Abstract
Background:
Chronic inflammation, innate immune activation, T-cell imbalance and endothelial activation have been linked with lung diseases. We sought to determine whether markers of these pathophysiologic pathways were associated with spirometry and chest CT abnormalities among adolescents living with HIV (ALWH).
Setting:
Coptic Hope Center for Infectious Diseases in Nairobi, Kenya
Methods:
We performed a cross-sectional study of ALWH (10–19 years old). Participants underwent chest CT, spirometry and venipuncture for serum biomarkers. We also collected demographic, anthropometric, T-cell subset, antiretroviral therapy, and exposure data. We compared characteristics and biomarkers by airflow obstruction (post-bronchodilator FEV1/FVC z-score [zFEV1/FVC] < −1.64). We used multivariable linear regression to determine associations of log10-transformed biomarkers and chest CT abnormalities with lower post-bronchodilator zFEV1/FVC (airflow limitation). We performed exploratory principal components analysis on biomarkers, and determined associations of factors with post-bronchodilator zFEV1/FVC and chest CT abnormalities.
Results:
Of 47 participants with acceptable quality spirometry, 21 (45%) were female, median age was 13 years and 96% had perinatally-acquired HIV. Median CD4 was 672 cells/μL. Overall, 28% had airflow obstruction and 78% had a chest CT abnormality; airflow obstruction was associated with mosaic attenuation (p=0.001). Higher endothelial activation (sVCAM-1, sICAM-1), inflammation and innate immune activation (SAA, sTREM-1, sCD163), and T-cell imbalance (lower CD4/CD8) markers were associated with airflow limitation. Factors comprising endothelial and innate immune activation were associated with airflow limitation.
Conclusions:
Endothelial activation, innate immune activation, T-cell imbalance, and chronic inflammation are associated with airflow limitation and obstruction, providing insights into chronic lung disease pathophysiology among ALWH.
Keywords: endothelial activation, innate immune activation, T-cell imbalance, HIV, adolescents, airflow limitation/obstruction
INTRODUCTION
Chronic lung diseases (CLD) are increasingly recognized as important complications of HIV across the lifespan. While chronic obstructive pulmonary disease (COPD) and emphysema have been identified as predominant disease manifestations among adults living with HIV,1,2 processes such as bronchiectasis, obliterative (constrictive) bronchiolitis, and asthma may contribute to CLD among older children and adolescents living with HIV (ALWH).3–5 These CLD manifestations as well as impaired lung function are increasingly documented among ALWH, particularly in low- and middle-income countries (LMICs) where HIV diagnosis and antiretroviral therapy (ART) initiation are often delayed.3–7
A growing body of evidence suggests that chronic inflammation, immune activation and endothelial activation are associated with the comorbid lung, cardiovascular, renal, neurocognitive and metabolic diseases that may be more prevalent in HIV.8–12 ALWH in the US, particularly those with perinatally-acquired HIV, have elevated markers of innate immune activation (soluble CD14 [sCD14]) and endothelial activation (soluble vascular cell adhesion molecule-1 [sVCAM-1]) as well as evidence of endothelial dysfunction based on peripheral arterial tonometry.13 In Kenya, ALWH exhibit higher sCD14 and lower CD4/CD8 ratio despite ART initiation, and these markers are associated with HIV disease progression.14 Importantly, these innate immune activation and T-cell imbalance markers are also associated with mortality among adults in high-income settings.15–18
Nonetheless, pathophysiologic mechanisms underlying the link between HIV and CLD manifestations remain uncertain and have not yet been investigated among ALWH. A recent study in Uganda reported that biomarkers of immune activation and systemic inflammation, specifically soluble CD163 (sCD163) and interleukin-6 (IL-6), respectively, are associated with impaired lung function defined as reduced forced expiratory volume in one second (FEV1) and/or forced vital capacity (FVC) among adults living with HIV,19 similar to associations observed in high-income settings.8,20 Additional studies in high-income countries link elevated sCD14 and a low CD4/CD8 ratio to emphysema among adults living with HIV.21,22
Building on existing evidence, we sought to determine whether circulating markers of chronic inflammation (IL-6, C-reactive protein [CRP], serum amyloid-A [SAA]), innate immune activation (soluble triggering receptor expressed on myeloid cells-1 [sTREM-1], sCD14, sCD163), T-cell imbalance (CD4/CD8 ratio), and endothelial activation (sVCAM-1, intercellular adhesion molecule-1 [ICAM-1], angiopoietin-2 to angiopoietin-1 ratio [Ang-2/Ang-1], endothelin-1) were associated with CLD as defined by spirometry and chest computed tomography (CT) abnormalities among primarily perinatally-infected ALWH in Nairobi, Kenya.
METHODS
Study Design and Cohort
The Biomarkers and REspiratory imaging among Adolescents – The Hope Center Experience (BREATHE) I Study is a cross-sectional study of 50 ALWH that was nested in a larger, prospective study of CLD that included pre- and post-bronchodilator spirometry among people living with HIV in Nairobi, Kenya, which has been described in detail elsewhere.5 Adolescents aged 10–19 years who participated in the parent study were invited to participate in BREATHE I. Individuals with acute respiratory symptoms or infections in the four weeks preceding study enrollment, recent tuberculosis, other illnesses too severe to allow participation or pregnancy were excluded. Fifty-three adolescents were still in clinical follow-up at the Hope Center and met inclusion criteria, of whom 50 agreed to participate. BREATHE I participants underwent chest CT scan and peripheral blood collection for biomarker ascertainment between September 2015 and April 2016. The median time between parent study and BREATHE I study enrollment was 1.8 years (interquartile range [IQR], 1.7 – 2.1). All participants provided written informed consent/assent for participation. Ethics approvals were obtained from the Kenyatta National Hospital/University of Nairobi and the University of Washington (UW).
Clinical Characteristics
All participants underwent standardized questionnaires and clinical assessment to ascertain exposure to CLD risk factors.5 Questionnaires assessed cigarette smoke exposure, indoor combustible fuel use, prior pulmonary infections, respiratory symptoms, age at ART initiation, and ART and co-trimoxazole use. Historical CD4 and CD8 cell counts and confirmation of ART and co-trimoxazole use were obtained from the Hope Center’s clinical research electronic medical record.
Spirometry
Pre- and post-bronchodilator spirometry was performed using an ndd EasyOne spirometer (ndd Medizintechnik AG, Zurich, Switzerland) according to American Thoracic Society standards and only spirometry results of acceptable quality were included in analyses.23 We used African American/black ethnicity Global Lung Initiative (GLI) equations to determine z-scores for FEV1, FVC and the FEV1/FVC ratio.24 A known limitation of existing spirometry reference equations is that sub-Saharan Africa lung function data has not historically been available for inclusion in these equations; nonetheless, the use of GLI reference values for children in sub-Saharan Africa has been recommended, particularly in the evaluation of the FEV1/FVC ratio.25 We defined airflow obstruction – a binary variable – as post-bronchodilator FEV1/FVC z-score (zFEV1/FVC) < −1.64 (i.e., one standard deviation below the population mean), and refer to lower continuous post-bronchodilator FEV1/FVC z-score as airflow limitation.24–26
Chest Computed Tomography without Contrast
All participants underwent non-contrast high-resolution chest CT scanning using a standardized protocol with a radiation dose of <3 mSv. CT exams were acquired during a breath-hold at end inspiration using a multidetector CT scanner calibrated daily (Siemens Definition Edge, Siemens Medical Solutions, Forsheim, Germany) at the adjacent Coptic Hospital. To minimize radiation exposure, we limited z-axis coverage, ensured appropriate patient positioning, tolerated a moderate noise level, and used thyroid and abdominal/pelvic shielding.27 Urine pregnancy tests confirmed negative pregnancy status. CT scans were interpreted by a board-certified radiologist with expertise in thoracic radiology blinded to clinical data. Parenchymal, airway, and pulmonary vascular abnormalities were categorized using established Fleischner Society definitions.28
Biomarker Measurement
Serum aliquots from peripheral venipuncture samples were frozen and stored at −80C until shipment on dry ice to the UW Pulmonary Research Laboratory in Seattle, WA. Samples were tested for endothelial activation, inflammation, and immune activation biomarkers. Notably, the biomarkers we measured are stable at room temperature for ≥24 hours and resistant to up to four freeze-thaw cycles,29–31 and this testing constituted the first thaw for each aliquot used.
Serum concentrations of sTREM-1 and endothelin-1 were measured by ELISA (R&D Systems Duoset kits, Minneapolis, MN) according to manufacturer’s instructions using 50 μL of sample per well. Concentrations were interpolated from 4-parameter-fit standard curves. Serum concentrations of IL-6, CRP, SAA, sCD14, sCD163, sVCAM-1, sICAM-1, Ang-1, and Ang-2 were measured by Meso Scale Discovery assays (Meso Scale Diagnostics [MSD], Rockville, MD) according to manufacturer’s instructions. All assays except for sCD14 and sCD163 were standard MSD assays. The sCD14 and sCD163 assays were custom plates manufactured using a DuoSet kit from R&D. Samples were diluted to fit the dynamic ranges of the assays.
Statistical Analysis
We compared characteristics, including demographic, anthropometric, clinical, and biomarker data across groups with and without post-bronchodilator airflow limitation, using Chi-square, Fisher’s exact or Wilcoxon rank sum tests, as appropriate, among participants with acceptable quality post-bronchodilator spirograms. Biomarker values were log10-transformed to approximate a normal distribution. We excluded the three values that represented outliers based on values that were greater than the sum of 1.5x the interquartile range (IQR) and the third quartile, or less than the first quartile minus 1.5x the IQR.32 Pearson correlation coefficients were then used to evaluate correlations among biomarker values and between biomarkers and post-bronchodilator zFEV1/FVC. Simple and adjusted linear regression models were used to determine associations of biomarkers as well as chest CT abnormalities with post-bronchodilator zFEV1/FVC. We used Fisher’s exact tests to evaluate associations between chest CT abnormality outcomes.
Because many biomarker levels were correlated based on Pearson correlation coefficients and because many of these biomarkers are closely related biologically, we performed an exploratory principal components analysis on these biomarkers to determine if unobservable groupings, or factors, best describe these data, using the factor, pcf option in Stata 14.1. We retained factors with Eigenvalues greater than one, and examined rotated factor loadings for patterns to determine the relevance of each variable in the factor. As no variables had a high uniqueness value (>80%), all examined variables were retained in the factor analysis. Results of the final factor analysis were used to predict values of each factor for each participant. We then used simple and adjusted linear and logistic regression to determine associations of factors with post-bronchodilator zFEV1/FVC and chest CT abnormalities, respectively.
As we detected a strong association between mosaic attenuation on chest CT and lower post-bronchodilator zFEV1/FVC, in secondary analyses, we generated an outcome comprised of adolescents who had both mosaic attenuation and airflow limitation, reflective of the clinical structural and physiologic criteria of obliterative (constrictive) bronchiolitis.33 We used simple and adjusted logistic regression to determine associations of factors with this outcome.
Finally, because perinatal and behavioral acquisition of HIV may differentially impact pathways and development of CLD, we performed a sensitivity analysis excluding the two adolescents with behaviorally-acquired HIV infection.
All analyses were conducted using Stata version 14.1 (StataCorp, College Station, TX). A two-sided p-value <0.05 was considered statistically significant.
RESULTS
Clinical characteristics
All 50 BREATHE I participants underwent chest CT scans and contributed serum samples for biomarker analysis, but only 47 had acceptable quality post-bronchodilator spirometry and are included in Table 1. Median age was 13 years, and 21 (45%) were female. Most (89%) lived in households that used indoor combustible fuels. Approximately one-quarter had a prior pulmonary infection, had stunted growth or were malnourished. Eighty-three percent reported at least one respiratory symptom, the most common being persistent cough. Nearly all (96%) had delayed diagnosis of perinatally-acquired HIV, with ART initiation at a median age of eight years. Sixteen (34%) had WHO HIV Stage 3 or 4, and median nadir CD4 was 269 cells/μL. Importantly, over 90% were currently receiving ART and co-trimoxazole. The median recent CD4 was 672 cells/μL in our cohort. Overall, 13 (28%) had airflow obstruction (post-bronchodilator zFEV1/FVC < −1.64). Participants with airflow obstruction were more likely to be girls or young women and had started ART at a later age than participants without airflow obstruction. Girls/young women initiated ART at a median age of nine years (IQR, 4 – 12) compared to boys/young men who initiated ART at six years (IQR, 4 – 9; p=0.32). Other clinical characteristics and serum biomarkers were similar when stratified by airflow obstruction.
Table 1.
Characteristics of all participants with adequate quality post-bronchodilator (post-BD) spirometry
| Overall (n=47) | Post-BD airflow obstruction [zFEV1/FVC < −1.64], n=13 | No post-BD airflow obstruction [zFEV1/FVC ≥ −1.64], n=34 | p-value | |
|---|---|---|---|---|
| Age, years, median (IQR) | 13 (11 – 15) | 13 (11 – 15) | 13 (11 – 15) | 0.95 |
| Female, n (%) | 21 (45) | 9 (69) | 12 (35) | 0.05 |
| Ever lived with a smoker, n (%) | 16 (34) | 4 (31) | 12 (35) | 1.0 |
| Energy source for heating/cooking‡, n (%) | ||||
| Any combustible fuel | 42 (89) | 12 (92) | 30 (88) | 1.0 |
| Paraffin/kerosene | 20 (43) | 6 (46) | 14 (41) | 1.0 |
| Wood | 9 (19) | 2 (15) | 7 (21) | 0.5 |
| Charcoal | 30 (64) | 7 (54) | 23 (68) | 1.0 |
| Self-reported prior pulmonary infection, n (%) | ||||
| Pneumonia (presumed bacterial) | 17 (36) | 5 (38) | 12 (35) | 1.0 |
| Tuberculosis | 11 (23) | 4 (31) | 7 (21) | 0.47 |
| Respiratory symptoms, n (%) | ||||
| Any respiratory symptom^ | 39 (83) | 12 (92) | 27 (79) | 0.41 |
| Cough | 30 (64) | 10 (77) | 20 (59) | 0.32 |
| Wheeze | 17 (37) | 5 (42) | 12 (35) | 0.74 |
| Stunted growth (ht-for-age Z-score < −2) | 11 (23) | 4 (31) | 7 (21) | 0.47 |
| Malnourished (BMI-for-age Z-score < −2) | 11 (23) | 5 (38) | 6 (18) | 0.25 |
| HIV-related variables* | ||||
| Perinatal HIV acquisition, n (%) | 45 (96) | 12 (92) | 33 (97) | 0.48 |
| ART initiation age (yrs), med (IQR) | 8 (4 – 11) | 11 (7 – 12) | 6 (4 – 10) | 0.04 |
| WHO HIV Clinical Stage 3/4, n (%) | 16 (34) | 5 (38) | 11 (32) | 0.74 |
| Recent CD4, cells/μL, median (IQR) | 672 (453 – 951) | 672 (188 – 840) | 667 (507 – 953) | 0.42 |
| Recent CD8, cells/μL, median (IQR) | 1005 (838 – 1341) | 1090 (916 – 1532) | 977 (829 – 1290) | 0.44 |
| Nadir CD4, cells/μL, median (IQR) | 269 (153 – 471) | 297 (135 – 542) | 263 (207 – 469) | 0.89 |
| Current ART use, n (%) | 44 (94) | 12 (92) | 32 (94) | 0.49 |
| Current co-trimoxazole use, n (%) | 45 (96) | 12 (92) | 33 (97) | 0.28 |
| Serum biomarkers, median (IQR) | ||||
| Inflammation, innate immune activation and T-cell imbalance | ||||
| Interleukin-6 (IL-6), pg/mL | 0.47 (0.25 – 0.95) | 0.57 (0.30 – 0.83) | 0.45 (0.25 – 0.95) | 0.62 |
| C-reactive protein (CRP), μg/mL | 1.15 (0.33 – 3.99) | 1.17 (0.79 – 10.4) | 1.08 (0.33 – 3.32) | 0.26 |
| Serum Amyloid-A (SAA), ng/mL | 1,619 (530 – 5,063) | 3,843 (846 – 10,400) | 1,608 (297 – 3,511) | 0.26 |
| sTREM-1, pg/mL | 50.4 (38.8 – 70.7) | 62.0 (43.5 – 81.8) | 50.4 (34.4 – 64.3) | 0.17 |
| sCD14, ng/mL | 2,344 (2,017 – 2,697) | 2,607 (2,231 – 3,261) | 2,288 (2,004 – 2,492) | 0.11 |
| sCD163, ng/mL | 302 (222 – 482) | 312 (222– 454) | 293 (237 – 482) | 0.79 |
| CD4/CD8 ratio (recent)* | 0.69 (0.35 – 0.94) | 0.63 (0.13 – 0.96) | 0.72 (0.42 – 0.94) | 0.31 |
| Endothelial activation | ||||
| sVCAM-1, ng/mL | 475 (376 – 602) | 474 (405 – 643) | 481 (360 – 577) | 0.46 |
| sICAM-1, ng/mL | 395 (308 – 447) | 419 (351 – 434) | 383 (298 – 447) | 0.26 |
| Angiopoietin-1 (Ang-1), ng/mL | 33.0 (25.3 – 41.1) | 32.9 (17.4 – 36.4) | 33.1 (25.4 – 42.3) | 0.35 |
| Angiopoietin-2 (Ang-2), ng/mL | 9.44 (7.95 – 15.0) | 9.44 (7.95 – 15.6) | 9.95 (7.82 – 14.4) | 0.79 |
| Ang-2/Ang-1 ratio | 0.34 (0.23 – 0.57) | 0.41 (0.24 – 0.56) | 0.34 (0.23 – 0.57) | 0.43 |
| Endothelin-1, pg/mL | 1.38 (1.16 – 1.71) | 1.33 (1.17 – 1.67) | 1.39 (1.16 – 1.72) | 0.67 |
Participants could have reported use of >1 energy source.
Any respiratory symptom includes report of cough, wheeze, breathlessness and/or chest tightness.
CD4 and CD8 T-cell counts missing for one participant with FEV1/FVC z-score < −1.64. Of note, although adherence to ART and HIV-related clinic follow-up is high, a few individuals are transiently not adherent to ART and/or follow-up; non-adherence to ART is often related to side effects or self-initiated “ART breaks”.
Biomarker correlations and factor analysis
Log10-transformed biomarkers, including IL-6, CRP, SAA, sTREM-1, CD4/CD8 ratio, sVCAM-1, sICAM-1 and Ang-2/Ang-1 ratio were highly correlated with one or more other biomarkers (Table S1, Supplemental Digital Content). Exploratory principal components analysis of these biomarkers identified four factors representing markers of chronic inflammation (Factor 1), endothelial and innate immune activation (Factor 2), primarily endothelin-1 (Factor 3), and primarily sCD14 (Factor 4) (Figure 1). For Factor 1, SAA, IL-6, CRP and sCD163 had the highest loadings, followed by sTREM-1, sVCAM-1, sICAM-1, Ang-2/Ang-1 and sCD14. For Factor 2, sCD163, sVCAM-1, sICAM-1 and Ang-2/Ang-1 had the highest factor loadings. Factors 1, 2, 3 and 4 together explained 72% of the total variance.
Figure 1. Heat map distribution demonstrates four independent factors that are differentiated by: 1) chronic inflammation, 2) endothelial and immune activation, 3) endothelin-1, and 4) sCD14.
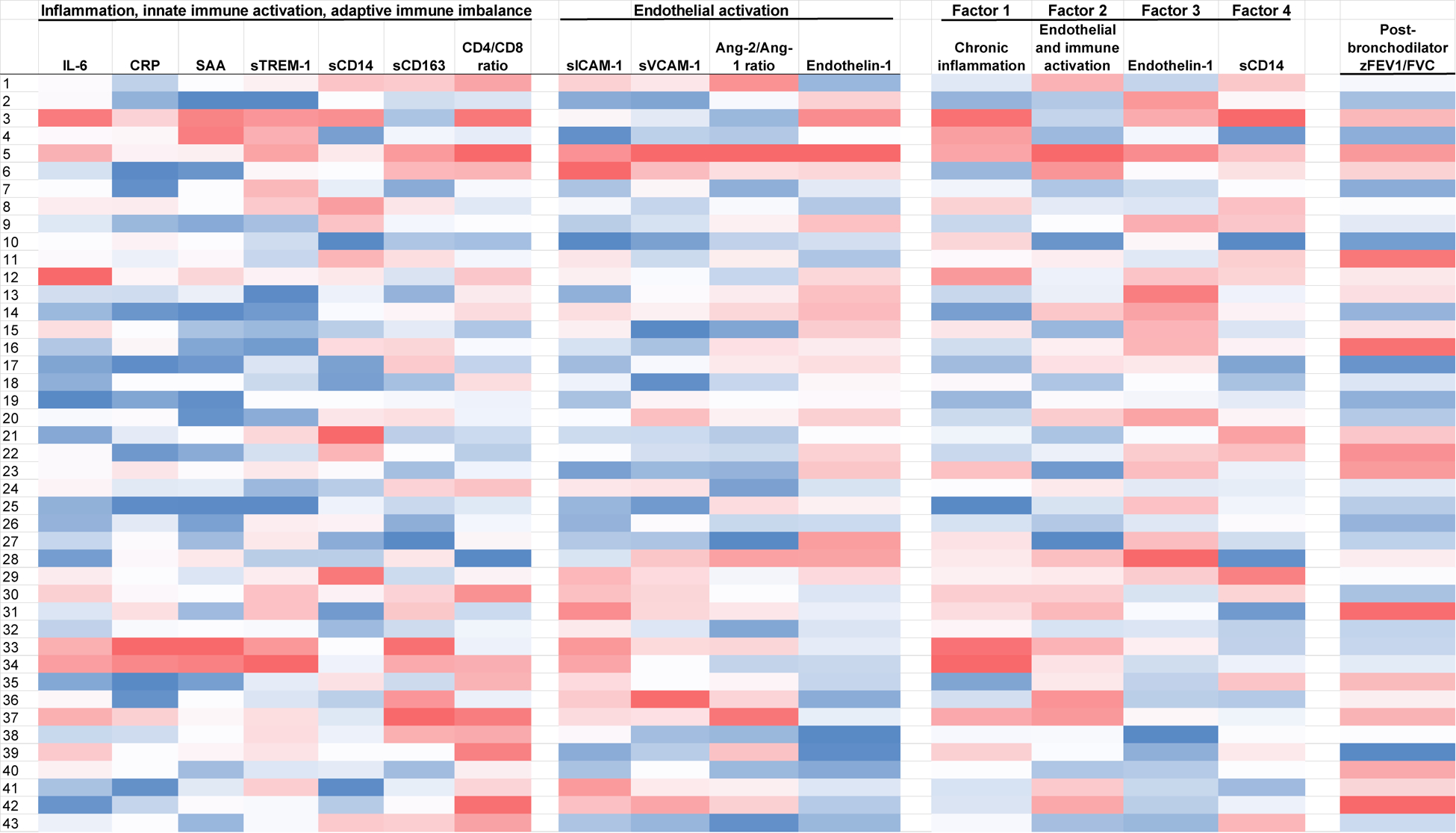
The darker the shade of red, the higher (or worse) the biomarker, derived factor or outcome value is, with the exception of the CD4/CD8 ratio and the zFEV1/FVC ratio, as lower CD4/CD8 and zFEV1/FVC represent worse values. The opposite is true for darker shades of blue.
Biomarker associations with airflow limitation
Several individual biomarkers of innate immune activation, T-cell imbalance, and endothelial activation varied across post-bronchodilator zFEV1/FVC (Figure 2). Higher log10 sVCAM-1 and log10 sICAM-1 values were associated with lower post-bronchodilator zFEV1/FVC (i.e., airflow limitation) in bivariate linear regression models (Table 2). When bivariate associations were adjusted for age and sex, higher log10 SAA, log10 sTREM-1 and log10 sCD163 values were also associated with lower post-bronchodilator zFEV1/FVC, and lower log10 CD4/CD8 was associated with lower post-bronchodilator zFEV1/FVC.
Figure 2. Distribution of log10-transformed biomarkers across post-bronchodilator FEV1/FVC z-score.
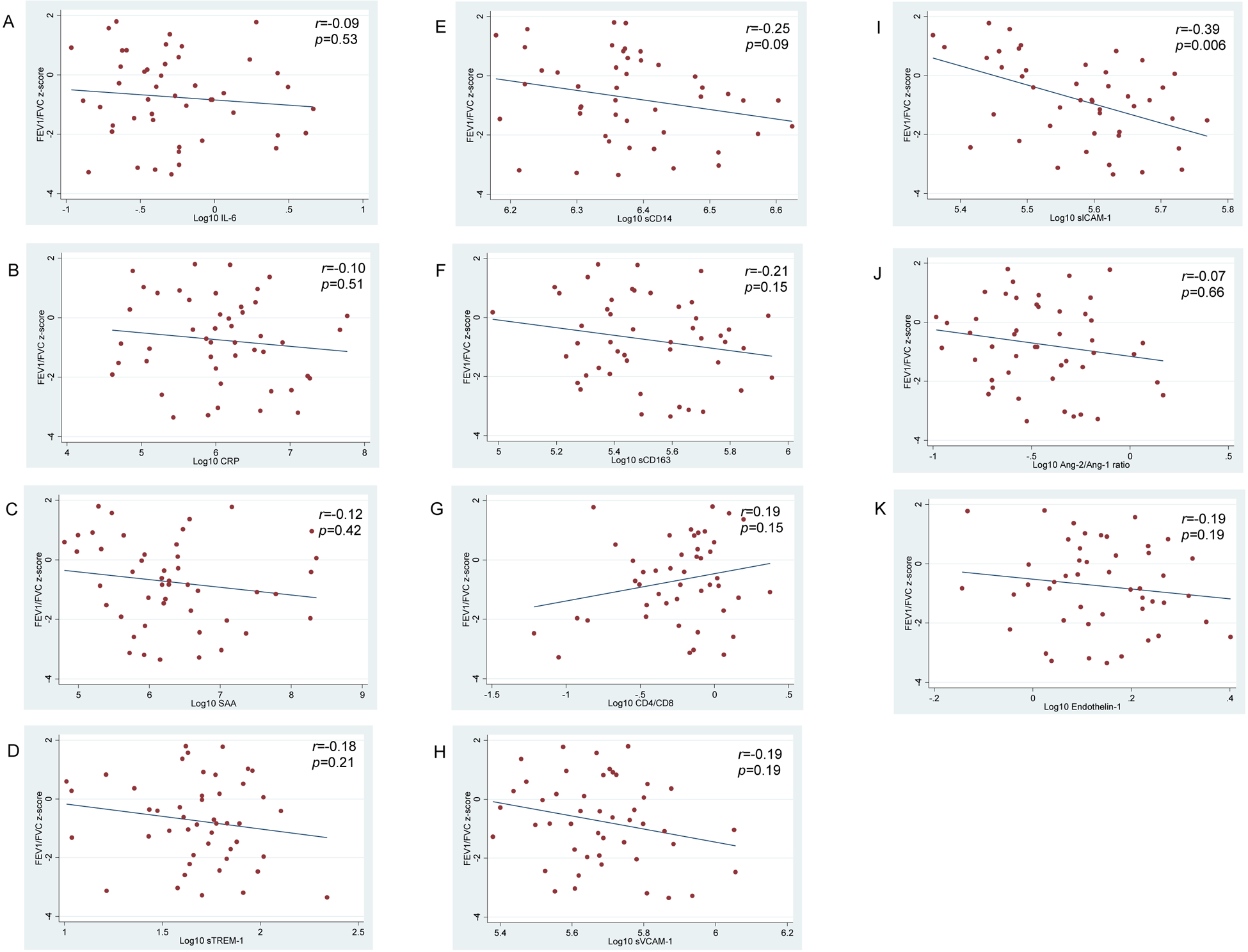
Solid circles represent individual data points for inflammation and immune activation markers (A-G) and endothelial activation markers (H-K); outlier data points for sCD14 and sICAM-1 are not included in the respective figures. Lines through the data points represent the unadjusted linear association of each log10-transformed biomarker with post-bronchodilator FEV1/FVC z-score (i.e., the bivariate associations presented in Table 2). The r and associated p-value represent Pearson correlation coefficients of the log10-transformed biomarker data points with FEV1/FVC z-score.
Table 2.
Biomarker correlates of post-bronchodilator FEV1/FVC z-score*
| Serum biomarkers | Bivariate associations (β, 95% CI) | p-value | Associations adjusted for age and sex (β, 95% CI) | p-value |
|---|---|---|---|---|
| Inflammation, innate immune activation, T-cell imbalance | ||||
| Log10 IL-6 | −0.35 (−1.32 – 0.62) | 0.47 | −0.67 (−1.76 – 0.42) | 0.22 |
| Log10 CRP | −0.23 (−0.74 – 0.28) | 0.37 | −0.34 (−0.82– 0.15) | 0.17 |
| Log10 SAA | −0.26 (−0.72 – 0.20) | 0.26 | −0.46 (−0.90 – −0.02) | 0.04 |
| Log10 sTREM-1 | −0.86 (−2.39 – 0.67) | 0.26 | −1.50 (−2.96 – −0.04) | 0.04 |
| Log10 sCD14 | −3.23 (−6.72 – 0.25) | 0.07 | −2.50 (−6.15 – 1.15) | 0.17 |
| Log10 sCD163 | −1.30 (−2.89 – 0.29) | 0.11 | −1.66 (−3.26 – −0.06) | 0.04 |
| Log10 CD4/CD8 | 0.92 (−0.41 – 2.25) | 0.17 | 1.59 (0.10 – 3.09) | 0.04 |
| Endothelial activation | ||||
| Log10 sVCAM-1 | −2.22 (−4.56 – 0.12) | 0.06 | −3.59 (−6.16 – −1.02) | 0.007 |
| Log10 sICAM-1 | −6.47 (−10.3 – −2.68) | 0.001 | −7.82 (−11.4 – −4.29) | <0.001 |
| Log10 Ang-1 | 1.24 (−0.58 – 3.06) | 0.18 | 1.11 (−0.97 – 3.19) | 0.29 |
| Log10 Ang-2 | −0.66 (−2.64 – 1.32) | 0.51 | −0.85 (−2.84 – 1.13) | 0.39 |
| Log10 Ang-2/Ang-1 | −0.91 (−2.15 – 0.32) | 0.14 | −0.94 (−2.37 – 0.49) | 0.19 |
| Log10 Endothelin-1 | −1.66 (−4.97 – 1.65) | 0.32 | −2.18 (−6.16 – 1.80) | 0.28 |
Linear regression analyses (n=46 for log10 CD4/CD8, n=46 for log10 sCD14 and n=45 for log10 sICAM-1; n=47 for all others)
NOTE: β represents the change in FEV1/FVC z-score associated with a one unit increase in log10-transformed biomarker values
In a multivariable linear regression model including the four identified factors, higher values of Factor 2 (endothelial and innate immune activation) were significantly associated with a lower post-bronchodilator zFEV1/FVC (Table 3A). The association of higher values of Factor 4 (sCD14) with lower post-bronchodilator zFEV1/FVC approached statistical significance.
Table 3.
Biomarker factor correlates of airflow limitation (3A), mosaic attenuation (3B), and combined mosaic attenuation and airflow obstruction outcome [representing obliterative bronchiolitis] (3C)
| Serum biomarkers* | Multivariable model (β, 95% CI) | p-value | Multivariable model adjusted for age and sex (β, 95% CI) | p-value |
|---|---|---|---|---|
| Factor 1 – Inflammation (SAA, IL-6, CRP, sTREM-1) | −0.15 (−0.50 – 0.21) | 0.41 | −0.34 (−0.77 – 0.10) | 0.13 |
| Factor 2 – Endothelial and innate immune activation (sCD163, sVCAM-1, sICAM-1, Ang-2/Ang-1 ratio) | −0.42 (−0.78 – −0.06) | 0.02 | −0.56 (−0.92 – −0.21) | 0.003 |
| Factor 3 – Endothelin-1 | −0.13 (−0.53 – 0.27) | 0.51 | −0.12 (−0.56 – 0.31) | 0.57 |
| Factor 4 – sCD14 | −0.36 (−0.72 – 0.01) | 0.06 | −0.30 (−0.60 – 0.00) | 0.05 |
| 3B. Biomarker factor correlates of mosaic attenuation, multivariable logistic regression analyses | ||||
| Serum biomarkers* | Multivariable model (OR, 95% CI) | p-value | Multivariable model adjusted for age and sex (OR, 95% CI) | p-value |
| Factor 1 – Inflammation (SAA, IL-6, CRP, sTREM-1) | 1.09 (0.60 – 1.98) | 0.78 | 0.78 (0.39 – 1.56) | 0.49 |
| Factor 2 – Endothelial and innate immune activation (CD163, sVCAM-1, sICAM-1, Ang-2/Ang-1 ratio) | 1.24 (0.68 – 2.29) | 0.48 | 1.11 (0.58 – 2.12) | 0.75 |
| Factor 3 – Endothelin-1 | 1.02 (0.55 – 1.91) | 0.94 | 1.19 (0.59 – 2.41) | 0.63 |
| Factor 4 – sCD14 | 1.56 (0.84 – 2.89) | 0.16 | 1.84 (0.93 – 3.64) | 0.08 |
| 3C. Biomarker factor correlates of combined mosaic attenuation and airflow obstruction outcome (representing obliterative bronchiolitis), multivariable logistic regression analyses | ||||
| Serum biomarkers* | Multivariable model (OR, 95% CI) | p-value | Multivariable model adjusted for age and sex (OR, 95% CI) | p-value |
| Factor 1 – Inflammation (SAA, IL-6, CRP, sTREM-1) | 1.22 (0.60 – 2.48) | 0.58 | 1.07 (0.44 – 2.60) | 0.87 |
| Factor 2 – Endothelial and innate immune activation (CD163, sVCAM-1, sICAM-1, Ang-2/Ang-1 ratio) | 1.51 (0.60 – 3.80) | 0.39 | 1.72 (0.75 – 3.92) | 0.20 |
| Factor 3 – Endothelin-1 | 1.31 (0.62 – 2.77) | 0.47 | 2.05 (0.68 – 6.18) | 0.74 |
| Factor 4 – sCD14 | 3.42 (1.25 – 9.30) | 0.02 | 4.47 (1.50 – 13.4) | 0.007 |
Log10-transformed biomarkers were used for factor analyses
NOTE: n = 43 for these multivariable analyses; airflow limitation = lower post-bronchodilator FEV1/FVC z-score; airflow obstruction = post-bronchodilator FEV1/FVC z-score < −1.64
Biomarker and airflow limitation and obstruction associations with chest CT abnormalities
Among the 50 adolescents who underwent chest CT (including the three with poor quality spirometry data), at least one abnormal finding was present in 78% (n=39). The most common findings included: mosaic attenuation (n=24), bronchial wall thickening (n=10), groundglass opacities (n=10), any emphysema (n=5), tree-in-bud opacities (n=5), and bronchiectasis (n=4) (Figure S1, Supplemental Digital Content). Importantly, mosaic attenuation was associated with lower post-bronchodilator zFEV1/FVC in unadjusted and adjusted bivariate analyses (β, −1.32, 95% confidence interval [CI] −2.05 to −0.60, p=0.001). Bronchiectasis was also associated with lower post-bronchodilator zFEV1/FVC (−1.49, 95% CI −2.59 to −0.38, p=0.01). The four adolescents with bronchiectasis also had mosaic attenuation, and these two radiographic findings were associated (p=0.046). We did not detect any additional associations of radiographic findings with mosaic attenuation.
Because mosaic attenuation was observed in nearly half of our cohort and the absolute numbers of other CT abnormalities were relatively few, we conducted secondary analyses of predictors of this outcome. While we detected no significant associations in these analyses, the association of Factor 4 (sCD14) with mosaic attenuation neared significance (Table 3B). In additional secondary analyses of associations with an outcome comprising mosaic attenuation combined with post-bronchodilator airflow obstruction that may reflect obliterative bronchiolitis (n=10), Factor 4 was a significant predictor, but confidence intervals were wide (Table 3C).
Sensitivity Analysis
Conclusions were largely unchanged in analyses excluding the two adolescents with behaviorally-acquired HIV despite more recent HIV acquisition and lower CD4 counts than those with perinatally-acquired HIV.
DISCUSSION
Our study is the first to report that circulating biomarkers of endothelial activation, innate immune activation and T-cell imbalance with related chronic inflammation are associated with CLD manifestations among ALWH. Specifically, log10-transformed sVCAM-1, sICAM-1, sCD163, sTREM-1, CD4/CD8 ratio and SAA were associated with lower post-bronchodilator zFEV1/FVC, representing airflow limitation. When these and other related biomarkers were incorporated into an exploratory factor analysis, associations of endothelial and innate immune activation with the degree of airflow limitation persisted, representing potential mechanistic pathways. Additionally, the derived factor primarily comprising sCD14 was associated with combined mosaic attenuation on chest CT and post-bronchodilator airflow obstruction, which may be consistent with obliterative (constrictive) bronchiolitis.
Chronic HIV infection has been linked with endothelial activation, chronic inflammation and immune activation despite viral suppression.34 Plasma levels of sICAM-1, sVCAM-1, and the Ang-2/Ang-1 ratio increase soon after acquisition of HIV,35 and elevated plasma sVCAM-1 and Ang-2 concentrations are associated with greater risk for HIV disease progression and mortality.35–37 Among ALWH, those individuals who acquired HIV perinatally have higher levels of innate immune activation compared to those with behaviorally-acquired HIV.13 In adults living with HIV, chronic inflammation associated with innate immune activation has been shown to be associated with HIV disease progression and development of comorbid illnesses, including CLDs, such as COPD and emphysema, as well as lung function impairment.21,38–41 Our data suggest that these mechanisms may also impact the development of CLD among ALWH, as airflow limitation and obstruction are common.
Although ALWH likely manifest CLD subtypes that differ from adults living with HIV, the associations of endothelial and innate immune activation pathways with post-bronchodilator airflow limitation are in line with some of the commonly proposed mechanisms of obstructive lung disease and impaired lung function identified in adults. For instance, an elevated Ang-2/Ang-1 ratio, an indicator of endothelial activation, has been detected in COPD.42 Additionally, HIV-induced endothelial activation may result in pulmonary vascular injury, as HIV membrane proteins stimulate activation of human pulmonary artery endothelial cells in vitro with release of the endothelial activation marker, sVCAM-1.43 The innate immune activation biomarker, sCD163, is associated with worse lung function and administrative codes for incident CLD in adults living with HIV.8,19,44 While studies in adults living with HIV have linked chronic inflammation, predominantly measured by IL-6 and CRP, with impaired lung function and COPD,8,19,20,45 we did not identify an association of these individual markers or the derived factor representing inflammation with either spirometric or radiographic manifestations of CLD in our cohort; notably, median IL-6 levels in our cohort of ALWH approximated normal values. We did, nonetheless, find that higher SAA, an inflammatory marker, was associated with airflow limitation. Interestingly, we found that the derived factor comprising primarily sCD14 was associated with the radiographic abnormality mosaic attenuation. Among adults living with HIV, elevated sCD14 is associated with radiographic emphysema.21
Together with existing data, our findings suggest that endothelial and innate immune activation may represent common pathways of CLD development in adolescents and adults living with HIV, although it is not clear how or whether these similar pathways may manifest as different CLD subtypes. Endothelial cell activation sets the stage for and leads to a number of processes including innate immune activation and chronic inflammation.36,46,47 Activated endothelial cells recruit pericytes, which are mesenchymal cells that surround endothelial cells along microvessels, including in the pulmonary microvasculature. Pericytes are involved in microvascular homeostasis and remodeling, and signal to endothelial cells via the Ang/Tie2 receptor axis.48 Ang-2 overexpression is associated with pericyte loss,48 and high expression of ICAM-1 is associated with gaps between pericytes.49 Higher sICAM-1 was associated with lower post-bronchodilator zFEV1/FVC (i.e., airflow limitation) in our cohort. The results of our factor analysis were also consistent with this potential pathway, as the factor that included elevated sICAM-1, Ang-2/Ang-1 and other markers of endothelial and innate immune activation was strongly associated with airflow limitation. Our results also reflect the complex interplay between inflammation, endothelial activation and innate immune activation, as individual biomarkers of these mechanisms and factors encompassing a plurality of these biomarkers were associated with airflow limitation. A growing body of evidence suggests that pericytes, together with endothelial cells, are necessary for mediating microvascular integrity, and that perturbations of the pericyte-endothelial cell relationship, along with chronic inflammation, may be related to development of CLDs such as asthma, COPD and fibrotic lung diseases,8,48,50,51 each of which may play a role in the lung health of ALWH.
Post-bronchodilator airflow obstruction was identified in 28% of our cohort and may reflect several CLD subtypes that involve the large and small airways. While asthma likely plays a role in CLD among ALWH, poor reversibility of airflow obstruction as observed in our cohort is unexpected in classic asthma, although it may be a feature of longstanding or poorly controlled asthma.52 Endothelial activation and inflammation have been linked with asthma, but whether these relationships have unique implications in HIV is unknown.53–55 Post-bronchodilator airflow obstruction is also in line with forms of chronic obstructive pulmonary disease (COPD), asthma-COPD overlap,56,57 bronchiectasis and obliterative bronchiolitis; however, only 8% of ALWH in our cohort had radiographic evidence of bronchiectasis. As post-bronchodilator airflow obstruction was associated with mosaic attenuation, we posit that obliterative bronchiolitis represents the most likely etiology of CLD to explain these findings.6
In secondary analyses, innate immune activation, potentially driven by monocyte/macrophage activation (manifested as elevated sCD14 as the primary component of Factor 4) was associated with an outcome encompassing concurrent spirometric post-bronchodilator airflow obstruction and radiographic mosaic attenuation, supporting the concept that these strongly associated physiologic and structural manifestations of CLD may share a common mechanistic pathway. Importantly, monocyte/macrophage activation was also associated with the individual components of airflow limitation and mosaic attenuation, and these detected associations neared statistical significance (p=0.05 and p=0.08). Over the past decade, data have emerged that highlight the potential role of obliterative bronchiolitis, an inflammatory disorder that results in fibrotic narrowing of the small airways, as an important etiology of CLD among ALWH.3,5,6,33 Spirometry findings are characterized by airflow limitation that is minimally, if at all, responsive to bronchodilator therapy, and chest CT findings demonstrate evidence of air trapping on exhalation, which can manifest as interspersed areas of varying or mosaic attenuation on inspiratory scans most consistent with obliterative bronchiolitis.
The pathophysiology of obliterative bronchiolitis remains incompletely understood, particularly among ALWH. Development of obliterative bronchiolitis has been linked to viral respiratory infections, long-standing exposure to high levels of air pollutants, gastroesophageal reflux and chronic rejection after stem-cell or lung transplantation.33 Our study was not powered to examine the role of prior pulmonary infections, air pollution exposure, or other potential contributors such as delayed ART initiation. However, higher values of Factor 4, primarily driven by innate immunity activation (sCD14), were strongly associated with the concurrent physiologic and structural manifestations that may represent obliterative bronchiolitis. Higher sCD14 levels are associated with lower FEV1, lower diffusing capacity and emphysema in adults living with HIV.8,21,22 Profound innate immune activation along with low CD4/CD8 persist despite ART among Kenyan perinatally HIV-infected children and adolescents.14 In our cohort of Kenyan ALWH who primarily acquired HIV perinatally, the CD4/CD8 ratio was low and innate immune activation was associated with CLD manifestations despite long-term ART and CD4 T-cell reconstitution/preservation, suggesting that HIV acquisition during critical phases of lung and immune development may be a driver of both spirometric and radiographic manifestations of CLD.
Our study has several limitations. There was a time lag between spirometry and subsequent chest CT and serum samples, but among children and adolescents with CLD in medical care as these adolescents were, little deviation in spirometry results is expected in up to 3-year follow-up.58 Nonetheless, we analyzed spirometry and imaging results separately for our main analyses. Additionally, CTs were interpreted using visual assessment by one radiologist, and reproducibility of readings was not assessed. We lacked an HIV-uninfected comparison group, precluding our ability to make comparisons with healthy adolescents, including for biomarker values. Our sample size was small and only included two adolescents with behaviorally-acquired HIV. Including data from these two adolescents did not substantially alter associations and generally improved power to detect associations. We still lacked sufficient statistical power to account for potential confounders and covariates. Nonetheless, we detected plausible associations of biomarkers with spirometry and imaging abnormalities in our cross-sectional analyses. Future longitudinal analyses would be informative, as rising biomarkers of inflammation may be associated with occurrence of non-AIDS-defining events.59 A notable strength of our study is that we performed low-dose chest CT in all participants rather than only symptomatic adolescents or those with abnormal spirometry.
In conclusion, persistent airflow obstruction and limitation as well as mosaic attenuation, were common in our cohort of primarily perinatally HIV-infected adolescents in Nairobi, Kenya. Our findings that systemic biomarkers of endothelial activation, innate immune activation and related chronic inflammation are associated with the degree of post-bronchodilator airflow limitation both individually and when analyzed in combination as derived factors, and that a derived factor potentially representing monocyte/macrophage activation is linked with manifestations of obliterative bronchiolitis may provide critical insights into CLD development and pathophysiology among ALWH. These pathways may offer novel targets for the prevention and mitigation of the CLD burden in this population.60
Supplementary Material
ACKNOWLEDGEMENTS:
We thank the patients, parents and caregivers for participating in this study. We remain indebted to the staff and clinicians of the Coptic Hope Center for Infectious Disease in Nairobi, Kenya for providing high quality HIV care and creating an environment conducive to clinical research, and to the Coptic Hospital Department of Radiology staff, technologists and physicians. We would also like to thank the study nurses, research personnel and data management teams of the Nairobi and Seattle-based offices of the University of Washington Teaching, Research and Expert Education (TREE) program; we are especially grateful for the dedication and tireless efforts of Norah Matheka, Grace Achieng, Dr. Sameh Sakr and Bishop Paul Yowakim. We are also thankful to Dr. Mark Wurfel, Lara Lovelace-Macon and Deirdre Ducken for their generous assistance in analyzing the biomarkers in the UW Pulmonary Research Laboratory. We also thank Dr. Jim Stout and Spirometry 360 for providing quality assurance and quality control for spirometry testing.
Footnotes
Parts of the Data were Presented at: The International AIDS Society Pediatric Workshop, Paris, France, July 2017
Conflicts of Interest and Source of Funding: E.F.A. has received the following funding for research reported in this publication: National Heart, Lung, And Blood Institute of the National Institutes of Health (F32 HL123031); INTERSECT-Ellison Fellowship; University of Washington Thomas Francis, Jr. Global Health Travel Fellowship; University of Washington Division of Pulmonary and Critical Care Medicine Lakshminarayan Fellow Fund Endowment Grant; Thrasher Research Fund Early Career Award (TRF12792). S.M.G. was supported by the Robert W. Anderson Endowed Professorship in Medicine. For the remaining authors, none were declared.
REFERENCES
- 1.Crothers K, Huang L, Goulet JL, et al. HIV infection and risk for incident pulmonary diseases in the combination antiretroviral therapy era. Am J Respir Crit Care Med. 2011;183:388–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crothers K, Thompson BW, Burkhardt K, et al. HIV-associated lung infections and complications in the era of combination antiretroviral therapy. Proc Am Thorac Soc. 2011;8:275–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferrand RA, Desai SR, Hopkins C, et al. Chronic lung disease in adolescents with delayed diagnosis of vertically acquired HIV infection. Clin Infect Dis. 2012;55:145–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mwalukomo T, Rylance SJ, Webb EL, et al. Clinical Characteristics and Lung Function in Older Children Vertically Infected With Human Immunodeficiency Virus in Malawi. J Pediatric Infect Dis Soc. 2016;5:161–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Attia EF, Maleche-Obimbo E, West TE, et al. Adolescent age is an independent risk factor for abnormal spirometry among people living with HIV in Kenya. AIDS. 2018;32:1353–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Desai SR, Nair A, Rylance J, et al. Human Immunodeficiency Virus-Associated Chronic Lung Disease in Children and Adolescents in Zimbabwe: Chest Radiographic and High-Resolution Computed Tomographic Findings. Clin Infect Dis. 2018;66:274–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Githinji LN, Gray DM, Hlengwa S, et al. Longitudinal changes in Spirometry in perinatally HIV-infected South African adolescents on antiretroviral therapy. Clin Infect Dis. 2019. April 2 pii: ciz255. doi: 10.1093/cid/ciz255. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fitzpatrick ME, Nouraie M, Gingo MR, et al. Novel relationships of markers of monocyte activation and endothelial dysfunction with pulmonary dysfunction in HIV-infected persons. AIDS. 2016;30:1327–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deeks SG, Tracy R, Douek DC. Systemic effects of inflammation on health during chronic HIV infection. Immunity. 2013;39:633–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zanni MV, Awadalla M, Toribio M, et al. Immune Correlates of Diffuse Myocardial Fibrosis and Diastolic Dysfunction Among Aging Women With Human Immunodeficiency Virus. J Infect Dis. 2019. May 17 pii: jiz184. doi: 10.1093/infdis/jiz184. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosenthal J, Tyor W. Aging, comorbidities, and the importance of finding biomarkers for HIV-associated neurocognitive disorders. J Neurovirol. 2019. March 13. doi: 10.1007/s13365-019-00735-0. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Utay NS, Hunt PW. Role of immune activation in progression to AIDS. Curr Opin HIV AIDS. 2016;11:131–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dirajlal-Fargo S, Sattar A, Kulkarni M, Bowman E, Funderburg N, McComsey GA. HIV-positive youth who are perinatally infected have impaired endothelial function. AIDS. 2017;31:1917–1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alvarez P, Mwamzuka M, Marshed F, et al. Immune activation despite preserved CD4 T cells in perinatally HIV-infected children and adolescents. PLoS One. 2017;12:e0190332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sandler NG, Wand H, Roque A, et al. Plasma Levels of Soluble CD14 Independently Predict Mortality in HIV Infection. J Infect Dis. 2011;203:780–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Serrano-Villar S, Sainz T, Lee SA, et al. HIV-infected individuals with low CD4/CD8 ratio despite effective antiretroviral therapy exhibit altered T cell subsets, heightened CD8+ T cell activation, and increased risk of non-AIDS morbidity and mortality. PLoS Pathog. 2014;10:e1004078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuller LH, Tracy R, Belloso W, et al. Inflammatory and Coagulation Biomarkers and Mortality in Patients with HIV Infection. PLoS Med. 2008;5:e203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McBride JA, Striker R. Imbalance in the game of T cells: What can the CD4/CD8 T-cell ratio tell us about HIV and health? PLoS Pathog. 2017;13(11):e1006624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.North CM, Muyanja D, Kakuhikire B, et al. Brief Report: Systemic Inflammation, Immune Activation, and Impaired Lung Function Among People Living With HIV in Rural Uganda. J Acquir Immune Defic Syndr. 2018;78:543–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fitzpatrick ME, Singh V, Bertolet M, et al. Relationships of pulmonary function, inflammation, and T-cell activation and senescence in an HIV-infected cohort. AIDS. 2014;28:2505–2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Attia EF, Akgun KM, Wongtrakool C, et al. Increased Risk of Radiographic Emphysema in HIV Is Associated With Elevated Soluble CD14 and Nadir CD4. Chest. 2014;146:1543–1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Triplette M, Attia EF, Akgun KM, et al. A Low Peripheral Blood CD4/CD8 Ratio Is Associated with Pulmonary Emphysema in HIV. PLoS One. 2017;12:e0170857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319–338. [DOI] [PubMed] [Google Scholar]
- 24.Quanjer PH, Stanojevic S, Cole TJ, et al. Multi-ethnic reference values for spirometry for the 3–95-yr age range: the global lung function 2012 equations. Eur Respir J. 2012;40:1324–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arigliani M, Canciani MC, Mottini G, et al. Evaluation of the Global Lung Initiative 2012 Reference Values for Spirometry in African Children. Am J Respir Crit Care Med. 2017;195:229–236. [DOI] [PubMed] [Google Scholar]
- 26.Quanjer PH, Weiner DJ. Interpretative consequences of adopting the Global Lungs 2012 reference equations for spirometry for children and adolescents. Pediatr Pulmonol. 2014;49:118–125. [DOI] [PubMed] [Google Scholar]
- 27.ICRP, 2013. Radiological protection in paediatric diagnostic and interventional radiology. ICRP Publication 121. Ann. ICRP 42(2). [DOI] [PubMed] [Google Scholar]
- 28.Hansell DM, Bankier AA, MacMahon H, et al. Fleischner Society: glossary of terms for thoracic imaging. Radiology. 2008;246:697–722. [DOI] [PubMed] [Google Scholar]
- 29.Hartweg J, Gunter M, Perera R, et al. Stability of soluble adhesion molecules, selectins, and C-reactive protein at various temperatures: implications for epidemiological and large-scale clinical studies. Clin Chem. 2007;53:1858–1860. [DOI] [PubMed] [Google Scholar]
- 30.Lukasz A, Hellpap J, Horn R, et al. Circulating angiopoietin-1 and angiopoietin-2 in critically ill patients: development and clinical application of two new immunoassays. Crit Care. 2008;12:R94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McKay HS, Margolick JB, Martinez-Maza O, et al. Multiplex assay reliability and long-term intra-individual variation of serologic inflammatory biomarkers. Cytokine. 2016;90:185–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mowbray FI, Fox-Wasylyshyn SM, El-Masri MM. Univariate Outliers: A Conceptual Overview for the Nurse Researcher. Can J Nurs Res. 2019;51:31–37. [DOI] [PubMed] [Google Scholar]
- 33.Barker AF, Bergeron A, Rom WN, et al. Obliterative bronchiolitis. N Engl J Med. 2014;370:1820–1828. [DOI] [PubMed] [Google Scholar]
- 34.Funderburg NT. Markers of coagulation and inflammation often remain elevated in ART-treated HIV-infected patients. Curr Opin HIV AIDS. 2014;9:80–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Graham SM, Rajwans N, Jaoko W, et al. Endothelial activation biomarkers increase after HIV-1 acquisition: plasma vascular cell adhesion molecule-1 predicts disease progression. AIDS. 2013;27:1803–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Graham SM, Rajwans N, Tapia KA, et al. A prospective study of endothelial activation biomarkers, including plasma angiopoietin-1 and angiopoietin-2, in Kenyan women initiating antiretroviral therapy. BMC Infect Dis. 2013;13:263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Larranaga GF, Bocassi AR, Puga LM, et al. Endothelial markers and HIV infection in the era of highly active antiretroviral treatment. Thromb Res. 2003;110:93–98. [DOI] [PubMed] [Google Scholar]
- 38.d’Ettorre G, Paiardini M, Ceccarelli G, et al. HIV-associated immune activation: from bench to bedside. AIDS Res Hum Retroviruses. 2011;27:355–364. [DOI] [PubMed] [Google Scholar]
- 39.Alcaide ML, Parmigiani A, Pallikkuth S, et al. Immune Activation in HIV-Infected Aging Women on Antiretrovirals – Implications for Age-Associated Comorbidities: A Cross-Sectional Pilot Study. PLoS ONE. 2013;8:e63804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hazenberg MD, Otto SA, van Benthem BH, et al. Persistent immune activation in HIV-1 infection is associated with progression to AIDS. AIDS. 2003;17:1881–1888. [DOI] [PubMed] [Google Scholar]
- 41.Saracino A, Bruno G, Scudeller L, et al. Chronic Inflammation in a Long-Term Cohort of HIV-Infected Patients According to the Normalization of the CD4:CD8 Ratio. AIDS Res Hum Retroviruses. 2014;30:1178–84. [DOI] [PubMed] [Google Scholar]
- 42.McDonald DM. Angiogenesis and remodeling of airway vasculature in chronic inflammation. Am J Respir Crit Care Med. 2001;164(10 Pt 2):S39–S45. [DOI] [PubMed] [Google Scholar]
- 43.Liu K, Chi DS, Li C, et al. HIV-1 Tat protein-induced VCAM-1 expression in human pulmonary artery endothelial cells and its signaling. Am J Physiol Lung Cell Mol Physiol. 2005;289:L252–260. [DOI] [PubMed] [Google Scholar]
- 44.Kirkegaard-Klitbo DM, Mejer N, Knudsen TB, et al. Soluble CD163 predicts incident chronic lung, kidney and liver disease in HIV infection. AIDS. 2017;31:981–988. [DOI] [PubMed] [Google Scholar]
- 45.MacDonald DM, Zanotto AD, Collins G, et al. Associations between baseline biomarkers and lung function in HIV-positive individuals. AIDS. 2019;33:655–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Armah KA, McGinnis K, Baker J, et al. HIV Status, Burden of Comorbid Disease, and Biomarkers of Inflammation, Altered Coagulation, and Monocyte Activation. Clin Infect Dis. 2012;55:126–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Graham SM, Mwilu R, Liles WC. Clinical utility of biomarkers of endothelial activation and coagulation for prognosis in HIV infection: a systematic review. Virulence. 2013;4:564–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rowley JE, Johnson JR. Pericytes in chronic lung disease. Int Arch Allergy Immunol. 2014;164:178–188. [DOI] [PubMed] [Google Scholar]
- 49.Wang S, Voisin MB, Larbi KY, et al. Venular basement membranes contain specific matrix protein low expression regions that act as exit points for emigrating neutrophils. J Exp Med. 2006;203:1519–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wanner A, Mendes ES. Airway Endothelial Dysfunction in Asthma and Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med. 2010;182:1344–1351. [DOI] [PubMed] [Google Scholar]
- 51.Oelsner EC, Pottinger TD, Burkart KM, et al. Adhesion molecules, endothelin-1 and lung function in seven population-based cohorts. Biomarkers. 2013. May;18:196–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Konstantellou E, Papaioannou AI, Loukides S, et al. Persistent airflow obstruction in patients with asthma: Characteristics of a distinct clinical phenotype. Respir Med. 2015;109:1404–1409. [DOI] [PubMed] [Google Scholar]
- 53.Hakansson L, Bjornsson E, Janson C, et al. Increased adhesion to vascular cell adhesion molecule-1 and intercellular adhesion molecule-1 of eosinophils from patients with asthma. J Allergy Clin Immunol. 1995;96(6 Pt 1):941–950. [DOI] [PubMed] [Google Scholar]
- 54.Stanciu LA, Djukanovic R. The role of ICAM-1 on T-cells in the pathogenesis of asthma. Eur Respir J. 1998;11:949–957. [DOI] [PubMed] [Google Scholar]
- 55.Gingo MR, Wenzel SE, Steele C, et al. Asthma diagnosis and airway bronchodilator response in HIV-infected patients. J Allergy Clin Immunol. 2012;129:708–714.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cosio BG, Dacal D, Perez de Llano L. Asthma-COPD overlap: identification and optimal treatment. Ther Adv Respir Dis. 2018;12:1753466618805662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Morgan BW, Grigsby MR, Siddharthan T, et al. Epidemiology and Risk Factors of Asthma-COPD Overlap in Low- and Middle-Income Countries. J Allergy Clin Immunol. 2019;143:1598–1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kapur N, Masters IB, Chang AB. Longitudinal growth and lung function in pediatric non-cystic fibrosis bronchiectasis: what influences lung function stability? Chest. 2010;138:158–164. [DOI] [PubMed] [Google Scholar]
- 59.Angelidou K, Hunt PW, Landay AL, et al. Changes in Inflammation but Not in T-Cell Activation Precede Non-AIDS-Defining Events in a Case-Control Study of Patients on Long-term Antiretroviral Therapy. J Infect Dis. 2018;218:239–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hunt PW. Soluble CD163 and Clinical Outcomes in Treated HIV Infection: Insights Into Mechanisms. J Infect Dis. 2016;214:1132–1133. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


