Abstract
Corallivorous crown-of-thorns starfishes (Acanthaster spp.) can decimate coral assemblages on Indo-Pacific coral reefs during population outbreaks. While initial drivers of population irruptions leading to outbreaks remain largely unknown, subsequent dispersal of outbreaks appears coincident with depletion of coral prey. Here, we used in situ time-lapse photography to characterize movement of the Pacific crown-of-thorns starfish (Acanthaster cf. solaris) in the northern and southern Great Barrier Reef in 2015, during the fourth recorded population outbreak of the starfish, but prior to widespread coral bleaching. Daily tracking of 58 individuals over a total of 1117 h revealed all starfish to move a minimum of 0.52 m, with around half of all tracked starfish showing negligible daily displacement (less than 1 m day−1), ranging up to a maximum of 19 m day−1. Movement was primarily nocturnal and daily displacement varied spatially with variation in local availability of Acropora spp., which is the preferred coral prey. Two distinct behavioural modes emerged: (i) homing movement, whereby tracked paths (as tested against a random-walk-model) involved short displacement distances following distinct ‘outward' movement to Acropora prey (typically displaying ‘feeding scars') and ‘homebound' movement to nearby shelter; versus (ii) roaming movement, whereby individuals showed directional movement beyond initial tracking positions without return. Logistic modelling revealed more than half of all tracked starfish demonstrated homing when local abundance (percentage cover) of preferred Acropora coral prey was greater than 33%. Our results reveal facultative homing by Acanthaster with the prey-dependent behavioural switch to roaming forays providing a mechanism explaining localized aggregations and diffusion of these population irruptions as prey is locally depleted.
Keywords: coral reefs, predation, behaviour, movement, time-lapse photography, random-walk-model
1. Background
Spatio-temporal variability in the abundance and function of consumers can have important consequences for ecosystem structure, especially for consumers that impact habitat-forming organisms [1–3]. Examples of dramatic consumer-driven impacts include effects of ungulates across Savanna landscapes (e.g. [4]), overgrazing of kelp forests by sea urchins (reviewed by [5]) and control of seagrass by coral reef fishes (e.g. [6]) and turtles (e.g. [7]) in marine environments. Consumers can exhibit plasticity in diet, enabling them to adapt to changing prey availability (e.g. [8,9]), else they must emigrate in search of new prey resources as local resources are diminished.
Crown-of-thorns starfishes (Acanthaster spp.) are one of the largest and most efficient consumers of coral on Indo-Pacific reefs (reviewed in [10]). Mostly, Acanthaster spp. occur at low densities and have negligible impact on populations and assemblages of coral prey, however at high densities during population outbreaks, Acanthaster spp. are a major contributor to extensive, widespread and sustained coral loss throughout the Indo-Pacific (e.g. [11–15]). The ecological impacts of crown-of-thorns starfish are unequivocally linked to their feeding patterns [16]. However, aside from feeding preferences (e.g. [15,17]) and maximum movement rates (e.g. [18]), relatively little is known of their behavioural ecology, which is a major limitation for understanding the dynamics of population irruptions and approaches to mitigate their impacts on coral reefs. While it has been suggested that different behavioural modes occur during population irruptions versus benign non-irruptive periods [19], the behavioural mechanisms of Acanthaster foraging and possible triggers of behavioural shifts during population irruptions has not been explored.
On Australia's Great Barrier Reef (GBR), there have been four documented waves of population irruptions leading to outbreaks of the Pacific crown-of-thorns starfish (Acanthaster cf. solaris) since the 1960s. Each of these irruptions (starting in 1962, 1979, 1993 and 2009) appeared to be initiated on mid-shelf reefs in the north-central region and then propagated southwards, through larval dispersion to downstream reefs [20,21]. Despite recent instances of mass-coral bleaching [22] and increasing disturbances on the GBR [23], population irruptions of crown-of-thorns starfish remain one of the major causes of coral loss on the GBR [24,25]. Accordingly, to alleviate cumulative anthropogenic pressures impacting coral reef ecosystems, there are concerted efforts to manage starfish irruptions on the GBR, both directly and indirectly [26–28]. The effectiveness of direct control measures is reliant on improved understanding of the spatial and temporal dynamics of starfish populations, and especially cryptic and emergent behavioural dynamics which determine their susceptibility to, and the efficiency of, in situ culling by divers [29].
In this study, we use time-lapse photography to characterize daily movement behaviour of A. cf. solaris in the northern and southern GBR in 2015. Initially, we hypothesized that movement would be primarily nocturnal based on night-time diver observations [19,30]. Then based on readily observable starfish ‘feeding scars' on preferred Acropora prey and nearby sheltering in the presence of this coral on the GBR (e.g. [17,31–33]; reviewed by [10]), we tested our chief hypothesis that movement behaviour would depend on the availability of this preferred coral prey. By revealing the movement dynamics of A. cf. solaris using time-lapse photography, our overarching aim was to define behavioural modes and identify possible behavioural switches to inform the control of starfish population irruptions and their destructive impact on corals.
2. Methods
(a). Study locations
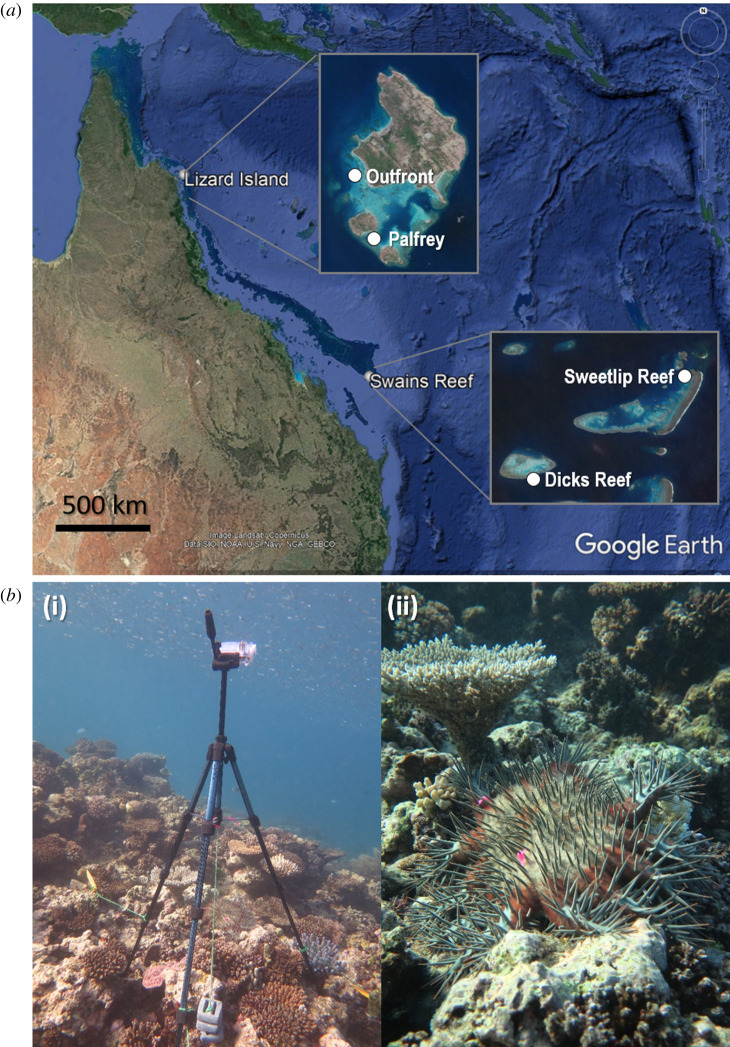
To characterize movement patterns of Acanthaster, time-lapse monitoring of individual starfish was performed at two sites in northern (Lizard Island) and southern (Swains Reefs) locations of the Great Barrier Reef (figure 1a). In each location, adult starfish (greater than 180 mm total diameter) were monitored over a range of coral reef types including reef crest and forereef ranging from 1.8 to 8.5 m depth; with overall cover of live Acropora spp. coral averaging 13.1% ± 2.4 s.e. and ranging from 0 to 75% cover in the surrounding square metre of monitored starfish (table 1). As estimated from counts by SCUBA divers along 100 m2 belt transects (n = 5 per site), densities of Acanthaster at the study reefs, ranged from 10 to 220 individuals per hectare (table 1). Note that 15 starfish per hectare is considered the trigger point of the initiation of a ‘population outbreak' (i.e. population irruption) [29].
Figure 1.
(a) Map of study locations and sites at Lizard Island and the Swains System on the Great Barrier Reef, Australia; locations were separated by 1100 km, while sites within each location were separated by 2 km and 10 km at Lizard Island and Swains Reef, respectively. (b) Photographs of experimental set-up at the Swains Reefs location: (i) example of time-lapse camera standing approximately 1.5 m high and set squarely above an individual Acanthaster cf. solaris tagged with small pieces of pink flagging tape; (ii) close-up view of pink flagging tape tags, which spanned approx. 350 mm, that were placed gently over dorsal spines of the starfish enabling individuals to be identified and displacement measured the following day. (Online version in colour.)
Table 1.
Summary of Acanthaster tracked and averages (±s.e.) of starfish density, depth and percentage cover of live Acropora spp. at the study sites.
| location | site | no. tracked individuals |
Acanthaster density (no. hectare) |
depth (m) |
% cover of live Acropora |
|---|---|---|---|---|---|
| Lizard Island | Outfront | 9 | 10 ± 10 | 2.8 ± 0.2 | 3.2 ± 3.0 |
| Palfrey | 20 | 50 ± 27 | 3.1 ± 0.2 | 20.6 ± 5.4 | |
| total | 29 | 30 ± 15 | 3.0 ± 0.2 | 15.2 ± 4.1 | |
| Swains Reefs | Dicks Reef | 18 | 220 ± 58 | 3.7 ± 0.1 | 13.4 ± 3.0 |
| Sweetlip Reef | 11 | 50 ± 22 | 5.6 ± 0.6 | 7.1 ± 3.6 | |
| total | 29 | 135 ± 41 | 4.4 ± 0.3 | 10.9 ± 2.3 | |
| grand total | 58 | 83 ± 24 | 3.7 ± 0.2 | 13.1 ± 2.4 |
(b). Tracking of Acanthaster movement
Movement of Acanthaster was tracked using time-lapse photography in the period May–June 2015 during the fourth recorded ‘population outbreak’ of the starfish, but prior to widespread recurrent coral bleaching on the GBR [34]. Individual starfish were visibly tagged by skewering 20 mm lengths of flagging tape on to their spines in unique arrangements. A Panasonic Lumix camera (model FT4 with underwater housing) was then held squarely above individual Acanthaster by attaching the camera to 1.5 m tall tripods achieving an approximate 1.0 m by 0.9 m field-of-view (FOV; figure 1b). Up to four cameras were deployed simultaneously in each location within the same depth range and over the same spatial extent as the transect surveys, with a minimum separation of 25 m between camera deployments and each focused on a different starfish. Cameras were set to ‘time-lapse shot’ mode with auto flash on. Images taken under low light conditions and at night were exposed to the cameras' flash which, at a frequency of 20 min, provided minimal local light disturbance and based on the limits of battery power, enabled movement to be tracked over 20 h depending on the fidelity of individuals within the camera FOV. A 150 mm plastic peg was used as a scale-bar and was squarely framed within the camera's FOV and photographed at the start of each image sequence to enable calibration of the FOV dimensions. For starfish no longer occurring within the FOV by the conclusion of the monitoring period, these individuals were considered to have emigrated (i.e. displaced by on average greater than 0.72 m from the centre of the FOV) and were searched for using a radial search pattern from the FOV until the individual was located. All monitored starfish were re-located. The distance of the starfish from the centre of the FOV was then recorded as the displacement over the monitoring period and expressed as daily displacement. Note that maximum daily displacement of tracked starfish was less than 20 m, which was less than the distance between neighbouring FOVs when multiple cameras were deployed over uniquely identifiable starfish individuals occurring on the same local reef.
(c). Cover of live coral
Cover of live coral within each FOV was assessed by importing images into ImageJ (v. 1.47, National Institutes of Health, USA) and tracing polygons around live coral colonies to determine the percentage of the FOV occupied by each coral taxon. Live coral taxa constituting at least approximately 1% cover on average across all FOVs, included the preferred genera Acropora [35,36], plus Porites, Stylophora, Pocillopora, Seriatopora, Goniastrea, Favites. Feeding of Acanthaster on coral was also noted for every time-lapse image (i.e. where a starfish either moved to or was observed to have formed a ‘feeding scar' on a coral colony). At the start of each image sequence, electivity of Acanthaster feeding for each coral taxon within the FOV was calculated by tracing the area of live coral taxa (i.e. proportion of each coral taxon occurring in the environment p) and tracing the area of apparent Acanthaster feeding scars for each coral taxon (i.e. proportion of each taxon in the diet of Acanthaster r). Proportions ‘r' and ‘p' were summed for each taxon in each FOV and analysed using Ivlev's electivity index (bounded between −1 and +1, with diet items closer to −1 representing avoided items, 0 consumption in proportion to abundance and +1 highly preferred items) to determine diet preference using the R package ‘electivity'. Differences in mean Ivlev's electivity indices between coral taxa were tested using one-way ANOVA on 38 replicate FOVs that contained live corals after appropriately transforming the data to stabilize variances as determined using the Box-Cox procedure (available in the R package ‘MASS').
(d). Movement analysis
Time-lapse image sequences were imported to ImageJ and the FOV was calibrated for each sequence. The ‘Manual Tracking' plugin for ImageJ was used to track the position of starfish through time. Individual paths were divided into a series of steps, stops and moves. A step was defined as the vector connecting successive positions (20 min apart), a stop as an interval in which an individual remained stationary for at least two frames (40 min) and a move as the vector between two successive stops (see [37] for a detailed explanation). An arbitrary minimum step length of 10 mm was used, below which movement was considered to be measurement error or indicating local spine movement of otherwise stationary individuals (after [38]).
The net displacement from start to end of each time-lapse sequence (cm h−1) and moving speed (cm min−1) was calculated for individual Acanthaster within each independent FOV. To test the hypothesis that Acanthaster is nocturnal, one-way analysis of variance (ANOVA) was undertaken to test the factor ‘time’, i.e. day versus night, excluding crepuscular ‘dawn' and ‘dusk' periods. Where data were heteroscedastic, the transformation used to stabilize variances was determined using the Box-Cox procedure. Predictors of daily displacement (over the 20 h monitoring period) were examined using additive multiple regression models to examine the effects of the predictor variables of cover of preferred live Acropora coral, location, depth, starfish size, site nested within location and location by cover of Acropora.
(e). Testing Acanthaster movement patterns against a random-walk model
For those Acanthaster remaining within FOV, observed movement paths were compared with paths simulated by a random-walk-model. The saturated correlation random walk (RW) model of Kareiva & Shigaseda [39] was used as per the recommendations of Flukes et al. [38]. The mean cosine of turning angles was found to be close to zero (F1,69 = 0.17, p > 0.6) and uniformly distributed, indicating no directional persistence. Thus, the model used for analysis was reduced to a simple RW equation: ; where is the net-squared displacement of a starfish's path composed of n moves, and m2 is the mean of the squared move length.
The RW model assumes no autocorrelation between either the length or direction of consecutive moves, so turning angles were tested for first- and second-order autocorrelation (see [40,41]). The presence of first-order autocorrelation between successive move lengths was also tested by Spearman rank tests [37,42]. Very weak to no autocorrelation was detected in the length of successive moves for Acanthaster across locations (Spearman rank correlation, rs(15) = 0.059, p > 0.6). No first- or second-order autocorrelation in turning angles was detected (χ2, p > 0.26 for both first- and second-order), so it was appropriate to proceed with the RW analysis.
It was necessary to pool paths across recording dates and sites to obtain sufficient sample size. Individual paths were compared with 1,000 paths simulated by the RW model using MATLAB R2019a as described by Flukes et al. [38]. A sample size (n) of 5 individuals was chosen as the minimum threshold for simulating the RW. This meant that individual paths simulated by the RW model could have a maximum of 5 moves per path. Once 1000 simulated paths were obtained, the mean net squared displacement () was calculated for every value of n as the mean of these 1000 paths. Variation around the expected was examined using the technique recommended by Turchin [40], with 95% confidence intervals estimated using the percentile method [36,40,43].
(f). Probability of emigration versus local movement
The probability of Acanthaster emigration (i.e. the binomial response of staying (0) or leaving (1) the FOV), was analysed with respect to the live cover of preferred Acropora coral using logistic regression. The probability of binomial emigration response was also analysed using logistic regression with respect to the observed time spent feeding as estimated from time-lapse imagery. Local movement suggests that starfish either move in a restricted fashion centred on a focal point (i.e. a ‘home site') or, alternatively, that they move with distinct ‘outwards' and ‘inwards' phases away from and then back to a home crevice (i.e. homing behaviour). Given these two possibilities, time-lapse sequences were re-assessed for evidence of homing. To assess for the presence of homing behaviour, time-lapse sequences were converted to movies so that starfish movement patterns could be visualized. Movement was binomially classified as homing, or not, depending on whether the starfish returned to the same home location within the FOV following a distinct outward then homeward movement. The probability of homing, i.e. homing (1) or non-homing (0), was analysed with respect to the cover of preferred live coral cover using logistic regression.
3. Results
(a). Tracking of Acanthaster movement
A total of 58 Acanthaster were tracked throughout at least one complete diel cycle across the northern and southern GBR, representing greater than 1117 h of observations. Movement was observed for all monitored starfish, with the distribution of daily displacement of tracked individuals skewed towards negligible (less than 1 m) displacement: 26 individuals stayed within 1 m of their original position over 1 day, but with a long tail of displacement distances up to a maximum of 19 m (figure 2a). Of those 26 individuals, 15 starfish remained within the FOV for the entirety of monitoring (and thus could be used for calculations of total movement distance). The maximum total distance moved by these individuals within a day was 3.2 m, with a mean of 1.1 m and minimum of 0.5 m day−1.
Figure 2.
(a) Frequency distribution of daily displacement of 58 individually tracked Acanthaster individuals across northern and southern Great Barrier Reef, May/June 2015; the hatched bar for the 0–1 m bin indicates those individuals largely remaining within the camera field-of-view for approximately 20 h of time-lapse monitoring at 20-min intervals. (b) Average speed of Acanthaster across the diel cycle (midnight to midnight) derived from time-lapse photography of tracked individuals for starfish trackable for at least 1 h within time-lapse tracking field-of-view (n = 48). Lightly shaded regions indicate crepuscular periods (dawn and dusk) and dark shading indicates night-time during May/June. Data are averages (±s.e.) of all individual speed estimates occurring within bins of 0.01-day fractions (i.e. every 14.4 min from 00.01 to 24.00 h); the number of individuals in each day fraction bin (i.e. number of observations per bin) is shown as a grey trace on lower panel.
Pooled across all individuals, general diel movement patterns of Acanthaster within the two study locations revealed peak rates of movement during crepuscular periods, with moderate movement occurring at night followed by minimal movement during an apparent morning ‘sleep in' phase, and generally low movement throughout daylight hours which ramped-up in the late afternoon (figure 2b). Testing mean movement speeds pooled across all time-lapsed tracked individuals, revealed significantly higher movement at night (6.19 cm h−1 ± 0.11 s.e.; n = 402) compared to day (3.28 cm h−1 ± 0.07 s.e., n = 410) using one-way ANOVA (transformation = speed0.2; F1,85 = 5.96, p = 0.017); notably for crepuscular times (i.e. dawn and dusk), movement was approximately twice that observed at night at a mean speed of 12 ± 0.24 cm h−1, n = 117.
Analysis of the electivity of Acanthaster feeding, as evidenced by scarring of colonies, identified Acropora spp. as the preferred prey of starfish, with a positive and significantly higher mean Ivlev's electivity index than all other coral genera which were non-preferred as evidenced by negative electivity (figure 3a; one-way ANOVA; transformation = log (electivity +1.1); F6,84 = 14.07; p < 0.0001).
Figure 3.
(a) Boxplot of Ivlev's electivity index for different coral genera averaged across replicate time-lapse camera fields-of-view centred on Acanthaster individuals; y-axis indicates range of values revealing preference to avoidance using this index, non-overlapping bars on x-axis indicate significant groupings at alpha less than 0.001 based on Tukeys HSD. (b) Acanthaster displacement versus live cover of Acropora species; locations are shown as different symbols, see legend.
Examination of daily displacement of Acanthaster across factors of location, Acropora cover, depth, and starfish size, revealed significant effects of location and Acropora cover on daily displacement (table 2). The location effect on Acanthaster daily displacement was explained by higher mean displacement for the Swains Reefs (3.85 m day−1 ± 0.68 s.e.) compared to the Lizard Island reefs (2.52 m day−1 ± 0.88 s.e.). Increasing Acropora cover had a negative effect on Acanthaster daily displacement, with a stronger effect observed for starfish at Lizard Island compared to the Swains Reefs (figure 3b).
Table 2.
Linear additive model predicting Acanthaster daily displacement for factors of location, Acropora cover, depth, and starfish size and sites (within location), and the interaction of location by Acropora cover. Log transformation (+0.01) was required to stabilize variance. Values in italics indicate significant effects at α = 0.05.
| source | d.f. | sum Sq | mean Sq | F-value | Pr(>F) |
|---|---|---|---|---|---|
| location | 1 | 20.81 | 20.81 | 12.15 | 0.001 |
| Acropora | 1 | 13.76 | 13.76 | 8.03 | 0.007 |
| depth | 1 | 0.07 | 0.07 | 0.04 | 0.838 |
| size | 1 | 0.01 | 0.01 | 0.00 | 0.945 |
| site (location) | 2 | 3.26 | 1.63 | 0.95 | 0.393 |
| location * Acropora | 1 | 5.20 | 5.20 | 3.04 | 0.088 |
| residuals | 50 | 85.66 | 1.71 |
Logistic regression revealed that more than half of all Acanthaster remained localized within the FOV when preferred live Acropora coral exceeded approximately 23% cover, and three-quarters of all starfish remained localized when preferred live Acropora coral exceeded 43% cover; three-quarters of all starfish emigrated as live Acropora declined to approximately 2% cover (figure 4a). Similarly, logistic regression also revealed Acanthaster to have a high probability of emigration when spending little time feeding locally within the FOV, with three quarters of all starfish emigrating when time spent feeding was approx. 3 h or less (or approx. 15% of the day); whereas only one quarter of all starfish ultimately emigrated the FOV when feeding occurred for more than 7 h or greater than 35% of the day (figure 4b).
Figure 4.

Probability of Acanthaster emigrating from field-of-view relative to (a) cover of preferred Acropora prey, and (b) relative to time spent feeding. Inset values represent the cover of live Acropora coral prey representing a ‘lethal dose' of emigration for the population at LD75, LD50, LD25, respectively. The grey band gives the standard error for predictions about the fitted curve. (Online version in colour.)
(b). Testing movement patterns against a random walk model
Of all the starfish trackable by time-lapse imagery (48 of 58 individuals in total across both locations), 17 paths were composed of at least three moves (12 in Lizard Island, 5 in Swains Reefs) and were thus appropriate for use in the RW analysis. Despite the relatively low number of individuals, this subset of starfish movement was highly localized and was indicative of homing behaviour (figure 5a). Additionally, binomial categorization from time-lapse movies revealed 15 of 58 starfish to observably demonstrate homing behaviour and that the probability of homing behaviour increased with increasing cover of live preferred coral prey (figure 5b). That is, homing behaviour was more likely than not (probability greater than 0.50) when preferred live Acropora coral exceeded 33% cover. Homing occurred with a probability of 0.75 when preferred live coral exceeded 48% cover; while homing was almost certain (probability of 0.95) when preferred coral exceeded 74% cover. Conversely, the probability of non-homing behaviour was greater than 0.75 when preferred coral fell below 18% cover.
Figure 5.

(a) Examination of Acanthaster movement relative to predictions of a random walk model; mean net squared-displacement is calculated over a maximum of five paths from predicted (solid line) and observed (closed circles) movement paths; dashed lines are 95% confidence limits for the predicted net squared-displacement based on a random walk model. Numbers in parentheses above the closed circles indicate the number of individuals observed, with most individuals within each step falling below the predicted random-walk line, i.e. with 94%, 100%, 88%, 100% and 100% of individuals falling below the predicted line for respective steps 1 to 5; indicating highly localized movement. (b) Probability of homing behaviour versus per cent cover of live Acropora sp.; homing was determined by visually inspecting time-lapse movies to determine if starfish returned to the same shelter within the field-of-view, plot as per figure 4 but with inclusion of LD95. (Online version in colour.)
4. Discussion
(a). Diel patterns of starfish movement
Our characterization of Acanthaster movement throughout the diel cycle revealed daily displacement distances to be highly skewed. While all starfish moved during diel observations, the net daily displacement for approximately half of all focal individuals was less than 1 m while 8.6% of all individuals (i.e. 5 of 58 starfish) displaced greater than 10 m. This indicates generally localized movement within reefs, but also distinct behavioural modes characterized as either highly localized versus roaming movement. While our estimates of daily displacement varied across the two GBR locations, overall it was also dependent on the local cover of preferred Acropora prey; a preference that has been widely documented [17,31,32]. Consistent with our findings, Keesing [32] concluded that movement was constrained (about 1 m day−1) in areas of high coral cover but increased to 10 m day−1 in areas with limited coral cover.
Despite the apparent dichotomy in daily displacement between local and roaming movement, Acanthaster are reported to be largely nocturnal (e.g. [19,30,44]). In our study, peak movement was observed during crepuscular periods, with moderate movement occurring at night and low movement throughout daylight hours. While Acanthaster was not exclusively nocturnal, it displayed greater overall activity at night while sheltering was most frequently observed during daylight hours. The predominance of nocturnal movement by Acanthaster spp. may be related to the elevated risk of predation to diurnally active predators, such as large predatory fishes (reviewed in [45]). Notably, however, diel shifts in behavioural modes for Acanthaster were not as marked as the en masse nocturnal emergence typical of obligate homing diadematid sea urchins, which generally remain entirely cryptic and/ or wedge into deep crevices during daylight hours as a predator avoidance strategy [38,46–49].
(b). Homing behaviour
The expression of homing, as distinct from highly local movements, was tested using a random-walk-model. For tracked individuals remaining within time-lapse camera FOV, testing of movement paths relative to expectations of a random-walk-model indicated highly localized behaviour, with distinct ‘outward' movement to, and bouts of feeding upon, preferred Acropora coral (typically possessing a ‘feeding scar') followed by retreat to high-relief ‘home site' shelters. Notably, both branching and tabular Acropora, as described by Ormond et al. [31], provided shelter for Acanthaster during non-feeding phases. Homing behaviour per se is previously unreported for Acanthaster spp., but it is commonly reported among echinoderms (e.g. [38,49,50]). While generalizing our intensive observations from two locations on the GBR to other regions requires caution, we note that coral scars formed as a result of successive feeding bouts by Acanthaster spp., plus the proximate sheltering of starfish, is commonly observed across the Indo-Pacific [13,14,17,29,33], suggesting widespread occurrence of homing behaviour.
The presence of facultative homing is important in understanding the population dynamics of Acanthaster spp., as well as their impacts on local coral assemblages. The homing mode is likely to be highly important in promoting aggregations of adult starfish [51], which are fundamental in enhancing reproductive success, especially among low-density populations [52,53]. Our results show that three-quarters of all starfish will remain localized when preferred live Acropora coral cover exceeds approximately 55%, with approximately half of all starfish likely to emigrate from the local area of the FOV when preferred coral cover is below 28%. This suggests that once preferred coral prey is locally consumed, the likelihood of roaming movements will increase. This could lead to accelerated local extirpation of preferred coral prey and may also promote aggregation of starfish in distinct areas with high abundance of preferred prey corals. Haywood et al. [54] suggested that localized aggregations of A. planci on reefs in northwest Australia resulted from moderate coral bleaching in 2010/11 and 2012/13, which greatly reduced the broad scale abundance of live corals. As such, aggregations of crown-of-thorns starfish formed and persisted in restricted areas of high coral cover, especially where there was an abundance of preferred prey. This highlights the potential for the cumulative and synergistic effects of mass coral bleaching and infestations of crown-of-thorns starfish to result in a catastrophic decline of coral reefs, as well as impacting recovery potential following such disturbances [15].
(c). Implications for starfish control
The diurnal timing of activity and the local prey-dependent switching between modes of behaviour, informs the direct control of starfish populations by culling programmes [55]. Most starfish increased activity during the afternoon/evening when, for homing individuals, the outward phase of movement towards prey occurred. Feeding then predominantly occurred during the night and movement activity again peaked during the homebound inward phase of movement, with reduced activity after sunrise and through the morning. For individuals homing within high relief reef habitat, this indicates that the starfish will be most detectable by divers on the reef surface during the afternoon/evening and during the night, as opposed to the morning.
On the GBR, the most direct and assured way to minimize local densities of crown-of-thorns starfish and associated coral loss is through recurrent culling at fixed locations [28]. Equivalent manual control programs have however had mixed success throughout the Indo-Pacific [56,57] and effectiveness of manual control is critically dependent on detectability of crown-of-thorns starfish [58]. Based on our findings, culling efforts at target sites should focus on late afternoon/evening sessions to increase the local efficiency of starfish culling by divers particularly for structurally complex reefs with Acropora exceeding approximately 30% live cover. Even if it is logistically challenging to undertake culling during periods of peak activity and exposure, as was originally suggested by Vine [55], surveillance activities should be conducted, or indeed concentrated, during these periods to more accurately assess the local abundance of Acanthaster spp.
5. Conclusion
Our results indicate that availability of preferred coral prey is a key determinant of Acanthaster behaviour. Behaviour evidently switches between a localized homing mode centred on preferred coral prey and a roaming prey-searching mode that involves displacements of up to approximately 20 m day−1 across coral reefs. Behavioural switching from homing to roaming movements as outbreaks proceed and corals are consumed, or when live coral becomes limiting following widespread bleaching events (e.g. [15,54]), therefore emerges as a behavioural mechanism capable of explaining diffusion of localized aggregations of Acanthaster during population irruptions.
Supplementary Material
Data accessibility
The datasets supporting this article are available via the following link: https://metadata.imas.utas.edu.au/geonetwork/srv/eng/metadata.show?uuid=27ef761e-cce8-4abc-ae71-b069fdbf5592.
Authors' contributions
S.D.L. conceived and designed the research. S.D.L., Z.-L.C., J.B. and M.S.P. performed field sampling; S.D.L. and E.B.F. analysed data; S.D.L. and M.S.P. wrote the manuscript; all authors provided editorial advice.
Competing interests
We declare we have no competing interests.
Funding
This study was supported by the NESP Tropical Water Quality Hub (M.P.), the ARC Centre of Excellence for Coral Reef Studies (Z.-L.C. and M.P.) and the Australian Research Council (S.D.L.). J.B. participation in this study was supported by the Spanish Ministry of Economy and Competitiveness grant no. EEBB-I-2015-09823.
References
- 1.Schmitz OJ, Krivan V, Ovadia O. 2004. Trophic cascades: the primacy of trait-mediated indirect interactions. Ecol. Lett. 7, 153–163. ( 10.1111/j.1461-0248.2003.00560.x) [DOI] [Google Scholar]
- 2.Babcock RC, Shears NT, Alcala AC, Barrett NS, Edgar GJ, Lafferty KD, McClanahan TR, Russ GR. 2010. Decadal trends in marine reserves reveal differential rates of change in direct and indirect effects. Proc. Natl Acad. Sci. USA 107, 18 256–18 261. ( 10.1073/pnas.0908012107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Graham NA, Wilson SK, Carr P, Hoey AS, Jennings S, MacNeil MA. 2018. Seabirds enhance coral reef productivity and functioning in the absence of invasive rats. Nature 559, 250–253. ( 10.1038/s41586-018-0202-3) [DOI] [PubMed] [Google Scholar]
- 4.Le Roux E, Kerley GI, Cromsigt JP. 2018. Megaherbivores modify trophic cascades triggered by fear of predation in an African savanna ecosystem. Curr. Biol. 28, 2493–2499. ( 10.1016/j.cub.2018.05.088) [DOI] [PubMed] [Google Scholar]
- 5.Ling SD, et al. 2015. Global regime shift dynamics of catastrophic sea urchin grazing. Phil. Trans. R. Soc. B 370, 20130269 ( 10.1098/rstb.2013.0269) [DOI] [Google Scholar]
- 6.Madin EM, Madin JS, Booth DJ. 2011. Landscape of fear visible from space. Sci. Rep. 1, 14 ( 10.1038/srep00014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fourqurean JW, Manuel S, Coates KA, Kenworthy WJ, Smith SR. 2010. Effects of excluding sea turtle herbivores from a seagrass bed: overgrazing may have led to loss of seagrass meadows in Bermuda. Mar. Ecol. Prog. Ser. 419, 223–232. ( 10.3354/meps08853) [DOI] [Google Scholar]
- 8.Estes JA, Tinker MT, Williams TM, Doak DF. 1998. Killer whale predation on sea otters linking oceanic and nearshore ecosystems. Science 282, 473–476. ( 10.1126/science.282.5388.473) [DOI] [PubMed] [Google Scholar]
- 9.Ling SD, Johnson CR. 2009. Population dynamics of an ecologically important range-extender: kelp beds versus sea urchin barrens. Mar. Ecol. Prog. Ser. 374, 113–125. ( 10.3354/meps07729) [DOI] [Google Scholar]
- 10.Pratchett MS, Caballes CF, Rivera-Posada JA, Sweatman HPA. 2014. Causes and consequences of outbreaks of crown-of-thorns starfishes (Acanthaster spp. Oceanogr. Mar. Biol. Annu. Rev. 52, 133–200. [Google Scholar]
- 11.Bruno JF, Selig ER. 2007. Regional decline of coral cover in the Indo-Pacific: timing, extent, and subregional comparisons. PLoS ONE 2, e711 ( 10.1371/journal.pone.0000711) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De'ath G, Fabricius KE, Sweatman H, Puotinen M. 2012. The 27–year decline of coral cover on the Great Barrier Reef and its causes. Proc. Natl Acad. Sci. USA 109, 17 995–17 999. ( 10.1073/pnas.1208909109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kayal M, et al. 2012. Predator crown-of-thorns starfish (Acanthaster planci) outbreak, mass mortality of corals, and cascading effects on reef fish and benthic communities. PLoS ONE 7, e47363 ( 10.1371/journal.pone.0047363) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baird AH, Pratchett MS, Hoey AS, Herdiana Y, Campbell SJ. 2013. Acanthaster planci is a major cause of coral mortality in Indonesia. Coral Reefs 32, 803–812. ( 10.1007/s00338-013-1025-1) [DOI] [Google Scholar]
- 15.Keesing JK, Thomson DP, Haywood MD, Babcock RC. 2019. Two-time losers: selective feeding by crown-of-thorns starfish on corals most affected by successive coral-bleaching episodes on western Australian coral reefs. Mar. Biol. 166, 72 ( 10.1007/s00227-019-3515-3) [DOI] [Google Scholar]
- 16.Pratchett MS, Schenk TJ, Baine M, Syms C, Baird AH. 2009. Selective coral mortality associated with outbreaks of Acanthaster planci L. in Bootless Bay, Papua New Guinea. Mar. Environ. Res. 67, 230–236. ( 10.1016/j.marenvres.2009.03.001) [DOI] [PubMed] [Google Scholar]
- 17.De'ath G, Moran PJ. 1998. Factors affecting the behaviour of crown-of-thorns starfish (Acanthaster planci L.) on the Great Barrier Reef: 2: feeding preferences. J. Exp. Mar. Biol. Ecol. 220, 107–126. ( 10.1016/S0022-0981(97)00100-7) [DOI] [Google Scholar]
- 18.Pratchett MS, Cowan ZL, Nadler LE, Caballes CF, Hoey AS, Messmer V, Fletcher CS, Westcott DA, Ling SD. 2017. Body size and substrate type modulate movement by the western Pacific crown-of-thorns starfish, Acanthaster solaris. PLoS ONE 12, e0180805 ( 10.1371/journal.pone.0180805) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moran PJ. 1986. The Acanthaster phenomenon. Oceanogr. Mar. Biol. Annu. Rev. 24, 379–480. [Google Scholar]
- 20.Black KP, Moran PJ. 1991. Influence of hydrodynamics on the passive dispersal and initial recruitment of larvae of Acanthaster planci (Echinodermata: Asteroidea) on the Great Barrier Reef. Mar. Ecol. Prog. Ser. 69, 55–65. ( 10.3354/meps069055) [DOI] [Google Scholar]
- 21.Uthicke S, Doyle J, Duggan S, Yasuda N, McKinnon AD. 2015. Outbreak of coral-eating crown-of-thorns creates continuous cloud of larvae over 320 km of the Great Barrier Reef. Sci. Rep. 5, 16885 ( 10.1038/srep16885) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hughes TP, et al. 2018. Global warming transforms coral reef assemblages. Nature 556, 492–496. ( 10.1038/s41586-018-0041-2) [DOI] [PubMed] [Google Scholar]
- 23.Matthews SA, Mellin C, MacNeil A, Heron SF, Skirving W, Puotinen M, Devlin MJ, Pratchett M. 2019. High-resolution characterization of the abiotic environment and disturbance regimes on the Great Barrier Reef, 1985–2017. Ecology 100, e02574 ( 10.1002/ecy.2574) [DOI] [PubMed] [Google Scholar]
- 24.Mellin C, et al. 2019. Spatial resilience of the Great Barrier Reef under cumulative disturbance impacts. Glob. Change Biol. 25, 2431–2445. [DOI] [PubMed] [Google Scholar]
- 25.MacNeil MA, Mellin C, Matthews S, Wolff NH, McClanahan TR, Devlin M, Drovandi C, Mengersen K, Graham NA. 2019. Water quality mediates resilience on the Great Barrier Reef. Nat. Ecol. Evol. 3, 620–627. ( 10.1038/s41559-019-0832-3) [DOI] [PubMed] [Google Scholar]
- 26.Hoey J, Campbell ML, Hewitt CL, Gould B, Bird R. 2016. Acanthaster planci invasions: applying biosecurity practices to manage a native boom and bust coral pest in Australia. Manage. Biol. Invasions 7, 213–220. ( 10.3391/mbi.2016.7.3.01) [DOI] [Google Scholar]
- 27.Pratchett MS, Cumming GS. 2019. Managing cross-scale dynamics in marine conservation: pest irruptions and lessons from culling of crown-of-thorns starfish (Acanthaster spp. Biol. Conserv. 238, 108211 ( 10.1016/j.biocon.2019.108211) [DOI] [Google Scholar]
- 28.Westcott DA, Fletcher CS, Kroon FJ, Babcock RC, Plagányi EE, Pratchett MS, Bonin MC. 2020. Relative efficacy of three approaches to mitigate crown-of-thorns starfish outbreaks on Australia's Great Barrier Reef. Sci. Rep. 10, 12594 ( 10.1038/s41598-020-69466-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Great Barrier Reef Marine Park Authority. 2017. Crown-of-thorns starfish control guidelines, 2nd edn Townsville, Australia: GBRMPA. [Google Scholar]
- 30.Weber JN. 1970. Ecological studies of the coral predator Acanthaster planci in the South Pacific. Mar. Biol. 6, 12–17. ( 10.1007/BF00352602) [DOI] [Google Scholar]
- 31.Ormond RFG, Campbell AC, Head SH, Moore RJ, Rainbow PR, Saunders AP. 1973. Formation and breakdown of aggregations of the crown-of-thorns starfish, Acanthaster planci (L.). Nature 246, 167–168. ( 10.1038/246167a0) [DOI] [Google Scholar]
- 32.Keesing JK. 1990. Feeding biology of the crown-of-thorns starfish, Acanthaster planci (L.). Unpublished PhD thesis, James Cook University, Townsville, Australia. [Google Scholar]
- 33.De'ath G, Moran PJ. 1998. Factors affecting the behaviour of crown-of-thorns starfish (Acanthaster planci L.) on the Great Barrier Reef. 1. Patterns of activity. J. Exp. Mar. Biol. Ecol. 220, 83–106. ( 10.1016/S0022-0981(97)00085-3) [DOI] [Google Scholar]
- 34.Hughes TP, et al. 2017. Global warming and recurrent mass bleaching of corals. Nature 543, 373–377. ( 10.1038/nature21707) [DOI] [PubMed] [Google Scholar]
- 35.Ormond RFG, Hanscomb NJ, Beach DH. 1976. Food selection and learning in the crown-of-thorns starfish, Acanthaster planci (L). Mar. Freshwater Behav. Physiol. 4, 93–105. ( 10.1080/10236247609386944) [DOI] [Google Scholar]
- 36.Crowley PH. 1992. Resampling methods for computation-intensive data analysis in ecology and evolution. Annual review of ecology. Evol. System. 23, 405–447. [Google Scholar]
- 37.Dumont CP, Himmelman JH, Robinson SMC. 2007. Random movement pattern of the sea urchin Strongylocentrotus droebachiensis. J. Exp. Mar. Biol. Ecol. 340, 80–89. ( 10.1016/j.jembe.2006.08.013) [DOI] [Google Scholar]
- 38.Flukes EB, Johnson CR, Ling SD. 2012. Forming sea urchin barrens from the inside out: an alternative pattern of overgrazing. Mar. Ecol. Prog. Ser. 464, 179–194. ( 10.3354/meps09881) [DOI] [Google Scholar]
- 39.Kareiva P, Shigesada N. 1983. Analyzing insect movement as a correlated random walk. Oecologia 56, 234–238. ( 10.1007/BF00379695) [DOI] [PubMed] [Google Scholar]
- 40.Turchin P. 1998. Quantitative analysis of movement: measuring and modeling population redistribution in plants and animals. Sunderland, MA: Sinauer Associates. [Google Scholar]
- 41.Conradt L, Roper T. 2006. Nonrandom movement behavior at habitat boundaries in two butterfly species: implications for dispersal. Ecology 87, 125–132. ( 10.1890/05-0413) [DOI] [PubMed] [Google Scholar]
- 42.Zar JH. 1999. Biostatistical analysis. Chennai, India: Pearson Education India. [Google Scholar]
- 43.Manly BF. 2006. Randomization, bootstrap and Monte Carlo methods in biology. Boca Raton, FL: Chapman and Hall/CRC. [Google Scholar]
- 44.Burn D, Matthews S, Caballes CF, Chandler JF, Pratchett MS. 2020. Biogeographical variation in diurnal behaviour of Acanthaster planci versus Acanthaster cf. solaris. PLoS ONE 15, e0228796 ( 10.1371/journal.pone.0228796) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cowan ZL, Pratchett MS, Messmer V, Ling SD. 2017. Known predators of crown-of-thorns starfish (Acanthaster spp.) and their role in mitigating, if not preventing, population outbreaks. Diversity 9, 7 ( 10.3390/d9010007) [DOI] [Google Scholar]
- 46.Ogden JC, Brown RA, Salesky N. 1973. Grazing by the echinoid Diadema antillarum Philippi: formation of halos around West Indian patch reefs. Science 182, 715–717. ( 10.1126/science.182.4113.715) [DOI] [PubMed] [Google Scholar]
- 47.Nelson B, Vance R. 1979. Diel foraging patterns of the sea urchin Centrostephanus coronatus as a predator avoidance strategy. Mar. Biol. 51, 251–258. ( 10.1007/BF00386805) [DOI] [Google Scholar]
- 48.Bernstein B, Williams B, Mann K. 1981. The role of behavioral responses to predators in modifying urchins (Strongylocentrotus droebachiensis) destructive grazing and seasonal foraging patterns. Mar. Biol. 63, 39–49. ( 10.1007/BF00394661) [DOI] [Google Scholar]
- 49.Ling SD, Mahon I, Marzloff MP, Pizarro O, Johnson CR, Williams SB. 2016. Stereo-imaging AUV detects trends in sea urchin abundance on deep overgrazed reefs. Limnol. Oceanogr. Methods 14, 293–304. ( 10.1002/lom3.10089) [DOI] [Google Scholar]
- 50.Purcell SW, Piddocke TP, Dalton SJ, Wang YG. 2016. Movement and growth of the coral reef holothuroids Bohadschia argus and Thelenota ananas. Mar. Ecol. Prog. Ser. 551, 201–214. ( 10.3354/meps11720) [DOI] [Google Scholar]
- 51.Scheibling RE. 1980. Homing movements of Oreaster reticulatus (L.) (Echinodermata: Asteroidea) when experimentally translocated from a sand patch habitat. Mar. Freshwater Behav. Phyisol. 7, 213–223. ( 10.1080/10236248009386982) [DOI] [Google Scholar]
- 52.Ling SD, Johnson CR, Mundy CN, Morris A, Ross DJ. 2012. Hotspots of exotic free-spawning sex: manmade environment facilitates success of an invasive seastar. J. Appl. Ecol. 49, 733–741. ( 10.1111/j.1365-2664.2012.02133.x) [DOI] [Google Scholar]
- 53.Rogers JG, Pláganyi ÉE, Babcock RC. 2017. Aggregation, Allee effects and critical thresholds for the management of the crown-of-thorns starfish Acanthaster planci. Mar. Ecol. Prog. Ser. 578, 99–114. ( 10.3354/meps12252) [DOI] [Google Scholar]
- 54.Haywood MDE, et al. 2019. Crown-of-thorns starfish impede the recovery potential of coral reefs following bleaching. Mar. Biol. 166, 99 ( 10.1007/s00227-019-3543-z) [DOI] [Google Scholar]
- 55.Vine PJ. 1973. Crown of thorns (Acanthaster planci) plagues: the natural causes theory. Atoll. Res. Bull. 166, 1–10. ( 10.5479/si.00775630.166.1) [DOI] [Google Scholar]
- 56.Yamaguchi M. 1986. Acanthaster planci infestations of reefs and coral assemblages in Japan: a retrospective analysis of control efforts. Coral Reefs 5, 23–30. ( 10.1007/BF00302168) [DOI] [Google Scholar]
- 57.Bos AR, Gumanao GS, Mueller B, Saceda-Cardoza MM. 2013. Management of crown-of-thorns sea star (Acanthaster planci L.) outbreaks: removal success depends on reef topography and timing within the reproduction cycle. Ocean Coast. Manage. 71, 116–122. ( 10.1016/j.ocecoaman.2012.09.011) [DOI] [Google Scholar]
- 58.MacNeil MA, Mellin C, Pratchett MS, Hoey J, Anthony KR, Cheal AJ, Moon S. 2016. Joint estimation of crown of thorns (Acanthaster planci) densities on the Great Barrier Reef. PeerJ 4, e2310 ( 10.7717/peerj.2310) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets supporting this article are available via the following link: https://metadata.imas.utas.edu.au/geonetwork/srv/eng/metadata.show?uuid=27ef761e-cce8-4abc-ae71-b069fdbf5592.