Abstract
Monoclonal immunoglobulins (MIg) may play a causal role in C3G by impairing regulation of the alternative pathway of complement. Ninety-five C3G patients were tested for MIg of which 36 (37.9%) were positive. Mean age at diagnosis was 60 years; among patient’s ≥50 years 65.1% had a MIg. At presentation, median serum creatinine and proteinuria were 1.9 mg/dL and 3.0 g/24 hours. Hematuria was present in 32 (88.9%) patients. Twelve (34.3%) patients had low C3 levels. C3Nef was detected in 45.8% patients; pathogenic variants in complement protein genes were rare. Hematologic evaluation revealed MGRS in 26, MM in 5, SMM in 2, CLL in 1, lymphoma in 1, and type I cryoglobulin in 1patient. After a median follow-up of 43.6 months, median serum creatinine and proteinuria was 1.4 mg/dL and 0.8g/24 hours. Nine (25%) patients developed ESRD. Sixteen patients received MIg-targeted treatment, 17 patients received non-targeted treatment; 3 patients were managed conservatively. Of the 16 patients receiving MIg-targeted treatment 10 achieved complete/very good/partial hematologic response, of these 7 (70%) also achieved complete/partial/stable renal response. Five (31.3%) patients receiving targeted treatment did not achieve hematologic response; none of these had a renal response. Patients receiving targeted treatment were more likely to have MM/SMM, while patients receiving non-targeted treatment were more likely to have MGRS. To summarize, C3G with MIg is seen in older patients. C3Nef is the most common autoantibody detected in these patients. MIg-targeted treatment may result in remission and stabilization of the kidney function in a subset of these patients.
Keywords: C3 glomerulopathy, C3 glomerulonephritis, dense deposit disease, monoclonal Ig, alternative pathway of complement
C3 glomerulopathy (C3G) is a rare disease entity that is characterized by accumulation of complement factors in the glomeruli due to over activation and abnormal regulation of the alternative pathway of complement.1-4 The deposition of complement factors drives glomerular inflammation resulting in a proliferative glomerulonephritis.5,6 Abnormal control of the alternative pathway of complement may be due to acquired or genetic abnormalities of the complement regulating proteins. The characteristic kidney biopsy finding is that of bright glomerular staining for C3 with minimal or no immunoglobulins on immunofluorescence studies. C3G is further subdivided into C3 glomerulonephritis (C3GN) and dense deposit disease (DDD) based on the ultrastructural findings. 1,7 C3GN is characterized by mesangial and capillary wall electron dense deposits, whereas DDD is characterized by dense osmiophilic intramembranous and mesangial deposits.
One of the abnormalities commonly associated with C3G is the detection of a monoclonal gammopathy.8-13 Monoclonal gammopathies consist of a heterogeneous group of disorders characterized by clonal proliferation of immunoglobulin (Ig) producing B-lymphocytes or plasma cells.14-17 The clinical spectrum of monoclonal gammopathies is wide and includes classic malignancies such as multiple myeloma and Waldenström macroglobulinemia; clonal, but nonmalignant, paraprotein related disorders such as light chain (AL) amyloidosis; and the incidentally detected premalignant plasma cell dyscrasia termed monoclonal gammopathy of undetermined significance (MGUS).15 The terminology MGRS (monoclonal gammopathy of renal significance) is used to denote an MGUS that is associated with a renal disease.18
Most of the reports on the clinical characteristics, pathology, complement evaluation, treatment and outcomes of C3G associated with monoclonal Ig are based on individual cases or small series of C3GN or DDD patients, except for a recent series by Chauvet et al. 2,13,19-22 In this study, we describe the clinical and pathology findings, evaluation of hematologic disease, complement abnormalities, treatment and renal outcomes of C3G associated with monoclonal gammopathy in a large series of patients seen over a 10-year period (2007-2016) at the Mayo Clinic, Rochester, MN.
RESULTS
Of the 114 patients with C3G seen at our institution, 95 (83.3%) patients were evaluated for a monoclonal Ig. Of these, 36 (37.9%) were positive for a monoclonal Ig, 32 had C3 glomerulonephritis (C3GN) and 4 had dense deposit disease (DDD). Among patients ≥50 years (n=43), 28 (65.1%) were positive for monoclonal Ig. The details of the 95 patients evaluated for monoclonal Ig are given in the following sections:
Clinical and laboratory characteristics
C3G with monoclonal Ig:
The median age at diagnosis was 60 years (range: 20-85), 25 (69.4%) males and 11 (30.6%) females, respectively. At diagnosis, the median serum creatinine was 1.9 mg/dL (range: 0.8-14.7), the median eGFR was 39.5 ml/min/1.73 m2 (range: 3-60) and the median proteinuria was 3.0 g/24 hours (range: 0.2-15.0). Hematuria was present in 32 (88.9%) patients. Twelve (34.3%) patients had low C3 and 4 (11.4%) had low C4, respectively. Of the 36 patients, 7 patients had CKD stage 2, 5 patients had CKD stage 3a, 13 patients had CKD stage 3b, 9 patients had CKD stage 4, and 2 patients had CKD stage 5.
C3G without monoclonal Ig:
In comparison, in C3G patients without a monoclonal Ig the median age at diagnosis was 28 years (range 4-84). The median serum creatinine at onset as 1.3 mg/dL (range: 0.3-7.9), the median eGFR was 52 ml/min/1.73 m2 (range: 7-106) and the median proteinuria was 1.7 g/24 hours (range: 0.3-24.2). Fifty one (86.4%) patients had hematuria. Twenty-eight (48.3%) patients had low C3 and 7 (12.5%) had low C4, respectively. Of the 59 patients, 1 patient had CKD stage 1, 26 patients had CKD stage 2, 6 patients had CKD stage 3a, 12 patients had CKD stage 3b, 11 patients had CKD stage 4, and 3 patients had CKD stage 5.
The clinical and laboratory features of C3G patients with and without a monoclonal Ig are described in Table 1.
Table 1:
Clinical and laboratory findings
| Data Variable | C3G with monoclonal Ig n=36 |
C3G without monoclonal Ig n=59 |
|---|---|---|
| Age at diagnosis (years) | Median: 60 | Median: 28 |
| Range: 20-85 | Range: 4-84 | |
| Sex (M/F) | Male: 25 (69.4%) | Male: 28 (47.5%) |
| Female: 11 (30.6%) | Female: 31 (52.5%) | |
| Race | White: 30 (83.3%) | White: 46 (78.0%) |
| Black: 2 (5.6%) | Black: 1 (1.7%) | |
| Asian: 0 | Asian: 2 (3.4%) | |
| American Indian/Alaskan Native: 1 (2.8%) | American Indian/Alaskan Native: 0 | |
| Other: 1(2.8%) | Other: 3 (5.1%) | |
| Unknown: 2 (5.6%) | Unknown: 7 (11.9%) | |
| Ethnicity | Hispanic or Latino: 1 (2.8%) | Hispanic or Latino: 0 |
| Non-Hispanic or Latino: 30 (83.3%) | Non-Hispanic or Latino: 51(86.4%) | |
| Unknown: 5 (13.9%) | Unknown: 8 (13.6%) | |
| Blood Urea Nitrogen (mg/dL) | Median: 37.5 | Median: 18 |
| Range: 15-95.8 | Range: 5.5-72 | |
| Serum Creatinine (mg/dL) at diagnosis | Median: 1.9 | Median: 1.3 |
| Range: 0.8-14.7 | Range: 0.3-7.9 | |
| Serum albumin at diagnosis (g/dL) | Median: 3.3 | Median: 3.5 |
| Range: 2.4-4.6 | Range: 1.2-4.5 | |
| Hematuria | Positive: 32 (88.9%) | Positive: 51 (86.4%) |
| Negative: 4 (11.1%) | Negative: 8 (13.6%) | |
| Proteinuria (g/24 hours) | Median: 3.0 | Median: 1.7 |
| Range: 0.2-15.0 | Range: 0.3-24.2 | |
| Immunology | ||
| Anti-GBM antibody | Positive: 0; Negative: 16; NA: 20 | Positive: 1*; Negative: 21; NA: 37 |
| ANCA/PR-3/MPO | Positive: 1; Negative: 23; NA: 12 | Positive: 0; Negative: 46; NA: 13 |
| Anti-streptolysin | Positive: 0; Negative: 6; NA: 30 | Positive: 2; Negative: 15; NA: 42 |
| Anti-DNase B | Positive:0; Negative: 4; NA: 32 | Positive: 0, Negative: 6; NA: 53 |
| ANA | Positive: 4; Negative: 24; NA: 8 | Positive:7; Negative: 50; NA: 2 |
| Anti-ds DNA | Positive: 1; Negative: 17; NA: 18 | Positive: 4; Negative: 43; NA: 12 |
| Cryoglobulins | Positive: 2#; Negative: 19; NA: 15 | Positive: 1@; Negative: 36; NA:22 |
| Cryofibrinogen | Positive: 0; Negative: 21; NA: 15 | Positive: 0; Negative: 35; NA: 24 |
| Complement | ||
| C3 | Low: 12; Normal: 23; NA: 1 | Low: 28; Normal: 30; NA: 1 |
| C4 | Low: 4; Normal: 31; NA: 1 | Low: 7; Normal: 49; NA: 3 |
Abbreviations: ANA: antinuclear antibody, ANCA, anti-neutrophil cytoplasmic antibodies; GBM, glomerular basement membrane; NA, not available; PR-3, anti-proteinase antibodies; MPO, anti-myeloperoxidase antibodies
Anti-GBM antibody: 6.6 EU/mL (results up to 50 EU/mL may occur in patients without anti-GBM disease and considered as equivocal).
Type 3 cryoglobulinemia
Type 1/Type 2 cryoglobulinemia
Hematological evaluation of C3G with monoclonal Ig
The hematologic diseases associated with C3G are outlined in figure 1. Twenty-six patients were classified as MGRS, 5 as multiple myeloma (MM), 2 as smoldering multiple myeloma (SMM), 1 as chronic lymphocytic leukemia (CLL), 2 patients were positive for cryoglobulins (one type 1 and one type 2). The patient with type 1 cryoglobulins showed (IgG kappa) on immunofixation studies of the cryoprecipitate; serum and urine electrophoresis were negative and free light chain ratio was within normal range. The patient with type 2 mixed cryoglobulinemia had a history of mucosal associated lymphoid tissue (MALT) lymphoma of the stomach and a history of Sjögren syndrome. Serum and urine electrophoresis were negative for monoclonal Ig in this patient. The distribution of heavy and light chains was IgG in 31 (88.6%), IgM in 3 (8.6%), IgA in 1 (2.8%), kappa (κ) in 26 (72.2%) and lambda (λ) in 10 (27.8%) patients, respectively. The commonest monoclonal Ig was an IgG kappa (63.9%). During follow-up, one patient with MGRS and one with SMM progressed to multiple myeloma, and two MGRS patients progressed to SMM.
Figure 1:
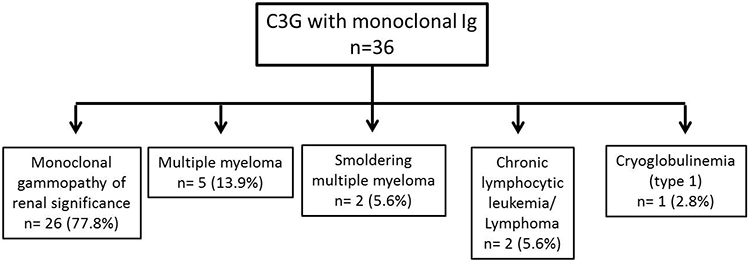
Hematologic evaluation of the C3G associated with monoclonal Ig. MGRS is the most common condition associated with C3G. *One monoclonal gammopathy of renal significance and one smoldering multiple myeloma progressed to multiple myeloma and two monoclonal gammopathy of renal significance progressed to smoldering multiple myeloma during the course of C3 glomerulopathy.
Evaluation of alternative pathway of complement
C3G with monoclonal Ig:
A total of 26 (72.2%) patients were evaluated for abnormalities of the alternative pathway of complement. Eleven (45.8%, n=24) patients had C3 nephritic factor and 3 (12.5%, n=24) patients were positive for other autoantibodies: 2 patients with autoantibodies to CFH and one to CFB. None of the patients tested (n=21) had a genetic variant/mutation in CFH, CFB, CFI, or MCP-1 genes, although 1 patient had a genetic variant/ heterozygous mutation in C3 and 1 in CFHR5 gene. Fifteen (100%, n=15) patients tested had polymorphisms associated with C3G. CFH gene polymorphism p.His 402 and/or p.Val62 (n=13) were the most common among these patients tested. Four (19.0%, n=21) patients were positive for other variant/mutations of unknown C3G pathogenicity including APCS, C1QA, F5, DGKE, FCN1 and PLG. The median serum C5b-9 level was 0.33 mg/L (range: 0.08-2.86, n= 23) (normal < 0.3 mg/L).
C3G without monoclonal Ig:
Forty-two (71.2%) patients without a monoclonal Ig were evaluated for abnormalities of the alternate complement pathway. Fourteen (37.8%, n=37) patients had C3 nephritic factor, 6 (16.7%, n=36) patients were positive for other autoantibodies that included 1 patient with autoantibodies to CFH, 3 to CFB , 1 to CFB and C4Nef, and 1 patient had C5Nef autoantibody, respectively. Twenty –four (58.5%, n=41) patients were positive for genetic variant/mutations, of which the most common was a heterozygous variant/mutation in CFH (10 patients), followed by C3 (5 patients), CFI (2 patients), CFB (1 patient), C5 (1 patient), C8 (4 patients) C9 (1 patient), CFHR2 (1 patient) and CFHR5 (3 patients) genes. CFH gene polymorphism p.His 402 and/or p.Val62 were present in 27(96.4%, n=28) patients tested. The median serum C5b-9 level was 0.27 mg/L (range: 0.01-2.00, n= 39) (normal < 0.3 mg/L).
Kidney biopsy findings in C3G with monoclonal Ig:
The mean number of glomeruli was 17.4 (range: 4-45), of which an average of 3.6 were sclerosed (range: 0-15). The most common pattern of injury was membranoproliferative glomerulonephritis (n=23, 63.9%), of which 5 were associated with crescents. Ten (27.8%) patients had a mesangial proliferative glomerulonephritis, of which 1 patient had crescents. Interstitial fibrosis and tubular atrophy was minimal (<10%) in 4 (11.1%), mild (10-25%) in 24 (66.7%), and moderate (26-50%) in 8 (22.2%) patients, respectively. None of the biopsies showed severe (>50%) interstitial fibrosis and tubular atrophy. Arteriosclerosis was mild in 14 (38.9%) and moderate in 13 (36.1%) patients, respectively. Immunofluorescence studies were positive for bright staining for C3 (2-3+/out of 3) in all cases. Positive Ig (trace-1+) was present in 14 (38.9%) patients. Fifteen (41.7%) biopsies had pronase IF studies performed on paraffin embedded material to rule out masked Ig deposits. Only 1 case showed minimal masked Ig deposits, all others were negative for monoclonal Ig on pronase studies. The one positive case showed IgG (1+), IgM (1-2+, likely in a sclerotic area) and kappa (1+), but showed intense C3 (3+) staining. Electron microscopy studies showed mesangial deposits in all 32 C3GN cases and capillary wall deposits (intramembranous and subendothelial deposits) in 31 C3GN cases, among which 13 also had hump-like deposits. Electron microscopy studies showed intramembranous dense deposits in all DDD cases. The kidney biopsy findings comparing C3G with and without monoclonal Ig are described in Table 2.
Table 2:
Kidney Biopsy Findings of C3G with and without monoclonal Ig
| Kidney biopsy findings | C3G with monoclonal Ig, n=36 | C3G without monoclonal Ig, n=59 |
|---|---|---|
| Total Glomeruli | Mean: 17.4 | Mean: 18.9 |
| Range: 4-45 | Range: 4-51 | |
| Number of globally sclerosed | Mean: 3.6 | Mean: 4.2 |
| Range: 0-15 | Range: 0-23 | |
| Glomerular pattern of injury | MPGN: 23 (63.9%) | MPGN: 29 (49.2%) |
| MES: 10 (27.8%) | MES: 19 (32.2%) | |
| CRES: 2 (5.6) | CRES: 4 (6.8%) | |
| DPGN: 1 (2.8%) | DPGN: 5 (8.5%) | |
| SCL: 0 | SCL: 2 (3.3%) | |
| Crescents/Fibrinoid necrosis | ≥40% crescents: 2 (5.6%) | ≥40% crescents: 4 (6.8%) |
| <40% crescents: 5 (13.9%) | >40% crescents: 7 (11.9%) | |
| FSGS | Present: 13 (36.1%) | Present: 19 (32.2%) |
| Absent: 23 (63.9%) | Absent: 40 (67.8%) | |
| IFTA | Minimal: 4 (11.1%) | Minimal: 16 (27.1%) |
| Mild: 24 (66.7%) | Mild: 27 (45.8%) | |
| Moderate: 8 (22.2%) | Moderate: 12 (20.3%) | |
| Severe: 0 | Severe: 4 (6.8%) | |
| Arteriosclerosis | None: 9 (25%) | None: 27 (45.8%) |
| Mild: 14 (38.9%) | Mild: 20 (33.9%) | |
| Moderate: 13 (36.1%) | Moderate: 10 (16.9%) | |
| Severe: 0 | Severe: 2 (3.4%) | |
| Immunofluorescence microscopy | C3: 36 (100%), 2+: 6; 3+: 30 | C3: 59 (100%), 2+: 8; 3+: 51 |
| Immunoglobulins: 14 (38.9%) | Immunoglobulins: 33 (55.9%) | |
| IgA: Trace: 1 | IgA: Trace: 2; 1+: 2 | |
| IgM: Trace: 4, 1+: 5 | IgM: Trace:6; 1+: 14 | |
| IgG: 1+: 4 | IgG: Trace: 4 , 1+:11 | |
| Electron microscopy | C3GN: Mesangial deposits: 32 | C3GN: Mesangial deposits: 56 |
| Capillary wall deposits: 31 | Capillary wall deposits: 56 | |
| With humps: 13 | With humps: 27 | |
| Subendothelial fluff: 1 | Subendothelial fluff:1 | |
| DDD: intramembranous deposits: 4 | DDD: intramembranous deposits: 3 |
Abbreviations: CRES, crescentic; DPGN, diffuse proliferative glomerular nephritis; FSGS, focal segmental glomerular sclerosis; MPGN, membranoproliferative glomerular nephritis; MES, mesangial; SCL, sclerotic; IFTA- interstitial fibrosis and tubular atrophy: grading: Minimal (<10%); Mild (10-25%); Moderate (26-50%); Severe (>50%), IF, Immunofluorescence; EM, electron microscopy.
Treatment and follow-up of C3G with monoclonal Ig
1. Renal outcomes
For C3G patients with a monoclonal Ig, after a median follow-up of 43.6 months (range: 1.8-128), nine (25%) progressed to end stage renal disease (ESRD). In comparison, only 7 (11.9%) C3G patients without a monoclonal Ig progressed to ESRD (table 3). However, Kaplan-Meier survival analysis demonstrated no statistically significant difference in ESRD-free renal survival among C3G patients with and without a monoclonal Ig (p-value: 0.0791, Figure 3). Renal outcomes were classified into complete renal response, partial renal response, no renal response and stable disease, respectively (methods). As some patients had progression of underlying hematological condition during the course of C3G, the type of monoclonal gammopathy at follow-up was considered. Treatment and outcomes of patients with and without a monoclonal Ig are described in table 3. In addition, the renal outcomes of patients with and without monoclonal Ig targeted therapy are shown in figure 4A.
Table 3:
Outcomes of C3 Glomerulopathy with and without monoclonal Ig
| Treatment and follow-up | C3 glomerulopathy with monoclonal Ig (n=36) |
C3 glomerulopathy without monoclonal Ig (n=59) |
|---|---|---|
| Duration of follow-up (months) | Median: 43.6 | Median: 36 |
| Range: 1.8-128 | Range: 0.3-251.3 | |
| Steroids only | 8 | 9 |
| Other immunosuppressants# with/without steroids | 25* | 28 |
| ACE-I/ARB | 26 | 48 |
| ESRD | 9 | 7 |
| Kidney transplant | 2 | 5 |
| Recurrent C3G post-transplant | 1 | 1 |
| Serum Creatinine at final follow-up (mg/dL) | Median: 1.4 (n=27; 9 ESRD) | Median: 1.6 (n=51, 1 NA, 7 ESRD) |
| Range: 0.9-3.8 | Range: 0.6-3.6 | |
| Proteinuria at final follow-up (g/24 hours) | Median: 0.8 (n=25) | Median: 0.9 (n=43) |
| Range: 0.4-7.0 | Range: 0.8-22.6 |
Abbreviations: ACE-I, angiotensin converting enzyme inhibitors; ARB, angiotensin receptor blockers; NA; not available
Sixteen patients received targeted therapy to monoclonal Ig that included drugs such as Velcade (Bortezomib)/Revlimid (Lenalidomide)/Rituximab/Vincristine/Pomalidomide/Daratumumab/ Melphalan/Carfilzomib/Thalidomide/Chlorambucil.
The other immunosuppressive drugs in patients with and without monoclonal Ig included mycophenolate mofetil, cyclophosphamide, tacrolimus, azathioprine, cyclosporine and eculizumab.
Figure 3:
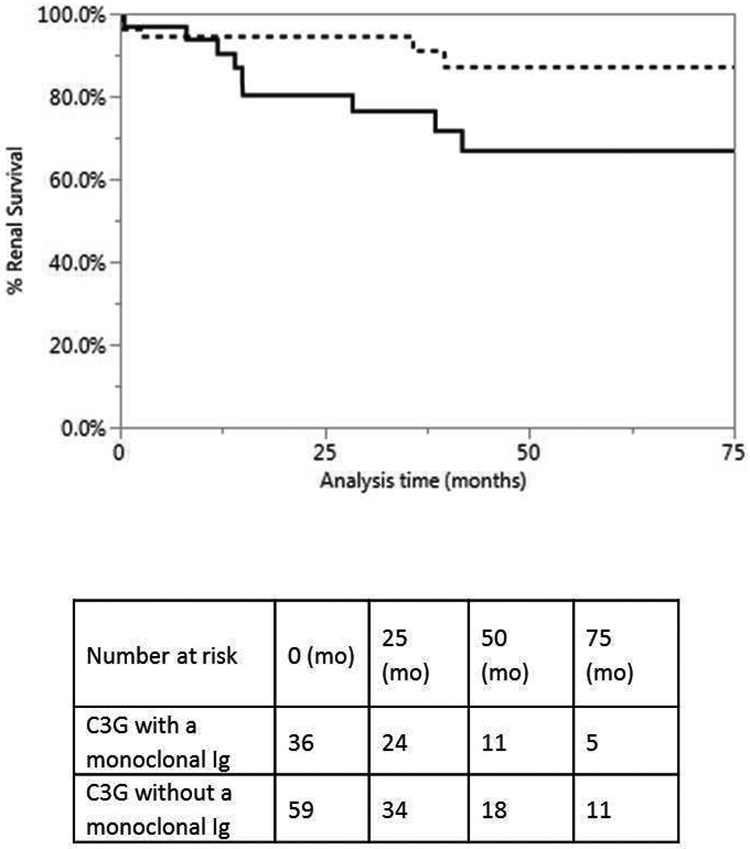
Kaplan-Meier analysis of renal survival in C3G patients with and without a monoclonal Ig. Black line represents C3G with monoclonal Ig and dotted line represents C3G without monoclonal Ig; mo: months. The analysis demonstrates no significant difference in ESRD free renal survival in C3G patients with and without monoclonal Ig (p= 0.0791).
Figure 4:
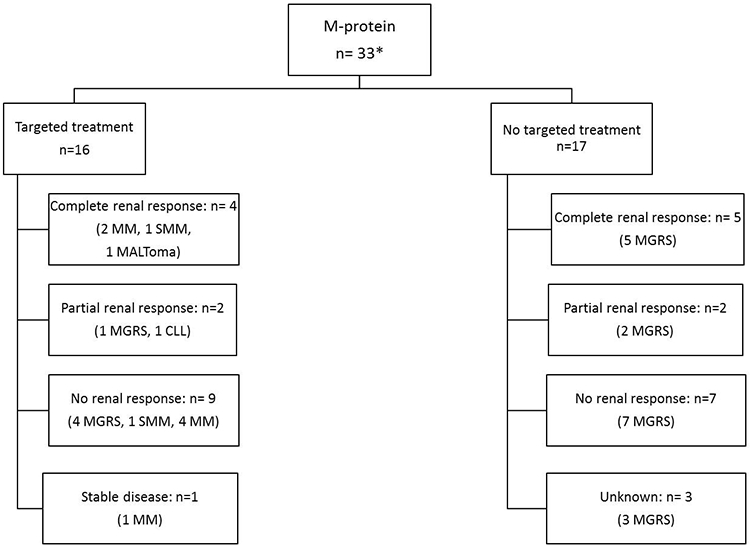
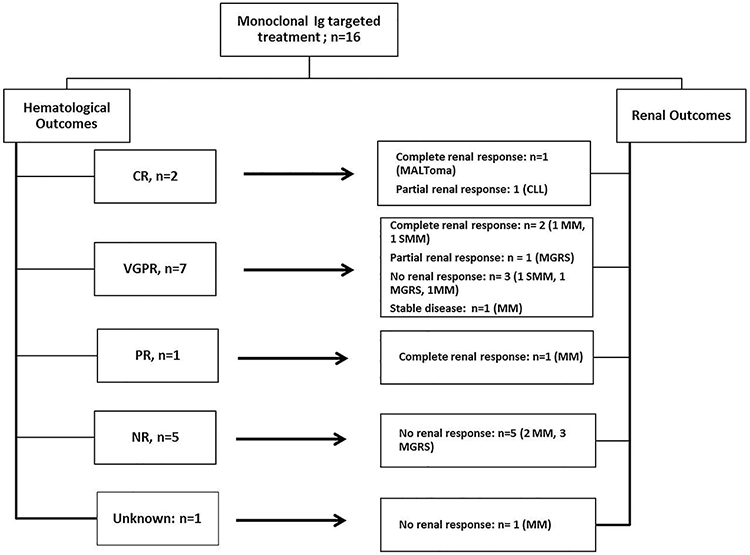
C3G with a monoclonal Ig: A) Renal outcomes of patients with and without targeted therapy; *3 received conservative therapy only; B) Renal and Hematological outcomes of patients with targeted therapyAbbreviations; CR, complete response; PR, partial response; VGPR, very good partial response; NR, no response; CLL, chronic lymphocytic leukemia; MGRS, monoclonal gammopathy of renal significance; SMM: smoldering multiple myeloma; MM, multiple myeloma.
C3G with monoclonal Ig:
Of 36 patients, 3 received conservative therapy, 16 received targeted therapy for monoclonal Ig and the remaining 17 received non-targeted therapy, respectively.
Conservative treatment: Of the 3 patients with conservative management only, all had MGRS (Supplemental table 1a). One patient had no renal response to therapy. One patient was lost to follow-up. One progressed to SMM and ESRD after a follow-up of 13.4 months and received kidney transplant with recurrence of C3G (DDD) at 4 months post-transplant.
Non-targeted treatment: Of the 17 patients with non-targeted therapy, all had MGRS (Supplemental table 1b). Among these, 5 achieved complete renal response, 2 partial renal response, 7 no renal response and 3 were unknown/ lost to follow-up. Of the 7 no renal response patients, 3 reached ESRD.
Targeted Treatment: Of the 16 patients receiving targeted treatment, 5 had MGRS, 7 had MM, 2 had SMM, 1 CLL and 1 type II cryoglobulinemia (Supplemental table 1c). Among these, the renal outcomes of 5 patients with MGRS were 1 partial renal response and 4 no renal response. For patients with SMM (n=2), 1 had complete renal response and 1 had no response. While for patients with MM (n=7), the renal outcomes were 2 complete, 4 no renal response and 1 stable disease. One patient had type 2 cryoglobulinemia with complete renal response at final follow-up, and one patient had CLL with partial renal response at final follow-up. Overall, of the 9 patients with no renal response, 5 reached ESRD.
On comparing renal survival in patients that received monoclonal protein targeted therapy (n=16) versus those without targeted therapy (n=17), Kaplan-Meier survival curve demonstrated no statistically significant difference (p-value: 0.5516).
C3G without a monoclonal Ig:
On the other hand, among the 59 patients without a monoclonal protein, 20 patients were managed conservatively of which 1 progressed to ESRD and of the remaining 19- 6 achieved CR, 2 PR, 6 NR and 5 were unknown. Two patients presented with ESRD at diagnosis and received kidney transplant at 2.3 and 65 months from diagnosis, although -one had recurrence of C3G (DDD) post-transplant and continued on dialysis at follow-up. Thirty-seven patients received steroids with/without other immunosuppressants. Of these 37 patients, 4 progressed to ESRD, among which 3 received kidney transplant. Of the remaining 33 patients, 4 achieved complete renal response, 9 partial renal response, 14 no renal response, 3 had stable disease and 3 were unknown.
2. Hematological Outcomes:
Of the 16 C3G patients with targeted monoclonal Ig therapy, 2 achieved complete hematologic response (CR), 7 achieved very good partial response (VGPR), 1 achieved partial response (PR), 5 had no response (NR) and 1 was unknown/lost to follow-up (Supplemental table 2). Of the 10 patients with hematological response (CR/VGPR/PR), the renal outcomes were complete renal response in 4 (25%) patients, partial renal response in 2 (12.5%) patients, stable disease in 1 (6.3%) patient and no renal response (18.8%) in 3 patients, respectively (Figure 4B). On the other hand, all 5 patients with no hematological response (NR), also had no renal response (31.3%).
DISCUSSION
The association between C3G and monoclonal gammopathy has been clearly docummented.8-10,23 However, most series showing the association are small, and detailed clinical, pathologic, hematologic and complement evaluation, treatment and outcomes are lacking. In this study, we provide a comprehensive analysis of patients with C3G associated with monoclonal gammopathy, and also compare the findings with C3G and without monoclonal gammopathy.
Our study shows a high prevalence of monoclonal Ig in C3G patients, in particular in the older patients, where 65.1% of C3G patient’s ≥ 50 years had a monoclonal gammopathy. This is approximately 16 times higher than the prevalence (4.2%) of monoclonal gammopathy in the general population.17,24 As expected, C3G with monoclonal Ig tend to be older (median age 60 years) when compared to C3G without monoclonal Ig (median age 28 years). Patients with C3G with monoclonal Ig also tend to have higher serum creatinine and proteinuria at presentation. Only 34.3% C3G patients with monoclonal Ig had low C3 levels, which was less than patients without monoclonal Ig (48.3%). This is somewhat surprising in that complement activation with low C3 levels is seen in the setting of monoclonal Ig and GN, and low C3 levels were noted to be associated with recurrence of GN associated with monoclonal Ig.25,26
The kidney biopsy of C3G with monoclonal Ig showed MPGN was the most common pattern of injury followed by mesangial proliferative glomerulonephritis. Immunofluorescence studies showed the hallmark bright glomerular C3 staining in all biopsies. Trace-1+ immunoglobulins were present in 38.9% biopsies. This is in keeping with other studies that also found low intensity positive Ig staining in a significant number of C3GN and DDD kidney biopsies 27,28 Electron microscopy revealed subepithelial hump-like deposits in 36.1% of the C3GN biopsies, indicating that they are not restricted to infection-related glomerulonephritis. We also ruled out masked Ig deposits in 15 C3GN biopsies using pronase based immunofluorescence methods. We did not include Mayo Clinic pronase positive cases that were part of the masked Ig study by Larsen et al.29
Hematologic evaluation showed that MGRS was the most common underlying hematologic disease, followed by multiple myeloma and smoldering multiple myeloma. Since C3G is a slowly progressive disease, conversion of 2 cases (1 MGRS and 1 SMM) to multiple myeloma and 2 cases of MGRS to SMM was noted during follow-up.
Evaluation of the alternative pathway of complement showed a high (46.1%) prevalence of occurrence of C3 nephritic factor and other autoantibodies. On the other hand, none of patients had genetic variants or mutations in CFH, CFI, CFB, or MCP-1 genes. These findings are highly suggestive that the monoclonal Ig likely acts as an autoantibody to C3 convertase or as an autoantibody to other complement regulating proteins. This is in contrast to C3G patients without monoclonal Ig who showed both autoantibodies and genetic variants/mutations in complement regulating proteins. In C3G patients without monoclonal Ig, a genetic variant/mutation in complement regulating proteins was detected in 58.5% of the patients and autoantibodies to complement regulating proteins were detected in 43.2% of the patients. It should be pointed out that even though many C3G patients with monoclonal Ig showed high prevalence of C3 nephritic factor and other autoantibodies, no abnormalities of the complement pathway was detected in many patients. This suggests that the monoclonal Ig may act by other mechanisms that activate the alternative pathway or that some of the autoantibodies to complement regulating proteins are not detected by the current methodology.
Targeted treatment of the monoclonal Ig resulted in complete or partial response or stable renal function in 43.8% of the patients, while 31.3% had no renal response. However, of patients achieving a hematological response (CR/PR/VGPR), 70% patients showed better renal outcomes (complete renal response/partial renal response/stable disease). On the other hand, all patients without a hematological response (n=5, 31.3%) failed to show a renal response. This is similar to the results presented in a recent study by Chauvet et al that showed a beneficial effect in renal outcomes of targeted treatment in monoclonal gammopathy-associated C3G.13 However, compared to the study by Chauvet et al, we provide additional data in comparing C3G with and without monoclonal Ig, and provide detailed renal pathology findings. We also perform EM in all cases and perform pronase based IF studies in a large number of cases.
It is important to point that targeted treatment was given preferentially to patients who had multiple myeloma, SMM or CLL while non-targeted treatment was preferred for patients with MGRS. In our study, 17 patients who received non targeted treatment had MGRS (figure 4). On the other hand, only 5 (31.2%) of 16 patients receiving targeted treatment had MGRS. Thus, patients receiving targeted treatment in general had more advanced hematologic disease. The fact that hematological response was associated with good renal response suggests that targeted therapy should also be considered for patients without advanced hematological conditions, since renal disease is progressive even in patients with MGRS, although further studies are needed to evaluate its efficacy in halting renal disease progression in these patients.
One of the strengths of the study is that all patients were seen at a single institution and detailed clinical, laboratory and kidney biopsy finding, treatment and follow-up data was available for study. A limitation of the study is that the detailed studies to elucidate the mechanism how the monoclonal Ig dysregulates the AP of complement were not done.. Thus, in some patients the monoclonal Ig may act as C3 nephritic factor stabilizing the C3 convertase and in others it may act as an autoantibody to complement pathway regulating proteins such as CFH or CFB.30,31
Taken together, these findings suggest that older patients with C3G appear to be a distinct subgroup of patients that are more likely to have a monoclonal Ig. Evaluation of the alternative pathway of complement is more likely to reveal C3 nephritic factor and/or autoantibodies to complement regulating proteins than genetic variants/mutations in the complement regulating proteins. This is in contrast to younger patients with C3G who are more likely to have genetic variant/mutations and/or autoantibodies to complement regulating proteins and do not have monoclonal Ig. Furthermore, targeted treatment of the monoclonal Ig may result in improvement of renal outcomes in a subset of these patients.
MATERIAL AND METHODS
We identified 114 patients seen at the Mayo Clinic from 2007-2016 with a diagnosis of C3 glomerulopathy (C3GN or DDD) in native kidney biopsies. SS reviewed all kidney biopsies. Complement evaluation was done as previously described.32 We included patients with the following criteria: i) diagnosis of C3 glomerulopathy and ii) tested for a monoclonal Ig with serum/urine protein electrophoresis and immunofixation studies. Clinical information was obtained from the charts. The study was approved by the Mayo Clinic Institutional Review Board and conducted in accordance to the Declaration of Helsinki. Statistical analyses were performed using JMP Pro software version 10.0.2 (SAS Institute Inc., Cary, NC). Complement evaluation was performed as previously described.32 Charts were reviewed for clinical data. Renal survival probabilities were determined using the Kaplan Meier method and group comparisons for survival were performed using the log-rank test. Renal outcomes were defined by the following criteria: i) Complete renal response (CRR) was defined by proteinuria levels of ≤ 0.5 g/24 hours and no more than 10% decrease in eGFR from baseline value; ii) Partial renal response (PRR) was defined by post-treatment proteinuria of≥ 50% reduction from baseline value; iii) No renal response (NRR) was defined by < 50% reduction in proteinuria among patients with proteinuria ≥ 1.5 g/24 hours and/or 25% increase in serum creatinine; iv) Stable disease (SD) was defined as eGFR within 10% of baseline with stable proteinuria of <1.5 g/24 hours from onset to completion of treatment. Hematological response was assessed according to the International Myeloma Working Group for patients with symptomatic MM.33 In patients with MGRS, response criteria was adapted from the 2012 International Society of Amyloidosis criteria 34: complete hematological response (CR) was defined by negative immunofixation of serum and urine with normal free light chain (FLC) ratio, very good partial response (VGPR) was defined by a 90% or more reduction of serum MIg and/or a 90% or more decrease in the difference between the concentration of the involved and uninvolved FLC (dFLC), and partial hematological response (PR) was defined by a 50% or more reduction of serum MIg and/or a 50% or more dFLC decrease. Patients who did not meet any of the hematological criteria were classified as no response (NR). Patients who were lost to follow-up or for whom lab values to determine response were not available were classified as “Unknown response.”
Supplementary Material
Supplemental Table 1: Renal Outcomes of C3G patients associated with a monoclonal gammopathy (N=36). A. Conservative therapy, B. Other (non-targeted) therapy, and C. targeted treatment.
Supplemental Table 2: Hematological and Renal Outcomes of C3G patients with Targeted therapy.
Figure 2:
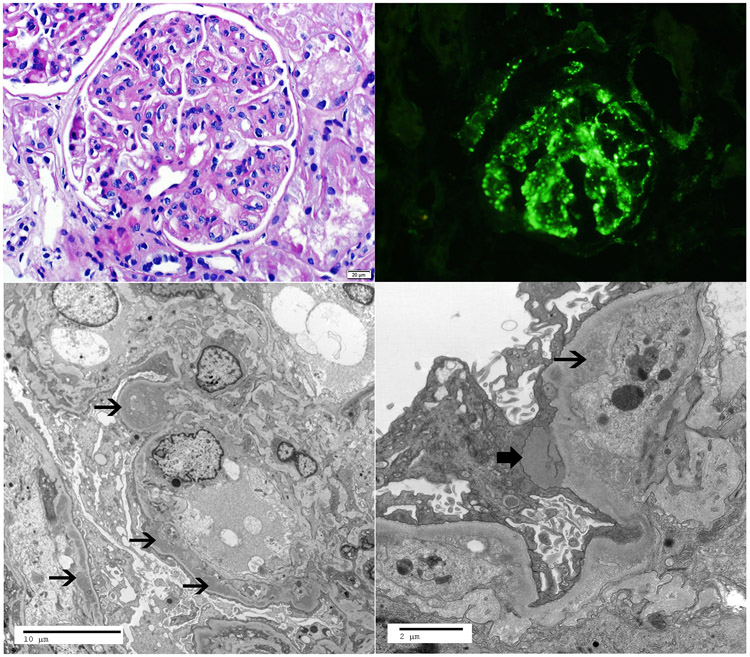
Representative kidney biopsy of C3G with monoclonal Ig. A. Light microscopy showing a membranoproliferative glomerulonephritis (periodic acid Schiff stain, 40x), B. Immunofluorescence microscopy showing bright mesangial and capillary wall staining for C3 (40x), C-D. Electron microscopy showing subendothelial deposits (thin arrows) and a subepithelial deposit (thick arrow) (C-2900x, D-9300x).
Footnotes
Statement of competing financial interests: None
Disclosure: None
Conflict of Interest: None
Supplementary information is available at KI Report's website.
References
- 1.Pickering MC, D'Agati VD, Nester CM, et al. C3 glomerulopathy: consensus report. Kidney Int 2013;84:1079–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sethi S, Fervenza FC, Zhang Y, et al. C3 glomerulonephritis: clinicopathological findings, complement abnormalities, glomerular proteomic profile, treatment, and follow-up. Kidney Int 2012;82:465–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Vriese AS, Sethi S, Van Praet J, Nath KA, Fervenza FC. Kidney Disease Caused by Dysregulation of the Complement Alternative Pathway: An Etiologic Approach. Journal of the American Society of Nephrology 2015;26:2917–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Angioi A, Fervenza FC, Sethi S, et al. Diagnosis of complement alternative pathway disorders. Kidney International 2016;89:278–88. [DOI] [PubMed] [Google Scholar]
- 5.Servais A, Noel L-H, Roumenina LT, et al. Acquired and genetic complement abnormalities play a critical role in dense deposit disease and other C3 glomerulopathies. Kidney Int 2012;82:454–64. [DOI] [PubMed] [Google Scholar]
- 6.Appel GB, Cook HT, Hageman G, et al. Membranoproliferative Glomerulonephritis Type II (Dense Deposit Disease): An Update. J Am Soc Nephrol 2005;16:1392–403. [DOI] [PubMed] [Google Scholar]
- 7.Sethi S, Nester CM, Smith RJH. Membranoproliferative glomerulonephritis and C3 glomerulopathy: resolving the confusion. Kidney Int 2012;81:434–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zand L, Kattah A, Fervenza FC, et al. C3 Glomerulonephritis Associated With Monoclonal Gammopathy: A Case Series. American Journal of Kidney Diseases 2013;62:506–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sethi S, Sukov WR, Zhang Y, et al. Dense Deposit Disease Associated With Monoclonal Gammopathy of Undetermined Significance. American Journal of Kidney Diseases 2010;56:977–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bridoux F, Desport E, Frémeaux-Bacchi V, et al. Glomerulonephritis With Isolated C3 Deposits and Monoclonal Gammopathy: A Fortuitous Association? Clinical Journal of the American Society of Nephrology 2011;6:2165–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sethi S, Rajkumar SV. Monoclonal Gammopathy–Associated Proliferative Glomerulonephritis. Mayo Clinic Proceedings 2013;88:1284–93. [DOI] [PubMed] [Google Scholar]
- 12.Sethi S, Fervenza FC, Rajkumar SV. Spectrum of manifestations of monoclonal gammopathy-associated renal lesions. Current Opinion in Nephrology and Hypertension 2016;25:127–37. [DOI] [PubMed] [Google Scholar]
- 13.Chauvet S, Frémeaux-Bacchi V, Petitprez F, et al. Treatment of B-cell disorder improves renal outcome of patients with monoclonal gammopathy–associated C3 glomerulopathy. Blood 2017;129:1437. [DOI] [PubMed] [Google Scholar]
- 14.Kyle RA, Therneau TM, Rajkumar SV, et al. A Long-Term Study of Prognosis in Monoclonal Gammopathy of Undetermined Significance. New England Journal of Medicine 2002;346:564–9. [DOI] [PubMed] [Google Scholar]
- 15.Kyle RA, Rajkumar SV. Criteria for diagnosis, staging, risk stratification and response assessment of multiple myeloma. Leukemia 2008;23:3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rajkumar SV, Kyle RA, Therneau TM, et al. Serum free light chain ratio is an independent risk factor for progression in monoclonal gammopathy of undetermined significance. Blood 2005;106:812–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dispenzieri A, Katzmann JA, Kyle RA, et al. Prevalence and risk of progression of light-chain monoclonal gammopathy of undetermined significance: a retrospective population-based cohort study. The Lancet 2010;375:1721–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bridoux F, Leung N, Hutchison CA, et al. Diagnosis of monoclonal gammopathy of renal significance. Kidney Int 2015;87:698–711. [DOI] [PubMed] [Google Scholar]
- 19.Servais A, Fremeaux-Bacchi V, Lequintrec M, et al. Primary glomerulonephritis with isolated C3 deposits: a new entity which shares common genetic risk factors with haemolytic uraemic syndrome. Journal of Medical Genetics 2007;44:193–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sethi S, Fervenza FC, Zhang Y, et al. Proliferative Glomerulonephritis Secondary to Dysfunction of the Alternative Pathway of Complement. Clinical Journal of the American Society of Nephrology 2011;6:1009–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nasr SH, Valeri AM, Appel GB, et al. Dense Deposit Disease: Clinicopathologic Study of 32 Pediatric and Adult Patients. Clinical Journal of the American Society of Nephrology 2009;4:22–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hamzi MA, Zniber A, Badaoui GE, et al. C3 glomerulopathy associated to multiple myeloma successfully treated by autologous stem cell transplant. Indian Journal of Nephrology 2017;27:141–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lloyd IE, Gallan A, Huston HK, et al. C3 glomerulopathy in adults: a distinct patient subset showing frequent association with monoclonal gammopathy and poor renal outcome. Clinical Kidney Journal 2016;9:794–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kyle RA, Therneau TM, Rajkumar SV, et al. Prevalence of Monoclonal Gammopathy of Undetermined Significance. New England Journal of Medicine 2006;354:1362–9. [DOI] [PubMed] [Google Scholar]
- 25.Zand L, Lorenz EC, Cosio FG, et al. Clinical Findings, Pathology, and Outcomes of C3GN after Kidney Transplantation. Journal of the American Society of Nephrology 2014;25:1110–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sethi S, Zand L, Leung N, et al. Membranoproliferative Glomerulonephritis Secondary to Monoclonal Gammopathy. Clinical Journal of the American Society of Nephrology 2010;5:770–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hou J, Markowitz GS, Bomback AS, et al. Toward a working definition of C3 glomerulopathy by immunofluorescence. Kidney Int 2014;85:450–6. [DOI] [PubMed] [Google Scholar]
- 28.Larsen CP, Walker PD. Redefining C3 glomerulopathy: /‘C3 only/' is a bridge too far. Kidney Int 2013;83:331–2. [DOI] [PubMed] [Google Scholar]
- 29.Larsen CP, Messias NC, Walker PD, et al. Membranoproliferative glomerulonephritis with masked monotypic immunoglobulin deposits. Kidney International 2015;88:867–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meri S, Koistinen V, Miettinen A, Tornroth T, Seppala I. Activation of the alternative pathway of complement by monoclonal lambda light chains in membranoproliferative glomerulonephritis. . J Exp Med 1992;175:939–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jokiranta T, Solomon A, Pangburn M, Zipfel P, Meri S. Nephritogenic lambda light chain dimer: a unique human miniautoantibody against complement factor H. J Immunol 1999;163:4590–6. [PubMed] [Google Scholar]
- 32.Zhang Y, Meyer NC, Wang K, et al. Causes of Alternative Pathway Dysregulation in Dense Deposit Disease. Clinical Journal of the American Society of Nephrology 2012;7:265–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Durie BG, Harousseau JL, Miguel JS, et al. International uniform response criteria for multiple myeloma. Leukemia 2006;20:1467–73. [DOI] [PubMed] [Google Scholar]
- 34.Palladini G, Dispenzieri A, Gertz MA, et al. New criteria for response to treatment in immunoglobulin light chain amyloidosis based on free light chain measurement and cardiac biomarkers: impact on survival outcomes. J Clin Oncol 2012;30:4541–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1: Renal Outcomes of C3G patients associated with a monoclonal gammopathy (N=36). A. Conservative therapy, B. Other (non-targeted) therapy, and C. targeted treatment.
Supplemental Table 2: Hematological and Renal Outcomes of C3G patients with Targeted therapy.