Abstract
Sperm cells experience considerable post-ejaculation environmental variation. However, little is known about whether this affects their molecular composition, probably owing to the assumption that sperm are transcriptionally quiescent. Nevertheless, recent evidence shows sperm have distinct RNA profiles that affect fertilization and embryo viability. Moreover, RNAs are expected to be highly sensitive to extracellular changes. One such group of RNAs are heat shock protein (hsp) transcripts, which function in stress responses and are enriched in sperm. Here, we exploit the experimental tractability of the mussel Mytilus galloprovincialis by exposing paired samples of ejaculated sperm to ambient (19°C) and increased (25°C) temperatures, then measure (i) sperm motility phenotypes, and (ii) messenger RNA (mRNA) levels of two target genes (hsp70 and hsp90) and several putative reference genes. We find no phenotypic changes in motility, but reduced mRNA levels for hsp90 and the putative reference gene gapdh at 25°C. This could reflect either decay of specific RNAs, or changes in translation and degradation rates of transcripts to maintain sperm function under stress. These findings represent, to our knowledge, the first evidence for changes in sperm RNA profiles owing to post-ejaculation environments, and suggest that sperm may be more vulnerable to stress from rising temperatures than currently thought.
Keywords: sperm RNA, sperm gene expression, sperm phenotype, external fertilization, climate change, paternal effects
1. Introduction
Sperm cells are traditionally thought to function as DNA-delivery machines, with fixed phenotypes determined by males during spermatogenesis [1]. This view was formed primarily from the study of sexual reproduction in internal fertilizers, where sperm follow a predictable path from one homeostatically-controlled environment (the male reproductive tract) to another (the female reproductive tract). However, it has recently become clear that sperm undergo numerous post-ejaculatory modifications, both at the phenotypic and molecular levels, as they move through the reproductive environment (reviewed in [2]). Even for internal fertilizers these modifications can be variable, for example, depending on the identity of the female with which the male mates (e.g. [3]). However, in the case of external fertilizers, sperm have no homeostatic protection once they have been released from the male; to achieve fertilization they must navigate an environment that fluctuates considerably, both abiotically and biotically [4,5]. We would therefore also expect highly variable post-ejaculatory modifications in these taxa.
Superimposed on natural environmental variation, externally spawned gametes also face substantial human-induced changes to abiotic conditions. Importantly, rapid warming owing to anthropogenic climate change is likely to have a disproportionate effect on external fertilizers in the marine environment, which has absorbed over 90% of the Earth's warming in the last 50 years [6,7]. Climate change has led to rises in both mean sea surface temperature and the frequency of extreme heatwave events (e.g. [8–10]). So far, studies of sperm responses to rising post-ejaculation temperatures have focused on swimming behaviour and fertilization rates. Sperm of external fertilizers tend to swim more quickly at higher temperatures, possibly owing to a trade-off between sperm velocity and longevity (e.g. [11]), and fertilization rates of some broadcast spawners (where both sexes release gametes externally) may be largely unaffected by predicted changes to mean ocean temperatures (reviewed in [12]). Nevertheless, decreases in fertilization success in response to rising temperatures have also been reported, particularly at the more extreme temperatures that reflect expected conditions during heatwaves (e.g. [13–15]). Therefore, the full effects of external warming on sperm remain unclear.
Focusing on broad patterns of sperm behaviour or fertilization success might obscure cellular-level responses of sperm to environmental changes such as heat stress. We now know that sperm cells are much more than simple DNA-delivery packages; importantly, they contain complex populations of both coding and non-coding RNA transcripts [16]. Traditionally, it has been assumed that these transcripts are loaded into the cell during spermatogenesis, with the sperm genome itself in a transcriptionally quiescent state [17]. Nevertheless, there is evidence that sperm can translate proteins from messenger RNA (mRNA) transcripts prior to fertilization [18,19] and that transcript profiles covary with male fertility [20]. This raises the possibility that sperm cells could potentially adjust transcript profiles and protein production to maintain function in unpredictable or stressful environments. Changes to sperm RNA profiles have previously been reported in the absence of phenotypic changes [21]. Moreover, coding and non-coding RNAs can be transferred to fertilized eggs and influence gene expression and development in early embryos (e.g. [22–24]), and sperm might alter transcript profiles following environmental stress to prepare embryos for similar conditions (see [25]). Even accidental or non-adaptive changes to transcript profiles under stress (e.g. through cellular damage) could have implications for embryo viability. However, few studies of sperm RNA have been conducted on external fertilizers (but see [26–28]), and we are not aware of any that have examined whether abundance of sperm transcripts changes in response to variable post-ejaculation environments.
The broadcast spawning blue mussel Mytilus galloprovincialis is an ecologically dominant member of temperate subtidal communities in both hemispheres [29] and an ideal candidate model for evaluating the post-ejaculatory responses to rising sea temperatures. Australian populations of M. galloprovincialis, which contain signatures of both a native southern lineage and more recently introduced northern individuals [30–32], are distributed across the southern coastline where they experience relatively high rates of sea surface warming, and have also been subject to several prolonged heatwave events in recent years where nearshore sea surface temperatures of up to 6°C above average have consistently been recorded [8,10]. Such temperature anomalies can have substantial effects on fitness across various life stages in M. galloprovincialis, including fertilization success, larval viability and adult mortality [33–35]. These factors, as well as the tractability of M. galloprovincialis for spawning and gamete manipulation experiments (e.g. [35,36]), make it an excellent model system for exploring the effects of ocean warming on sperm. A previous study found that sperm of M. galloprovincialis appeared to maintain normal swimming behaviour and fertilization competence under short-term exposure to high temperatures [37], although decreased fertilization rates have also been reported at elevated temperatures [35]. Importantly, the potential for molecular changes to the sperm cells has yet to be explored in this or any other system, which as we note above, could underlie the maintenance of normal phenotypic function under stresses such as heat shock. Uncovering such molecular changes in sperm would provide key experimental support for the hypothesis that post-ejaculatory environmental changes can affect fertilized eggs and embryos.
In this study, we conduct, to our knowledge, the first investigation of changes in sperm transcript levels in response to post-ejaculation temperatures. To achieve this we employ a split-ejaculate design to expose samples of sperm from individual males of M. galloprovincialis to both ambient and experimentally increased seawater temperatures. At each temperature, we measured sperm motility phenotypes (experiment 1) to clarify whether sperm can maintain normal phenotypic function under heat stress. We then measured mRNA abundance of candidate genes (experiment 2) to explore putative underlying molecular responses to high temperatures. Specifically, we target mRNA derived from the heat shock protein genes hsp70 and hsp90. These genes code for molecular chaperone proteins that maintain protein stability and transport, and are typically upregulated by cells under stressful conditions such as heat shock [38–40]. Previous studies in M. galloprovincialis have shown that upregulation of these genes in somatic tissues is induced by temperature changes that reflect recent heatwaves, i.e. 5–6°C above mean ambient conditions [41,42]. Transcripts from hsp70 and hsp90 are enriched in sperm of disparate taxa, including M. galloprovincialis [27,43], and studies of human and livestock sperm suggest mRNA and protein dynamics of these genes are linked to capacitation and fertility [44–46]. Heat shock proteins are also crucial in early embryo development [47], and mRNAs coding for them could be transferred from sperm to fertilized eggs [46,48]. They are, therefore, highly relevant targets for exploring molecular changes to sperm induced by heat shock.
2. Methods
(a). Mussel collection and spawning
Live mussels were collected from Woodman Point, Western Australia (32°14′03.6″ S, 115°76′25″ E) during the 2018 spawning season (June–September), and used in experimental trials on the day of collection. Many other marine organisms use mussel shells as substrates, which could potentially contaminate RNA preparations (as used in experiment 2; see below) with transcripts from non-target species. Therefore, for mussels collected during experiment 2, encrusting organisms were cleaned from shells prior to spawning. To ensure there were no contaminants in the seawater that could affect RNA preparation and analyses (see below), we prepared experimental seawater synthetically via a multi-step filtration protocol prior to using it for gamete collection (hereafter filtered seawater, FSW; see the electronic supplementary material, Supplementary methods and results).
Spawning was induced by placing mussels in a heated water bath (28°C), as is standard for M. galloprovincialis ([36], e.g. [49,50]). We note that while there is a possibility that the temperature shock experienced by whole mussels could affect gametes and fertilization, all mussels were treated to this procedure prior to gametes being collected, and thus before intra-ejaculate samples were split across experimental temperature treatments (see ‘Experimental overview and temperature treatments’). This means that any effect of the spawning temperature would not confound gamete treatment effects. Moreover, as soon as a mussel began spawning, it was removed from the spawning tank, washed in FSW to remove any gametes that had already been spawned, and placed into an individual jar of FSW at 19°C (reflecting ambient sea surface temperatures) for gamete collection. Therefore, spawned gametes used in experiments were not directly exposed to FSW at elevated temperatures prior to the application of experimental treatments.
After approximately 30 min of spawning, we removed the mussels from their individual jars and estimated gamete concentrations. Sperm in Mytilus spp. can remain fertilization competent for longer than 11 h [51], and studies in M. galloprovincialis have found sperm are fully motile after 3 h trials [52,53]; therefore, sperm ageing is likely to be negligible over the 30 min period of spawning. Egg concentrations were estimated by counting the number of cells in a homogenized 5 µl subsample, and sperm concentrations were estimated in subsamples fixed with 1% formalin using an improved Neubauer haemocytometer (Hirschmann Laborgeräte, Eberstadt, Germany). Gametes were then adjusted to the concentrations required for experimental trials (see below).
(b). Experimental overview and temperature treatments
In both experiments, sperm were exposed to two temperature treatments (prepared with water baths in a temperature-controlled room): (i) ambient (i.e. average temperature during the spawning season), 19°C; and (ii) high, 25°C. The latter temperature was chosen to reflect thermal stress from a heatwave event [8]. For logistical reasons, it was not always possible to collect both sperm motility and RNA data from the same males; although a subset of males did have both sets of data collected (see Results). For both sperm motility (experiment 1) and heat shock gene RNA assays (experiment 2), we used a paired (split-ejaculate) design in which two separate aliquots of each male's ejaculate were collected and exposed to the different temperature treatments. In the dense, highly competitive spawning events typical of M. galloprovincialis [54,55], acute effects of short-term heat stress on sperm are likely to be most relevant to reproductive fitness. Therefore, we suspended tubes containing the ejaculate aliquots in the respective water bath treatments for a period of 10 min before downstream assays.
(c). Experiment 1: sperm motility
Sperm motility was measured using computer-assisted sperm analysis (CASA; CEROS, Hamilton-Thorne, Beverley, MA) for paired ejaculate samples in each treatment from n = 23 males. For logistical purposes, these males were measured across four spawning days (referred to as ‘blocks’). For each male, two separate 10 ml aliquots of sperm at 5.0 × 106 cells ml−1 were collected. This concentration was chosen to ensure sufficient motile cells would be present in the field of view, while remaining well within the recommended maximum for CASA [56]. Sperm motility was measured immediately after temperature treatment by placing 2 µl of the sample onto an individual well of a 12-well multi-test slide, which had previously been washed with 1% polyvinyl alcohol to prevent sperm sticking to the slide. Elsewhere we have measured sperm motility both in seawater and in the presence of egg-derived substances that change swimming patterns [57]. Here, however, we confine the measures to seawater, as we would not have been able to separate the effects of high temperature treatments on the sperm themselves, from altered bioactivity of egg-derived substances. Threshold values for defining static cells were set to 19.9 µm s−1 for average path velocity (VAP) and 4 µm s−1 for straight-line velocity (VSL). The following motility parameters were recorded (which are highly repeatable within samples for M. galloprovincialis; [50]): VAP, VSL, curvilinear velocity (VCL), linearity (LIN), straightness (STR), beat cross frequency (BCF) and amplitude of lateral head displacement (ALH). We also calculated the proportion of motile sperm (PM) from the motile and total cell counts. These traits have previously been shown to be predictive of sperm success during both non-competitive and competitive fertilizations in M. galloprovincialis [50,53,57,58].
(d). Experiment 2: sperm RNA extraction and RNA abundance assays
For gene expression assays, paired 10 ml ejaculate samples were collected from n = 18 males over three spawning days (‘blocks’). Sperm cells contain very small quantities of RNA, and large numbers of cells are required for downstream analyses [59]; therefore, the two ejaculate samples from each male were prepared at 1.0 × 108 cells ml−1. Following temperature treatment, these samples were immediately centrifuged to pellet the sperm samples (10 min at 2500 r.p.m. (1258g) and a constant temperature of 19°C) and snap frozen in liquid nitrogen. The temperature of 19°C for the spinning step was chosen to ensure that sperm from the ambient treatment did not receive any thermal shock during spinning, and thus that any differences in transcript counts between treatments could be attributed to temperatures experienced prior to centrifugation.
RNA was extracted from the sperm samples using the RNeasy Plus Universal Mini Kit (Qiagen, Melbourne, Australia) according to the manufacturer's instructions, with the following modifications: (i) sperm cells were lysed by adding 100 µl of glass beads to the sample and lysis reagent, and samples were shaken for 45 s using a FastPrep benchtop homogenizer (MP Biomedicals, Perth, Australia); (ii) during phase separation, samples were transferred to 1.5 ml phase-lock gel heavy tubes (VWR International, Brisbane, Australia), avoiding the transfer of any glass beads, and centrifuged at 20 000g for 30 min; (iii) on-column DNA digestion was performed using Qiagen DNase. RNA quantity and purity was assessed using a Qubit® 2.0 Fluorometer (ThermoFisher Scientific, Melbourne, Australia). Concentrations ranged from 12.2 to 45.4 ng µl−1. In sperm cells, much of the ribosomal RNA (rRNA) is degraded, and they contain intact 18S rRNA, but not 28S rRNA [60]. Therefore, to check that there was no contamination by non-sperm cells, we examined rRNA peaks using a LabChip GXII Automated Electrophoresis System (PerkinElmer, Melbourne, Australia). All samples contained a single rRNA peak in the size range expected for 18S, with no larger peaks that would correspond to 28S rRNA (electronic supplementary material, figure S1), indicating that our samples did not contain any somatic contamination.
We converted 200 ng of each RNA sample to complementary DNA (cDNA) in a 20 µl reaction volume using a High-Capacity cDNA Reverse Transcription Kit (ThermoFisher Scientific, Melbourne, Australia) following the manufacturer's instructions. We assayed the RNA abundance of the heat shock genes hsp70 and hsp90, with primer pairs designed from the relevant GenBank sequences using the ThermoFisher Scientific Custom Gene Expression Assay Design Tool (see the electronic supplementary material, table S1). Additionally, we tested three putative reference genes, α-tubulin, actin, and glyceraldehyde-3-phosphate-dehydrogenase (gapdh) using previously described primers for M. galloprovincialis (electronic supplementary material, table S1; [61,62]). However, for some samples α-tubulin either failed to amplify or amplified non-specific products. Therefore, we only retained actin and gapdh as putative reference genes for further analysis.
RNA from a non-experimental mussel sperm sample was used to prepare standard cDNA curves of fivefold serial dilutions (10, 2, 0.4, 0.08 and 0.016 ng µl−1). These curves produced slopes ranging from −3.394 to −3.108 and R2 ≥ 0.943, corresponding to acceptable amplification efficiencies between 1.97 and 2.1 (electronic supplementary material, table S1). For all genes, 10 ng µl−1 of cDNA produced cycle threshold (CT) values between 20.75 and 27.14; therefore, we chose this quantity as the input for further assays.
Assays were set up in triplicate for each gene-sample combination, using iTaq Universal SYBR Green Supermix (Bio-Rad, Sydney, Australia) in 10 µl reactions containing 2 µl of cDNA template and 200 nM each of forward and reverse primer (actin primers were at 100 nM). Assays were run on a StepOne Plus Real-Time PCR system (ThermoFisher Scientific, Melbourne, Australia) using the following cycling conditions: a hold stage of 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 60 s, and finally a melt curve stage with a gradual increase of 1.6°C s−1 from 60°C to 95°C. For each male, all assays in both temperature treatment samples were run on a single compatible 96-well plate, along with negative controls for each gene. The amplification results were analysed via STEPONE software v. 2.3 (ThermoFisher Scientific, Melbourne, Australia).
(e). Data analyses
Statistical analyses were carried out in R v. 3.6.0 [63]. We reduced the seven highly correlated sperm motility measures from experiment 1 to principle components (PCs) and retained the first two PCs, which had eigenvalues greater than 1 [64] and together accounted for greater than 90% of variance in the original traits (table 1). For the analysis of experiment 1, these PC scores were used as response variables in separate linear mixed-effects models (LMMs) in the package ‘lme4’ [65], with a fixed effect of temperature treatment (ambient or high) and random effects for male and block identity (ID). Model assumptions were checked via residual versus fitted value and quantile–quantile plots; additionally, formal tests revealed no evidence for heteroskedasticity of errors (Levene's tests, p > 0.5 for each model). The proportion of motile sperm was analysed using a generalized linear mixed-effects model (GLMM) with binomial error distribution (logit link function), again including a fixed temperature treatment effect and random effects for male and block. There was evidence of overdispersion in the initial GLMM (residual deviance = 104.43 on 42 degrees of freedom, dispersion factor = 2.49); therefore, the model was re-run with an observation-level random effect (resulting in residual deviance = 8.82 on 41 degrees of freedom, dispersion factor = 0.215). In all models, we tested the significance of the fixed temperature treatment effect with Wald χ2 tests. Note that in the LMM for motility PC2 and the GLMM for proportion of motile sperm, ‘block’ was excluded from the final models, as the among-block variance was close to zero, which affected model convergence (this had no qualitative effect, and little quantitative effect, on tests of the fixed temperature treatment).
Table 1.
The first two principle components generated from sperm motility traits, showing original trait loadings, eigenvalues and cumulative per cent of variance explained by composite trait variables.
| trait | PC1 | PC2 |
|---|---|---|
| ALH: amplitude of lateral head displacement | −0.958 | 0.176 |
| BCF: beat cross frequency | 0.654 | −0.393 |
| LIN: linearity | 0.765 | 0.635 |
| STR: straightness | 0.816 | 0.565 |
| VAP: average path velocity | −0.621 | 0.766 |
| VCL: curvilinear velocity | −0.734 | 0.619 |
| VSL: straight-line velocity | 0.466 | 0.878 |
| eigenvalue | 3.738 | 2.650 |
| cumulative per cent of variance explained | 53.400 | 91.251 |
Transcript abundance of target genes in each treatment for experiment 2 was compared using the package ‘MCMC.qpcr’ [66]. Briefly, this method converts CT scores to molecule counts and models them using Bayesian GLMMs, with Poisson-lognormal errors and default uninformative prior distributions from the ‘MCMCglmm’ package of Hadfield [67]. Effects are estimated using a Markov chain Monte Carlo (MCMC) algorithm to sample from the joint posterior distribution. This mixed modelling approach can correctly account for variation among samples in template loading, even in the absence of reference genes [66]. This makes the method particularly useful when there is no previous information on the stability of reference genes, as is the case in our study (i.e. our putative reference genes had not previously been tested for M. galloprovincialis sperm samples at different temperatures). We initially fit a ‘naive’ model, without specifying any of the targets as reference genes, with a fixed treatment effect and random effects of male and block ID. From this model, we confirmed that the abundance of actin was stable across temperature treatments (log2(fold change) = 0.265, fold change = 1.202, PMCMC = 0.356); however, the abundance of gapdh was significantly lower in the high treatment than in the ambient treatment (log2(fold change) = 1.617, fold change = −3.068, PMCMC < 0.001). Moreover, the abundance of gapdh was considerably less stable across all samples (CT mean = 26.67, s.d. = 3.16) than actin (CT mean = 27.36, s.d. = 1.68). Therefore, we fit a second ‘informed’ model, specifying actin (but not gapdh) as a control gene. The informed model is slightly more powerful than a naive model, but still allows the control gene to vary slightly in response to fixed factors. This allowable variation is specified by the ‘m.fix’ parameter, which by default corresponds to an average fold change very similar to our point estimate for actin (default allowable fold change = 1.2; [66]).
3. Results
(a). Experiment 1: sperm motility
The first PC was strongly loaded in the positive direction by LIN and STR, and negatively by ALH and VCL (table 1). There were also moderate loadings of BCF in the positive direction and VAP in the negative direction. The second PC had strong positive loadings of VAP and VSL, and moderate positive loadings of VCL and LIN (table 1). In other words, high scores on the PC1 axis describe straight and slow swimming sperm, while high scores on PC2 primarily describe fast sperm. Sperm swimming motility did not differ significantly between temperature treatments, either for PC1 (Wald χ12 = 1.173, p = 0.279) or PC2 (Wald χ12 = 1.694, p = 0.193). These findings were reflected for linear mixed effects models using the individual raw motility trait (electronic supplementary material, table S2). The proportion of motile sperm was also the same in both temperature treatments (Wald χ12 = 1.266, p = 0.261), with a mean of 66.42% of sperm recorded as motile across all samples. Given the non-significant results for all motility tests, we used a simulation procedure to determine the power of our analyses to detect differences in motility traits across temperature treatments (taking into account the structure of the data; see the electronic supplementary material, Supplementary methods and results). These revealed our analyses had power greater than 0.8 to detect a mean difference of less than 10% for all traits (except BCF, for which a power of 0.8 fell between 10% and 15% mean difference; electronic supplementary material, table S3).
(b). Experiment 2: sperm RNA abundance
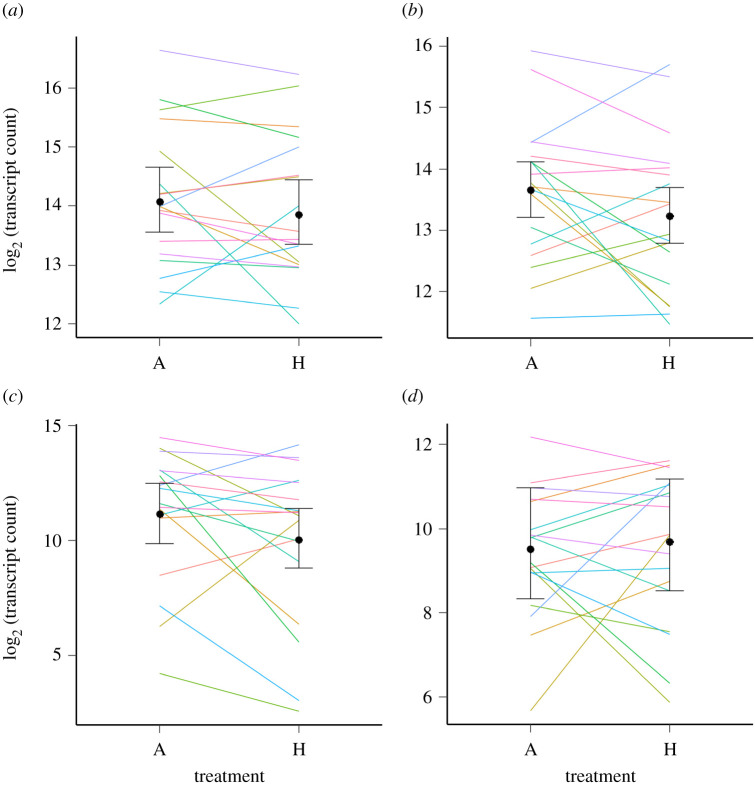
In the naive model, the mRNA count of target gene hsp90 was significantly lower at the high temperature treatment (log2 (fold change) = −0.589, fold change = −1.504, PMCMC = 0.002), as was the mRNA count of putative reference gene gapdh (see Methods). There was a non-significant negative fold change in the abundance of hsp70 in the high temperature treatment (log2(fold change) = −0.290, fold change = −1.222, PMCMC = 0.115). Similar results were obtained using an informed model, where actin was specified as a reference gene, but allowing some variability across fixed effects (figure 1). Again, hsp90 (log2(fold change) = −0.523, fold change = −1.508, PMCMC = 0.001) and gapdh (log2(fold change) = −1.620, fold change = −3.075, PMCMC < 0.001) had significantly lower mRNA in the high temperature treatment, while there was a non-significant negative fold change in hsp70 (log2(fold change) = −0.287, fold change = −1.220, PMCMC = 0.121). It is also noteworthy that although most fold changes were negative across replicate males for hsp90 and gapdh, they were not always in the same direction (figure 1).
Figure 1.
Transcript counts (log base 2 transformed) of the two target genes, (a) hsp70 and (b) hsp90, and the two putative reference genes, (c) gapdh and (d) actin, at the two temperature treatments (A = ambient, 19°C; H = high, 25°C). Lines show the log2 transcript counts for each individual male. Points show Bayesian posterior means and bars show 95% highest probability density intervals, calculated using an ‘informed’ model in the package ‘MCMC.qpcr’ ([66]; see Methods), with actin specified as a reference gene (results were qualitatively the same for a ‘naive’ model without any reference genes specified; see Results). Estimates for actin itself are from the naive model. (Online version in colour.)
We further compared the sperm motility and RNA response (i.e. the change between temperature treatments) with correlation tests for each motility PC with hsp70, hsp90 and gapdh molecule counts, for those males that had both motility and RNA data collected (n = 11). We found no significant correlations between the change in either motility PC with changes in RNA abundance (electronic supplementary material, table S4).
4. Discussion
Our investigation of the phenotypic and molecular effects of temperature on the sperm of an external fertilizer show that sperm appear able to maintain normal swimming behaviour under temperatures experienced during heatwave events; in contrast, however, sperm RNA profiles change at high temperatures. Specifically, the number of hsp90 and gapdh mRNA transcripts were approximately 1.5 and 3.1 times higher, respectively, at ambient conditions than at high temperatures, which are biologically relevant fold changes for cellular function [68]. These findings offer, to our knowledge, the first evidence for altered mRNA profiles in sperm as a result of variation in post-ejaculation environmental conditions. As we discuss below, such molecular changes could represent a cellular response to heat stress owing to translation of proteins that allow the sperm to sustain normal phenotypic function. Standard cellular responses to increased temperatures, involving increased metabolism and faster movement, could be detrimental to fitness in sperm of broadcast spawners, where swimming slowly in seawater is critical for successfully tracking eggs [50,58]. Moreover, regardless of the functional implications to the sperm themselves, the changes to RNA profiles could impact embryo development and fitness (e.g. [23,24]). Our findings have important implications for our understanding of the effects of environmental changes on gametes and reproduction; most previous studies of such effects have focused on sperm phenotypes and fertilization rates [12]. Importantly, our results indicate that the lack of phenotypic changes to sperm under factors such as increased temperature can mask underlying cellular-level changes to sperm.
We envisage two possible explanations for the altered abundances of target gene mRNAs: (i) changes in the transcriptional dynamics of these genes; and (ii) changes in the stability and degradation rate of mRNAs. We consider transcription dynamics unlikely to explain our results, given the bulk of the sperm genome is expected to be transcriptionally quiescent [1]. Even if sperm possess the capability to actively transcribe some genes (as has recently been suggested, [69–71]), we would expect sperm to upregulate the production of hsp transcripts under heat shock (e.g. [72–74]), contrary to our findings of downregulation (although there appears to be some among-individual variation). However, we cannot conclusively rule out transcriptional changes as an explanation, and suggest that future studies would benefit from testing this potential mechanism by employing transcription inhibitors (e.g. [75,76]). Nevertheless, we consider the second explanation, regarding changes in mRNA stability and degradation rate, more likely; this possibility is therefore the focus of our present discussion.
Changes in the dynamics of mRNA degradation and stability under heat shock could reflect either global or gene-specific effects. An overall increase in mRNA decay under thermal stress is certainly a possibility, for example through denaturation of ribonucleoprotein complexes, leaving transcripts unprotected [77]. However, it is unclear whether our observed downregulation effect is global; at least one of the genes we assayed (the putative reference gene actin) was stable across treatments (the trend was also non-significant for hsp70). The alternative hypothesis of gene-specific changes in mRNA stability could be either non-functional, for example if some transcripts are simply more susceptible to heat damage than others, or functional. The observed downregulation effects might represent a functional change if mRNA degradation is co-translational; i.e. if mRNA degradation is initiated by the process of translation [78], then our observations of a decrease in transcript abundance might represent an increase in translation of those genes into proteins (and consequently higher degradation rates for the associated transcripts). This would imply that the proteins encoded by genes such as hsp90 are important in sperm responding to heat stress and preserving cellular function, possibly explaining the apparent ability of sperm to maintain normal behaviour (as we report here) and fertility following thermal shock. This could also explain the variable responses following exposure to high treatment among replicate males, although our experiment was not designed to statistically test among-individual differences. Nevertheless, if the responses were simply heat-induced mRNA decay we would have expected that transcript abundance would universally decline across replicates; the apparent among-male variation in the RNA change suggests a biological response to high temperatures (e.g. [11,35]).
Interestingly, one of our putative reference genes, gapdh, also exhibited a downregulation effect. This gene is typically involved in catalysing glycolysis (although it may also be involved in a range of other cellular functions), and a previous study in corals found that gapdh and other genes related to carbon metabolism are upregulated in somatic tissues following heat stress [79]. There is some debate over the extent to which sperm cells depend on glycolysis (i.e. anaerobic metabolism) versus oxidative phosphorylation (OXPHOS; aerobic metabolism) [80]; in broadcast spawners, where the availability of external nutrients is presumably minimal, some authors have argued that sperm might rely entirely on aerobic metabolism [81]. Nevertheless, there is evidence of glycolytic activity in broadcast spawner sperm (e.g. [82]), and recent work suggests bivalve sperm might switch between OXPHOS and glycolysis during periods of sustained, fast swimming [83]. Based on our initial findings here of changes in abundance of hsp90 and gapdh under sperm heat shock, it could be highly revealing to: (i) conduct full transcriptomic sequencing to determine whether the downregulation effect is general or specific; and (ii) determine whether there are corresponding changes in protein expression.
Regardless of whether the observed changes in RNA profiles are functional for sperm, they could have implications for early embryo viability. Sperm mRNAs can be delivered to the oocyte at fertilization, and correlations between sperm mRNA profiles and embryo cleavage rates suggest that paternally delivered transcripts may affect early zygote development prior to activation of the embryonic genome [22,48,84]. The importance of heat shock proteins in early embryos is well established [47], and previous authors have suggested that the relatively high amounts of hsp70 and hsp90 mRNAs that typify sperm cells might have a role in early embryonic translation (although this has yet to be experimentally investigated) [46]. Moreover, if the downregulation effects we report are representative of a general decay of sperm RNAs, a range of epigenetic factors that influence embryo development could be affected; for example, there is strong experimental evidence that alterations to sperm micro RNAs (miRNAs) caused by the environments experienced by fathers can modify offspring phenotypes [23]. Our results suggest a potential mechanism by which the post-ejaculation environment experienced by the sperm themselves might influence offspring fitness (e.g. [25]).
In conclusion, we provide novel experimental evidence that RNA profiles in externally spawned sperm can be altered by abiotic changes in the post-ejaculation environment. These provisional findings indicate that at least some mRNAs have reduced abundance following exposure of sperm to temperatures that reflect heatwave events. We propose three key areas for future research to expand on and clarify these findings: (i) sequencing of the full sperm transcriptome at different temperatures (as well as non-coding RNAs such as miRNAs), to determine the generality of the downregulation effect; (ii) comparison of transcript abundance changes to protein expression changes, to explore the functional implications for sperm; and (iii) determining whether embryo development is affected by alterations to sperm RNAs under heat shock. Importantly, our findings suggest that externally spawned sperm may be more sensitive than typically thought to environmental changes such as warming, and highlight the importance of considering molecular as well as phenotypic sperm responses to changing conditions.
Supplementary Material
Acknowledgements
We thank Cameron Duggin, Jacob Berson, Jessica Hadlow and Elizabeth Speechley for practical help with mussel collections and experiments, Sherralee Lukehurst for assistance with qPCR assays, Julia Grassl and Laura Boykin for molecular laboratory space, and the Cockburn Power Boats Association for access to jetties. We also thank two anonymous referees for helpful comments on an earlier draft of the manuscript.
Data accessibility
Data associated with this manuscript are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.8pk0p2nkh [85].
Authors' contributions
R.A.L., J.P.E. and W.J.K. conceived of the study and designed the experiments. R.A.L. conducted the experiments, data collection and statistical analyses. R.A.L. wrote the first draft of the manuscript, and all authors contributed to the final version. All authors gave final approval for publication.
Competing interests
The authors have no competing interests.
Funding
This work was supported by an Australian Research Council grant awarded to J.P.E. and W.J.K. (grant no. DP170103290).
References
- 1.Hecht NB. 1998. Molecular mechanisms of male germ cell differentiation. Bioessays 20, 555–561. () [DOI] [PubMed] [Google Scholar]
- 2.Pitnick S, Wolfner MF, Dorus S. 2020. Post-ejaculatory modifications to sperm (PEMS). Biol. Rev. 95, 365–392. ( 10.1111/brv.12569) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gasparini C, Pilastro A. 2011. Cryptic female preference for genetically unrelated males is mediated by ovarian fluid in the guppy. Proc. R. Soc. B 278, 2495–2501. ( 10.1098/rspb.2010.2369) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levitan DR. 1998. Sperm limitation, gamete competition, and sexual selection in external fertilizers. In Sperm competition and sexual selection (eds Birkhead TR, Møller AP), pp. 175–217. San Diego, CA: Academic Press. [Google Scholar]
- 5.Evans JP, Sherman CDH. 2013. Sexual selection and the evolution of egg-sperm interactions in broadcast-spawning invertebrates. Biol. Bull. 224, 166–183. ( 10.1086/BBLv224n3p166) [DOI] [PubMed] [Google Scholar]
- 6.Church JA, et al. 2011. Revisiting the Earth's sea-level and energy budgets from 1961 to 2008. Geophys. Res. Lett. 38, L18601 ( 10.1029/2011GL048794) [DOI] [Google Scholar]
- 7.Levitus S, et al. 2012. World ocean heat content and thermosteric sea level change (0–2000 m), 1955–2010. Geophys. Res. Lett. 39, L10603 ( 10.1029/2012GL051106) [DOI] [Google Scholar]
- 8.Pearce AF, Feng M. 2013. The rise and fall of the ‘marine heat wave’ off Western Australia during the summer of 2010/2011. J. Mar. Syst. 111–112, 139–156. ( 10.1016/j.jmarsys.2012.10.009) [DOI] [Google Scholar]
- 9.Le Nohaïc M, Ross CL, Cornwall CE, Comeau S, Lowe R, McCulloch MT, Schoepf V. 2017. Marine heatwave causes unprecedented regional mass bleaching of thermally resistant corals in northwestern Australia. Sci. Rep. 7, 14999 ( 10.1038/s41598-017-14794-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oliver ECJ, Benthuysen JA, Bindoff NL, Hobday AJ, Holbrook NJ, Mundy CN, Perkins-Kirkpatrick SE. 2017. The unprecedented 2015/16 Tasman Sea marine heatwave. Nat. Commun. 8, 16101 ( 10.1038/ncomms16101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Purchase CF, Butts IAE, Alonso-Fernández A, Trippel EA. 2010. Thermal reaction norms in sperm performance of Atlantic cod (Gadus morhua). Can. J. Fish. Aquat. Sci. 67, 498–510. ( 10.1139/F10-001) [DOI] [Google Scholar]
- 12.Byrne M. 2011. Impact of ocean warming and ocean acidification on marine invertebrate life history stages: vulnerabilities and potential for persistence in a changing ocean. Oceanogr. Mar. Biol. Annu. Rev. 49, 1–42. ( 10.1201/b11009-2) [DOI] [Google Scholar]
- 13.Byrne M, Ho M, Selvakumaraswamy P, Nguyen HD, Dworjanyn SA, Davis AR. 2009. Temperature, but not pH, compromises sea urchin fertilization and early development under near-future climate change scenarios. Proc. R. Soc. B 276, 1883–1888. ( 10.1098/rspb.2008.1935) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Foo SA, Dworjanyn SA, Poore AGB, Harianto J, Byrne M. 2016. Adaptive capacity of the sea urchin Heliocidaris erythrogramma to ocean change stressors: responses from gamete performance to the juvenile. Mar. Ecol. Prog. Ser. 556, 161–172. ( 10.3354/meps11841) [DOI] [Google Scholar]
- 15.Chirgwin E, Marshall DJ, Monro K. 2020. Physical and physiological impacts of ocean warming alter phenotypic selection on sperm morphology. Funct. Ecol. 34, 646–657. ( 10.1111/1365-2435.13483) [DOI] [Google Scholar]
- 16.Dadoune JP. 2009. Spermatozoal RNAs: what about their functions? Microsc. Res. Tech. 72, 536–551. ( 10.1002/jemt.20697) [DOI] [PubMed] [Google Scholar]
- 17.Hosken DJ, Hodgson DJ. 2014. Why do sperm carry RNA? Relatedness, conflict, and control. Trends Ecol. Evol. 29, 451–455. ( 10.1016/j.tree.2014.05.006) [DOI] [PubMed] [Google Scholar]
- 18.Gur Y, Breitbart H. 2008. Protein synthesis in sperm: dialog between mitochondria and cytoplasm. Mol. Cell. Endocrinol. 282, 45–55. ( 10.1016/j.mce.2007.11.015) [DOI] [PubMed] [Google Scholar]
- 19.Fischer BE, Wasbrough E, Meadows LA, Randlet O, Dorus S, Karr TL, Russell S. 2012. Conserved properties of Drosophila and human spermatozoal mRNA repertoires. Proc. R. Soc. B 279, 2636–2644. ( 10.1098/rspb.2012.0153) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jodar M, Selvaraju S, Sendler E, Diamond MP, Krawetz SA. 2013. The presence, role and clinical use of spermatozoal RNAs. Hum. Reprod. Update 19, 604–624. ( 10.1093/humupd/dmt031) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Siklenka K, et al. 2015. Disruption of histone methylation in developing sperm impairs offspring health transgenerationally. Science 350, aab2006 ( 10.1126/science.aab2006) [DOI] [PubMed] [Google Scholar]
- 22.Ostermeier GC, Miller D, Huntriss JD, Diamond MP, Krawetz SA. 2004. Delivering spermatozoan RNA to the oocyte. Nature 429, 154 ( 10.1038/429154a) [DOI] [PubMed] [Google Scholar]
- 23.Gapp K, Jawaid A, Sarkies P, Bohacek J, Pelczar P, Prados J, Farinelli L, Miska E, Mansuy IM. 2014. Implication of sperm RNAs in transgenerational inheritance of the effects of early trauma in mice. Nat. Neurosci. 17, 667–669. ( 10.1038/nn.3695) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gross N, Strillacci MG, Peñagaricano F, Khatib H. 2019. Characterization and functional roles of paternal RNAs in 2–4 cell bovine embryos. Sci. Rep. 9, 20347 ( 10.1038/s41598-019-55868-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ritchie H, Marshall DJ. 2013. Fertilisation is not a new beginning: sperm environment affects offspring developmental success. J. Exp. Biol. 216, 3104–3109. ( 10.1242/jeb.087221) [DOI] [PubMed] [Google Scholar]
- 26.Riesco MF, Oliveira C, Soares F, Gavaia PJ, Dinis MT, Cabrita E. 2017. Solea senegalensis sperm cryopreservation: new insights on sperm quality. PLoS ONE 12, e0186542 ( 10.1371/journal.pone.0186542) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Piscopo M, Notariale R, Rabbito D, Ausió J, Olanrewaju OS, Guerriero G. 2018. Mytilus galloprovincialis (Lamarck, 1819) spermatozoa: hsp70 expression and protamine-like protein property studies. Environ. Sci. Pollut. Res. 25, 12 957–12 966. ( 10.1007/s11356-018-1570-9) [DOI] [PubMed] [Google Scholar]
- 28.Lettieri G, Maione M, Ranauda MA, Mele E, Piscopo M. 2019. Molecular effects on spermatozoa of Mytilus galloprovincialis exposed to hyposaline conditions. Mol. Reprod. Dev. 86, 650–660. ( 10.1002/mrd.23141) [DOI] [PubMed] [Google Scholar]
- 29.Daguin C, Borsa P. 2000. Genetic relationships of Mytilus galloprovincialis Lamarck populations worldwide: evidence from nuclear-DNA markers. In Evolutionary biology of the Bivalvia (eds Harper EM, Taylor JD, Crame JA), pp. 389–397. Bath, UK: Geological Soc. Publishing House. [Google Scholar]
- 30.Westfall KM, Gardner JPA. 2010. Genetic diversity of Southern Hemisphere blue mussels (Bivalvia: Mytilidae) and the identification of non-indigenous taxa. Biol. J. Linn. Soc. 101, 898–909. ( 10.1111/j.1095-8312.2010.01549.x) [DOI] [Google Scholar]
- 31.Dias PJ, Fotedar S, Snow M. 2014. Characterisation of mussel (Mytilus sp.) populations in Western Australia and evaluation of potential genetic impacts of mussel spat translocation from interstate. Mar. Freshw. Res. 65, 486–496. ( 10.1071/mf13179) [DOI] [Google Scholar]
- 32.Popovic I, Matias AMA, Bierne N, Riginos C. 2020. Twin introductions by independent invader mussel lineages are both associated with recent admixture with a native congener in Australia. Evol. Appl. 13, 515–532. ( 10.1111/eva.12857) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vihtakari M, Hendriks IE, Holding J, Renaud PE, Duarte CM, Havenhand JN. 2013. Effects of ocean acidification and warming on sperm activity and early life stages of the Mediterranean mussel (Mytilus galloprovincialis). Water 5, 1890–1915. ( 10.3390/w5041890) [DOI] [Google Scholar]
- 34.Gazeau F, Alliouane S, Bock C, Bramanti L, Correa ML, Gentile M, Hirse T, Pörtner HO, Ziveri P. 2014. Impact of ocean acidification and warming on the Mediterranean mussel (Mytilus galloprovincialis). Front. Mar. Sci. 1, 62 ( 10.3389/fmars.2014.00062) [DOI] [Google Scholar]
- 35.Eads AR, Evans JP, Kennington WJ. 2016. Plasticity of fertilization rates under varying temperature in the broadcast spawning mussel, Mytilus galloprovincialis. Ecol. Evol. 6, 6578–6585. ( 10.1002/ece3.2375) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lymbery RA, Kennington WJ, Cornwall CE, Evans JP. 2019. Ocean acidification during prefertilization chemical communication affects sperm success. Ecol. Evol. 9, 12 302–12 310. ( 10.1002/ece3.5720) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eads AR, Kennington WJ, Evans JP. 2016. Interactive effects of ocean warming and acidification on sperm motility and fertilization in the mussel Mytilus galloprovincialis. Mar. Ecol. Prog. Ser. 562, 101–111. ( 10.3354/meps11944) [DOI] [Google Scholar]
- 38.Morimoto RI. 1998. Regulation of the heat shock transcriptional response: cross talk between a family of heat shock factors, molecular chaperones, and negative regulators. Genes Dev. 12, 3788–3796. ( 10.1101/gad.12.24.3788) [DOI] [PubMed] [Google Scholar]
- 39.Feder ME, Hofmann GE. 1999. Heat-shock proteins, molecular chaperones, and the stress response: evolutionary and ecological physiology. Annu. Rev. Physiol. 61, 243–282. ( 10.1146/annurev.physiol.61.1.243) [DOI] [PubMed] [Google Scholar]
- 40.Kregel KC. 2002. Heat shock proteins: modifying factors in physiological stress response and acquired thermotolerance. J. Appl. Physiol. 92, 2177–2186. ( 10.1152/japplphysiol.01267.2001) [DOI] [PubMed] [Google Scholar]
- 41.Dutton JM, Hofmann GE. 2009. Biogeographic variation in Mytilus galloprovincialis heat shock gene expression across the eastern Pacific range. J. Exp. Mar. Biol. Ecol. 376, 37–42. ( 10.1016/j.jembe.2009.06.001) [DOI] [Google Scholar]
- 42.Ioannou S, Anestis A, Pörtner HO, Michaelidis B. 2009. Seasonal patterns of metabolism and the heat shock response (HSR) in farmed mussels Mytilus galloprovincialis. J. Exp. Mar. Biol. Ecol. 381, 136–144. ( 10.1016/j.jembe.2009.09.014) [DOI] [Google Scholar]
- 43.Lymbery RA.2018. Sperm competition and gamete interactions in a marine broadcast spawner. PhD thesis, University of Western Australia, Crawley, Western Australia, Australia.
- 44.Spinaci M, Volpe S, Bernardini C, De Ambrogi M, Tamanini C, Seren E, Galeati G. 2005. Immunolocalization of heat shock protein 70 (Hsp 70) in boar spermatozoa and its role during fertilization. Mol. Reprod. Dev. 72, 534–541. ( 10.1002/mrd.20367) [DOI] [PubMed] [Google Scholar]
- 45.Li K, Xue Y, Chen A, Jiang Y, Xie H, Shi Q, Zhang S, Ni Y. 2014. Heat shock protein 90 has roles in intracellular calcium homeostasis, protein tyrosine phosphorylation regulation, and progesterone-responsive sperm function in human sperm. PLoS ONE 9, e115841 ( 10.1371/journal.pone.0115841) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zannoni A, Bernardini C, Zaniboni A, Ferlizza E, Ventrella D, Bacci ML, Forni M. 2017. Relative abundance of heat shock proteins and clusterin transcripts in spermatozoa collected from boar routinely utilised in an artificial insemination centre: preliminary results. Vet. Res. Commun. 41, 233–239. ( 10.1007/s11259-017-9689-6) [DOI] [PubMed] [Google Scholar]
- 47.Neur A, Spandorfer SD, Giraldo P, Dieterle S, Rosenwaks Z, Witkin SS. 2000. The role of heat shock proteins in reproduction. Hum. Reprod. Update 6, 149–159. ( 10.1093/humupd/6.2.149) [DOI] [PubMed] [Google Scholar]
- 48.Boerke A, Dieleman SJ, Gadella BM. 2007. A possible role for sperm RNA in early embryo development. Theriogenology 68S, S147–S155. ( 10.1016/j.theriogenology.2007.05.058) [DOI] [PubMed] [Google Scholar]
- 49.Obata M, Komaru A. 2005. Specific location of sperm mitochondria in mussel Mytilus galloprovincialis zygotes stained by MitoTracker. Dev. Growth Differ. 47, 255–263. ( 10.1111/j.1440-169X.2005.00801.x) [DOI] [PubMed] [Google Scholar]
- 50.Fitzpatrick JL, Simmons LW, Evans JP. 2012. Complex patterns of multivariate selection on the ejaculate of a broadcast spawning marine invertebrate. Evolution 66, 2451–2460. ( 10.1111/j.1558-5646.2012.01627.x) [DOI] [PubMed] [Google Scholar]
- 51.Sprung M, Bayne BL. 1984. Some practical aspects of fertilizing the eggs of the mussel Mytilus edulis L. ICES J. Mar. Sci. 41, 125–128. ( 10.1093/icesjms/41.2.125) [DOI] [Google Scholar]
- 52.Evans JP, García-González F, Almbro M, Robinson O, Fitzpatrick JL. 2012. Assessing the potential for egg chemoattractants to mediate sexual selection in a broadcast spawning marine invertebrate. Proc. R. Soc. B 279, 20120181 ( 10.1098/rspb.2012.0181) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Oliver M, Evans JP. 2014. Chemically moderated gamete preferences predict offspring fitness in a broadcast spawning invertebrate. Proc. R. Soc. B 281, 20140148 ( 10.1098/rspb.2014.0148) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wilson BR, Hodgkin EP. 1967. A comparative account of the reproductive cycles of five species of marine mussels (Bivalvia: Mytilidae) in the vicinity of Fremantle, Western Australia. Mar. Freshw. Res. 18, 175–203. ( 10.1071/MF9670175) [DOI] [Google Scholar]
- 55.Villalba A. 1995. Gametogenic cycle of cultured mussel, Mytilus galloprovincialis, in the bays of Galicia (N.W. Spain). Aquaculture 130, 269–277. ( 10.1016/0044-8486(94)00213-8) [DOI] [Google Scholar]
- 56.Lu JC, Huang YF, Lü NQ. 2014. Computer-aided sperm analysis: past, present and future. Andrologia 46, 329–338. ( 10.1111/and.12093) [DOI] [PubMed] [Google Scholar]
- 57.Hadlow JH, Evans JP, Lymbery RA. 2020. Egg-induced changes to sperm phenotypes shape patterns of multivariate selection on ejaculates. J. Evol. Biol. 33, 797–807. ( 10.1111/jeb.13611) [DOI] [PubMed] [Google Scholar]
- 58.Lymbery RA, Kennington WJ, Evans JP. 2018. Multivariate sexual selection on ejaculate traits under sperm competition. Am. Nat. 192, 94–104. ( 10.1086/697447) [DOI] [PubMed] [Google Scholar]
- 59.Goodrich RJ, Anton E, Krawetz SA. 2013. Isolating mRNAs and small noncoding RNAs from human sperm. In Spermatogenesis: methods and protocols (eds Carrell DT, Aston KI), pp. 385–396. New York, NY: Humana Press. [DOI] [PubMed] [Google Scholar]
- 60.Cappallo-Obermann H, Schulze W, Jastrow H, Baukloh V, Spiess AN. 2011. Highly purified spermatozoal RNA obtained by a novel method indicates an unusual 28S/18S rRNA ratio and suggests impaired ribosome assembly. Mol. Hum. Reprod. 17, 669–678. ( 10.1093/molehr/gar037) [DOI] [PubMed] [Google Scholar]
- 61.Moreira R, Pereiro P, Costa MM, Figueras A, Novoa B. 2014. Evaluation of reference genes of Mytilus galloprovincialis and Ruditapes philippinarum infected with three bacteria strains for gene expression analysis. Aquat. Living Resour. 27, 147–152. ( 10.1051/alr/2014015) [DOI] [Google Scholar]
- 62.Martínez-Escauriaza R, Lozano V, Pérez-Parallé ML, Pazos AJ, Sánchez JL. 2018. Validation of reference genes in mussel Mytilus galloprovincialis tissues under the presence of okadaic acid. J. Shellfish Res. 37, 93–101. ( 10.2983/035.037.0108) [DOI] [Google Scholar]
- 63.R Core Team. 2019. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; See https://www.R-project.org/. [Google Scholar]
- 64.Reynolds RJ, Childers DK, Pajewski NM. 2010. The distribution and hypothesis testing of eigenvalues from the canonical analysis of the gamma matrix of quadratic and correlational selection gradients. Evolution 64, 1076–1085. ( 10.1111/j.1558-5646.2009.00874.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bates D, Macechler M, Bolker B, Walker S. 2015. Fitting linear mixed-effects models using lme4. J. Stat. Soft. 67, 1–48. ( 10.18637/jss.v067.i01) [DOI] [Google Scholar]
- 66.Matz MV, Wright RM, Scott JG. 2013. No control genes required: Bayesian analysis of qRT-PCR data. PLoS ONE 8, e71448 ( 10.1371/journal.pone.0071448) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hadfield JD. 2010. MCMC methods for multi-response generalized linear mixed models: the MCMCglmm R package. J. Stat. Softw. 33, 1–22. ( 10.18637/jss.v033.i02)20808728 [DOI] [Google Scholar]
- 68.Vallejos CA, Richardson S, Marioni JC. 2016. Beyond comparisons of means: understanding changes in gene expression at the single-cell level. Genome Biol. 17, 70 ( 10.1186/s13059-016-0930-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ren X, Chen X, Wang Z, Wang D. 2017. Is transcription in sperm stationary or dynamic? J. Reprod. Dev. 63, 439–443. ( 10.1262/jrd.2016-093) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bohacek J, Rassoulzadegan M. 2020. Sperm RNA: Quo vadis? Semin. Cell Dev. Biol. 97, 123–130. ( 10.1016/j.semcdb.2019.07.005) [DOI] [PubMed] [Google Scholar]
- 71.Santiago J, Santos MAS, Fardilha M, Silva JV. 2020. Stress response pathways in the male germ cells and gametes. Mol. Hum. Reprod. 26, 1–13. ( 10.1093/molehr/gaz063) [DOI] [PubMed] [Google Scholar]
- 72.Franzellitti S, Fabbri E. 2005. Differential HSP70 gene expression in the Mediterranean mussel exposed to various stressors. Biochem. Biophys. Res. Commun. 336, 1157–1163. ( 10.1016/j.bbrc.2005.08.244) [DOI] [PubMed] [Google Scholar]
- 73.Farcy É, Voiseux C, Lebel JM, Fiévet B. 2009. Transcriptional expression levels of cell stress marker genes in the pacific oyster Crassostrea gigas exposed to acute thermal stress. Cell Stress Chaperones 14, 371–380. ( 10.1007/s12192-008-0091-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Runcie DE, Garfield DA, Babbitt CC, Wygoda JA, Mukherjee S, Wray GA. 2012. Genetics of gene expression responses to temperature stress in a sea urchin gene network. Mol. Ecol. 21, 4547–4562. ( 10.1111/j.1365-294X.2012.05717.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Grigull J, Mnaimneh S, Pootoolal J, Robinson MD, Hughes TR. 2004. Genome-wide analysis of mRNA stability using transcription inhibitors and microarrays reveals posttranscriptional control of ribosome biogenesis factors. Mol. Cell. Biol. 24, 5534–5547. ( 10.1128/mcb.24.12.5534-5547.2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vibranovski MD, Chalopin DS, Lopes HF, Long MY, Karr TL. 2010. Direct evidence for postmeiotic transcription during Drosophila melanogaster spermatogenesis. Genetics 186, 431–433. ( 10.1534/genetics.110.118919) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Valcarce DG, Cartón-García F, Herráez MP, Robles V. 2013. Effect of cryopreservation on human sperm messenger RNAs crucial for fertilization and early embryo development. Cryobiology 67, 84–90. ( 10.1016/j.cryobiol.2013.05.007) [DOI] [PubMed] [Google Scholar]
- 78.Huch S, Nissan T. 2014. Interrelations between translation and general mRNA degradation in yeast. WIREs RNA 5, 747–763. ( 10.1002/wrna.1244) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Leggat W, Seneca F, Wasmund K, Ukani L, Yellowlees D, Ainsworth TD. 2011. Differential responses of the coral host and their algal symbiont to thermal stress. PLoS ONE 6, e26687 ( 10.1371/journal.pone.0026687) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.du Plessis SS, Agarwal A, Mohanty G, Van Der Linde M. 2015. Oxidative phosphorylation versus glycolysis: what fuel do spermatozoa use? Asian J. Androl. 17, 230–235. ( 10.4103/1008-682X.135123) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Milani L, Ghiselli F. 2015. Mitochondrial activity in gametes and transmission of viable mtDNA. Biol. Direct 10, 22 ( 10.1186/s13062-015-0057-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Boulais M, Soudant P, Le Goïc N, Quéré C, Boudry P, Suquet M. 2015. Involvement of mitochondrial activity and OXPHOS in ATP synthesis during the motility phase of spermatozoa in the Pacific oyster, Crassostrea gigas. Biol. Reprod. 93, 118 ( 10.1095/biolreprod.115.128538) [DOI] [PubMed] [Google Scholar]
- 83.Bettinazzi S, Nadarajah S, Dalpé A, Milani L, Blier PU, Breton S. 2020. Linking paternally inherited mtDNA variants and sperm performance. Phil. Trans. R. Soc. B 375, 20190177 ( 10.1098/rstb.2019.0177) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hwang JY, Mulligan BP, Kim HM, Yang BC, Lee CK. 2013. Quantitative analysis of sperm mRNA in the pig: relationship with early embryo development and capacitation. Reprod. Fertil. Dev. 25, 807–817. ( 10.1071/RD12160) [DOI] [PubMed] [Google Scholar]
- 85.Lymbery RA, Evans JP, Kennington WJ.2020. Data from: Post-ejaculation thermal stress causes changes to the RNA profile of sperm in an external fertilizer. Dryad Digital Repository. ( ) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Lymbery RA, Evans JP, Kennington WJ.2020. Data from: Post-ejaculation thermal stress causes changes to the RNA profile of sperm in an external fertilizer. Dryad Digital Repository. ( ) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Data associated with this manuscript are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.8pk0p2nkh [85].