Abstract
A European goldfinch (Carduelis carduelis), a canary (Serinus canaria), and a lovebird (Agapornis roseicollis) captive-bred at three different private aviaries in Spain were submitted for necropsy with a history of weakness and ruffled feathers, weight loss associated with glossitis, and respiratory disease, respectively. Microscopically, enterocytes in the jejunum and ileum contained colonies of gram- and Stamp-positive, oval to elliptical microorganisms within parasitophorous vacuoles in the apical cytoplasm. Nested PCR using MSP primers that target microsporidian RNA genes produced amplicons of expected size for Encephalitozoon species, and analysis of forward and reverse DNA sequences confirmed the presence of Encephalitozoon hellem in all cases. The main cause of death of all three birds consisted of concurrent infections. However, intestinal encephalitozoonosis may have contributed to exacerbated catabolism. Encephalitozoonosis (or microsporidiosis) has been rarely described in passerine birds.
Keywords: Agapornis roseicollis, CarduelisCarduelis, Encephalitozoonosis, Microsporidiosis, Serinus canaria
The phylum Microsporidia contains a diverse group of obligate intracellular fungal-like parasites including 144 genera and over 1200 species that infect a wide range of vertebrate and invertebrate hosts (Didier and Weiss, 2011). Microsporidia have a unique organelle known as the polar filament that is used to infect the host cell and distinguishes these organisms from other protists (Wasson and Peper, 2000).
Pathogenic microsporidians affecting humans such as Enterocytozoon bieneusi and Encephalitozoon species (E. cuniculi, E. intestinalis and Encephalitozoon hellem) have been detected in avian tissues and fecal samples, particularly during the last decade in Europe. Species commonly involved in these studies include urban pigeons, raptors, wild waterfowl, and psittacine birds (Powell et al., 1989; Snowden et al., 2000; Barton et al., 2003; Phalen et al., 2006; Müller et al., 2008; Sak et al., 2010; Malčeková et al., 2011; Lallo et al., 2012; Malčeková et al., 2013). E. hellem is one of the most commonly described microsporidian species in birds and mixed infections with the other species have been reported (Snowden et al., 2000; Barton et al., 2003; Phalen et al., 2006). In passerine birds, microsporidiosis has been documented in tricolor parrot (Erythrura tricolor) and Gouldian (E. gouldiae) finches in Australia (Carlisle et al., 2002; Gelis and Raidal, 2006). This report describes intestinal E. hellem infection in aviary passerine and psittacine birds in Spain.
An adult female European goldfinch (Carduelis carduelis) (case No. 1), an adult male canary (Serinus canaria) (case No. 2), and a young female lovebird (Agapornis roseicollis) (case No. 3) from different private aviaries in Spain were submitted for necropsy (cases Nos. 1 and 2) or histopathology (case No. 3) to a commercial diagnostic pathology laboratory with a history of weakness and ruffled feathers (case No. 1), weight loss (cases Nos. 1 and 2), glossitis (case No. 2), and dyspnea (case No. 3). Grossly, severe atrophy of adipose tissue and prominent dilatation with thickening of the duodenum was observed in the European goldfinch. The canary had severe atrophy of adipose tissue and moderate generalized muscular atrophy, as well as a slightly raised whitish plaque in the tongue, and cloacal dilatation with urate stasis. Gross lesions in the lovebird were not reported by the referring veterinarian. Necropsy tissues from all three birds were fixed in buffered 10% formalin, embedded in paraffin, sectioned at 5-μm, and stained with hematoxylin and eosin. Gram (all cases), Ziehl–Neelsen (case No. 1), and Stamp (cases No. 2 and 3) stains were also performed. A touch imprint of intestinal mucosa was obtained in case No. 2 and stained with a Diff-Quick stain. In situ hybridization for psittacine beak and feather disease was performed on formalin-fixed, paraffin-embedded tissues of the lovebird.
Cytological examination of intestinal mucosa imprint of case No. 2 revealed oval to elliptical organisms with a clear cytoplasm and an eccentric nucleus in the apical cytoplasm of some enterocytes (Fig. 1A). Upon histopathological examination, enterocytes in the jejunum and ileum of all three birds contained colonies of oval to elliptical microorganisms measuring approximately 2 × 1–1.5 μm and located within parasitophorous vacuoles in the apical cytoplasm that were suggestive of microsporidia (Fig. 1B). These organisms were most abundant in the apical portion of villi and stained gram-positive in all cases (Fig. 1C and D) and Stamp-positive in cases Nos. 2 and 3 (Fig. 1E and F); nevertheless, some of the organisms did not pick up these stains, particularly in cases Nos. 2 and 3, which is common for the less mature pre-spore stages of microsporidia. In case No. 3, these organisms were occasionally found in the lamina propria of the mucosa, as well (Fig. 1F). Intestinal lesions were only noted in case No. 3 and consisted of severe atrophy and fusion of villi in most of the intestinal sections examined. Apical enterocytes in affected villi contained chlamydial-like, gram-negative, Stamp-positive basophilic granular intracytoplasmic inclusions and exfoliated enterocytes with these inclusions were numerous in the lumen. The lamina propria was mildly infiltrated with heterophils, lymphocytes, and plasma cells.
Fig. 1.
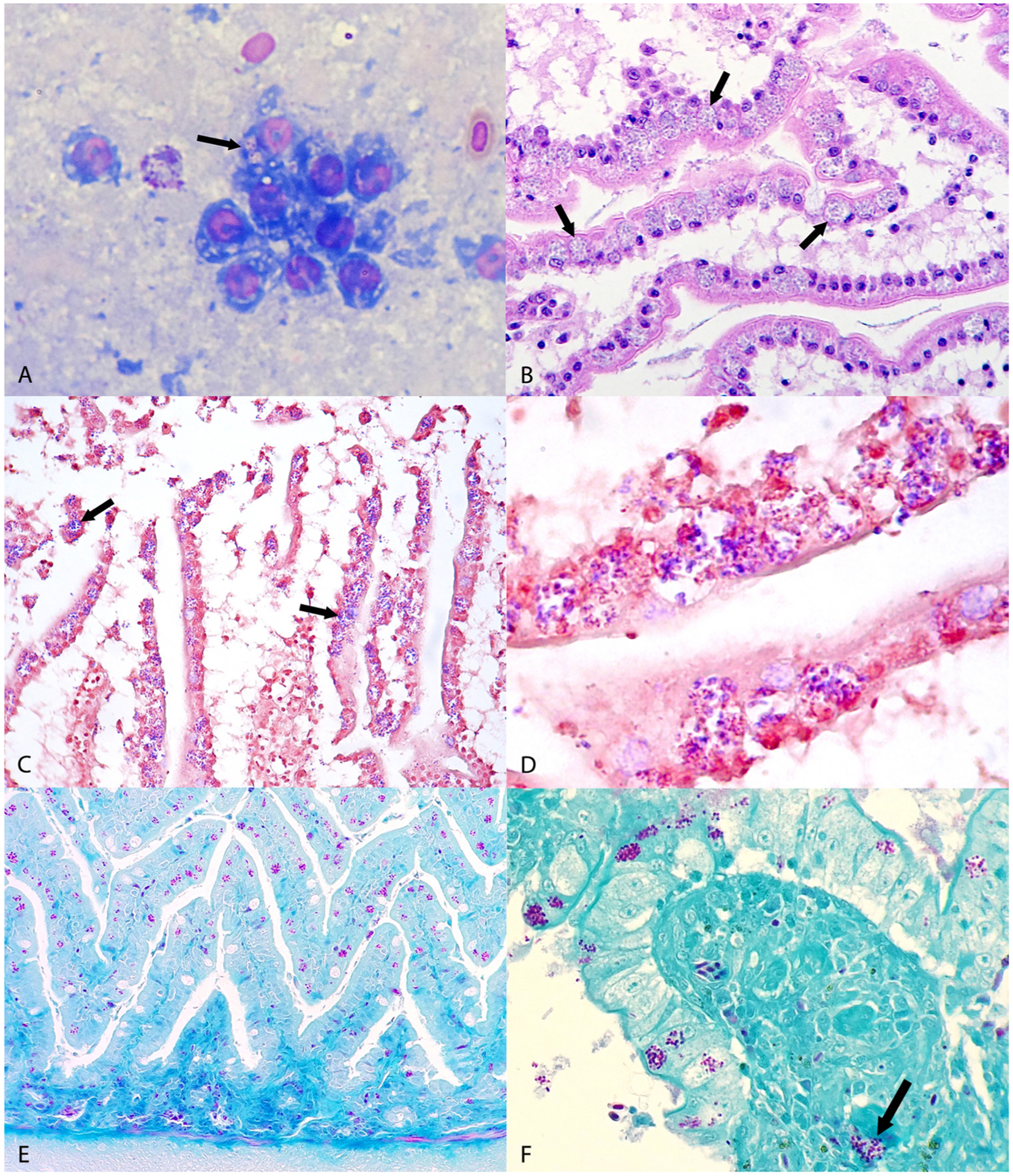
Microscopic findings from avian tissues. (A) Jejunum; canary, case No. 2. Oval to elliptical microorganisms with a clear cytoplasm and an eccentric nucleus compatible with microsporidian parasite are observed in the apical cytoplasm of enterocytes (arrows). Diff Quick. (B) Jejunum; European goldfinch, case No. 1. Note intense infection of the apical cytoplasm of enterocytes lining villi with microsporidial-like colonies located within parasitophorous vacuoles (arrows). HE. (C) Jejunum, European goldfinch, case No. 1. The microsporidial-like colonies in Fig. 1B stain gram positive (arrows). Gram. (D) Jejunum, European goldfinch, case No. 1. Higher magnification of gram positive microsporidial spores shown in Fig. 1C. Gram. (E) Jejunum, canary, case No. 2. Note abundant purple microsporidial colonies within the cytoplasm of enterocytes. Stamp. (F) Small intestine, lovebird, case No. 3. Note purple microsporidial colonies within the apical cytoplasm of enterocytes and in the lamina propria of villi (arrow). Stamp.
The other main microscopic findings consisted mostly of concurrent infections.
The European goldfinch had systemic granulomatous (histiocytic) inflammation with intralesional acid-fast bacilli involving mostly the duodenum, duodenal visceral adipose tissue, adrenal gland, and ovary. The canary had severe acute diffuse necrotizing and proliferative glossitis with intralesional poxviral intracytoplasmic inclusion bodies in keratinocytes and bacteria. Numerous granular basophilic intracytoplasmic inclusions were found in enterocytes and cloacal epithelial cells. Some of these inclusions contained Stamp-positive and gram-positive oval to elliptical microorganisms similar to those described above, but many were negative for these stains. In the lovebird, severe proventricular and ventricular macrorhabdiosis, severe hypertrophy and vacuolation of interrenal cells in the adrenal glands, as well as kariomegaly with intranuclear basophilic inclusion bodies in the renal tubular epithelium were also noted. All these concurrent lesions and disease processes did not contain intralesional microsporidial colonies according to routine and special stains. All three birds had severe atrophy of the adipose tissue.
Paraffin-embedded, formalin-fixed sections of intestinal tissue from all three cases were incubated in ATL Buffer (Qiagen, Valencia, CA, USA) at 90°C for one hour to partially reverse the effects of formaldehyde on nucleic acids. DNA was extracted from the tissue using the QIAmp DNA Mini Kit (Qiagen). Nested PCR was applied with MSP primers that target the internal transcribed sequence and flanking region of the large and small subunits of the rDNA genes of Encephalitozoon, Enterocytozoon, Vairimorpha and Nosema species of microsporidia as previously described (Katzwinkel-Wladarsch et al.,1996). The reaction contained 2.5 units of PuReTaq DNA polymerase, 10 mM Tris–HCl, 50 mM KCl, 1.5 mM MgCl2, 200 μM of each dNTP, stabilizers, and BSA using the PuReTaq Ready-To-Go Beads (Amersham; Piscataway, NJ, USA) along with 1 μM of each primer and 1 μl of template DNA in a final reaction final volume of 25 μl. The PCR first-round primers were 5′-TGA ATG KGT CCC TGT-3′ (MSP-1), 5′-TCA CTC GCC GCT ACT-3′ (MSP-2A) and 5′-GTT CAT TCG CAC TAC T-3′ (MSP-2B). The second-round primers were 5′-GGA ATT CAC ACC GCC CGT CRY TAT-3′ (MSP-3), 5′-CCA AGC TTA TGC TTA AGT YMAARG GGT-3′ (MSP-4A) and 5′-CCA AGC TTA TGC TTA AGT CCAGGG AG-3′ (MSP-4B). Thermal cycler conditions were 5 min at 95 °C followed by 36 cycles of: 1 min at 95 °C, 1.5 min at 55 °C, 3 min at 72 °C and then a final primer extension at 72 °C for 10 min. Amplicons were electrophoresed in a 1% agarose gel and stained with ethidium bromide. PCR amplicons were excised from the gel and purified using the Wizard SV Gel and PCR Clean-Up System (Promega Corporation, Madison, WI, Catalog #A9281). Products were submitted to the GeneLab at Louisiana State University School of Veterinary Medicine in Baton Rouge, LA, USA for sequencing, and sequences of forward and reverse amplicons were evaluated by BLASTn analysis (http://blast.ncbi.nlm.nih.gov/Blast.cgi?PROGRAM=blastn&BLAST_SPEC=WGS&BLAST_PROGRAMS=megaBlast&PAGE_TYPE=BlastSearch). In all three birds, amplicons of 289–305 bp, equivalent to products expected for Encephalitozoon species, were observed after electrophoresis of the nested PCR assay from each of the ten individual intestinal tissue sections processed. DNA nucleotide sequencing of the amplicons and BLASTn analysis demonstrated 99% identity with E. hellem (several Genbank accession numbers including CP002713.1 and AF338367.1).
The lovebird tested negative for psittacine beak and feather disease (PBFD) virus infection by in situ hybridization.
The overall results of this report identified severe intestinal microsporidiosis caused by E. hellem in a European goldfinch, a canary, and a lovebird based on histopathological and histochemical findings, and PCR with nucleotide sequencing from paraffin-embedded intestinal tissue.
To the authors’ knowledge, microsporidiosis has been only described in passerine birds on two occasions in Australia (Carlisle et al., 2002; Gelis and Raidal, 2006). In one study, tricolor parrot finches (Erythrura tricolor) had a nodular to diffuse granulomatous inflammation of the serosal surfaces of the gastrointestinal tract, perirenal air sacs and connective tissue, bone marrow, and conjunctiva with intralesional microsporidia that were not typed (Gelis and Raidal, 2006). The other report described E. hellem infection of small intestinal enterocytes in a Gouldian finch (Erythrura [Chloebia] gouldiae) (Carlisle et al., 2002). The cases reported herein add European goldfinches and canaries to the list of avian species susceptible to E. hellem infection. Two previous and two subsequent cases of mortality in European goldfinches from the same aviary as case No. 1 had no evidence of microsporidiosis. Also, no additional cases of microsporidiosis were found in 426 passerine bird submissions to Noah’s Path from June 2008 to November 2014. Microsporidiosis has been reported previously in lovebirds (Powell et al., 1989; Snowden et al., 2000; Barton et al., 2003) but microsporidia were not typed except in one study of fecal shedding in which E. hellem was identified (Barton et al., 2003).
Avian microsporidial infections may present with nonspecific signs and affected birds may die without premonitory signs. Previously described lesions in birds with microsporidiosis include multifocal necrotizing hepatitis, biliary hyperplasia, lymphocytic nephritis and enteritis, hypertrophy and necrosis of intestinal villi, and keratoconjuntivitis (Powell et al., 1989; Phalen et al., 2006). Microsporidian spores not associated with inflammation can be found in several tissues of affected birds, including the intestinal mucosa, renal tubular, bile duct, and corneal epithelium (Snowden et al., 2000; Carlisle et al., 2002; Phalen et al., 2006). In the European goldfinch and canary of this report, microsporidia only infected enterocytes of the jejunum and ileum and were not associated with inflammation or any other lesions. In the canary, the granular inclusions in enterocytes and cloacal epithelial cells that inconsistently stained gram- and Stamp-positive may correspond in part to microsporidial meronts (Wasson and Peper, 2000). In the lovebird, the intestinal lesions were strongly associated with abundant gram-negative, Stamp-positive, granular basophilic intracytoplasmic inclusions in enterocytes similar to chlamydial inclusions, but electron microscopy was not performed for an ultrastructural confirmation. In this animal, E. hellem might be contributory to intestinal lesions.
The cause of death or main diseases contributing to death of these three birds consisted of concurrent infections: disseminated mycobacteriosis (case No. 1); poxviral glossitis with secondary bacterial infection (case No. 2); enteric disease associated with chlamydial-like inclusions, and severe gastric macrorhabdiosis (case No. 3). Despite the absence of lesions associated directly with E. hellem infection at least in the passerine birds in this report, an intense microsporidial infection of intestinal absorptive epithelial cells may contribute to malabsorption. It is thus possible that E. hellem infection in these birds contributed to exacerbated catabolism. The occurrence of concurrent infections in all three birds suggests the possibility of underlying immunosuppression or interaction between pathogens. In this regard, the possibility of underlying PBFD virus infection was evaluated in the lovebird but in situ hybridization was negative. This animal had an intense morphological response of chronic stress in the adrenal glands and this may have favored infectious disease.
Supplementary Material
Acknowledgments
We would like to acknowledge support from the National Institutes of Health to the Tulane National Primate Research Center (OD011104). We also thank Dr. Juan Moreno de la Espada (Clínica Veterinaria Santa Ana, Chiclana de la Frontera, Cádiz, Spain) for submission of and permission to include the lovebird in this study.
Footnotes
Conflict of interest
The authors have no conflicts of interest.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.vetpar.2016.01. 022.
References
- Barton CE, Phalen DN, Snowden KF, 2003. Prevalence of microsoridian spores shed by asymptomatic lovebirds: evidence for a potential emerging zoonosis. J. Avian Med. Surg 17, 197–202. [Google Scholar]
- Carlisle MS, Snowden K, Gill J, Jones M, O’Donoghue P, Prociv P, 2002. Microsporidiosis in a Gouldian finch (Erythrura [Chloebia] gouldiae). Aust. Vet. J 80, 41–44. [DOI] [PubMed] [Google Scholar]
- Didier ES, Weiss LM, 2011. Microsporidiosis: not just in AIDS patients. Curr. Opin. Infect. Dis 24, 490–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelis S, Raidal SR, 2006. Microsporidiosis in a flock of tricolor parrot finches (Erythrura tricolor). Vet. Clin. North Am. Exot. Anim. Pract 9, 481–486. [DOI] [PubMed] [Google Scholar]
- Katzwinkel-Wladarsch S, Lieb M, Helse W, Löscher T, Rinder H, 1996. Direct amplification and species determination of microsporidian DNA from stool specimens. Trop. Med. Int. Health 1, 373–378. [DOI] [PubMed] [Google Scholar]
- Lallo MA, Calábria P, Milanelo L, 2012. Encephalitozoon and Enterocytozoon (Microsporidia) spores in stool from pigeons and exotic birds: microsporidia spores in birds. Vet. Parasitol 190, 418–422. [DOI] [PubMed] [Google Scholar]
- Malčeková B, Valenčáková A, Luptaková L, Molnar L, Ravaszova P, Novotny F, 2011. First detection and genotyping of Encephalitozoon cuniculi in a new host species: gyrfalcon (Falco rusticolus). Parasitol. Res 108, 1479–1482. [DOI] [PubMed] [Google Scholar]
- Malčeková B, Valenčáková A, Molnár L, Kočišová A, 2013. First detection and genotyping of human-associated microsporidia in wild waterfowl of Slovakia. Acta Parasitol. 58, 13–17. [DOI] [PubMed] [Google Scholar]
- Müller MG, Kinne J, Schuster RK, Walochnik J, 2008. Outbreak of microsporidiosis caused by Enterocytozoon bieneusi in falcons. Vet. Parasitol 152, 67–78. [DOI] [PubMed] [Google Scholar]
- Phalen DN, Logan KS, Snowden KF, 2006. Encephalitozoon hellem infection as the cause of a unilateral chronic keratoconjunctivitis in an umbrella cockatoo (Cacatua alba). Vet. Ophthalmol 9, 59–63. [DOI] [PubMed] [Google Scholar]
- Powell S, Tang K, Chandler F, Parks D, Hood C, 1989. Microsporidiosis in a lovebird. J. Vet. Diagn. Invest 1, 69–71. [DOI] [PubMed] [Google Scholar]
- Sak B, Kasicková D, Kvác M, Kvetonová D, Ditrich O, 2010. Microsporidia in exotic birds: intermittent spore excretion of Encephalitozoon spp. in naturally infected budgerigars (Melopsittacus undulatus). Vet. Parasitol 168, 196–200. [DOI] [PubMed] [Google Scholar]
- Snowden KF, Logan K, Phalen DN, 2000. Isolation and characterization of an avian isolate of Encephalitozoon hellem. Parasitology 121, 9–14. [DOI] [PubMed] [Google Scholar]
- Wasson K, Peper L, 2000. Mammalian microsporidiosis. Vet. Pathol 50, 113–128. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


