Abstract
Major evolutionary transitions can be triggered by behavioural novelty, and are often associated with ‘adaptive suites’, which involve shifts in multiple co-adapted traits subject to complex interactions. Heliconius butterflies represent one such example, actively feeding on pollen, a behaviour unique among butterflies. Pollen feeding permits a prolonged reproductive lifespan, and co-occurs with a constellation of behavioural, neuroanatomical, life history, morphological and physiological traits that are absent in closely related, non-pollen-feeding genera. As a highly tractable system, supported by considerable ecological and genomic data, Heliconius are an excellent model for investigating how behavioural innovation can trigger a cascade of adaptive shifts in multiple diverse, but interrelated, traits. Here, we synthesize current knowledge of pollen feeding in Heliconius, and explore potential interactions between associated, putatively adaptive, traits. Currently, no physiological, morphological or molecular innovation has been explicitly linked to the origin of pollen feeding, and several hypothesized links between different aspects of Heliconius biology remain poorly tested. However, resolving these uncertainties will contribute to our understanding of how behavioural innovations evolve and subsequently alter the evolutionary trajectories of diverse traits impacting resource acquisition, life history, senescence and cognition.
Keywords: behaviour, diet, brain size, life history, senescence, novelty
1. Introduction: behaviour and the evolution of adaptive suites
Major evolutionary transitions can be triggered by behavioural novelty [1] and are often associated with ‘adaptive suites’ that incorporate multiple co-adapted traits [2]. These transitions involve complex interactions, including mutual dependency between traits [2]. In these circumstances, disentangling the causal relationships between behavioural and morphological traits involved in major transitions is challenging.
The butterfly genus Heliconius (Nymphalidae: Heliconiinae) presents an excellent system for investigating how adaptive suites evolve. Uniquely among butterflies, Heliconius supplement their nectar diet by actively collecting and feeding on pollen [3]. Heliconius gather pollen by probing flowers and collecting it as a lumped mass on the proboscis (figure 1a). The pollen load is mixed with saliva and externally digested to release amino acids that are drawn up the proboscis [3]. Pollen is collected primarily from a limited number of plant species [8], and Heliconius from at least the melpomene clade (figure 1g) show a particular preference for certain pollen-rich cucurbitaceous vines [8–10], with which they are hypothesized to have coevolved [11,12].
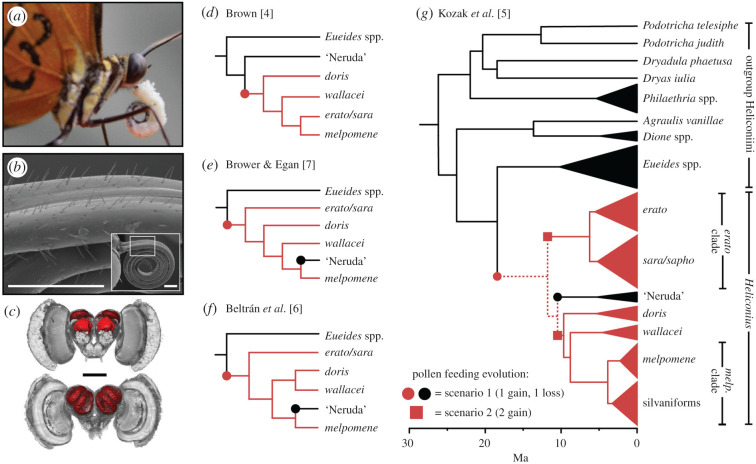
Figure 1.
Evolution of pollen feeding. (a) A captive H. hecale with pollen load (white) affixed to its proboscis. (b) Elongated sensory bristles on the proboscis of H. melpomene. (c) A three-dimensional reconstruction of a H. hecale brain showing the mushroom bodies (red) and rest of the brain (grey) from the posterior (top) and anterior (bottom) view. Scale bars, 500 µm in (b) and (c). (d–g) How phylogenetic hypotheses for Heliconiini have changed through time, and how that affects predicted gains (red circle/square) and loss (black circle) of pollen feeding. Pollen feeding lineages are shown in red, dotted lines indicate branches with higher uncertainty. Note the changing position of the Neruda clade, a group of four non-pollen feeding species. (d) Adapted from [4] and based on morphological data. (e–g) Adapted from [5–7] and based on molecular data. (Online version in colour.)
This dietary innovation provides adults with a consistent supply of amino acids, permitting a prolonged reproductive lifespan [13], and co-occurs with behavioural, neuroanatomical, life history, physiological and morphological changes that are absent in closely related, non-pollen-feeding Heliconiini [14] (figure 2). Among these putative adaptations, apparent specializations in foraging behaviour have received particular attention. Heliconius establish ‘traplines’, foraging routes along which specific plants are regularly visited, suggesting a sophisticated capacity for spatial navigation, probably using learnt visual landmarks [11,15,16].
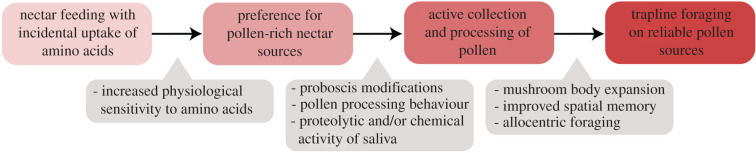
Figure 2.
Hypothesized transition for a butterfly from nectarivory to trapline pollen feeding, showing plausible intermediary stages and possible key adaptations. (Online version in colour.)
Pollen feeding evolved relatively recently, with Heliconius and its sister genus Eueides diverging approximately 18 Ma [5]. The close phylogenetic relatedness and ecological similarity with other Heliconiini therefore provides a potentially powerful framework for conducting comparative analyses. However, the suite of traits associated with pollen feeding is widely assumed to have evolved only once [6] (figure 1d–g), presenting difficulties in separating evolutionary cause and effect. As such, many hypothesized links between different aspects of Heliconius biology are poorly tested. Moreover, no physiological, morphological or molecular traits have been specifically linked to the origin of pollen feeding in this genus. Nevertheless, as a major system for studying the genomic bases of adaptation [14], considerable genomic resources have been developed for Heliconius [17]. Together with recently developed genetic techniques and comparative methods [18–20], this provides a clear route towards understanding the evolution of this behavioural innovation.
This review synthesizes current knowledge of pollen feeding in Heliconius, highlighting gaps in our understanding of several putatively linked traits, and their interactions, which are probably key to the origin of pollen feeding. We identify key areas of investigation that can contribute to central questions in evolutionary biology including: (i) how dietary shifts can alter energic constraints, re-shaping life history and reproductive trade-offs; (ii) how evolutionary innovations co-opt ancestral molecular and morphological traits; and (iii) how brains accommodate cognitive enhancements.
2. Pollen-feeding in Heliconius butterflies: an energetic payload
Populations are often under energetic constraint, resulting in investment trade-offs between competing tissues, traits, or strategies [21]. Shifts in diet quality have dramatic effects on evolutionary trajectories by increasing individual energy budgets. For example, dietary innovations have been linked to larger body sizes in carnivores [22], brain expansion in primates [23] and increased fecundity in butterflies [24]. However, comparative life-history studies often compare phylogenetically and ecologically diverse species, leading to difficulties in determining the precise role of dietary shifts in macro-evolutionary dynamics (see [25]).
Like most holometabolous insects, Lepidoptera experience a profound shift in nutrient intake between the larval and adult stages. Lepidopteran larvae are generally herbivorous, whereas adults of most species feed predominantly on nectar, which is carbohydrate-rich, but protein-poor [26,27]. Consequently, reproductive output is largely constrained by protein acquired during the larval stage [24,26]. While some Lepidoptera also exploit alternative nutrient sources including mud, fruit, dung and carrion [27], pollen feeding in Heliconius is perhaps the clearest example of a change in adult diet being linked to major shifts in life history. Pollen feeding provides Heliconius with a consistent source of essential amino acids during the adult stage [3]. Compared to other Heliconiini, Heliconius exhibit a pronounced delay in female reproductive senescence [13]. Heliconius females generally collect more pollen than males [9,28], incorporating pollen-derived amino acids into eggs [29], and suffer a marked decline in fecundity when deprived of pollen [13]. This reflects a potential physiological convergence with honeybees, where colonies cease brood rearing when denied access to pollen [30].
Heliconius butterflies have dramatically extended lifespans, living for up to six months in the wild, without diapause [3,15], compared to a maximum of four to six weeks in Dryas iulia, a non-pollen-feeding Heliconiini [13]. However, the causal relationship between pollen feeding and longevity is not well quantified. One study reports that pollen-deprived Heliconius charithonia are shorter-lived [13], but this difference was not tested statistically. In adult honeybees, pollen feeding is associated with increased longevity [31], providing reason to expect a similar effect in Heliconius.
The energetic payload provided by pollen feeding has clearly had a major impact on reproductive output in Heliconius. This provides several opportunities for investigating the effects of foraging innovations on life histories, for example: (i) how do novel diets alter energetic trade-offs between life stages? (ii) what physiological mechanisms control the shifting balance of these trade-offs and the use of new resources? and (iii) what physiological mechanisms underpin increased longevity?
3. Origins of a novel trait
The processes and underlying conditions that give rise to evolutionary novelties are incompletely understood [32]. Although evolutionary novelties can arise from the emergence of new ecological opportunities [33], this is not always the case [34]. Importantly, evolutionary trajectories appear constrained by pre-existing variation [35,36], suggesting contingency plays a substantial role in the emergence of novel traits. For example, complex behaviours can be achieved through the integration of simpler, pre-existing behavioural modules [37]. However, the relative importance of behaviour, morphology and physiology as the ultimate drivers of novelty is debated [32]. Understanding fitness benefits during intermediate stages, and the timing of trait acquisition, is therefore key to disentangling the origins of an innovation.
(a). Reconstructing evolutionary shifts in pollen feeding
Except for the four species of the ‘Neruda’ clade (figure 1e–g), all Heliconius species feed on pollen and appear to possess the complete suite of associated traits, presenting a challenge to reconstructing the origin of pollen feeding. As the only non-pollen-feeding Heliconius, the four Neruda species may offer the possibility of decoupling pollen feeding and its associated adaptations, helping to resolve the timing of these shifts, and the relationships between traits. Regrettably, such analyses are currently limited by the scarcity of data on Neruda biology and lingering uncertainty regarding their phylogenetic position. Long considered a separate genus [4] (figure 1d), recent molecular phylogenies have positioned Neruda within Heliconius [5–7] (figure 1e–g). Hence, whether pollen feeding evolved once in Heliconius and was secondarily lost in the Neruda, or evolved multiple times within Heliconius, with Neruda retaining the ancestral state, is unclear, with these two scenarios being equally parsimonious (figure 1g) [5]. In addition, given evidence of widespread introgression throughout the evolution of the genus [17], which could mislead the species tree, it also remains possible that the Neruda are, after all, a sister clade to Heliconius, as suggested by morphological data (figure 1d). Nevertheless, the absence of pollen feeding in other Heliconiini suggests that a single gain within Heliconius is likely, with or without a loss in the Neruda. However, discordance between any single Heliconiini species tree and underlying gene trees may present persistent difficulties in resolving this question [38].
More broadly, the scarcity of pollen feeding across the approximately 180 000 described species of Lepidoptera marks Heliconius as particularly peculiar. Although wild-caught butterflies from several genera have been reported as having pollen affixed to their proboscis [39], active pollen feeding is unknown in other Lepidoptera, with only a few exceptions, all of which are separated from Heliconius by large phylogenetic distances. Two families of basal moths, Heterobathmiidae and Micropterigidae, feed on pollen as adults [40]. These groups, however, lack a proboscis and use plesiomorphic mandibles to collect and crush pollen [40]. Two species of Gelechiidae moths are the only other proboscis-bearing Lepidoptera reported to feed on pollen, purportedly by dissolving the pollen wall with an unidentified salivary agent [41]. However, the ecology and life history of these species, and the prevalence of pollen feeding across Gelechiidae, are poorly understood, making it difficult to assess the feasibility of comparative analyses within this group.
(b). Fitness during evolutionary transitions: effects of adult amino acid intake
The presence of amino acids in nectar [42], often derived from contaminating pollen grains [43], suggests that pollen feeding may have originated with the incidental intake of amino acids while nectar feeding. This may have selected for an increased sensitivity to amino acids, and a preference for pollen-rich plants (figure 2). The importance of nectar-derived amino acids to adult butterflies is supported by comparisons showing that plants pollinated by butterfly species tend to have higher amino acid concentrations in their nectar [42]. Additionally, several butterfly species show a preference for nectars containing amino acids [44,45]. However, the benefits derived from amino acids in the adult diet vary widely between Lepidopteran species. In several species, no association has been found between fecundity and adult amino acid intake [46–48]. Yet, for other species, it has been linked to increases in egg quantity [49] and size [50]. Importantly, the transfer of nectar-derived amino acids to eggs has been directly demonstrated in some species [51,52]. The effects on male fecundity have received less attention, however, male Coenonympha pamphilus produced larvae with larger hatching masses when provided with amino acids, probably owing to enhanced spermatophore quality [53]. Adult amino acid intake also increases longevity in some species, but has no effect in others [46,54]. Interestingly, lifespans comparable to Heliconius have been recorded in some fruit-feeding butterflies [55], raising the possibility that fruit-derived amino acids may similarly facilitate an extended lifespan.
While the effects appear to be highly species-dependent, evidence that adult amino acid intake can lead to improvements in longevity or fecundity supports the hypothesis that pollen feeding in Heliconius probably originated from passive uptake of pollen-derived amino acids during nectar feeding (figure 2). If so, it remains puzzling that pollen feeding is so rare. Central to answering why, is identifying the adaptations necessary for the transition to pollen feeding.
(c). Using old traits for new purposes
Comparative studies across Heliconiini suggest that pollen collection does not involve any novel morphological structures [56]. However, Heliconius do have elongated proboscises compared to non-pollen-feeding Heliconiini, with longer and more numerous bristles at the proximal- and mid-regions (figure 1b), which may assist in affixing pollen grains [56]. In addition, the intrinsic muscles involved in coiling movements are more numerous and extend further into the proboscis [57]. Pollen collection also involves the same sequence of probing movements as nectarivorous butterflies [27,58], but Heliconius probe with higher frequency and handle individual flowers for longer, with handling time increasing in the presence of pollen [27,58]. Additionally, pollen processing may be derived from proboscis grooming behaviours, which similarly involve the release of saliva and the repetitive coiling of the proboscis [59].
Saliva appears to play a key role in pollen feeding by helping to bind pollen to the proboscis and facilitating external digestion. Indeed, the salivary glands of Heliconius are larger than in other nymphalids [60], and Heliconius release greater quantities of saliva during feeding [58]. Although the saliva of Heliconius contains proteases [61,62], it is unknown how it differs from that of other Heliconiini. However, proteolytic activity of the saliva does increase when the proboscis is stimulated with pollen, and is generally higher in females [61]. Two of the proteases identified in Heliconius melpomene saliva also show close homology with the serine protease cocoonase [62,63], which is secreted from the proboscis of silkworms to weaken the cocoon during eclosion [64]. Like all butterflies, Heliconius lack cocoons, and it has been suggested that cocoonase homologues may have been co-opted for use in digesting pollen proteins [62,63], potentially weakening the pollen wall. Cocoonase underwent several duplications along the lineage leading to Heliconius and their sister genus, Eueides, with an additional duplication specific to Heliconius and a further duplication in H. melpomene [63]. However, a functional role for cocoonase in pollen feeding has not yet been directly demonstrated. Importantly, it is unclear how proteolysis would break down sporopollenin, the primary component of pollen exines, as it is not composed of proteins [65]. Pollen grains are, in fact, visibly damaged after processing by Heliconius [66]. However, it is unknown if this is achieved solely by mechanical digestion, or through biochemicals capable of breaking down sporopollenin, as is claimed for Gelechiidae moths [41]. The role of specific salivary proteins in pollen digestion therefore requires further comparative studies that include a broader representation of the non-pollen-feeding Heliconiini.
Pollen feeding illustrates how evolutionary innovation can occur through the co-option and modification of pre-existing anatomical, behavioural and physiological traits for new purposes. Although it remains unclear why pollen feeding arose in Heliconius but not other butterflies, this question can potentially be answered by combining functional genetics, physiology and anatomy.
4. Increases in behavioural sophistication and neural elaboration
Dietary innovations not only involve adaptations in the processing and use of a resource but often co-occur with changes in foraging behaviour as determined by the quality, and spatial and temporal distribution of food sources [67]. These parameters impose demands on perception, learning and memory, and can favour investment in associated brain structures [68]. In Heliconius, the interactions between butterflies and their pollen sources may have led to notable refinements in both the brain and behaviour [11,16,69,70].
(a). Exploitation of a novel resource: plant–animal interactions and foraging strategies
Many species of Heliconius collect pollen predominantly from cucurbitaceous vines, particularly the relatively rare, but pollen rich Psiguria and Gurania, which show evidence of coevolution with Heliconius [8–12]. Heliconius are the primary pollinators of Psiguria, visiting more plants, and depositing more pollen, over greater distances, than even hummingbirds [71]. Indeed, several species of Gurania and Psiguria appear to have evolved lower nectar production and smaller flowers to promote visitation from Heliconius over hummingbirds, and older plants may even switch from producing male to female flowers once Heliconius visitation is established [72].
Psiguria flowers contain large amounts of pollen, and inflorescences generally produce a new flower every 1–3 days [11]. Individual plants can flower continuously for up to a year [11], in contrast to the seasonal pollen production common for neotropical angiosperms [73]. A single Psiguria plant is therefore potentially a reliable pollen resource for the entire lifespan of an individual. Heliconius use this dependable, but scarce, resource by establishing ‘traplines’, foraging routes along which specific plants are visited with a high degree of spatial and temporal regularity [11,15,16]. This suggests Heliconius possess a capacity for navigation using learnt visual landmarks, similar to behaviours observed in certain bees [74,75]. Heliconius traplines centre on a limited home range of 100 m2 to 1 km2, within which individuals return to the same roosting locations at night, located using visual cues [16,71,76]. Although other butterfly species, including the heliconiine Agraulis vanillae, are reported to temporarily establish home ranges [77], Heliconius seem peculiar in maintaining long-term, stable home ranges with high roost-site fidelity [16,71]. Site fidelity is presumably a pre-requisite for trap lining, which, together with the central role of pollen resources in Heliconius trap lines, suggests that these behaviours are linked to the acquisition of pollen feeding. However, the lack of data on whether non-pollen feeding Heliconiini use spatial information during foraging, means this hypothesis is yet to be formally tested.
Despite the role of Psiguria and Gurania in Heliconius foraging behaviour, there is considerable variation in the exploitation of pollen sources between species. Although these differences are partly explained by habitat preference, there appears to be a division between the two main Heliconius clades [8]. Species of the melpomene clade tend to forage more intensively on Psiguria, while erato clade species predominantly exploit non-cucurbitaceous plants, such as Lantana [8–10] (figure 1g). Notably, Heliconius erato do trapline on specific patches of Lantana [16]. Hence, the role of cucurbitaceous pollen sources in the transition to pollen feeding is unclear. One possibility is that pollen feeding arose in the context of coevolution with certain cucurbitaceous vines, with members of the erato clade subsequently pushed towards other pollen resources owing to competitive exclusion. Alternatively, pollen feeding may have originated as a more opportunistic, generalist strategy, retained in the erato clade, with specialization on Cucurbitaceae secondarily emerging in the melpomene group.
(b). Neural basis of a cognitive adaptation
Behavioural innovations are generally associated with changes in the structure and function of the brain [78]. For example, foraging innovations are linked to brain expansion in several vertebrate groups [23,79]. Trapline foraging in Heliconius represents a degree of behavioural sophistication rarely reported among the Lepidoptera, and is suggestive of enhancements in visually oriented spatial memory and long-term memory retention [11]. The cognitive abilities of Heliconius relative to non-pollen-feeding Heliconiini are, however, yet to be experimentally assessed. Nevertheless, the apparent cognitive demands of traplining are predicted to be associated with elaborations in the Heliconius nervous system [69,70].
Indeed, Heliconius have dramatically enlarged mushroom bodies (figure 1c), which are three to four times larger than is typical of Lepidoptera, including two closely related Heliconiini, Dryas iulia and Agraulis vanillae [69,70]. Mushroom bodies are paired, central brain structures that receive visual and/or olfactory information, and play an important role in learning and memory [80]. Mushroom body function varies across insects with different ecologies, which is reflected in the relative importance of visual and olfactory inputs [80]. In Heliconius, the demands of trapline foraging is hypothesized to have driven mushroom body expansion [69,70]. However, direct evidence for a functional link between the mushroom bodies and visually oriented spatial memory is limited to a handful of ablation experiments in cockroaches [81] and ants [82,83]. Indirect evidence also comes from comparative data from Hymenoptera, showing that expansion of the mushroom bodies coincided with the evolution of parasitoidism [84], which relies on spatial memory for host location [85], and plasticity experiments in a desert ant that show visually guided foraging experience affects mushroom body maturation [86]. Though suggestive, these data are relatively impoverished compared to our understanding of the role of the central complex, another sensory-motor integration structure in the central brain, in insect spatial learning and orientation [87,88].
Increases in Heliconius mushroom body size have also been speculatively linked to host plant use [89]. Heliconius lay exclusively on Passiflora plants, with varying degrees of specialization. Passiflora display a remarkable diversity of leaf shape, and host plant use in Heliconius appears to be, in part, based on leaf shape recognition and learning through associative conditioning [90]. Mushroom body expansion may, therefore, support a greater array of search images and enhanced shape-learning abilities, facilitating improved visual identification of host plants [89]. Indeed, there are indications that, for some butterflies, mushroom body plasticity is shaped by experience with host plants [91,92]. However, the current lack of data on variation in mushroom body size within Heliconius and across Heliconiini prohibits tests of these hypotheses. Likewise, a better understanding of the foraging behaviours of non-pollen-feeding Heliconiini is crucial to understanding the drivers behind mushroom body expansion in Heliconius.
The impacts of pollen feeding in Heliconius clearly extend beyond direct effects on fecundity, shaping their foraging strategy and probably changing the types and complexity of information processed and stored by the brain. Heliconius therefore offer a highly tractable system for investigating how behavioural innovations can alter a species' cognitive ecology.
5. Ripple effects following evolutionary innovations
Profound shifts in a major trait can have substantial knock-on effects on the selection regimes governing both developmental and evolutionary processes [2] (figure 3). In Heliconius, the evolution of pollen feeding has been linked to an extensive suite of life history, reproductive and phenotypic traits. However, the interactions and dependencies between these traits are poorly explored. Here, we highlight three areas in which pollen feeding is hypothesized to have altered Heliconius biology (figure 3), and which illustrate the broader impacts of behavioural innovations.
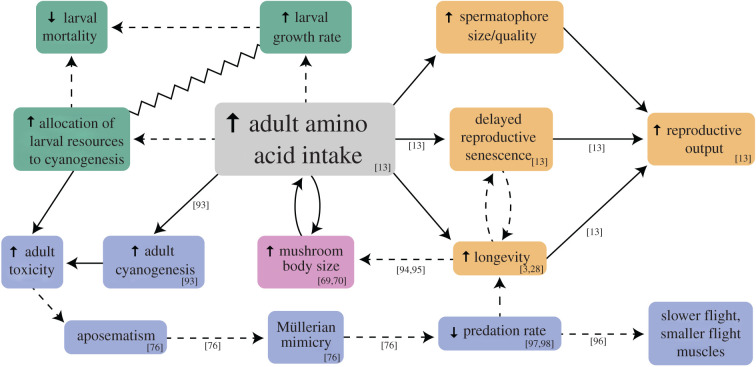
Figure 3.
Summary of the hypothesized consequences of increased adult amino acid intake in Heliconius, divided into: (i) changes in the allocation of larval resources, green; (ii) increased longevity and delayed reproductive senescence, yellow; (iii) increases in cyanogenesis, adult distastefulness and aposematism, blue; and (iv) increases in behavioural sophistication and neural elaboration, pink. Solid arrows represent amino acid investment, dashed arrows represent selective pressures, and the zig-zag line represents a developmental trade-off. Footnotes indicate supporting evidence for specific traits or relationships. (Online version in colour.)
First, dietary improvements in adults can alter how energetically expensive investments are provisioned during the larval stage [28] (figure 3, green). As larvae, Heliconiini sequester cyanogenic compounds and synthesize cyanogens de novo from host plant (Passiflora) derived amino acids [99], making them distasteful to predators. A pollen-rich adult diet may allow Heliconius to allocate a greater proportion of larval resources towards chemical defence, rather than energy reserves, with the shortfall in reproductive investment being recouped during the adult stage [100]. However, although Heliconius tend to emerge with higher cyanogen concentrations than most other Heliconiini, the non-pollen-feeding Agraulis vanillae and Eueides spp. show levels similar to Heliconius [100,101]. Such comparisons are, however, complicated by adult cyanogen profiles being influenced by larval diet [99,102], which varies between and within Heliconiini species. Moreover, cyanogen concentration is an imperfect proxy for distastefulness due to interspecific variation in chemical profile [101]. Pollen feeding has also been suggested to allow Heliconius to reduce time spent in the vulnerable larval stage [3,12] by trading off increased growth rates against investment in nitrogen reserves. Although larval growth rates across the Heliconiini have not yet been compared, females Dryas iulia emerge with a higher proportion of abdominal nitrogen allocated to reproductive reserves than Heliconius [28]. Similarly, compared with D. iulia, H. charithonia females emerge with smaller ovaries containing fewer oocytes, further suggesting a reduction in larval investment in reproduction [13].
Second, it has been suggested that Heliconius may also appropriate pollen-derived amino acids for increased cyanogenesis during the adult stage [12]. Together with a potential increased allocation of larval resources to chemical defence, this could strengthen selection for aposematic wing patterns and Müllerian mimicry [76] (figure 3, blue). Heliconius do experience lower predation rates than sympatric non-pollen-feeding Heliconiini [97,98], indicating greater unpalatability and salience of warning cues, which, in butterflies, is also associated with decreased investment in flight muscle [96]. However, the role of pollen intake in adult cyanogenesis remains ambiguous. Restriction of dietary amino acids reportedly has no effect on cyanogen levels in two-week old Heliconius ethilla and Heliconius hecale [100]. However, an effect was seen in H. melpomene after two to four weeks, suggesting pollen feeding could prolong adult cyanogenesis [93]. Heliconius also invest large amounts of cyanogens in eggs and spermatophores [99], and pollen feeding may help replenish cyanogens lost through reproduction. Indeed, when deprived of amino acids, H. charithonia show depressed cyanogen levels relative to freshly eclosed adults, suggesting that cyanogens are re-appropriated under stressful conditions [100].
Finally, a decrease in adult mortality owing to predation, together with a delay of reproductive senescence, probably strengthened selection for extended lifespans (figure 3, yellow). An increase in longevity would also favour investment in learning, long-term memory and their neural correlates (figure 3, pink), which is less rewarding for shorter-lived species [94,95]. However, this interaction may itself require concomitant investment trade-offs or physiological adaptations, as the costs of learning can cause reductions in longevity [103], and fecundity [104]. While the above hypotheses remain poorly tested, interactions between these diverse traits may be crucial to the evolution of pollen feeding [25].
6. Disentangling the origins of a singular adaptation
Since it was first described nearly 50 years ago [3], pollen feeding in Heliconius has been shown to involve a complex suite of adaptations (figure 2), with substantial knock-on effects on life history (figure 3). We propose one evolutionary scenario that describes plausible transitional stages in moving from nectarivory to trapline pollen feeding (figures 2 and 3), highlighting important ecological and evolutionary interactions. However, the order in which these traits were acquired, and therefore how they interact, is unresolved. Nevertheless, the comparatively recent phylogenetic scale over which these shifts occurred (approx. 18 Ma [5]) positions Heliconius as a valuable model for exploring a number of core questions in evolutionary biology. Importantly, Heliconius are already a highly tractable system, readily reared in insectaries and amenable to behavioural experimentation [90], and supported by broad genomic resources [17] and tools for studying candidate genes [18,20]. Below, we identify five fundamental questions in evolutionary biology that can be addressed through further investigation of pollen feeding in Heliconius.
(a). How quickly do behavioural innovations alter selection on related traits?
Heliconius are distinguished from their non-pollen feeding relatives by several traits. However, whether these traits evolved in a short burst of multi-trait adaptive evolution, or were assembled gradually through time, is unclear. Variation in the degree of specialization on cucurbit vines across Heliconius may provide one avenue to investigate ties between pollen feeding and foraging behaviours. However, as the only non-pollen-feeding Heliconius, the four Neruda species offer the possibility of separating pollen feeding from its associated adaptations. Although little is known about Neruda biology, particularly their foraging behaviour, there is evidence that they exhibit some ancestral traits. For example, the proboscis of Heliconius aeode lacks the increased density and length of bristles seen in other Heliconius [56]. However, the extent to which they share other traits with non-Heliconius Heliconiini is unclear. Unresolved questions include whether the Neruda trapline forage or possess expanded mushroom bodies, and the level of proteolytic activity in their saliva.
(b). What molecular mechanisms underlie functional innovations?
The nature of the molecular bases of adaptation is a central question in evolutionary biology, and identifying the genetic basis of a trait can help understand adaptive novelty [20]. For Heliconius, it is possible that developing the molecular ‘toolkit’ to digest pollen was a key innovation, unlocking a new ecological resource that triggered subsequent adaptive evolution in other traits. Although a number of proteases have been identified in Heliconius saliva [61,62], their role in pollen digestion is undemonstrated, and the mechanisms by which Heliconius saliva can break down pollen remain unclear. Comparisons of salivary compounds and gene expression in the mouthparts of Heliconius and other Heliconiini may lead to the identification of genes key to the evolution of pollen feeding, offering important insight into how novelty evolves.
(c). How do biological trade-offs shape the evolution of novel traits?
Novel traits often involve considerable costs and may only confer fitness benefits under certain conditions, resulting in unequal landscapes of adaptive opportunity between species [25,32]. Despite pollen feeding offering large reproductive benefits, Heliconius are the only butterflies to have evolved this ability. It is possible that reliable collection of pollen can only be achieved through increased investment in neural tissue and learning, both of which can be costly [105,106]. The benefits of increased reproductive longevity may also depend on high adult survival, which is supported by Müllerian mimicry in Heliconius. These interactions could be approached using mathematical models that formalize their interdependencies [107], or agent-based simulations that reveal a hierarchy of competitive advantages provided by different traits [108]. This provides one route to reconstructing the order in which these traits changed, and their inter-dependencies, highlighting which were key to the origins of pollen-feeding and which were consequences of it.
(d). What selective pressures and constraints shape brain evolution?
Expansion of specific brain regions is restricted by the energetic costs of neural tissue [106] and constraints that can limit the independent evolution of component parts [109]. Yet, region-specific changes can underpin behavioural innovation [109]. In Heliconius, increased behavioural sophistication putatively co-occurs with an expansion of the mushroom bodies. This provides an opportunity to study how selection for behavioural innovation can shape brain evolution, and how ancestral neural structures can be co-opted for new functions. Difficulties associated with testing comparative hypotheses where phenotypic shifts occur in a single lineage [110] may be overcome with more flexible phylogenetic methods that incorporate rate heterogeneity in trait evolution to reveal distinct shifts in correlated traits across time [19].
(e). What mechanisms permit delayed reproductive senescence and extended lifespans?
Age-related declines in fitness are widespread among animals, but these effects can vary dramatically between closely related taxa and within species [111]. However, our understanding of these processes remains incomplete. The greatly extended lifespans of Heliconius indicate a remarkable ability to delay bodily senescence [13]. Additionally, the ability to maintain long-term memories of foraging routes suggest that Heliconius may mitigate cognitive senescence observed in other insects [112,113]. Uncovering the mechanisms by which Heliconius delay senescence, and the potential role of pollen-derived amino acids, could provide valuable insight into ageing processes.
7. Conclusion
In conclusion, we highlight pollen feeding in Heliconius as a remarkable example of a behavioural innovation triggering an adaptive shift across a suite of multiple, interrelated traits, with the potential to become a highly informative, textbook case of the causes and consequences of behavioural evolution. By exploring the questions set out above, we can make progress towards better understanding how behavioural novelties arise, and subsequently lead to profound changes across diverse aspects of an animal's biology.
Supplementary Material
Acknowledgements
We thank the Montgomery and McMillan laboratories, Richard Merrill, Chi-Yun Kuo, Érika Pinheiro de Castro and Amar Sarkar for discussion and comments on the manuscript, Wyatt Toure for the image in figure 1a, and Harald Krenn for the image in figure 1b.
Data accessibility
This article has no additional data.
Authors' contributions
F.J.Y. and S.H.M. conceived of and wrote the manuscript.
Competing interests
We declare we have no competing interests.
Funding
This work was supported by a Trinity College (Cambridge) Studentship to F.J.Y., a NERC IRF grant no. (NE/N014936/1) and an ERC Starter grant no. (758508) to S.H.M.
References
- 1.Zuk M, Bastiaans E, Langkilde T, Swanger E. 2014. The role of behaviour in the establishment of novel traits. Anim. Behav. 92, 333–344. ( 10.1016/j.anbehav.2014.02.032) [DOI] [Google Scholar]
- 2.Pianka ER. 1981. Resource acquisition and allocation among animals. In Physiological ecology: an evolutionary approach to resource use (eds Calow P, Townsend CR), pp. 300–314. Sunderland, MA: Sinauer. [Google Scholar]
- 3.Gilbert LE. 1972. Pollen feeding and reproductive biology of Heliconius butterflies. Proc. Natl Acad. Sci. USA 69, 1403–1407. ( 10.1073/pnas.69.6.1403) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown KS. 1981. The biology of Heliconius and related genera. Annu. Rev. Entomol. 26, 427–457. ( 10.1146/annurev.en.26.010181.002235) [DOI] [Google Scholar]
- 5.Kozak KM, Wahlberg N, Neild AFE, Dasmahapatra KK, Mallet J, Jiggins CD. 2015. Multilocus species trees show the recent adaptive radiation of the mimetic Heliconius butterflies. Syst. Biol. 64, 505–524. ( 10.1093/sysbio/syv007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beltrán M, Jiggins CD, Brower AVZ, Bermingham E, Mallet J. 2007. Do pollen feeding, pupal-mating and larval gregariousness have a single origin in Heliconius butterflies? Inferences from multilocus DNA sequence data. Biol. J. Linn. Soc. 92, 221–239. ( 10.1111/j.1095-8312.2007.00830.x) [DOI] [Google Scholar]
- 7.Brower AVZ, Egan MG. 1997. Cladistic analysis of Heliconius butterflies and relatives (Nymphalidae: Heliconiiti): a revised phylogenetic position for Eueides based on sequences from mtDNA and a nuclear gene. Proc. R. Soc. Lond. B 264, 969–977. ( 10.1098/rspb.1997.0134) [DOI] [Google Scholar]
- 8.Estrada C, Jiggins CD. 2002. Patterns of pollen feeding and habitat preference among Heliconius species. Ecol. Entomol. 27, 448–456. ( 10.1046/j.1365-2311.2002.00434.x) [DOI] [Google Scholar]
- 9.Cardoso MZ. 2001. Patterns of pollen collection and flower visitation by Heliconius butterflies in southeastern Mexico. J. Trop. Ecol. 17, 763–768. ( 10.1017/S0266467401001572) [DOI] [Google Scholar]
- 10.Boggs CL, Smiley JT, Gilbert LE. 1981. Patterns of pollen exploitation by Heliconius butterflies. Oecologia 48, 284–289. ( 10.1007/BF00347978) [DOI] [PubMed] [Google Scholar]
- 11.Gilbert LE. 1975. Ecological consequences of a coevolved mutualism between butterflies and plants. In Coevolution of animals and plants (eds Gilbert LE, Raven PH), pp. 210–240. Austin, TX: University of Texas Press. [Google Scholar]
- 12.Gilbert LE. 1991. Biodiversity of a Central American Heliconius community: pattern, process, and problems. In Plant–animal interactions: evolutionary ecology in tropical and temperate regions (eds Price PW, Lewinsohn TM, Fernandes GW, Benson WW), pp. 403–427. New York, NY: Wiley. [Google Scholar]
- 13.Dunlap-Pianka H, Boggs CL, Gilbert LE. 1977. Ovarian dynamics in Heliconiine butterflies: programmed senescence versus eternal youth. Science 197, 487–490. ( 10.1126/science.197.4302.487) [DOI] [PubMed] [Google Scholar]
- 14.Jiggins CD. 2017. The ecology and evolution of Heliconius butterflies, 1st edn Oxford, UK: Oxford University Press. [Google Scholar]
- 15.Ehrlich PR, Gilbert LE. 1973. Population structure and dynamics of the tropical butterfly, Heliconius ethilla. Biotropica 5, 69–83. ( 10.2307/2989656) [DOI] [Google Scholar]
- 16.Mallet J. 1986. Gregarious roosting and home range in Heliconius butterflies. Natl Geogr. Res. 2, 198–215. ( 10.2307/5054) [DOI] [Google Scholar]
- 17.Edelman NB, et al. 2019. Genomic architecture and introgression shape a butterfly radiation. Science 366, 594–599. ( 10.1126/science.aaw2090) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lewis JJ, van der Burg KRL, Mazo-Vargas A, Reed RD. 2016. ChIP-seq-annotated Heliconius erato genome highlights patterns of cis-regulatory evolution in Lepidoptera. Cell Rep. 16, 2855–2863. ( 10.1016/j.celrep.2016.08.042) [DOI] [PubMed] [Google Scholar]
- 19.Nunn CL, Zhu L. 2014. Phylogenetic prediction to identify ‘evolutionary singularities'. In Modern phylogenetic comparative methods and their application in evolutionary biology: concepts and practice (ed. Garamszegi LZ.), pp. 481–514. Berlin, Germany: Springer. [Google Scholar]
- 20.Concha C, et al. 2019. Interplay between developmental flexibility and determinism in the evolution of mimetic Heliconius wing patterns. Curr. Biol. 29, 3996–4009; e4. ( 10.1016/j.cub.2019.10.010) [DOI] [PubMed] [Google Scholar]
- 21.Stearns S. 1989. Trade-offs in life-history evolution. Br. Ecol. Soc. 3, 259–268. ( 10.2307/2389364) [DOI] [Google Scholar]
- 22.Carbone C, Mace GM, Roberts SC, Macdonald DW. 1999. Energetic constraints on the diet of terrestrial carnivores. Nature 402, 286–288. ( 10.1038/46266) [DOI] [PubMed] [Google Scholar]
- 23.DeCasien AR, Williams SA, Higham JP. 2017. Primate brain size is predicted by diet but not sociality. Nat. Ecol. Evol. 1, 1–7. ( 10.1038/s41559-017-0112) [DOI] [PubMed] [Google Scholar]
- 24.Swanson EM, Espeset A, Mikati I, Bolduc I, Kulhanek R, White WA, Kenzie S, Snell-Rood EC. 2016. Nutrition shapes life-history evolution across species. Proc. R. Soc. B 283, 20152764 ( 10.1098/rspb.2015.2764) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dunbar RIM, Shultz S. 2017. Why are there so many explanations for primate brain evolution? Phil. Trans. R. Soc. B 372, 20160244 ( 10.1098/rstb.2016.0244) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Slansky F, Scriber JM. 1985. Food consumption and utilization. In Comprehensive insect physiology, biochemistry and pharmacology (eds Kerkut GA, Gilbert LI), pp. 87–163. Oxford, UK: Pergamon Press. [Google Scholar]
- 27.Krenn HW. 2008. Feeding behaviours of neotropical butterflies (Lepidoptera, Papilionoidea). Stapfia (Linz) 88, 295–304. [Google Scholar]
- 28.Boggs CL. 1981. Nutritional and life-history determinants of resource allocation in holometabolous insects. Am. Nat. 117, 692–709. ( 10.2307/2460754) [DOI] [Google Scholar]
- 29.O'Brien DM, Boggs CL, Fogel ML. 2003. Pollen feeding in the butterfly Heliconius charitonia: isotopic evidence for essential amino acid transfer from pollen to eggs. Proc. R. Soc. B 270, 2631–2636. ( 10.1098/rspb.2003.2552) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haydak MH. 1935. Brood rearing by honeybees confined to a pure carbohydrate diet. J. Econ. Entomol. 28, 657–660. ( 10.1093/jee/28.4.657) [DOI] [Google Scholar]
- 31.Schmidt JO, Thoenes SC, Levin MD. 1987. Survival of honey bees, Apis mellifera (Hymenoptera: Apidae), fed various pollen sources. Ann. Entomol. Soc. Am. 80, 176–183. ( 10.1093/aesa/80.2.176) [DOI] [Google Scholar]
- 32.Martin CH, McGirr JA, Richards EJ, St. John ME. 2019. How to investigate the origins of novelty: insights gained from genetic, behavioral, and fitness perspectives. Integr. Organismal Biol. 1, obz018 ( 10.1093/iob/obz018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stroud JT, Losos JB. 2016. Ecological opportunity and adaptive radiation. Annu. Rev. Ecol. Evol. Syst. 47, 507–532. ( 10.1146/annurev-ecolsys-121415-032254) [DOI] [Google Scholar]
- 34.Wilson GP, Evans AR, Corfe IJ, Smits PD, Fortelius M, Jernvall J. 2012. Adaptive radiation of multituberculate mammals before the extinction of dinosaurs. Nature 483, 457–460. ( 10.1038/nature10880) [DOI] [PubMed] [Google Scholar]
- 35.Blount ZD, Borland CZ, Lenski RE. 2008. Historical contingency and the evolution of a key innovation in an experimental population of Escherichia coli. Proc. Natl Acad. Sci. USA 105, 7899–7906. ( 10.1073/pnas.0803151105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meyer JR, Dobias DT, Weitz JS, Barrick JE, Quick RT, Lenski RE. 2012. Repeatability and contingency in the evolution of a key innovation in phage lambda. Science 335, 428–432. ( 10.1126/science.1214449) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weber JN, Peterson BK, Hoekstra HE. 2013. Discrete genetic modules are responsible for complex burrow evolution in Peromyscus mice. Nature 493, 402–405. ( 10.1038/nature11816) [DOI] [PubMed] [Google Scholar]
- 38.Hahn MW, Nakhleh L. 2016. Irrational exuberance for resolved species trees: Commentary. Evolution 70, 7–17. ( 10.1111/evo.12832) [DOI] [PubMed] [Google Scholar]
- 39.DeVries PJ. 1979. Pollen-feeding rainforest Parides and Battus butterflies in Costa Rica. Biotropica 11, 237–238. ( 10.2307/2388045) [DOI] [Google Scholar]
- 40.Krenn HW. 2010. Feeding mechanisms of adult Lepidoptera: structure, function, and evolution of the mouthparts. Annu. Rev. Entomol. 55, 307–327. ( 10.1146/annurev-ento-112408-085338) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Luo S, Li Y, Chen S, Zhang D, Renner SS. 2011. Gelechiidae moths are capable of chemically dissolving the pollen of their host plants: first documented sporopollenin breakdown by an animal. PLoS ONE 6, e19219 ( 10.1371/journal.pone.0019219) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baker HG, Baker I. 1973. Amino-acids in nectar and their evolutionary significance. Nature 242, 117–118. ( 10.1038/227680a0) [DOI] [PubMed] [Google Scholar]
- 43.Erhardt A, Baker I. 1990. Pollen amino acids - an additional diet for a nectar-feeding butterfly? Plant Syst. Evol. 169, 111–121. ( 10.1007/BF00935989) [DOI] [Google Scholar]
- 44.Alm J, Ohnmeiss TE, Lanza J, Vriesenga L. 1990. Preference of cabbage white butterflies and honey bees for nectar that contains amino acids. Oecologia 84, 53–57. ( 10.1007/BF00665594) [DOI] [PubMed] [Google Scholar]
- 45.Mevi-Schütz J, Erhardt A. 2004. Mating frequency influences nectar amino acid preference of Pieris napi. Proc. R. Soc. B 271, 153–158. ( 10.1098/rspb.2003.2579) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hill CJ, Pierce NE. 1989. The effect of adult diet on the biology of butterflies - 1. The common imperial blue, Jalmenus evagoras. Oecologia 81, 249–257. ( 10.1007/BF00379812) [DOI] [PubMed] [Google Scholar]
- 47.Molleman F, Ding J, Wang J-L, Brakefield PM, Carey JR, Zwaan BJ. 2008. Amino acid sources in the adult diet do not affect life span and fecundity in the fruit-feeding butterfly Bicyclus anynana. Ecol. Entomol. 33, 429–438. ( 10.1111/j.1365-2311.2008.00986.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.O'Brien DM, Schrag DP, Martínez Del Rio C. 2000. Allocation to reproduction in a hawkmoth: a quantitative analysis using stable carbon isotopes. Ecology 81, 2822–2831. ( 10.1890/0012-9658(2000)081[2822:ATRIAH]2.0.CO;2) [DOI] [Google Scholar]
- 49.Murphy DD, Launer AE, Ehrlich PR. 1983. The role of adult feeding in egg production and population dynamics of the checkerspot butterfly Euphydryas editha. Oecologia 56, 257–263. ( 10.1007/BF00379699) [DOI] [PubMed] [Google Scholar]
- 50.Mevi-Schütz J, Erhardt A. 2005. Amino acids in nectar enhance butterfly fecundity: a long-awaited link. Am. Nat. 165, 411–419. ( 10.1086/429150) [DOI] [PubMed] [Google Scholar]
- 51.Boggs CL. 1997. Dynamics of reproductive allocation from juvenile and adult feeding: radiotracer studies. Ecology 78, 192–202. ( 10.1890/0012-9658(1997)078[0192:DORAFJ]2.0.CO;2) [DOI] [Google Scholar]
- 52.Levin E, McCue MD, Davidowitz G. 2017. More than just sugar: allocation of nectar amino acids and fatty acids in a Lepidopteran. Proc. R. Soc. B 284, 20162126 ( 10.1098/rspb.2016.2126) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cahenzli F, Erhardt A. 2013. Nectar amino acids enhance reproduction in male butterflies. Oecologia 171, 197–205. ( 10.1007/s00442-012-2395-8) [DOI] [PubMed] [Google Scholar]
- 54.Beck J. 2007. The importance of amino acids in the adult diet of male tropical rainforest butterflies. Oecologia 151, 741–747. ( 10.1007/s00442-006-0613-y) [DOI] [PubMed] [Google Scholar]
- 55.Molleman F, Zwaan B, Brakefield P, Carey J. 2007. Extraordinary long life spans in fruit-feeding butterflies can provide window on evolution of life span and aging. Exp. Gerontol. 42, 472–482. ( 10.1016/j.exger.2007.01.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Krenn HW, Penz CM. 1998. Mouthparts of Heliconius butterflies (Lepidoptera: Nymphalidae): a search for anatomical adaptation to pollen-feeding behavior. Int. J. Insect Morphol. Embryol. 27, 301–309. ( 10.1016/S0020-7322(98)00022-1) [DOI] [Google Scholar]
- 57.Bauder J, Krenn HW. 2009. Muskelanordnung im Rüssel von pollenfressenden und nektrasaugenden Heliconiini. Entomol. Austriaca 16, 159–160. [Google Scholar]
- 58.Penz CM, Krenn HW. 2000. Behavioral adaptations to pollen-feeding in Heliconius butterflies (Nymphalidae, Heliconiinae): an experiment using Lantana flowers. J. Insect Behav. 13, 865–880. [Google Scholar]
- 59.Hikl A-L, Krenn HW. 2011. Pollen processing behavior of Heliconius butterflies: a derived grooming behavior. J. Insect Sci. 11, 1–13. ( 10.1673/031.011.9901) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Eberhard SH, Nemeschkal HL, Krenn HW. 2009. Biometrical evidence for adaptations of the salivary glands to pollen feeding in Heliconius butterflies (Lepidoptera: Nymphalidae). Biol. J. Linn. Soc. 97, 604–612. ( 10.1111/j.1095-8312.2009.01243.x) [DOI] [Google Scholar]
- 61.Eberhard SH, Hrassnigg N, Crailsheim K, Krenn HW. 2007. Evidence of protease in the saliva of the butterfly Heliconius melpomene (L.) (Nymphalidae, Lepidoptera). J. Insect Physiol. 53, 126–131. ( 10.1016/j.jinsphys.2006.11.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Harpel D, Cullen DA, Ott SR, Jiggins CD, Walters JR. 2015. Pollen feeding proteomics: salivary proteins of the passion flower butterfly, Heliconius melpomene. Insect Biochem. Mol. Biol. 63, 7–13. ( 10.1016/j.ibmb.2015.04.004) [DOI] [PubMed] [Google Scholar]
- 63.Smith G, Macias-Muñoz A, Briscoe AD. 2016. Gene duplication and gene expression changes play a role in the evolution of candidate pollen feeding genes in Heliconius butterflies. Genome Biol. Evol. 8, 2581–2596. ( 10.1093/gbe/evw180) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kafatos F, Tartakoff A, Law J. 1967. Preliminary characterization of a proteolytic enzyme from silk moths. J. Biol. Chem. 242, 1477–1487. [PubMed] [Google Scholar]
- 65.Mackenzie G, Boa AN, Diego-Taboada A, Atkin SL, Sathyapalan T. 2015. Sporopollenin, the least known yet toughest natural biopolymer. Front. Mater. 2, 66 ( 10.3389/fmats.2015.00066) [DOI] [Google Scholar]
- 66.Krenn HW, Eberhard MJB, Eberhard SH, Hikl AL, Huber W, Gilbert LE. 2009. Mechanical damage to pollen aids nutrient acquisition in Heliconius butterflies (Nymphalidae). Arthropod-Plant Interact. 3, 203–208. ( 10.1007/s11829-009-9074-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hassell MP, Southwood TRE. 1978. Foraging strategies of insects. Annu. Rev. Ecol. Syst. 9, 75–98. ( 10.1146/annurev.es.09.110178.000451) [DOI] [Google Scholar]
- 68.Smid HM, Vet LEM. 2016. The complexity of learning, memory and neural processes in an evolutionary ecological context. Curr. Opin. Insect Sci. 15, 61–69. ( 10.1016/j.cois.2016.03.008) [DOI] [PubMed] [Google Scholar]
- 69.Sivinski J. 1989. Mushroom body development in Nymphalid butterflies: a correlate of learning? J. Insect. Behav. 2, 277–283. ( 10.1007/BF01053299) [DOI] [Google Scholar]
- 70.Montgomery SH, Merrill RM, Ott SR. 2016. Brain composition in Heliconius butterflies, posteclosion growth and experience-dependent neuropil plasticity. J. Comp. Neurol. 524, 1747–1769. ( 10.1002/cne.23993) [DOI] [PubMed] [Google Scholar]
- 71.Murawski DA, Gilbert LE. 1986. Pollen flow in Psiguria warscewiczii: a comparison of Heliconius butterflies and hummingbirds. Oecologia 68, 161–167. ( 10.1007/BF00384782) [DOI] [PubMed] [Google Scholar]
- 72.Condon MA, Gilbert LE. 1990. Reproductive biology and natural history of the neotropical vines Gurania and Psiguria. In Biology and utilization of the cucurbitaceae (eds Bates DM, Robinson RW, Jeffrey C), pp. 150–166. Ithaca, NY: Cornell University Press. [Google Scholar]
- 73.Zimmerman JK, Wright SJ, Calderón O, Pagan MA, Paton S. 2007. Flowering and fruiting phenologies of seasonal and aseasonal neotropical forests: the role of annual changes in irradiance. J. Trop. Ecol. 23, 231–251. ( 10.1017/S0266467406003890) [DOI] [Google Scholar]
- 74.Heinrich B. 1979. Resource heterogeneity and patterns of movement in foraging bumblebees. Oecologia 40, 235–245. ( 10.1007/BF00345321) [DOI] [PubMed] [Google Scholar]
- 75.Janzen DH. 1971. Euglossine bees as long-distance pollinators of tropical plants. Science 171, 203–205. ( 10.1126/science.171.3967.203) [DOI] [PubMed] [Google Scholar]
- 76.Benson WW. 1972. Natural selection for Müllerian mimicry in Heliconius erato in Costa Rica. Science 176, 936–939. ( 10.1126/science.176.4037.936) [DOI] [PubMed] [Google Scholar]
- 77.Mallet J, Longino JT, Murawski D, Murawski A, Gamboa ASD, Journal T, Jun N. 1987. Handling effects in Heliconius: where do all the butterflies go? J. Anim. Ecol. 56, 377–386. [Google Scholar]
- 78.Logan CJ, et al. 2018. Beyond brain size: uncovering the neural correlates of behavioral and cognitive specialization. Comp. Cogn. Behav. Rev. 13, 55–89. ( 10.3819/CCBR.2018.130008) [DOI] [Google Scholar]
- 79.Sherry DF, Vaccarino AL, Buckenham K, Herz RS. 1989. The hippocampal complex of food-storing birds. Brain Behav. Evol. 34, 308–317. ( 10.1159/000116516) [DOI] [PubMed] [Google Scholar]
- 80.Farris SM. 2013. Evolution of complex higher brain centers and behaviors: behavioral correlates of mushroom body elaboration in insects. Brain Behav. Evol. 82, 9–18. ( 10.1159/000352057) [DOI] [PubMed] [Google Scholar]
- 81.Mizunami M, Weibrecht JM, Strausfeld NJ. 1998. Mushroom bodies of the cockroach: their participation in place memory. J. Comp. Neurol. 402, 520–537. () [DOI] [PubMed] [Google Scholar]
- 82.Kamhi JF, Barron AB, Narendra A. 2020. Vertical lobes of the mushroom bodies are essential for view-based navigation in Australian Myrmecia ants. Curr. Biol. 30, 1–6. ( 10.1016/j.cub.2020.06.030) [DOI] [PubMed] [Google Scholar]
- 83.Buehlmann C, Wozniak B, Goulard R, Webb B, Graham P, Niven JE. 2020. Mushroom bodies are required for learned visual navigation, but not for innate visual behavior, in ants. Curr. Biol. 30, 3438–3443; e2. ( 10.1016/j.cub.2020.07.013) [DOI] [PubMed] [Google Scholar]
- 84.Farris SM, Schulmeister S. 2011. Parasitoidism, not sociality, is associated with the evolution of elaborate mushroom bodies in the brains of hymenopteran insects. Proc. R. Soc. B 278, 940–951. ( 10.1098/rspb.2010.2161) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.van Nouhuys S, Kaartinen R. 2008. A parasitoid wasp uses landmarks while monitoring potential resources. Proc. R. Soc. B 275, 377–385. ( 10.1098/rspb.2007.1446) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kühn-Bühlmann S, Wehner RW. 2006. Age-dependent and task-related volume changes in the mushroom bodies of visually guided desert ants, Cataglyphis bicolor. J. Neurobiol. 66, 511–521. ( 10.1002/neu) [DOI] [PubMed] [Google Scholar]
- 87.Pfeiffer K, Homberg U. 2014. Organization and functional roles of the central complex in the insect brain. Annu. Rev. Entomol. 59, 165–184. ( 10.1146/annurev-ento-011613-162031) [DOI] [PubMed] [Google Scholar]
- 88.Varga AG, Kathman ND, Martin JP, Guo P, Ritzmann RE. 2017. Spatial navigation and the central complex: sensory acquisition, orientation, and motor control. Front. Behav. Neurosci. 11, 4 ( 10.3389/fnbeh.2017.00004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.de Castro ÉCP, Zagrobelny M, Cardoso MZ, Bak S. 2017. The arms race between heliconiine butterflies and Passiflora plants: new insights on an ancient subject. Biol. Rev. 93, 555–573. ( 10.1111/brv.12357) [DOI] [PubMed] [Google Scholar]
- 90.Dell'Aglio DD, Losada ME, Jiggins CD. 2016. Butterfly learning and the diversification of plant leaf shape. Front. Ecol. Evol. 4, 81 ( 10.3389/FEVO.2016.00081) [DOI] [Google Scholar]
- 91.van Dijk LJA, Janz N, Schapers A, Gamberale-Stille G, Carlsson MA. 2017. Experience-dependent mushroom body plasticity in butterflies: consequences of search complexity and host range. Proc. R. Soc. B 284, 20171594 ( 10.1098/rspb.2017.1594) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Snell-Rood EC, Papaj DR, Gronenberg W. 2009. Brain size: a global or induced cost of learning? Brain Behav. Evol. 73, 111–128. ( 10.1159/000213647) [DOI] [PubMed] [Google Scholar]
- 93.Nahrstedt A, Davis RH. 1985. Biosynthesis and quantitative relationships of the cyanogenic glucosides, linamarin and lotaustralin, in genera of the Heliconiini (Insecta: Lepidoptera). Comp. Biochem. Phys. B 82, 745–749. ( 10.1016/0305-0491(85)90519-X) [DOI] [Google Scholar]
- 94.DeCasien AR, Thompson NA, Williams SA, Shattuck MR. 2018. Encephalization and longevity evolved in a correlated fashion in Euarchontoglires but not in other mammals. Evolution 72, 2617–2631. ( 10.1111/evo.13633) [DOI] [PubMed] [Google Scholar]
- 95.Minias P, Podlaszczuk P. 2017. Longevity is associated with relative brain size in birds. Ecol. Evol. 7, 3558–3566. ( 10.1002/ece3.2961) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chai P, Srygley RB. 1990. Predation and the flight, morphology, and temperature of neotropical rain-forest butterflies. Am. Nat. 135, 748–765. ( 10.1086/285072) [DOI] [Google Scholar]
- 97.Chai P. 1990. Relationships between visual characteristics of rainforest butterflies and responses of a specialized insectivorous bird. In Adaptive coloration in invertebrates (ed. Wicksten M.), pp. 31–60. Galveston, TX: Texas A&M University. [Google Scholar]
- 98.Brower LP, Brower JVZ, Collins CT. 1963. Experimental studies of mimicry: relative palatability and Müllerian mimicry among neotropical butterflies of the subfamily Heliconiinae. Zoolog. New York 48, 65–84. [Google Scholar]
- 99.de Castro ÉCP, Demirtas R, Orteu A, Olsen CE, Motawie MS, Cardoso MZ, Zagrobelny M, Bak S. 2020. The dynamics of cyanide defences in the life cycle of an aposematic butterfly: biosynthesis versus sequestration. Insect Biochem. Mol. Biol. 116, 103259 ( 10.1016/j.ibmb.2019.103259) [DOI] [PubMed] [Google Scholar]
- 100.Cardoso MZ, Gilbert LE. 2013. Pollen feeding, resource allocation and the evolution of chemical defence in passion vine butterflies. J. Evol. Biol. 26, 1254–1260. ( 10.1111/jeb.12119) [DOI] [PubMed] [Google Scholar]
- 101.de Castro ÉCP, Zagrobelny M, Zurano JP, Cardoso MZ, Feyereisen R, Bak S. 2019. Sequestration and biosynthesis of cyanogenic glucosides in passion vine butterflies and consequences for the diversification of their host plants. Ecol. Evol. 9, 5079–5093. ( 10.1002/ece3.5062) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sculfort O, de Castro ÉCP, Kozak KM, Bak S, Elias M, Nay B, Llaurens V. 2020. Variation of chemical compounds in wild Heliconiini reveals ecological factors involved in the evolution of chemical defenses in mimetic butterflies. Ecol. Evol. 10, 2677–2694. ( 10.1002/ece3.6044) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Burger JMS, Kolss M, Pont J, Kawecki TJ. 2008. Learning ability and longevity: a symmetrical evolutionary trade-off in Drosophila. Evolution 62, 1294–1304. ( 10.1111/j.1558-5646.2008.00376.x) [DOI] [PubMed] [Google Scholar]
- 104.Snell-Rood EC, Davidowitz G, Papaj DR. 2011. Reproductive tradeoffs of learning in a butterfly. Behav. Ecol. 22, 291–302. ( 10.1093/beheco/arq169) [DOI] [Google Scholar]
- 105.Burns JG, Foucaud J, Mery F. 2011. Costs of memory: lessons from ‘mini’ brains. Proc. R. Soc. B 278, 923–929. ( 10.1098/rspb.2010.2488) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Niven JE, Laughlin SB. 2008. Energy limitation as a selective pressure on the evolution of sensory systems. J. Exp. Biol. 211, 1792–1804. ( 10.1242/jeb.017574) [DOI] [PubMed] [Google Scholar]
- 107.Pyke GH. 2010. Optimal foraging theory: introduction. In Encyclopedia of animal behavior (eds Breed MD, Moore J), pp. 601–603. Burlington, MA: Elsevier. [Google Scholar]
- 108.Grimm V, Railsback S. 2005. Individual-based modeling and ecology. Princeton, NJ: Princeton University Press. [Google Scholar]
- 109.Montgomery SH, Mundy NI, Barton RA. 2016. Brain evolution and development: adaptation, allometry and constraint. Proc. R. Soc. B 283, 20160433 ( 10.1098/rspb.2016.0433) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Uyeda JC, Zenil-Ferguson R, Pennell MW. 2018. Rethinking phylogenetic comparative methods. Syst. Biol. 67, 1091–1109. ( 10.1093/sysbio/syy031) [DOI] [PubMed] [Google Scholar]
- 111.Nussey DH, Froy H, Lemaitre J-F, Gaillard J-M, Austad SN. 2013. Senescence in natural populations of animals: widespread evidence and its implications for bio-gerontology. Ageing Res. Rev. 12, 214–225. ( 10.1016/j.arr.2012.07.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Brown S, Strausfeld NJ. 2009. The effect of age on a visual learning task in the American cockroach. Learn. Mem. 16, 210–223. ( 10.1101/lm.1241909) [DOI] [PubMed] [Google Scholar]
- 113.Tofilski A. 2000. Senescence and learning in honeybee (Apis mellifera) workers. Acta Neurobiol. Exp. 60, 35–39. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This article has no additional data.