Abstract
Evolutionary reversals, including re-evolution of lost structures, are commonly found in phylogenetic studies. However, we lack an understanding of how these reversals happen mechanistically. A snake-like body form has evolved many times in vertebrates, and occasionally a quadrupedal form has re-evolved, including in Brachymeles lizards. We use body form and locomotion data for species ranging from snake-like to quadrupedal to address how a quadrupedal form could re-evolve. We show that large, quadrupedal species are faster at burying and surface locomotion than snake-like species, indicating a lack of expected performance trade-off between these modes of locomotion. Species with limbs use them while burying, suggesting that limbs are useful for burying in wet, packed substrates. Palaeoclimatological data suggest that Brachymeles originally evolved a snake-like form under a drier climate probably with looser soil in which it was easier to dig. The quadrupedal clade evolved as the climate became humid, where limbs and large size facilitated fossorial locomotion in packed soils.
Keywords: elongation, lizard, locomotion, re-evolution
1. Background
How complex traits evolve has long been a key question in biology, particularly when their evolution reverses course [1–3]. Dollo's law has received considerable attention because it argues against the re-evolution of complex traits to their ancestral state after they are lost [4–7]. It is thought that, as mutational changes in the genetic architecture of a lost trait accumulate, its re-evolution becomes increasingly unlikely [8]. Yet, there is considerable phylogenetic evidence for re-evolution of lost traits. Examples include the re-evolution of nipples in mammals [9], teeth in frogs [10], wings in insects [11] and limb elements in lizards [12–14]. However, this subject remains controversial because of a lack of mechanistic understanding of how these instances of re-evolution occur from a functional or developmental perspective [15,16]. Here, we address the functional underpinnings behind the re-evolution of a quadrupedal form from a snake-like form.
The evolution of a snake-like form involves the elongation of the body and the loss and reduction in size of digits and limbs [17]. Its evolution and reversion to a quadrupedal form is an excellent opportunity for understanding functional mechanisms of evolutionary reversal. Snake-like body forms have evolved in most major vertebrate clades, including at least 25 times in squamates (lizards and snakes) [17–24]. The prevalent hypothesis is that snake-like forms evolved as an adaptation for fossoriality [25–27]. Indeed, many snake-like species are fossorial, and snakes are thought to have evolved from a fossorial ancestor [24,26].
The fossoriality hypothesis predicts that more snake-like species are better at fossorial locomotion but worse at surface locomotion than quadrupedal species, resulting in a performance trade-off that is related to body form [28]. The existence of multiple extant intermediate forms in the transition between quadrupedal and snake-like is critical to testing this hypothesis. Few clades satisfy this criterion, and the Australian skink genus Lerista is the most diverse, with a unidirectional evolution from quadrupedal to snake-like forms [29–31]. Like most snake-like skinks, Lerista live in loose, dry sand into which they bury themselves to escape predators [32]. Recently, Morinaga & Bergmann [28] showed that snake-like species of Lerista were faster at burying themselves, but that the performance of surface locomotion was unrelated to body form, supporting the fossoriality hypothesis but without the expected trade-off.
The work on Lerista establishes how locomotion changes during the evolution of a snake-like form but cannot address implications for locomotion when the reverse happens. We study this using skinks of the genus Brachymeles, for which we have strong phylogenetic evidence for the re-evolution of a quadrupedal, pentadactyl form, suggesting a violation of Dollo's law [14,33]. Currently, there are 41 species of Brachymeles recognized primarily from the Philippines that together display a full spectrum of body forms from snake-like to quadrupedal, with a clade of 17 species having re-evolved a short body with pentadactyl limbs [14,34,35]. Unlike Lerista, which inhabit dry, sandy environments, Brachymeles occur in rainforests with wet, packed soil. Therefore, one possible explanation is that this re-evolved body form allows pentadactyl Brachymeles to attain high fossorial and surface locomotor performance in these environments—a facilitation, as opposed to a trade-off [36]. Quadrupedal species of Brachymeles have fewer and more robust finger phalanges than skinks with ancestral front limb morphologies [33], suggesting a role of the front limbs when digging in wet, packed soil. Such a facilitation hypothesis postulates a dryer climate with loose, dry soil when Brachymeles evolved to be snake-like, followed by changes to a wet climate with wet soil, selecting for the re-evolution of a quadrupedal form.
Here, we integrate body form, locomotion, ecological and palaeoclimatological data with our phylogeny to understand how Brachymeles could have re-evolved a quadrupedal phenotype from a snake-like form. First, we test whether species that are pentadactyl are faster at burying themselves and at surface locomotion using 13 species of Brachymeles ranging from pentadactyl to limbless (figure 1). We then compare these data to those for Lygosoma (Subduloceps) bowringii, a burrowing skink that is ancestrally pentadactyl [37]. We predict that Lygosoma will be worse at burying than pentadactyl Brachymeles, but a better runner because it evolved from a surface-dwelling ancestor. Finally, we overlay published palaeoclimatological data with our phylogeny to assess whether changes in climate correspond with evolutionary changes in body form, where snake-like Brachymeles evolved under a drier climate similar to Lerista, and subsequently re-evolved limbs with the onset of wet, monsoonal conditions.
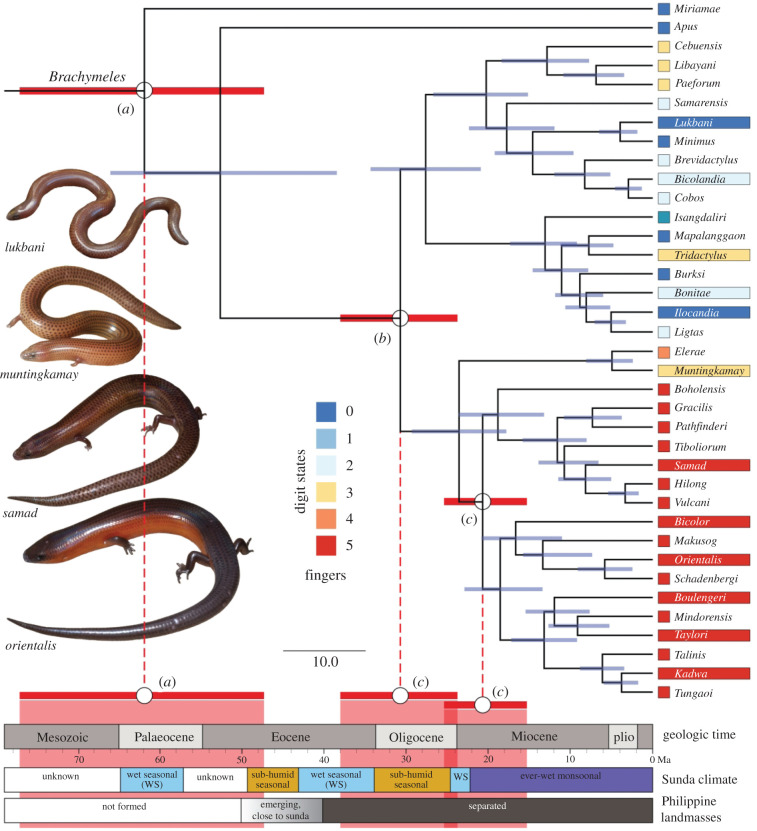
Figure 1.
Dated phylogeny of Brachymeles with palaeoclimatological reconstructions, stages in the formation of the Philippines, and the geological time scale mapped on. Key events in the evolution of Brachymeles are also mapped on, with 95% confidence intervals, including the origin of Brachymeles (a), the origin of Brachymeles on the Philippines (b) and the origin of the pentadactyl clade (c). The number of front digits, climate on the Sunda Peninsula and status of Philippines are colour-coded (see legends in the figure). Species represented in the locomotion dataset have their names highlighted with their digit number colour. Phylogeny is from Wagner et al. [33]. (Online version in colour.)
2. Methods
(a). Animal collection and morphometric data
We conducted locomotor trials with 147 individuals belonging to 13 species of Brachymeles plus Lygosoma bowringii in the Philippines and Thailand (electronic supplementary material, table S1). We captured animals using pitfall traps and by hand while raking leaf litter and detritus to uncover the animals [38]. Capture locations were marked using a handheld GPS unit (Garmin Ltd., Olathe, KS, USA). We used a pocket penetrometer (LR-281, Forestry Suppliers, Jackson, MS, USA) to measure load-bearing capacity of the soil [39] five times (taking the average) within a 50 cm radius of the exact site of capture for each animal. We also measured percentage soil moisture using a Kelway soil tester (Kel Instruments Co., Wyckoff, NJ, USA) for 161 specimens belonging to nine species of Brachymeles. We completed trials within 3 days of capture at base camp. Animals were then preserved and housed in the collections of the Sam Noble Museum of Natural History, Oklahoma, the Kansas University Biodiversity Institute, or the Zoological Museum of Kasetsart University, Thailand (electronic supplementary material, table S2).
We collected basic morphometric data from each preserved specimen. We counted the number of fingers and toes and took measurements using a Mitutoyo (Kanagawa, Japan) digital caliper to the nearest 0.01 mm. Measurements followed [40] and included: head length, width and height; snout–vent (SVL), tail, front and hind limb lengths; and body width. All measurements were taken in triplicate, using the average for analysis. We also photographed the head of each specimen from dorsal and lateral views using a Nikon D90 (Tokyo, Japan) camera with a Nikkor 60 mm macro lens. We then used ImageJ 1.52p [41] to measure head width and internasal distance from dorsal view, and head height and nasal height from a lateral view, similar to [42], to quantify snout pointiness and slope. Pointiness was calculated as internasal distance ÷ head width from dorsal view and nasal height ÷ head height from a lateral view, with smaller ratios indicating pointier snouts. These are the inverse of ‘rostral lateral decline' and ‘rostral angulation’ defined by [42]. The slope was calculated as (head width − internasal distance) ÷ head length from dorsal view, and (head height − nasal height) ÷ head length from a lateral view. Smaller slopes represented more gradually tapering snouts.
(b). Locomotor trials
We painted dots on each animal using non-toxic white paint dorsally at the occiput, pectoral girdle, mid-body, pelvic girdle, cloaca, elbows and knees [43]. The mid-body was the mid-point between the pectoral and pelvic girdles. Limbless species had subtle depressions where limb buds once developed, allowing us to identify girdle positions externally.
We conducted at least three running and three burying trials per individual on a fine and coarse substrate, resulting in over 2000 high-speed videos from the 147 individuals. Substrates were obtained by sieving (Hubbard #548, Forestry Suppliers Inc., Jackson, MS, USA) soil from the animals' habitat that we had first dried in the sun to ensure the same moisture content. The fine and coarse substrates were composed of particles 0.25–0.50 mm and 3–5 mm in diameter, respectively.
For running trials, we used a 15 cm wide by 1 m long field-portable racetrack with the substrate to a depth of 0.5 cm. For burying trials, we used a 31 × 18 cm plastic tub filled to a depth of 8 cm with the substrate. We recorded trials from dorsal view with a field of view of approximately 30 cm long, at a resolution of 512 × 384 pixels at 240 Hz and a shutter speed of 1/640 s using Casio Exilim cameras (Tokyo, Japan, models EX-ZR850 and EX-ZR1700). We coaxed animals to run or bury by gently tapping their hindquarters. A trial was successful when the animal either ran across the field of view without touching the walls of the track or buried continuously until the pelvic dot disappeared below the substrate. We conducted trials only when the ambient temperature was ≥27°C and measured body temperature of each animal immediately after each successful trial using an infrared thermometer (Raytek MT6, Santa Cruz, CA, USA) [28].
We also measured the penetration force exerted by each animal as it burrowed into each substrate at least three times. We used a 16 cm long plastic block with a 20 mm diameter tunnel drilled into it. One end of the tunnel was blocked with an aluminium plate mounted on a single element piezoelectric Kistler type 9203 force transducer connected to a Type 5995 charge amplifier (Kistler Instrument Corp., Amherst, NY, USA). The tunnel was filled with 5 cm of the substrate and the animal coaxed to burrow horizontally into this substrate, measuring the compressive force it exerted while burrowing at least 2 cm into the substrate [28,44].
(c). Quantifying running and burying performance and kinematics
We used DLTdv5 (Hedrick, 2008) in MatLab 2017a (MathWorks, Natick, MA, USA) to digitize the painted dots, providing us with xy coordinates for each point in each frame. The number of pixels per metre was calculated in ImageJ using a Philippine one Peso coin that was recorded in each video. We then calculated time from frame number and cumulative distance moved by the pelvic dot from the beginning of the video to each frame [43]. The pelvic dot approximated the centre of mass (CoM). We then used the curve-fitting toolbox in MatLab to fit a sixth-order smoothing spline to the cumulative distance and time data [39]. We adjusted the smoothing of the spline until secondary oscillations in the second derivative were removed and its maximum stabilized [43,45]. The first derivative provided us with frame-by-frame velocity estimates, from which we calculated maximum and average velocity while the animal was moving. We selected the trial with the highest average velocity for each individual per a mode of locomotion per substrate to avoid pseudoreplication and calculated kinematic variables for these trials.
We focused on axial kinematic variables because some species were limbless. For running, we calculated the average frequency, wavelength and amplitude of lateral undulations for the pectoral, mid-body and pelvic dots. We did this in R, using the pectoral point to model a linear path that the animal travelled and calculating the undulatory variables based on that path, as in [46]. Animals do not undulate regularly during burying and disappear from view as it progresses, so for this behaviour, we tallied the proportion of trials that each individual used its front and hind limbs just before each set of limbs disappeared beneath the substrate [47].
(d). Statistical analysis
We conducted all analyses in R v. 3.5.1 [48]. All analyses were phylogenetically informed and we used the most recent dated phylogeny of Brachymeles, which was based on a multi-locus gene dataset, and with most clades being supported with posterior probabilities of one [33]. A phylogenetic principal component analysis (pPCA) was used to study patterns of morphometric variation using the ‘phytools' package [49,50]. The pPCA was done using a correlation matrix, simultaneously estimating λ [51], a measure of phylogenetic signal in the data, including the number of digits (equal in front and hind limbs in our species), ln-transformed head length, and relative SVL, body width, and front and hind limb lengths. We calculated relative lengths by dividing each by head length, following other studies of body elongation and limb reduction [14,29,52]. We chose not to standardize by body mass because we found that in Brachymeles, body mass correlated highly with body form, as represented by pPC-1 (R2 = 0.738, slope = −0.049 ± 0.008, t = −5.81, p < 0.001) and made the pPCA difficult to interpret. Head length correlated strongly with body mass (R2 = 0.959, slope = 3.048 ± 0.182, t = 16.71, p < 0.001), indicating that it was an appropriate measure of body size. To understand how intraspecific variation mapped on our species PC space, we projected individuals onto the phylomorphospace [53]. There was relatively little intraspecific variation along the pPC-1 axis (electronic supplementary material, figure S1). Our pPCA results closely matched those obtained using a covariance matrix, and one where we decomposed the hind limbs into their constituent segments, supporting the robustness of the analysis (electronic supplementary material, table S3). Hence, we used pPC-1 as an index of body form for subsequent analyses.
We also modelled the evolution of size, represented by head length, for 40 species of Brachymeles and our phylogeny [33,40]. We did this by fitting a series of continuous trait evolution models in the ‘OUwie' package [54] and comparing them using AIC corrected for small sample size, interpretting a ΔAIC > 2 as support for one model over another [55]. To test whether the pentadactyl clade evolved towards a different size optimum than the limbless and limb-reduced species, we fitted a single rate Brownian motion model, a single optimum OU model and two OU models with multiple optima. The first had an optimum for the pentadactyl clade, including its most recent common ancestor and an optimum for all limbless and limb-reduced species. The second had three optima: one for the pentadactyl clade, one for limb-reduced species and one for limbless species. We then compared whether the optimum size for the pentadactyl clade differed from the optimum for the other species using a t-test.
We tested for relationships between variables using phylogenetic generalized least-squares regression (PGLS) while estimating λ of the residuals [56], using the ‘caper' package [57]. For analyses of locomotor variables, we conducted separate regressions for coarse and fine substrates. We tested for relationships among pPC-1 factor scores, head shape indices, locomotor and soil variables using PGLS. Locomotor velocity, and the amplitude and wavelength of body undulations could be affected by body size, so we also repeated analyses with these variables divided by HL to adjust for size. Although our regressions were planned a priori, there were many comparisons, so we corrected for multiple comparisons while accounting for false discovery rate [58,59]. Because this is not implemented in ‘caper', we did these corrections post hoc, so some p-values that we present are <0.05 yet are interpreted as not significant.
3. Results and discussion
(a). Evolution of body form in Brachymeles
Our pPCA characterized body form variation in Brachymeles, with the first component explaining 77% of this variation. pPC-1 provided an index of body form ranging from lizard-like to snake-like. Species with high pPC-1 scores were small and had relatively long, narrow bodies and relatively short limbs with few digits, so were more snake-like than species with lower scores (electronic supplementary material, table S3). Species with low pPC-1 scores were large, had short, thick bodies and were pentadactyl. Projection of individual data into the species PC space revealed that intraspecific shape variation was small along the pPC-1 axis, further supporting its use as an index of body form (electronic supplementary material, figure S1).
These body form variables are related similarly in other squamate clades [14,24,40,60], and the elongation of the body and reduction of appendages may be the primary axis of body form variation in vertebrates [61,62]. However, body size and body form relate differently across exemplar clades. In Lerista, both limbless snake-like and quadrupedal pentadactyl species are diminutive while intermediate species are large [29]. By contrast, Brachymeles head length, the proxy for size in elongate squamates, was correlated tightly with pPC-1 (PGLS regression: R2 = 0.631, slope = −0.137 ± 0.030, t = −4.53, p < 0.001), indicating that more lizard-like species were larger than more snake-like ones.
In investigating the role of body size in the re-evolution of a quadrupedal, pentadactyl body form, the Ornstein–Uhlenbeck (OU) model with two selective optima best fit the data, followed by the OU model with three optima (electronic supplementary material, table S4), both indicating that the pentadactyl clade, including its ancestor, evolved towards a significantly higher size optimum (HL = 25.6 ± 5.7 mm) than species with reduced or absent limbs (HL = 5.1 ± 0.9 mm, t = 3.58, p < 0.001). Thus, body size appears to be an important part of the story behind the re-evolution of a quadrupedal, pentadactyl form in Brachymeles.
(b). Locomotion and the re-evolution of quadrupedal form
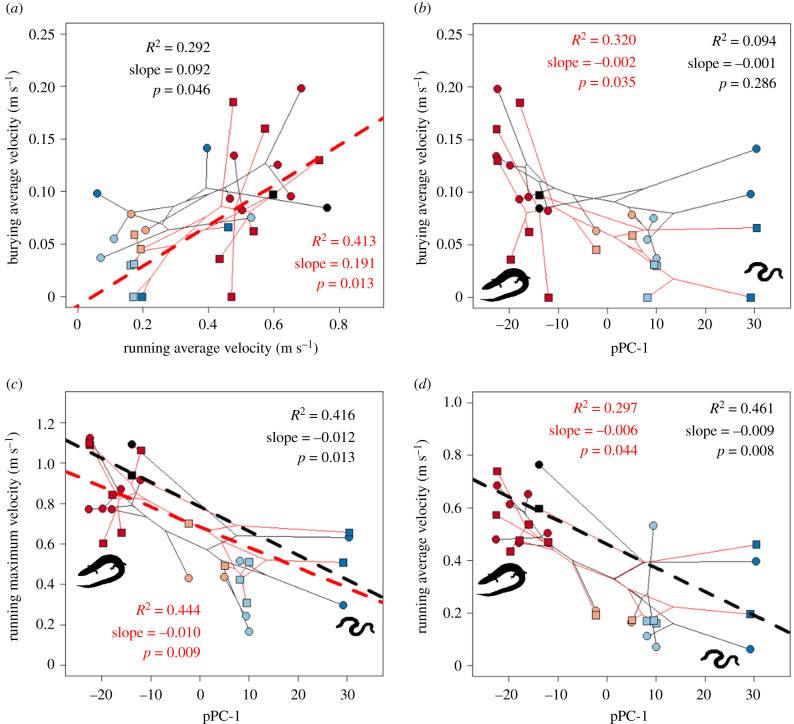
To study the coevolution of locomotion and body form in Brachymeles, we first tested whether there was a correlation between burying and surface locomotion performance. We found support for the facilitation hypothesis because there was a significant positive relationship between average burying and surface velocity on the coarse substrate, with a similar, marginally non-significant trend on the fine substrate (figure 2a; electronic supplementary material, table S5A). This was driven largely by increased maximum and average surface velocity in more lizard-like species (figure 2c,d; electronic supplementary material, table S5B), because there was only a non-significant trend between average burying velocity and body form (figure 2b; electronic supplementary material, table S5C). These results were unchanged when we analysed size-corrected locomotion data (electronic supplementary material, table S5D). Opposite to what we show here for Brachymeles, in Lerista, body form is strongly related to burying performance but not surface locomotion [28].
Figure 2.
Phylomorphospaces relating locomotor performance and body form (pPC-1: pentadactyl to snake-like, shown by silhouettes) on different substrates. Diamonds and black lines show data for the fine substrate and circles and red lines show data for the coarse substrate. Number of digits is indicated by symbol colour: 5—red, 3—pink, 2—light blue, 0—dark blue, Lygosoma bowringii—black. Dashed lines show significant PGLS regressions between variables; no line indicates no significant relationship after correction for multiple comparisons. (Online version in colour.)
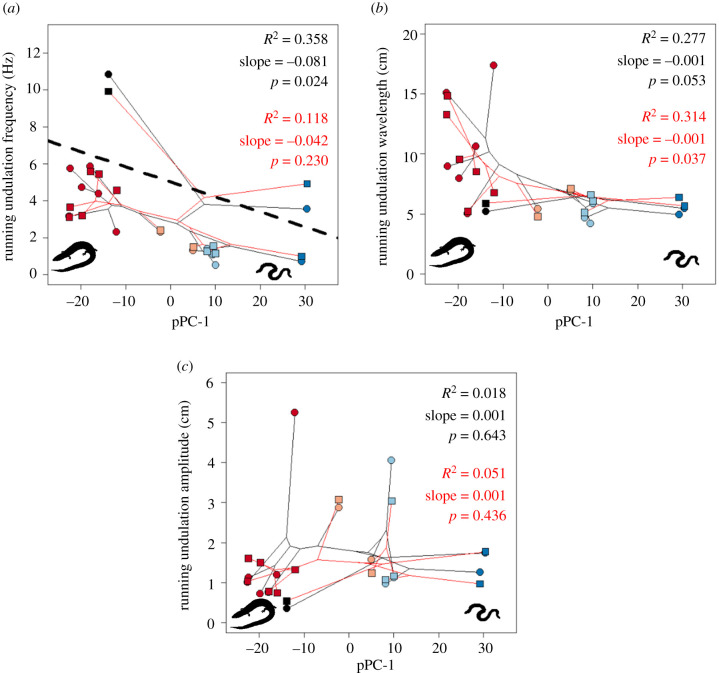
Animals increase surface locomotor speed by increases in the stride or undulatory frequency of the locomotor cycle, or the distance moved during that cycle [63,64]. To understand how more lizard-like species moved faster on the surface, we related body form to the characteristics of lateral undulation of the animals. We found that more lizard-like species adopted higher-frequency undulations, but with no change in undulatory amplitude or wavelength, resulting in their higher velocity compared with more snake-like species (figure 3; electronic supplementary material, table S5B). These patterns were also unchanged when considering size-adjusted amplitude and wavelength (electronic supplementary material, table S5D).
Figure 3.
Phylomorphospaces relating running kinematics of the body mid-point and body form on different substrates. Symbols and colours as for figure 2. (Online version in colour.)
Locomotor data for Lygosoma bowringii also supported the facilitation hypothesis. This species had the lowest average burying velocity of any pentadactyl species on the coarse substrate, a mid-range burying velocity on the fine substrate, yet very high average surface locomotor velocity and undulatory frequency on both substrates (figure 2a, 3a). This makes sense because L. bowringii evolved from a cursorial, non-burrowing ancestor [65,66], while ancestors of pentadactyl Brachymeles have been estimated as snake-like burrowers using phylogenetic evidence [14,33].
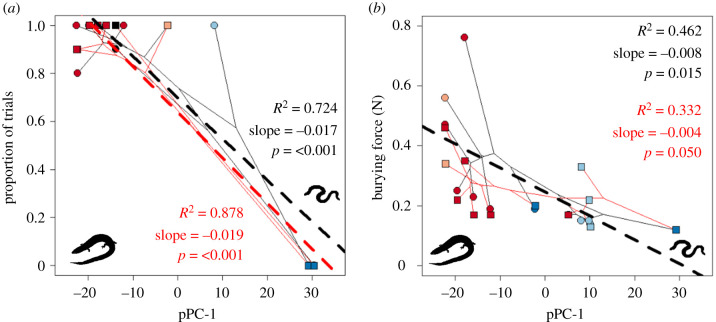
Our facilitation hypothesis posits that re-evolved limbs might aid in fossorial locomotion in tougher soils. Wet substrates, like those inhabited by Brachymeles, have at least a 400% higher resistive force than comparable dry substrates [67]. Supporting our hypothesis, we found that species with limbs used them in the vast majority of trials during burying. This was especially true for the front limbs (figure 4a; electronic supplementary material, table S5C). Although L. bowringii has ancestral limb morphology, it lives in wet substrates similar to Brachymeles [37], and usually used its front limbs (>90% of trials, figure 4a), but rarely its hind limbs (<20% of trials, electronic supplementary material, figure S2A) while burying. The phalanges of limbed Brachymeles re-evolved to be more robust, possibly allowing them to exert more force on the substrate, something not seen in Lygosoma [33]. Although some lizard species fold their limbs against their bodies while burrowing [63], Chalcides occelatus, a skink that often inhabits damp sand, also uses its front limbs while burying [67]. All of these lines of evidence suggest that wet substrates may impose different selective pressures on fossorial animals than dry substrates.
Figure 4.
Phylomorphospaces relating the proportion of trials for each species during which the front limbs were used during burrowing (a) and burrowing force (b) to body form on different substrates. Symbols and colours as for figure 2. (Online version in colour.)
We found that more snake-like species of Brachymeles were able to initiate burying while exerting less force, particularly on the fine substrate (figure 4b). Narrower objects require less force to penetrate a substrate [68,69], and snake-like Brachymeles have narrower heads (electronic supplementary material, figure S2B). Other aspects of head shape may also affect burying performance. For example, the sandfish skink (Scincus scincus) and South American gymnophthalmid species that burrow in looser soil have pointy, shovel-like snouts [42,70,71]. We tested this idea and found that more snake-like species had more gently sloping snouts (electronic supplementary material, figure S2C), but that pointiness was unrelated to body form (electronic supplementary material. figure S2D, table S5E). Despite the gradation in the slope of the snout, the head shape was unrelated to burying velocity or penetration force (electronic supplementary material, table S5F).
One reason for the lack of relationship between head shape and burying performance may be that all species inhabit equally firm substrates. Indeed, we found no relationship between either of the load-bearing capacity or the moisture of the substrate and body form (pPC-1), snout pointiness or slope, supporting this hypothesis. This is unlike the situation in gymnophthalmids [42,72], but Brachymeles lives in wetter soils with more plant debris.
(c). Palaeoclimate change and the re-evolution of quadrupedal form
Our locomotion data suggest a scenario in which Brachymeles evolved to be snake-like when the climate and resulting substrate properties were more amenable to fossoriality, and subsequently re-evolved a quadrupedal, pentadactyl form when the substrate became more wet and packed. Many other clades of snake-like lizards live in arid areas with dry substrates. For example, snake-like species of gymnophthalmids live in more arid regions with drier soils than quadrupedal species [72]. The same appears to be the case with South African Scelotes scincid lizards [73].
To investigate the relationship between body form evolution and climate change, we compared published palaeoclimatological and geographical data with our latest dated phylogeny of Brachymeles (figure 1) [33]. Our phylogeny estimates that Brachymeles evolved 62 ± 15 Ma, which was before the Philippines formed (figure 1), indicating that its origin was on mainland Southeast Asia when it extended to present-day Borneo [74]. Palaeoclimatological data are sparse for this time period, but suggest a drier and more seasonal climate than today [75]. From 50 MA to present, oxygen isotope, temperature, pollen and fossil algae data are available, resulting in more reliable reconstructions [75]. These data show that between 50 and 30 Ma the climate tended to be subhumid and seasonal with coniferous trees expanding during cooler periods (figure 1) [75,76]. Hence, Brachymeles probably evolved in habitats that were much drier, with looser substrates that were easier to penetrate than today.
Brachymeles’s origin in the Philippines was 31 ± 6 Ma, shortly after many Philippine islands separated from the Sunda Shelf [74] (figure 1). At this time, the climate remained hot and dry [75,77]. However, by 25 Ma, the climate had shifted to be much wetter, with the monsoons becoming established [75–77]. This change in climate would have resulted in wet, packed soils with more vegetation that are more difficult to penetrate [67], similar to today's environments. The pentadactyl clade evolved around 21 ± 5 Ma (figure 1), coinciding with this transition to a monsoonal ‘ever-wet' climate, when robust limbs and larger body size would facilitate burrowing in such soil, as our locomotion data show. These large-scale palaeoclimatological data cannot take into account microhabitat selection of drier or wetter soils as the climate changed. However, our field soil moisture and load-bearing capacity data showed no relationship with body form, despite a possible expectation of more snake-like species inhabiting less compacted or drier soils.
(d). On the persistence of snake-like forms
We showed that limbless species of Brachymeles are poorer surface and fossorial locomotors than quadrupedal species and live in soil that is not well suited to their locomotion. These species are often syntopic with large pentadactyl species [34], and so an outstanding question is how they persist. Our observations in the field suggest that the diminutive, snake-like species are more secretive and slower moving than large pentadactyl species. The snake-like species exclusively feed on arthropods that live in the leaf litter, while the pentadactyl species also prey on vertebrates, including lizards and snakes [78,79]. We also primarily captured pentadactyl species in pitfall traps, suggesting that they are more surface dwelling than snake-like species. Hence, high levels of performance may be less important to limbless Brachymeles than pentadactyl species on account of the limbless species being less frequently be exposed to predators due to their fossorial and secretive nature [80,81].
Size and dietary differences among species differing in body form may have also allowed for the persistence of intermediate forms in Australian Lerista skinks, but in that clade, the intermediate forms that are largest [29]. Quadrupedal species of Lerista tend to have diets of more surface-dwelling insects than more snake-like species [82] and tend to eat smaller prey [83], so in both clades, there appears to be a greater reliance on surface locomotion in quadrupedal species, further supporting a link between snake-like forms and fossoriality.
Divergence in size and activity patterns may have led to a divergence in a dietary niche, where high locomotor performance is necessary to capture small vertebrates. Many vertebrate radiations initially diverge in microhabitat followed by trophic morphology [84], but snake-like and quadrupedal, pentadactyl species of Brachymeles can be found in the same rotten log, yet may have diverged in size, diet and associated locomotor behaviour. Further work is needed to test this hypothesis, but similar patterns of diversification and coexistence have been documented in cichlid fishes [85].
4. Conclusion
There are many well-supported examples of evolutionary reversal, including the re-evolution of lost complex structures [7,10,11,13]. Here, we showed that the re-evolution of strong, functional limbs and a short body can be explained by the re-evolved limbs allowing both enhanced fossorial and surface locomotion in habitats with wet, packed substrates, particularly with coarse particles. This change in habitat was probably driven by changes in climate during the evolution of the pentadactyl clade. Divergence in size, diet and microhabitat among different lineages of Brachymeles probably allowed for persistence of different body forms along the lizard-like/snake-like axis. We conclude that the integration of evolutionary morphology, biomechanics, ecology and palaeoclimatology is critical for understanding patterns of body shape evolution.
Supplementary Material
Acknowledgements
We are particularly grateful to J. Fernandez and our Philippine field team members for assistance during expeditions. We are also grateful to E. G. Schaper, S. Reed, S. D. Mann and M. Azeez for help with data collection. We also thank S.-M. Chaw of the Academia Sinica, Taipei for help in understanding the Southeast Asian palaeoclimate.
Ethics
M. Lim, C. Custodio, J. de Leon and A. Tagtag of the Biodiversity Management Bureau (BMB) of the Philippine Department of Environment and Natural Resources (DENR) helped facilitate collecting and export permits. Fieldwork in the Philippines was conducted under the Memorandum of Agreement with the Protected Areas and Wildlife Bureau of the Philippines (2015–2020), and Gratuitous Permit to Collect #260 & 273 (renewals). The National Research Council of Thailand allowed fieldwork under permit #54/60. The IACUC of Clark University (protocol 017R) and the University of Oklahoma (protocols R13-011, R13-012 and R17-019) approved work with animals.
Data accessibility
The data and phylogenies, as well as code used to analyse the data in this article, are available at the Dryad Digital Repository [86].
Authors' contributions
P.J.B., C.D.S., G.P.W. and D.J.I. conceived of the study. All authors collected data in the field. P.J.B. processed and analysed data, and wrote the first draft of the manuscript. All authors contributed to the manuscript and gave final approval for publication.
Competing interests
The authors have no competing interests to declare.
Funding
This work was supported by the National Science Foundation (grant nos. IOS-1353703, 1353743, 1353691 and 1353683 to P.J.B., G.P.W., D.J.I. and C.D.S., respectively), by a Fulbright Fellowship to E.S.F. and by Clark University.
References
- 1.Goldberg EE, Igic B. 2008. On phylogenetic tests of irreversible evolution. Evolution 62, 2727–2741. ( 10.1111/j.1558-5646.2008.00505.x) [DOI] [PubMed] [Google Scholar]
- 2.Muller HJ. 1939. Reversibility in evolution considered from the standpoint of genetics. Biol. Rev. Camb. Phil. Soc. 14, 261–280. ( 10.1111/j.1469-185X.1939.tb00934.x) [DOI] [Google Scholar]
- 3.Simpson GG. 1953. The major features of evolution. New York, NY: Columbia University Press. [Google Scholar]
- 4.Dollo L. 1893. Les lois de l'evolution. Bulletin de la Societé Belge de Geologie, de Paleontologie et d'Hydrologie7, 164–166. [Google Scholar]
- 5.Dollo L. 1922. Los cephalopodes deroules et l'irreversibilite de l'evolution. Bijdragen tot de Dierkunde 1922, 215–227. ( 10.1163/26660644-02201030) [DOI] [Google Scholar]
- 6.Gould SJ. 1970. Dollo on Dollo's law: irreversibility and the status of evolutionary laws. J. Hist. Biol. 3, 189–212. ( 10.1007/BF00137351) [DOI] [PubMed] [Google Scholar]
- 7.Pagel M. 2004. Limpets break Dollo's law. Trends Ecol. Evol. 19, 278–280. ( 10.1016/j.tree.2004.03.020) [DOI] [PubMed] [Google Scholar]
- 8.Marshall CR, Raff EC, Raff RA. 1994. Dollo's law and the death and resurrection of genes. Proc. Natl Acad. Sci. USA 91, 12 283–12 287. ( 10.1073/pnas.91.25.12283) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sherman PW, Braude S, Jarvis JUM. 1999. Litter sizes and mammary numbers of naked mole-rats: breaking the one-half rule. J. Mammal. 80, 720–733. ( 10.2307/1383241) [DOI] [Google Scholar]
- 10.Wiens JJ. 2011. Re-evolution of lost mandibular teeth in frogs after more than 200 million years, and re-evaluating Dollo's Law. Evolution 65, 1283–1296. ( 10.1111/j.1558-5646.2011.01221.x) [DOI] [PubMed] [Google Scholar]
- 11.Whiting MF, Bradler S, Maxwell T. 2003. Loss and recovery of wings in stick insects. Nature 421, 264–267. ( 10.1038/nature01313) [DOI] [PubMed] [Google Scholar]
- 12.Brandley MC, Huelsenbeck JP, Wiens JJ. 2008. Rates and patterns in the evolution of snake-like body form in squamate reptiles: evidence for repeated re-evolution of lost digits and long-term persistence of intermediate body forms. Evolution 62, 2042–2064. ( 10.1111/j.1558-5646.2008.00430.x) [DOI] [PubMed] [Google Scholar]
- 13.Kohlsdorf T, Wagner GP. 2006. Evidence for the reversibility of digit loss: a phylogenetic study of limb evolution in Bachia (Gymnophthalmidae: Squamata). Evolution 60, 1896–1912. ( 10.1111/j.0014-3820.2006.tb00533.x) [DOI] [PubMed] [Google Scholar]
- 14.Siler CD, Brown RM. 2011. Evidence for repeated acquisition and loss of complex body-form characters in an insular clade of southeast Asian semi-fossorial skinks. Evolution 65, 2641–2663. ( 10.1111/j.1558-5646.2011.01315.x) [DOI] [PubMed] [Google Scholar]
- 15.Galis F, Artzen JW, Lande R. 2010. Dollo's law and the irreversibility of digit loss in Bachia. Evolution 64, 2466–2476. [DOI] [PubMed] [Google Scholar]
- 16.Kohlsdorf T, Lynch VJ, Rodrigues MT, Brandley MC, Wagner GP. 2010. Data and data interpretation in the study of limb evolution: a reply to Galis et al. on the reevolution of digits in the lizard genus Bachia. Evolution 64, 2477–2485. [Google Scholar]
- 17.Lande R. 1978. Evolutionary mechanisms of limb loss in tetrapods. Evolution 32, 73–92. ( 10.1111/j.1558-5646.1978.tb01099.x) [DOI] [PubMed] [Google Scholar]
- 18.Bejder L, Hall BK. 2002. Limbs in whales and limblessness in other vertebrates: mechanisms of evolutionary and developmental transformation and loss. Evol. Dev. 4, 445–458. ( 10.1046/j.1525-142X.2002.02033.x) [DOI] [PubMed] [Google Scholar]
- 19.Bergmann PJ, Mann SDW, Morinaga G, Freitas ES, Siler CD. 2020. Convergent evolution of elongate forms in craniates and of locomotion in elongate squamate reptiles. Integr. Comp. Biol. 60, 190–201. ( 10.1093/icb/icaa015) [DOI] [PubMed] [Google Scholar]
- 20.Caputo V, Lanza B, Palmieri R. 1995. Body elongation and limb reduction in the genus Chalcides Laurenti 1768 (Squamata Scincidae): a comparative study. Tropical Zool. 8, 95–152. ( 10.1080/03946975.1995.10539275) [DOI] [Google Scholar]
- 21.Law CJ, Slater GJ, Mehta RS. 2019. Shared extremes by ectotherms and endotherms: body elongation in musteloids is associated with small size and reduced limbs. Evolution 73, 735–749. ( 10.1111/evo.13702) [DOI] [PubMed] [Google Scholar]
- 22.Wake DB, Wake MH, Specht CD. 2011. Homoplasy: From detecting pattern to determining process and mechanism of evolution. Science 331, 1032–1035. ( 10.1126/science.1188545) [DOI] [PubMed] [Google Scholar]
- 23.Ward AB, Brainerd EL. 2007. Evolution of axial patterning in elongate fishes. Biol. J. Linnean Soc. 90, 97–116. ( 10.1111/j.1095-8312.2007.00714.x) [DOI] [Google Scholar]
- 24.Wiens JJ, Brandley MC, Reeder TW. 2006. Why does a trait evolve multiple times within a clade? Repeated evolution of snakelike body form in squamate reptiles. Evolution 60, 123–141. [PubMed] [Google Scholar]
- 25.Gans C. 1975. Tetrapod limlessness: evolution and functional corollaries. Am. Zool. 15, 455–467. ( 10.1093/icb/15.2.455) [DOI] [Google Scholar]
- 26.Rieppel O. 1988. A review of the origin of snakes. In Evolutionary biology (ed. Hecht MK.), pp. 37–130. New York, NY: Plenum Press. [Google Scholar]
- 27.Simões BF, et al. 2015. Visual system evolution and the nature of the ancestral snake. J. Evol. Biol. 28, 1309–1320. ( 10.1111/jeb.12663) [DOI] [PubMed] [Google Scholar]
- 28.Morinaga G, Bergmann PJ. 2020. Evolution of fossorial locomotion in the transition from tetrapod to snake-like in lizards. Proc. R. Soc. B 287, 20200192 ( 10.1098/rspb.2020.0192) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morinaga G, Bergmann PJ. 2017. Convergent body shapes have evolved via deterministic and historically contingent pathways in Lerista lizards. Biol. J. Linnean Soc. 121, 858–875. ( 10.1093/biolinnean/blx040) [DOI] [Google Scholar]
- 30.Skinner A, Lee MSY. 2009. Body-form evolution in the scincid lizard clade Lerista and the mode of macroevolutionary transitions. Evol. Biol. 36, 292–300. ( 10.1007/s11692-009-9064-9) [DOI] [Google Scholar]
- 31.Skinner A, Lee MSY, Hutchinson MN. 2008. Rapid and repeated limb loss in a clade of scincid lizards. BMC Evol. Biol. 8, 310–318. ( 10.1186/1471-2148-8-310) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilson S, Swan G. 2005. A complete guide to the reptiles of Australia. Sydney, Australia: Reed New Holland Publishers. [Google Scholar]
- 33.Wagner GP, Griffith OW, Bergmann PJ, Bello-Hellegouarch G, Kohlsdorf T, Bhullar A, Siler CD. 2018. Are there general laws for digit evolution in squamates? The loss and re-evolution of digits in a clade of fossorial lizards (Brachymeles. Scincinae). J. Morphol. 279, 1104–1119. ( 10.1002/jmor.20834) [DOI] [PubMed] [Google Scholar]
- 34.Siler CD, Diesmos AC, Alcala AC, Brown RM. 2011. Phylogeny of Philippine slender skinks (Scincidae: Brachymeles) reveals underestimated species diversity, complex biogeographical relationships, and cryptic patterns of lineage diversification. Mol. Phylogenet. Evol. 59, 53–65. ( 10.1016/j.ympev.2010.12.019) [DOI] [PubMed] [Google Scholar]
- 35.Uetz P. 2019. The reptile database. See http://www.reptile-database.org.
- 36.Walker JA. 2010. An integrative model of evolutionary covariance: a symposium on body shape in fishes. Integr. Comp. Biol. 50, 1051–1056. ( 10.1093/icb/icq014) [DOI] [PubMed] [Google Scholar]
- 37.Heitz BB, Diesmos AC, Freitas ES, Ellsworth ED, Grismer LL, Aowphol A, Brown RM, Siler CD. 2016. A new supple skink, genus Lygosoma (Reptilia: Squamata: Scincidae), from the Western Philippines. Herpetologica 72, 352–361. ( 10.1655/Herpetologica-D-16-00023.1) [DOI] [Google Scholar]
- 38.Bergmann PJ, Irschick DJ. 2010. Alternate pathways of body shape evolution translate into common patterns of locomotor evolution in two clades of lizards. Evolution 64, 1569–1582. ( 10.1111/j.1558-5646.2009.00935.x) [DOI] [PubMed] [Google Scholar]
- 39.Korff WL, McHenry MJ. 2011. Environmental differences in substrate mechanics do not affect sprinting performance in sand lizards (Uma scoparia and Callisaurus draconoides). J. Exp. Biol. 214, 122–130. ( 10.1242/jeb.045682) [DOI] [PubMed] [Google Scholar]
- 40.Bergmann PJ, Morinaga G. 2019. The convergent evolution of snake-like forms by divergent evolutionary pathways in squamate reptiles. Evolution 73, 481–496. ( 10.1111/evo.13651) [DOI] [PubMed] [Google Scholar]
- 41.Rasband WS. 2019. Image J. See http://imagej.nih.gov/ij/.
- 42.Barros FC, Herrel A, Kohlsdorf T. 2011. Head shape evolution in Gymnophthalmidae: does habitat use constrain the evolution of cranial design in fossorial lizards? J. Evol. Biol. 24, 2423–2433. ( 10.1111/j.1420-9101.2011.02372.x) [DOI] [PubMed] [Google Scholar]
- 43.Bergmann PJ, Pettinelli KJ, Crockett ME, Schaper EG. 2017. It's just sand between the toes: how particle size and shape variation affect running performance and kinematics in a generalist lizard. J. Exp. Biol. 220, 3706–3716. ( 10.1242/jeb.161109) [DOI] [PubMed] [Google Scholar]
- 44.Vanhooydonck B, Boistel R, Fernandez V, Herrel A. 2011. Push and bite: trade-offs between burrowing and biting in a burrowing skink (Acontias percivali). Biol. J. Linnean Soc. 102, 91–99. ( 10.1111/j.1095-8312.2010.01563.x) [DOI] [Google Scholar]
- 45.Umberger CM, de Buron I, Roumillat WA, McElroy EJ. 2013. Effects of a muscle-infecting parasitic nematode on the locomotor performance of their fish host. J. Fish Biol. 82, 1250–1258. ( 10.1111/jfb.12061) [DOI] [PubMed] [Google Scholar]
- 46.Morinaga G, Bergmann PJ. 2019. Angles and waves: intervertebral joint angles and axial kinematics of limbed lizards, limbless lizards, and snakes. Zoology 134, 16–26. ( 10.1016/j.zool.2019.04.003) [DOI] [PubMed] [Google Scholar]
- 47.Benesch AR, Withers PC. 2002. Burrowing performance and the role of limb reduction in Lerista (Scincidae, Lacertilia). Senckenbergiana Lethaea 82, 107–114. ( 10.1007/BF03043776) [DOI] [Google Scholar]
- 48.R Core Team. 2019. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 49.Revell LJ. 2009. Size-correction and principal components for interspecific comparative studies. Evolution 63, 3258–3268. ( 10.1111/j.1558-5646.2009.00804.x) [DOI] [PubMed] [Google Scholar]
- 50.Revell LJ. 2012. Phytools: an R package for phylogenetic comparative biology (and other things). Methods Ecol. Evol. 3, 217–223. ( 10.1111/j.2041-210X.2011.00169.x) [DOI] [Google Scholar]
- 51.Pagel M. 1997. Inferring evolutionary processes from phylogenies. Zoologica Scripta 26, 331–348. ( 10.1111/j.1463-6409.1997.tb00423.x) [DOI] [Google Scholar]
- 52.Wiens JJ, Singluff JL. 2001. How lizards turn into snakes: a phylogenetic analysis of body form evolution in anguid lizards. Evolution 55, 2303–2318. ( 10.1111/j.0014-3820.2001.tb00744.x) [DOI] [PubMed] [Google Scholar]
- 53.Revell LJ. 2018. Computing phylogenetic PCA scores for individual data, when PCs were extracted from the correlation matrix & with Pagel's λ. See http://blog.phytools.org/2018/11/computing-phylogenetic-pca-scores-for.html.
- 54.Beaulieu JM, Jhueng D-J, Boettiger C, O'Meara BC. 2012. Modeling stabilizing selection: Expanding the Ornstein-Uhlenbeck model of adaptive evolution. Evolution 66, 2369–2383. ( 10.1111/j.1558-5646.2012.01619.x) [DOI] [PubMed] [Google Scholar]
- 55.Burnham KP, Anderson DR. 2002. Model selection and multimodel inference: a practical information-theoretic approach, 2nd edn New York, NY: Springer. [Google Scholar]
- 56.Revell LJ. 2010. Phylogenetic signal and linear regression on species data. Methods Ecol. Evol. 1, 319–329. ( 10.1111/j.2041-210X.2010.00044.x) [DOI] [Google Scholar]
- 57.Orme D, Freckleton RP, Thomas G, Petzoldt T, Fritz S, Isaac N, Pearse W. 2013. The caper package: comparative analysis of phylogenetics and evolution in R. R package version 0.5.2. See https://CRAN.R-project.org/package=caper.
- 58.Benjamini Y, Hochberg Y. 1995. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. B 57, 289–300. [Google Scholar]
- 59.Williams VSL, Jones LV, Tukey JW. 1999. Controlling error in multiple comparisons, with examples from state-to-state differences in educational achievement. J. Educ. Behav. Stat. 24, 42–69. ( 10.3102/10769986024001042) [DOI] [Google Scholar]
- 60.Bergmann PJ, Irschick DJ. 2012. Vertebral evolution and the diversification of squamate reptiles. Evolution 66, 1044–1058. ( 10.1111/j.1558-5646.2011.01491.x) [DOI] [PubMed] [Google Scholar]
- 61.Claverie T, Wainwright PC. 2014. A morphospace for reef fishes: elongation is the dominant axis of body shape evolution. PLoS ONE 9, e112732 ( 10.1371/journal.pone.0112732) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Collar DC, Quintero M, Buttler B, Ward AB, Mehta RS. 2016. Body shape transformation along a shared axis of anatomical evolution in labyrinth fishes (Anabantoidei). Evolution 70, 555–567. ( 10.1111/evo.12887) [DOI] [PubMed] [Google Scholar]
- 63.Maladen RD, Ding Y, Li C, Goldman DI. 2009. Undulatory swimming in sand: Subsurface locomotion of the sandfish lizard. Science 325, 314–318. ( 10.1126/science.1172490) [DOI] [PubMed] [Google Scholar]
- 64.Vanhooydonck B, Van Damme R, Aerts P. 2002. Variation in speed, gait characteristics and microhabitat use in lacertid lizards. J. Exp. Biol. 205, 1037–1046. [DOI] [PubMed] [Google Scholar]
- 65.Freitas ES, Datta-Roy A, Karanth P, Grismer LL, Siler CD. 2019. Multilocus phylogeny and a new classification for African, Asian and Indian supple and writhing skinks (Scincidae: Lygosominae). Zool. J. Linn. Soc. 186, 1067–1096. ( 10.1093/zoolinnean/zlz001) [DOI] [Google Scholar]
- 66.Zheng Y, Wiens JJ. 2016. Combining phylogenomic and supermatrix approaches, and a time-calibrated phylogeny for squamate reptiles (lizards and snakes) based on 52 genes and 4162 species. Mol. Phylogenet. Evol. 94, 537–547. ( 10.1016/j.ympev.2015.10.009) [DOI] [PubMed] [Google Scholar]
- 67.Sharpe SS, Kuckuk RM, Goldman DI. 2015. Controlled preparation of wet granular media reveals limits to lizard burial ability. Phys. Biol. 12, 046009 ( 10.1088/1478-3975/12/4/046009) [DOI] [PubMed] [Google Scholar]
- 68.Albert I, Sample JG, Morss AJ, Rajagopalan S, Barabasi AL, Schiffer P. 2001. Granular drag on a discrete object: shape effects on jamming. Phys. Rev. E 64, 061303 ( 10.1103/PhysRevE.64.061303) [DOI] [PubMed] [Google Scholar]
- 69.Goldman DI, Umbanhowar P. 2008. Scaling and dynamics of sphere and disk impact into granular media. Phys. Rev. E 77, 021308 ( 10.1103/PhysRevE.77.021308) [DOI] [PubMed] [Google Scholar]
- 70.Baumgartner W, Fidler F, Weth A, Habbecke M, Jakob P, Butenweg C, Bohme W. 2008. Investigating the locomotion of the sandfish in desert sand using NMR-imaging. PLoS ONE 3, e3309 ( 10.1371/journal.pone.0003309) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Holovacs NT, Daza JD, Guerra C, Stanley EL, Montero R. 2020. You can't run, but you can hide: the skeleton of the sand-swimmer lizard Calyptommatus leiolepis (Squamata: Gymnophthalmidae). Anatomical Record 303, 1305–1326. ( 10.1002/ar.24246) [DOI] [PubMed] [Google Scholar]
- 72.Grizante MB, Brandt R, Kohlsdorf T. 2012. Evolution of body elongation in gymnophthalmid lizards: relationships with climate. PLoS ONE 7, e49772 ( 10.1371/journal.pone.0049772) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Branch W. 1998. Field guide to the snakes and other reptiles of Southern Africa. Sanibel Island, FL: Ralph Curtis Publishing. [Google Scholar]
- 74.Hall R. 2002. Cenozoic geological and plate tectonic evolution of SE Asia and SW Pacific: computer-based reconstructions, model and animations. J. Asian Earth Sci. 20, 353–431. ( 10.1016/S1367-9120(01)00069-4) [DOI] [Google Scholar]
- 75.Morely RJ. 2012. A review of the Cenozoic palaeoclimate history of Southeast Asia. In Biotic evolution and environmental climate in Southeast Asia (ed. Gower DJ.), pp. 79–114. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 76.Guo ZT, et al. 2008. A major reorganization of Asian climate by the early Miocene. Clim. Past 4, 153–174. ( 10.5194/cp-4-153-2008) [DOI] [Google Scholar]
- 77.Ramstein G, Fluteau F, Besse J, Joussaume S. 1997. Effect of orogeny, plate motion and land-sea distribution on Eurasian climate change over the past 30 million years. Nature 386, 788–795. ( 10.1038/386788a0) [DOI] [Google Scholar]
- 78.Schaper EG, Bergmann PJ, Morinaga G, Fernandez J, Smith SN, Ellsworth ED, Siler CD. 2018. Brachymeles bicolor (Philippine Slender Skink) diet. Herpetol. Rev. 49, 740–741. [Google Scholar]
- 79.Siler CD, Alviola P, Baniqued RD, Duya MR. 2012. Brachymeles boulengeri (Philippine Slender Skink) diet. Herpetol. Rev. 41, 130. [Google Scholar]
- 80.Irschick DJ, Losos JB. 1998. A comparative analysis of the ecological significance of maximal locomotor performance in Caribbean Anolis lizards. Evolution 52, 219–226. ( 10.1111/j.1558-5646.1998.tb05155.x) [DOI] [PubMed] [Google Scholar]
- 81.Irschick DJ, Meyers JJ, Husak JF, Le Galliard JF. 2008. How does selection operate on whole-organism functional performance capacities? A review and synthesis. Evol. Ecol. Res. 10, 177–196. [Google Scholar]
- 82.Kendrick PG. 1991. The phylogenetics and comparative ecology of Lerista bell, 1983; patterns of evolution in a genus of sand-swimming skinks Doctoral thesis, University of Western Australia, Perth, Australia. [Google Scholar]
- 83.Pough FH, Preest MR, Fusari M. 1997. Prey-handling and the evolutionary ecology of sand-swimming lizards (Lerista: Scincidae). Oecologia 112, 351–361. ( 10.1007/s004420050320) [DOI] [PubMed] [Google Scholar]
- 84.Streelman JT, Danley PD. 2003. The stages of vertebrate evolutionary radiation. Trends Ecol. Evol. 18, 126–131. ( 10.1016/S0169-5347(02)00036-8) [DOI] [Google Scholar]
- 85.Muschick M, Indermaur A, Salzburger W. 2012. Convergent evolution within an adaptive radiation of cichlid fishes. Curr. Biol. 22, 2362–2368. ( 10.1016/j.cub.2012.10.048) [DOI] [PubMed] [Google Scholar]
- 86.Bergmann PJ, Morinaga G, Freitas ES, Irschick DJ, Wagner GP, Siler CD. 2020. Data from: Locomotion and palaeoclimate explain the re-evolution of quadrupedal body form in Brachymeles lizards. Dryad Digital Repository ( 10.5061/dryad.bcc2fqz9w) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Bergmann PJ, Morinaga G, Freitas ES, Irschick DJ, Wagner GP, Siler CD. 2020. Data from: Locomotion and palaeoclimate explain the re-evolution of quadrupedal body form in Brachymeles lizards. Dryad Digital Repository ( 10.5061/dryad.bcc2fqz9w) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
The data and phylogenies, as well as code used to analyse the data in this article, are available at the Dryad Digital Repository [86].