Abstract
Top-down effects of apex predators are modulated by human impacts on community composition and species abundances. Consequently, research supporting top-down effects of apex predators occurs almost entirely within protected areas rather than the multi-use landscapes dominating modern ecosystems. Here, we developed an integrated population model to disentangle the concurrent contributions of a reintroduced apex predator, the grey wolf, human hunting and prey abundances on vital rates and abundance of a subordinate apex predator, the puma. Increasing wolf numbers had strong negative effects on puma fecundity, and subadult and adult survival. Puma survival was also influenced by density dependence. Overall, puma dynamics in our multi-use landscape were more strongly influenced by top-down forces exhibited by a reintroduced apex predator, than by human hunting or bottom-up forces (prey abundance) subsidized by humans. Quantitatively, the average annual impact of human hunting on equilibrium puma abundance was equivalent to the effects of 20 wolves. Historically, wolves may have limited pumas across North America and dictated puma scarcity in systems lacking sufficient refugia to mitigate the effects of competition.
Keywords: competition, puma, survival, wolf, yellowstone
1. Introduction
The reintroduction of large carnivores to areas in which they were previously extirpated has provided opportunities to study and quantify the top-down effects of apex predators within ecological communities (e.g. [1,2]). The strength of the various ecological effects of apex predators, however, is modulated by jurisdiction and appears to be obscured in unprotected landscapes where they are overshadowed by human impacts on community composition and species abundances [3–6]. Human activities and social tolerance for large carnivores, for example, determine carnivore distribution and abundances [7], and therefore the potential strength of top-down effects attributable to apex predators in most ecosystems. Further, human agricultural practices subsidize lower trophic levels and increase bottom-up effects in many modified systems [4,8]. For these reasons, results demonstrating strong top-down effects of apex predators on subordinate predators and their prey almost entirely come from studies conducted inside protected areas rather than the much more common multi-use landscapes dominating modern ecosystems [4,5,9].
Traditionally, ‘top-down effects' described effects across trophic levels, but more recently they have also been used to describe the effects of dominant competitors on subordinate competitors that share trophic levels in more complex food webs (e.g. [10]). Pumas (Puma concolor) are subordinate, wide-ranging, solitary carnivores and their population dynamics exemplify the difficulties in differentiating top-down from bottom-up effects. Pumas live at low densities and exhibit life histories typical of long-lived species, making it difficult to obtain sample sizes needed for complex analyses aimed at understanding drivers of their population dynamics [11]. Contemporary puma population dynamics in western North America are also dominated by anthropogenic top-down effects in the form of legal hunting [12,13] and other anthropogenic impacts (e.g. road mortality, conflict management and depredation permits [14]). Like other apex carnivores, theory predicts that the abundance of pumas in areas without human hunting is determined by prey availability [15–17]. Pumas, however, are also subordinate to four dominant competitors across their range: grey wolves (Canis lupus), grizzly bears (Ursus arctos), American black bears (U. americanus) and jaguars (Panthera onca) [18]. These species compete with pumas for prey, usurp their kills (i.e. kleptoparasitism) and sometimes kill them. Therefore, pumas are clearly susceptible to additional top-down forces beyond those exerted by humans. Evidence suggests that grey wolves, in particular, impact numerous puma behaviours, including puma habitat use and prey selection [19,20], but researchers still lack direct evidence that wolves affect the abundance of pumas on the landscape [18].
Wolves were reintroduced in Yellowstone National Park in the USA in 1995, shortly after which they expanded into adjacent multi-use landscapes. Between 2000 and 2015, the puma population in the southern Greater Yellowstone Ecosystem (GYE) declined by 48%, as explained by three primary causes of mortality: regulated human hunting of adult and subadult pumas, grey wolves killing puma kittens, and increased starvation across age classes, but especially subadults [21]. The southern GYE is a mosaic of variable human perturbations influencing local wildlife, including legal hunting of predators and ungulates, and subsidized primary production through watering grasslands and agriculture on private ranches and public lands. Wildlife managers also subsidize bottom-up effects through supplemental feeding programmes on public lands aimed at supporting wintering elk (Cervus canadensis) populations and mitigating elk conflicts with local ranchers [22].
Here, we combine 16 years of monitoring data from 147 individual pumas, their associated estimates of survival and fecundity, as well as abundance estimates of pumas, wolves and prey (elk) in an integrated population model (IPM) to link observed patterns of mortality with declines in puma abundance. Integrated population models provide the opportunity to include multiple types of data and allow researchers to simultaneously examine the abundance and demographic drivers underlying changes in abundance [23,24]. Such insights will support conservation management of pumas and wolves, given the current expansion of both species in North America due to reintroduction efforts for wolves and the evolution of wildlife management encouraging coexistence strategies with large carnivores following the cessation of predator bounty hunting. Such work may also prove useful in deciphering historic ecological systems in North America, when pumas and wolves were sympatric across nearly all of the puma range. Recent research has highlighted that coyote (C. latrans) expansion in North America, for example, is in part due to wolf eradication efforts that occurred a century ago [2,25].
Based upon research in other carnivore guilds highlighting the impacts that dominant competitors have on the abundance of subordinate competitors (e.g. African lion (Panthera leo) and wild dog (Lycaon pictus) [26]; tiger (Panthera tigris) and leopard (Panthera pardus) [27]; wolf and coyote [25]), we predicted that reintroduced wolves would have a population-level effect on puma abundance. To begin with, we accounted for the effects of human hunting on pumas that might obscure wolf effects. Then we tested several a priori models to determine whether top-down (human hunting or wolf abundance) or bottom-up (prey abundance) or some combination of top-down and bottom-up forces best fit puma vital rates and changes in puma abundance determined over 16 years of fieldwork (the time period during which wolves completely recolonized the study area). Finally, we used the most parsimonious model explaining observed fluctuations in puma population size to project future potential puma populations in the region, essential for the conservation of this charismatic predator and the maintenance of its diverse contributions to healthy ecosystems [28–30].
2. Methods
(a). Study area and wolf reintroductions
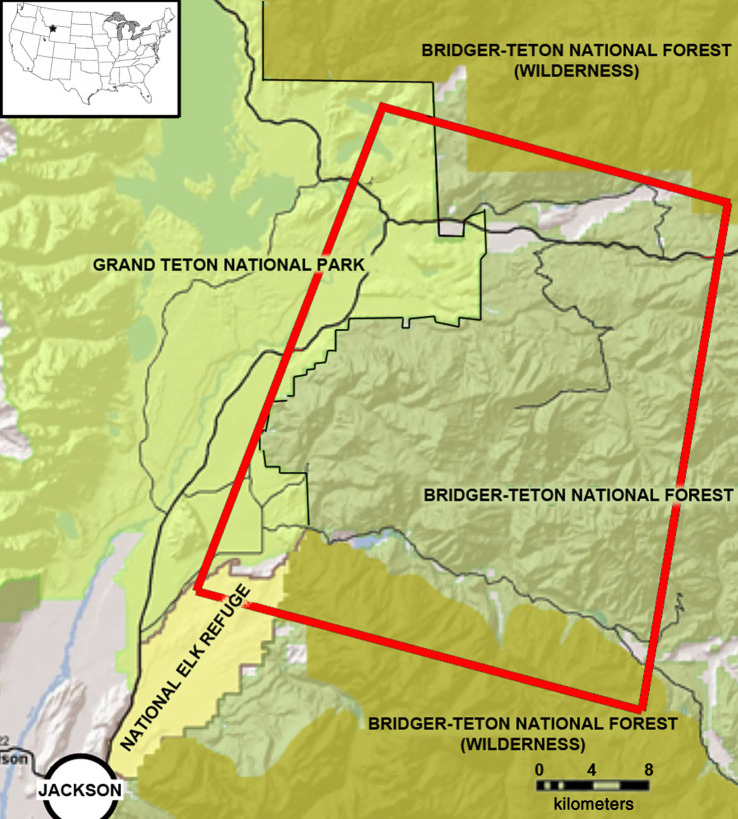
Our research ran from late 2000 until 2017, and our study area encompassed approximately 2300 km2 of the southern GYE in northwest Wyoming, USA, northeast of the town of Jackson (figure 1; WGS84 43.60671, -110.41182). Our study area overlapped different types of public lands reflecting various, species-specific management. To the west, the study area included approximately 475 km2 of the Grand Teton National Park, where wildlife were fully protected, except elk, which were subject to an Autumn hunt in managed subsections of the National Park. To the south, wildlife were also fully protected on the 100 km2 National Elk Refuge (NER), except bison (Bison bison) and elk, which were subject to limited harvest during an Autumn hunt. The remaining 75% of the study area was composed of lands managed by the United States Forest Service (USFS), which allowed legal hunting and trapping of diverse mammals following guidelines set by the Wyoming Game and Fish Department (WGFD). This included hunting of carnivores and competitors, including American black bears and coyotes, and ungulate prey, including elk, mule deer (Odocoileus hemionus), moose (Alces alces), pronghorn (Antilocapra americana) and bighorn sheep (Ovis canadensis). Grizzly bears were fully protected during the study, except bears killed due to human safety or livestock conflict issues on public or private lands.
Figure 1.
The location of our study area in northwest Wyoming in the USA in the inset, and a larger map delineating land ownership. The rectangle is the portion of the study area in which we annually captured pumas. (Online version in colour.)
Wolves were first reintroduced north of our study area in Yellowstone National Park in 1995 [31]. The first breeding pair settled in our study area in 1999, and annual estimates for the numbers of wolves and wolf packs in the study area have since been determined by the United States Fish and Wildlife Service (USFW). Wolves were protected from legal hunting during our study excepting 2012 and 2013, when a limited quota hunt was permitted from October 1 to December 31 of each year. Over the duration of our research, the number of wolves in the study area ranged between 10 and 91 individuals, the peak of which occurred in 2010 [21].
Elk in our study area were part of the migratory Jackson herd and cooperatively managed by the WGFD, National Park Service and the NER. Portions of the Jackson elk herd travel long distances, but the entire herd congregates near Jackson, in winter, where they receive supplemental feeding on the NER and adjacent USFS lands on feed lots managed by the WGFD. Our study occurred during the time period in which managers implemented liberal hunting quotas across jurisdictional boundaries to reduce the Jackson herd from 16 000 in 2000 to 11 000 animals [22,32].
Our study area also included large private inholdings surrounded by public lands (e.g. ranches), as well as development on the fringes of local communities, all of which subsidized primary production directly through agriculture (hay or alfalfa or other crops), watering pastures for livestock or lawns, and/or feed provisioning meant for livestock but used by wildlife. Additional descriptions of climate, topography and habitat are presented in Elbroch et al. [33].
(b). Puma captures, monitoring and age classifications
We included puma monitoring data beginning in 2001, when a sufficient proportion of the population had been captured to justify analyses. We followed puma capture and immobilization protocols described in Elbroch et al. [33] and approved by the Jackson Institutional Animal Care and Use Committee (Protocol 027-10EGDBS-060210) and National Park Service IACUC (Protocol IMR_GRTE_Elbroch_Cougar_2013-2015). Pumas were fitted with a VHF (Telonics, Mesa, AZ) or GPS (Telonics, Mesa, AZ; Televilt, Lindesberg, Sweden; Vectronics, Berlin, Germany; Lotek Wireless, Ontario, Canada) collar. We counted kittens in known dens, when possible within 3 weeks of their birth, and then hand-captured kittens between 5 and 7 weeks old without the aid of immobilization drugs. Any kittens we managed to capture were fitted with custom-made, lightweight, expandable VHF collars (Telonics, Mesa, AZ).
We attempted to locate kittens wearing VHF collars every 2 days until they were 10- to 12-months old, when collars dropped on their own. All other pumas wearing VHF collars were located at minimum weekly from the ground and monthly from aircraft. Location data were acquired by GPS collars 4–12 times per day. All collars were equipped with mortality sensors, which alerted researchers when an individual had not moved for greater than or equal to 8 h. We investigated mortality sites and determined the cause of death through interpreting field signs (e.g. bite marks, footprints), necropsies conducted with a veterinarian and based on blood and tissue samples analysed by the Wyoming Game and Fish Wildlife Health Laboratory [34].
(c). Estimating annual puma density
Each year, we determined the minimum puma density in our study area based on overlapping home ranges [35]. Annual home ranges for adult pumas were determined using fixed-kernel density estimators [36] in ArcGIS 10, and isopleth calculations in the Geospatial Modeling Environment [37]; methods are further described in Lendrum et al. [38]. We determined the boundaries of the area in which we consistently searched for pumas each winter, and in which we believed we had captured all resident pumas. In ArcGIS 10, we created a polygon of our capture area and quantified each puma's residency within this polygon [35]. ‘Minimum puma densities' (i.e. excluding transients or residents we did not capture) were then determined by summing the residency estimates for all adult pumas with overlapping home ranges for each year. Kitten estimates were those kittens that accompanied marked, resident females we monitored.
(d). Integrated population model
We estimated future puma abundance using a demographic model inclusive of seasonal fecundity and stage-specific survival rates operating on a six-month time step. Following Elbroch et al. [21], we split each year into two six-month seasons, one in which there was regulated legal hunting for pumas, and the other during which hunting was not permitted. These seasons captured variation in effects due to human-caused mortality as well as other mortality: (i) pumas were legally hunted during a ‘hunting season' running from 1 October to 31 March of the following year. The hunting season also captured additional ecological variation: elk migrations to low-elevation winter ranges where they aggregated in large herds near supplementary feeding stations, mule deer migrations out of the study area, increased competition between wolves and pumas, and deep snows and cold temperatures influencing puma movements and energetics [20]. (ii) Puma hunting was closed during the ‘non-hunting season' from 1 April to 30 September of each year, during which elk migrated to high-elevation summer ranges and spread out, mule deer returned to the study area, temperatures warmed, and ungulate and puma parturitions occurred [21,38].
We defined puma life-history stages based on differences in behaviour and survival reported in the literature. We defined kittens as 0–6 months, subadults as 7–18 months and adults as greater than or equal to 18 months of age. Kittens (defined as stage K) were completely dependent on their mothers and experienced high mortality from both predation and starvation [39,40]. Subadults were dependent on their mothers, less susceptible to predation [39], but more susceptible to starvation. Subadults could be legally hunted once they were 1-year old and separate from their mothers [41], and they experienced additional risks associated with dispersal [11,42]. While we assumed that all subadults had the same vital rates, we distinguished between subadults less than a year old that could not be hunted (defined as stage S1) from older individuals that could be hunted (defined as stage S2). We pooled all individuals greater than or equal to 18 months old into an adult age class (defined as stage A) when pumas were expected to establish stable territories and become reproductively active [11,39].
Our models assumed a birth pulse in the non-hunting season [38], thus fecundity was only modelled for the non-hunting season. We did, however, allow some kittens to recruit the following non-hunting season, to better reflect the fact that kittens may be born late in the non-hunting season, and on occasion, at the onset of the hunting season [43]. We modelled the number of kittens that recruited at the end of the non-hunting season as a fixed proportion, π, of the total number of kittens birthed in that calendar year.
Survival probabilities were informed by integrating an abundance model with a multistate capture–mark–recapture model. The abundance model described the seasonal abundances as lognormal random variables. The mean of each was modelled using the transition matrices described in equations (2.1) and (2.2). We modelled the variance of the abundances using a stage-specific variance term that can be interpreted as the environmental variation experienced by each stage [44]. The capture–recapture data was modelled using a multistate survival model that accounted for death due to legal hunting, or death due to other causes, hereafter ‘other mortality’. Other causes of death in our study included starvation, disease, predation, poaching, capture-related mortalities and undetermined mortality [21]. We allowed harvested animals to remain in the model until the end of the six-month harvest period to compete for resources but removed these harvested individuals from the reproductive pool so that they were not producing offspring.
The survival models for each stage and season were coupled to a fecundity model in a stage-structured matrix [45] to quantify seasonal changes in population abundance. A life cycle diagram describing all possible state transitions is given in the electronic supplementary material, figure S1. We denote the natural survival terms in the model as ϕ, with a subscript indicating the life-history stage and season, harvest probabilities as pharvest, and fecundity as f. Kittens (stage K) were surveyed soon after birth, so we were able to estimate true fecundity; all other stages were surveyed at the end of each census period. Our model for the non-hunting season in year y included the birth pulse of kittens, the survival of subadults and adults, and recruitment of all stages. In equation (2.1), we assumed that kittens recruited to become subadults with probability π, accounting for the fact that some proportion of kittens may be born late in the non-hunting season, and on occasion, at the onset of the hunting season.
The hunting season model was similar to the non-hunting season though it did not have any fecundity term (equation 2.2). We modelled annual natural survival rates (ϕ(y)) using a logit link that incorporated covariates associated with each of our hypotheses (described below). Recapture (i.e. detection) probabilities were modelled with year (y) as a random effect. Individuals that dispersed from the study area during the study were censored.
Integrated population models are robust to some assumptions (e.g. dependence of abundance and recapture data [46]), but fragile to others, such as tag-loss [47]. We only had one dropped collar over the course of the study, and we accounted for heterogeneity in mortality with a stage-structured model. Transient individuals that are never monitored and only spend a short time in the study area also have the potential to increase intraspecific competition that could bias model outputs. However, in a land-tenure species like pumas [15], we expect that
| 2.1 |
| 2.2 |
bias to be minimal because negative effects of competition between residents and transients primarily impact transients.
We considered four continuous covariates as potential drivers of seasonal changes in puma abundances: (i) annual minimum puma densities to test for possible density-dependent effects (estimated as resident adults/890 km2); (ii) annual elk counts for the Jackson herd as reported by WGFD; (iii) annual elk counts of the number of animals wintering off the NER, and which are more reflective of true prey availability for local pumas [48] and (iv) annual wolf counts for our study system, as reported by the USFW (electronic supplementary material, table S1 [21]). Two covariates were different elk metrics representing prey availability and bottom-up effects, as elk abundance had previously proven highly predictive of cause-specific mortality rates for pumas in the study system [21]. We tested for multicollinearity among predictor variables and report them in electronic supplementary material, figure S2.
We used the above covariates to construct six hypotheses about the ecological factors driving puma vital rates: Model 1 (hereafter the ‘null model') assumed that all demographic rates in the models defined by equations (2.1) and (2.2) were constant through time. Model 2 (hereafter the ‘density-dependent model') varied all vital rates depending upon adult puma densities from the previous season. We constructed three additional models that had density-dependent variables plus one of three covariates representing top-down versus bottom-up effects on puma vital rates: Model 3 included annual wolf counts for our study area as reported by the USFW (hereafter the ‘density + wolf model’). Model 4 included annual counts for the Jackson elk herd as reported by WGFD (hereafter the ‘density + elk model’), and Model 5 included annual counts of elk in the Jackson elk herd that wintered off the NER as reported by the WGFD and NER (hereafter the ‘density + off-refuge elk model'). Model 6 combined elements from these covariates into a hypothesis we generated based on the literature about puma ecology and our own observations in the field. This model included density-dependent and off-refuge elk effects on puma fecundity, wolf effects on kitten survival, and both off-refuge elk and wolf effects on adult survival (hereafter referred to as the ‘Local Perceptions' model). We did not include a model with both wolves and elk, because of the high correlations between the variables (R2 = 0.71, 0.78; electronic supplementary material, figure S2). Our initial diagnostic figures also illustrated patterns of density dependence in population counts, and therefore, we did not test models without puma density in our initial a priori models. However, we did run post hoc models that included the effects of individual covariates without density-dependent effects. We standardized all covariates so that the magnitudes of parameter estimates could be directly compared within and among models.
We modelled survival rate of age class S, season X, in year y (denoted as ϕS,X(y)) using a logit model with covariate effects that could include the effects of density dependence and a covariate. This full form of this model was logit(ϕS,X(y)) = βS,X + βS,P · AX(y) + βS,C · C(y), where βS,X was the intercept term, βS,P was a regression coefficient that gives the effect of the adults puma density, AX(y), in season X and year y, and βS,C was a regression coefficient that determined the effect of a covariate, C(y), in year y, on age class S. For the null model, we set βS,P = 0 and βS,C = 0, while for the density-dependent model we set βS,C = 0.
The full annual fecundity model for year y was, , where α0 is the log-fecundity and αP and αC were regression coefficients that accounted for the effects of puma adults density in the hunting season and other covariates, respectively. As in the survival model, we set the appropriate coefficients to 0 for the null and density-dependent models. The vital rate models for each of our candidate models are reported in electronic supplementary material, table S3.
All estimates were conducted using MCMC in JAGS [49]. We ran models with four chains, each chain had a burn-in of 104 iterations followed by 104 draws from the posterior distribution. We determined convergence of the MCMC chains by a visual inspection of each posterior distribution and by examining Gelman's statistic. We used the Watababe–Akaike criterion (WAIC) to rank relative model performance [50].
(e). Projecting future puma populations
We simulated potential puma populations 25 years into the future using parameter estimates from our most parsimonious model under two scenarios. For both scenarios, we simulated the effects of wolf abundance on puma populations, but in only one did we include human harvest. We let wolf abundance range from the minimum observed value (10 wolves in 2001) to the maximum (91 wolves in 2010) over a range of 15 evenly spaced values, assuming that wolf densities were constant over the simulation period. In the first scenario, we included the effect of hunting mortality at historic levels, using the estimated value for the probability of an animal being hunted (pHarvest). In the second scenario, we completely removed the effects of hunting (i.e. we set pHarvest = 0). We simulated dynamics using the mean posterior estimates under each wolf abundance 104 times and calculated the mean puma abundance in the hunting season. The population simulations were initiated using the puma population abundances from 2016, the last full year of the study.
3. Results
(a). Puma monitoring
We monitored 147 individual pumas (86 kittens, 22 subadults, 39 adults) and estimated minimum annual puma densities based on 4.5 (1.8 s.d.) adult pumas monitored each year. Adult puma densities in the 890 km2 portion of the study area for which we determined density varied between 2.5 and 8.9 resident adults, or 0.28–1.0 adults/100 km2 (electronic supplementary material, table S1). Over the course of the study, we recorded 115 mortalities. Eighteen mortalities were from legal hunting, eight of which were censored from the analyses because they dispersed beyond the study area, and 10 pumas were killed by wolves (table 1). Information on annual wolf and elk abundances is found in electronic supplementary material, table S1.
Table 1.
Cause of mortality for pumas at age of death, as opposed to age at start of monitoring.
| hunting | other anthropogenica | undetermined | starvation | other natural | predation | |
|---|---|---|---|---|---|---|
| kitten | 4 | 10 | 8 | 6 | 12 | |
| subadult | 1 | 1 | 12 | 4 | 3 | 8 |
| adult | 17b | 5 | 6 | 10 | 3 | 6 |
aThree were translocated by the state wildlife agency and their fates are unknown.
bEight harvested outside the study area.
(b). Integrated population model
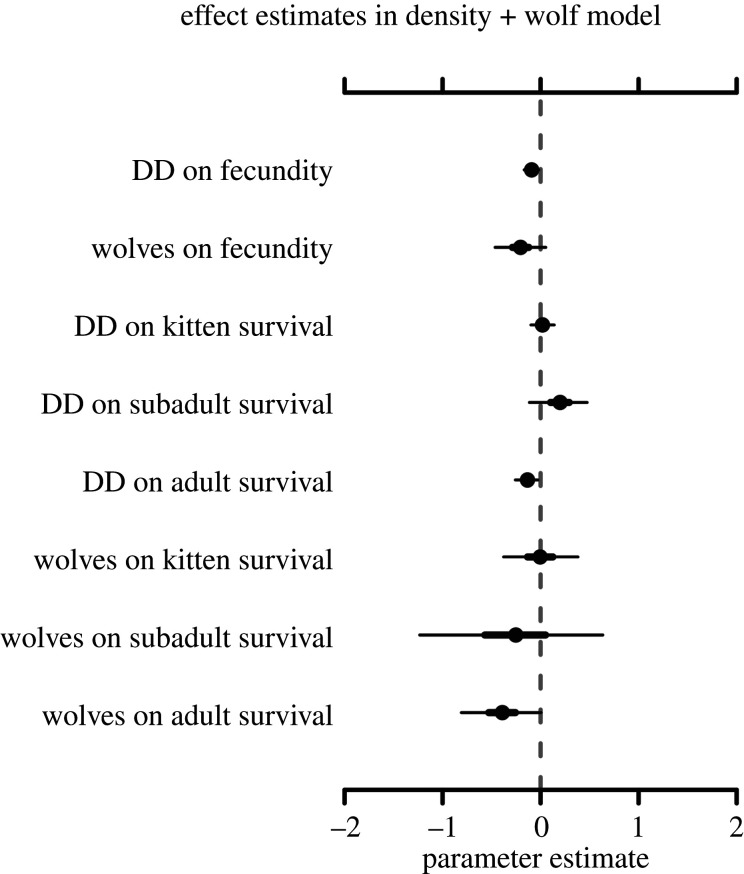
Our analyses demonstrated that observed changes in population abundance of pumas were best described by a model that included puma density and wolf abundance (density + wolf) as predictors of survival and fecundity (table 2). This model estimated (estimates reported as (mean, probability of direction (pD) [51]) that both puma density (αP = −0.09, pD = 0.99) and wolves (αW = −0.20, pD = 0.98) negatively affected puma fecundity, and that wolves also negatively influenced adult puma survival (βA,W = −0.36, pD = 0.99) and subadult survival (βS,W = −0.24, pD = 0.70); the effects on subadult survival, however, had high uncertainty. We also found that the impact of puma densities on adult puma survival was negative (βA,P = −0.14, pD = 0.99), consistent with a density-dependent effect on survival. All other covariate effects in these models were near zero and had a pD less than 0.95. All parameter estimates and credible intervals from this model are reported in electronic supplementary material, table S2. Support for this model garnered more than five times the empirical support of the second-ranked model that included the effects of puma density and off-range elk [52].
Table 2.
Ranked results of model selection. Density refers to annual puma density, wolf to annual wolf abundance, off-refuge elk to annual elk in the Jackson herd wintering off the National Elk Refuge, and elk to annual elk in the Jackson herd wintering on the National Elk Refuge.
| model | number of parameters | ΔWAIC | WAICw |
|---|---|---|---|
| density + wolf | 19 | 0.00 | 0.67 |
| density + off-refuge elk | 19 | 3.52 | 0.12 |
| density only | 15 | 3.99 | 0.09 |
| density + elk | 19 | 4.21 | 0.08 |
| null | 11 | 6.54 | 0.03 |
| local perceptions modela | 15 | 7.19 | 0.02 |
aThe ‘local perceptions' model included density dependent and off-refuge elk effects on puma fecundity, wolf effects on kitten survival, and both off-refuge elk and wolf effects on adult survival.
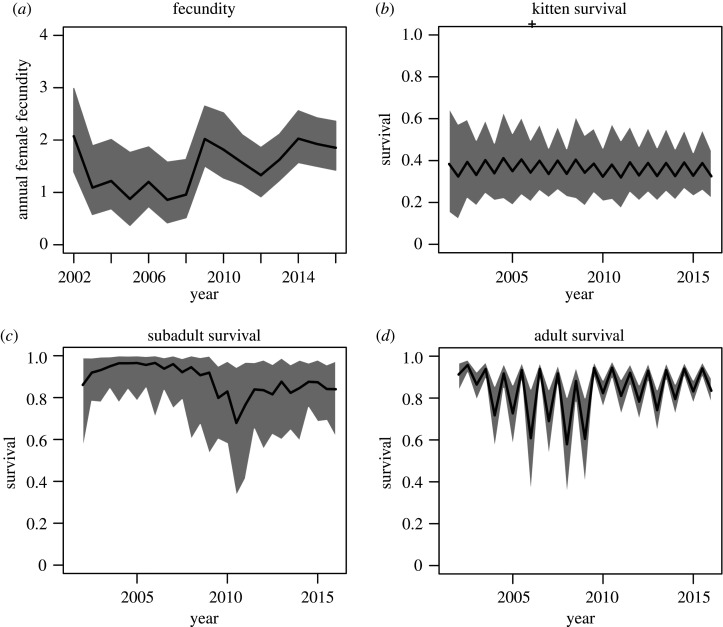
In our best model, we estimated the average annual recapture probability of a puma to be 0.90 (0.17 s.d.). All rates varied substantially between hunting and non-hunting seasons and in some cases by year as well (figure 2). The estimated annual fecundity (reported as posterior mean) was 1.53 (0.51 s.d.) kittens per female per year. Predicted fecundity had substantial temporal variation due to the strong effects of both density dependence and wolves (figure 3). Our six-month survival estimates for kittens were 0.36 (0.10 s.d.) in the non-hunting season and 0.28 (0.08 s.d.) in the hunting season (figure 2b). The subadult survival rates, including only other mortality, were 0.93 (0.10 s.d.) in the non-hunting season and 0.82 (0.17 s.d.) in the hunting season (figure 2c). The adult survival rates, including only other mortality, were 0.90 (0.03 s.d.) in the non-hunting season and 0.86 (0.03 s.d.) in the hunting season (figure 2d). Finally, we estimated the annual probability of mortality in subadults and adults due to hunting as 0.04 (0.02 s.d.).
Figure 2.
Estimated annual vital rates of pumas throughout the study, excepting hunting mortality. Black lines denote estimated mean, grey shaded area denotes the 95% credible interval.
Figure 3.
Posterior density estimates for each covariate in the density (DD) + wolf model. Dots denote median estimates, thick horizontal lines denote the 50% credible interval and thin black lines denote the 95% credible interval.
(c). Simulated future puma populations
Our projections under our best model predicted a threefold (CI 1.4–4.3) decrease in the local puma population over the range of observed wolf abundances (reported as median (95% CI) (figure 4). Removing legal hunting mortality increased puma abundance by approximately 30% (CI −21%–106%), which translated to roughly two adult pumas at low wolf abundance and one adult puma at high wolf abundance. The relative impact of removing puma hunting corresponded to a change approximately equivalent to removing 20 wolves from the system.
Figure 4.
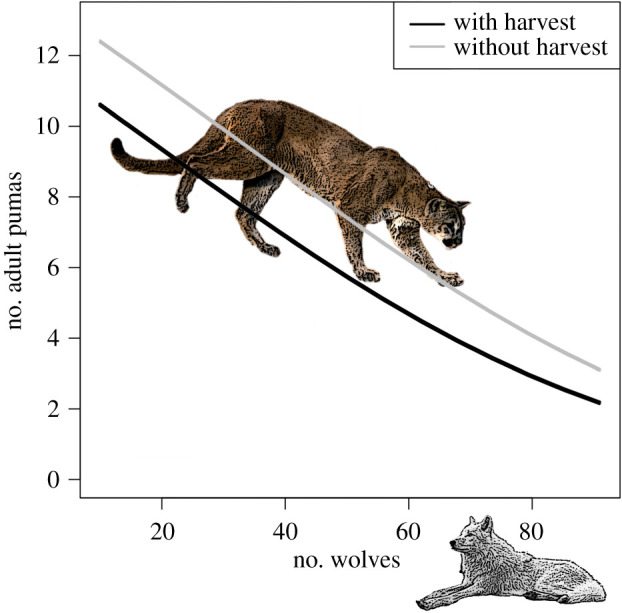
Effect of changing wolf abundance and the effect of hunting mortality on the average adult puma abundance during the hunting season for the 890 km2 area for which we estimated puma density.
4. Discussion
Our results suggested that puma abundance in the southern GYE is more strongly influenced by top-down forces (i.e. competition) exhibited by a reintroduced apex predator, than by top-down forces exhibited by human hunting or bottom-up forces (prey abundance) subsidized by humans promoting and providing primary production through agriculture and supplemental feeding programmes. Our earlier analytical approach, in which we determined local puma survival rates using multistate capture–mark–recapture models [21], supported previous research emphasizing that top-down forces are obscured by stronger bottom-up forces outside protected areas [4]. Here, our IPM combining vital rates and abundance data provided novel insights into this complex system and helped us further parse out the competitive effects of wolves and the bottom-up effects of elk on different puma age classes. Our modelling framework, in which we separate human hunting from the effects of apex predators, may also allow other researchers to more realistically assess top-down effects outside protected landscapes, where we see mixed human effects and increased bottom-up forces. In the era of the Anthropocene, mixed scenarios occurring along a spectrum of completely protected to completely developed landscapes are increasingly likely to occur [5].
Predominantly, our study system was not protected from wildlife exploitation. Grey wolves, however, were protected in all but 2 years of our study, during which there was limited harvest. Grey wolves are distinctive because they can exhibit strong top-down effects that initiate trophic cascades in natural systems [1,7,53]. In our study, increasing wolf numbers had strong negative effects on puma fecundity, subadult survival and adult survival. These effects were near parallel to effects previously assigned to changing elk densities off the NER rather than wolves in an earlier analysis we conducted with multistate capture–mark–recapture models and a subset of the puma data herein [21]. The effects of increasing wolves and decreasing off-refuge elk, however, are highly correlated and difficult to tease apart (R2 = 0.71; electronic supplementary material, figure S2). In fact, wildlife managers suspect changing elk distributions are at least in part explained by increasing wolves in the system, and that elk are seeking to reduce predation risk from wolves by selecting more open habitat on the NER than they did historically [32]. Further evidence for this complexity is found in interpreting the potential cause of puma starvation, which nearly equalled mortality attributed to predation (table 1). Puma starvation may have increased over the study due to the declining elk herd (i.e. bottom-up effects), decreased accessibility to elk, as mediated by exploitive and interference competition with wolves (top-down effects), or both [21].
Puma survival was also influenced by density dependence and decreasing puma abundance over the course of the study. Density-dependent effects negatively impacted fecundity as well as adult and subadult survival. Subadult survival appeared to increase with decreasing puma numbers, which is not surprising for a land-tenure species that exhibits territoriality [15]. Given the uncertainty in this estimate (electronic supplementary material, table S2), however, we are cautious about how we interpret this result. ‘Juvenile delinquent theory' predicts that when hunting removes adult males, the number of independent subadults in puma populations increases, creating unstable social dynamics [54,55], but at this time, it is unknown whether there is a threshold level of hunting that results in these dynamics.
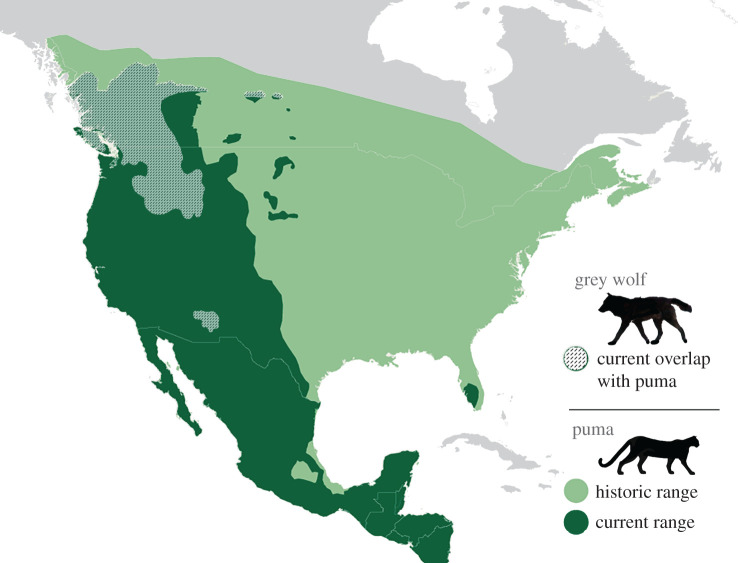
Our results supported previous research emphasizing the additive effects of hunting on puma mortality [12,13]. Uniquely, we were able to estimate that the average annual impact of human hunting on puma abundance in this system was approximately equivalent to the effects of 20 wolves (figure 4). We would emphasize that human hunting was low in our study system as compared to other areas of the USA, and that human hunting does not replace wolf effects. Wolves and human hunting directly and indirectly influence puma sex and age classes very differently. Nevertheless, these results have implications for how we might interpret the current versus historic distribution and abundance of pumas in what was wolf range. It may be that historically, wolves limited pumas heavily across North America, where the species were entirely sympatric except in southern Mexico and Central American countries. Historic wolf abundance may have also dictated puma scarcity in systems lacking sufficient refugia to mitigate the effects of competition (e.g. plains grasslands [56,57]). Further, current puma abundance in parts of western North America may be high not only due to the cessation of puma bounties in the mid-twentieth century, but also to competitive release due to the widespread extirpation of wolves in the USA (figure 5).
Figure 5.
A map of North America depicting the historic and current ranges of pumas in green, and the current range in which they overlap with grey wolves in dashed lines. Historically, wolves covered nearly the entirety of North America, excepting southernmost Mexico and Central America. (Online version in colour.)
Most importantly, our research emphasizes that when hunting is used as a management tool on subordinate predators in systems with other apex predators, population declines can happen quickly. This is an especially cautionary note for managers in regions where apex predators are recovering or being reintroduced [18]. This puma population dropped by 48% while wolves repopulated the study area and increased in abundance [21]. In another example, leopards decreased by 79% over 4 years as tiger numbers increased; researchers assumed that leopard numbers decreased due to competition reducing leopard foraging opportunities, as well as spatial displacement driving leopards into areas where conflict with people increased leopard mortality [27]. Thus, we recommend that in systems with recovering apex predators, managers evaluate subordinate predator hunting limits pre-emptively rather than post hoc as they did in our system, to compensate for the effects of dominant competitors on subordinate guild members. In Wyoming, wolf hunting has recently been legalized again, and as an unintended byproduct, this action will likely facilitate the maintenance of a higher density of pumas in the study system.
Supplementary Material
Acknowledgements
We thank our agency cooperators S. Cain (NPS), E. Cole (NER), T. Fuchs (WGFD) and K. Murphy (USFS). We thank B. Smith, S. Smith, and the numerous others for fieldwork and data collection. We thank T. Riecke, M. Hebblewhite, and several anonymous reviewers for critical feedback on earlier drafts of the manuscript.
Ethics
We followed puma capture and immobilization protocols suggested by the American Society of Mammalogists and approved by the Jackson Institutional Animal Care and Use Committee (Protocol 027-10EGDBS-060210) and National Park Service IACUC (Protocol IMR_GRTE_Elbroch_Cougar_2013-2015).
Data accessibility
All data not presented in the manuscript, as well as code for our analyses, are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.5qfttdz31 [58].
Authors' contributions
L.M.E. designed the study, coordinated the study, collected data, participated in data analysis and led the writing of the manuscript; J.F. designed and conducted the analyses, and drafted the manuscript; H.Q. designed the study, coordinated the study and collected data; D.C. designed the study, coordinated the study and collected data; D.T. provided project support and collected data. H.W. participated in data analysis and drafted the manuscript. All authors gave final approval for publication and agree to be held accountable for the work performed therein.
Competing interests
We declare we have no competing interests
Funding
This work was supported by the Summerlee Foundation, Charles Engelhard Foundation, Eugene V. and Clare E. Thaw Charitable Trust, Laura Moore Cunningham Foundation, Richard King Mellon Foundation, National Geographic Society (grant no. C236–13), Connemara Fund, the Community Foundation of Jackson Hole, the Lee and Juliet Folger Fund, L. Westbrook, the Scully Family, R. and L. Haberfeld, Hogan LLC, L. and R. Holder, S. and L. Robertson, R. and L. Heskett, F. and B. Burgess, J. Morgan, T. Thomas, A. Smith, Ecotour Adventures, and D. Bainbridge.
References
- 1.Hebblewhite M, White CA, Nietvelt CG, McKenzie JA, Hurd TE, Fryxell JM, Bayley SE, Paquet PC. 2005. Human activity mediates a trophic cascade caused by wolves. Ecology 86, 2135–2144. ( 10.1890/04-1269) [DOI] [Google Scholar]
- 2.Berger KM, Gese EM. 2007. Does interference competition with wolves limit the distribution and abundance of coyotes? J. Anim. Ecol. 76, 1075–1085. ( 10.1111/j.1365-2656.2007.01287.x) [DOI] [PubMed] [Google Scholar]
- 3.Ellis EC. 2011. Anthropogenic transformation of the terrestrial biosphere. Proc. R. Soc. A 369, 1010–1035. ( 10.1098/rsta.2010.0331) [DOI] [PubMed] [Google Scholar]
- 4.Muhly TB, Hebblewhite M, Paton D, Pitt JA, Boyce MS, Musiani M. 2013. Humans strengthen bottom-up effects and weaken trophic cascades in a terrestrial food web. PLoS ONE 8, e64311 ( 10.1371/journal.pone.0064311) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuijper DPJ, Sahlén E, Elmhagen B, Chamaillé-Jammes S, Sand H, Lone K, Cromsigt JPGM. 2016. Paws without claws? Ecological effects of large carnivores in anthropogenic landscapes. Proc. R. Soc. B 283, 20161625 ( 10.1098/rspb.2016.1625) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brodie J, et al. 2013. Relative influence of human harvest, carnivores, and weather on adult female elk survival across western North America. J. Appl. Ecol. 50, 295–305. ( 10.1111/1365-2664.12044) [DOI] [Google Scholar]
- 7.Ripple WJ, et al. 2014. Status and ecological effects of the world's largest carnivores. Science 343, 151–163. ( 10.1126/science.1241484) [DOI] [PubMed] [Google Scholar]
- 8.Dorresteijn I, Schultner J, Nimmo DG, Fischer J, Hanspach J, Kuemmerle T, Kehoe L, Ritchie EG. 2015. Incorporating anthropogenic effects into trophic ecology: predator–prey interactions in a human-dominated landscape. Proc. R. Soc. B 282, 20151602 ( 10.1098/rspb.2015.1602) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Melis C, et al. 2009. Predation has a greater impact in less productive environments, variation in roe deer, Capreolus capreolus, population density across Europe. Global Ecol. Biogeogr. 18, 724–734. ( 10.1111/j.1466-8238.2009.00480.x) [DOI] [Google Scholar]
- 10.Levi T, Wilmers CC. 2012. Wolves–coyotes–foxes: a cascade among carnivores. Ecology 93, 921–929. ( 10.1890/11-0165.1) [DOI] [PubMed] [Google Scholar]
- 11.Quigley H, Hornocker M. 2009. Cougar population dynamics. In Cougar: ecology and conservation (eds Hornocker M., Negri S.), pp. 59–75. Chicago, IL: University of Chicago Press. [Google Scholar]
- 12.Cooley HS, Wielgus RS, Koehler GM, Robinson HS, Maletzke BT. 2009. Does hunting regulate cougar populations? A test of the compensatory mortality hypothesis. Ecology 90, 2913–2921. ( 10.1890/08-1805.1) [DOI] [PubMed] [Google Scholar]
- 13.Wolfe ML, Koons DN, Stoner DC, Terletsky P, Gese EM, Choate DM, Aubry LM. 2015. Is anthropogenic cougar mortality compensated by changes in natural mortality in Utah? Insight from long-term studies. Biol. Conserv. 182, 187–196. ( 10.1016/j.biocon.2014.12.008) [DOI] [Google Scholar]
- 14.Vickers TW, et al. 2015. Survival and mortality of pumas (Puma concolor) in a fragmented, urbanizing landscape. PLoS ONE 10, e0131490 ( 10.1371/journal.pone.0131490) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hornocker MG. 1969. Winter territoriality in mountain lions. J. Wildl. Manage. 33, 457–464. ( 10.2307/3799367) [DOI] [Google Scholar]
- 16.Soria-Díaz L, Fowler MS, Monroy-Vilchis O. 2017. Top-down and bottom-up control on cougar and its prey in a central Mexican natural reserve. Eur. J. Wildl. Res. 63, 73 ( 10.1007/s10344-017-1129-y) [DOI] [Google Scholar]
- 17.Logan K. 2019. Puma population limitation and regulation: what matters in puma management? J. Wildl. Manage. 83, 1652–1666. ( 10.1002/jwmg.21753) [DOI] [Google Scholar]
- 18.Elbroch LM, Kusler AL. 2017. Are pumas subordinate carnivores, and does it matter? PeerJ 6, e4293 ( 10.7717/peerj.4293) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kortello AD, Hurd TE, Murray DL. 2007. Interactions between cougars (Puma concolor) and gray wolves (Canis lupus) in Banff National Park, Alberta. Ecoscience 14, 214–222. ( 10.2980/1195-6860(2007)14[214:IBCPCA]2.0.CO;2) [DOI] [Google Scholar]
- 20.Ruth TK, Murphy K. 2010. In competition with other carnivores for prey. In Cougar: ecology and conservation (eds Hornocker M, Negri S), pp. 138–162. Chicago, IL: University of Chicago Press. [Google Scholar]
- 21.Elbroch LM, Marescot L, Quigley Q, Craighead D, Wittmer HU. 2018. Multiple anthropogenic interventions drive puma survival following wolf recovery in the Greater Yellowstone Ecosystem. Ecol. Evol. 8, 7236–7245. ( 10.1002/ece3.4264) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cole EK, Foley AM, Warren JM, Smith BL, Dewey SR, Brimeyer DG, Fairbanks WS, Sawyer H, Cross PC. 2015. Changing migratory patterns in the Jackson elk herd. J. Wildl. Manage. 79, 877–886. ( 10.1002/jwmg.917) [DOI] [Google Scholar]
- 23.Arnold TW, Clark RG, Koons DN, Schaub M. 2018. Integrated population models facilitate ecological understanding and improved management decisions. J. Wildl. Manage. 82, 266–274. ( 10.1002/jwmg.21404) [DOI] [Google Scholar]
- 24.Horne JS, Ausband DE, Hurley MA, Struthers J, Berg JE, Groth K. 2019. Integrated population model to improve knowledge and management of Idaho wolves. J. Wildl. Manage. 83, 32–42. ( 10.1002/jwmg.21554) [DOI] [Google Scholar]
- 25.Newsome TM, et al. 2017. Top predators constrain mesopredator distributions. Nat. Communications 8, 15469 ( 10.1038/ncomms15469) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Swanson A, Caro T, Davies-Mostert H, Mills MG, Macdonald DW, Borner M, Masenga E, Packer C. 2014. Cheetahs and wild dogs show contrasting patterns of suppression by lions. J. Anim. Ecol. 83, 1418–1427. ( 10.1111/1365-2656.12231) [DOI] [PubMed] [Google Scholar]
- 27.Harihar A, Pandav B, Goyal SP. 2011. Responses of leopard Panthera pardus to the recovery of a tiger Panthera tigris population. J. Appl. Ecol. 48, 806–814. ( 10.1111/j.1365-2664.2011.01981.x) [DOI] [Google Scholar]
- 28.Elbroch LM, Peziol M, O'Malley C, Quigley H. 2017. Vertebrate diversity benefiting from carrion subsidies provided by subordinate carnivores. Biol. Conserv. 215, 123–131. ( 10.1016/j.biocon.2017.08.026) [DOI] [Google Scholar]
- 29.Wolf C, Ripple WJ. 2018. Rewilding the world's large carnivores. R. Soc. Open Sci. 5, 172235 ( 10.1098/rsos.172235) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barry JM, Elbroch LM, Aiello-Lammens ME, Sarno RJ, Seelye L, Kusler A, Quigley HB, Grigione MM. 2019. Pumas as ecosystem engineers: ungulate carcasses support beetle assemblages in the Greater Yellowstone Ecosystem. Oecologia 189, 577–586. ( 10.1007/s00442-018-4315-z) [DOI] [PubMed] [Google Scholar]
- 31.Smith DW, Ferguson G. 2012. Decade of the wolf: returning the wild to Yellowstone. New York, NY: Lyons Press. [Google Scholar]
- 32.Wyoming Game and Fish Department (WGFD). 2016. Big game job completion report. See https://wgfd.wyo.gov/WGFD/media/content/PDF/Hunting/JCRS/JCR_BGJACKSON_ELK_2015.pdf.
- 33.Elbroch LM, Lendrum PE, Newby J, Quigley H, Craighead D. 2013. Seasonal foraging ecology of non-migratory cougars in a system with migrating prey. PLoS ONE 8, e83375 ( 10.1371/journal.pone.0083375) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Elbroch LM, Vickers TW, Quigley H. 2020. Plague, pumas, and potential zoonotic exposure in the Greater Yellowstone Ecosystem. Environ. Conserv. 47, 75–78. ( 10.1017/S0376892920000065) [DOI] [Google Scholar]
- 35.Rinehart K, Elbroch LM, Wittmer HU. 2014. Common biases in density estimation based on home range overlap with reference to pumas in Patagonia. Wildl. Biol. 20, 19–26. ( 10.2981/wlb.12100) [DOI] [Google Scholar]
- 36.Worton BJ. 1989. Kernel methods for estimating the utilization distribution in home-range studies. Ecology 70, 164–168. ( 10.2307/1938423) [DOI] [Google Scholar]
- 37.Beyer HL. 2012. Geospatial modeling environment. See http://www.spatialecology.com/gme/.
- 38.Elbroch LM, Lendrum PE, Alexander P, Quigley H. 2015. Cougar den site selection in the Southern Yellowstone Ecosystem. Mamm. Res. 60, 89–96. ( 10.1007/s13364-015-0212-6) [DOI] [Google Scholar]
- 39.Logan KA, Sweanor LL. 2001. Desert cougar: evolutionary ecology and conservation of an enduring carnivore. Washington, DC: Island Press. [Google Scholar]
- 40.Ruth TK, Haroldson MA, Murphy KM, Buotte PC, Hornocker MG, Quigley HB. 2011. Cougar survival and source–sink structure on Greater Yellowstone's Northern Range. J. Wildl. Manage. 75, 1381–1398. ( 10.1002/jwmg.190) [DOI] [Google Scholar]
- 41.Wyoming Game and Fish Department. 2006. Mountain lion management plan. Cheyenne, WY: Wyoming Game and Fish Department. [Google Scholar]
- 42.Stoner DC, Wolfe ML, Rieth WR, Bunnell KD, Durham SL, Stoner LL. 2013. De facto refugia, ecological traps and the bio-geography of anthropogenic cougar mortality in Utah. Divers. Distrib. 19, 1114–1124. ( 10.1111/ddi.12035) [DOI] [Google Scholar]
- 43.O'Malley C, Elbroch LM, Kusler A, Peziol M, Quigley H. 2018. Aligning mountain lion hunting seasons to mitigate orphaning dependent kittens. Wildl. Soc. B. 42, 438–443. ( 10.1002/wsb.902) [DOI] [Google Scholar]
- 44.Ferguson JM, Ponciano JM. 2014. Predicting the process of extinction in experimental microcosms and accounting for interspecific interactions in single-species time series. Ecol. Lett. 17, 251–259. ( 10.1111/ele.12227) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Caswell H. 2001. Matrix population models: construction, analysis, and interpretation. Sunderland, MA: Sinauer Associates. [Google Scholar]
- 46.Abadi F, Gimenez O, Arlettaz R, Schaub M. 2010. An assessment of integrated population models: bias, accuracy, and violation of the assumption of independence. Ecology 91, 7–14. ( 10.1890/08-2235.1) [DOI] [PubMed] [Google Scholar]
- 47.Riecke TV, Williams PJ, Behnke TL, Gibson D, Leach AG, Sedinger BS, Street PA, Sedinger JS. 2019. Integrated population models: model assumptions and inference. Methods Ecol. Evol. 10, 1072–1082. ( 10.1111/2041-210X.13195) [DOI] [Google Scholar]
- 48.Elbroch LM, Lendrum PE, Robinson H, Quigley H. 2016. Individual- and population-level prey selection by a solitary predator, as determined with two estimates of prey availability. Can. J. Zool. 94, 275–282. ( 10.1139/cjz-2015-0092) [DOI] [Google Scholar]
- 49.Plummer M. 2003. JAGS: a program for analysis of Bayesian graphical models using Gibbs sampling. In Proc. of the 3rd Int. Workshop on Distributed Statistical Computing (DSC 2003), 20–22 March, Vienna, Austria (eds Hornik K, Leisch F, Zeileis A). See https://www.r-project.org/conferences/DSC-2003/Proceedings/Plummer.pdf. [Google Scholar]
- 50.Watanabe S. 2010. Asymptotic equivalence of Bayes cross validation and widely applicable information criterion in singular learning theory. J. Mach. Learn. Res. 11, 3571–3594. [Google Scholar]
- 51.Makowski D, Ben-Shachar MS, Chen SHA, Lüdecke D. 2019. Indices of effect existence and significance in the Bayesian framework. Front. Psychol. 10, 2767 ( 10.3389/fpsyg.2019.02767) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Burnham KP, Anderson DR, Huyvaert KP. 2011. AIC model selection and multimodel inference in behavioural ecology: some background, observations, and comparisons. Behav. Ecol. Soc. 65, 23–35. ( 10.1007/s00265-010-1029-6) [DOI] [Google Scholar]
- 53.Flagel DG, Belovsky GE, Beyer DE Jr. 2016. Natural and experimental tests of trophic cascades: gray wolves and white-tailed deer in a Great Lakes forest. Oecologia 180, 1183–1194. ( 10.1007/s00442-015-3515-z) [DOI] [PubMed] [Google Scholar]
- 54.Robinson HS, Wielgus RB, Cooley HS, Cooley SW. 2008. Sink populations in carnivore management: cougar demography and immigration in a hunted population. Ecol. Appl. 18, 1028–1037. ( 10.1890/07-0352.1) [DOI] [PubMed] [Google Scholar]
- 55.Peebles KA, Wielgus RB, Maletzke BT, Swanson ME. 2013. Effects of remedial sport hunting on cougar complaints and livestock depredations. PLoS ONE 8, e79713 ( 10.1371/journal.pone.0079713) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Riley SJ, Nesslage GM, Maurer BA. 2004. Dynamics of early wolf and cougar eradication efforts in Montana: implications for conservation. Biol. Conserv. 119, 575–579. ( 10.1016/j.biocon.2004.01.019) [DOI] [Google Scholar]
- 57.Laundré J. 2012. Phantoms of the prairie: the return of cougars to the midwest. Madison, WI: University of Wisconsin Press. [Google Scholar]
- 58.Elbroch LM, Ferguson JM, Quigley H, Craighead D, Thompson DJ, Wittmer HU.2020. Data from: Reintroduced wolves and hunting limit the abundance of a subordinate apex predator in a multi-use landscape. Dryad Digital Repository. ( ) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Elbroch LM, Ferguson JM, Quigley H, Craighead D, Thompson DJ, Wittmer HU.2020. Data from: Reintroduced wolves and hunting limit the abundance of a subordinate apex predator in a multi-use landscape. Dryad Digital Repository. ( ) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
All data not presented in the manuscript, as well as code for our analyses, are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.5qfttdz31 [58].