Abstract
Background
In the current SARS-CoV-2 pandemic, there has been worldwide debate on the use of corticosteroids in COVID-19. In the recent RECOVERY trial, evaluating the effect of dexamethasone, a reduced 28-day mortality in patients requiring oxygen therapy or mechanical ventilation was shown. Their results have led to considering amendments in guidelines or actually already recommending corticosteroids in COVID-19. However, the effectiveness and safety of corticosteroids still remain uncertain, and reliable data to further shed light on the benefit and harm are needed.
Objectives
The aim of this systematic review and meta-analysis was to evaluate the effectiveness and safety of corticosteroids in COVID-19.
Methods
A systematic literature search of RCTS and observational studies on adult patients was performed across Medline/PubMed, Embase and Web of Science from December 1, 2019, until October 1, 2020, according to the PRISMA guidelines. Primary outcomes were short-term mortality and viral clearance (based on RT-PCR in respiratory specimens). Secondary outcomes were: need for mechanical ventilation, need for other oxygen therapy, length of hospital stay and secondary infections.
Results
Forty-four studies were included, covering 20.197 patients. In twenty-two studies, the effect of corticosteroid use on mortality was quantified. The overall pooled estimate (observational studies and RCTs) showed a significant reduced mortality in the corticosteroid group (OR 0.72 (95%CI 0.57–0.87). Furthermore, viral clearance time ranged from 10 to 29 days in the corticosteroid group and from 8 to 24 days in the standard of care group. Fourteen studies reported a positive effect of corticosteroids on need for and duration of mechanical ventilation. A trend toward more infections and antibiotic use was present.
Conclusions
Our findings from both observational studies and RCTs confirm a beneficial effect of corticosteroids on short-term mortality and a reduction in need for mechanical ventilation. And although data in the studies were too sparse to draw any firm conclusions, there might be a signal of delayed viral clearance and an increase in secondary infections.
Keywords: COVID-19, SARS-CoV-2, Coronavirus, Corticosteroids, Mortality, Viral clearance, Mechanical ventilation
Background
Since the start of the outbreak, Coronavirus disease 2019 (COVID-19), caused by the novel coronavirus SARS-CoV-2, has spread globally from Wuhan, China. A total of 40,559,736 cases have been reported, and 1,121,499 people have died as of October 19. [1] Many countries have been affected, causing immense stress on healthcare systems worldwide. This is the third epidemic caused by a coronavirus, after severe acute respiratory syndrome (SARS) in 2002 and Middle East respiratory syndrome (MERS) in 2012 [2, 3]. The clinical presentation ranges from asymptomatic or mild disease to severe pneumonia in which the most severe cases deteriorate with acute respiratory distress syndrome (ARDS) requiring prolonged mechanical ventilation, or even extracorporeal membrane oxygenation (ECMO) [4, 5]. Approximately 16–35% develop severe pneumonia, 2–17% need mechanical ventilation, of whom up to 15% need ECMO therapy, [6–8] and the case fatality rate is 1.4–15% [5, 9, 10]. In the pathophysiology of severe COVID-19, the host immune response plays a key role and it has become evident that COVID-19 pneumonia is associated with both hyper inflammation and immunoparalysis [11]. A clinical presentation of massive vascular inflammation, disseminated coagulation, shock and ARDS is frequently triggered [9–11].
Though many therapies aiming at mitigation of the inflammatory response are being evaluated, strong evidence of benefit is lacking. Corticosteroids might have beneficial effects in overcoming both hyperinflammation and ARDS [4, 15–17]. Furthermore, they could serve as an easily accessible and affordable treatment option. On the other hand, there are known adverse effects of corticosteroid use, such as delayed viral clearance, opportunistic infections and suppression of the hypothalamic-pituitary-adrenal axis [2, 18, 19]. Earlier studies done in MERS-CoV and SARS-CoV showed delayed viral clearance, opportunistic infections and hyperglycemia [20–22]. Therefore, a high number of observational studies and randomized controlled trials (RCT) on corticosteroids for COVID-19 have been initiated and reported, and the signal is a beneficial effect. The RECOVERY trial was the first to report that the use of dexamethasone as opposed to usual care reduced 28-day mortality in patients requiring oxygen therapy or mechanical ventilation [23]. And a prospective meta-analysis of seven randomized clinical trials showed that administration of corticosteroids was associated with lower 28-day all-cause mortality [24]. And while initially the World Health Organization (WHO) recommended against corticosteroid treatment, as of September 2, 2020, the WHO recommends systemic corticosteroids rather than no systemic corticosteroids for the treatment of patients with severe and critical COVID-19 [15, 25]. Also, the Surviving Sepsis Guideline on management of COVID-19 recommends administration of steroids in patients with severe COVID-19 on mechanical ventilation with ARDS and in patients with COVID-19 and refractory shock [26].
However, the effectiveness and safety of corticosteroids still remain uncertain, because of scarcity of RCTs and inconclusive observational studies, and reliable data to further shed light on the benefit and harm are needed. Therefore, the aim of this systematic review and meta-analysis of observational studies and RCTs was to evaluate the effectiveness and safety of corticosteroids in COVID-19.
Methods
Data sources and search strategy
A systematic review according to the PRISMA guidelines was conducted [27]. The meta-analysis was retrospectively registered under number 38752 at ISRCTN.org. A comprehensive systematic search was conducted for published studies in Medline/PubMed, Embase and Web of Science from December 1, 2019, to October 1, 2020. The search strategy consisted of the components “COVID-19,” “intensive care” and “corticosteroids” (Additional file 1).
Eligibility
RCTs and observational cohort studies assessing the effect of corticosteroids in COVID-19 were eligible if they met the following inclusion criteria: adult patients (age ≥ 18 years), COVID-19 patients diagnosed by reverse transcriptase polymerase chain reaction (RT-PCR), reporting on outcome measures in relation to corticosteroid treatment, corticosteroids not restricted with respect to type, dose and duration. Studies concerning pregnant women or children, reviews, case series including less than 15 patients and articles that were not available in English were excluded [28].
Definition of primary and secondary outcomes
The primary outcomes were short-term mortality (i.e., short-term mortality as defined in the study, including 28-day, 30-day and hospital mortality) and viral clearance (i.e., as defined by the study, based on RT-PCR in respiratory specimens). Secondary outcomes were: mechanical ventilation (i.e., as defined by the study: need for invasive mechanical ventilation, duration of mechanical ventilation, ventilator-free days or other oxygen therapy), length of hospital stay (LOS-hospital) and secondary infections. For exact used definitions see Additional file 2.
Study selection
Suitable studies were selected in two stages. First, six independent reviewers screened all selected titles and abstracts (JvP, JV, EH, KN, PB, SA). If there was consensus that a study was unsuitable for inclusion, it was excluded. Next, the full-text articles were screened independently by two authors and included if both authors agreed. If needed, the article was discussed with the third reviewer until consensus was reached.
Data extraction and quality analysis
After selection, data were extracted by one and checked by a second investigator (JvP, JV, EH, KN, PB). For each study, the author, journal, country, city and hospital in which the study was conducted, date of start of inclusion, study population, study groups, type, dose, route of administration of corticosteroids, median time before corticosteroid initiation, duration of administration, primary and secondary outcomes and adverse events at any time point after admission were extracted in a standardized data extraction form (Additional file 2).
For each individual study, the quality was assessed. For RCTs, the risk of bias was assessed on six domains (random sequence generation, concealment of allocation, blinding, selective outcome reporting, incomplete outcome data and other) [29, 30]. The Newcastle Ottawa Scale was used for validity assessment of observational studies [31, 32]. The NOS score ranges from 0 (low quality) to 9 (high quality) points.
Data analysis and reporting
For the effect of corticosteroids on mortality, a pooled estimate was calculated and graphically summarized in a forest plot. Data from observational studies were analyzed separately from the RCTs, and both the separate results and the overall combined outcomes were calculated and summarized in the plot. When available, the adjusted odds ratio (OR) or relative risk (RR) from the cohort studies were used for pooling to reduce confounding. Since the endpoint (mortality) occurred relative infrequently, the OR will be close to the RR and therefore we decided to pool both RR and OR estimates of the individual studies [33]. Furthermore, a pooled estimate was calculated and graphically summarized in a forest plot for need for mechanical ventilation.
To allow studies to have a different underlying effect, a random effects model was used. I2 statistics was used to quantify heterogeneity. Furthermore, for the pooled estimate of effect on mortality, tau2 was used to assess the variance of the true effects. The GRADE approach was used to assess the quality of the evidence for the effect of corticosteroids on mortality. STATA 16.0 was used to perform data analysis.
Results
Study selection
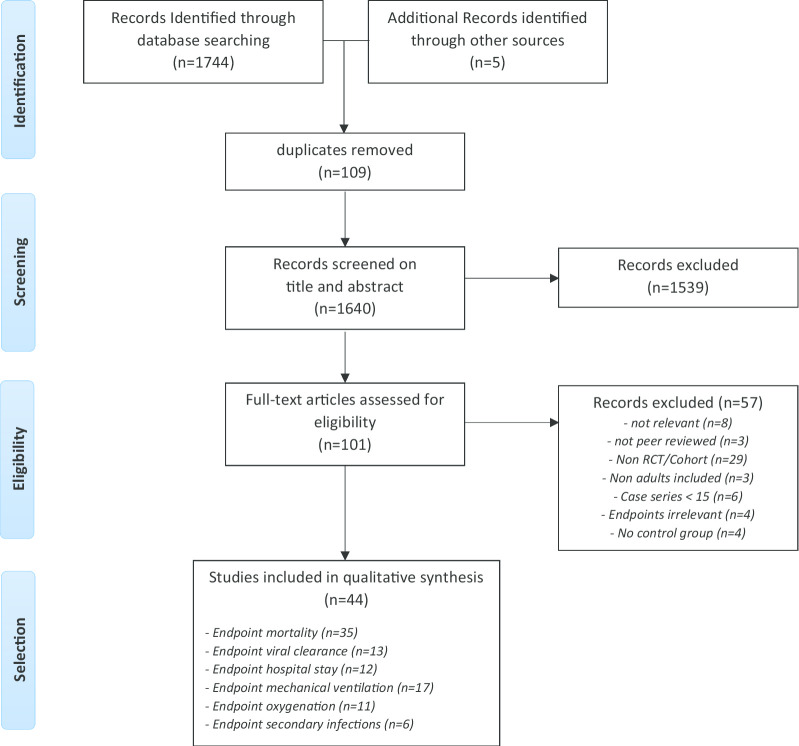
Our search yielded 1640 unique studies. After qualification of title and abstract, 101 studies were selected for full review. Based on exclusion criteria, 57 additional studies were excluded (references in Additional file 3). The remainder of 44 studies, comprising 20.197 patients, was included in this systematic review and meta-analysis. (Fig. 1).
Fig. 1.
Flowchart article selection.docx
Study characteristics (Table 1 and Additional file 4)
Table 1.
Study characteristics
| Author | Reference | Study type | Type—dose c corticosteroids | Sample size | CoVID—Study population | Reporting outcomea | Quality scoreδ (Risk of bias or NOS) | Main findings | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | V | H | R | O | I | |||||||||
| 1 | Angus | 34 | REMAPt b | Hydrocortisone < 1 mg/kg ED | 403 | ICU patients | x | x | x | Risk of biasd | Two hydorcoritsone dosing resulted high probabilities of superiority with regard to the odds of improvement in organ support–free days within 21 days, compared to standard of care | |||
| 2 | Bani-Sadr | 39 | Cohort with historical controls | Prednisolone or Methylprednisolone ≥ 1 mg/kg ED | 319 | Hospitalized patients | x | x | x | 4 | Addition of corticosteroids to our institution’s COVID-19 treatment protocol was associated with a significant reduction in hospital mortality in the “after” period | |||
| 3 | Cao | 80 | Retrospective Observational | Unknown | 102 | Hospitalized patients | x | 5 | Patient characteristics seen more frequently in those who died were development of systemic complications following onset of the illness and the severity of disease requiring admission to the ICU | |||||
| 4 | Chen Zu | Retrospective Observational | Unknown | 267 | Hospitalized patients | x | x | 7 | Corticosteroid treatment is associated with prolonged viral RNA shedding and should be used with caution | |||||
| 5 | Chroboczek | 72 | Retrospective Observational | Unknown | 70 | Hospitalized patients | x | 6 | Corticosteroids therapy affected the risk of intubation with a risk difference of − 47.1% (95% CI − 71.8 to − 22.5) | |||||
| 6 | Dequin | 35 | Randomized controlled trial | Methylprednisolone or hydrocortisone < 1 mg/kg ED | 149 | ICU patients with respiratory failure | x | x | x | x | Risk of Biasd | Low-dose hydrocortisone, compared with placebo, did not significantly reduce treatment failure (defined as death or persistent respiratory support) at day 21 in critically ill patients | ||
| 7 | Fadel | 38 | Quasi experimental | Methylprednisolone ≥ 1 mg/kg ED | 213 | Moderate-to-severe CoVID patients | x | x | x | 6 | An early short course of methylprednisolone in patients with moderate-to-severe COVID-19 reduced escalation of care and improved clinical outcomes | |||
| 8 | Fang Mei | 40 | Retrospective Observational | Methylprednisolone < 1 mg/kg ED | 78 | Hospitalized patients | x | 5 | Low-dose corticosteroid therapy may not delay viral clearance in patients with COVID-19 | |||||
| 9 | Feng Ling | 66 | Retrospective Observational | Unknown | 476 | Hospitalized patients | x | x | 5 | Differences in AT II receptor inhibitors use were associated with different severities of disease. Multiple lung lobes involvement and pleural effusion were associated with the severity of COVID-19. Advanced age (> 75 yr) was a risk factor for mortality | ||||
| 10 | Fernandez | 41 | Retrospective Observational | Methylprednisolone ≥ 1 mg/kg ED | 463 | Patients with ARDS hyperinflammation | x | x | 5 | Glucocorticoid use is associated with increased survival and improved mortality rates in severe CoVID-19 patients | ||||
| 11 | Gazzaruso | 42 | Retrospective Observational | Methylprednisolone or prednisone < 1 mg/kg ED | 219 | Hospitalized patients | x | x | 3 | Antirheumatic drugs, probably steroids included, may modulate inflammation and avoid a hyperinflammation that leads to severe complications and death in subjects with COVID-19 | ||||
| 12 | Gong Guan | 43 | Retrospective Observational | Methylprednisolone ≥ 1 mg/kg ED | 34 | Hospitalized Patients < 50 years | x | x | 6 | Corticosteroids therapy can effectively release COVID‐19 symptoms, improve oxygenation and prevent disease progression. However, it can prolong the negative conversion of nucleic acids | ||||
| 13 | Horby | 23 | Randomized controlled trial | Dexamethasone < 1 mg/kg ED | 6425 | Hospitalized patients | x | x | x | Risk of biasd | The use of dexamethasone resulted in lower 28-day mortality among those who were receiving either invasive mechanical ventilation or oxygen alone at randomization but not among those receiving no respiratory support | |||
| 15 | Hu Wang | 44 | Retrospective Observational | Prednisolone or methylprednisolone > 1 mg/kg ED | 308 | Hospitalized patients | x | x | 4 | Glucocorticoid therapy did not significantly influence the outcomes nor the adverse events of COVID-19 pneumonia | ||||
| 16 | Huang Song | 45 | Retrospective Observational | Methylprednisolone 2 study groups: High: ≥ 1 mg/kg ED Low: < 1 mg/kg ED | 64 | Hospitalized patients | x | 4 | There were no significant differences in the duration of severe illness or the number of days on high level respiratory support between low-dose and high-dose methylprednisolone group. The mean number of days in the hospital was higher in the high-dose group | |||||
| 14 | Huang Yang | 81 | Retrospective Observational | Unknown | 60 | Severe CoVID patients | x | 5 | There were no statistically significant differences in immunoglobulin therapy and GCs therapy between the improvement and deterioration subgroups | |||||
| 17 | Jeronimo | 36 | Randomized controlled trial | Methylprednisolone < 1 mg/kg ED | 393 | Hospitalized patients | x | x | x | x | Risk of biasd | Results showed no overall reduction in mortality in 28 days. Patients over 60 years presented a lower mortality in a subgroup analysis | ||
| 18 | Keller | 73 | Retrospective Observational | Unknown | 1806 | Early hospitalized patients | x | x | 6 | In high CRP group, glucocorticoids show significantly reduced risk of mortality or mechanical ventilation (odds ratio, 0.23; 95% CI, 0.08–0.70). In low CRP group, glucocorticoids were associated with significantly increased risk of mortality or mechanical ventilation (OR, 2.64; 95% CI,1.39–5.03) | ||||
| 19 | Li Hu | 46 | Retrospective Observational | Methylprednisolone high and low ED | 203 | Hospitalized patients | x | 5 | A dose response relation is suggested for corticosteroids on viral shedding. In addition, high-dose but not low-dose corticosteroids were found to potentially increase mortality in severe patients | |||||
| 20 | Li Li | 47 | Retrospective Observational | Methylprednisolone or prednisone < 1 mg/kg ED | 475 | Non-severe CoVID patients | x | x | x | x | 5 | Early, low-dose, and short-term corticosteroids therapy was associated with worse clinical outcomes | ||
| 21 | Li Zhou | 48 | Retrospective Observational | Methylprednisolone > 1 mg/kg ED | 187 | Radiologically progressive CoVID patients | x | x | 6 | Short-term, low-to-moderate-dose corticosteroids benefits patients with LDH levels of less than two times the ULN, who may be in the early phase of excessive inflammation | ||||
| 22 | Lui Fang | 49 | Retrospective Observational | Methylprednisolone ≥ 1 mg/kg ED | 101 | Hospitalized patients | x | 3 | The majority of patients present primarily with fever and typical manifestations on chest imaging. Middle-aged and elderly patients with underlying comorbidities are susceptible to respiratory failure and may have a poorer prognosis | |||||
| 23 | Liu Zhang | 81 | Retrospective Observational | Unknown | 1190 | Hospitalized patients | x | 5 | Treatment with glucocorticoids increased the risk of progression from not severe to severe disease (OR 3.79, 95% CI 2.39–6.01) | |||||
| 24 | Liu Zheng | 50 | Retrospective Observational | Methylprednisolone ≥ 1 mg/kg ED | 101 | Hospitalized patients | x | x | 5 | Timely and appropriate application of methylprednisolone in severe and critical patients may improve outcomes and lung function without negative impacts on specific SARS-CoV-2 IgG production | ||||
| 25 | Lu Chen | 51 | Retrospective Observational | Methylprednisolone, hydrocortisone or dexamethasone > 1 mg/kg ED | 244 | Hospitalized patients | x | x | 7 | Limited effect of corticosteroid therapy could pose to overall survival of critically ill patients with COVID-19. Given the adverse effects, corticosteroid therapy must be commenced with caution, and prudent dosage should be promoted under certain circumstances | ||||
| 26 | Ma Qi | 52 | Retrospective Observational |
Methylprednisolone 2 study groups: High: ≥ 1 mg/kg ED Low: < 1 mg/kg ED |
72 | Severe and critical patients | x | x | x | x | 6 | Corticosteroids cannot reduce the hospital mortality and is not associated with delayed viral clearance, but it could relieve the inflammatory storm and improve clinical symptoms in brief. Patients with severe COVID-19 could benefit from low-dose corticosteroids | ||
| 27 | Ma Zeng | 53 | Retrospective Observational | Methylprednisolone ≥ 1 mg/kg ED | 450 | Severe and non-severe patients | x | x | x | x | x | 4 | Corticosteroids use may be accompanied by increased use of antibiotics, longer hospitalization, and prolonged viral shedding | |
| 28 | Majmundar | 54 | Retrospective Observational |
Prednisolone, dexamethasone, methylprednisolone > 1 mg/kg ED |
205 | Hospitalized patients | x | x | x | x | 6 | Corticosteroids were associated with a significantly lower risk of the ICU transfer, intubation, or in-hospital death, | ||
| 29 | Mikulska | 55 | Retrospective Observational | Methylprednisolone high and low ED | 215 | Hospitalized non-intubated patients | x | x | 6 | Early adjunctive treatment with tocilizumab, methylprednisolone or both may improve outcomes in non-intubated patients | ||||
| 30 | Nelson | 56 | Retrospective Observational | Methylprednisolone ≥ 1 mg/kg ED | 117 | ICU patients on mechanical ventilation | x | x | x | 8 | Methylprednisolone was associated with increased ventilator-free days and higher probability of extubation in a propensity-score matched cohort | |||
| 31 | Rodriquez | 57 | Retrospective Observational | Methylprednisolone ≥ 1 mg/kg ED | 1014 | Hospitalized patients | x | x | x | 7 | Tocilizumab should be prioritized for being tested in randomized trials targeting patients with data suggestive of a hyperinflammatory state. The results for PDC were less consistent but are also encouraging | |||
| 32 | Rubio | 68 | Retrospective Observational | Unknown | 92 | ICU and general ward patients | x | x | 5 | The early use of GC pulses could reduce the use of tocilizumab and might decrease events such as intubation and death | ||||
| 33 | Salton | 58 | Retrospective Observational | Methylprednisolone ≥ 1 mg/kg ED | 173 | ARDS patients | x | x | x | 8 | Per-protocol administration of prolonged low-dose methylpred-nisolone treatment is associated with a significantly lower hazard of death, reduced ICU burden and decreased ventilator dependence | |||
| 34 | Shen Zheng | 59 | Retrospective Observational | Methylprednisolone unknown dose | 325 | Hospitalized patients | x | 4 | COVID-19 cases in Shanghai were imported. Rapid identification and effective control measures helped to contain the outbreak and prevent community transmission | |||||
| 35 | Shi Wu | 71 | Retrospective Observational | Unknown | 99 | Hospitalized patients | x | 4 | SARS-CoV-2 RNA clearance time was associated with sex, disease severity and lymphocyte function. The current antiviral protocol and low-to-moderate dosage of corticosteroid had little effect on the duration of viral excretion | |||||
| 36 | Tomazini | 37 | Randomized controlled trial |
Dexamethasone > 1 mg/kg ED |
299 | ICU patients with moderate-to-severe ARDS | x | x | x | Risk of biasd | Dexamethasone plus standard care compared with standard care alone resulted in a significant increase in the number of ventilator-free days (days alive and free of mechanical ventilation) over 28 days | |||
| 37 | Wang Jiang | 60 | Retrospective Observational |
Methylprednisolone > 1 mg/kg ED |
46 | Severe hospitalized patients | x | x | x | 7 | early, low-dose and short-term application of methylprednisolone was associated with better clinical outcomes in severe CoVID-19 patients and should be considered before onset of ARDS | |||
| 38 | Wang Yang | 67 | Retrospective Observational | Unknown | 69 | Hospitalized patients | x | 4 | COVID-19 shows frequently fever, dry cough, and increase of inflammatory cytokines, and induced a mortality rate of 7.5%. Older patients or those with comorbidities are at higher risk of death | |||||
| 38 | Wang Zhang | 69 | Retrospective Observational | Unknown | 548 | Not Reported | x | 6 | Low-dose or no glucocorticoid treatment was associated with a lower hazard compared with high-dose treatment (≥ 1 mg/kg) for 15 days in hospital death | |||||
| 40 | Wu Chen | 61 | Retrospective Observational | Methylprednisolone unknown dose | 201 | Hospitalized patients | x | 4 | Treatment with methylprednisolone may be beneficial for patients who develop ARDS | |||||
| 41 | Wu Huang | 62 | Retrospective Observational |
Methylprednisolone < 1 mg/kg ED |
1763 | Severe or critical patients | x | 7 | Corticosteroid use was not associated with beneficial effect in reducing in-hospital mortality for severe or critical cases in Wuhan | |||||
| 42 | Xu Chen | 63 | Retrospective Observational |
Methylprednisolone < 1 mg/kg ED |
113 | Hospitalized patients | x | x | 5 | Prolonged SARS-CoV-2 RNA shedding was associated with male sex (P = .009), old age (P = .033), concomitant hypertension (P = .009), delayed admission to hospital after illness onset (P = .001), severe illness at admission (P = .049), invasive mechanical ventilation (P = .006) and corticosteroid treatment (P = .025) | ||||
| 43 | Yang Lipes | 64 | Retrospective Observational |
Methylprednisolone, hydrocortisone or dexamethasone > 1 mg/kg ED |
15 | ICU patients | x | x | 6 | Possible short-term clinical improvements with corticosteroid. Emphasis the urgent need for high-quality studies on steroids and outcome in critically ill COVID-19 patients | ||||
| 44 | Zha Li | 65 | Retrospective Observational |
Methylprednisolone < 1 mg/kg ED |
31 | Hospitalized patients | x | x | x | 5 | No evidence of clinical benefit of corticosteroids was found for those without acute respiratory distress syndrome. Virus clearance may be slower in people with chronic HBV infections | |||
aM = mortality; V = viral clearance; H = length of hospital stay; R = mechanical ventilator/respirator; O = oxygenation; I = secondary infections
bRandomized Embedded Multifactorial Adaptive Platform trial
cED = Prednisolone Equivalent Dose
d Newcastle Ottawa Scale (N.O.S.) for retrospective observational studies. Risk of bias (R.O.B.) for randomized controlled trials: see Fig. 2
Thirty-one of the 44 studies originated in China, 11 in Europe, five in North America, two in South America and one study were multi-continental. The inclusion period of patients ranged from late December 2019 until August 20, 2020. The majority of studies were retrospective observational studies (37/44), five were RCTs [23, 34–37], and there were two studies with historical controls [38, 40]. The study population varied from hospitalized patients (28/44) to patients admitted to the Intensive Care Unit (ICU) (15/44), and one study included discharged patients for viral clearance assessment. The median age of patients ranged from 34 to 75 years.
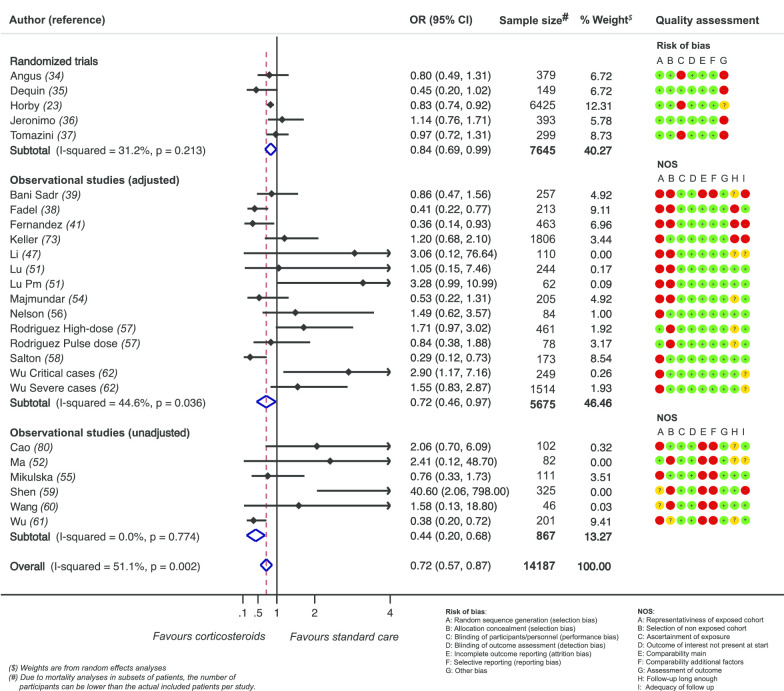
For the observational studies, the median NOS score was 5 (2–8) points (Additional file 5). For the RCT, the risk of bias table is depicted in Fig. 2.
Fig. 2.
Effect of corticosteroids on mortality
Corticosteroid regimen (Table 1 and Additional file 6)
In the 44 studies, very diverse corticosteroid strategies were used. If reported (n = 35), methylprednisolone was the most frequently prescribed (n = 28) [35, 36, 38–65]. Prednisone (n = 5) and dexamethasone (n = 5) and hydrocortisone (n = 4) were also used, some in studies that allowed multiple corticosteroid regimens (n = 9).
The indication to start corticosteroids was described in 12 studies (Additional file 6): In three studies, corticosteroids were started at diagnosis/hospital admission. [38, 41, 56] In five studies, ICU admission or respiratory deterioration were the indications to start, either randomized according to study protocol [23, 34, 35, 37] or not randomized [38, 48, 49, 60, 64].
In 29 studies, the dose of corticosteroids was reported: In 16 studies, an equivalent dose of > 1 mg/kg prednisolone was used [37–39, 41, 43, 44, 48–51, 53, 54, 56–58, 64] and in 11 studies a lower equivalent dose than 1 mg/kg prednisolone [23, 34–36, 40, 42, 47, 52, 62, 63, 65]. In two studies, a low- and high-dose group were present [45, 46]. The duration of therapy varied within a range of 5–10 days, in observational studies frequently dependent on clinical condition of patients.
Effect of steroids on primary and secondary outcomes (Table 2, Additional file 7)
Table 2.
Summary of findings
| Outcomes | Total no events/total no of patients | Relative effect (95% CI) |
No of participants (studies) | Certainty of evidence (Grade a) |
Comments | |
|---|---|---|---|---|---|---|
| Standard care | Corticosteroids | |||||
| Effect of corticosteroids in hospitalized CoVID-19 patients. Intervention: Corticosteroids; Comparison: Standard of Care | ||||||
| In-hospital mortality |
1547/9080 (17.0%) |
1173/5234 (22.4%) |
Estimate 0.72 (0.57–0.87) |
14.187b (22) |
RCT: moderate Non RCT: Very low |
Corticosteroids reduce mortality in CoVID-19 hospitalized patients |
| Requirement of mechanical ventilation |
124/467 (26,6%) |
89/472 (18,9%) |
Estimate 0.70 (0.54–0.91) |
939 (7) |
All studies: Very low |
17 studies reported on mechanical ventilation, but effects could only be quantified in 7 studies |
| Descriptive results: Data too heterogeneous for quantification of effect | ||||||
| Viral Clearance | In corticosteroid group viral clearance time ranged from 10 to 29 days in corticosteroids group and from 8 to 24 days in standard of care group |
2.556 (13) |
0 × RCT 13 × retrospective observational study |
Heterogeneous outcome reporting. Corticosteroids are associated with a probable delay in viral clearance | ||
| Length of hospital stay | Conflicting results both in favor and against the use of corticosteroids |
9.433 (12) |
2 × RCT, 10 × retrospective observational study |
Effect of corticosteroids on length of hospital stay is uncertain | ||
| Mechanical ventilation | In 14 out of 17 studies, corticosteroids therapy is associated with beneficial effects on ventilator-free days, on respiratory failure requiring mechanical ventilation and time on mechanical ventilator |
12.114 (17) |
5 × RCT, 12 × retrospective observational study |
Beneficial effects of corticosteroids on mechanical ventilation different definitions used) | ||
| Oxygenation | Outcome reporting in saturation, p/F ratio and oxygen demand. Conflicting results in favor and against the use of corticosteroids |
3.211 (11) |
1 × RCT, 10 × retrospective observational study |
Outcome definition too heterogeneous to draw conclusions | ||
| Secondary infections | In five out of six studies, secondary infections and antibiotic use are increased |
2.145 (6) |
3 × RCT 3 × retrospective observational study |
Corticosteroids are associated with an increase in infectious complications | ||
a Details on GRADE score are available in Additional file 10
b Due to mortality analyses in subsets of patients, this number of participants is lower than the sum of sample sizes from the included study
Thirty-five of 44 studies reported on Mortality. Thirteen of these could not be integrated in the meta-analysis due to only overall mortality reporting (n = 5), [45, 63, 64, 66, 67] or only descriptive reporting (n = 8), i.e., of a trend toward better outcome (n = 3), [42, 68, 69], no effect (n = 3) [44, 49, 65] or negative effect on outcome (n = 2) [50, 52]. For the remainder of 22 studies, a pooled estimate was calculated and graphically summarized in a forest plot (Fig. 2). The mortality reported in these studies was mainly 28-day mortality (11 studies), in six studies in-hospital mortality of shorter duration and in five studies there was an unreported follow up period (Additional file 7). The overall risk estimate (OR) was 0.72 (95%CI 0.57–0.87), suggesting a beneficial effect of steroids use in COVID-19 patients hospitalized with moderate or severe respiratory failure on mortality. Studies were heterogeneous (overall I2 of 51.1%, p = 0.002) with a between-study variance (tau2) of 0.048. For the subset of RCTs, the risk estimate was 0.84 (95%CI 0.72–0.96) and I2 and tau2 were 31.2% (p = 0.213) and 0.0096, corresponding to less heterogeneity and less between-study variance.
Thirteen of 44 studies reported on viral clearance, which most frequently was defined as two consecutive negative RT-PCR on nasopharyngeal swabs, or a cycle time value of 40 or more. In the corticosteroid group, viral clearance time ranged from 5 to 29 days, in the standard of care group from 8 to 24 days. In nine of 13 studies, viral shedding was delayed in the corticosteroid group. [40, 43, 46, 47, 53, 59, 63, 65, 70] In the other four studies, viral clearance was equal (n = 2) [50, 71] or even better in the corticosteroid group (n = 2) [44, 52]. The numbers are too small to quantify the effect of corticosteroids on viral shedding, or to compare viral shedding duration in subgroups of severity of COVID illness, dose, type or timing of corticosteroids administered. (Additional file 8).
In twelve studies, length of hospital stay was compared in corticosteroid versus non-corticosteroid groups. The outcomes varied between studies: six reported longer hospital stay in the corticosteroid group [36, 47, 53, 56, 66] and five reported the opposite [23, 34, 38, 52, 54] or no effect on hospital stay [58].
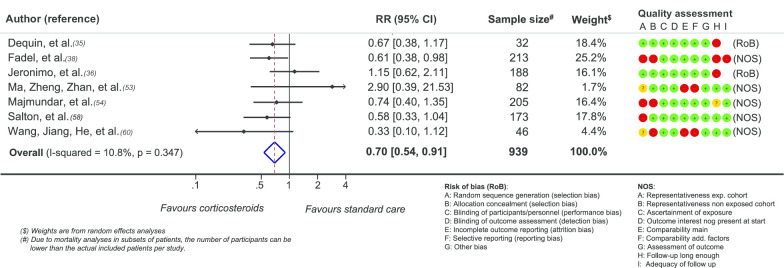
Fourteen of 17 studies reported a positive effect of corticosteroids on ventilator-free days [34, 37, 56], on the number of patient requiring mechanical ventilation for respiratory insufficiency [23, 35, 38, 48, 54, 57, 58, 60, 68, 72] or on the time on mechanical ventilator [52]. In the pooled analyses fewer patients required mechanical ventilation in the corticosteroids group (RR 0.71 (95%CI 0.54–0.97) (Fig. 3) though only seven studies supplied sufficient data for this analysis. Jeronimo and Keller failed to demonstrate significant differences [36, 73] and one study reported the opposite effect [53]. The dose of corticosteroids could not be related to respiratory outcomes.
Fig. 3.
Effect of corticosteroids on need for mechanical ventilation
Eleven studies reported on the effect of corticosteroids on oxygenation. Various definitions were used: liters per minute of oxygen needed, oxygen saturation, PaO2/FiO2 ratio. The effect of corticosteroids on oxygenation was very heterogeneous: In four studies, there was no significant effect [41, 42, 51, 55], in three studies significant improvement was described [50, 60, 64] and in four studies worse outcome was observed. [35, 39, 54, 57]
Six studies addressed secondary infections. More frequently broad spectrum antibiotics were used in the corticosteroid group [39, 47, 53] and more secondary infections or sepsis episodes were described [35, 36]. Only Tomazini found a lower percentage of secondary infections in the corticosteroid group [37]. A dose effect of steroids on development of infections or antibiotic need could not be demonstrated.
Discussion
In this systematic review and meta-analysis on effectiveness and safety of corticosteroids in COVID-19 patients, the pooled estimate of the observational retrospective studies and the RCTs supported the positive effect of corticosteroids therapy on mortality in COVID-19 disease as first reported in the RECOVERY trial. [23] Furthermore, in already respiratory compromised COVID-19 patients, the need for mechanical ventilation was lower in corticosteroid treated COVID-19 patients. And although data in the studies were too sparse to draw any firm conclusions, there might be a signal of delayed viral clearance and an increase in antibiotic use and infections in the corticosteroid group. However, this did not seem to lead to prolonged hospital stay or increased mortality.
Besides reviews extrapolating knowledge on SARS-CoV or MERS-CoV [21] or on non-viral ARDS [4], or combining studies on SARS-CoV and MERS-CoV [2, 18, 74], to our knowledge, only three other meta-analyses on this subject were conducted with the conflicting results. [24, 75, 76] Sarkar et al. found low‐quality evidence with high variability, showing that in patients with COVID‐19 corticosteroids may be associated with an around twofold increase in mortality [75]. Tlayjeh et al. [76] found no significant difference in mortality or mechanical ventilation need, at the cost of a prolonged viral clearance time. The investigators explained that the discordance in studies was due to bias in the large number of non-RCTs. In the third, very robust, prospective meta-analysis of published and pending trials (inclusion has pretty much stopped since the recovery trial was published), Sterne et al. [24], found that in critically ill patients with COVID-19, the administration of systemic corticosteroids, compared with usual care or placebo, was associated with lower 28-day all-cause mortality. A downside of this rather robust study was that almost 60 percent of the population consisted of the RECOVERY study population and a reasonable amount of data was generated from unpublished, unfinished studies.
Compared to these other systematic reviews on corticosteroids and COVID-19, ours was able to include the largest number of studies and COVID-19 patients. Furthermore, we included both observational studies and RCTs to be able to assess adverse effects such as viral clearance and risk of infections. To obtain the highest possible quality, we excluded non-peer reviewed pre-published manuscripts and furthermore, if available, we included adjusted estimates in the meta-analysis, reducing bias by incongruent study groups (Additional file 9).
Our review has several limitations. The first is that we retrospectively registered our systematic review and meta-analysis. Indeed, it is very important, especially in COVID-19 pandemic times with a high number of publications on COVID-19, to register beforehand to avoid redundancy and inefficiency and to prevent flooding. The review arose from a clinical point of view to gather all literature on corticosteroids and COVID-19, as we clinicians were in doubt whom to administer this drug to. Doing so, we thought it would be best to summarize our findings in a review, since we presumed other clinicians would be struggling with the same questions.
Furthermore, most of the included studies were retrospective cohort studies with increased risk of bias and lower level of evidence, as we confirmed by the GRADE classification (Table 2, Additional file 10). Besides that, large heterogeneity in the studies was present (i.e., study population, type, dose, initiation and duration of corticosteroids and outcome measures) and we emphasize that definitions of primary and secondary outcome measures varied substantially per included article and pooled data from this review should be interpreted cautiously. However, we decided beforehand to only include short-term mortality, i.e., 28-day or closely related short-term in-hospital mortality. Furthermore, we decided to carefully note the applied definitions in the studies in our data extraction tables and include only outcomes as defined by the investigators if they filled our inclusions criteria. We agree that the remaining variation in definitions is indeed a drawback of this review. And although the pooled data from this review should therefore be interpreted cautiously, they represent the effect of corticosteroids on short-term 28-day mortality and the pooled estimates for RCTs and adjusted and unadjusted observational studies pointed toward the same direction, i.e., of a beneficial effect. In many studies, confounding by indication was evidently present: two studies described that corticosteroid administration was “at the discretion of the treating physician” [40, 41] and four reported that severe patients were more likely to receive corticosteroid treatment. [40, 49, 60, 66] Many studies had incomplete follow-up and a considerable amount of patients did not reach definite endpoints. However, our conscious exclusion of non-peer-reviewed studies, the focus on a measurable and quantifiable endpoint, and, if possible, inclusion of risk estimates corrected for confounders and propensity matched, increased the validity of the retrospective evidence supporting the RECOVERY trial. Furthermore, from the included studies, 26 originated in China, with 13 from the hotspot regions (Wuhan, Hubei and Shanghai). This might impair generalizability but although overlapping study populations were present within the included studies (see table in Additional file 4.), this was only incidentally the case for secondary outcome measures. For the main outcome, multiple publication bias was unlikely (Additional file 11). Furthermore, 42% of the study population was included from outside China. Moreover, in terms of generalizability, the median age from the included patients in this review ranged from 34 to 72 years. However, data from the CDC state that 42.9% of hospitalized patients in the USA are > 65 years and European numbers from the European Centre for Disease Prevention and Control (ECDC) show that 54.2% hospitalized patients are > 65 years with great variation between countries. [77, 78] Despite aforementioned limitations, still, this systematic review and meta-analysis confirms the conclusion of the meta-analysis of the RCTs that critically ill COVID-19 patients hospitalized for moderate or severe respiratory failure, with or without mechanical ventilation, should receive corticosteroids.
Severe COVID-19 patients are faced with a twofold problem. On the one hand, there is the hyperinflammatory response, resulting in pulmonary thrombosis, extravasation of cell debris and acute lung injury or even ARDS [79]. On the other hand, there is a need to clear the viral infection itself. This primary phenomenon suggests a possible target for corticosteroids [17]. Thus, the confirmation that there is predominantly a beneficial effect of corticosteroids on mortality is congruent with pathophysiological reasoning and prior knowledge. In our study we, found a signal of delayed viral clearance, but data in the studies were too sparse to draw any firm conclusions. Therefore, what is lacking is knowledge on the optimal start of corticosteroid administration after the start of illness, specific subpopulations and type, dose and duration of corticosteroids. RCTs so far reported a strongly beneficial effect on mortality but did not investigate optimal timing and indication of corticosteroid administration [24], and our study was not able to provide an answer to the latter issues, either. Therefore, future research should focus on which patient characteristics, laboratory and radiological markers can be used to guide indication and timing of corticosteroid treatment, particularly in relation to safety (e.g., delayed viral clearance and increased incidence of secondary infections).
Conclusion
Our findings from both observational studies and RCTs confirm a beneficial effect of corticosteroids on short-term mortality and a reduction in the need for mechanical ventilation. And although data in the studies were too sparse to draw any firm conclusions, there might be a signal of delayed viral clearance and an increase in secondary infections related to corticosteroid use. Optimal timing, dose and duration of corticosteroids, in relation to safety, remain subject for further investigation. Since corticosteroids are affordable and easily accessible in healthcare systems quivering under the pressure of the global outbreak of this rapidly spreading coronavirus, this field of research should be a universal priority.
Supplementary information
Additional file 2. Data extraction form.
Additional file 4. Data extraction General Information.
Additional file 6. Data extraction Treatment.
Additional file 7 . Data extraction Outcome.
Additional file 8. Viral Clearance time.
Additional file 9. Mechanical ventilation.
Additional file 10. Grade classification.
Acknowledgements
We would like to express our gratitude to C. Pees, librarian, for her efforts in designing the search strategies used in collecting data. We would also like to express our gratitude to Olaf M. Dekkers for his aid in the statistical analysis, i.e., calculating pooled estimate and constructing the forest plots.
Abbreviations
- ARDS
Acute respiratory distress syndrome
- CDC
Centers for Disease Control and Prevention
- CI
Confidence interval
- COVID-19
Coronavirus disease 2019
- CT
Computed tomography
- ECDC
European Centre for Disease Prevention and Control
- FiO2
Inspiratory oxygen fraction
- HR
Hazard ratio
- ICU
Intensive care Unit
- IQR
Interquartile range
- LOS
Length of stay
- MERS-CoV
Middle East respiratory syndrome coronavirus
- OR
Odds ratio
- NOS
Newcastle Ottawa Scale
- NR
Not reported
- OR
Odds ratio
- PaO2
Arterial oxygen tension
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- RNA
Ribonucleic acid
- RR
Rate ratio
- RT-PCR
Reverse transcription polymerase chain reaction
- SARS-CoV
Severe acute respiratory syndrome coronavirus
- SD
Standard deviation
- Steroids
Glucocorticoids or corticoids
- SpO2
Plasma oxygen saturation
- WHO
World Health Organization
Authors’ contributions
SA created the study project. JvP, JV, PB, EH, KN and SA extracted and analyzed the data. JvP and SA performed the statistical analyses with the aid of OD (in acknowledgements). JvP, PB, EH, KN and SA wrote the draft and all co-authors critically revised the manuscript and approved the final version for publication. All authors have read and approved the final manuscript.
Funding
Not applicable.
Availability of data and materials
Not applicable.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
All persons who meet authorship criteria are listed as authors. The manuscript has been seen and approved by all authors. On behalf of all authors, the corresponding author states that there is no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s13054-020-03400-9.
References
- 1.European Centre for Disease Prevention and Control. Accessed October 14, 2020 at https://www.ecdc.europa.eu/en/geographical-distribution-2019-ncov-cases
- 2.Li H, Chen C, Hu F, et al. Impact of corticosteroid therapy on outcomes of persons with SARS-CoV-2, SARS-CoV, or MERS-CoV infection: a systematic review and meta-analysis. Leukemia. 2020;34(6):1503–1511. doi: 10.1038/s41375-020-0848-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang C, Horby PW, Hayden FG, Gao GF. A novel coronavirus outbreak of global health concern. Lancet. 2020;395(10223):470–473. doi: 10.1016/S0140-6736(20)30185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Villar J, Confalonieri M, Pastores SM, et al. Rationale for prolonged corticosteroid treatment in the acute respiratory distress syndrome caused by coronavirus disease 2019. Crit Care Explor. 2020;2(4):e0111. doi: 10.1097/CCE.0000000000000111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grasselli G, Zangrillo A, Zanella A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region. Italy JAMA. 2020;323(16):1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ziehr DR, Alladina J, Petri CR, et al. Respiratory Pathophysiology of Mechanically Ventilated Patients with COVID-19: A Cohort Study. Am J Respir Crit Care Med. 2020;201(12):1560–1564. doi: 10.1164/rccm.202004-1163LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guan W-j, Ni Z-y, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. New Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jamilloux Y, Henry T, Belot A, et al. Should we stimulate or suppress immune responses in COVID-19? Cytokine and anti-cytokine interventions. Autoimmun Rev. 2020;19(7):102567. doi: 10.1016/j.autrev.2020.102567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Channappanavar R, Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin Immunopathol. 2017;39(5):529–539. doi: 10.1007/s00281-017-0629-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mehta P, McAuley DF, Brown M, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang W, Zhao Y, Zhang F, et al. The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): the perspectives of clinical immunologists from China. Clin Immunol. 2020;214:108393. doi: 10.1016/j.clim.2020.108393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alijotas-Reig J, Esteve-Valverde E, Belizna C, et al. Immunomodulatory therapy for the management of severe COVID-19. Beyond the anti-viral therapy: a comprehensive review. Autoimmun Rev. 2020;19(7):102569. doi: 10.1016/j.autrev.2020.102569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang S, Liu T, Hu Y, et al. Efficacy and safety of glucocorticoids in the treatment of severe community-acquired pneumonia: a meta-analysis. Medicine (Baltimore) 2019;98(26):e16239. doi: 10.1097/MD.0000000000016239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Villar J, Ferrando C, Martínez D, et al. Dexamethasone treatment for the acute respiratory distress syndrome: a multicentre, randomised controlled trial. Lancet Respir Med. 2020;8(3):267–276. doi: 10.1016/S2213-2600(19)30417-5. [DOI] [PubMed] [Google Scholar]
- 18.Singh AK, Majumdar S, Singh R, et al. Role of corticosteroid in the management of COVID-19: A systemic review and a Clinician's perspective. Diabetes Metab Syndr. 2020;14(5):971–978. doi: 10.1016/j.dsx.2020.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Veronese N, Demurtas J, Yang L, et al. Use of corticosteroids in coronavirus disease 2019 pneumonia: a systematic review of the literature. Front Med (Lausanne) 2020;7:170. doi: 10.3389/fmed.2020.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arabi YM, Mandourah Y, Al-Hameed F, et al. Corticosteroid therapy for critically ill patients with Middle East Respiratory Syndrome. Am J Respir Crit Care Med. 2018;197(6):757–767. doi: 10.1164/rccm.201706-1172OC. [DOI] [PubMed] [Google Scholar]
- 21.Russell CD, Millar JE, Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020;395(10223):473–475. doi: 10.1016/S0140-6736(20)30317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stockman LJ, Bellamy R, Garner P. SARS: systematic review of treatment effects. PLoS Med. 2006;3(9):e343. doi: 10.1371/journal.pmed.0030343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horby P, Lim WS, Emberson JR, et al. Dexamethasone in hospitalized patients with covid-19: preliminary report. N Engl J Med. 2020 doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sterne JAC, Murthy S, Diaz JV, et al. Association between administration of systemic corticosteroids and mortality among critically Ill patients with COVID-19: a meta-analysis. JAMA. 2020. [DOI] [PMC free article] [PubMed]
- 25.World Health Organization. Accessed October 14, 2020 at https://www.who.int/publications/i/item/clinical-management-of-covid-19
- 26.Alhazzani W, Møller MH, Arabi YM, et al. Surviving Sepsis Campaign: guidelines on the management of critically ill adults with Coronavirus Disease 2019 (COVID-19) Intensive Care Med. 2020;46(5):854–887. doi: 10.1007/s00134-020-06022-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4(1):1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sterne JA, Gavaghan D, Egger M. Publication and related bias in meta-analysis: power of statistical tests and prevalence in the literature. J Clin Epidemiol. 2000;53(11):1119–1129. doi: 10.1016/S0895-4356(00)00242-0. [DOI] [PubMed] [Google Scholar]
- 29.Dekkers OM. Meta-analysis: key features, potentials and misunderstandings. Res Pract Thromb Haemost. 2018;2(4):658–663. doi: 10.1002/rth2.12153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;18(343):d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Coding manual for case-control studies. GA Wells BS, D O'Connell, J Peterson, et al. Accessed October 18, 2020 at http://www.ohri.ca/programs/clinical_epidemiology/nos_manual.pdf
- 32.Newcastle-Ottowa quality assessment scale case control studies. GA Wells BS, D O'Connell, J Peterson, et al. Accessed at Octobre 18, 2020 at http://www.ohri.ca/programs/clinical_epidemiology/nosgen.pdf
- 33.Egger M, Smith GD, Phillips AN. Meta-analysis: principles and procedures. BMJ. 1997;315:1533–1537. doi: 10.1136/bmj.315.7121.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Angus DC, Derde L, Al-Beidh F, et al. Effect of hydrocortisone on mortality and organ support in patients with severe COVID-19: the REMAP-CAP COVID-19 corticosteroid domain randomized clinical trial. JAMA. 2020;324(13):1317–1329. doi: 10.1001/jama.2020.17022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dequin PF, Heming N, Meziani F, et al. Effect of hydrocortisone on 21-day mortality or respiratory support among critically ill patients with COVID-19: a randomized clinical trial. JAMA. 2020;324(13):1–9. doi: 10.1001/jama.2020.16761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jeronimo CMP, Farias MEL, Val FFA, et al. Methylprednisolone as adjunctive therapy for patients hospitalized with COVID-19 (metcovid): a randomised, double-blind, phase IIb, placebo-controlled trial. Clin Infect Dis. 2020. [DOI] [PMC free article] [PubMed]
- 37.Tomazini BM, Maia IS, Cavalcanti AB, et al. Effect of dexamethasone on days alive and ventilator-free in patients with moderate or severe acute respiratory distress syndrome and COVID-19: the CoDEX randomized clinical trial. JAMA. 2020. [DOI] [PMC free article] [PubMed]
- 38.Fadel R, Morrison AR, Vahia A, et al. Early short course corticosteroids in hospitalized patients with COVID-19. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bani-Sadr F, Hentzien M, Pascard M, et al. Corticosteroid therapy for patients with COVID-19 pneumonia: a before-after study. Int J Antimicrob Agents. 2020;56(2):106077. doi: 10.1016/j.ijantimicag.2020.106077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fang X, Mei Q, Yang T, et al. Low-dose corticosteroid therapy does not delay viral clearance in patients with COVID-19. J Infect. 2020;81(1):147–178. doi: 10.1016/j.jinf.2020.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fernández-Cruz A, Ruiz-Antorán B, Muñoz-Gómez A, et al. A retrospective controlled cohort study of the impact of glucocorticoid treatment in SARS-CoV-2 infection mortality. Antimicrob Agents Chemother. 2020;64:9. doi: 10.1128/AAC.01168-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gazzaruso C, Carlo Stella N, Mariani G, et al. Impact of anti-rheumatic drugs and steroids on clinical course and prognosis of COVID-19. Clin Rheumatol. 2020;39(8):2475–2477. doi: 10.1007/s10067-020-05239-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gong Y, Guan L, Jin Z, et al. Effects of methylprednisolone use on viral genomic nucleic acid negative conversion and CT imaging lesion absorption in COVID-19 patients under 50 years old. J Med Virol. 2020. [DOI] [PMC free article] [PubMed]
- 44.Hu Y, Wang T, Hu Z, et al. Clinical efficacy of glucocorticoid on the treatment of patients with COVID-19 pneumonia: a single-center experience. Biomed Pharmacother. 2020;130:110529. doi: 10.1016/j.biopha.2020.110529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang H, Song B, Xu Z, et al. Predictors of coronavirus disease 2019 severity: a retrospective study of 64 cases. Jpn J Infect Dis. 2020. [DOI] [PubMed]
- 46.Li Q, Li W, Jin Y, et al. Efficacy evaluation of early, low-dose, short-term corticosteroids in adults hospitalized with non-severe COVID-19 pneumonia: a retrospective cohort study. Infect Dis Therapy. 2020. [DOI] [PMC free article] [PubMed]
- 47.Li S, Hu Z, Song X. High-dose but not low-dose corticosteroids potentially delay viral shedding of patients with COVID-19. Clin Infect Dis. 2020. [DOI] [PMC free article] [PubMed]
- 48.Li Y, Zhou X, Li T, et al. Corticosteroid prevents COVID-19 progression within its therapeutic window: a multicentre, proof-of-concept, observational study. Emerg Microbes Infect. 2020;9(1):1869–1877. doi: 10.1080/22221751.2020.1807885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu J, Zheng X, Huang Y, et al. Successful use of methylprednisolone for treating severe COVID-19. J Allergy Clin Immunol. 2020;146(2):325–327. doi: 10.1016/j.jaci.2020.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu K, Fang Y-Y, Deng Y, et al. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chin Med J (Engl) 2020;133(9):1025–1031. doi: 10.1097/CM9.0000000000000744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lu X, Chen T, Wang Y, et al. Adjuvant corticosteroid therapy for critically ill patients with COVID-19. Crit Care. 2020;24(1):241. doi: 10.1186/s13054-020-02964-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ma Q, Qi D, Deng XY, et al. Corticosteroid therapy for patients with severe novel Coronavirus disease 2019. Eur Rev Med Pharmacol Sci. 2020;24(15):8194–8201. doi: 10.26355/eurrev_202008_22508. [DOI] [PubMed] [Google Scholar]
- 53.Ma Y, Zeng H, Zhan Z, et al. Corticosteroid use in the treatment of COVID-19: a multicenter retrospective study in hunan, China. Front Pharmacol. 2020;11:1198. doi: 10.3389/fphar.2020.01198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Majmundar M, Kansara T, Lenik JM, et al. Efficacy of corticosteroids in non-intensive care unit patients with COVID-19 pneumonia from the New York Metropolitan region. PLoS ONE. 2020;15(9):e0238827. doi: 10.1371/journal.pone.0238827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mikulska M, Nicolini LA, Signori A, et al. Tocilizumab and steroid treatment in patients with COVID-19 pneumonia. PLoS ONE. 2020;15(8):e0237831. doi: 10.1371/journal.pone.0237831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nelson BC, Laracy J, Shoucri S, et al. Clinical outcomes associated with methylprednisolone in mechanically ventilated patients with COVID-19. Clin Infect Dis. 2020. [DOI] [PMC free article] [PubMed]
- 57.Rodríguez-Baño J, Pachón J, Carratalà J, et al. Treatment with tocilizumab or corticosteroids for COVID-19 patients with hyperinflammatory state: a multicentre cohort study (SAM-COVID-19). Clin Microbiol Infect. 2020. [DOI] [PMC free article] [PubMed]
- 58.Salton F, Confalonieri P, Meduri GU, et al. Prolonged low-dose methylprednisolone in patients with severe COVID-19 pneumonia. Open Forum Infect Dis. 2020;7:10. doi: 10.1093/ofid/ofaa421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shen Y, Zheng F, Sun D, et al. Epidemiology and clinical course of COVID-19 in Shanghai. China Emerg Microbes Infect. 2020;9(1):1537–1545. doi: 10.1080/22221751.2020.1787103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang Y, Jiang W, He Q, et al. A retrospective cohort study of methylprednisolone therapy in severe patients with COVID-19 pneumonia. Signal Transduct Target Ther. 2020;5(1):57. doi: 10.1038/s41392-020-0158-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan. China JAMA Intern Med. 2020;180(7):934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wu J, Huang J, Zhu G, et al. Systemic corticosteroids and mortality in severe and critical COVID-19 patients in Wuhan. China. J Clin Endocrinol Metab. 2020;105:12. doi: 10.1210/clinem/dgz136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xu K, Chen Y, Yuan J, et al. Factors associated with prolonged viral RNA shedding in patients with coronavirus disease 2019 (COVID-19) Clin Infect Dis. 2020;71(15):799–806. doi: 10.1093/cid/ciaa351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yang SS, Lipes J. Corticosteroids for critically ill COVID-19 patients with cytokine release syndrome: a limited case series. Can J Anaesth. 2020;67(10):1462–1464. doi: 10.1007/s12630-020-01700-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zha L, Li S, Pan L, et al. Corticosteroid treatment of patients with coronavirus disease 2019 (COVID-19) Med J Aust. 2020;212(9):416–420. doi: 10.5694/mja2.50577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Feng Y, Ling Y, Bai T, et al. COVID-19 with different severities: a multicenter study of clinical features. Am J Respir Crit Care Med. 2020;201(11):1380–1388. doi: 10.1164/rccm.202002-0445OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang Z, Yang B, Li Q, et al. Clinical features of 69 cases with coronavirus disease 2019 in Wuhan. China Clin Infect Dis. 2020;71(15):769–777. doi: 10.1093/cid/ciaa272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Callejas Rubio JL, Luna Del Castillo JD, de la Hera FJ, et al. Effectiveness of corticoid pulses in patients with cytokine storm syndrome induced by SARS-CoV-2 infection. Med Clin (Barc) 2020;155(4):159–161. doi: 10.1016/j.medcli.2020.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang K, Zhang Z, Yu M, et al. 15-day mortality and associated risk factors for hospitalized patients with COVID-19 in Wuhan, China: an ambispective observational cohort study. Intensive Care Med. 2020;46(7):1472–1474. doi: 10.1007/s00134-020-06047-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen X, Zhu B, Hong W, et al. Associations of clinical characteristics and treatment regimens with the duration of viral RNA shedding in patients with COVID-19. Int J Infect Dis. 2020;98:252–260. doi: 10.1016/j.ijid.2020.06.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shi D, Wu W, Wang Q, et al. Clinical characteristics and factors associated with long-term viral excretion in patients with severe acute respiratory syndrome coronavirus 2 infection: a single-Center 28-day study. J Infect Dis. 2020;222(6):910–918. doi: 10.1093/infdis/jiaa388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chroboczek T, Lacoste M, Wackenheim C, et al. Corticosteroids in patients with COVID-19: what about the control group? Clin Infect Dis. 2020. [DOI] [PMC free article] [PubMed]
- 73.Keller MJ, Kitsis EA, Arora S, et al. Effect of systemic glucocorticoids on mortality or mechanical ventilation in patients with COVID-19. J Hosp Med. 2020;15(8):489–493. doi: 10.12788/jhm.3497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yang Z, Liu J, Zhou Y, et al. The effect of corticosteroid treatment on patients with coronavirus infection: a systematic review and meta-analysis. J Infect. 2020;81(1):e13–e20. doi: 10.1016/j.jinf.2020.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sarkar S, Khanna P, Soni KD. Are the steroids a blanket solution for COVID-19? A systematic review and meta-analysis. J Med Virol. 2020. [DOI] [PubMed]
- 76.Tlayjeh H, Mhish OH, Enani MA, et al. Association of corticosteroids use and outcomes in COVID-19 patients: a systematic review and meta-analysis. Journal of Infection and Public Health. 2020. [DOI] [PMC free article] [PubMed]
- 77.COVID-NET. Centers for Disease Control and Prevention. Accessed at October 14, 2020 at https://gis.cdc.gov/grasp/COVIDNet/COVID19_5.html
- 78.European Centre for Disease Prevention and Control. Accessed at October 14, 2020 at https://qap.ecdc.europa.eu/public/extensions/COVID-19/COVID-19.html
- 79.Polak SB, Van Gool IC, Cohen D, et al. A systematic review of pathological findings in COVID-19: a pathophysiological timeline and possible mechanisms of disease progression. Mod Pathol. 2020 doi: 10.1038/s41379-020-0603-3:1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cao J, Tu WJ, Cheng W, et al. Clinical features and short-term outcomes of 102 patients with coronavirus disease 2019 in Wuhan. China Clin Infect Dis. 2020;71(15):748–755. doi: 10.1093/cid/ciaa243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Huang M, Yang Y, Shang F, et al. Clinical characteristics and predictors of disease prgression in severe patients with COVID-19 infection in jiangsu province, China: a discriptive study. Am J Med Sci. 2020;360(2):120–128. doi: 10.1016/j.amjms.2020.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liu J, Zhang S, Wu Z, et al. Clinical outcomes of COVID-19 in Wuhan, China: a large cohort study. Ann Intensive Care. 2020;10(1):99. doi: 10.1186/s13613-020-00706-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 2. Data extraction form.
Additional file 4. Data extraction General Information.
Additional file 6. Data extraction Treatment.
Additional file 7 . Data extraction Outcome.
Additional file 8. Viral Clearance time.
Additional file 9. Mechanical ventilation.
Additional file 10. Grade classification.
Data Availability Statement
Not applicable.