Abstract
Immune checkpoint inhibitor (ICI) therapy is now in widespread clinical use for the treatment of lung cancer. Although patients with autoimmune disease and other comorbidities were excluded from initial clinical trials, emerging real-world experience suggests that these promising treatments may be administered safely to individuals with inactive low-risk autoimmune disease such as rheumatoid arthritis or psoriasis, mild-to-moderate renal and hepatic dysfunction, and certain chronic viral infections. Considerations for ICI in autoimmune disease populations include exacerbations of the underlying autoimmune disease, increased risk of ICI-induced immune-related adverse events, and potential for compromised efficacy if patients are receiving chronic immunosuppression. ICI use in higher-risk autoimmune conditions, such as myasthenia gravis or multiple sclerosis, requires careful evaluation on a case-by-case basis. ICI use in individuals with solid organ transplant carries a substantial risk of organ rejection. Ongoing research into the prediction of ICI efficacy and toxicity may help in patient selection, treatment, and monitoring.
Keywords: autoimmune disease, immune checkpoint inhibitors, immune-related adverse events, immunosuppression, transplant
Immune checkpoint inhibitors (ICI) are now in widespread clinical use for the treatment of lung cancer and multiple other malignancies. Indeed, it is estimated that approximately 44% of patients with advanced cancer may be candidates for ICI-based therapy (1). In some cases, ICI have truly revolutionized cancer treatment. In metastatic melanoma, a cancer highly resistant to conventional chemotherapy, combination ICI results in profound and prolonged responses in a majority of patients (2). In a minority of patients, ICI has complicated the assessment of efficacy, as clinicians may need to distinguish between progression, hyperprogression, and pseudoprogression (3).
From a clinician’s standpoint, it is the potential for autoimmune toxicity that truly sets ICI apart from chemotherapy and molecularly targeted therapies. So-termed immune-related adverse events (irAE) occur when ICI-induced immune cell activation cross-reacts with normal tissues. irAE may affect almost any organ, including the brain, pituitary, eyes, thyroid, lungs, heart, liver, pancreas, colon, kidneys, adrenal, skin, joints, and muscles (4). In contrast to the well-characterized onset of classical chemotherapy toxicities—such as alopecia, nausea/vomiting, and myelosuppression—irAE may occur at almost any point during ICI treatment. In some cases, they may not develop until months after the last ICI dose (4).
As have individual clinicians, the field of immuno-oncology has gained experience and wisdom related to irAE. In early clinical trials of anti-programmed death 1 (PD1) and anti-PD1 ligand (PDL-1) therapies in lung cancer, nearly 3% of patients developed fatal autoimmune pneumonitis (5). Now that medical oncologists and their colleagues immediately consider the possibility of this irAE in patients with new clinical or radiographic respiratory findings, lethal cases are exceedingly rare (6). Similarly, ICI effects on complex physiologic pathways such as the hypothalamic-pituitary-adrenal axis have led to recommendations for routine monitoring of endocrine function and early consultation with relevant experts.
Occasionally, combination therapies have revealed largely unanticipated toxicities. For instance, a study of combined atezolizumab (anti-PDL1) and the epidermal growth factor receptor inhibitor osimertinib demonstrated a pulmonary toxicity rate exceeding 50%, even though each agent independently causes pneumonitis in fewer than five percent of cases (7). In contrast, certain combinations anticipated to cause substantial toxicity have been surprisingly well tolerated. In a phase 3 clinical trial, the administration of consolidation durvalumab (anti-PDL1) for up to one year after chemoradiation for locally advanced non-small cell lung cancer (NSCLC) led to high-grade pneumonitis in only 3% of patients, comparable to rates seen with chemoradiation alone (8).
Although ICI has introduced new toxicities, in other areas ICI therapy clearly presents fewer risks than other treatments. The classic toxicities of cytotoxic chemotherapy are alopecia, nausea/vomiting, and myelosuppression leading to cytopenias. These events occur only rarely with ICI. Particularly relevant to patient safety, effects on bone marrow or circulating blood cells occur in well under five percent of individuals (9).
Approach to comorbidities
Based on these observations and experiences, it has become abundantly clear that patient selection and monitoring for ICI differ substantially from those for chemotherapy and molecularly targeted therapy. Nevertheless, which clinical factors truly influence ICI efficacy and safety remain unclear. We searched clinicaltrials.gov for completed and resulted lung cancer trials using the terms immune checkpoint inhibitors, checkpoint inhibitors, ICI, nivolumab, ipilimumab, pembrolizumab, atezolizumab, avelumab, and durvalumab. As shown in Table 1 and Supplemental Table 1, eligibility criteria vary widely among these trials. We also searched clinicaltrials.gov with the same search terms to identify currently recruiting trials to characterize recent trends in eligibility criteria (Supplemental Table 2). Over time, we found relatively little change in trial eligibility, with frequent and arbitrary exclusion of autoimmune disease (AID), immunosuppression, organ dysfunction, and chronic viral infections. Although expansion of ICI to these populations represents an area of major interest to researchers and clinicians, we identified only five active or forthcoming trials specifically investigating ICI use in patients with comorbidities (NCT 04499053, 04108026, 04473703, 04514484, 03313544).
Table 1.
Selected lung cancer immune checkpoint inhibitor trials without chemotherapy and comparison of eligibility criteria for comorbidities, based on clinicaltrials.gov.
| Trial / NCT# / Reference | Phase | Year | Autoimmune disease |
Systemic Immune suppression |
End-organ function | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Renal | Liver | Bone Marrow | Interstitial Lung Disease |
Heart Failure |
HIV | Viral Hepatitis |
|||||
| Atezolizumab as First-line Monotherapy for Advanced or Metastatic NSCLC (B-F1RST) (NCT02848651) | II | 2016 | Excluded | Immunosuppression within 14 days excluded | ** | ** | ** | Excluded | Excluded | Excluded | Excluded |
| Combined Ionizing Radiation and Ipilimumab in Metastatic NSCLC (NCT02221739) | II | 2014 | Excluded | Excluded | Creatinine ≤ 3.0 x ULN | AST/ALT
≤ 2.5 x ULN ≤ 5 x ULN if liver metastases present. Bilirubin ≤ 3 x ULN |
WBC ≥
2 x 109/ L ANC ≥ 1 x 109/ L Platelets ≥ 50 x 109/ L Hemoglobin ≥ 8 g/dL |
||||
| Pembrolizumab in Participants With Advanced NSCLC (MK-3475-025/KEYNOTE-025) (NCT02007070) (114) | IB | 2013 | AID requiring steroids or immunosup pression excluded | Immunosuppression within 3 days excluded | Excluded | ||||||
| Atezolizumab in Participants With PD-L1 Positive Locally Advanced or Metastatic NSCLC (BIRCH) (NCT02031458) | II | 2013 | Excluded | Excluded | Excluded | Excluded | |||||
| Atezolizumab in Participants With PD-L1 Positive Locally Advanced or Metastatic NSCLC [FIR] (NCT01846416) | II | 2013 | ** | ** | ** | ||||||
| Ipilimumab in Previously Untreated Subjects With NSCLC or SCLC (NCT00527735) | II | 2017 | Excluded | Chronic steroid use excluded | Serum
creatinine <2.5 x ULN; CrCl ≥ 50 mL/min |
Excluded for:
Total bilirubin level >2 x ULN, or ≥2.5 x ULN if liver
metastases present, or AST/ALT ≥2.5 x ULN or ≥5 x ULN if liver metastases present |
Hemoglobin
≥ 9 g/dL ANC ≥ 1.5 x 109/ L Platelet count ≥ 100 x 109 /L |
Excluded | Excluded | ||
| Avelumab in NSCLC (JAVELIN Lung 200) (NCT02395172) (115) | III | 2015 | Selected AID allowed | Steroid equivalent > 10 mg prednisone/day excluded | CrCl ≥ 30 mL/min | Bilirubin
≤ 1.5 x ULN AST and ALT ≤ 2.5 x ULN |
WBCs ≥
2.5 × 109/L ANC ≥ 1.5 × 10^9/L Lymphocytes ≥ 0.5 × 10^9/L Platelets ≥100 × 10^9/L Hemoglobin ≥ 9 g / dL |
||||
| Pembrolizumab in Locally Advanced or Metastatic Carcinoma, Melanoma, or NSCLC (P07990/MK-3475-001/KEYNOTE-001) (KEYNOTE-001) (NCT01295827) (116) | I | 2011 | Excluded | Excluded | ** | ** | ** | Excluded | Excluded | Excluded | |
| First-Line Nivolumab Versus Investigator’s Choice Chemotherapy for Stage IV or Recurrent PD-L1+ NSCLC (CheckMate 026) (NCT02041533) (117) | III | 2014 | Excluded | ||||||||
Parameters required but not specified in clinicaltrials.gov
Empty cells indicate no information listed in clinicaltrials.gov
AID – autoimmune disease
ALT – alanine aminotransferase
ANC – absolute neutrophil count
AST – aspartate aminotransferase
CrCl – creatinine clearance
NSCLC – non-small cell lung cancer
SCLC – small cell lung cancer
ULN – upper-limit of normal
WBC – white blood cell
Understanding the efficacy and safety of ICI in diverse patient populations will be critical to realizing the full potential of these therapies. It is estimated that stringent eligibility criteria may exclude up to 70% of patients with lung cancer from ICI clinical trials (10, 11). Lung cancer populations may be particularly susceptible to such exclusions. In the U.S., the average at diagnosis is over 70 years, substantially older than average age for other common malignancies such as breast and colorectal cancer (12). Directly relevant to ICI considerations, incidence of AID increases with age (13). Renal function also decreases with age (14). Separately, more than 80% of individuals with lung cancer are current or former smokers (15), an exposure that conveys risk of both chronic pulmonary conditions and autoimmune diseases (16, 17).
To fill the immense gap between rarefied ICI clinical trial populations and actual lung cancer patients seen in clinical practice, numerous observational studies from real-world settings have emerged. Some of these reveal clear differences with trial reports. For instance, single-agent anti-PD1/PDL1 trials generally report rates of pneumonitis between 3-5% (18). However, observational patient series describe incidence closer to 20% (18). Whether this discrepancy reflects characteristics of the treated populations or the inherent challenges of diagnosing and characterizing irAE (19) is not clear. Additionally, retrospective data—particularly for small case series and case reports—may be susceptible to publication bias, with authors and editors choosing to publish events with unexpectedly good or particularly poor outcomes (20).
For this review, we identified relevant publications by performing Ovid MEDLINE and Ovid Embase searches of articles published from inception through June 2020 using the terms lung, pulmonary, respiratory, cancer, neoplasm, tumor, malignancy, comorbidity, autoimmune disease, immunotherapy, checkpoint inhibitor, ipilimumab, nivolumab, pembrolizumab, atezolizumab, avelumab, durvalumab, anti-PD1, anti-PDL1, anti-CTLA4, infection, and performance status. Because concerns such as flare of pre-existing AID or irAE do not appear to reflect tumor biology, we included reports of all cancer types. Based on this literature, we provide an overview of the medical comorbidities that have potential relevance to ICI therapy, and discuss how these conditions influence trial design, treatment selection, and clinical monitoring.
Autoimmune disease
An estimated 20 to 50 million individuals in the U.S. have an AID, including up to 14 to 25% of patients with lung cancer (21). While these conditions have not generally represented a major consideration for conventional chemotherapy or molecularly targeted therapies, they are generally restricted or excluded entirely from ICI clinical trials. Some trials have excluded patients with “active” AID (such as those requiring corticosteroids equivalent to prednisone >10 mg daily) (22). Some studies have excluded patients with any history of autoimmune disease (23). Other trials determine eligibility according to the potential morbidity of the AID, allowing enrollment of patients with low-risk AID, such as psoriasis and vitiligo (24).
Complicating these considerations is the inherent challenge of diagnosing AID. Establishing a cancer diagnosis is generally a straightforward process relying on pathologic evaluation of biopsy material. By contrast, an AID diagnosis may incorporate clinical history, physical examination findings, laboratory, radiology, and histologic data. Individual diagnostic components may be neither sufficiently sensitive nor specific. For instance, antinuclear antibodies—a characteristic finding of such AID as lupus, scleroderma, Sjogren’s, and dermatomyositis/polymyositis—may be elevated in more than one quarter of healthy adults (25). The impact of this diagnostic uncertainty is apparent in the wide range of estimated AID cases among the general population and among individuals with cancer (21).
AID-related concerns center on potential toxicity, including heightened rates of irAE and exacerbation of the underlying AID. AID flares represent a wide spectrum of potential clinical severity. Worsening of conditions such as rheumatoid arthritis and psoriasis are unlikely to be life threatening. However, an acute exacerbation of myasthenia gravis could result in phrenic nerve paresis, diaphragmatic dysfunction, and respiratory failure. Similarly, a flare of multiple sclerosis could have immediate and profound effects on critical functions such as vision.
Given the almost universal exclusion of patients with AID from ICI clinical trials, clinicians have published a number of observational series of patients with AID treated with approved ICI regimens (Table 2). These studies vary in size, cohort characteristics, and outcomes. One study reported occurrence of AID flare in 23% of patients, but no apparent increase in risk of ICI-associated irAE (26). Most AID flares were minor and responded to immunosuppression; none required ICI therapy discontinuation (26). Another series identified increased incidence of grade 1 and 2 irAE in those with a history of AID (including both “active” and “inactive” cases), but no increased risk of grade 3 and 4 irAE (27). Other observational studies have similarly found increased risk of irAE in those with pre-existing AID (28, 29). Patients with an isolated high anti-nuclear antibody titer without associated clinical features of AID appear to tolerate ICI therapy, although those with titers ≥1:320 may require heightened monitoring due to increased risk of irAE (30).
Table 2.
Summary of published reports of immune checkpoint inhibitor use in patients with autoimmune disease.
| Autoimmune Disease Category |
Summary of Findings | Reference |
|---|---|---|
| Gastrointestinal (Inflammatory Bowel Disease) |
Case reports including five patients (3 CD, 2 UC), multiple cancer and ICI types, concurrent immunosuppression, two with AID flare, no irAE, three with anti-tumor response. | (47, 48, 118, 119) |
| Observational study with six patients (3 CD, 3 UC), NSCLC, treated with anti-PD-(L)1 therapy, none with AID flare, three with irAE. Individual efficacy not reported. | (26) | |
| Observational studies with nine patients (5 UC, 4 CD), melanoma treated with ipilimumab, two with AID flare, two with irAE, one with anti-tumor response. | (120, 121) | |
| Observational study with six patients (3 CD, 2 UC, 1 Celiac disease), melanoma treated with anti-PD-(L)1 therapy, none with AID flare. Individual efficacy and irAE not reported. | (122) | |
| Observational study with one patient with UC, melanoma treated with anti-PD-(L)1 therapy, with AID flare, without irAE, with anti-tumor response. | (123) | |
| Observational studies with 17 patients (14 unspecified, 3 CD), multiple cancers treated with multiple ICI therapies, 11 with AID flare, seven with irAE. Individual efficacy not reported. | (27, 124) | |
| Overall: 44 patients, 16 with AID flare, 12 with irAE, 20 with no irAE, 5 with anti-tumor response, 8 with no anti-tumor response. | ||
| Neurologic | Observational study with three patients (2 MS, 1 MG), NSCLC treated with anti-PD-(L)1 therapy, none with AID flare, two with irAE. Individual efficacy not reported. | (26) |
| Observational studies with four patients (3 MS, 1 LETM), melanoma treated with ipilimumab, one with AID flare, two with irAE, none with anti-tumor response. | (120, 121) | |
| Observational study with five patients (2 GBS, 1 MG, 1 CIDP, 1 Bell’s palsy), melanoma treated with anti-PD-(L)1 therapy, none with AID flare. Individual efficacy and irAE not reported. | (122) | |
| Review of 13 patients with MG, multiple cancer types treated with anti-PD-1 therapy, 11 with AID flare. Individual efficacy and irAE not reported. | (125) | |
| Case reports of two patients with MS, melanoma treated with ipilimumab, with AID flare, no irAE, with anti-tumor response. | (126, 127) | |
| Observational study with two patients (1 MS, 1 GBS), melanoma treated with anti-PD-(L)1 therapy, none with AID flare, one with irAE, none with anti-tumor response. | (123) | |
| Observational studies with five patients (3 MS, 2 MG), multiple cancer and ICI types, none with AID flare. Individual efficacy and irAE not reported. | (124, 128) | |
| Observational study with one patient with optic neuritis, unspecified cancer and ICI type, with AID flare, with with irAE. Individual efficacy not reported. | (27) | |
| Overall: 35 patients, 14 with AID flare, 4 with irAE, 7 with no irAE, 1 with anti-tumor response, 5 with no anti-tumor response. | ||
| Rheumatologic | Observational study with 25 patients (11 RA, 5 PMR, 4 seronegative arthritis, 2 scleroderma, 2 psoriatic arthritis, 1 SLE), NSCLC treated with anti-PD-(L)1 therapy, 10 with AID flare, six with irAE. Individual efficacy not reported. | (26) |
| Observational studies with 30 patients (20 RA, 4 sarcoidosis, 3 spondylitis, 2 SLE, 1 CREST syndrome) melanoma treated with ipilimumab, 15 with AID flare, 12 with irAE, eight with anti-tumor response. | (120, 121, 129) | |
| Observational study with 27 patients (13 RA, 3 sarcoidosis, 3 PMR, 2 SLE, 2 scleroderma, 2 psoriatic arthritis, 2 SS), melanoma treated with anti-PD-(L)1 therapy, 14 with AID flare. Individual efficacy and irAE not reported. | (122) | |
| Case reports with two patients with RA, melanoma treated with multiple ICI types, no AID flare, no irAE, two with anti-tumor response. | (126, 130) | |
| Observational study with seven patients (2 sarcoidosis, 2 spondylitis, 1 RA, 1 PMR, 1 myositis), melanoma treated with anti-PD-(L)1 therapy, five with AID flare, two with irAE, three with anti-tumor response. | (123) | |
| Observational study with seven patients (4 SS, 2 RA, 1 PMR), multiple cancer and ICI types, one with AID flare. Individual efficacy and irAE not reported. | (128) | |
| Observational studies with 62 patients (24 RA, 9 PMR, 9 SLE, 5 spondylitis, 4 sarcoidosis, 4 systemic sclerosis, 3 SS, 1 APS, 1 DM, 1 unspecified CTD), multiple cancer and ICI types, 19 with AID flare, 20 with irAE. Individual efficacy not reported. | (27, 124) | |
| Observational study with 16 patients (5 RA, 5 PMR, 2 SS, 2 SLE, 1 spondylitis, 1 sarcoidosis), multiple cancer and ICI types, two with AID flare, five with irAE, six with anti-tumor response. | (131) | |
| Overall: 176 patients, 66 with AID flare, 45 with irAE, 97 with no irAE, 19 with anti-tumor response, 33 with no anti-tumor response. | ||
| Endocrine | Observational study with nine patients (5 Graves’ thyroiditis, 4 Hashimoto’s thyroiditis), NSCLC treated with anti-PD-(L)1 therapy, one with AID flare, three with irAE. Individual efficacy not reported. | (26) |
| Observational studies with 17 patients (12 unspecified thyroiditis, 5 Hashimoto’s thyroiditis) melanoma treated with ipilimumab, two with AID flare, two with irAE, one with anti-tumor response. | (120, 121) | |
| Observational study with four patients with Grave’s disease, melanoma treated with anti-PD-(L)1 therapy, one with AID flare. Individual efficacy and irAE not reported. | (122) | |
| Observational study with six patients with autoimmune thyroiditis, melanoma treated with anti-PD-(L)1 therapy, one with AID flare, one with irAE, one with anti-tumor response. | (123) | |
| Observational studies with 17 patients (8 autoimmune thyroiditis, 4 Grave’s disease, 3 Hashimoto’s thyroiditis, 1 type 1 diabetes, 1 autoimmune hypophysitis), multiple cancer and ICI types, one with AID flare. Individual efficacy and irAE not reported. | (124, 128) | |
| Observational study with 51 patients (10 Grave’s disease, 41 autoimmune thyroiditis), multiple cancer and ICI types, 22 with AID flare, 19 with irAE. Individual efficacy not reported. | (27) | |
| Overall: 104 patients, 28 with AID flare, 29 with irAE, 54 with no irAE, 1 with anti-tumor response, 22 with no anti-tumor response. | ||
| Dermatologic | Observational study with 16 patients (14 psoriasis, 1 alopecia areata, 1 discoid lupus), NSCLC treated anti-PD-(L)1 therapy, four with AID flare, seven with irAE. Individual efficacy not reported. | (26) |
| Observational studies with 14 patients (12 psoriasis, 2 autoimmune urticaria), melanoma treated with ipilimumab, four with AID flare, six with irAE, five with anti-tumor response. | (120, 121) | |
| Observational study with eight patients (6 psoriasis, 1 eczema, 1 erythema nodosum), melanoma treated with anti-PD-(L)1 therapy, three with AID flare. Individual efficacy and irAE not reported. | (122) | |
| Case report of patient, melanoma treated with sequential ipilimumab and pembrolizumab, severe AID flare, no irAE, with anti-tumor response. | (132) | |
| Observational study with three patients with psoriasis, melanoma treated with anti-PD-(L)1 therapy, one with AID flare, none with irAE, none with anti-tumor response. | (123) | |
| Observational study with 33 patients (17 vitiligo, 12 psoriasis, 4 unspecified), multiple cancer and ICI types, eight with AID flare. Individual efficacy and irAE not reported. | (128) | |
| Observational study with 45 patients (45 psoriasis), multiple cancer and ICI types, 28 with AID flare, 17 with irAE. Individual efficacy not reported. | (27, 124) | |
| Overall: 120 patients, 49 with AID flare, 30 with irAE, 50 with no irAE, 6 with anti-tumor response, 13 with no anti-tumor response. | ||
| Overall | Overall: 479 patients, 171 with AID flare, 120 with irAE, 228 with no irAE, 32 with anti-tumor response, 81 with no anti-tumor response. |
AID – autoimmune disease
APS – anti-phospholipid syndrome
BD – Behcet’s disease
CD – Crohn’s disease
CIDP – chronic inflammatory demyelinating polyneuropathy
CTD – connective tissue disease
DM - dermatomyositis
GBS – Guillain-Barre syndrome
ICI – immune checkpoint inhibitor
irAE – immune-related adverse event
LETM – longitudinal extensive transverse myelitis
MG – myasthenia gravis
MS – multiple sclerosis
NSCLC – non-small cell lung cancer
PMR – polymyalgia rheumatica
PD-(L) – programmed death-(ligand)
RA – rheumatoid arthritis
SLE – systemic lupus erythematosus
SS – Sjogren’s syndrome
UC – ulcerative colitis
There is relatively little data on ICI use in high-risk AID. ICI administration may induce relapse of multiple sclerosis, in some instances leading to rapid neurological progression and death (31). A case report has described the feasibility of ICI in a patient with granulomatosis with polyangiitis (a form of vasculitis affecting the respiratory and renal systems), although the disease was not clinically active and the patient was receiving immunosuppression (32).
Relevant to ICI administration and monitoring, some cancer diagnoses predispose patients to autoimmune phenomena. Autoimmune paraneoplastic conditions may affect multiple organ systems, including the nervous system, connective tissue and skin, and blood cells. These events arise from immune cross-reactivity between tumor cells and healthy tissue. For autoimmune paraneoplastic neurologic syndromes (eg, limbic encephalitis, cerebellar degeneration, and myasthenia gravis), overrepresented cancer types tend to either (1) produce neuroendocrine proteins (eg, small cell lung cancer, neuroblastoma), (2) contain neuronal components (eg, teratoma), (3) involve immunoregulatory organs (eg, thymic tumors), or (4) affect immunoglobulin production (eg, lymphoma, myeloma) (33). The safety profile of ICI in these malignancies varies widely. Despite one of the highest associations with paraneoplastic neurologic syndromes of any cancer, small cell lung cancer treated with ICI has rates of irAE and other autoimmune phenomena comparable to those of other lung cancer subtypes (34, 35). By contrast, ICI trials in thymic tumors have demonstrated rates of grade ≥3 irAE up to 40% (36-38), suggesting that, independent of a patient’s history of autoimmune disease, these rare malignancies represent at least a relative contraindication to ICI use.
Immunosuppression
Use of immunosuppressive therapies in patients with lung cancer is relatively common. AID and solid organ transplant are common indications for chronic immunosuppression. These regimens may include corticosteroids, calcineurin inhibitors, mammalian target of rapamycin inhibitors, and antimetabolites. Additionally, corticosteroids are frequently employed for their anti-inflammatory and anti-emetic properties in this population, with specific indications including management of brain metastasis, spinal cord compression, dyspnea, fatigue, decreased appetite, chronic obstructive pulmonary disease, and prevention of nausea and vomiting (39, 40).
Given the immunosuppressive effects of corticosteroids, in particular negative effects on T-cell function (41, 42), there is concern that concurrent steroid use may reduce the efficacy of ICI. Accordingly, patients taking steroids above specified thresholds (eg, prednisone equivalent ≥10 mg/day) are frequently excluded from ICI clinical trials. Early observational studies found statistically significant and clinically meaningful associations between baseline corticosteroid use and worse outcomes in patients receiving ICI therapy, with an overall survival hazard ratio 1.7 for those receiving baseline steroids (95% confidence interval 1.3-2.2) (43, 44). Subsequent analyses have found that the poor prognosis associated with steroid indications—such as neurologic symptoms or anorexia—may drive these observations, with no difference in progression-free or overall survival between patients receiving less than 10 mg prednisone equivalent per day versus those receiving greater than 10 mg prednisone equivalent per day for non-cancer related indications (45). In contrast, treatment of ICI-induced immune-related adverse events (irAE) with corticosteroids does not appear to worsen outcomes (46). It seems plausible that these findings reflect the clear link between irAE and ICI benefit, which may counteract the potential negative effects of ICI interruption and corticosteroid administration.
Case reports have described patients with active AID receiving immunosuppression achieving good outcomes with cancer immunotherapy (47, 48), but evidence is limited and subject to publication bias. To optimize ICI efficacy, some experts advocate transitioning from non-selective immunosuppression (eg, corticosteroids) to targeted agents (such as infliximab and tocilizumab) for specific AIDs prior to ICI initiation (49), an approach that would require validation in prospective trials.
ICI use in individuals with solid organ transplants appears to convey substantial clinical risk (Table 3). One review of these cases found that transplant rejection was common (37%) and was the most common cause of death (50). Nevertheless, there are several reports of ICI administration resulting in anti-tumor efficacy without inducing graft rejection. However, the potential for reporting and publication bias in these instances seems quite high. Accordingly, ICI use in solid organ transplant recipient should be approached with extreme caution. On a case-by-case basis, some clinicians may distinguish between organ transplants for which there is routinely available alternate support (eg, hemodialysis for kidney transplant) and those lacking such options (eg, heart, lung, liver transplant) when considering potential use of ICI in this population.
Table 3.
Summary of Evidence for ICI use in organ transplant.
| Organ | Total Cases | Graft Rejection | Anti-tumor Response |
Comment |
|---|---|---|---|---|
| Kidney | 32 | 13 | 10 | Most responses (6) occurred in patients with no graft rejection. |
| Liver | 20 | 7 | 5 | All 5 responses occurred in patients with no graft rejection. |
| Heart | 5 | 1 | 1 | Single response occurred in patient with no graft rejection. |
Adapted from Fisher et al (50).
End-organ dysfunction
For conventional chemotherapy and molecularly targeted therapies, laboratory evidence of end-organ dysfunction (eg, elevated creatinine, elevated bilirubin, or reduced blood counts indicating renal, hepatic, and bone marrow dysfunction, respectively) may require avoidance or adjustment of certain agents. Specific thresholds are regularly included among clinical trial eligibility criteria (51). Such guidance reflects the potential for increased toxicity on already compromised organ systems (pharmacodynamic effects) or reduced clearance of the anti-neoplastic agent (pharmacokinetic effects). For ICI therapies, these pharmacokinetic considerations may not apply, as their clearance is not substantially impacted by renal or hepatic function, and instead relies predominantly on non-specific degradation within plasma and tissues (52). However, baseline organ function may be relevant to pharmacodynamic concerns.
The most widely used measure of medical comorbidities is the Charlson comorbidity index, which includes conditions such as heart disease and cancer, and predicts one year mortality (53). Trials specifically evaluating ICI therapy in patients with high comorbidity scores or organ dysfunction are limited (53). A small observational study evaluating ICI therapy use in those with mild cardiac, renal, and/or liver dysfunction found that ICI was well tolerated did not worsen baseline organ function (54).
Pulmonary
Because most patients with lung cancer are current or former smokers, a substantial proportion have comorbid pulmonary disease. A number of retrospective studies have demonstrated that pre-existing interstitial lung disease (ILD) increases risk of immune-mediated pneumonitis in patients receiving ICI (55-57). A single-center series of 102 patients also showed a trend towards increased risk of ICI-associated pneumonitis in patients with pre-existing chronic obstructive pulmonary disease (COPD) (58). This observation may reflect the inflammatory nature of COPD, which features increased CD8 T-cell numbers and PD-L1 expression (59). However, other studies suggest no detrimental effect on pulmonary function or symptoms in patients with COPD receiving ICI (60).
Cardiac
Cardiac irAE are well characterized but quite rare, with myocarditis estimated to occur in fewer than 1% of patients treated with ICI (61). Although one case series found myocarditis occurs more frequently in those with underlying cardiovascular risk factors, most patients who develop myocarditis have previously normal cardiac function (61). Unfortunately, further data on the safety of these therapies in individuals with underlying cardiac disease is limited (62). Nevertheless, recommendations for monitoring for these events in this population have emerged, including baseline cardiology evaluation and assessment of cardiac enzymes (including high-sensitivity troponin) every six weeks for at least twelve weeks after ICI initiation (63). While it is not clear whether individuals with reduced ejection fraction at baseline have increased risk of ICI-induced myocarditis, these patients do have less functional reserve should such as event occur.
Liver
ICI therapy may cause autoimmune hepatitis. While anti-PD1 therapy has been studied in and is now approved for hepatocellular carcinoma, reports of ICI use in patients with underlying liver failure or cirrhosis are extremely limited (64). ICI use in patients with viral hepatitis is discussed in the infectious disease section below.
Renal
The development of clinically significant ICI-induced nephrotoxicity is relatively rare (approximately 2% of irAEs (65)), and risk factors for immune-mediated nephritis are poorly understood (65). Furthermore, it is unclear if renal disease impacts the development of other irAE. A study of 78 patients found increased risk of irAE in those with stage 3 to 4 chronic kidney disease (CrCl < 60 mL/min) (28), but this finding has not been replicated in other studies. A retrospective analysis of 414 patients found worse baseline renal function and use of proton pump inhibitors were associated with ICI-induced acute kidney injury (66).
Performance status/Age
Historically, patients with poor performance status (Eastern Cooperative Oncology Group [ECOG] ≥2) and elderly patients have been excluded from or underrepresented in clinical trials, even though these individuals account for more than 50% of the overall lung cancer population (67). Although early ICI clinical trials restricted enrollment to ECOG 0-1 performance status (PS), more recent studies have included patients with worse functional status. The phase II CheckMate 171 trial of nivolumab in advanced squamous NSCLC reported no difference in treatment-related adverse events in ECOG 2 patients compared to the ECOG 0-1 population; however, ECOG 2 patients had inferior overall survival (median 5.4 months in ECOG PS 2 cohort versus 9.9 months in total cohort)(68), consistent with historical data in this population (69). Comparable findings were reported in the phase III/IV CheckMate 153 study of nivolumab for previously treated advanced NSCLC (70). A meta-analysis across cancer types found no difference in outcomes between patients with ECOG 0 and ECOG 1-2, although there were relatively few patients in the ECOG 2 category (71). Other observational studies reported no safety concerns in patients with worse PS, but again identified inferior survival (72-77). However, one single retrospective evaluation of 190 patients identified an increased risk of high-grade irAE in those with ECOG PS 2 to 3 (78). There are clearly substantial challenges to determining the benefit of ICI therapy in patients with poor functional status, as they tend to have inherently poor prognosis independent of treatment (53, 79). The phase III eNERGY trial (NCT 03351361) comparing first-line combination ICI therapy to cytotoxic therapy in elderly patients and those with poor performance status has completed enrollment and will provide more insights into these under-studied populations.
A single-center study of 75 patients found that older patients (age ≥70 years) tolerated ICI therapy without an increased safety signal, and that the poor overall survival in this group was driven by poor PS (median survival 13.7 months for ECOG 0 to 1, compared to 3.8 months for ECOG ≥2) (80). A larger study of 245 patients found no increased risk of toxicity with age, although patients age >80 years had substantially lower overall survival (median 3.6 months) compared to those age 70 to 79 years (median 12.9 months) (81). Additional observational evidence found no new safety signals in older patients receiving ICI therapy (82, 83). Furthermore, two meta-analyses of ICI clinical trials found that patients age ≥65 years derive similar clinical benefit to younger patients, and actually may have a lower incidence of grade 3 and 4 irAE (84, 85). These findings suggest that advanced age alone should not be considered a contraindication to ICI therapy.
Infectious disease
A number of studies have evaluated ICI use in individuals with human immunodeficiency virus (HIV) infection. Hypothetically, administration of immunotherapy to a population with suppressed and dysregulated immunity raises both efficacy and safety concerns. Nevertheless, a growing body of evidence supports the safety and efficacy of ICI therapy in patients infected with HIV (86-88). Specifically, ICI responses have been documented in patients with low CD4+ counts (89). While a single study identified a heightened risk of irAE, particularly pneumonitis (24%), in patients with HIV (90), other studies have not replicated this observation (91). Ongoing clinical trials are evaluating ICI use in this population prospectively (92).
To date, relatively little information is available on ICI use in chronic viral hepatitis. Limited case series have demonstrated ICI safety and efficacy in patients with hepatitis B and hepatitis C (93, 94).
Historically, acute infections have been considered relative contraindications to the administration of chemotherapy due to concerns that myelosuppression could worsen severity and prolong duration of the infection. For ICI, there have been few studies addressing this clinical question. It has been observed that concurrent diagnosis of lung cancer and respiratory tract infection is associated with worse ICI outcomes, although may be due to reduced treatment exposure (95). Recent or ongoing infection may also influence efficacy of ICI through possible alteration of host microbiome and immune system (96). Additionally, there appears to be a link between antibiotic exposure and ICI outcomes (97). The underlying mechanism appears to be dysregulation of the fecal microbiome, which may persist for months after antibiotic use. Microbiome changes appear not only to influence ICI efficacy, but may also increase the risk or irAE, in particular colitis (97-99).
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic has implications on ICI therapy for patients with lung cancer. Patients with lung cancer are at particular risk of Coronavirus Disease 2019 (COVID-19) given their underlying malignancy and frequent comorbidities (100). Diagnosis of COVID-19 in lung cancer patients receiving ICI is particularly difficult, as its non-specific symptoms of dyspnea, cough, and fever could also represent tumor progression, pulmonary embolism, exacerbation of respiratory comorbidities such as COPD, or immune-mediated pneumonitis (which may have similar radiographic features as COVID-19) (100, 101). ICI administration raises hypothetical concerns about increasing COVID-19 severity, as morbidity and death may result from cytokine storm (102). Indeed, tocilizumab (an interleukin-6 receptor antibody occasionally used to treat severe ICI-induced irAE) has been investigated as a potential treatment for COVID-19 (102). At this time, there is limited clinical evidence of the safety and efficacy of ICI therapy in patients with COVID-19 infection. For patients currently on ICI therapy without COVID-19 infection, it may be reasonable to continue therapy, with consideration of increasing the dosing interval (such as pembrolizumab 400 mg every 6 weeks) to minimize exposure risk (100). Some experts have recommended ICI treatment breaks for patients with long-term disease control during the COVID-19 pandemic (100). Nevertheless, recent observations suggest that patients with lung cancer and recent COVID-19 infection treated with ICI had outcomes comparable to similar patients receiving other therapies (103). In newly diagnosed, treatment-naive patients with history of COVID-19 infection, the optimal therapeutic approach is even less clear. Based on the hypothetical risk of cytokine storm, some authors have suggested initially withholding ICI therapy in favor of cytotoxic chemotherapy (100).
Published guideline recommendations
While oncology society guidelines infrequently address specific comorbidities, treatment recommendations routinely incorporate functional status. In many cases, this guidance reflects the data available from clinical trials. The most recent European Society for Medical Oncology (ESMO) guidelines recommend first-line ICI therapy for ECOG 0-1 patients, but they extend ICI recommendations to those with ECOG 2 in the second-line setting (104). Likewise, the latest American Society of Clinical Oncology (ASCO) guidelines endorse first-line ICI therapy for ECOG 0-1 patients, but do not comment on those with worse performance status (105). The ESMO and ASCO guidelines do not address specific comorbidities.
The National Comprehensive Cancer Network (NCCN) guidelines consider AID, use of immunosuppression, or presence of a driver oncogene (which may correlate with lack of therapeutic benefit from ICI) as potential contraindications to ICI therapy (106). The NCCN does not specify a PS threshold for single-agent ICI therapy in those with tumor PD-L1 expression ≥50%, but does restrict recommendations for combination ICI and chemotherapy to ECOG 0 to 1 patients (106). For patients with squamous non-small cell lung cancer and ECOG 2, the NCCN recommends cytotoxic chemotherapy rather than ICI therapy.
Discussion and Recommendations
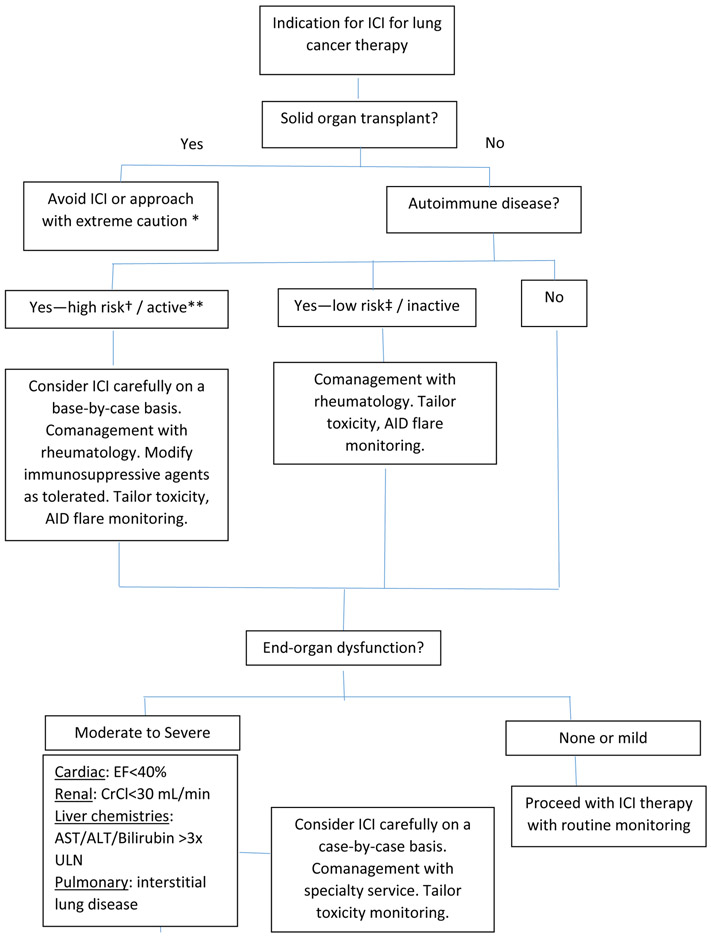
Although ICI therapy has been in widespread use for lung cancer and other malignancies for several years, relatively little is known about the use of these agents in patients with comorbidities. Based on the available evidence, we have developed a suggested approach to ICI therapy in this population (Figure 1).
Figure 1.
Algorithm for treating lung cancer with ICI in patients with comorbidities.
*In rare cases, potential exception for kidney transplant (see text).
**Receiving prednisone ≥10 mg daily or other immunosuppressive agents.
†Eg, myasthenia gravis, multiple sclerosis
‡Eg, psoriasis, rheumatoid arthritis
In considering the relevance of medical comorbidities to ICI administration, it is important to note clear differences between these agents and conventional chemotherapy and targeted therapies: (1) the clearance of immune checkpoint inhibitors does not depend on renal and hepatic function to the extent that other treatments do; (2) ICI almost never cause cytopenias, substantial nausea and vomiting, or alopecia—the classic triad of chemotherapy toxicities; (3) ICI have a unique mechanism of action, relying on the patient’s own immune system to induce cancer cell death; (4) they have also introduced novel toxicities, namely immune-related adverse events; (5) there is no option for dose modification of ICI; these drugs are either given at full dose, temporary withheld, or permanently discontinued.
An important consideration in the use of ICI is the role of clinical monitoring. Currently, recommended laboratory assessments include renal, hepatic, thyroid, pituitary, and adrenal function. Radiographic surveillance for pulmonary toxicities is not routinely performed, so regular thoracic imaging may only occur if indicated for assessment of response to therapy. Nor is routine monitoring for cardiac or intestinal toxicities undertaken. As clinicians consider administering ICI to patients at higher risk for toxicities, adjusting the components and frequency of monitoring may mitigate potential risk. Additionally, biomarkers for the prediction of immune-related adverse events represent an area of ongoing investigation (107-110).
Perhaps more so than for other cancer treatments, comorbidities may affect the efficacy of ICI, as well as safety. Conditions requiring chronic immunosuppression may render an individual less likely to benefit from immune checkpoint inhibition. Similarly, antibiotic exposure may reduce ICI efficacy through modification of the microbiome. Understanding the influence of these clinical factors is important because lung cancer patients who progress on an initially selected therapy may not be able to receive subsequent treatment if they decline clinically at the time of progression. Additionally, ICI are costly therapies that may lead to substantial financial burden on patients and their families (111). Indeed, some economic analyses suggest that many of these agents are not cost-effective for their standard of care indications (112, 113).
In conclusion, at this point in the clinical development of ICI for lung cancer treatment, there are few prospective studies evaluating the safety and efficacy of these therapies in patients with comorbidities. While trials in selected populations such as certain AID are currently underway, clinicians must make real-world decisions now about which patients to offer these promising but potentially toxic treatments. Retrospective, observational studies suggest that ICI administration may be feasible in the elderly, individuals with HIV, HCV, or HBV infection, and patients with low-risk and inactive AID. Use of ICI in transplant recipients and patients with high-risk AID (such as myasthenia gravis and multiple sclerosis) appears to convey substantial morbidity, is unlikely to be studied in future clinical trials, and cannot be routinely recommended.
Supplementary Material
Supplemental Table 1. Selected lung cancer immune checkpoint inhibitor trials with chemotherapy and comparison of eligibility criteria for comorbidities, based on clinicaltrials.gov.
Supplemental Table 2. Selected planned novel lung cancer immune checkpoint inhibitor trials in 2020 without chemotherapy and comparison of eligibility criteria for comorbidities, based on clinicaltrials.gov.
Acknowledgements:
The authors thank Ms. Dru Gray for assistance with manuscript preparation.
Disclosures/ funding information: Funded in part by a National Cancer Institute Midcareer Investigator Award in Patient-Oriented Research (K24 CA201543-01), the National Institute of Allergy and Infectious Disease (1U01AI156189-01), an American Cancer Society-Melanoma Research Alliance Team Award (MRAT-18-114-01-LIB), a V Foundation Robin Roberts Cancer Survivorship Award (DT2019-007), the University of Texas Lung Cancer Specialized Program in Research Excellence (SPORE, P50-CA-070907-08S1), and The University of Texas Southwestern Medical Center - Dallas holds a Physician-Scientist Institutional Award from the Burroughs Wellcome Fund.
Footnotes
Conflicts of interests: The authors report no relevant conflicts of interest.
References
- 1.Haslam A, Gill J, Prasad V. Estimation of the Percentage of US Patients With Cancer Who Are Eligible for Immune Checkpoint Inhibitor Drugs. JAMA Netw Open. 2020;3(3):e200423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eggermont AMM, Crittenden M, Wargo J. Combination Immunotherapy Development in Melanoma. Am Soc Clin Oncol Educ Book. 2018;38:197–207. [DOI] [PubMed] [Google Scholar]
- 3.Popat V, Gerber DE. Hyperprogressive disease: a distinct effect of immunotherapy? J Thorac Dis. 2019;11(Suppl 3):S262–S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Postow MA, Sidlow R, Hellmann MD. Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. N Engl J Med. 2018;378(2):158–68. [DOI] [PubMed] [Google Scholar]
- 5.Gettinger SN, Horn L, Gandhi L, Spigel DR, Antonia SJ, Rizvi NA, et al. Overall Survival and Long-Term Safety of Nivolumab (Anti-Programmed Death 1 Antibody, BMS-936558, ONO-4538) in Patients With Previously Treated Advanced Non-Small-Cell Lung Cancer. J Clin Oncol. 2015;33(18):2004–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mok TSK, Wu YL, Kudaba I, Kowalski DM, Cho BC, Turna HZ, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet. 2019;393(10183):1819–30. [DOI] [PubMed] [Google Scholar]
- 7.Oshima Y, Tanimoto T, Yuji K, Tojo A. EGFR-TKI-Associated Interstitial Pneumonitis in Nivolumab-Treated Patients With Non-Small Cell Lung Cancer. JAMA Oncol. 2018;4(8):1112–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N Engl J Med. 2017;377(20):1919–29. [DOI] [PubMed] [Google Scholar]
- 9.Michot JM, Lazarovici J, Tieu A, Champiat S, Voisin AL, Ebbo M, et al. Haematological immune-related adverse events with immune checkpoint inhibitors, how to manage? Eur J Cancer. 2019;122:72–90. [DOI] [PubMed] [Google Scholar]
- 10.Yoo SH, Keam B, Kim M, Kim TM, Kim DW, Heo DS. Generalization and representativeness of phase III immune checkpoint blockade trials in non-small cell lung cancer. Thoracic Cancer. 2018;9(6):736–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schulkes KJ, Nguyen C, van den Bos F, van Elden LJ, Hamaker ME. Selection of Patients in Ongoing Clinical Trials on Lung Cancer. Lung. 2016;194(6):967–74. [DOI] [PubMed] [Google Scholar]
- 12.National Cancer Institute B, MD. SEER Cancer Stat Facts: Lung and bronchus cancer Accessed on August 13, 2020 [Available from: https://seer.cancer.gov/statfacts/html/lungb.html.
- 13.Goronzy JJ, Weyand CM. Immune aging and autoimmunity. Cell Mol Life Sci. 2012;69(10):1615–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weinstein JR, Anderson S. The aging kidney: physiological changes. Adv Chronic Kidney Dis. 2010;17(4):302–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walser T, Cui X, Yanagawa J, Lee JM, Heinrich E, Lee G, et al. Smoking and lung cancer: the role of inflammation. Proc Am Thorac Soc. 2008;5(8):811–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Speyer CB, Costenbader KH. Cigarette smoking and the pathogenesis of systemic lupus erythematosus. Expert Rev Clin Immunol. 2018;14(6):481–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang K, Yang SM, Kim SH, Han KH, Park SJ, Shin JI. Smoking and rheumatoid arthritis. Int J Mol Sci. 2014;15(12):22279–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suresh K, Voong KR, Shankar B, Forde PM, Ettinger DS, Marrone KA, et al. Pneumonitis in Non-Small Cell Lung Cancer Patients Receiving Immune Checkpoint Immunotherapy: Incidence and Risk Factors. J Thorac Oncol. 2018;13(12):1930–9. [DOI] [PubMed] [Google Scholar]
- 19.Hsiehchen D, Watters MK, Lu R, Xie Y, Gerber DE. Variation in the Assessment of Immune-Related Adverse Event Occurrence, Grade, and Timing in Patients Receiving Immune Checkpoint Inhibitors. JAMA Netw Open. 2019;2(9):e1911519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nissen T, Wynn R. The clinical case report: a review of its merits and limitations. BMC Res Notes. 2014;7:264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khan SA, Pruitt SL, Xuan L, Gerber DE. Prevalence of Autoimmune Disease Among Patients With Lung Cancer: Implications for Immunotherapy Treatment Options. JAMA Oncol. 2016;2(11):1507–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hammers HJ, Plimack ER, Infante JR, Rini BI, McDermott DF, Lewis LD, et al. Safety and Efficacy of Nivolumab in Combination With Ipilimumab in Metastatic Renal Cell Carcinoma: The CheckMate 016 Study. J Clin Oncol. 2017;35(34):3851–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horn L, Spigel DR, Vokes EE, Holgado E, Ready N, Steins M, et al. Nivolumab Versus Docetaxel in Previously Treated Patients With Advanced Non-Small-Cell Lung Cancer: Two-Year Outcomes From Two Randomized, Open-Label, Phase III Trials (CheckMate 017 and CheckMate 057). J Clin Oncol. 2017;35(35):3924–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sharma P, Retz M, Siefker-Radtke A, Baron A, Necchi A, Bedke J, et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial. Lancet Oncol. 2017;18(3):312–22. [DOI] [PubMed] [Google Scholar]
- 25.Wandstrat AE, Carr-Johnson F, Branch V, Gray H, Fairhurst AM, Reimold A, et al. Autoantibody profiling to identify individuals at risk for systemic lupus erythematosus. J Autoimmun. 2006;27(3):153–60. [DOI] [PubMed] [Google Scholar]
- 26.Leonardi GC, Gainor JF, Altan M, Kravets S, Dahlberg SE, Gedmintas L, et al. Safety of Programmed Death-1 Pathway Inhibitors Among Patients With Non-Small-Cell Lung Cancer and Preexisting Autoimmune Disorders. J Clin Oncol. 2018;36(19):1905–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cortellini A, Buti S, Santini D, Perrone F, Giusti R, Tiseo M, et al. Clinical Outcomes of Patients with Advanced Cancer and Pre-Existing Autoimmune Diseases Treated with Anti-Programmed Death-1 Immunotherapy: A Real-World Transverse Study. Oncologist. 2019;24(6):e327–e37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kartolo A, Sattar J, Sahai V, Baetz T, Lakoff JM. Predictors of immunotherapy-induced immune-related adverse events. Current Oncology. 2018;25(5):e403–e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Danlos FX, Voisin AL, Dyevre V, Michot JM, Routier E, Taillade L, et al. Safety and efficacy of anti-programmed death 1 antibodies in patients with cancer and pre-existing autoimmune or inflammatory disease. European Journal of Cancer. 2018;91:21–9. [DOI] [PubMed] [Google Scholar]
- 30.Yoneshima Y, Tanaka K, Shiraishi Y, Hata K, Watanabe H, Harada T, et al. Safety and efficacy of PD-1 inhibitors in non-small cell lung cancer patients positive for antinuclear antibodies. Lung cancer (Amsterdam, Netherlands). 2019;130:5–9. [DOI] [PubMed] [Google Scholar]
- 31.Garcia CR, Jayswal R, Adams V, Anthony LB, Villano JL. Multiple sclerosis outcomes after cancer immunotherapy. Clinical & translational oncology : official publication of the Federation of Spanish Oncology Societies and of the National Cancer Institute of Mexico. 2019;21(10):1336–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamada T, Masuda T, Yamaguchi K, Sakamoto S, Horimasu Y, Miyamoto S, et al. Non-small Cell Lung Cancer Treated by an Anti-programmed Cell Death-1 Antibody without a Flare-up of Preexisting Granulomatosis with Polyangiitis. Internal medicine (Tokyo, Japan). 2019;58(21):3129–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pelosof LC, Gerber DE. Paraneoplastic syndromes: an approach to diagnosis and treatment. Mayo Clin Proc. 2010;85(9):838–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paz-Ares L, Dvorkin M, Chen Y, Reinmuth N, Hotta K, Trukhin D, et al. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet. 2019;394(10212):1929–39. [DOI] [PubMed] [Google Scholar]
- 35.Horn L, Mansfield AS, Szczesna A, Havel L, Krzakowski M, Hochmair MJ, et al. First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer. N Engl J Med. 2018;379(23):2220–9. [DOI] [PubMed] [Google Scholar]
- 36.Cho J, Kim HS, Ku BM, Choi YL, Cristescu R, Han J, et al. Pembrolizumab for Patients With Refractory or Relapsed Thymic Epithelial Tumor: An Open-Label Phase II Trial. J Clin Oncol. 2019;37(24):2162–70. [DOI] [PubMed] [Google Scholar]
- 37.Giaccone G, Kim C, Thompson J, McGuire C, Kallakury B, Chahine JJ, et al. Pembrolizumab in patients with thymic carcinoma: a single-arm, single-centre, phase 2 study. Lancet Oncol. 2018;19(3):347–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arun Rajan CRH, Susan Perry, Corrine Keen, Mammen Andrew L, Berman Arlene W., Stefania Pittaluga, Lauren Marissa Lepone, Renee Nicole Donahue, Italia Grenga, Jeffrey Schlom, Raffit Hassan, Gulley James L.. Safety and clinical activity of anti-programmed death-ligand 1 (PD-L1) antibody (ab) avelumab (MSB0010718C) in advanced thymic epithelial tumors (TETs). DOI: 101200/JCO20163415_supple20106 Journal of Clinical Oncology 34, no 15_suppl Published online May 20, 2016. [Google Scholar]
- 39.Ryken TC, McDermott M, Robinson PD, Ammirati M, Andrews DW, Asher AL, et al. The role of steroids in the management of brain metastases: a systematic review and evidence-based clinical practice guideline. J Neurooncol. 2010;96(1):103–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ben-Aharon I, Gafter-Gvili A, Paul M, Leibovici L, Stemmer SM. Interventions for alleviating cancer-related dyspnea: a systematic review. J Clin Oncol. 2008;26(14):2396–404. [DOI] [PubMed] [Google Scholar]
- 41.Pallet N, Fernandez-Ramos AA, Loriot MA. Impact of Immunosuppressive Drugs on the Metabolism of T Cells. Int Rev Cell Mol Biol. 2018;341:169–200. [DOI] [PubMed] [Google Scholar]
- 42.Libert C, Dejager L. How steroids steer T cells. Cell Rep. 2014;7(4):938–9. [DOI] [PubMed] [Google Scholar]
- 43.Taniguchi Y, Tamiya A, Isa SI, Nakahama K, Okishio K, Shiroyama T, et al. Predictive factors for poor progression-free survival in patients with non-small cell lung cancer treated with nivolumab. Anticancer Research. 2017;37(10):5857–62. [DOI] [PubMed] [Google Scholar]
- 44.Arbour KC, Mezquita L, Long N, Rizvi H, Auclin E, Ni A, et al. Impact of Baseline Steroids on Efficacy of Programmed Cell Death-1 and Programmed Death-Ligand 1 Blockade in Patients With Non-Small-Cell Lung Cancer. J Clin Oncol. 2018;36(28):2872–8. [DOI] [PubMed] [Google Scholar]
- 45.Ricciuti B, Dahlberg SE, Adeni A, Sholl LM, Nishino M, Awad MM. Immune Checkpoint Inhibitor Outcomes for Patients With Non-Small-Cell Lung Cancer Receiving Baseline Corticosteroids for Palliative Versus Nonpalliative Indications. J Clin Oncol. 2019;37(22):1927–34. [DOI] [PubMed] [Google Scholar]
- 46.Horvat TZ, Adel NG, Dang TO, Momtaz P, Postow MA, Callahan MK, et al. Immune-Related Adverse Events, Need for Systemic Immunosuppression, and Effects on Survival and Time to Treatment Failure in Patients With Melanoma Treated With Ipilimumab at Memorial Sloan Kettering Cancer Center. J Clin Oncol. 2015;33(28):3193–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Frohne CC, Llano EM, Perkovic A, Cohen RD, Luke JJ. Complete response of metastatic melanoma in a patient with Crohn’s disease simultaneously receiving anti-alpha4beta7 and anti-PD1 antibodies. J Immunother Cancer. 2019;7(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Uemura M, Trinh VA, Haymaker C, Jackson N, Kim DW, Allison JP, et al. Selective inhibition of autoimmune exacerbation while preserving the anti-tumor clinical benefit using IL-6 blockade in a patient with advanced melanoma and Crohn’s disease: a case report. J Hematol Oncol. 2016;9(1):81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Haanen J, Ernstoff MS, Wang Y, Menzies AM, Puzanov I, Grivas P, et al. Autoimmune diseases and immune-checkpoint inhibitors for cancer therapy: review of the literature and personalized risk-based prevention strategy. Ann Oncol. 2020. [DOI] [PubMed]
- 50.Fisher J, Zeitouni N, Fan W, Samie FH. Immune checkpoint inhibitor therapy in solid organ transplant recipients: A patient-centered systematic review. J Am Acad Dermatol. 2020;82(6):1490–500. [DOI] [PubMed] [Google Scholar]
- 51.Lichtman SM, Harvey RD, Damiette Smit MA, Rahman A, Thompson MA, Roach N, et al. Modernizing Clinical Trial Eligibility Criteria: Recommendations of the American Society of Clinical Oncology-Friends of Cancer Research Organ Dysfunction, Prior or Concurrent Malignancy, and Comorbidities Working Group. J Clin Oncol. 2017;35(33):3753–9. [DOI] [PubMed] [Google Scholar]
- 52.Centanni M, Moes D, Troconiz IF, Ciccolini J, van Hasselt JGC. Clinical Pharmacokinetics and Pharmacodynamics of Immune Checkpoint Inhibitors. Clin Pharmacokinet. 2019;58(7):835–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Friedlaender A, Banna GL, Buffoni L, Addeo A. Poor-Performance Status Assessment of Patients with Non-small Cell Lung Cancer Remains Vague and Blurred in the Immunotherapy Era. Current Oncology Reports. 2019;21(12):107. [DOI] [PubMed] [Google Scholar]
- 54.Kanz BA, Pollack MH, Johnpulle R, Puzanov I, Horn L, Morgans A, et al. Safety and efficacy of anti-PD-1 in patients with baseline cardiac, renal, or hepatic dysfunction. J Immunother Cancer. 2016;4:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yamaguchi T, Shimizu J, Hasegawa T, Horio Y, Inaba Y, Yatabe Y, et al. Pre-existing pulmonary fibrosis is a risk factor for anti-PD-1-related pneumonitis in patients with non-small cell lung cancer: A retrospective analysis. Lung cancer (Amsterdam, Netherlands). 2018;125:212–7. [DOI] [PubMed] [Google Scholar]
- 56.Kiyofumi Shimoji TM, Yu Nakanishi, Kakuhiro Yamaguchi, Shinjiro Sakamoto, Yasushi Horimasu, Taku Nakashima, Shintaro Miyamoto, Hiroshi Iwamoto, Kazunori Fujitaka, Hironobu Hamada, Noboru Hattori. Pre-existing interstitial lung abnormalities are risk factors for immune checkpoint inhibitor-induced interstitial lung disease in non-NSCLC cancers. DOI: 101200/JCO20203815_supple15171 Journal of Clinical Oncology 38, no 15_suppl May 25 2020. [Google Scholar]
- 57.Nakanishi Y, Masuda T, Yamaguchi K, Sakamoto S, Horimasu Y, Nakashima T, et al. Pre-existing interstitial lung abnormalities are risk factors for immune checkpoint inhibitor-induced interstitial lung disease in non-small cell lung cancer. Respir Investig. 2019;57(5):451–9. [DOI] [PubMed] [Google Scholar]
- 58.Okada N, Matsuoka R, Sakurada T, Goda M, Chuma M, Yagi K, et al. Risk factors of immune checkpoint inhibitor-related interstitial lung disease in patients with lung cancer: a single-institution retrospective study. Sci Rep. 2020;10(1):13773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wilkinson TMA. Immune checkpoints in chronic obstructive pulmonary disease. Eur Respir Rev. 2017;26(144). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Suzuki Y, Inui N, Karayama M, Imokawa S, Yamada T, Yokomura K, et al. Effect of PD-1 inhibitor on exhaled nitric oxide and pulmonary function in non-small cell lung cancer patients with and without COPD. Int J Chron Obstruct Pulmon Dis. 2019;14:1867–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Upadhrasta S, Elias H, Patel K, Zheng L. Managing cardiotoxicity associated with immune checkpoint inhibitors. Chronic Dis Transl Med. 2019;5(1):6–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Delgobo M, Frantz S. Heart failure in cancer: role of checkpoint inhibitors. J Thorac Dis. 2018;10(Suppl 35):S4323–S34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Spallarossa P, Sarocchi M, Tini G, Arboscello E, Toma M, Ameri P, et al. How to Monitor Cardiac Complications of Immune Checkpoint Inhibitor Therapy. Frontiers in Pharmacology. 2020;11:972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lombardi A, Mondelli MU. Review article: immune checkpoint inhibitors and the liver, from therapeutic efficacy to side effects. Aliment Pharmacol Ther. 2019;50(8):872–84. [DOI] [PubMed] [Google Scholar]
- 65.Shingarev R, Glezerman IG. Kidney Complications of Immune Checkpoint Inhibitors: A Review. Am J Kidney Dis. 2019;74(4):529–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cortazar FB, Kibbelaar ZA, Glezerman IG, Abudayyeh A, Mamlouk O, Motwani SS, et al. Clinical Features and Outcomes of Immune Checkpoint Inhibitor-Associated AKI: A Multicenter Study. Journal of the American Society of Nephrology : JASN. 2020;31(2):435–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lilenbaum RC, Cashy J, Hensing TA, Young S, Cella D. Prevalence of poor performance status in lung cancer patients: implications for research. J Thorac Oncol. 2008;3(2):125–9. [DOI] [PubMed] [Google Scholar]
- 68.Felip E, Ardizzoni A, Ciuleanu T, Cobo M, Laktionov K, Szilasi M, et al. CheckMate 171: A phase 2 trial of nivolumab in patients with previously treated advanced squamous non-small cell lung cancer, including ECOG PS 2 and elderly populations. Eur J Cancer. 2020;127:160–72. [DOI] [PubMed] [Google Scholar]
- 69.Popat S AA, Ciuleanu T, et al. Nivolumab in previously treated patients with metastatic squamous NSCLC: Results of a European single-arm, phase 2 trial (CheckMate 171) including patients aged 70 years and with poor performance status. Ann Oncol 28, 2017. (abstr 1303PD). [Google Scholar]
- 70.Spigel D SL, Waterhouse D, et al. Is nivolumab safe and effective in elderly and PS2 patients with non-small cell lung cancer (NSCLC)? Results of CheckMate 153. J Thorac Oncol 12:S1287–S1288, 2017. (suppl; abstr P302c-026). [Google Scholar]
- 71.Bersanelli M, Brighenti M, Buti S, Barni S, Petrelli F. Patient performance status and cancer immunotherapy efficacy: a meta-analysis. Med Oncol. 2018;35(10):132. [DOI] [PubMed] [Google Scholar]
- 72.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Five-Year Survival and Correlates among Patients with Advanced Melanoma, Renal Cell Carcinoma, or Non-Small Cell Lung Cancer Treated with Nivolumab. JAMA Oncology. 2019;5(10):1411–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fujimoto D, Yoshioka H, Kataoka Y, Morimoto T, Kim YH, Tomii K, et al. Efficacy and safety of nivolumab in previously treated patients with non-small cell lung cancer: A multicenter retrospective cohort study. Lung Cancer. 2018;119:14–20. [DOI] [PubMed] [Google Scholar]
- 74.Garassino MC, Gelibter AJ, Grossi F, Chiari R, Soto Parra H, Cascinu S, et al. Italian Nivolumab Expanded Access Program in Nonsquamous Non-Small Cell Lung Cancer Patients: Results in Never-Smokers and EGFR-Mutant Patients. J Thorac Oncol. 2018;13(8):1146–55. [DOI] [PubMed] [Google Scholar]
- 75.Montana M, Garcia ME, Ausias N, Jeanpierre M, Meiffren M, Giorgi R, et al. Efficacy and safety of nivolumab in patients with non-small cell lung cancer: a retrospective study in clinical practice. Journal of Chemotherapy. 2019;31(2):90–4. [DOI] [PubMed] [Google Scholar]
- 76.Lang D, Huemer F, Rinnerthaler G, Horner A, Wass R, Brehm E, et al. Therapy Line and Associated Predictors of Response to PD-1/PD-L1-Inhibitor Monotherapy in Advanced Non-small-Cell Lung Cancer: A Retrospective Bi-centric Cohort Study. Targeted Oncology. 2019;14(6):707–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ahn BC, Pyo KH, Xin CF, Jung D, Shim HS, Lee CY, et al. Comprehensive analysis of the characteristics and treatment outcomes of patients with non-small cell lung cancer treated with anti-PD-1 therapy in real-world practice. Journal of Cancer Research and Clinical Oncology. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ksienski D, Wai ES, Croteau N, Freeman AT, Chan A, Fiorino L, et al. Pembrolizumab for advanced nonsmall cell lung cancer: Efficacy and safety in everyday clinical practice. Lung Cancer. 2019;133:110–6. [DOI] [PubMed] [Google Scholar]
- 79.Passaro A, Spitaleri G, Gyawali B, de Marinis F. Immunotherapy in non-small-cell lung cancer patients with performance status 2: Clinical decision making with scant evidence. Journal of Clinical Oncology. 2019;37(22):1863–7. [DOI] [PubMed] [Google Scholar]
- 80.Muchnik E, Loh KP, Strawderman M, Magnuson A, Mohile SG, Estrah V, et al. Immune Checkpoint Inhibitors in Real-World Treatment of Older Adults with Non-Small Cell Lung Cancer. Journal of the American Geriatrics Society. 2019;67(5):905–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lichtenstein MRL, Nipp RD, Muzikansky A, Goodwin K, Anderson D, Newcomb RA, et al. Impact of Age on Outcomes with Immunotherapy in Patients with Non-Small Cell Lung Cancer. Journal of Thoracic Oncology. 2019;14(3):547–52. [DOI] [PubMed] [Google Scholar]
- 82.Joris S, Pieters T, Sibille A, Bustin F, Jacqmin L, Kalantari HR, et al. Real life safety and effectiveness of nivolumab in older patients with non-small cell lung cancer: Results from the Belgian compassionate use program. Journal of geriatric oncology. 2020;11(5):796–801. [DOI] [PubMed] [Google Scholar]
- 83.Galli G, De Toma A, Pagani F, Randon G, Trevisan B, Prelaj A, et al. Efficacy and safety of immunotherapy in elderly patients with non-small cell lung cancer. Lung Cancer. 2019;137:38–42. [DOI] [PubMed] [Google Scholar]
- 84.Marur S, Singh H, Mishra-Kalyani P, Larkins E, Keegan P, Sridhara R, et al. FDA analyses of survival in older adults with metastatic non-small cell lung cancer in controlled trials of PD-1/PD-L1 blocking antibodies. Seminars in Oncology. 2018;45(4):220–5. [DOI] [PubMed] [Google Scholar]
- 85.Elias R, Hartshorn K, Rahma O, Lin N, Snyder-Cappione JE. Aging,immune senescence,and immunotherapy:A comprehensive review. Semin Oncol. 2018;45(4):187–200. [DOI] [PubMed] [Google Scholar]
- 86.Spano JP, Veyri M, Gobert A, Guihot A, Perre P, Kerjouan M, et al. Immunotherapy for cancer in people living with HIV: Safety with an efficacy signal from the series in real life experience. AIDS. 2019;33(11):F13–F9. [DOI] [PubMed] [Google Scholar]
- 87.Ostios-Garcia L, Faig J, Leonardi GC, Adeni AE, Subegdjo SJ, Lydon CA, et al. Safety and Efficacy of PD-1 Inhibitors Among HIV-Positive Patients With Non-Small Cell Lung Cancer. Journal of Thoracic Oncology. 2018;13(7):1037–42. [DOI] [PubMed] [Google Scholar]
- 88.Lavole A, Guihot A, Veyri M, Lambotte O, Autran B, Cloarec N, et al. PD-1 blockade in HIV-infected patients with lung cancer: A new challenge or already a strategy? Annals of Oncology. 2018;29(4):1065–6. [DOI] [PubMed] [Google Scholar]
- 89.Shah NJ, Al-Shbool G, Blackburn M, Cook M, Belouali A, Liu SV, et al. Safety and efficacy of immune checkpoint inhibitors (ICIs) in cancer patients with HIV, hepatitis B, or hepatitis C viral infection. J Immunother Cancer. 2019;7(1):353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chang E, Sabichi AL, Kramer JR, Hartman C, Royse KE, White DL, et al. Nivolumab Treatment for Cancers in the HIV-infected Population. Journal of Immunotherapy. 2018;41(8):379–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cook MR, Kim C. Safety and Efficacy of Immune Checkpoint Inhibitor Therapy in Patients With HIV Infection and Advanced-Stage Cancer: A Systematic Review. JAMA Oncol. 2019;5(7):1049–54. [DOI] [PubMed] [Google Scholar]
- 92.Sahin IH, Kane SR, Brutcher E, Guadagno J, Smith KE, Wu C, et al. Safety and Efficacy of Immune Checkpoint Inhibitors in Patients With Cancer Living With HIV: A Perspective on Recent Progress and Future Needs. JCO Oncol Pract. 2020;16(6):319–25. [DOI] [PubMed] [Google Scholar]
- 93.Kothapalli A, Khattak MA. Safety and efficacy of anti-PD-1 therapy for metastatic melanoma and non-small-cell lung cancer in patients with viral hepatitis: A case series. Melanoma Research. 2018;28(2):155–8. [DOI] [PubMed] [Google Scholar]
- 94.Pu D, Yin L, Zhou Y, Li W, Huang L, Cai L, et al. Safety and efficacy of immune checkpoint inhibitors in patients with HBV/HCV infection and advanced-stage cancer: A systematic review. Medicine (Baltimore). 2020;99(5):e19013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nagy A, Muller V, Kolonics-Farkas AM, Eszes N, Vincze K, Horvath G. Worse lung cancer outcome in patients with lower respiratory tract infection confirmed at time of diagnosis. Thoracic Cancer. 2019;10(9):1819–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hanada S, Pirzadeh M, Carver KY, Deng JC. Respiratory Viral Infection-Induced Microbiome Alterations and Secondary Bacterial Pneumonia. Frontiers in immunology. 2018;9:2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Helmink BA, Khan MAW, Hermann A, Gopalakrishnan V, Wargo JA. The microbiome, cancer, and cancer therapy. Nat Med. 2019;25(3):377–88. [DOI] [PubMed] [Google Scholar]
- 98.Chaput N, Lepage P, Coutzac C, Soularue E, Le Roux K, Monot C, et al. Baseline gut microbiota predicts clinical response and colitis in metastatic melanoma patients treated with ipilimumab. Ann Oncol. 2017;28(6):1368–79. [DOI] [PubMed] [Google Scholar]
- 99.Dubin K, Callahan MK, Ren B, Khanin R, Viale A, Ling L, et al. Intestinal microbiome analyses identify melanoma patients at risk for checkpoint-blockade-induced colitis. Nat Commun. 2016;7:10391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dingemans AC, Soo RA, Jazieh AR, Rice SJ, Kim YT, Teo LLS, et al. Treatment Guidance for Patients With Lung Cancer During the Coronavirus 2019 Pandemic. J Thorac Oncol. 2020;15(7):1119–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Passaro A, Peters S, Mok TSK, Attili I, Mitsudomi T, de Marinis F. Testing for COVID-19 in lung cancer patients. Ann Oncol. 2020;31(7):832–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bersanelli M Controversies about COVID-19 and anticancer treatment with immune checkpoint inhibitors. Immunotherapy. 2020;12(5):269–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Garassino MC, Whisenant JG, Huang LC, Trama A, Torri V, Agustoni F, et al. COVID-19 in patients with thoracic malignancies (TERAVOLT): first results of an international, registry-based, cohort study. Lancet Oncol. 2020;21(7):914–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Planchard D, Popat S, Kerr K, Novello S, Smit EF, Faivre-Finn C, et al. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29(Suppl 4):iv192–iv237. [DOI] [PubMed] [Google Scholar]
- 105.Hanna NH, Schneider BJ, Temin S, Baker S Jr., Brahmer J, Ellis PM, et al. Therapy for Stage IV Non-Small-Cell Lung Cancer Without Driver Alterations: ASCO and OH (CCO) Joint Guideline Update. J Clin Oncol. 2020;38(14):1608–32. [DOI] [PubMed] [Google Scholar]
- 106.Ettinger DS, Wood DE, Aggarwal C, Aisner DL, Akerley W, Bauman JR, et al. NCCN Guidelines Insights: Non-Small Cell Lung Cancer, Version 1.2020. J Natl Compr Canc Netw. 2019;17(12):1464–72. [DOI] [PubMed] [Google Scholar]
- 107.von Itzstein MS, Khan S, Gerber DE. Investigational Biomarkers for Checkpoint Inhibitor Immune-Related Adverse Event Prediction and Diagnosis. Clin Chem. 2020;66(6):779–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.von Itzstein MS, Khan S, Popat V, Lu R, Khan SA, Fattah FJ, et al. Statin Intolerance, Anti-HMGCR Antibodies, and Immune Checkpoint Inhibitor-Associated Myositis: A “Two-Hit” Autoimmune Toxicity or Clinical Predisposition? Oncologist. 2020. [DOI] [PMC free article] [PubMed]
- 109.Khan S, von Itzstein MS, Lu R, Bermas BL, Karp DR, Khan SA, et al. Late-Onset Immunotherapy Toxicity and Delayed Autoantibody Changes: Checkpoint Inhibitor-Induced Raynaud’s-Like Phenomenon. Oncologist. 2020;25(5):e753–e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Auslander N, Zhang G, Lee JS, Frederick DT, Miao B, Moll T, et al. Robust prediction of response to immune checkpoint blockade therapy in metastatic melanoma. Nat Med. 2018;24(10):1545–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tran G, Zafar SY. Financial toxicity and implications for cancer care in the era of molecular and immune therapies. Ann Transl Med. 2018;6(9):166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Verma V, Sprave T, Haque W, Simone CB 2nd, Chang JY, Welsh JW, et al. A systematic review of the cost and cost-effectiveness studies of immune checkpoint inhibitors. J Immunother Cancer. 2018;6(1):128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chu JN, Choi J, Ostvar S, Torchia JA, Reynolds KL, Tramontano A, et al. Cost-effectiveness of immune checkpoint inhibitors for microsatellite instability-high/mismatch repair-deficient metastatic colorectal cancer. Cancer. 2019;125(2):278–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nishio M, Takahashi T, Yoshioka H, Nakagawa K, Fukuhara T, Yamada K, et al. KEYNOTE-025: Phase 1b study of pembrolizumab in Japanese patients with previously treated programmed death ligand 1-positive advanced non-small-cell lung cancer. Cancer Sci. 2019;110(3):1012–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Barlesi F, Vansteenkiste J, Spigel D, Ishii H, Garassino M, de Marinis F, et al. Avelumab versus docetaxel in patients with platinum-treated advanced non-small-cell lung cancer (JAVELIN Lung 200): an open-label, randomised, phase 3 study. Lancet Oncol. 2018;19(11):1468–79. [DOI] [PubMed] [Google Scholar]
- 116.Robert C, Ribas A, Wolchok JD, Hodi FS, Hamid O, Kefford R, et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet. 2014;384(9948):1109–17. [DOI] [PubMed] [Google Scholar]
- 117.Carbone DP, Reck M, Paz-Ares L, Creelan B, Horn L, Steins M, et al. First-Line Nivolumab in Stage IV or Recurrent Non-Small-Cell Lung Cancer. N Engl J Med. 2017;376(25):2415–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bergqvist V, Hertervig E, Gedeon P, Kopljar M, Griph H, Kinhult S, et al. Vedolizumab treatment for immune checkpoint inhibitor-induced enterocolitis. Cancer Immunol Immunother. 2017;66(5):581–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Pedersen M, Andersen R, Norgaard P, Jacobsen S, Thielsen P, Thor Straten P, et al. Successful treatment with Ipilimumab and Interleukin-2 in two patients with metastatic melanoma and systemic autoimmune disease. Cancer Immunol Immunother. 2014;63(12):1341–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Johnson DB, Sullivan RJ, Ott PA, Carlino MS, Khushalani NI, Ye F, et al. Ipilimumab Therapy in Patients With Advanced Melanoma and Preexisting Autoimmune Disorders. JAMA Oncol. 2015:1–7. [DOI] [PubMed] [Google Scholar]
- 121.Kahler KC, Eigentler TK, Gesierich A, Heinzerling L, Loquai C, Meier F, et al. Ipilimumab in metastatic melanoma patients with pre-existing autoimmune disorders. Cancer Immunol Immunother. 2018;67(5):825–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Menzies AM, Johnson DB, Ramanujam S, Atkinson VG, Wong ANM, Park JJ, et al. Anti-PD-1 therapy in patients with advanced melanoma and preexisting autoimmune disorders or major toxicity with ipilimumab. Ann Oncol. 2017;28(2):368–76. [DOI] [PubMed] [Google Scholar]
- 123.Gutzmer R, Koop A, Meier F, Hassel JC, Terheyden P, Zimmer L, et al. Programmed cell death protein-1 (PD-1) inhibitor therapy in patients with advanced melanoma and preexisting autoimmunity or ipilimumab-triggered autoimmunity. Eur J Cancer. 2017;75:24–32. [DOI] [PubMed] [Google Scholar]
- 124.Tison A, Quere G, Misery L, Funck-Brentano E, Danlos FX, Routier E, et al. Safety and Efficacy of Immune Checkpoint Inhibitors in Patients With Cancer and Preexisting Autoimmune Disease: A Nationwide, Multicenter Cohort Study. Arthritis Rheumatol. 2019;71(12):2100–11. [DOI] [PubMed] [Google Scholar]
- 125.Safa H, Johnson DH, Trinh VA, Rodgers TE, Lin H, Suarez-Almazor ME, et al. Immune checkpoint inhibitor related myasthenia gravis: single center experience and systematic review of the literature. J Immunother Cancer. 2019;7(1):319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kyi C, Carvajal RD, Wolchok JD, Postow MA. Ipilimumab in patients with melanoma and autoimmune disease. J Immunother Cancer. 2014;2(1):35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gettings EJ, Hackett CT, Scott TF. Severe relapse in a multiple sclerosis patient associated with ipilimumab treatment of melanoma. Mult Scler. 2015;21(5):670. [DOI] [PubMed] [Google Scholar]
- 128.Danlos FX, Voisin AL, Dyevre V, Michot JM, Routier E, Taillade L, et al. Safety and efficacy of anti-programmed death 1 antibodies in patients with cancer and pre-existing autoimmune or inflammatory disease. Eur J Cancer. 2018;91:21–9. [DOI] [PubMed] [Google Scholar]
- 129.Lee B, Wong A, Kee D, Neeson P, Shackleton M, McArthur G, et al. The use of ipilimumab in patients with rheumatoid arthritis and metastatic melanoma. Ann Oncol. 2016;27(6):1174–7. [DOI] [PubMed] [Google Scholar]
- 130.Puri A, Homsi J. The safety of pembrolizumab in metastatic melanoma and rheumatoid arthritis. Melanoma Res. 2017;27(5):519–23. [DOI] [PubMed] [Google Scholar]
- 131.Richter MD, Pinkston O, Kottschade LA, Finnes HD, Markovic SN, Thanarajasingam U. Brief Report: Cancer Immunotherapy in Patients With Preexisting Rheumatic Disease: The Mayo Clinic Experience. Arthritis Rheumatol. 2018;70(3):356–60. [DOI] [PubMed] [Google Scholar]
- 132.Beck KM, Dong J, Geskin LJ, Beltrani VP, Phelps RG, Carvajal RD, et al. Disease stabilization with pembrolizumab for metastatic acral melanoma in the setting of autoimmune bullous pemphigoid. J Immunother Cancer. 2016;4:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kim YJ, Keam B, Ock CY, Song S, Kim M, Kim SH, et al. A phase II study of pembrolizumab and paclitaxel in patients with relapsed or refractory small-cell lung cancer. Lung Cancer. 2019;136:122–8. [DOI] [PubMed] [Google Scholar]
- 134.Yang CJ, McSherry F, Mayne NR, Wang X, Berry MF, Tong B, et al. Surgical Outcomes After Neoadjuvant Chemotherapy and Ipilimumab for Non-Small Cell Lung Cancer. Ann Thorac Surg. 2018;105(3):924–9. [DOI] [PubMed] [Google Scholar]
- 135.Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, von Pawel J, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389(10066):255–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Horinouchi H, Yamamoto N, Fujiwara Y, Sekine I, Nokihara H, Kubota K, et al. Phase I study of ipilimumab in phased combination with paclitaxel and carboplatin in Japanese patients with non-small-cell lung cancer. Invest New Drugs. 2015;33(4):881–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Fehrenbacher L, Spira A, Ballinger M, Kowanetz M, Vansteenkiste J, Mazieres J, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet. 2016;387(10030):1837–46. [DOI] [PubMed] [Google Scholar]
- 138.Reck M, Luft A, Szczesna A, Havel L, Kim SW, Akerley W, et al. Phase III Randomized Trial of Ipilimumab Plus Etoposide and Platinum Versus Placebo Plus Etoposide and Platinum in Extensive-Stage Small-Cell Lung Cancer. J Clin Oncol. 2016;34(31):3740–8. [DOI] [PubMed] [Google Scholar]
- 139.Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csoszi T, Fulop A, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med. 2016;375(19):1823–33. [DOI] [PubMed] [Google Scholar]
- 140.Gandhi L, Rodriguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N Engl J Med. 2018;378(22):2078–92. [DOI] [PubMed] [Google Scholar]
- 141.Hellmann MD, Ciuleanu TE, Pluzanski A, Lee JS, Otterson GA, Audigier-Valette C, et al. Nivolumab plus Ipilimumab in Lung Cancer with a High Tumor Mutational Burden. N Engl J Med. 2018;378(22):2093–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Paz-Ares L, Luft A, Vicente D, Tafreshi A, Gumus M, Mazieres J, et al. Pembrolizumab plus Chemotherapy for Squamous Non-Small-Cell Lung Cancer. N Engl J Med. 2018;379(21):2040–51. [DOI] [PubMed] [Google Scholar]
- 143.Socinski MA, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N, et al. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N Engl J Med. 2018;378(24):2288–301. [DOI] [PubMed] [Google Scholar]
- 144.Jotte R, Cappuzzo F, Vynnychenko I, Stroyakovskiy D, Rodriguez-Abreu D, Hussein M, et al. Atezolizumab in Combination With Carboplatin and Nab-Paclitaxel in Advanced Squamous NSCLC (IMpower131): Results From a Randomized Phase III Trial. J Thorac Oncol. 2020;15(8):1351–60. [DOI] [PubMed] [Google Scholar]
- 145.Herbst RS, Baas P, Kim DW, Felip E, Perez-Gracia JL, Han JY, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387(10027):1540–50. [DOI] [PubMed] [Google Scholar]
- 146.Brahmer J, Reckamp KL, Baas P, Crino L, Eberhardt WE, Poddubskaya E, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med. 2015;373(2):123–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med. 2015;373(17):1627–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1. Selected lung cancer immune checkpoint inhibitor trials with chemotherapy and comparison of eligibility criteria for comorbidities, based on clinicaltrials.gov.
Supplemental Table 2. Selected planned novel lung cancer immune checkpoint inhibitor trials in 2020 without chemotherapy and comparison of eligibility criteria for comorbidities, based on clinicaltrials.gov.