the study. 4 patients from each group were lost to follow-up. Out of 4 patients who were lost to follow-up in PRP group, 2 discontinued due to severe pain and 2 discontinued due to lack of improvement. Out of 4 patients who were lost for follow-up in triamcinolone group, 2 stopped due to lack of improvement, and 2 because of migration from native place to abroad.
More patients were in the age group of 18–30 years, i.e. 50% in PRP group and 65% in triamcinolone group. While 10% in PRP group were more than 50 years of age, the remaining 35% of patients in ILS group were in the age group of 31–50 years. In PRP group, 55% were males and 45% were females, whereas in triamcinolone group, 90% were males and only 10% were females.
In both PRP and triamcinolone groups, 60% of patients had a disease duration less than 3 months, ranging from 7 days to 3 months. Disease duration was 3 months to 1 year in 25% and 35% of patients in PRP group and triamcinolonegroup, respectively. Only 15% and 5% of patients in PRP and ILS group, respectively, had the disease for more than 1 year.
In PRP group, 50%, 25%, 15%, and 10% of patients had lesions in vertex, occipital, temporal, and combination, respectively. In triamcinolone group, 35%, 30%, 30%, and 5% had lesions in vertex, occipital, temporal, and combination, respectively. Most of the patients included in the study had a single patch (75% in PRP group and 85% in triamcinolone group). 15% in PRP group and 10% in triamcinolone group had 2 patches, while the remaining had 3 patches.
There was no statistically significant difference between the SALT scores of both groups when compared at different intervals, however treatment response was better in PRP group than triamcinolone group A [Figure 1].
Figure 1.
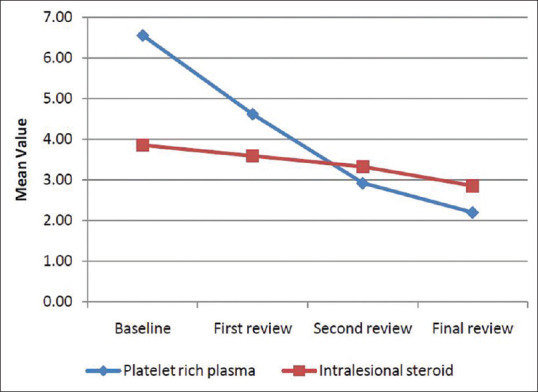
Comparison of SALT score at different intervals of time
While comparing the decrease in SALT score at different intervals of time, there was a significant difference in SALT score reduction during the second review between PRP group and triamcinolone group (P = 0.028). After the first and final review, results did not show any statistically significant difference between the two groups [Figure 2].
Figure 2.
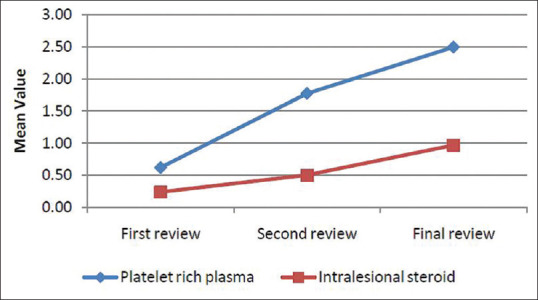
Comparison of decrease in SALT score at different intervals of time
While comparing the hair regrowth scale between treatments, there was no statistical significance. 12.5% patients in PRP group reported excellent response after final review (Scale 4), compared to none in triamcinolone group. 31.3% patients in PRP group reported good response after final review (Scale 3), as compared to 18.8% patients in triamcinolone group. 43.8% patients in triamcinolone group reported poor response after final review, while only 18.8% in PRP group reported poor response [Figure 3].
Figure 3.
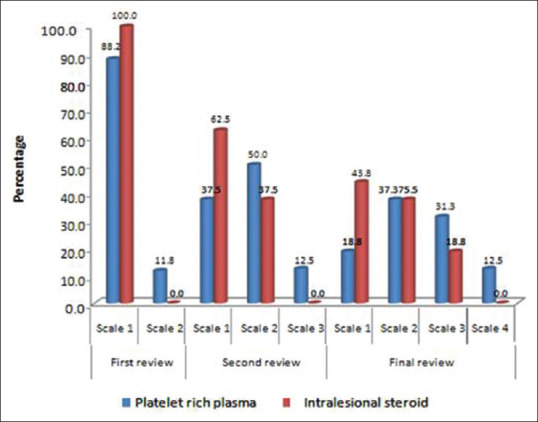
Comparison of hair regrowth scale between treatments
While comparing the side effects, there were no side effects of steroids like cutaneous atrophy, hypopigmentation, telangiectasia, or burning reported by the patients enrolled in triamcinolone group. Three patients had severe pain in PRP group while none reported severe pain in triamcinolone group. Photographs of AA patients before and after 3 PRP injections and 3 triamcinolone injections are shown as in Figure 4a-d, respectively.
Figure 4.
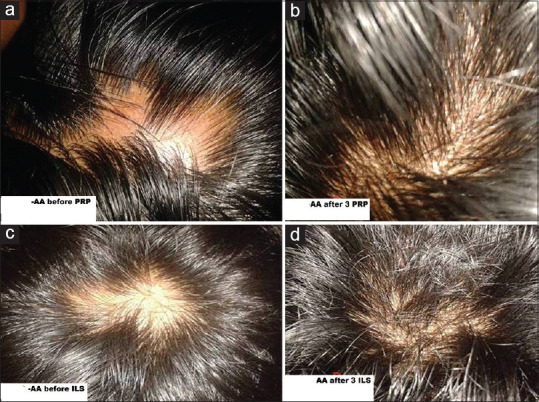
(a-d) Photographs of the AA patients before and after 3 PRP injections and 3 triamcinolone injections, respectively
Discussion
Alopecia areata (AA) is a chronic inflammatory disease that involves hair follicles and occassionally nails.[5] In this study, the efficacy of PRP was evaluated and compared with intralesional triamcinolone acetonide injections in alopecia areata patients. Majority of patients were in 18–30 years age group and most of them were males. Most of the patients had disease duration of less than 3 months and the progression of disease was mild for most of the patients. Most of them had involvement of vertex followed by involvement of occipital and temporal regions. In this study, most of the patients had a single patch of alopecia areata. While comparing SALT scores at different intervals of time, there was no statistically significant difference. The difference in the reduction of SALT score in patients who took PRP treatment was more when compared to triamcinolone but was not statistically significant. However, patients in PRP group showed a statistically significant (P = 0.028) reduction in SALT score during second review when compared to triamcinolone group.
PRP is autologous concentration of platelets contained in small volume of plasma which accelerates the rejuvenation of skin and hair follicles as it contains various growth factors and cellular adhesion molecules.[6] The principle of use of PRP in hair loss depends on its high concentration of growth factors.[7] Growth factors stimulate the formation of hair epithelium and the differentiation of stem cells into hair follicle cells, through an up-regulation of β-catenin.[8] They also prolong the anagen phase of hair cycle by increasing the expression of fibroblast growth factor-7.[9] Activated PRP leads to proliferation of dermal papillary cells by modulating fibroblast growth factor7 (FGF-7), β-catenin, extracellular signal-related kinase (ERK), and Akt signalling.[10]
Antiinflammatory effects of PRP have a significant role in the management of AA. Pathology of AA is characterized by lymphocyte infiltrate around the hair bulb, which may be responsible for secretion of various inflammatory cytokines. PRP with its potent anti-inflammatory effect may suppress cytokine release, limiting the local tissue inflammation. Activated PRP increases the levels of antiapoptotic protein Bcl-2, protecting cells from apoptosis.[9] Akt also has antiapoptotic effects. PRP increases the proliferation of epidermal and hair follicle bulge cells, evidenced by an increase in Ki-67 which is a marker for cell proliferation in androgenetic alopecia.[10] In AA too, an increase in Ki-67 was noted[2] and PRP appeared to act as a potent antiinflammatory agent, suppressing the release of inflammation cytokines.
As the platelets secrete growth factors within 10 min after activation and more than 95% of the growth factors are secreted within 1 hour, PRP should be used within 10 min of activation. Giusti et al. found that the optimal platelet concentration for the induction of angiogenesis was 1.5 million platelets per microliter and excessively high concentrations of platelets decrease the angiogenic potential.[11]
In a previous double-blind randomized placebo and active sided half head study by Trink et al., PRP treatment in alopecia areata showed a good response.[6] In our study also, PRP treatment in alopecia areata showed good response. In the previous study by Shumez et al., patients treated with PRP had an earlier response than patients treated with triamcinolone.[12] This difference was statistically insignificant, while the difference was statistically significant (P = 0.028) in our study during the second review. The treatment in both study groups was done once in 4 weeks in 3 sessions, and the response was monitored at 4th, 8th, and 12th weeks. There is no standardized protocol for an interval between treatment sessions and the duration of treatment in AA. Most of the studies published earlier have given treatment once in 3 weeks to 4 weeks and continued the treatment for variable time periods from 3 to 6 months or till clinical response was seen.[13,14,15,16,17,18] While comparing the hair regrowth scale between treatments, there was no statistically significant difference. However, 12.5% patients in PRP group reported excellent response after final review (Scale 4), compared to none in triamcinolone group. However, hair regrowth scale is more subjective and has greater possibility of observer bias. PRP has proliferation inducing effect and antiinflammatory effect, which suppress cytokine release and limit local tissue inflammation. As alopecia areata is characterized by a variety of inflammatory cytokines, it is probable that the antiinflammatory effects of PRP may be of significant benefit in this condition.
A study by Singh in 2015, including 20 patients with chronic AA treated with PRP, reported hair regrowth of his patients, with failure of only one patient.[19] Shumez et al. treated 48 patients with triamcinolone injections and 26 patients with PRP injections. PRP treated patients had an earlier response at the end of 6 weeks, but the difference was statistically insignificant.[12] At the end of 9 weeks, there was 100% improvement for all patients in both groups.
D'Ovidio and Roberto treated 25 patients affected by severe chronic AA with PRP. None of the patients achieved noticeable cosmetic results.[20] Of the nine patients who completed the study, at the eighth month, six had obtained regrowth of terminal pigmented hair; the others noted nonpigmented vellus after the second session of PRP.
In the study by El taieb et al., 90 patients were allocated into three groups; the first was treated with minoxidil 5% solution, the second with PRP injections, and the third with placebo.[21] They concluded that PRP is a more effective therapy for the treatment of AA than minoxidil 5% in the same period of treatment. PRP showed a significant decrease in yellow dots and short vellus hair, while minoxidil showed an increase in short vellus hair.
In the previous study by Cervelli et al., on the effect of autologous PRP injection in alopecia areata with clinical and histo morphometric evaluation, at the end of the 3 cycles of treatment, clinical improvement was noted in the mean number of hairs and density compared with baseline values.[10]
A study of intralesional corticosteroids showed the time from injection to visible hair growth was 2–4 weeks.[13] Any hair regrowth was seen within 3 months and the therapy should be stopped if there is no response by 6 months, as such individuals may lack adequate corticosteroid receptors in their scalp tissue.
In our study, PRP had no major adverse effects. While comparing the side effects, usual steroid-induced cutaneous atrophy, hypopigmentation, telangiectasia, or burning were not observed in triamcinolone group. Both the groups had pain during the procedure which was graded into mild, moderate, and severe. Three patients reported severe pain in PRP group, while none had severe pain in triamcinolone group. This study has some limitations including small study population and the average follow-up of patients was too short to draw conclusions about the effectiveness of long-term treatment. There was a selection bias, as the size of the lesion was not measured and only the number of lesions was considered for inclusion criteria. More studies are needed with longer duration of follow-up and with a larger sample size to evaluate efficacy and cost-effectiveness of PRP.
Conclusion
This was a comparative study, which evaluated the treatment outcome of intralesional platelet-rich plasma and triamcinolone acetonide injections for alopecia areata. Our study showed similar treatment response to PRP and ILS in alopecia areata at the end of 12 weeks. However, there was a statistically significant earlier response at 8 weeks with PRP when compared to triamcinolone. PRP is a safe, effective, steroid sparing, and suitable alternative in AA. Only side effect noted was pain during injection in both the groups. There is a need for further clinical studies with a larger number of patients to arrive at a definite conclusion about the role of PRP in AA.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patients have given their consent for their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Ross EK, Shapiro J. Management of hair loss. Dermatol Clin. 2005;23:227–43. doi: 10.1016/j.det.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 2.Trink A, Sorbellini E, Bezzola P. A randomized, double-blind, placebo and active-controlled, half-head study to evaluate the effects of platelet rich plasma on alopecia areata. Br J Dermatol. 2013;169:690–94. doi: 10.1111/bjd.12397. [DOI] [PubMed] [Google Scholar]
- 3.Cheng H, Zhang J. Platelet rich plasma stimulates angiogenesis in mice which may promote hair growth. Eur J Med Resp. 2017;22:39–45. doi: 10.1186/s40001-017-0278-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Olsen EA, Hordinsky MK, Price VH, Roberts JL, Shapiro J, Canfield D. Alopecia areata investigational assessment guidelines Part II National Alopecia Areata Foundation. J Am Acad Dermatol. 2004;51:440–47. doi: 10.1016/j.jaad.2003.09.032. [DOI] [PubMed] [Google Scholar]
- 5.De Berker DA, Messenger AG, Sinclair RD. Disorders of hair. In: Burns T, Breathnach S, Cox N, Griffiths C, editors. Rook's textbook of Dermatology. 8th ed. Edinburgh: Wiley Blackwell; 2010. pp. 6631–8. [Google Scholar]
- 6.Schippinger G, Prüller F, Divjak M, Mahla E, Fankhauser F, Rackemann S, et al. Autologous Platelet-rich plasma preparations: Influence of non steroidal anti-inflammatory drugs on platelet function. Orthop J Sports Med. 2015:3. doi: 10.1177/2325967115588896. doi: 2325967115588896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eppley BL, Woodell JE, Higgins J. Platelet quantification and growth factor analysis from platelet-rich plasma: Implications for wound healing. Plast Reconstr Surg. 2004;114:1502–8. doi: 10.1097/01.prs.0000138251.07040.51. [DOI] [PubMed] [Google Scholar]
- 8.Sohn KC, Shi G, Jang S, Choi DK, Lee Y, Yoon TJ, et al. Pitx2, a beta-catenin-regulated transcription factor, regulates the differentiation of outer root sheath cells cultured in vitro. J Dermatol Sci. 2009;54:6–11. doi: 10.1016/j.jdermsci.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 9.Li ZJ, Choi HI, Choi DK, Sohn KC, Im M, Seo YJ, et al. Autologous platelet-rich plasma: A potential therapeutic tool for promoting hair growth. Dermatol Surg. 2012;38:1040–6. doi: 10.1111/j.1524-4725.2012.02394.x. [DOI] [PubMed] [Google Scholar]
- 10.Cervelli V, Garcovich S, Bielli A, Cervelli G, Curcio BC, Scioli MG, et al. The effect of autologous activated platelet rich plasma (AA-PRP) injection on pattern hair loss: Clinical and histo morphometric evaluation. Bio Med Res Int. 2014;4:760–9. doi: 10.1155/2014/760709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giusti I, Rughetti A, D'Ascenzo S, Millimaggi D, Pavan A, Dell'Orso L, et al. Identification of an optimal concentration of platelet gel for promoting angiogenesis in human endothelial cells. Transfusion. 2009;49:771–8. doi: 10.1111/j.1537-2995.2008.02033.x. [DOI] [PubMed] [Google Scholar]
- 12.Shumez H, Prasad PVS, Kaviarasan PK, Deepika R. Intralesional platelet rich plasma vs intralesional triamcinolone in the treatment of alopecia areata: A comparative study. Int J Med Res Health Sci. 2015;4:118–22. [Google Scholar]
- 13.Porter D, Burton JL. A comparison of intra Iesional triamcinolone hexacetonide and triamcinolone acetonide in alopecia areata. Br J Dermatol. 1971;85:272–3. doi: 10.1111/j.1365-2133.1971.tb07230.x. [DOI] [PubMed] [Google Scholar]
- 14.Kubeyinje EP. Intralesional triamcinolone acetonide in alopecia areata amongst 62 Saudi Arabs. East Am Med J. 1994;71:674–5. [PubMed] [Google Scholar]
- 15.Abell E, Munro DD. Intralesional treatment of alopecia areata with triamcinolone acetonide by jet injector. Br J Dermatol. 1973;88:55–9. doi: 10.1111/j.1365-2133.1973.tb06672.x. [DOI] [PubMed] [Google Scholar]
- 16.Lahiry AK. Multiple cystic swellings of the scalp: A complication of intralesional steroid therapy. Indian Dermatol Venereol Leprol. 2003;69:345–6. [PubMed] [Google Scholar]
- 17.Marx RE, Carlson ER, Eichstaedt RM, Schimmele SR, Strauss JE, Georgeff KR. Platelet-rich plasma: Growth factor enhancement for bone grafts. Endodontology. 1998;85:638–46. doi: 10.1016/s1079-2104(98)90029-4. [DOI] [PubMed] [Google Scholar]
- 18.Khatu S, More Y, Gokhale N, Chavhan D, Bendsure N. Platelet-rich plasma in androgenic alopecia: Myth or an effective tool. J Cutan Aesthet Surg. 2014;7:107–10. doi: 10.4103/0974-2077.138352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singh S. Role of platelet-rich plasma in chronic alopecia areata: Our centre experience. Indian J Plast Surg. 2015;48:57–9. doi: 10.4103/0970-0358.155271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.d'Ovidio R, Roberto M. Limited effectiveness of platelet-rich-plasma treatment on chronic severe alopecia areata. Hair Ther Transplant. 2014;4:116–7. [Google Scholar]
- 21.El Taieb MA, Ibrahim H, Nada EA, Seif Al-Din M. Platelets rich plasma versus minoxidil 5% in treatment of alopecia areata: A trichoscopic evaluation. Dermatol Ther. 2017;30:1–6. doi: 10.1111/dth.12437. [DOI] [PubMed] [Google Scholar]


