Abstract
Introduction:
Alopecia areata (AA) is a chronic, non-scarring type of alopecia that presents as patchy hair loss over the scalp and other parts of the body. The diagnosis of AA can sometimes be challenging. Trichoscopy can be used to observe certain follicular patterns, shaft changes, and interfollicular pattern which help in diagnosing and determining the disease activity in AA.
Materials and Methods:
This study was a 1-year hospital-based observational cross-sectional study consisting of 60 patients clinically diagnosed with AA. Trichoscopic examination of the scalp and hair was performed using a videodermatoscope—Dinolite premier AM4113ZT model, trichoscopic images were recorded, and results were analyzed statistically.
Results:
AA was more common in males 39/60 cases (65%) with male to female ratio of 1.85:1. Scalp was the most frequently involved site, seen in 52/60 cases (86.67%) and patchy alopecia was the most frequent clinical pattern of presentation (83.33%). The characteristic follicular features noted were black dots, yellow dots, and empty hair follicles. Black dots were the commonest finding (63.33%) and represented a marker for active disease. The characteristic hair patterns noted were broken hair, micro-exclamation mark hair, coudability hair, all of which were commonly seen in active cases. 72% of cases that had clinically inactive disease showed active disease on trichoscopy.
Conclusion:
Trichoscopic features of AA are characteristic and they not only provide an important clue to the diagnosis in doubtful cases but also help in assessing disease activity in AA.
Keywords: Alopecia areata, dermoscopy, trichoscopy
Introduction
Alopecia areata (AA) is a chronic, non-scarring, immune-mediated disease of anagen hair. It presents as patchy hair loss over the scalp and other parts of the body.[1] The diagnosis of AA can sometimes be challenging and it becomes difficult to assess the disease activity.[2] Trichoscopy is a non-invasive method that is used to examine various patterns of skin, hair and subsurface skin structures that helps in diagnosing and determining the disease activity in AA.[1]
Materials and Methods
The study was a 1-year hospital-based cross-sectional observational study where a total of 60 consecutive consenting patients who were clinically diagnosed as having AA were included as per universal sampling, irrespective of age and sex. Those with concomitant androgenetic alopecia, trichotillomania, and other coexisting scalp disorders were excluded. Clinical photographs were taken and hair pull test was performed at the advancing edges of the alopecic patches by grasping approximately 20–60 hairs between the thumb, index, and middle fingers from the base of the hairs near the scalp and firmly, but not forcefully, tugged away from the scalp. If more than 10% of hairs are pulled away from the scalp, this was considered a positive pull test. Trichoscopic examination of the scalp and hair was performed using a videodermatoscope—Dinolite premier AM4113ZT model—with polarized light and a contact method (at times using ultrasound gel as interface fluid) under 50 × and 200 × magnifications, and trichoscopic images were recorded. The trichoscopic features were noted; the results were tabulated and statistically analyzed by SPSS version 25 software using the correlation method and Chi-square test wherever applicable.
Results
Of the 60 cases included in the study, 39/60 (65%) were male and 21/60 (35%) were female. Most patients belonged to the group of 21–30 years with the average age of the patients being 25.12 years.
The duration of onset of the disease in the cases ranged from 3 days to 10 years with a mean duration of disease onset being 471.92 days. Most patients in the study, (22/60;36.67%) had disease onset of fewer than 30 days, followed by 19/60 (31.67%) patients who had a duration of onset of more than 6 months.
The most common site involved was the scalp constituting 52/60 (86.67%) cases. The commonest feature at presentation was a single patch that constituted 27/60 (45%) cases.
Patchy hair loss was the most common clinical pattern seen in 50/60 (83.33%) patients followed by alopecia universalis in 5/60 (8.33%) patients, ophiasis pattern in 4/60 (6.67%) patients, and diffuse pattern in 1/60 (1.67%) patient.
Clinical activity of the disease was assessed mainly by positive hair pull test, presence of exclamation mark hair or black dots over the alopecic patches. Among the enrolled patients, 35/60 (58.33%) patients had clinically active disease and 25/60 (41.67%) patients showed inactive disease.
On trichoscopy, the follicular features noted predominantly were black dots [Figure 1], yellow dots [Figure 2]), empty follicles and white dots. The characteristic hair pattern noted was micro-exclamation mark hair (EMH) [Figure 3], coudability hair [Figure 4], and broken hair. Other less commonly noted patterns were tulip hair, pigtail hair, short vellus hair, and leucotrichia. The various follicular patterns, hair patterns and their incidence in our study are tabulated in Table 1.
Figure 1.
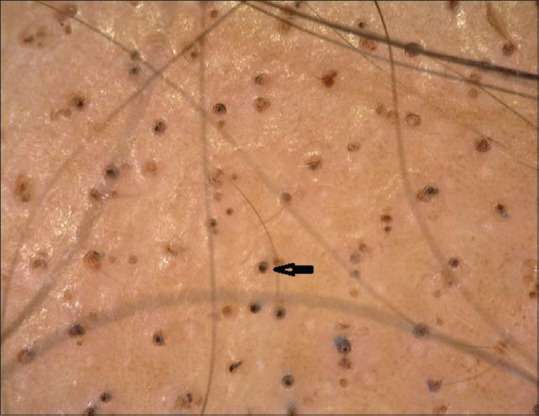
Multiple black dots (black arrow) on trichoscopy (50×; polarized mode)
Figure 2.
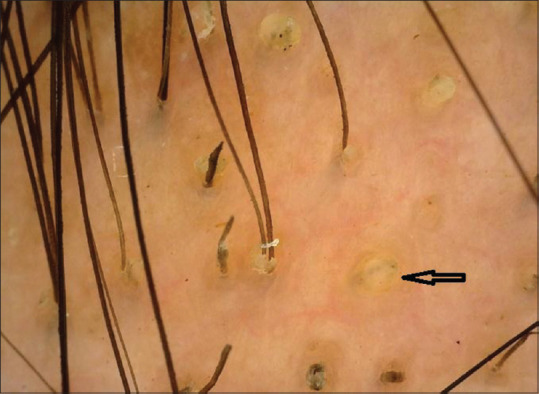
Multiple yellow dots (black arrow) on trichoscopy (50×; polarized mode)
Figure 3.
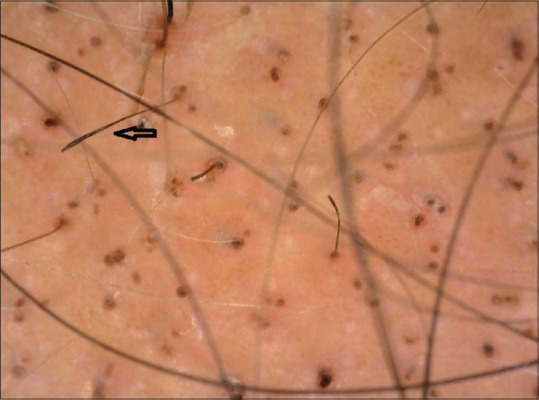
Micro-exclamation mark hair (black arrow) on trichoscopy (50×; polarized mode)
Figure 4.
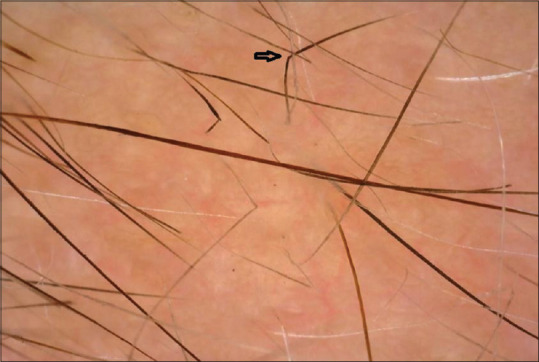
Coudability hair (black arrow) on trichoscopy (50×; polarized mode)
Table 1.
Follicular and interfollicular patterns on trichoscopy
| Follicular pattern | No. of cases present | % | No. of cases absent | % |
|---|---|---|---|---|
| Black dots | 38 | 63.33 | 22 | 36.67 |
| Yellow dots | 30 | 50.00 | 30 | 50.00 |
| White dots | 7 | 11.67 | 53 | 88.33 |
| Empty follicles | 20 | 33.33 | 40 | 66.67 |
| Micro-exclamation mark hair | 21 | 35 | 39 | 65 |
| Broken hair | 8 | 13.33 | 52 | 86.67 |
| Coudability hair | 31 | 51.67 | 29 | 48.33 |
| Short vellus hair | 27 | 45 | 33 | 55 |
| Upright regrowing hair | 25 | 41.67 | 35 | 58.33 |
| Pigtail hair | 11 | 18.33 | 49 | 81.67 |
| Monolitherex hair | 1 | 1.67 | 59 | 98.33 |
| I-hair | 2 | 3.33 | 58 | 96.67 |
| Tulip hair | 6 | 10 | 54 | 90 |
| Leucotrichia | 7 | 11.67 | 53 | 88.33 |
| Interfollicular pattern | Present | % | Absent | % |
| Honeycomb | 21 | 35.00 | 39 | 65.00 |
| Erythema | 23 | 38.33 | 37 | 61.67 |
| Arborizing blood vessels | 7 | 11.67 | 53 | 88.33 |
The interfollicular patterns on trichoscopy showed arborizing blood vessels in 7/60 (11.67%), honeycomb pattern in 21/60 (35%) and erythema in 23/60 (38.33%) cases.
When the activity of the disease on trichoscopy was studied, the presence of black dots, micro- exclamation mark hair, or coudability hair were considered to have active disease. 52/60 (86.67%) patients had active disease and 8/60 (13.33%) had inactive disease on trichoscopy. The association between the disease activity on trichoscopy with the follicular pattern on trichoscopy is depicted in Table 2. It was noted that black dots showed statistical significance (P = 0.0001) with respect to the disease activity. Amongst the various hair patterns noted, micro-EMH and coudability hair too showed statistical significance (P = 0.0260 and 0.0020, respectively) with respect to the disease activity as depicted in Table 3. Among the interfollicular pattern, in trichoscopically active cases, honeycomb pattern was noted in 20/21 (95.24%) cases, erythema in 21/23 (91.30%) cases and arborizing blood vessels in 6/7 (85.71%) cases.
Table 2.
Association between disease activity on trichoscopy and follicular patterns on trichoscopy
| Follicular pattern | No. of cases showing activity | % | No. of cases showing inactivity | % | Chi-Square | P |
|---|---|---|---|---|---|---|
| Black dots | 38 | 100.00 | 0 | 0.00 | 12.9520 | 0.0001* |
| Yellow dots | 26 | 86.67 | 4 | 13.33 | 0.0000 | 1.0000 |
| White dots | 7 | 100.00 | 0 | 0.00 | 1.2190 | 0.2700 |
| Empty follicles | 18 | 90.00 | 2 | 10.00 | 0.2880 | 0.5910 |
*P<0.05 is considered to be statistically significant
Table 3.
Association between hair patterns with activity of the disease on trichoscopy
| Hair pattern | Activity | % | Inactivity | % | Chi-square | P |
|---|---|---|---|---|---|---|
| Micro-exclamation hair | 21 | 100.00 | 0 | 0.00 | 4.9700 | 0.0260* |
| Broken hair | 8 | 100.00 | 0 | 0.00 | 1.4200 | 0.2330 |
| Coudability hair | 31 | 100.00 | 0 | 0.00 | 9.8670 | 0.0020* |
| Short vellus hair | 22 | 81.48 | 5 | 18.52 | 1.1420 | 0.2850 |
| Upright regrowing hair | 22 | 88.00 | 3 | 12.00 | 0.0660 | 0.7970 |
| Pigtail hair | 9 | 81.82 | 2 | 18.18 | 0.2740 | 0.6010 |
| Monolithrix hair | 1 | 100.00 | 0 | 0.00 | 0.1560 | 0.6920 |
| I-hair | 2 | 100.00 | 0 | 0.00 | 0.3180 | 0.5730 |
| Tulip hair | 5 | 83.33 | 1 | 16.67 | 0.0640 | 0.8000 |
| Leucotrichia | 6 | 85.71 | 1 | 14.29 | 0.0060 | 0.9370 |
*P<0.05 is considered to be statistically significant
With respect to the correlation between clinical activity and activity on trichoscopy, it was noted that 18/25 (72%) cases that had inactive disease clinically showed active disease on trichoscopy. The sensitivity and specificity of trichoscopy in evaluating the disease activity were found to be 97.14% and 28%, respectively. The correlation between clinical and trichoscopic activity is highlighted in Table 4.
Table 4.
Association between activity on trichoscopy and clinical activity
| Clinical activity | Activity on trichoscopy | |||||
|---|---|---|---|---|---|---|
| Activity | % | Inactivity | % | Total | % | |
| Active | 34 | 97.14 | 1 | 2.86 | 35 | 58.33 |
| Inactive | 18 | 72.00 | 7 | 28.00 | 25 | 41.67 |
| Total | 52 | 86.67 | 8 | 13.33 | 60 | 100.00 |
Chi-square with Yates’s correction=5.951 P=0.0150*. *P<0.05 Sensitivity-97.14% specificity-28%
Discussion
Clinical diagnosis of AA is not difficult at most times, however, sometimes it becomes difficult to differentiate it from other conditions such as trichotillomania, telogen effluvium, and tinea capitis. With respect to disease activity, there are situations where some patients may have hair pull test negative but have features of active disease trichoscopically. Also, black dots or exclamation mark hair can be missed clinically where trichoscopy would be able to detect them.
Trichoscopy shows characteristic patterns and these patterns are used not only to diagnose but also to assess the activity of the disease. Hence, we undertook this study to observe trichoscopy features in AA that correlate the various findings with the activity of the disease.
Of the 60 patients studied, a male predominance with 39/60 (65%) cases was noted. The age of patients was between 5 and 54 years with the mean age being 25.2 years. Patchy hair loss was the most frequent clinical pattern observed in 50/60 (83.3%) cases.
Follicular features on trichoscopy in our study showed yellow dots in 50% of cases. This was much more than the findings noted by Ankad et al.[2] (30%) but similar to the study by El-Taweel et al.[3] (55%) and lesser than that observed by Mane et al.[4] (81.8%). These yellow dots are representative of keratinous material in the hair follicle and are considered a sensitive marker for AA.[5]
Black dots represent hairs that break at the level of the surface of the scalp. In our study, black dots were seen in 38/60 (63.33%) cases, comparable with the study by El-Taweel et al. (65%) but much more than that reported by Mane et al. (44.66%) and Ankad et al. (20%). In our study, black dots were the most frequent follicular pattern seen.
The life cycle of the hair follicle has been divided into anagen, telogen and catagen phase. A further phase, exogen refers to the phase where hair shaft is extruded which is being coincident with the emergence of the new anagen hair. However, at times instead of being occupied by a new terminal hair, the follicle remains vacant.[6] This vacant stage is referred to as kenogen phase. Empty hair follicles have been previously described in AGA. Presence of this empty hair follicle suggests that the hair cycle is in kenogen stage. In our study, a higher incidence of empty hair follicles was noted in 20/60 (33.33%) cases as compared to the study by Nikam and Mehta where the incidence was 16%.[7]
Broken hair is considered a sign of active disease and commonly seen in severe cases.[5] Our study showed lower incidence, that is, in 8/60 (13.33%) cases compared to studies by Ankad et al. (30%), El-Taweel et al. (40.0%), and Mane et al. (55.4%) who noticed a higher incidence of broken hair.
Micro-EMH is seen commonly in active stages of AA, however, these are not a distinct feature of AA as they can be seen in other conditions.[7] This feature in our study was seen in 21/60 (35%) cases, and showed lower incidence than the study by Ankad et al. (60%), El-Taweel et al. (50%), and a higher incidence than the study done by Mane et al. (12.1%).
Coudability sign is proposed as a marker of disease activity in AA.[2,7,8] In our study, this feature was seen in 31/60 (51.67%) cases which was much higher when compared with the values in the study by Ankad et al. (10%). This variation may be due to more number of active cases encountered in our study.
Pigtail hair represents hair regrowth.[2] This feature in our study was seen in (18.33%) cases and was comparable to the study by El-Taweel, et al. (15%).
Monilethrix-like hair was seen in 1/60 (1.67%) cases which is comparable to the incidence in the study by Rudnicka et al. where incidence was 3%.[9] However, other studies have not mentioned this feature.
The incidence of tulip hair in AA in the present study was 10%. This significantly varied with the incidence in a study by Rakowska et al. which showed the incidence of 2%.[10]
In our study, “i hair” was noted in 2/60 (3.33%) patients. This feature has been mentioned in a study by Malakar et al.[11]
Amongst the follicular changes, in patients with clinically active disease black dots and yellow dots were present in 25/35 (65.79%) cases and 16/30 (53.33%) patients, respectively. In trichoscopically active disease, black dots were present in 38/38 (100%) cases and yellow dots were seen in 26/30 (86.67%) patients. Though in our study both yellow dots and black dots were seen in active disease, the presence of black dots showed a significant statistical correlation with the disease activity. Our results match with the study done by Inui et al.[5,7]
Among the hair shaft changes, in patients with clinically active disease, micro-EMH was present in 16/21 (76.19%) of patients and was statistically significant. In patients with trichoscopically active disease, all patients were found to have micro-EMH. This suggests that micro-EMH acts as a specific marker of active disease.
In patients with clinically active disease, broken hair, and coudability sign were seen in 62.5% and 67.74% patients, respectively. In trichoscopically active cases, broken hair, and coudability sign were also seen in all cases, however, only coudability sign showed a statistically significant correlation with the disease activity, suggesting that broken hair and coudability hair correlates positively with disease activity with coudability hair being more specific than broken hair.
In our study, the clinical activity of the disease was seen in 58.33%, however, the trichoscopic activity of the disease was seen in 86.67%. 18/25 (72%) patients who had inactive disease clinically showed active disease on trichoscopy. Also, the sensitivity of trichoscopy in assessing disease activity was 97.14% with specificity being 28%. Hence, trichoscopy can act as a reliable and sensitive tool in assessing the disease activity and thereby aid in early active intervention.
Conclusion
From our study, black dots and yellow dots were seen mainly in active disease and the presence of black dots on trichoscopy showed a significant correlation with the active disease. Micro-EMH, coudability hair, black dots, and broken hair showed a positive correlation with disease activity on trichoscopy. The presence of micro-EMH correlated significantly in both clinically and trichoscopically active disease. Though both broken hair and coudability hair correlated positively with disease activity, coudability hair was found to be more specific. Many patients who had clinically inactive disease were found to have an active disease on trichoscopy. Hence, trichoscopy would aid in diagnosing AA and acts as a highly accurate and sensitive method for detecting disease activity.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Jain N, Doshi B, Khopkar U. Trichoscopy in alopecias: Diagnosis simplified. Intl J Trichol. 2013;5:170–8. doi: 10.4103/0974-7753.130385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ankad B, Beergouder SL, Panchkattimath VS, Sheik Ahmed M. Trichoscopy of alopecia areata: A diagnostic aide. Hair Ther Transplant. 2014;4:3. [Google Scholar]
- 3.El-Taweel AE, El-Esawy F, Abdel-Salam O. Different trichoscopic deatures of tinea capitis and alopecia areata in pediatric patients dermatology research and practice. Dermatol Res Pract. 2014;2014:1848763. doi: 10.1155/2014/848763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mane M, Nath AK, Thappa DM. Utility of dermoscopy in alopecia areata. Indian J Dermatol. 2011;56:407–11. doi: 10.4103/0019-5154.84768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dias SE, Panicker VV, Thomas J, Jagadeesan S, Anjaneyan G, Lekshmi S. Trichoscopic features in diagnosis of alopecia areata and its relation to severity of alopecia tool (SALT) score. J Pak Assoc Dermatol. 2018;28:152–6. [Google Scholar]
- 6.Rebora A, Guarrera M. Kenogen? A new phase of the hair cycle. Dermatology. 2002;205:108–10. doi: 10.1159/000063908. [DOI] [PubMed] [Google Scholar]
- 7.Nikam VV, Mehta HH. A Nonrandomized study of trichoscopy patterns using nonpolarized (contact) and polarized (noncontact) dermatoscopy in hair and shaft disorders. Int J Trichol. 2014;6:54–62. doi: 10.4103/0974-7753.138588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Inui S, Nakajima T, Itami S. Coudability hairs: A revisited sign of alopecia areata assessed by trichoscopy. Clin Exp Dermatol. 2010;35:361–5. doi: 10.1111/j.1365-2230.2009.03510.x. [DOI] [PubMed] [Google Scholar]
- 9.Rudnicka L, Olszewska M, Rakowska A, Slowinska M. Trichoscopy update 2011. J Dermatol Case. 2011;5:82–8. doi: 10.3315/jdcr.2011.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rakowska A, Maj M, Zadurska M, Czuwara J, Warszawik-Henzel O, Olszewska M, et al. Trichoscopy of focal alopecia in children-New trichoscopic findings: Hair bulbs arranged radially along hair-bearing margins in aplasia cutis congenita. Skin Appendage Disord. 2016;2:1–6. doi: 10.1159/000445721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malakar S, Mehta PR. “i hair”: A prognostic marker in alopecia areata and trichotillomania. Indian J Dermatol. 2017;62:658–60. doi: 10.4103/ijd.IJD_337_17. [DOI] [PMC free article] [PubMed] [Google Scholar]


