Abstract
Background:
Diabetes mellitus places a considerable burden on the individual and the family with respect to lifestyle changes. There is a paucity of systematic studies in India examining the efficacy of self-management programs for diabetes. The study examined the impact of a brief self-management intervention (SMI) on primary outcome of HbA1c and secondary outcomes of quality of life (QOL), self-care, perceived barriers to self-care (BSC), perceptions regarding illness and mood in patients with type 2 diabetes mellitus.
Methods and materials:
Eighty patients with type 2 diabetes mellitus were randomly allocated to either a 4-session SMI or treatment as usual (TAU) and were assessed on HbA1c levels, QOL, self-care, BSC, illness perceptions, anxiety, and depression at baseline, postintervention , and at three-month postintervention follow-up.
Results:
Repeated measures analysis of variance indicated significant improvement in the SMI group from baseline to follow-up on HbA1c (P = 0.001), impact of diabetes on QOL (P = 0.006), self-care with respect to diet and exercise (Ps = 0.001), perceived barriers in adherence to diet, exercise, (P = 0.001), medication (P < 0.01), glucose testing (P = 0.04), general BSC (P = 0.001), total barriers (P = 0.001), illness perceptions-timeline or chronicity of illness (P = 0.002), personal control over illness, (P = 0.001), belief in effectiveness of treatment (P = 0.002), understanding of one’s illness (P = 0.001), and emotional representations regarding illness (P =0.001), depression, (P = 0.001), and anxiety (P = 0.001). In the SMI group, large effect sizes were obtained at the postintervention assessment and the three-month follow-up on most outcome measures.
Conclusions:
Brief psychological intervention is efficacious in patients with type 2 diabetes.
Keywords: Type 2 diabetes, self-management, illness perception, quality of life, perceived barriers
Key Messages:
Self-management is an important aspect of care in chronic medical conditions such as diabetes mellitus and is impacted by psychological factors. Providing brief, yet effective self-management interventions remain a challenge in the Indian setting. The findings of this study highlight the efficacy of a brief psychological intervention in adult patients with type 2 diabetes in an Indian setting.
Diabetes places significant demands on the patient, impacting the quality of life (QOL) and well-being.1 The prevalence of type 2 diabetes in India is higher than in the West.2–4 Self-management in diabetes is complex and critical in achieving glycemic control, and patients experience significant difficulties in maintaining the treatment regimen.5 Diabetes self-care refers to the execution of and adherence to self-management behaviors such as diet, exercise, and blood glucose monitoring.6
Psychological models of health behaviors attempt to understand factors impacting self-care. Bandura’s social cognitive theory identifies psychological factors, such as personal characteristics, arousal, coping, behavioral capacity, self-efficacy, expectancies, self-regulation, observational and experiential learning, and reinforcement as impacting health behaviors.7, 8 Several other models have also been extended to explain health behaviors on the basis of social cognitive processes and perceived barriers to self-care (BSC).9, 10 Recent recommendations in diabetes care suggest that psychosocial care must be integrated with collaborative medical care so as to improve glycemic control and QOL, with a focus on changing attitudes and improving health care.11 It is important to explore the perceived barriers to a specified behavior and address them in therapy to enhance adherence.12 Perception of barriers is a cognitive process, and several factors, including social, personal, environmental, and economic barriers, impact behaviors. Patients with diabetes are more likely to experience depression than the general population. They are reported to have higher blood glucose levels, poorer adherence, and lower QOL and well-being.13, 14 Concerns about mood and adherence are often not addressed adequately in routine care. Identification and treatment of comorbid mental health conditions in patients with type 2 diabetes are associated with improved metabolic outcomes.15 The Diabetes Attitudes, Wishes and Needs (DAWN) reports that many health care providers were underconfident about their ability to identify psychological problems or provide psychological support that the patients require.16, 17
Meta-analytic reviews of psychological interventions have focused on knowledge, lifestyle behaviors, skill development, glycemic control, self-monitoring of blood glucose, enhancing empowerment and self-efficacy, and promoting relaxation.8, 19, 20 Meaningful changes in metabolic status and mood with cognitive behavior therapy (CBT) have been demonstrated.21
Cultural and psychosocial factors must be considered in designing psychological interventions for persons with type 2 diabetes.22 There is a dearth of systematic studies on the efficacy of psychological therapies in improving their self-care, in the Indian context. Recent Indian studies noted that while there are evidence-based guidelines for the management of persons with diabetes, they do not adequately address psychological issues, with no systematic studies on the efficacy of these interventions.23 The feasibility and effectiveness of strategies developed in the West need to be empirically tested in the Indian setting. Studies on urban Indian patients with type 2 diabetes highlight the need for interventions that focus on self-care, mood, and illness perceptions.24–26 Following the diagnosis of a chronic medical condition, patients are likely to go through a period of psychological and emotional adjustment, before reaching an acceptance of the diagnosis.27 The need for culturally specific, sustainable self-management interventions (SMIs) for persons with diabetes in India has been highlighted.23 In view of these findings, the present study examined the efficacy of a brief psychological intervention to which cognitive and behavioral components were incorporated, keeping in mind the cultural context of the patients and their needs.
Materials and Methods
Design and Participants
A two-group randomized controlled design with baseline, postintervention, and three-month follow-up assessments was adopted. Eighty patients with type 2 diabetes were recruited from outpatient services of the Dept. of Endocrinology, St. John’s Teaching College and Hospital, Bangalore. Based on the primary outcome measure of HbA1c, an expected 80% power, and 5% significance, it was estimated that 37 participants would be required in each group. Inclusion criteria were aged 30–65 years, with at least one-year duration of illness, and HbA1c between 7.5% and 10.0% (moderate to poor range). Patients were required to have been on a stable dosage of the medication for a period of at least two months prior to recruitment. Those with co-morbid psychiatric conditions, gestational or drug-induced diabetes, myocardial infarction, cerebrovascular illness, or microvascular complications were excluded, as were patients who had received structured psychological intervention in the last year.
Randomization
Patients fulfilling study criteria were randomized to either a SMI group or treatment as usual (TAU) group, using a random number table. Allocation concealment was not done. The primary outcome measure was HbA1C, and secondary outcomes were QOL, self-care, perceived BSC, illness perceptions, and mood. Patients in both the groups were assessed on all measures at baseline, postintervention, and at three-month postintervention follow-up. Patients who required changes in medication dosage during the study were excluded from the analysis but continued to receive therapy.
Intervention
Participants in the SMI group received four individual sessions (modules), in addition to the routine advice on diet, exercise, and medication from the endocrinologist. The four modules of the SMI were developed based on interviews with 15 patients with type 2 diabetes, 15 caregivers, and 13 diabetes care professionals (physicians, endocrinologists, nurses, psychologists, and nutritionists). Interviews were carried out by the first author to identify the felt needs for diabetes SMI and their relevant components.28 The four modules are as follows:
Diabetes education and relaxation skills, with a brief individualized diabetes self-management module and training in deep breathing.
Goal setting, prioritizing, and promoting diabetes care, in which two personalized behavioral goals were set and strategies to prioritize and implement diabetes care activities were discussed. (e.g., planning exercise time and meals in advance, getting help from family for self-care).
Barrier identification and problem-solving skills, with respect to self-care, and their application to situations of diabetes self-care.
Managing diabetes-related distress and understanding its impact on self-care and metabolic status.
The first author conducted the sessions individually, at a general hospital setting, for a duration of 30–45 minutes per session held fortnightly. Patients were provided handouts and worksheets in English and vernacular languages, for self-monitoring and homework assignments. A brief telephonic contact to provide prompts and reinforcements for self-care behaviors were made at four time points during the SMI.
TAU consisted of regular care by the consultant endocrinologist, including monitoring of blood glucose levels, prescription of medication, and inputs on the diet, exercise, and medication from the endocrinologist and did not include structured psychological intervention.
Measures
HbA1c levels were recorded at baseline, postintervention, and three-month postintervention follow-up. The Diabetes Quality of Life Measure (DQOL)29 consists of 46 items rated on a 5-point Likert scale (1 = no impact, 5 = always affected, never satisfied). In the present study, the total score and a single item that assesses DQOL were considered.
Self-care was assessed using the summary of diabetes self-care activities schedule (SDSCA)30, 31; an 11 item, self-report measure of the frequency of performing diabetes self-care tasks over the preceding one week.
Perceived BSC were assessed using BSC,32 a 31-item self-report instrument. Subjects rate the frequency of barriers experienced across five subscales of diet, exercise, medication, glucose testing, and general barriers. An overall barrier score is also generated.
The illness perception questionnaire-revised (IPQ-R)33 assesses five components of illness, based on Leventhal’s (1987) self-regulatory model.34 It consists of 8 subscales—identity, timeline (how long illness will last), consequences, personal control (personal control over illness), treatment control (beliefs about treatment effectiveness), illness coherence (understanding about illness), cyclical timeline (predictability of illness), and emotional representations (emotional reactions to illness). The scales were translated to the local languages of Kannada and Hindi and back-translated into English.
Data Analysis
Data was analyzed using the Statistical Software Package for Social Sciences Version 15.0 (SPSS 15.0). Outcome variables were tested for normality of distribution using the Kolmogorov–Smirnov test. Nonparametric tests were applied for non-normal variables. Intention-to-treat (ITT) analysis was carried out. Additionally, the completer sample was also analyzed. Missing values were imputed using the last observation carried forward method, a conservative method that reduces the overestimation of intervention effects. Independent sample t-test was used to compare differences between groups at baseline. Repeated measures analysis of variance (RMANOVA) was carried out to evaluate changes across time points. Baseline differences between groups were examined using independent samples t-test. Paired t-test and independent samples t-tests were carried out to examine within and between-group differences, respectively. Effect sizes (ESs) for the outcome variables were calculated using Cohen’s d.35 The ES cutoffs are as follows: >0.20 = small ES, >0.50 = medium ES, >0.80 = large ES.
Ethical Approval
The study protocol was reviewed and approved by the two ethics committees of institutions (National Institute of Mental Health and Neurosciences and St. John’s Medical College and teaching hospital) involved in planning of the study and in data recruitment. Written informed consent was obtained from all patients in the study. The study was registered retrospectively in the clinical trial registry of the Indian Council of Medical Research (CTRI/2015/01/005415).
Results
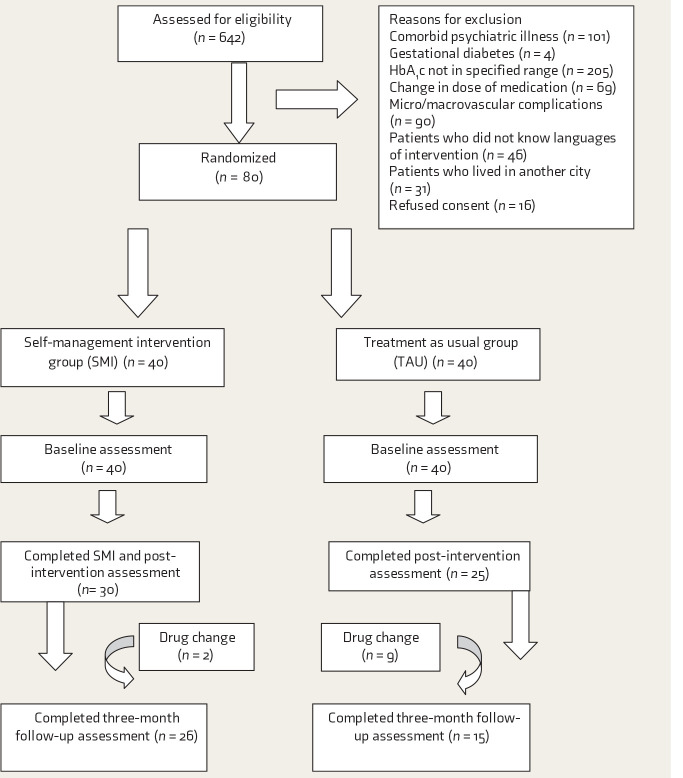
Six hundred and forty-two patients were screened during their follow-up visits to the endocrinologist. Of these, 562 did not meet the study criteria, for reasons mentioned in Figure 1. Eighty participants were randomized to the SMI group or the TAU group, using random numbers (Figure 1). Overall, attrition was 25%. Nonpharmacological interventions have reported diverse rates of attrition.18
Figure 1. Participant Flow.
At baseline, completers and noncompleters in SMI were comparable on all measures except DQOL-single item, SDSCA-foot care, and IPQ-identity.
At baseline, both groups were statistically comparable on demographic and clinical characteristics (Table 1). RMANOVA (Tables 2 and 3) was conducted to examine the efficacy of the treatment
TABLE 1. Baseline Sociodemographic and Clinical Characteristics.
| Variable | SMI Group (N = 40) | TAU Group (N = 40) | t/U value | Sig(2-Sided) | |
| Mean + SD | Mean (SD) | ||||
| Age | 50.4 ± 9.3 | 50.6 ± 8.3 | 0.08 | 0.93 | |
| Baseline HbA1c* | 8.58 ± .84 | 8.41 ± .73 | 720 | 0.44 | |
| Duration of illness* | 9.7 ± 6.8 | 9.8 ± 6.9 | 784 | 0.87 | |
| N (%) | N (%) | χ 2 | Sig (2-sided) | ||
| Gender | Male | 18 (44) | 17 (42) | 0.05 | 0.82 |
| Female | 22 (55) | 23 (58) | |||
| Education | Not literate | 2 (5) | 3 (8) | 1.26 | 0.56 |
| Less than 7 years | 7 (18) | 6 (15) | |||
| 8–12 years | 9 (22) | 13 (32) | |||
| Graduate | 9 (22) | 8 (20) | |||
| Postgraduate | 5 (13) | 4 (10) | |||
| Professional | 8 (20) | 6 (15) | |||
| Employment status | Employed | 21 (52) | 16 (40) | 1.26 | 0.53 |
| Unemployed | 16 (40) | 20 (50) | |||
| Retired | 3 (8) | 4 (10) | |||
| Marital status | Married | 34 (85) | 32 (80) | 0.34 | 0.55 |
| Unmarried and others | 6 (15) | 8 (20) | |||
| Family history of diabetes | Present | 28 (70) | 30 (75) | 0.25 | 0.61 |
| Absent | 12 (30) | 10 (25) | |||
| Type of treatment | OHGA only | 28 (70) | 26 (65) | 0.05 | 0.81 |
| Insulin + OHGA | 12 (30) | 14 (35) | |||
| Comorbid conditions | Hypertension | 14 (35) | 16 (40) | 4.43 | 0.21 |
| Dyslipidemia | 15 (38) | 7 (18) | |||
| Others | 8 (20) | 5 (13) | |||
| None | 12 (30) | 17 (43) | |||
*Mann–Whitney U test.
TABLE 2. Within- and Between-Group Differences (RMANOVA) on Primary and Secondary Outcome Measures at Different Time Points and Effect Sizes Noted in the SMI and TAU Groups.
| Variable | SMI | Within-Group Effects | Effect Size | TAU | Within-Group Effects | Effect Size | Between-Group Effects | |||||||||
| Pre | Post | F/U | F | Sig (2-Tailed) | Post | F/U | Pre | Post | F/U | F | Sig (2-Tailed) | Post | F/U | F | Sig (2-Tailed) | |
| HbA1c | 8.58 ± 0.84 | 8.19 ± 0.78 | 8.08 ± 0.81 | 32.92*** | 0.001 | 0.50* | 0.60* | 8.41 ± 0.73 | 8.70 ± 1.02 | 8.68 ± 0.99 | 7.50** | 0.007 | 0.32 | 0.21 | 2.90 | 0.09 |
| DQOL-SAT | 3.10 ± 0.38 | 3 ± 0.36 | 3.40 ± 3.19 | 0.52 | 0.47 | 0.27 | 0.13 | 2.98 ± 0.35 | 3.02 ± 0.37 | 3.02 ± 0.39 | 1.27 | 0.27 | 0.11 | 0.13 | 0.74 | 0.39 |
| DQOL-IMP | 2.74 ± 0.31 | 2.74 ± 0.29 | 2.66 ± 0.28 | 7.07** | 0.006 | 0.23 | 0.33 | 2.72 ± 0.27 | 2.74 ± 0.28 | 2.74 ± 0.28 | 2.82 | 0.09 | 0.07 | 0.07 | 0.12 | 0.72 |
| DQOL-1 | 2.88 ± 0.68 | 2.83 ± 0.67 | 2.38 ± 0.54 | 32.56*** | 0.001 | 0.07 | 0.81* | 2.80 ± 0.51 | 2.78 ± 0.62 | 2.83 ± 0.59 | 0.36 | 0.67 | 0.03 | 0.05 | 0.74 | 0.39 |
| DQOL total | 2.85 ± 0.31 | 2.80 ± 0.28 | 2.72 ± 0.25 | 14.48*** | 0.001 | 0.16 | 0.56* | 2.85 ± 0.31 | 2.83 ± 0.30 | 2.83 ± 0.30 | 0.11 | 0.80 | 0.03 | 0.03 | 0.32 | 0.56 |
| SDSCA-GD | 3.96 ± 1.99 | 4.92 ± 1.61 | 4.93 ± 1.60 | 17.59*** | 0.001 | 0.54 | 0.56* | 4.15 ± 1.82 | 4.33 ± 1.72 | 4.35 ± 1.69 | 4.35* | 0.02 | 0.10 | 0.11 | 0.78 | 0.38 |
| SDSCA-SD | 4.47 ± 1.56 | 5.53 ± 1.15 | 5.42 ± 1.32 | 15.74*** | 0.001 | 0.80* | 0.87* | 4.83 ± 1.30 | 5.05 ± 1.22 | 5.01 ± 1.21 | 2.11 | 0.15 | 0.17 | 0.14 | 0.36 | 0.54 |
| SDSCA-EX | 2.28 ± 2.26 | 3.53 ± 2.19 | 3.76 ± 2.13 | 29.85*** | 0.001 | 0.56* | 0.66* | 2.60 ± 2.44 | 2.71 ± 2.40 | 2.66 ± 2.38 | 0.54 | 0.58 | 0.04 | 0.04 | 1.16 | 0.28 |
| SDSCA-GT | 1.03 ± 0.58 | 1.1 ± 0.54 | 1.08 ± 0.52 | 1.47 | 0.23 | 0.12 | 0.09 | 1.35 ± 1.14 | 1.35 ± 1.14 | 1.35 ± 1.14 | 0.00 | 1.00 | 0.009 | 0.009 | 1.95 | 0.16 |
| SDSCA-FC | 0.45 ± 0.61 | 0.48 ± 0.72 | 0.41 ± 0.69 | 0.68 | 0.49 | 0.04 | 0.06 | 0.45 ± 1.22 | 0.45 ± 1.22 | 0.45 ± 1.22 | 0.00 | 1.00 | 0.008 | 0.008 | 0.001 | 1.00 |
HBA1C: glycosylated haemoglobin; DQOL=Diabetes quality of life, SDSCA: summary of diabetes self-care activities schedule; GD: general diet; SD: specific diet, EX: exercise; GT: glucose testing, FC: foot care. Higher scores indicate better QoL.
TABLE 3. Within- and Between-Group Differences (RMANOVA) on Measures at Different Time Points and Effect Sizes Noted in the SMI and TAU Groups on Barriers to Self-care, Illness Perceptions, and Mood.
| Variable | SMI Mean (SD) | Within-Group Effects | Effect Size | TAU Mean (SD) | Within-Group Effects | Effect Size | Between-Group Effects | |||||||||
| Pre | Post | F/U | F | Sig (2-Tailed) | Post | F/U | Pre | Post | F/U | F | Sig (2-Tailed) | Post | F/U | F | Sig (2-Tailed) | |
| BSC-diet | 4.45 ± 0.99 | 3.86 ± 0.91 | 3.78 ± 0.93 | 63.26*** | 0.001 | 0.62a | 0.69a | 4.24 ± 0.96 | 4.26 ± 0.98 | 4.28 ± 0.96 | 1.44 | 0.24 | 0.02 | 0.04 | 1.18 | 0.27 |
| BSC-exercise | 3.96 ± 0.89 | 3.48 ± 0.96 | 3.38 ± 0.99 | 40.93*** | 0.001 | 0.51a | 0.61a | 3.87 ± 0.92 | 3.94 ± 0.92 | 3.94 ± 0.94 | 4.35* | 0.02 | 0.02 | 0.07 | 2.22 | 0.14 |
| BSC-M | 2.14 ± 0.97 | 2.06 ± 0.89 | 2.01 ± 0.78 | 5.70** | 0.01 | 0.08 | 0.14 | 1.99 ± 0.67 | 2.01 ± 0.66 | 2.00 ± 0.65 | 0.22 | 0.63 | 0.01 | 0.01 | 0.20 | 0.65 |
| BSC-GT | 2.64 ± 1.29 | 2.52 ± 1.25 | 2.49 ± 1.24 | 3.68* | 0.04 | 0.10 | 0.07 | 2.73 ± 1.74 | 2.73 ± 1.74 | 2.73 ± 1.74 | 0.59 | 0.48 | 0.005 | 0.011 | 0.28 | 0.59 |
| BSC-GEN | 3.64 ± 0.68 | 3.37 ± 0.71 | 3.30 ± 0.77 | 18.58*** | 0.001 | 0.38 | 0.51a | 3.50 ± 0.86 | 3.51 ± 0.88 | 3.53 ± 0.87 | 0.59 | 0.50 | 0.011 | 0.03 | 0.19 | 0.66 |
| BSC-total | 3.34 ± 0.77 | 3.06 ± 0.72 | 3.00 ± 0.69 | 31.72*** | 0.001 | 0.37 | 0.50a | 3.26 ± 0.75 | 3.30 ± 0.75 | 3.31 ± 0.75 | 4.26* | 0.01 | 0.05 | 0.06 | 0.85 | 0.35 |
| IPQ-I | 3.57 ± 2.46 | 3.10 ± 1.94 | 2.98 ± 1.77 | 5.39** | 0.001 | 0.18 | 0.02 | 3.25 ± 2.38 | 3.60 ± 2.42 | 3.55 ± 2.34 | 6.52** | 0.007 | 0.14 | 0.13 | 0.26 | 0.60 |
| IPQ-T | 23.85 ± 3.66 | 25.17 ± 3.09 | 25.47 ± 2.44 | 8.69** | 0.002 | 0.38 | 0.54a | 23.63 ± 3.34 | 23.63 ± 3.24 | 23.62 ± 3.22 | 0.001 | 1.00 | 0.003 | 0.003 | 3.23 | 0.07 |
| IPQ-C | 24.28 ± 3.32 | 24.38 ± 3.15 | 24.38 ± 3.20 | 0.18 | 0.69 | 0.03 | 0.03 | 24.05 ± 2.81 | 23.97 ± 2.87 | 23.95 ± 2.75 | 0.45 | 0.59 | 0.02 | 0.03 | 0.27 | 0.60 |
| IPQ-PC | 19.50 ± 3.53 | 23.40 ± 3.35 | 23.93 ± 3.72 | 39.71*** | 0.001 | 1.14 | 1.23 | 20.47 ± 4.29 | 20.45 ± 4.27 | 20.50 ± 4.28 | 0.42 | 0.60 | 0.004 | 0.007 | 4.80 | 0.03* |
| IPQ-TC | 15.25 ± 2.18 | 18.10 ± 2.67 | 18.30 ± 2.77 | 45.45*** | 0.001 | 1.20a | 1.26a | 15.73 ± 2.44 | 15.62 ± 2.44 | 15.68 ± 2.47 | 0.49 | 0.50 | 0.017 | 0.02 | 8.85 | 0.004** |
| IPQ-IC | 14.70 ± 4.12 | 17.67 ± 2.93 | 17.97 ± 2.91 | 31.91*** | 0.001 | 0.83a | 0.92a | 15.25 ± 3.84 | 15.18 ± 4.08 | 15.20 ± 4.08 | 0.26 | 0.62 | 0.03 | 0.012 | 4.04 | 0.04* |
| IPQ- CYC | 13.30 ± 2.25 | 13.60 ± 2.43 | 13.18 ± 2.18 | 1.50 | 0.23 | 0.13 | 0.05 | 13.17 ± 2.65 | 13.27 ± 2.56 | 13.25 ± 2.55 | 1.08 | 0.32 | 0.03 | 0.03 | 0.05 | 0.81 |
| IPQ-ER | 22.72 ± 2.51 | 19.67 ± 2.57 | 18.78 ± 3.00 | 51.33*** | 0.001 | 1.21a | 1.42a | 22.57 ± 2.14 | 22.60 ± 2.45 | 22.73 ± 2.40 | 0.81 | 0.44 | 0.04 | 0.08 | 19.37 | 0.001*** |
| HADS-D | 4.15 ± 3.07 | 3.13 ± 2.59 | 2.60 ± 2.29 | 22.13*** | 0.001 | 0.36 | 0.58a | 4.18 ± 2.73 | 4.07 ± 2.65 | 4.18 ± 2.65 | 0.47 | 0.54 | 0.04 | 0.003 | 2.15 | 0.14 |
| HADS-A | 4.65 ± 1.96 | 3.90 ± 1.61 | 3.38 ± 1.42 | 30.63*** | 0.001 | 0.42 | 0.81a | 4.05 ± 2.98 | 4.05 ± 2.68 | 4.02 ± 2.61 | 0.02 | 0.92 | 0.003 | 0.010 | 0.91 | 0.89 |
BSC: barriers to self-care, IPQ: illness perception questionnaire, CYC: cyclical, T: timeline, C: consequences, PC: personal control, TC: treatment control, HADS: hospital anxiety and depression scale, D: depression, Anx: anxiety >0.20: small effect size, >0.50: medium effect size, >0.80: large effect size. aModerate to large effect size. Higher scores indicate better QoL. ***P < 0.001, **P < 0.01, *P < 0.05.
Within- and Between-Group Differences on HbA1c and QOL
There was a significant within-group difference on HbA1c from baseline (Mean = 8.58) to post intervention (Mean = 8.19) in the SMI group. A significant time effect was observed; there was a further decrease in HbA1c even after the termination of SMI (8.08; P = 0.001). In the TAU group, there was a significant increase in HbA1c level (P < 0.01), indicating a worsening of glycemic control. There were no significant between-group differences in the RMANOVA on HbA1c. However, between-groups comparison on the t-test at the postintervention and 3 month postintervention follow-up showed a significant difference in HbA1c (t = 2.51 and t = 2.95, Ps < 0.01). Larger ES (Tables 2 and 4) were noted for the completer sample on HbA1c in the SMI group at postintervention and follow-up points (0.63 and 1.06, respectively), as compared to TAU completer sample (0.19 and 0.12 at postintervention and three-month postintervention follow-up, respectively).
TABLE 4. Effect Sizes of the Primary Outcome Measures for the SMI and TAU Groups at Post-treatment and Three-Month Follow-Up (Completer Sample).
| Variable | SMI Group | TAU Group | ||
| Cohen’s d (Baseline to Post) (N = 30) |
Cohen’s d (Baseline to Follow-Up) (N = 26) |
Cohen’s d (Baseline to Post) (N = 25) |
Cohen’s d (Baseline to Follow-Up) (N = 15) |
|
| HbA1c | 0.63 | 1.06 | 0.19 | 0.12 |
| DQOL-SAT | 0.50 | 1.31 | 0.19 | 0.18 |
| DQOL-IMP | 0.07 | 0.02 | 0.22 | 0.04 |
| DQOL-1 | 0.14 | 1.39 | 0.22 | 0.01 |
| DQOL-TOT | 0.21 | 0.62 | 0.22 | 0.22 |
| SDSCA-GD | 0.93 | 0.96 | 0.26 | 0.22 |
| SDSCA-SD | 0.99 | 0.93 | 0.07 | 0.27 |
| SDSCA-EX | 0.84 | 1.27 | 0.18 | 0.11 |
| SDSCA-GT | 0.30 | 0.36 | 0.03 | 0.07 |
| SDSCA-FC | 0.09 | 0.09 | 0.02 | 0.02 |
| BSC-D | 0.91 | 0.99 | 0.10 | 0.12 |
| BSC-E | 0.69 | 0.95 | 0.13 | 0.32 |
| BSC-M | 0.10 | 0.17 | 0.01 | 0.01 |
| BSC-GT | 0.15 | 0.26 | 0.04 | 0.04 |
| BSC-GEN | 0.41 | 0.52 | 0.07 | 0.07 |
| BSC-TOT | 0.12 | 0.81 | 0.10 | 0.11 |
| IPQ-I | 0.29 | 0.40 | 0.16 | 0.12 |
| IPQ-T | 0.49 | 0.69 | 0.27 | 0.28 |
| IPQ-C | 0.03 | 0.05 | 0.02 | 0.05 |
| IPQ-PC | 1.68 | 2.13 | 0.02 | 0.01 |
| IPQ-TC | 1.84 | 2.14 | 0.003 | 0.04 |
| IPQ-IC | 1.19 | 1.40 | 0.06 | 0.10 |
| IPQ-CYC | 0.17 | 0.05 | 0.02 | 0.003 |
| IPQ-ER | 2.02 | 2.66 | 0.23 | 0.05 |
| HADS-D | 0.52 | 0.89 | 0.22 | 0.07 |
| HADS-A | 0.52 | 1.02 | 0.12 | 0.15 |
Effect size: >0.20—small effect size, >0.50—medium effect size; >0.80—large effect size.
Significant improvements were noted on DQOL-impact (P < 0.01), DQOL-single item (P = 0.001), and DQOL-total (P = 0.001) in the SMI group. Significant differences were observed at the 3-month postintervention follow-up on DQOl-single item (P = 0.001) in SMI. There was no significant within-group difference in the TAU group on DQOL-single item. There were no significant between-group differences on DQOL. However, large within-group ES was noted in the SMI (completer and ITT sample) as compared to the TAU group (Tables 2 and 4).
Within- and Between-Group Differences on Self-Care, Perceived Barriers, Illness Perceptions, and Mood (ITT)
There was an improvement in adherence on the domains of SDSCA (Ps < 0.001) in the SMI group (Table 3). There was a significant change in the perception of barriers with respect to diabetes self-care, including glucose testing (P < 0.05), general, and total barriers in the SMI group (P = 0.001). Large ESs were obtained for subscales of general diet (general recommendations for healthy eating) and specific diet (specific questions on consumption of healthy/ unhealthy food) and exercise for both ITT and completer samples at postintervention and three-month postintervention follow-up, ranging 0.54–0.87 (Tables 2 and 4). In the TAU group, there were significant differences on overall BSC, barriers to exercise, and general diet (Ps ≤ 0.05; Table 2).
The SMI group demonstrated a significant change in illness perceptions on most dimensions, except on perception of illness as being recurring and its long-term consequences. The TAU group demonstrated significant change on the identity domain of IPQ (P < 0.01). Large ES (ITT and completer samples) were obtained on the timeline (duration of illness), illness coherence (meaning regarding illness), personal and treatment control (control over illness and treatment effectiveness), and emotional representations (mood) in SMI group at postintervention and three-month postintervention follow-up (0.54–1.26 in ITT and 0.40–2.14 in completer sample, Tables 3 and 4).
Significant between-group differences at postintervention were noted on IPQ-R dimensions of personal and treatment control (P = 0.001), emotional representation, and meaning regarding illness (illness coherence) (P < 0.01), with the SMI group doing better on these domains (Table 3). Significant between-group differences were observed at the 3-month postintervention follow-up on these variables. Similar results were obtained on independent sample t-test for between-group differences on personal and treatment control, illness coherence, and emotional representation at postintervention and 3-month postintervention follow-up (P = 0.001).
There was a significant within-group difference in the SMI group on HADS (P = 0.001), which was not observed in the TAU group (Table 2). On HADS, larger ES was noted for both anxiety and depression at follow-up in the ITT sample of the SMI group than postintervention (Table 3). ES was larger in the completer sample (SMI) than the TAU (Table 4).
Discussion
Our findings support the effectiveness of SMI in improving HbA1c, QOL, self-care, perceived barriers, illness perceptions, and mood in persons with type 2 diabetes. Given the lower acceptance of psychological interventions for medical conditions in India, 75% of the participants enrolled were completers. This maybe an indicator of the feasibility and acceptability of the intervention.
There was a decrease in HbA1c levels at post-treatment, with a further decrease at 3-month postintervention follow-up, indicating continued improvement even after active SMI. The inclusion of techniques to improve diabetes self-care as a behavioral goal and practicing these activities may have resulted in a further decrease in the HbA1c levels. This is supported by medium ESs for HbA1c levels at post-treatment and follow-up. Results with respect to improvements in HbA1c following psychosocial interventions are equivocal.36, 37
Diabetes QOL improved significantly between post-treatment and 3-month postintervention follow-up. This could be attributed to possible changes in HbA1c, illness perceptions, perception of barriers, and mood. The ES on DQOL-total and DQOL-single item at post-treatment and three-month follow-up in the SMI group further support this finding. The brief nature of the intervention is likely to have been a reason for the absence of significant changes in other domains of the DQOL scale. Similar findings have been reported in the literature.38
The frequency of engaging in diabetes self-care activities increased significantly in the SMI group. ESs on domains of diet and exercise, in the SMI group, maybe a result of the focus of the intervention on improving dietary habits and physical activity. Our findings are in line with a large body of evidence supporting the efficacy of SMI in improving adherence to diabetes care regimens.26, 39–42
There was a significant decrease in perceived BSC, indicating a change in the cognitive process related to pursuing behavioral tasks following the SMI. This is further supported by the ESs on barriers to diet, exercise, general barriers such as being busy or having visitors, and total barriers. Participants in the SMI group were taught barrier identification and problem-solving skills. Findings at follow-up suggest that participants may have continued to apply the skills, resulting in a further reduction in perceived barriers during the follow-up. Changes in dimensions timeline, personal and treatment control, illness coherence, and emotional representations of the IPQ- R suggest that they may have developed more adaptive beliefs about the illness. The ESs obtained on the dimensions of the IPQ can be attributed to the content of the SMI.
The SMI was efficacious in reducing anxiety, distress, and depression. Diabetes distress may result from a perceived inability to cope with the demands of living with diabetes.43 Large to medium ES on HADS in the SMI group may be attributed to skills training in managing mood, an experience of well-being, and better metabolic control. Literature has documented ESs for mood and related constructs in the range of 0.32–0.78.18, 39-41
Attrition was an important factor in this study. Non-pharmacological interventions report variable rates of attrition. Attrition in psychotherapy research and, in particular, diabetes self-management programs is challenging, with rates up to 79%.18 Reasons for attrition in the present are similar to those reported globally, including work schedules, poor knowledge and confidence, apathy, and lack of familiarity with services.44 Better physician–patient communication and interventions to improve attitudinal and general barriers are important in enhancing adherence.
The study has some obvious limitations. It was carried out at a single center, and the increased therapist contact in the SMI group may have impacted findings to some extent. Patients who required changes in medication were not analyzed, as they did not fulfill study criteria, but due to ethical reasons, they continued to receive the psychological intervention, with no change in the allocation of the group. Recruitment was based on specified criteria, affecting the generalizability of the findings. The inclusion criterion of a specific range of HbA1c (7.5%–10.0%) and exclusion criteria of comorbid psychiatric conditions, substance dependence, and other microvascular complications resulted in the exclusion of a large number of participants screened for eligibility. Severely ill patients with multiple comorbid conditions were excluded, as a brief program would not be sufficient for their needs. A longer follow-up is necessary to examine the durability of change. Tools were translated and back-translated into local languages. However, translations were not validated in the present study. There is also a possibility of social desirability in responses as self-report measures were used. The inclusion of other outcomes related to medical comorbidities such as lipid profile, blood pressure, and BMI is important. The absence of an independent rater for psychological variables was another limitation. The use of the last observation brought forward method for missing data does not take into account the variability of the results and may be less suitable for a study of this nature.
Conclusions
The findings support the effectiveness of a culturally appropriate, brief intervention based on cognitive behavioral principles in improving self-care and reducing distress, with an improvement in HbA1C levels following the intervention. This brief, focused intervention could be delivered by health care professionals during regular follow-ups. Findings, however, need further replication, with larger samples and longer follow-up.
Acknowledgment
The authors would like to thank Dr DK Subba Krishna, Professor of Biostatistics (Retd), for his inputs on the research design and statistical procedures.
Declaration of Conflicting Interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Sprangers MAG, de Regt EB, Andries F. Which chronic conditions are associated with better or poorer quality of life? J Clin Epidemiol; 2000; 53: 895–907. [DOI] [PubMed] [Google Scholar]
- 2.Cavan D, Fernandes JR, Makaroff L. et al. eds. IDF diabetes atlas. 7th ed. Brussels, Belgium: International Diabetes Federation (IDF), 2015: 90–93. http://www.idf.org/idf-diabetes-atlas-seventh-edition. [Google Scholar]
- 3.Varghese C Riley N Harvey A eds.. Global report on diabetes. Geneva: World Health Organization, 2016: 25–29. http://www.who.int/diabetes/global-report/en/. [Google Scholar]
- 4.Jayawardena R, Ranasinghe P, Byrne NM. et al. Prevalence and trends of the diabetes epidemic in South Asia: a systematic review and meta-analysis. BMC Public Health; 2012; 12: 380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Delamater AM, Jacobson AM, Anderson BJ. et al. Psychosocial therapies in diabetes: report of the psychosocial therapies working group. Diabetes Care; 2001; 24: 1286–1292. [DOI] [PubMed] [Google Scholar]
- 6.Sridharan SG, Chittem M, Muppavaram N. A review of literature on diabetes self-management: Scope for research and practice in India. J Soc Health Diabetes; 2016; 4: 108–114. [Google Scholar]
- 7.Bandura A. Social foundations of thought and action: a social cognitive theory. 2nd ed. Englewood Cliffs, NJ: Prentice-Hall, 1986. [Google Scholar]
- 8.Baranowski T, Perry CL, Parcel GS. How individuals, environments, and health behavior interact: social cognitive theory. In: Glanz K, Lewis FM, Rimer B, eds. Health behavior and health education: theory, research, and practice. San Francisco, CA: Jossey-Bass, 1997: 165–184. [Google Scholar]
- 9.Armitage CJ, Conner M. Social cognition models and health behavior: a structured review. Psychic Health; 2007; 15: 173–189. [Google Scholar]
- 10.Glasgow RE. Perceived barriers to self-management and preventive behaviors. National Cancer Institute, 2003. http://cancercontrol.cancer.gov/brp/constructs/barriers/barriers.pdf Accessed July11, 2011. [Google Scholar]
- 11.Young-Hyman D, de Groot M, Hill-Briggs F. et al. Psychosocial care for people with diabetes: a position statement of the American Diabetes Association. Diabetes Care; 2016; 39: 2126–2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ciechanowski PS, Kason WJ, Russo JE. Depression and diabetes: impact of depressive symptoms on adherence, function, and costs. Arch Intern Med; 2000; 160: 3278–3285. [DOI] [PubMed] [Google Scholar]
- 13.Lustman PJ, Griffith LS, Freedland KE. et al. Fluoxetine for depression in diabetes. Diabetes Care; 2000; 23: 618–623. [DOI] [PubMed] [Google Scholar]
- 14.Wexler DJ, Grant RW, Wittenberg E. et al. Correlates of health related quality of life in type 2 diabetes. Diabetologia; 2006; 49: 1489–1497. [DOI] [PubMed] [Google Scholar]
- 15.Gonzalez JS, Steven A, Safren SA. et al. Depression, self-care, and medication adherence in type 2 diabetes relationships across the full range of symptom severity. Diabetes Care; 2007. Sep; 30(9): 2222–2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Funnell MM. The Diabetes, Attitudes, Wishes and Needs (DAWN) study. Clin Diabetes; 2006; 24: 154–155. [Google Scholar]
- 17.Kalra S, Brush MP. Discrimination and diabetes: insight from the second Diabetes Attitudes Wishes and Needs (DAWN2) study. J Soc Health Diabetes; 2015; 3: 56–57. [Google Scholar]
- 18.Norris SL, Engelgau MM, Narayan KMV. Effect of self-management training in type 2 diabetes. Diabetes Care; 2001; 24: 561–587. [DOI] [PubMed] [Google Scholar]
- 19.Peyrot M, Rubin RR. Behavioral and psychosocial interventions in diabetes. Diabetes Care; 2007; 30: 2433–2440. [DOI] [PubMed] [Google Scholar]
- 20.Ismail K, Winkley K, Rabe-Hesketh S. Systematic review and meta-analysis of randomised controlled trials of psychological interventions to improve glycaemic control in patients with type 2 diabetes. Lancet; 2004; 363: 1589–1597. [DOI] [PubMed] [Google Scholar]
- 21.Safren SA, Gonzalez JS, Wexler DJ. et al. A randomized controlled trial of cognitive behavioral therapy for adherence and depression (CBT-AD) in patients with uncontrolled type 2 diabetes. Diabetes Care; 2014; 37: 625–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tripp-Reimer Choi E, Kelley LS. et al. Cultural barriers to care: inverting the problem. Diabetes Spectr; 2001; 14: 13–22. [Google Scholar]
- 23.Gopichandran V, Lyndon S, Angel MK. et al. Diabetes self-care activities: a community-based survey in urban Southern India. Natl Med J India; 2012; 25: 14–17. [PubMed] [Google Scholar]
- 24.Abraham AM, Sudhir PM, Philip M. et al. Illness perceptions and perceived barriers to self-care in patients with type 2 diabetes mellitus: an exploratory study from India. Int J Diabetes Dev Ctries; 2015; 35: 1–18. [Google Scholar]
- 25.Kalra S, Sridhar GR, Balhara YP. et al. National recommendations: psychosocial management of diabetes in India. Indian J Endocrinol Metab; 2013; 17: 76–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Raveendranathan D, George J, Perumal NL. et al. The effectiveness of a brief psychological intervention for patients with diabetes-related distress. Indian J Psychol Med; 2019. Jul–Aug; 41(4): 357–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beeney LJ, Bakry AA, Dunn SM. Patient psychological and information needs when the diagnosis is diabetes. Patient Educ Couns; 1996. Oct; 29(1): 109–116. [DOI] [PubMed] [Google Scholar]
- 28.Abraham AM. Development and feasibility of a self-management intervention in type 2 diabetes mellitus [unpublished doctoral thesis]. Bengaluru: National Institute of Mental Health and Neurosciences, 2014 [Google Scholar]
- 29.Jacobson A, Barofsky I, Cleary P. et al. Reliability and validity of a diabetes quality-of-life measure for the diabetes control and complications trial (DCCT). Diabetes Care; 1988; 11: 725–732. [DOI] [PubMed] [Google Scholar]
- 30.Toobert DJ, Glasgow RE. Assessing diabetes self-management: the summary of diabetes self-care activities questionnaire. In: Bradley C, ed. Handbook of psychology and diabetes research and practice. Berkshire, England: Harwood Academic, 1994: 351–375. [Google Scholar]
- 31.Toobert DJ, Sampson SE, Glasgow RE. The summary of diabetes self-care measure: results from seven studies and a revised scale. Diabetes Care; 2000; 23: 943–950. [DOI] [PubMed] [Google Scholar]
- 32.Glasgow RE. Social-environmental factors in diabetes: barriers to diabetes self-care. In: Bradley C, ed. Handbook of psychology and diabetes research and practice. Berkshire, England: Harwood Academic, 1994: 335–349. [Google Scholar]
- 33.Moss- Morris R, Weinman J, Petrie KJ. et al. The revised illness perceptions questionnaire (IPQ-R). Psychol Health; 2002; 17: 1–16. [Google Scholar]
- 34.Leventhal H, Cameron L. Behavioral theories and the problem of compliance. Patient Educ Couns; 1987; 10: 117–138. [Google Scholar]
- 35.Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Hillsdale, NJ: Lawrence Earlbaum Associates, 1988. [Google Scholar]
- 36.Forjuoh SN, Bolin JN, Huber JC. et al. Behavioral and technological interventions targeting glycemic control in a racially/ethnically diverse population: a randomized controlled trial. BMC Public Health; 2014; 14:14–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Steed L, Cooke D, Newman SA. Systematic review of psychosocial outcomes following education, self-management and psychological interventions in diabetes mellitus. Patient Educ Couns; 2005; 51: 1–15. [DOI] [PubMed] [Google Scholar]
- 38.de Weerdt I, Visser A, Kok G. et al. Randomized controlled evaluation of an education programme for insulin treated patients with diabetes: effects on psychosocial variables. Patient Educ Couns; 1989; 14: 191–215. [Google Scholar]
- 39.Glasgow RE, Toobert D, Hampson SE. Effects of a brief office-based intervention to facilitate diabetes dietary self-management. Diabetes Care; 1996; 19: 835–842. [DOI] [PubMed] [Google Scholar]
- 40.Christian JG, Bessesen DH, Byers TE. et al. Clinic-based support to help overweight patients with type 2 diabetes increase physical activity and lose weight. Arch Intern Med; 2008; 168: 141–146. [DOI] [PubMed] [Google Scholar]
- 41.Sacco WP, Malone JI, Morrison AD. et al. Effect of a brief, regular telephone intervention by paraprofessionals for type 2 diabetes. J Behav Med; 2009; 32: 349–359. [DOI] [PubMed] [Google Scholar]
- 42.Tang TS, Funnell MM, Oh M. Lasting effects of a 2-year diabetes self-management intervention: outcomes at 1 year follow-up. Prev Chronic Dis; 2012; 9: 1103–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kalra S, Verma K, Singh Balhara YP. Management of diabetes distress. J Pak Med Assoc; 2017. Oct; 67(10): 1625–1627. [PubMed] [Google Scholar]
- 44.Gucciardi E, DeMelo M, Offenheim A. et al. Factors contributing to attrition behavior in diabetes self-management programs: a mixed method approach. BMC Health Serv Res; 2008; 8: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]