Abstract
Living true seals (phocids) are the most widely dispersed semi-aquatic marine mammals, and comprise geographically separate northern (phocine) and southern (monachine) groups. Both are thought to have evolved in the North Atlantic, with only two monachine lineages—elephant seals and lobodontins—subsequently crossing the equator. The third and most basal monachine tribe, the monk seals, have hitherto been interpreted as exclusively northern and (sub)tropical throughout their entire history. Here, we describe a new species of extinct monk seal from the Pliocene of New Zealand, the first of its kind from the Southern Hemisphere, based on one of the best-preserved and richest samples of seal fossils worldwide. This unanticipated discovery reveals that all three monachine tribes once coexisted south of the equator, and forces a profound revision of their evolutionary history: rather than primarily diversifying in the North Atlantic, monachines largely evolved in the Southern Hemisphere, and from this southern cradle later reinvaded the north. Our results suggest that true seals crossed the equator over eight times in their history. Overall, they more than double the age of the north–south dichotomy characterizing living true seals and confirms a surprisingly recent major change in southern phocid diversity.
Keywords: Monachinae, Monachini, phylogeny, fossil, Taranaki, biogeography
1. Introduction
The reinvasion of the oceans by marine tetrapods ultimately resulted in widespread global distributions from limited ancestral areas, as exemplified by true seals (phocids). Found from pole to pole, the biogeography of living true seals reflects their phylogeny: phocines inhabit Arctic and northern temperate oceans, whereas monachines are found in the (sub)tropics and throughout the Southern Ocean [1]. Despite this marked north–south dichotomy, prevailing biogeographic hypotheses propose that monachines originated and diversified alongside phocines in the North Atlantic [2–7]. Evidence for this scenario comes from a broad geographical overlap in their respective fossil records [1,8–10], as well as the exclusive Northern Hemisphere distribution of monk seals, the earliest diverging of the three extant monachine tribes [6,7,11]. Consequently, true seals are hypothesized to have crossed the thermal equatorial barrier a limited number of times, similar to eared seals [7,12–14].
Yet, current interpretations of true seal evolution have relied on fragmentary fossil specimens, and are challenged by recent taxonomic revisions [8,9,15], as well as the lack of a comprehensive phylogenetic framework [1]. In addition, matters are complicated by a historical research bias towards the Northern Hemisphere and global rarity of informative seal fossils [16], which has led to a notable collection gap in Antarctica and Australasia [1,17]. This lack of data is critical, considering that monachines today mostly inhabit the Southern Ocean, and have been present in southern waters since at least the late Miocene [18,19]. Therefore, despite decades of research, the evolution of this major group of marine mammals remains vastly underexplored. Here, we describe a new extinct monk seal from the Pliocene of Taranaki (New Zealand), based on one of the best-preserved and richest samples of true seal fossils worldwide. The new species is the first of its kind from the Southern Hemisphere, and has profound implications for phocid evolution and biogeography.
2. Material and methods
Our phylogenetic analysis includes five outgroups, all living phocids, the recently extinct Caribbean monk seal Neomonachus tropicalis, and 18 extinct operational taxonomic units, selected on the basis that at least 10% of characters could be coded. Our matrix combines 168 morphological characters (four ordered; electronic supplementary material, dataset S3) with 16 nuclear genes and 12 mitochondrial genes (or, where available, the whole mitochondrial sequence; electronic supplementary material, datasets S2, S3). Molecular data were downloaded from GenBank, and alignment and non-coding regions checked in Aliview [20]. Aligned sequences were imported into PartitionFinder 2 [21], and grouped into partitions according to the best model for each gene.
We employed MrBayes v. 3.2.6 [22] on the CIPRES Science Gateway [23] to run a total evidence fossilized birth–death analysis [24], using node and tip dating (with stratigraphic ranges for tips) to improve accuracy [25,26]. The species sampling strategy parameter (SampleStrat) was set to ‘random’, which allows fossils to be sampled serially along the birth–death tree and thus appear as sampled ancestors [24]. Morphological and molecular partitions were unlinked. The analysis was run for 50 million generations (four runs, four chains) sampling every 5000 generations, with convergence achieved at a standard deviation of split frequencies below 0.01. See electronic supplementary material, dataset S3 for specific settings and data. Concatenated tree outputs were produced using the Perl script ‘burntrees.pl’ [27] and rerun as a maximum clade credibility (MCC) tree with mean node depths using TreeAnnotator v. 2.3.2 [28]. The fossilized birth–death model has the potential to recover some taxa as ancestral to younger sister taxa, as indicated by zero-branch lengths. These taxa are interpreted as ancestors in the context of the posterior tree produced by this model.
For the biogeographic analysis, outgroup taxa were pruned from trees in Mesquite v. 3.51 [29] and zero-length branches were set to 0.1 Myr in RStudio v. 1.2.1335 (R v. 3.6.0) using the package ‘ape’ [30]. Analysis was performed in RStudio using the packages ‘BioGeoBEARS’, ‘rexpokit’, ‘cladoRcpp’ and ‘phytools’ [31,32]. See electronic supplementary material, datasets S4–S11 for specific settings. The best model was selected based on likelihood ratio tests and AICc (Akaike information criterion) model weights. The selected model was simplified for figure 3; full models and ancestral area probabilities are provided as electronic supplementary material.
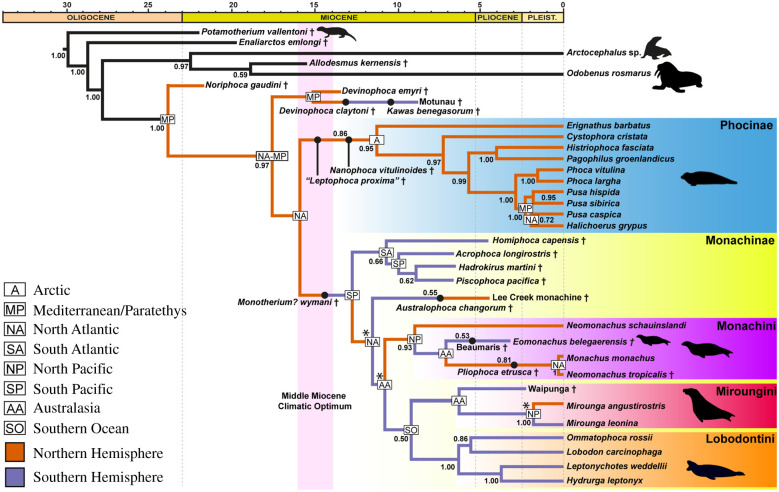
Figure 3.
Total evidence phylogeny (maximum clade credibility tree) of Phocidae, with DIVALIKE + J ancestral range estimation. Black lines indicate outgroup relationships, coloured lines indicate the (simplified) biogeographic estimation and dots on branches represent ancestors (as resolved by the FBD model). Numbers at nodes and on ancestors represent posterior probabilities above 0.5. Daggers represent extinct species. Middle Miocene Climatic optimum range from Song et al. [46]. Biogeographic node labels are only shown where the reconstructed range differs from the previous (basal) node. Ancestral area estimations with a probability of less than 50% are indicated with an asterisk (*). Posterior tree output and the full biogeographic model (including ancestral area support values) are provided as electronic supplementary material. (Online version in colour.)
3. Systematic palaeontology
Carnivora Bowdich, 1821.
Pinnipedia Illiger, 1811.
Phocidae Gray, 1821.
Monachinae Gray, 1869.
Monachini Gray, 1869.
Eomonachus belegaerensis. gen. et sp. nov.
(a). Etymology
From the ancient greek ‘’ (eos) meaning ‘dawn’ and Monachus, the generic name originally applied to all extant monk seals. The species name, ‘belegaerensis’ references the fictional ‘Great Sea’ Belegaer to the west of J. R. R. Tolkien's Middle-Earth [33], paying homage to both the Tasman Sea which the new species inhabited, and the role of New Zealand in bringing Middle-Earth to the big screen.
(b). Holotype
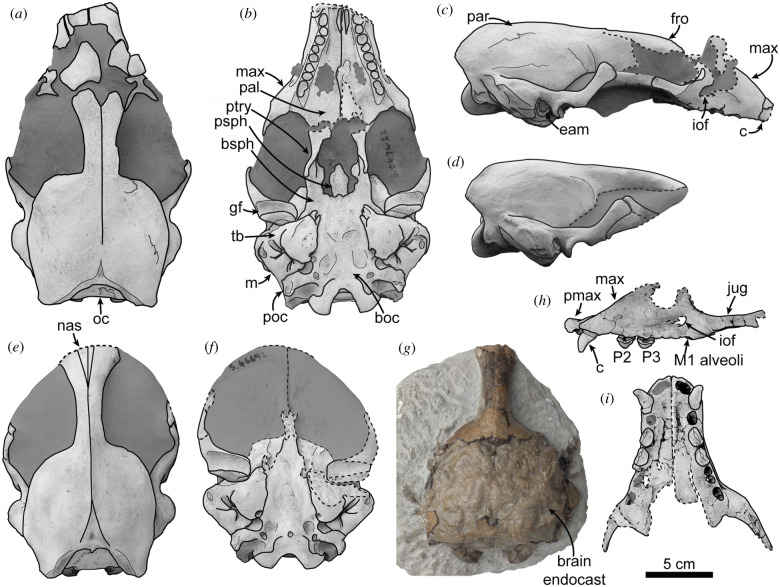
Museum of New Zealand Te Papa Tongarewa (NMNZ; Wellington, NZ) specimen S.047422, comprising a partial dorsoventrally distorted skull missing the anterior portion of the premaxilla, the jugals and the teeth (figure 1a–c; electronic supplementary material, figures S1, S2).
Figure 1.
New species of monk seal, Eomonachus belegaerensis from the Pliocene of Taranaki, New Zealand. Holotype NMNZ S.047422 in (a) dorsal, (b) ventral and (c) right lateral views. Paratype NMNZ S.046692 in (d) right lateral, (e) dorsal and (f) ventral views. Paratype CM 2017.62.7 in (g) dorsal view. Paratype CM 2020.74.1 in (h) left lateral and (i) ventral views. All fossils except CM 2017.62.7 were whitened with ammonium chloride. Darkened regions indicate mudstone, dashed lines indicate broken surfaces. Anatomical abbreviations: boc, basioccipital; bsph, basisphenoid; c, canine; eam, external auditory meatus; fro, frontal; gf, glenoid fossa; iof, infraorbital foramen; jug, jugal; m, mastoid; max, maxilla; nas, nasal; oc, occipital; pal, palatine; par, parietal; P, premolar; M, molar; pmax, premaxilla; poc, paroccipital process; psph, presphenoid; ptry, pterygoid; tb, tympanic bulla. (Online version in colour.)
(c). Paratypes
Paratypes are housed at NMNZ and Canterbury Museum (CM), Christchurch, New Zealand. NMNZ S.046692, partial cranium preserving the interorbital region and posterior zygomatic arches (figures 1d–f, 2g); CM 2017.62.7, partial cranium preserving the interorbital region, right posterior zygomatic arch, a natural brain endocast and the basicranium (figure 1g); CM 2020.74.1, rostrum with canines, P2s and P3s in situ (figures 1h–i, 2e); NMNZ S.047433, partial palate and nasal region; CM 2016.33.9, abraded cranium missing most of the dorsal surface; NMNZ S.047276 dorsoventrally crushed cranium (electronic supplementary material, figures S3–S7).
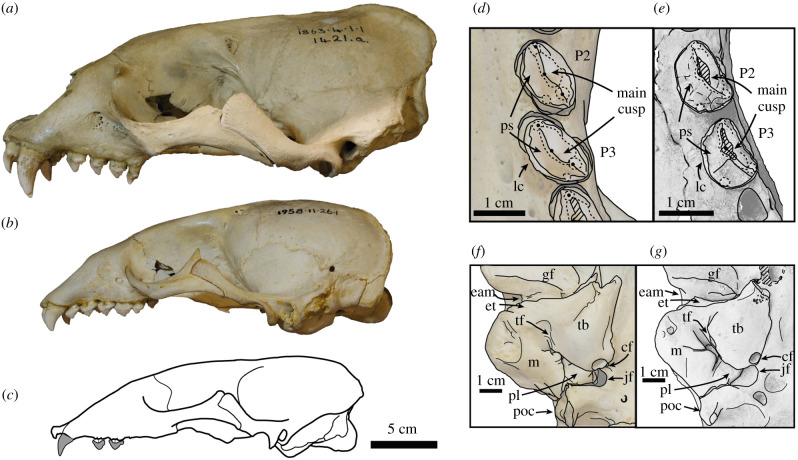
Figure 2.
Eomonachus belegaerensis compared with extant monk seals. Left lateral view of (a) Monachus monachus NHMUK 1863.4.1.1, (b) Neomonachus schauinslandi NHMUK 1958.11.26.1, and line reconstruction of Eomonachus belegaerensis. Postcanines of (d) Monachus monachus NHMUK 1894.7.27.2 and (e) Eomonachus belegaerensis CM 2020.74.1 in ventral view. Right ear regions of (f) Monachus monachus NHMUK 1894.7.27.1 and (g) Eomonachus belegaerensis NMNZ S.046692 in ventral view. Fossils were whitened with ammonium chloride. NHMUK: National History Museum London. Anatomical abbreviations: cf, carotid foramen; eam, external auditory meatus; et, ectotympanic tubercle; gf, glenoid fossa; jf, jugular foramen; lc, lingual cingulum; m, mastoid; P, premolar; M, molar; pl, petrosal lobe; poc, paroccipital process; ps, postcanine shelf; tb, tympanic bulla; tf, tympanohyal fossa. (Online version in colour.)
(d). Type locality and horizon
The holotype and paratypes were collected from sandy mudstone concretions northwest of Waihi Stream on Ohawe and Waihi Beaches, Taranaki, New Zealand. Concretions originate from the Tangahoe Mudstone Formation [34], a mid-Pliocene shallow marine sequence dated to 3.0–3.4 Ma [35].
(e). Diagnosis
Medium-sized monachine characterized by the carotid foramen being visible in ventral view, the mastoid being obscured in dorsal view, subparallel major axes of the glenoid fossa and the presence of four incisors. Differs from all other monachines in having a shortened ectotympanic tubercle (less than half the width of the postglenoid process) that is reflected dorsally to frame the external auditory meatus, a small fovea on the anteroventrolateral surface of the mastoid, a carotid foramen that is medially framed by the basioccipital (also present in Mirounga), and circular postcanines (in occlusal view) with a broad semicircular shelf making up half the crown with a small cusplet distolabial to the main cusp. Further differs from all monachines except Monachini in having a petrosal lobe extending between the mastoid and the tympanic bulla, and postcanines with a dominant main cusp, a broad lingual shelf, and no prominent accessory cusps; from Lobodontini and Miroungini in lacking a posterior extension of the tympanic posteromedial to the carotid foramen, and in having the M1 alveoli aligned with the infraorbital foramen; from Lobodontini in lacking a mastoid lip; from crown Miroungini in lacking a vaulted basicranium, and in having double-rooted P2–M1, separate auricular and external cochlear foramina, and a posteroventrally oriented mastoid; from crown Monachini in having the posterolateral mastoid obscured by the supramastoid ridge in dorsal view, and M1 alveoli that are almost as large as those for P2–P4; from Monachus in lacking a dorsoventrally thickened petrosal lobe; from Neomonachus in lacking an anteroposteriorly thick ectotympanic tubercle, and in having an inflated entotympanic that does not taper medially; and from N. tropicalis in lacking an opening on the posterodorsal surface of the bulla immediately lateral to the carotid foramen.
4. Results and discussion
(a). Fossil record of true seals
Owing to their poor fossil record, extinct true seals are often based on isolated postcranial material [16], and frequently restricted to just a single specimen. In this light, both the quality (skulls instead of postcrania) and abundance of the type series of Eomonachus are unprecedented [16] and substantially improves the historically poor fossil record of Australasia [36–41]. Specimens from Taranaki are often remarkably complete, thanks to being protected inside concretions. The latter aid three-dimensional preservation and increase resistance to erosion, and as such deserve attention as potential sources of exceptional fossil assemblages [42]. The position of Australasian seals in our phylogeny highlights the importance of the region in global marine tetrapod evolution, as also evident in the local diversity of whales, dolphins and seabirds [43–45]. Overall, Eomonachus demonstrates the potential of New Zealand and other underexplored parts of the Southern Hemisphere (e.g. Antarctica) to elucidate fundamental gaps in the evolutionary history of seals.
(b). True seal phylogeny
To determine the evolutionary relationships of our new fossil, we performed the most comprehensive total evidence analysis of true seal phylogeny to date (figure 3; electronic supplementary material). Our results recover both Eomonachus and an ancestral form from the latest Miocene of Beaumaris (Victoria, Australia) [36] as monk seals, and as such deeply nested within crown monachines.
True seals originated around 27.9 Ma, earlier than in most previous studies [7,47]. This was followed by the divergence of Noriphoca, which thus appears to be a stem phocid, rather than a monachine [8]. Other stem taxa include Devinophoca, in line with most previous analyses [48,49]; and Kawas from the Miocene of Argentina, previously thought to be the only known phocine from the Southern Hemisphere [8–10]. Its reidentification here as a stem phocid is significant, as it substantially reduces the geographical overlap between phocines and monachines in the fossil record.
Monachines are monophyletic and comprise five distinct lineages: a basal clade comprising Homiphoca, Acrophoca, Piscophoca and Hadrokirus; a second lineage comprising only the Lee Creek monachine and its (poorly supported) ancestor Australophoca; and crown monachines, including monk seals (Monachini), elephant seals (Miroungini) and lobodontins. Whereas previous analyses interpreted Homiphoca, Acrophoca, Piscophoca and Hadrokirus as either stem lobodontins [9,50,51] or a succession of stem monachines [8,15,49], our results cluster them into a novel, albeit poorly supported, extinct clade. With pinniped phylogeny remaining in flux, we refrain from formally naming this group. Nevertheless, it is tempting to speculate that monachines once included a fourth tribe, which coexisted with the others in the Southern Hemisphere.
Neomonachus schauinslandi is the earliest diverging monk seal, followed by Eomonachus, N. tropicalis and Monachus, with Pliophoca etrusca ancestral to the latter two (figure 3). The paraphyly of Neomonachus mirrors disagreements between previous studies [15,52,53] and probably reflects the limited amount of molecular information for the extinct Caribbean monk seal, N. tropicalis (just a single gene, cytb [53], compared with 28 genes for all other extant pinnipeds in our dataset). Despite the strong morphological arguments in favour of Neomonachus [53], additional molecular work is required to confirm the status of this genus.
(c). First monk seal from the Southern Hemisphere
Until now, monk seals had only been known from the Northern Hemisphere [1], with pre-Pleistocene fossils being restricted to the Mediterranean [15]. This pattern led to the assumption that they were an exclusively northern clade, and had dispersed to the Pacific via the Central American Seaway [15,53]. Eomonachus and its ancestor from Beaumaris now reveal a much broader geographical range, and necessitate a history of monk seal dispersal across the equator. Together, they represent the oldest evidence of monk seals in the world. Like their living relatives, both Eomonachus and the seal from Beaumaris appear to have inhabited warm waters, with local sea surface temperatures during the late Neogene rivalling those of the modern Mediterranean [54,55]. Monk seals appear to be sensitive to temperature fluctuations [56], and as such may have become locally extinct when temperatures cooled at the end of the Pliocene [57,58].
(d). Equatorial crossings in true seal evolution
The best-supported biogeographic model (DIVALIKE + J) implies that stem phocids and monachines crossed the equator eight times over the past 15 Ma (figure 3). This figure is far higher than previously assumed [2–7], and yet may still be an underestimate given the recent description of North Pacific monachine remains resembling fossils from Peru [59]. Even today, southern elephant seals have been noted north of the equator [60]. The apparent ease with which phocids cross the tropics is remarkable [12], and notably contrasts with the single dispersal event that gave rise to southern fur seals and sea lions (otariids) [13,14]. Perhaps their mobility was facilitated by a broad environmental tolerance, as reflected today in the contrast between the tropical monk seals and the polar phocines and lobodontins [1]. By contrast, otariids appear to be tied to cool, highly productive waters, which probably limited opportunities for equatorial dispersal [13]. This suggests that true seals, despite being semi-aquatic, have dispersal capabilities and environmental tolerances approaching those of cetaceans. Overall, this has enabled true seals to conquer more of the world's oceans than any other semi-aquatic tetrapod, such as walruses, eared seals, penguins, auks and living sea turtles.
(e). A southern cradle for monachine evolution
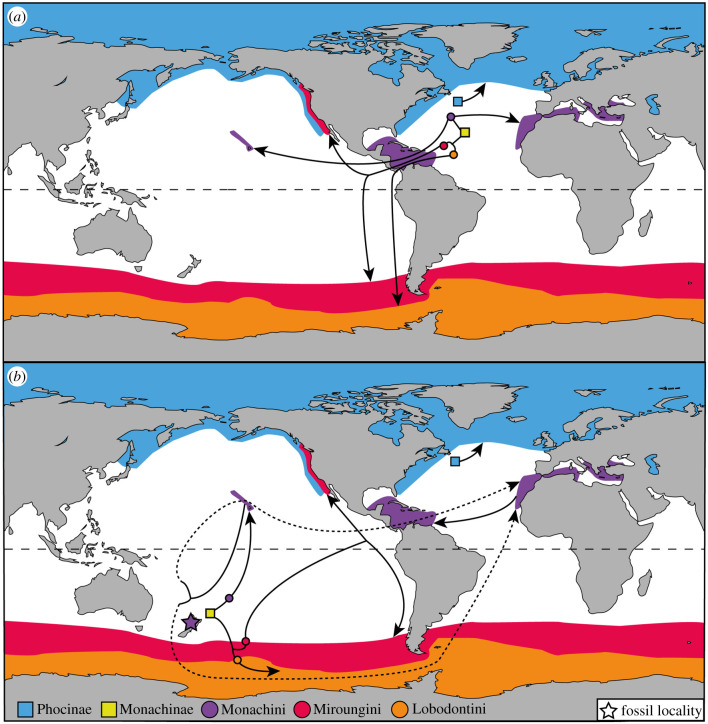
The DIVALIKE + J biogeographic model suggests that phocids originated in the Paratethys–Mediterranean, with phocines and monachines later diverging in the North Atlantic. While phocines continued to disperse entirely within the Northern Hemisphere (especially the Arctic), monachines quickly moved southwards across the equator (figure 3). It currently remains unclear whether this dispersal occurred through the Central American Seaway or via the South Atlantic (figure 4b), as perhaps indicated by the fragmentary remains of Properiptychus from the late Miocene of Argentina [18,19].
Figure 4.
Previous and new biogeographic hypotheses for global crown monachine origins and dispersal. (a) Previous hypothesis from Fulton & Strobeck [7], (b) current hypothesis, as informed by our new fossils and total evidence phylogeny. Extant ranges for phocines and each of the monachine tribes are mapped. Squares represent the divergences of crown Monachinae and Phocinae, respectively, while circles indicate estimated areas of origin of each monachine tribe. Dashed lines indicate alternative monk seal dispersal routes from Australasia to the North Atlantic. (Online version in colour.)
Contrasting with previous biogeographic hypotheses [2–7,11,61] (figure 4a), much of subsequent monachine evolution appears to have unfolded in the Southern Hemisphere. Stem monachines, monk seals, elephant seals and lobodontins all originated here, even though some of them later reinvaded the north (figures 3 and 4b). Rather than in the North Atlantic [7] (figure 4a), elephant seals and lobodontins diverged in the Southern Ocean, suggesting that monachines reached the Antarctic as early as 9.2 Ma. Elephant seals then diversified in Australasia, and crossed into the Northern Hemisphere during the Pleistocene, as previously proposed [41].
Monk seals emerged in Australasia around 10.8 Ma, but then seemingly diversified in the North Pacific. From here, they reinvaded Australasia, before spreading to the North Atlantic around 7.1 Ma, and finally to the Mediterranean around 3 Ma. This scenario corroborates a relatively long history of monachines in the Pacific [59], and questions earlier suggestions that monk seals dispersed westwards from the Atlantic via the Central American Seaway [53,59]. Instead, they either migrated in the opposite direction, or entered the Atlantic by dispersing around the southern tip of South America (figure 4b). Note, however, that this interpretation partly rests on the basal position of N. schauinslandi in our phylogeny. Future changes to this assumption might simplify the biogeographic scenario proposed here, especially if Neomonachus were found to be monophyletic [53] and extant monk seals formed a clade to the exclusion of Eomonachus.
Overall, our biogeographic scenario identifies the north–south split characterizing living phocids as a surprisingly ancient pattern stretching back to around 15 Ma. Both our divergence times and geographical estimates suggest that all three monachine tribes coexisted in the Southern Hemisphere for several million years, even though their fossil records remain relatively sparse and so far only partially overlap [37,41,62]. The disappearance of southern monk seals and stem monachines marks a major turnover in pinniped and, indeed, global marine megafauna diversity [58], during which southern phocids were broadly replaced by fur seals and sea lions [40,62,63]. The fact that the role of monk seals in these profound changes had so far gone unnoticed testifies to the still underexplored nature of the austral fossil record, and its potential to elucidate—and at times rewrite—marine mammal evolution.
Supplementary Material
Acknowledgements
We thank C. de Muizon for advice on specimens and morphological characters; H. L. Richards for advice on morphological characters; R. M. D. Beck, K. Rowe, D. S. Rovinsky, K. M. Thorn and M. Lee for advice on phylogenetic methods; A. Mannering and T. Ziegler for final fossil preparation; and staff at numerous museums for access to collections (T. Elder, T. Schultz, E. Ruigomez, T. Ziegler, K. Roberts, K. Date, R-L. Erickson, K. Rowe, D. Stemmer, R. Miguez, P. Jenkins, C. de Muizon, G. Billet, J. Mead, J. Ososky, M. McGowen, D. Lunde, D. Bohaska, N. Pyenson, S. Godfrey, J. Velez-Juarbe). Fossils came from the rohe of Ngāti Ruanui and Ngāruahine, and were collected and initially prepared by D. Allen, J. Buchanan-Brown, A. Johnson and K. Raubenheimer. We thank the editor, R. W. Boessenecker, and one anonymous reviewer for their constructive comments.
Data accessibility
Files and data used in the phylogenetic and biogeographic analyses are available at Figshare repository (dois: 10.6084/m9.figshare.12762836; 10.6084/m9.figshare.12762845; 10.6084/m9.figshare.12762905; 10.6084/m9.figshare.12762827; 10.6084/m9.figshare.12762851; 10.6084/m9.figshare.12762893; 10.6084/m9.figshare.12762869; 10.6084/m9.figshare.12763028; 10.6084/m9.figshare.12762830; 10.6084/m9.figshare.12762914; 10.6084/m9.figshare.12762917; 10.6084/m9.figshare.12762929; 10.6084/m9.figshare.12763055; 10.6084/m9.figshare.12762887; 10.6084/m9.figshare.12762923). Additional data are available in the electronic supplementary material file. The LSID (life science identifier) for Eomonachus is urn:lsid:zoobank.org:act:42C918B7-EF30-45CC-AF28-5E161415787A, and for Eomonachus belegaerensis is urn:lsid:zoobank.org:act:1BAFB80D-07B9-486C-99C0-5822563505B7.
Authors' contributions
J.P.R., J.W.A., F.G.M., A.R.E. and E.M.G.F. designed research; J.P.R., J.W.A., A.J.D.T., R.P.S. and E.M.G.F. collected data; J.P.R. and F.G.M. analysed data; J.P.R, J.W.A., F.G.M., A.R.E., A.J.D.T., R.P.S. and E.M.G.F. wrote the paper.
Competing interests
We declare we have no competing interests.
Funding
J.P.R. was funded by the Australian Government Research Training Program and a Robert Blackwood Partnership PhD scholarship, with additional support from the Monash Biomedical Discovery Institute, the Department of Anatomy and Developmental Biology and a Monash University Graduate Research Travel Grant.
References
- 1.Berta A, Churchill M, Boessenecker RW. 2018. The origin and evolutionary biology of pinnipeds: seals, sea lions, and walruses. Annu. Rev. Earth Planet. Sci. 46, 203–228. ( 10.1146/annurev-earth-082517-010009) [DOI] [Google Scholar]
- 2.Ray CE. 1976. Geography of phocid evolution. Syst. Biol. 25, 391–406. [Google Scholar]
- 3.Repenning CA, Ray CE, Grigorescu D. 1979. Pinniped biogeography. In Historical biogeography, plate tectonics, and the changing environment (eds Gray J, Boucot AJ), pp. 357–369. Corvallis, OR: Oregon State University Press. [Google Scholar]
- 4.de Muizon C. 1982. Phocid phylogeny and dispersal. Ann. S. Afr. Mus. 89, 175–213. [Google Scholar]
- 5.Deméré TA, Berta A, Adam PJ. 2003. Pinnipedimorph evolutionary biogeography. Bull. Am. Mus. Nat. Hist. 279, 32–76. () [DOI] [Google Scholar]
- 6.Fyler CA, Reeder TW, Berta A, Antonelis G, Aguilar A, Androukaki E. 2005. Historical biogeography and phylogeny of monachine seals (Pinnipedia : Phocidae) based on mitochondrial and nuclear DNA data. J. Biogeogr. 32, 1267–1279. ( 10.1111/j.1365-2699.2005.01281.x) [DOI] [Google Scholar]
- 7.Fulton TL, Strobeck C. 2010. Multiple fossil calibrations, nuclear loci and mitochondrial genomes provide new insight into biogeography and divergence timing for true seals (Phocidae, Pinnipedia). J. Biogeogr. 37, 814–829. ( 10.1111/j.1365-2699.2010.02271.x) [DOI] [Google Scholar]
- 8.Dewaele L, Lambert O, Louwye S. 2018. A critical revision of the fossil record, stratigraphy and diversity of the Neogene seal genus Monotherium (Carnivora. Phocidae). R. Soc. Open Sci. 5, 171669 ( 10.1098/rsos.171669) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dewaele L, Lambert O, Louwye S. 2017. On Prophoca and Leptophoca (Pinnipedia, Phocidae) from the Miocene of the North Atlantic realm: redescription, phylogenetic affinities and paleobiogeographic implications. PeerJ 5, e3024 ( 10.7717/peerj.3024) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cozzuol MA. 2001. A ‘northern’ seal from the Miocene of Argentina: implications for phocid phylogeny and biogeography. J. Vertebr. Paleontol. 21, 415–421. ( 10.1671/0272-4634(2001)021[0415:ANSFTM]2.0.CO;2) [DOI] [Google Scholar]
- 11.Arnason U, Gullberg A, Janke A, Kullberg M, Lehman N, Petrov EA, Väinölä R. 2006. Pinniped phylogeny and a new hypothesis for their origin and dispersal. Mol. Phylogenet. Evol. 41, 345–354. ( 10.1016/j.ympev.2006.05.022) [DOI] [PubMed] [Google Scholar]
- 12.Holt B, Marx FG, Fritz S, Lessard J-P, Rahbek C. 2020. Evolutionary diversification in the marine realm: a global case study with marine mammals. Front. Biogeogr. 12, e45184 ( 10.21425/F5FBG45184) [DOI] [Google Scholar]
- 13.Churchill M, Boessenecker RW, Clementz MT. 2014. Colonization of the Southern Hemisphere by fur seals and sea lions (Carnivora: Otariidae) revealed by combined evidence phylogenetic and Bayesian biogeographical analysis. Zool. J. Linn. Soc. 172, 200–225. ( 10.1111/zoj.12163) [DOI] [Google Scholar]
- 14.Yonezawa T, Kohno N, Hasegawa M. 2009. The monophyletic origin of sea lions and fur seals (Carnivora; Otariidae) in the Southern Hemisphere. Gene 441, 89–99. ( 10.1016/j.gene.2009.01.022) [DOI] [PubMed] [Google Scholar]
- 15.Berta A, Kienle S, Bianucci G, Sorbi S. 2015. A Reevaluation of Pliophoca etrusca (Pinnipedia, Phocidae) from the Pliocene of Italy: phylogenetic and biogeographic implications. J. Vertebr. Paleontol. 35, e889144 ( 10.1080/02724634.2014.889144) [DOI] [Google Scholar]
- 16.Valenzuela-Toro A, Pyenson ND. 2019. What do we know about the fossil record of pinnipeds? A historiographical investigation. R. Soc. Open Sci. 6, 191394 ( 10.1098/rsos.191394) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fordyce RE. 1989. Origins and evolution of Antarctic marine mammals. Geol. Soc. Spec. Publ. 47, 269–281. ( 10.1144/GSL.SP.1989.047.01.20) [DOI] [Google Scholar]
- 18.de Muizon C, Bond M. 1982. Le Phocidae (Mammalia) Miocene de la formation Parana (Entre Rios, Argentine). Bull. Mus. Natl. Hist. Nat. Sect. C Sci. 4, 165–207. [Google Scholar]
- 19.Péreza LM, Iturrería SFG, Griffini M. 2010. Paleoecological and paleobiogeographic significance of two new species of bivalves in the Paraná Formation (late Miocene) of Entre Ríos province, Argentina. Malacologia 53, 61–77. ( 10.4002/040.053.0104) [DOI] [Google Scholar]
- 20.Larsson A. 2014. AliView: a fast and lightweight alignment viewer and editor for large datasets. Bioinformatics 30, 3276–3278. ( 10.1093/bioinformatics/btu531) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lanfear R, Frandsen PB, Wright AM, Senfeld T, Calcott B. 2016. PartitionFinder 2: new methods for selecting partitioned models of evolution for molecular and morphological phylogenetic analyses. Mol. Biol. Evol. 34, 772–773. [DOI] [PubMed] [Google Scholar]
- 22.Ronquist F, et al. 2012. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 61, 539–542. ( 10.1093/sysbio/sys029) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller MA, Pfeiffer W, Schwartz T. 2010. Creating the CIPRES Science Gateway for inference of large phylogenetic trees In 2010 Gateway Computing Environments workshop (GCE), New Orleans, LA, 14 November, pp. 1–8. New York, NY: Institute of Electrical and Electronics Engineers. [Google Scholar]
- 24.Gavryushkina A, Heath TA, Ksepka DT, Stadler T, Welch D, Drummond AJ. 2017. Bayesian total-evidence dating reveals the recent crown radiation of penguins. Syst. Biol. 66, 57–73. ( 10.1093/sysbio/syw060) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O'Reilly JE, Donoghue PC. 2016. Tips and nodes are complementary not competing approaches to the calibration of molecular clocks. Biol. Lett. 12, 20150975 ( 10.1098/rsbl.2015.0975) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barido-Sottani J, Aguirre-Fernández G, Hopkins MJ, Stadler T, Warnock R. 2019. Ignoring stratigraphic age uncertainty leads to erroneous estimates of species divergence times under the fossilized birth–death process. Proc. R. Soc. B 286, 20190685 ( 10.1098/rspb.2019.0685) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nylander JAA. 2011. Burntrees v0. 1.9. Perl script for manipulating MrBayes trees and parameter files.
- 28.Rambaut A, Drummond A. 2002. Treeannotator: MCMC output analysis. Edinburgh, UK: Institute of Evolutionary Biology, University of Edinburgh. [Google Scholar]
- 29.Maddison W, Maddison D.. 2019. Mesquite: a modular system for evolutionary analysis. Version 3.51. 2018.
- 30.Paradis E, Schliep K. 2019. ape 5.0: an environment for modern phylogenetics and evolutionary analyses in R. Bioinformatics 35, 526–528. ( 10.1093/bioinformatics/bty633) [DOI] [PubMed] [Google Scholar]
- 31.Matzke NJ. 2013. BioGeoBEARS: BioGeography with Bayesian (and likelihood) evolutionary analysis in R Scripts. R package, version 02 1: 2013.
- 32.Matzke NJ. 2013. Probabilistic historical biogeography: new models for founder-event speciation, imperfect detection, and fossils allow improved accuracy and model-testing. Front. Biogeogr. 5, 242–248. ( 10.21425/F5FBG19694) [DOI] [Google Scholar]
- 33.Tolkien JRR. 1977. The Silmarillion. London, UK: George Allen and Unwin. [Google Scholar]
- 34.Hay R. 1967. Sheet 7—Taranaki. Geological map of New Zealand. 1:250000. Wellington, New Zealand: Department of Scientific and Industrial Research. [Google Scholar]
- 35.Naish TR, et al. 2005. An integrated sequence stratigraphic, palaeoenvironmental, and chronostratigraphic analysis of the Tangahoe Formation, southern Taranaki coast, with implications for mid-Pliocene (c. 3.4–3.0 Ma) glacio-eustatic sea-level changes. J. R. Soc. N. Z. 35, 151–196. ( 10.1080/03014223.2005.9517780) [DOI] [Google Scholar]
- 36.Fordyce RE, Flannery T. 1983. Fossil phocid seals from the late Tertiary of Victoria. Proc. R. Soc. Vic. 95, 99–100. [Google Scholar]
- 37.King JE. 1973. Pleistocene Ross Seal (Ommatophoca rossi) from New Zealand (Note). N. Z. J. Mar. Freshwater Res. 7, 391–397. ( 10.1080/00288330.1973.9515483) [DOI] [Google Scholar]
- 38.Rule JP, Adams JW, Fitzgerald EMG. In press. Colonization of the ancient southern oceans by small-sized Phocidae: new evidence from Australia. Zool. J. Linn. Soc. zlaa075 ( 10.1093/zoolinnean/zlaa075) [DOI] [Google Scholar]
- 39.Fitzgerald EMG. 2005. Pliocene marine mammals from the Whalers Bluff Formation of Portland, Victoria, Australia. Mem. Mus. Vic. 62, 67–89. ( 10.24199/j.mmv.2005.62.2) [DOI] [Google Scholar]
- 40.Rule JP, Hocking DP, Fitzgerald EMG. 2019. Pliocene monachine seal (Pinnipedia: Phocidae) from Australia constrains timing of pinniped turnover in the Southern Hemisphere. J. Vertebr. Paleontol. 39, e1734015 ( 10.1080/02724634.2020.1734015) [DOI] [Google Scholar]
- 41.Boessenecker RW, Churchill M. 2016. The origin of elephant seals: implications of a fragmentary late Pliocene seal (Phocidae: Miroungini) from New Zealand. N. Z. J. Geol. Geophys. 59, 544–550. ( 10.1080/00288306.2016.1199437) [DOI] [Google Scholar]
- 42.Lyson TR, et al. 2019. Exceptional continental record of biotic recovery after the Cretaceous–Paleogene mass extinction. Science 366, 977–983. ( 10.1126/science.aay2268) [DOI] [PubMed] [Google Scholar]
- 43.Thomas DB, Tennyson AJD, Scofield RP, Heath TA, Pett W, Ksepka DT. 2020. Ancient crested penguin constrains timing of recruitment into seabird hotspot. Proc. R. Soc. B 287, 20201497 ( 10.1098/rspb.2020.1497) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mayr G, Tennyson AJD. 2020. A small, narrow-beaked albatross from the Pliocene of New Zealand demonstrates a higher past diversity in the feeding ecology of the Diomedeidae. Ibis 162, 723–734. ( 10.1111/ibi.12757) [DOI] [Google Scholar]
- 45.Fordyce R. 1991. A new look at the fossil vertebrate record of New Zealand. In Vertebrate palaeontology of Australasia (eds P Vickers-Rich, JM Monaghan, RF Baird, TH Rich), pp. 1191–1316. Lilydale, Australia: Pioneer Design Studio. [Google Scholar]
- 46.Song Y, Wang Q, An Z, Qiang X, Dong J, Chang H, Zhang M, Guo X. 2018. Mid-Miocene climatic optimum: clay mineral evidence from the red clay succession, Longzhong Basin, Northern China. Palaeogeogr. Palaeoclimatol. Palaeoecol. 512, 46–55. ( 10.1016/j.palaeo.2017.10.001) [DOI] [Google Scholar]
- 47.Higdon JW, Bininda-Emonds OR, Beck RM, Ferguson SH. 2007. Phylogeny and divergence of the pinnipeds (Carnivora: Mammalia) assessed using a multigene dataset. BMC Evol. Biol. 7, 216 ( 10.1186/1471-2148-7-216) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Koretsky IA, Rahmat SJ. 2015. A new species of the subfamily Devinophocinae (Carnivora, Phocidae) from the Central Paratethys. Riv. Ital. Paleontol. Stratigr. 121, 31–47. [Google Scholar]
- 49.Paterson RS, Rybczynski N, Kohno N, Maddin HC. 2020. A total evidence phylogenetic analysis of pinniped phylogeny and the possibility of parallel evolution within a monophyletic framework. Front. Ecol. Evol. 7, 1–16. ( 10.3389/fevo.2019.00457) [DOI] [Google Scholar]
- 50.Amson E, de Muizon C. 2014. A new durophagous phocid (Mammalia: Carnivora) from the late Neogene of Peru and considerations on monachine seals phylogeny. J. Syst. Palaeontol. 12, 523–548. ( 10.1080/14772019.2013.799610) [DOI] [Google Scholar]
- 51.Govender R. 2015. Preliminary phylogenetics and biogeographic history of the Pliocene seal, Homiphoca capensis from Langebaanweg, South Africa. Trans. R. Soc. S. Afr. 70, 25–39. ( 10.1080/0035919X.2014.984258) [DOI] [Google Scholar]
- 52.Wyss AR. 1988. On ‘retrogression’ in the evolution of the Phocinae and phylogenetic affinities of the monk seals. Am. Mus. Novit. 2924, 1–38. [Google Scholar]
- 53.Scheel DM, Slater GJ, Kolokotronis SO, Potter CW, Rotstein DS, Tsangaras K, Greenwood AD, Helgen KM. 2014. Biogeography and taxonomy of extinct and endangered monk seals illuminated by ancient DNA and skull morphology. Zookeys 409, 21–33. ( 10.3897/zookeys.409.6244) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Warne MT. 2005. The global Mio–Pliocene climatic equability and coastal ostracod faunas of southeast Australia. Palaeogeogr. Palaeoclimatol. Palaeoecol. 225, 248–265. ( 10.1016/j.palaeo.2005.06.013) [DOI] [Google Scholar]
- 55.Hendy AJ, Kamp PJ, Vonk AJ. 2009. Late Miocene turnover of molluscan faunas, New Zealand: Taxonomic and ecological reassessment of diversity changes at multiple spatial and temporal scales. Palaeogeogr. Palaeoclimatol. Palaeoecol. 280, 275–290. ( 10.1016/j.palaeo.2009.06.010) [DOI] [Google Scholar]
- 56.Whittow G. 1987. Thermoregulatory adaptations in marine mammals: interacting effects of exercise and body mass. A review. Mar. Mammal. Sci. 3, 220–241. ( 10.1111/j.1748-7692.1987.tb00165.x) [DOI] [Google Scholar]
- 57.Boessenecker RW. 2013. A new marine vertebrate assemblage from the Late Neogene Purisima Formation in Central California, part II: Pinnipeds and Cetaceans. Geodiversitas 35, 815–940. ( 10.5252/g2013n4a5) [DOI] [Google Scholar]
- 58.Pimiento C, Griffin JN, Clements CF, Silvestro D, Varela S, Uhen MD, Jaramillo C. 2017. The Pliocene marine megafauna extinction and its impact on functional diversity. Nat. Ecol. Evol. 1, 1100–1106. ( 10.1038/s41559-017-0223-6) [DOI] [PubMed] [Google Scholar]
- 59.Velez-Juarbe J, Valenzuela-Toro AM. 2019. Oldest record of monk seals from the North Pacific and biogeographic implications. Biol. Lett. 15, 20190108 ( 10.1098/rsbl.2019.0108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Valenzuela-Toro AM, Zicos MH, Pyenson ND. 2020. Extreme dispersal or human-transport? The enigmatic case of an extralimital freshwater occurrence of a Southern elephant seal from Indiana. PeerJ 8, e9665 ( 10.7717/peerj.9665) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Koretsky IA, Barnes LG, Csiki Z. 2006. Pinniped evolutionary history and paleobiogeography. In Mesozoic and cenozoic vertebrates and paleoenvironments: tributes to the career of Professor Dan Grigorescu (ed. Csiki Z.), pp. 143–153. Bucharest, Romania: Ed. Ars Docendi. [Google Scholar]
- 62.Avery G, Klein RG. 2011. Review of fossil phocid and otariid seals from the southern and western coasts of South Africa. Trans. R. Soc. S. Afr. 66, 14–24. ( 10.1080/0035919X.2011.564490) [DOI] [Google Scholar]
- 63.Valenzuela-Toro AM, Gutstein CS, Varas-Malca RM, Suarez ME, Pyenson ND. 2013. Pinniped turnover in the South Pacific Ocean: new evidence from the Plio-Pleistocene of the Atacama Desert, Chile. J. Vertebr. Paleontol. 33, 216–223. ( 10.1080/02724634.2012.710282) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Files and data used in the phylogenetic and biogeographic analyses are available at Figshare repository (dois: 10.6084/m9.figshare.12762836; 10.6084/m9.figshare.12762845; 10.6084/m9.figshare.12762905; 10.6084/m9.figshare.12762827; 10.6084/m9.figshare.12762851; 10.6084/m9.figshare.12762893; 10.6084/m9.figshare.12762869; 10.6084/m9.figshare.12763028; 10.6084/m9.figshare.12762830; 10.6084/m9.figshare.12762914; 10.6084/m9.figshare.12762917; 10.6084/m9.figshare.12762929; 10.6084/m9.figshare.12763055; 10.6084/m9.figshare.12762887; 10.6084/m9.figshare.12762923). Additional data are available in the electronic supplementary material file. The LSID (life science identifier) for Eomonachus is urn:lsid:zoobank.org:act:42C918B7-EF30-45CC-AF28-5E161415787A, and for Eomonachus belegaerensis is urn:lsid:zoobank.org:act:1BAFB80D-07B9-486C-99C0-5822563505B7.