Abstract
Objective To comparatively examine differences in risk of clinical manifestations and death among people admitted to hospital with coronavirus disease 2019 (covid-19) and seasonal influenza.
Design Cohort study.
Setting US Department of Veterans Affairs.
Participants Patients admitted to hospital with covid-19 between 1 February 2020 and 17 June 2020 (n=3641) and seasonal influenza between 2017 and 2019 (n=12 676).
Main outcome measures Risks of clinical manifestations, healthcare resource use (including use of mechanical ventilation, admission to intensive care, and length of stay), and death, estimated using a doubly robust approach to build propensity scores that were then used along with covariates to adjust the outcome models.
Results Compared with seasonal influenza, covid-19 was associated with higher risk of acute kidney injury (odds ratio 1.52, 95% confidence interval 1.37 to 1.69), incident renal replacement therapy (4.11, 3.13 to 5.40), incident insulin use (1.86, 1.62 to 2.14), severe septic shock (4.04, 3.38 to 4.83), vasopressor use (3.95, 3.46 to 4.51), pulmonary embolism (1.50, 1.18 to 1.90), deep venous thrombosis (1.50, 1.20 to 1.88), stroke (1.62, 1.17 to 2.24), acute myocarditis (7.82, 3.53 to 17.36), arrythmias and sudden cardiac death (1.76, 1.40 to 2.20), elevated troponin (1.75, 1.50 to 2.05), elevated aspartate aminotransferase (3.16, 2.91 to 3.43), elevated alanine aminotransferase (2.65, 2.43 to 2.88), and rhabdomyolysis (1.84, 1.54 to 2.18). Compared with seasonal influenza, covid-19 was also associated with higher risk of death, mechanical ventilator use, and admission to intensive care (hazard ratio 4.97, (95% confidence interval 4.42 to 5.58), 4.01 (3.53 to 4.54), and 2.41 (2.25 to 2.59), respectively) and 3.00 (2.20 to 3.80) additional days of hospital stay. Differences in rates of death per 100 patients between covid-19 and seasonal influenza were most pronounced in people over 75 years of age with chronic kidney disease or dementia and those with black race and obesity, diabetes, or chronic kidney disease.
Conclusions Among people admitted to hospital, compared with seasonal influenza, covid-19 was associated with increased risk of extrapulmonary organ dysfunction, death, and increased health resource use. The findings may inform the global discussion about the comparative risks of covid-19 and seasonal influenza and may help the ongoing effort to manage the covid-19 global pandemic.
Introduction
The coronavirus disease 2019 (covid-19) global pandemic might be the most challenging health crisis of our lifetime. The number of infected people and number of deaths continue to grow worldwide.1 Comparisons of clinical manifestations and mortality between covid-19 and seasonal influenza have been drawn by public health officials, policy makers, and the public at large. However, these comparisons have so far relied on data and mortality statistics obtained by disparate methods and are not based on an “apples with apples” comparison.2 A robust comparative approach would allow stakeholders to develop a deeper understanding of the health risks of covid-19 (compared with the well known seasonal influenza) and to anticipate demand for healthcare services and project mortality with greater accuracy.
In this study, we leveraged the breadth and depth of the electronic healthcare databases of the US Department of Veterans Affairs (VA), which operates the largest nationally integrated healthcare delivery system in the US, to do a comparative evaluation of clinical manifestations and outcomes among US veterans admitted to hospital with covid-19 and seasonal influenza. This approach provides a comparative assessment between covid-19 and seasonal influenza and will inform the global discussion and effort to manage current and future waves of this global pandemic in which some waves might spatiotemporally coincide with seasonal influenza.
Methods
Setting
We used the electronic healthcare databases of the US Department of Veterans Affairs, which operates the largest nationally integrated healthcare system in the United States—a veteran specific national health service—to discharged veterans of the US armed forces. The US Department of Veterans Affairs provides care at 1255 healthcare facilities, including 170 VA medical centers (hospital systems) and 1074 outpatient sites of care of varying complexity (Veterans Health Administration outpatient clinics) to more than nine million veterans enrolled in the VA. All enrolled veterans have access to the Department of Veterans Affairs’ comprehensive medical benefits package including inpatient hospital care; outpatient services; preventive, primary, and specialty care; prescriptions; mental healthcare; home healthcare; geriatric and extended care; medical equipment; and prosthetics.
Cohort
We selected patients from the Department of Veterans Affairs healthcare system with a positive laboratory test result for influenza between 1 January 2017 and 31 December 2019 (n=54 281) or with a positive laboratory test for covid-19 between 1 February 2020 and 17 June 2020 (n=9125). We identified patients with a hospital admission between five days before and 30 days after the positive test result (n=12 748 in seasonal influenza group; n=3641 in covid-19 group). For participants with both seasonal influenza and covid-19 hospital admission records (n=72), we used their first covid-19 admission records in the analyses. The final cohort consisted of 16 317 participants, including 12 676 participants in the seasonal influenza group and 3641 participants in the covid-19 group (supplementary figure S1). We defined time zero as the date of admission to hospital and followed participants until the first occurrence of 60 days after admission, death, or end of study follow-up (17 June 2020). After 60 days of follow-up, 168 (1.33%) patients in the seasonal influenza group and 76 (2.08%) patients in the covid-19 group remained in hospital. The probability of patients remaining in hospital in the two groups is provided in supplementary figure S2.
Data sources
The Department of Veterans Affairs Corporate Data Warehouse (CDW) provided electronic health records from participants enrolled in the VA healthcare system.3 4 5 6 We used the CDW laboratory results domain to collect laboratory test information and the CDW inpatient encounters domains to collect information including diagnoses, procedures, and other aspects of healthcare use during a hospital admission.7 We used the CDW pharmacy domain and CDW bar code medication administration domain to collect medication data and the CDW vital signs domain to collect vital measurements. We collected demographic information from the CDW patient domain and VA vital status database, as well as disease history from both the CDW inpatient encounter and outpatient encounter domains.8 9 10
Exposures
Exposures in the study were admission to hospital with seasonal influenza and admission to hospital with covid-19, defined on the basis of a positive laboratory test result for seasonal influenza or covid-19 with a hospital admission within five days before the test or within 30 days after the test.
Outcomes
The primary outcome of the study was time until death since hospital admission. We defined clinical outcomes including acute kidney injury, incident renal replacement therapy, incident insulin use, severe septic shock, vasopressor use, pulmonary embolism, deep venous thrombosis, stroke, acute myocarditis, arrythmias and sudden cardiac death, elevated troponin, elevated aspartate aminotransferase, elevated alanine aminotransferase, and rhabdomyolysis by using definitions based on laboratory data and diagnostic and procedure codes.6 11 12 13 14 15 16 17 18 Outcomes related to healthcare use included time until mechanical ventilation, time until admission to intensive care unit (ICU), and length of stay in hospital, which we defined as days from admission until first discharge among patients discharged from hospital.
Covariates
Covariates in the study included demographics such as age, race (white, black, and other), and sex; history of diseases such as chronic lung disease, cancer, cardiovascular disease, cerebrovascular disease, dementia, diabetes mellitus, hypertension, and peripheral artery disease; history of use of drugs such as statins, angiotensin converting enzyme inhibitors or angiotensin receptor blockers, and non-steroidal anti-inflammatory drugs; smoking status (never, former, and current); and estimated glomerular filtration rate and body mass index (underweight <18.50, normal ≥18.50-<25, overweight ≥25-<30, and obese ≥30) calculated from height and weight. We ascertained disease history during the year before admission to hospital and defined outpatient drug use by a record of prescription that provided medication within the 30 days before the admission. We selected estimated glomerular filtration rate and body mass index from the measurement before and closest to the admission. When values of covariates were missing (0.18% of age, 2.56% of estimated glomerular filtration rate, and 2.02% of body mass index), we applied mean imputation.
We also collected vital signs at admission, including heart rate, temperature, respiratory rate, blood oxygen concentration, systolic and diastolic blood pressure, and laboratory measurements including white blood cell count, hemoglobin, serum albumin, blood urea nitrogen, platelet count, bicarbonate, and lymphocyte percentage, on the basis of measurements during the hospital admission and closest to admission date and time.
Statistical analyses
We present the mean and standard deviation, frequency and percentage, or median and interquartile range, as appropriate, of the characteristics for patients admitted to hospital with seasonal influenza and covid-19. We made between group comparisons using χ2 tests for counts and t tests for continuous variables.
We used logistic regressions to examine the difference in clinical manifestations between seasonal influenza and covid-19. We estimated excess events per 100 patients based on difference in the probability of the event occurring if all participants had a hospital admission for covid-19 compared with if all participants had a seasonal influenza related admission. To adjust for confounding, we used doubly robust estimation for all models. We built a propensity score based on covariates including age, sex, race, smoking status, body mass index, estimated glomerular filtration rate, chronic lung diseases, cancer, cardiovascular diseases, cerebrovascular disease, dementia, diabetes mellitus, hypertension, peripheral artery disease, and use of statins, angiotensin converting enzyme inhibitors or angiotensin receptor blockers, and non-steroidal anti-inflammatory drugs. Doubly robust estimation adjusted for both the covariates and the propensity score in the outcome models.19 20 To evaluate the likelihood of violation of the positivity assumption, we plotted the propensity score distributions in two exposure groups (supplementary figure S3). We examined the balance of baseline characteristics between the covid-19 group and seasonal influenza group, and the result suggested that balance was achieved with the application of the propensity score (supplementary figures S3 and S4; supplementary table S1).
We constructed survival models with doubly robust estimation to examine the difference in risk of death between hospital admission for covid-19 and hospital admission for seasonal influenza. We estimated risk of mechanical ventilator use and admission to ICU on the basis of the Fine and Gray survival model, in which death before the occurrence of the event was considered a competing risk.21 For all survival outcomes, we evaluated proportional hazard assumptions and observed no indication of violation (supplementary figure S5). We estimated excess outcomes per 100 patients on the basis of the differences in cumulative incidence at 60 days if all participants had a hospital admission for covid-19 compared with if all participants had a hospital admission for seasonal influenza.13 We also used linear regression to examine the difference in length of stay in patients discharged from hospital.
We aimed to identify groups of participants that were at the highest risk of death due to covid-19, as well as those groups that had the highest disparities between risk of death due to covid-19 and seasonal influenza. We did further survival analyses to investigate risk of death by comorbid condition and demographics (age ≤65, >65-≤75, >75; white, black race) pairs. We estimated death rates per 100 participants on the basis of the cumulative incidence function at 60 days if all participants had a hospital admission for covid-19 with a defined comorbid condition and demographic pair (for example, rate of death in those aged ≤65 and with cardiovascular disease). We then estimated differences in death rates between hospital admission for covid-19 and hospital admission for seasonal influenza under each condition pair. Results are presented in heat maps, colored by the rate of death.
We did sensitivity analyses to test the robustness of the results. We estimated the risk (hazard ratios) of death, mechanical ventilation, and admission to ICU for covid-19 versus seasonal influenza in each influenza season included in the cohort (influenza season 2016-17, 2017-18, 2018-19, and 2019-20). We then examined the risks (hazard ratios) for covid-19 versus influenza type A and type B, separately. Because influenza A subtypes may vary by season, we also examined the association of covid-19 versus influenza type A in each season. We examined the risks (hazard ratios) of outcomes for covid-19 versus influenza in patients with a record of vaccination for influenza and those without a record of vaccination. We estimated the risks within 30 days of admission to hospital and setting the time of positive test result as time zero. We examined the risks (hazard ratios) according to hospital bed occupancy and, separately, according to ICU bed occupancy in covid-19 patients. We updated the covariates during the follow-up and did time dependent analyses. We applied the overlap weighting method and the inverse probability weighting method. To remove potentially nosocomially acquired infections, we removed patients who had a first positive test after admission to hospital and then additionally removed those who had a first positive test on the first day of admission. We did analyses in subgroups based on sex, and, finally, we evaluated the association in subgroups according to smoking status and body mass index category.
We considered P values less than 0.05, 95% confidence intervals of the hazard ratio or odds ratio that did not cross 1, or 95% confidence intervals of the length of stay difference or event rate difference that did not cross 0 to be statistically significant. We used SAS Enterprise Guide version 7.1 for all analyses.
Patient and public involvement
No patients were involved in developing the hypothesis, the specific aims, or the research questions; nor were they involved in developing plans for design or implementation of the study. No patients were involved in the interpretation or writing up of results. The study was conceived, designed, and conducted under the exigent circumstances of the covid-19 global pandemic, which precluded patient and public engagement.
Results
A total of 12 676 people were admitted to hospital with seasonal influenza between 1 January 2017 and 31 December 2019, and 3641 were admitted with covid-19 between 1 February 2020 and 17 June 2020. Table 1 describes the baseline demographic and health characteristics. The percentage of black patients admitted with covid-19 (49.8%) was more than double the percentage admitted with seasonal influenza (22.0%); the percentages of patients with obesity, dementia, and diabetes mellitus were higher in the covid-19 group. The percentages of people who were current smokers or who had cancer, cardiovascular disease, chronic lung disease, and use of angiotensin converting enzyme inhibitors or angiotensin receptor blockers and statins was higher in the seasonal influenza group (table 1).
Table 1.
Baseline and admission characteristics of patients admitted to hospital with seasonal influenza and coronavirus disease 2019 (covid-19). Values are numbers (percentages) unless stated otherwise
| Characteristics | Overall (n=16 317) | Seasonal influenza (n=12 676; (77.7%) | Covid-19 (n=3641; 22.3%) | P value |
|---|---|---|---|---|
| Baseline characteristics | ||||
| Mean (SD) age, years | 69.98 (12.91) | 70.25 (12.8) | 69.03 (13.4) | <0.001 |
| Sex: | 0.65 |
|||
| Male | 15 432 (94.6) | 11 994 (94.6) | 3438 (94.4) | |
| Female | 885 (5.42 | 682 (5.4) | 203 (5.6) | |
| Race: | <0.001 |
|||
| White | 10 975 (67.3) | 9318 (73.5) | 1657 (45.5) | |
| Black | 4599 (28.2) | 2787 (22.0) | 1812 (49.8) | |
| Other | 743 (4.6) | 571 (4.5) | 172 (4.7) | |
| Smoking: | <0.001 |
|||
| Never | 7673 (47.0) | 5694 (44.9) | 1979 (54.4) | |
| Former | 4420 (27.1) | 3315 (26.2) | 1105 (30.4) | |
| Current | 4224 (25.9) | 3667 (28.9) | 557 (15.3) | |
| Body mass index: | <0.001 |
|||
| Underweight (<18.5) | 550 (3.4) | 441 (3.5) | 109 (3.0) | |
| Normal (18.5 to <25) | 4093 (25.1) | 3333 (26.3) | 760 (20.9) | |
| Overweight (25 to <30) | 4872 (29.9) | 3829 (30.2) | 1043 (28.7) | |
| Obese (≥30) | 6802 (41.7) | 5073 (40.0) | 1729 (47.5) | |
| Mean (SD) eGFR, mL/min/1.73m2 | 63.51 (27.3) | 63.57 (26.6) | 63.30 (29.6) | 0.63 |
| Chronic kidney disease | 6994 (42.9) | 5425 (42.8) | 1569 (43.1) | 0.75 |
| Cancer | 2788 (17.1) | 2258 (17.8) | 530 (14.6) | <0.001 |
| Cerebrovascular disease | 2373 (14.5) | 1817 (14.3) | 556 (15.37) | 0.16 |
| Cardiovascular disease | 6134 (37.6) | 5007 (39.5) | 1127 (31.0) | <0.001 |
| Dementia | 2403 (14.7) | 1693 (13.4) | 710 (19.5) | <0.001 |
| Diabetes mellitus | 7135 (43.7) | 5418 (42.7) | 1717 (47.2) | <0.001 |
| Hypertension | 11 931 (73.1) | 9277 (73.2) | 2654 (72.9) | 0.72 |
| Chronic lung disease | 6160 (37.8) | 5271 (41.6) | 889 (24.4) | <0.001 |
| Peripheral artery disease | 764 (4.7) | 610 (4.8) | 154 (4.2) | 0.14 |
| Statins | 7511 (46.0) | 6114 (48.2) | 1397 (38.4) | <0.001 |
| ACE/ARB | 5579 (34.2) | 4603 (36.3) | 976 (26.8) | <0.001 |
| NSAIDs | 1842 (11.29) | 1439 (11.4) | 403 (11.1) | 0.63 |
| Selected vital signs on admission * | ||||
| Median (IQR) heart rate, beats per min | 87 (75-100) | 88 (76-101) | 85 (75-97) | <0.001 |
| Median (IQR) temperature, °C | 36.9 (36.6-37.5) | 36.9 (36.6-37.5) | 37.1 (34.4-36.7) | <0.001 |
| Temperature >38°C | 2161 (13.3) | 1627 (12.9) | 534 (14.9) | 0.002 |
| Median (IQR) respiratory rate, breaths per min | 18 (18-20) | 18 (18-20) | 18 (18-20) | <0.001 |
| Median (IQR) oxygen concentration, % | 95 (93-97) | 95 (93-97) | 96 (94-98) | <0.001 |
| Oxygen concentration <95% | 6104 (38.2) | 4969 (40.0) | 1135 (32.2) | <0.001 |
| Median (IQR) systolic blood pressure, mm Hg | 131 (116-148) | 131 (116-148) | 131 (117-146) | 0.24 |
| Median (IQR) diastolic blood pressure, mm Hg | 74 (65-82) | 74 (65-82) | 75 (67-83) | <0.001 |
| Selected laboratory measurements on admission * | ||||
| Median (IQR) white blood cell count, ×109/L | 7.1 (5.3-9.7) | 7.4 (5.5-10.0) | 6.2 (4.7-8.3) | 0.001 |
| Median (IQR) hemoglobin, g/L | 131 (116-145) | 131 (116-145) | 131 (115-144) | 0.02 |
| Median (IQR) serum albumin, g/L | 36 (31-39) | 36 (32-39) | 35 (30-38) | <0.001 |
| Median (IQR) blood urea nitrogen, mmol/L | 6.8 (5.0-10.0) | 6.8 (5.0-9.6) | 7.1 (5.0-12.1) | <0.001 |
| Median (IQR) platelet count, ×109/L | 185 (144-237) | 182 (142-233) | 193 (149-252) | <0.001 |
| Median (IQR) bicarbonate, mmol/L | 25 (23-27) | 25 (23-27) | 25 (23-28) | <0.001 |
| Median (IQR) lymphocytes, % | 12.7 (7.8-20.2) | 11.9 (7.3-19.0) | 15.8 (10.0-23.4) | <0.001 |
ACE/ARB=angiotensin converting enzyme inhibitors/angiotensin II receptor blockers; eGFR=estimated glomerular filtration rate; IQR=interquartile range; NSAID=non-steroidal anti-inflammatory drug.
Vital signs and laboratory measurements recorded after and closest to admission date and time.
Differences in clinical manifestations of covid-19 versus seasonal influenza
We then comparatively evaluated the clinical manifestations of covid-19 versus those of seasonal influenza. Table 2 shows the number of events in each group, the unadjusted and adjusted odds ratios, and the excess number of outcomes per 100 patients admitted to hospital with covid-19 compared with seasonal influenza. Compared with people admitted to hospital with seasonal influenza, we observed a graded association between covid-19 and risk of acute kidney injury; the odds of the association increased with increasing severity of acute kidney injury, and we also saw an increased risk of incident renal replacement therapy. Covid-19 was also associated with increased risk of incident insulin use, severe septic shock, vasopressor use, pulmonary embolism, deep venous thrombosis, stroke, acute myocarditis, arrythmias and sudden cardiac death, elevated troponin, elevated aspartate aminotransferase, elevated alanine aminotransferase, and rhabdomyolysis.
Table 2.
Clinical manifestations in patients admitted to hospital with seasonal influenza and coronavirus disease 2019 (covid-19)
| Clinical manifestations | Seasonal influenza (n=12 676)—No (%) | Covid-19 (n=3641)—No (%) | Unadjusted OR* (95% CI) | Adjusted OR* † (95% CI) | Excess outcomes per 100 patients* ‡ (95% CI) |
|---|---|---|---|---|---|
| Any acute kidney injury | 3670 (29.0) | 1355 (37.2) | 1.46 (1.35 to 1.57) | 1.52 (1.37 to 1.69) | 5.81 (5.35 to 6.27) |
| Acute kidney injury stage 2 or above | 638 (5.0) | 538 (14.8) | 3.27 (2.90 to 3.69) | 3.16 (2.74 to 3.64) | 8.06 (6.49 to 9.85) |
| Acute kidney injury stage 3 | 408 (3.2) | 397 (10.9) | 3.68 (3.19 to 4.25) | 3.43 (2.90 to 4.06) | 5.97 (4.57 to 7.66) |
| Incident renal replacement therapy§ | 113/12 128 (0.9) | 160/3430 (4.7) | 5.20 (4.08 to 6.64) | 4.11 (3.13 to 5.40) | 2.77 (1.63 to 4.63) |
| Incident insulin use¶ | 1048/8484 (12.4) | 555/2412 (23.0) | 2.12 (1.89 to 2.38) | 1.86 (1.62 to 2.14) | 9.35 (8.31 to 10.29) |
| Severe septic shock | 300 (2.4) | 319 (8.8) | 3.96 (3.37 to 4.66) | 4.04 (3.38 to 4.83) | 6.36 (4.48 to 8.87) |
| Vasopressor use | 613 (4.8) | 595 (16.3) | 3.84 (3.41 to 4.33) | 3.95 (3.46 to 4.51) | 11.47 (9.10 to 14.22) |
| Pulmonary embolism | 262 (2.1) | 118 (3.2) | 1.59 (1.27 to 1.98) | 1.50 (1.18 to 1.90) | 1.00 (0.59 to 1.69) |
| Deep venous thrombosis | 285 (2.3) | 141 (3.9) | 1.75 (1.43 to 2.15) | 1.50 (1.20 to 1.88) | 1.13 (0.69 to 1.83) |
| Stroke | 124 (1.0) | 69 (1.9) | 1.96 (1.45 to 2.63) | 1.62 (1.17 to 2.24) | 0.62 (0.30 to 1.25) |
| Acute myocarditis | 10 (0.1) | 23 (0.6) | 8.05 (3.83 to 16.92) | 7.82 (3.53 to 17.36) | 0.53 (0.11 to 2.63) |
| Arrythmias and sudden cardiac death | 275 (2.2) | 138 (3.8) | 1.78 (1.44 to 2.19) | 1.76 (1.40 to 2.20) | 1.57 (0.97 to 2.51) |
| Troponin >0.4 ng/mL | 654 (5.2) | 311 (8.5) | 1.73 (1.51 to 1.99) | 1.75 (1.50 to 2.05) | 3.37 (2.50 to 4.47) |
| Aspartate aminotransferase: | |||||
| >40 IU/L | 3694 (29.1) | 2104 (57.8) | 3.33 (3.08 to 3.59) | 3.16 (2.91 to 3.43) | 27.00 (26.00 to 27.71) |
| >5 times upper limit of normal** | 279 (2.2) | 204 (5.6) | 2.64 (2.19 to 3.17) | 2.38 (1.94 to 2.91) | 2.90 (1.90 to 4.35) |
| Alanine aminotransferase: | |||||
| >40 IU/L | 3154 (24.9) | 1697 (46.6) | 2.64 (2.44 to 2.84) | 2.65 (2.43 to 2.88) | 21.12 (19.81 to 22.26) |
| >5 times upper limit of normal †† | 311 (2.5) | 309 (8.5) | 3.46 (2.95 to 4.06) | 3.33 (2.80 to 3.97) | 5.54 (3.90 to 7.76) |
| Rhabdomyolysis: | |||||
| CPK >1000 IU/L | 411 (3.2) | 274 (7.5) | 2.43 (2.08 to 2.84) | 1.84 (1.54 to 2.18) | 2.65 (1.87 to 3.72) |
| CPK >5000 IU/L | 94 (0.7) | 43 (1.2) | 1.60 (1.11 to 2.30) | 1.20 (0.81 to 1.77) | 0.15 (−0.06 to 0.42) |
| CPK >5× upper limit of normal ‡‡ | 288 (2.3) | 181 (5.0) | 2.25 (1.86 to 2.72) | 1.66 (1.35 to 2.04) | 1.53 (0.98 to 2.35) |
CPK=creatine phosphokinase; OR=odds ratio.
Seasonal influenza group served as reference.
Models adjusted for age, sex, race, smoking status, body mass index, estimated glomerular filtration rate, chronic lung disease, cancer, cardiovascular disease, cerebrovascular disease, dementia, diabetes mellitus, hypertension, peripheral artery disease, and use of statins, angiotensin converting enzyme inhibitors/angiotensin II receptor blockers, and non-steroidal anti-inflammatory drugs.
Excess outcomes per 100 patients due to covid-19 compared with seasonal influenza.
Among patients without history of renal replacement therapy before hospital admission (n=15 558).
Among patients without history of diabetes treatment before hospital admission (n=10 896).
Defined as 240 IU/L for males and 215 IU/L for females.
Defined as 165 IU/L for males and 125 IU/L for females.
Defined as 1540 IU/L for males and 960 IU/L for females.
Differences in risk of death and health resource use for covid-19 versus seasonal influenza
Table 3 and table 4 show the number of deaths in each group, unadjusted and adjusted hazard ratios, and the number of excess deaths per 100 patients admitted to hospital with covid-19 compared with seasonal influenza. Compared with people admitted to hospital with seasonal influenza, admission with covid-19 was associated with increased risk of death; we observed 16.85 (95% confidence interval 14.85 to 18.99) excess deaths per 100 patients due to covid-19. Health resource use was much more pronounced for covid-19 compared with seasonal influenza. Covid-19 was associated with significantly higher risk of mechanical ventilation use, admission to ICU, and prolonged length of hospital stay (table 3, table 4, fig 1, and fig 2).
Table 3.
Risks of death and healthcare resource use in patients admitted to hospital with coronavirus disease 2019 (covid-19) versus seasonal influenza
| Outcomes | Measure | Seasonal influenza (n=12 676) | Covid-19 (n=3641) | Unadjusted HR* (95% CI) | Adjusted HR* † (95% CI) | Excess outcomes per 100 patients* ‡ (95% CI) |
|---|---|---|---|---|---|---|
| Death | No (%) | 674 (5.3) | 676 (18.6) | 4.55 (4.09 to 5.07) | 4.97 (4.42 to 5.58) | 16.85 (14.85 to 18.99) |
| Median (IQR) days till event§ | 18 (6-17) | 10 (6-17) | ||||
| Mechanical ventilator use¶ | No (%) | 529 (4.2) | 545 (15.0) | 3.93 (3.49 to 4.42) | 4.01 (3.53 to 4.54) | 11.29 (9.62 to 13.14) |
| Median (IQR) days till event** | 1 (0-4) | 2 (0-5) | ||||
| ICU admission¶ | No (%) | 2353 (18.6) | 1341 (36.8) | 2.30 (2.16 to 2.46) | 2.41 (2.25 to 2.59) | 19.80 (17.81 to 21.87) |
| Median (IQR) days till event†† | 1 (0-5) | 0 (0-4) |
HR=hazard ratio; ICU=intensive care unit; IQR=interquartile range.
Seasonal influenza group served as reference.
Models adjusted for age, sex, race, smoking status, body mass index, estimated glomerular filtration rate, chronic lung disease, cancer, cardiovascular disease, cerebrovascular disease, dementia, diabetes mellitus, hypertension, peripheral artery disease, and use of statins, angiotensin converting enzyme inhibitors/angiotensin II receptor blockers, and non-steroidal anti-inflammatory drugs.
Excess outcome per 100 patients due to covid-19 compared with seasonal influenza.
Among patients who died.
Death before event was considered as competing risk.
Among patients who used mechanical ventilation.
Among patients who had ICU admission.
Table 4.
Length of hospital stay in patients admitted to hospital with coronavirus disease 2019 (covid-19) versus seasonal influenza, among patients discharged from hospital (n=15 009)
| Outcome | Measure | Seasonal influenza (n=12 388) | Covid-19 (n=2621) | Unadjusted days difference* (95% CI) | Adjusted days difference* † (95% CI) |
|---|---|---|---|---|---|
| Length of hospital stay, days | Median (IQR) | 3 (2-6) | 6 (3-12) | 3.11 (2.36 to 3.87) | 3.00 (2.20 to 3.80) |
| Mean (SD) | 7 (19.00) | 10 (10.99) |
IQR=interquartile range.
Seasonal influenza group served as reference.
Models adjusted for age, sex, race, smoking status, body mass index, estimated glomerular filtration rate, chronic lung disease, cancer, cardiovascular disease, cerebrovascular disease, dementia, diabetes mellitus, hypertension, peripheral artery disease, and use of statins, angiotensin converting enzyme inhibitors/angiotensin II receptor blockers, and non-steroidal anti-inflammatory drugs.
Fig 1.
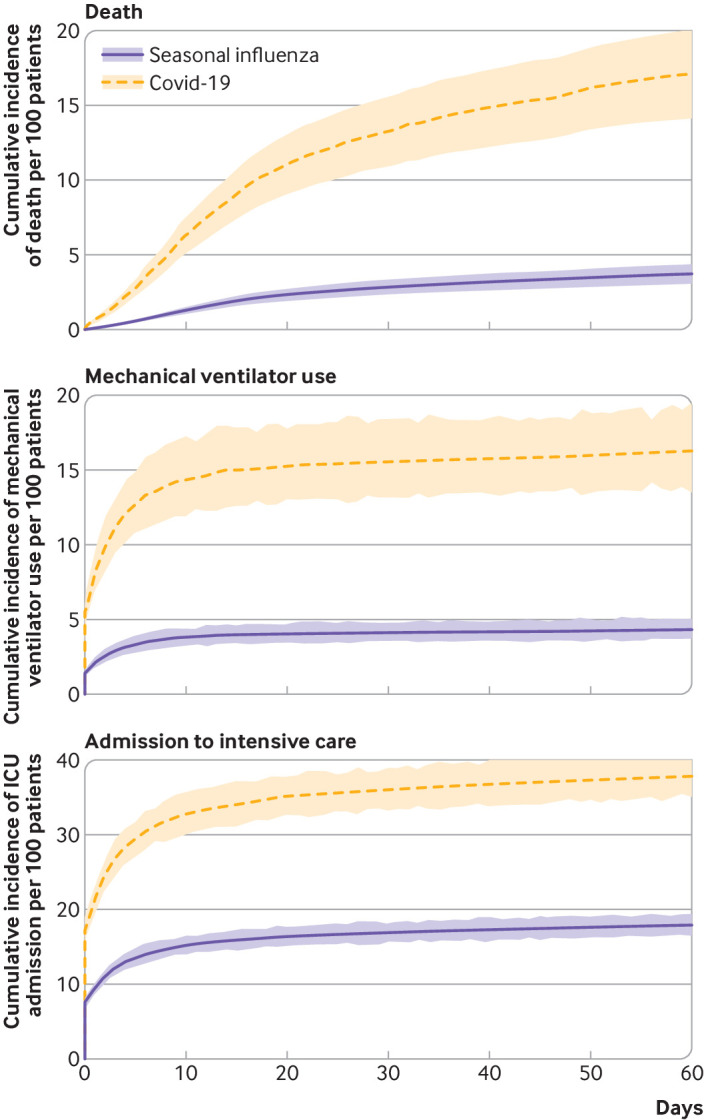
Adjusted cumulative incidence rate (with 95% CI) of death, mechanical ventilator use, and admission to intensive care unit (ICU) per 100 patients admitted to hospital with seasonal influenza and coronavirus disease 2019 (covid-19)
Fig 2.
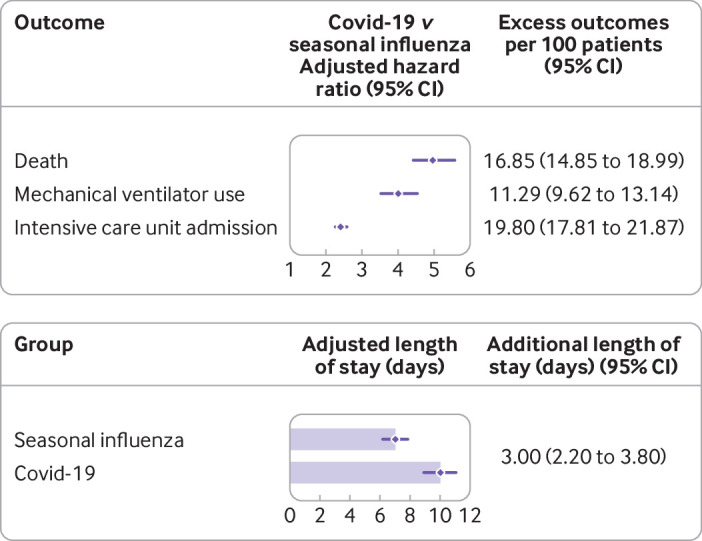
Risks of death and healthcare resource use in patients admitted to hospital with coronavirus disease 2019 (covid-19) versus seasonal influenza. Models were adjusted for age, sex, race, smoking status, body mass index, estimated glomerular filtration rate, chronic lung disease, cancer, cardiovascular disease, cerebrovascular disease, dementia, diabetes mellitus, hypertension, peripheral artery disease, and use of statins, angiotensin converting enzyme inhibitors/angiotensin II receptor blockers and non-steroidal anti-inflammatory drugs. Seasonal influenza group served as reference for each model. Length of hospital stay was calculated within patients discharged from hospital (n=15 009)
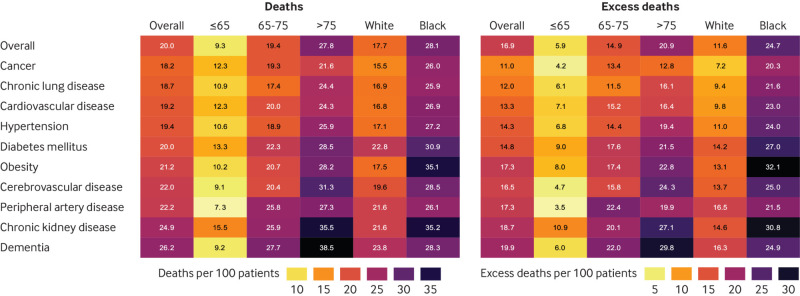
Rates of death by age, race, and comorbid condition
Figure 3 and supplementary table S2a show estimated rates of death with covid-19 by age, race, and comorbid condition. Death rates were generally highest in older and black individuals. Excess rates of death per 100 people with covid-19 (compared with seasonal influenza) were most pronounced in adults older than 75 years with chronic kidney disease or dementia and in black people with obesity, diabetes, or chronic kidney disease (fig 3 and supplementary table S2b).
Fig 3.
Rates of death in patients admitted to hospital with coronavirus disease 2019 (covid-19) (left), and excess deaths compared with patients admitted with seasonal influenza (right), by age (≤65, 65-75, >75 years), race (white, black), and comorbid condition. Number in each cell represents rate of death or excess deaths per 100 patients admitted with covid-19, given conditions. Lighter colored cells indicate lower death rates or smaller differences, and darker colored cells indicate higher death rates or larger differences
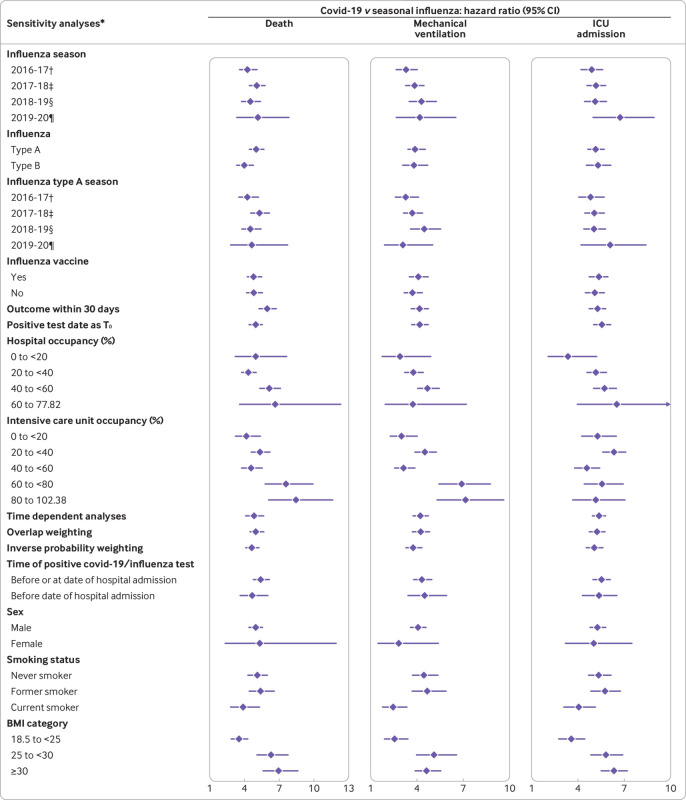
Sensitivity analyses
We did several sensitivity analyses to test the robustness of our results (fig 4 and supplementary table S3). Firstly, because mortality from seasonal influenza may vary by season, we developed sensitivity analyses to examine the risk of death and health resource use in covid-19 patients compared with those admitted with influenza in each season (2016-17, 2017-18, 2018-19, 2019-20) separately; the results were consistent. Secondly, the results were also consistent when we evaluated the risk of death and resource use for covid-19 compared with influenza A and B, separately. Thirdly, because subtypes of influenza A may have differential effects on health outcomes and because the predominant subtype of influenza A may vary by season, we examined the association of covid-19 versus influenza A in each season; the results suggested that compared with influenza A, covid-19 was associated with increased risk of health resource use and death in each of the examined seasons (supplementary table S4). Fourthly, the increased risk in covid-19 versus seasonal influenza was observed in analyses in which the reference group was those influenza patients who received an influenza vaccine and, separately, those who did not receive an influenza vaccine. Fifthly, analyses that evaluated the risk of death in the first 30 days and, separately, analyses that considered the date of testing positive for influenza or covid-19 as time zero yielded consistent results.
Fig 4.
Risks of death and healthcare resource use in patients admitted to hospital with coronavirus disease 2019 (covid-19) versus seasonal influenza. Seasonal influenza group served as reference. BMI=body mass index; ICU=intensive care unit; T0=time zero. *Models adjusted for age, sex, race, smoking status, BMI, estimated glomerular filtration rate, chronic lung disease, cancer, cardiovascular disease, cerebrovascular disease, dementia, diabetes mellitus, hypertension, peripheral artery disease, use of statins, angiotensin converting enzyme inhibitors/angiotensin II receptor blockers, and non-steroidal anti-inflammatory drugs. †Included seasonal influenza patients between 1 Jan 2017 and 31 Aug 2017 in cohort. ‡Included seasonal influenza patients between 1 Sept 2017 and 31 Aug 2018 in cohort. §Included seasonal influenza patients between 1 Sept 2018 and 31 Aug 2019 in cohort. ¶Included seasonal influenza patients between 1 Sept 2019 and 31 Dec 2019 in cohort
Sixthly, because a putative relation may exist between hospital bed occupancy—an indicator of how overwhelmed the hospital system is—and health outcomes, we examined the risk of death and health resource use for covid-19 versus seasonal influenza according to hospital bed occupancy (<20%, 20-<40%, 40-<60%, and 60-77.82%, the maximum observed hospital bed occupancy in our cohort) and, separately, according to ICU bed occupancy (<20%, 20-<40%, 40-<60%, 60-<80%, and 80-102.38%, the maximum observed ICU bed occupancy in our cohort); the results were consistent with the primary analyses. The distributions of occupancy of hospital beds and ICU beds are provided in supplementary figure S6. Seventhly, the results were also consistent in time dependent analyses in which covariates were updated during follow-up. Eighthly, we considered the overlap weighting method and the inverse probability weighting method as two alternatives to our primary approach (the doubly robust method); both alternative methods yielded estimates consistent with the primary approach. Ninthly, to eliminate concern about nosocomially acquired seasonal influenza or covid-19 infections, we did analyses in which we removed patients who had a positive test while in hospital and restricted cohort entry to those with a positive influenza or covid-19 test before and including the first day of hospital admission; results were consistent with those in the primary analyses and were also consistent in analyses considering patients with a positive test before admission. Tenthly, because our cohort comprised mostly male US veterans, and to examine whether the observed associations were also evident among female patients, we did analyses in subgroups based on sex; the results were consistent in both male and female subgroups. Finally, to test whether the observed associations were evident in smokers and non-smokers and, separately, among people with normal weight and obesity, we evaluated the association in subgroups according to smoking status and body mass index category; the results suggested that compared with seasonal influenza, covid-19 was associated with increased risk of death and health resource use regardless of smoking and body mass index status (fig 4 and supplementary table S3).
Discussion
In this work, we provide a comparative evaluation of the clinical manifestations and outcomes in a national cohort of patients admitted to hospital with covid-19 or seasonal influenza. Our results suggest that compared with seasonal influenza, covid-19 was associated with higher risk of acute kidney injury, incident renal replacement therapy, incident insulin use, severe septic shock, vasopressor use, pulmonary embolism, deep venous thrombosis, stroke, acute myocarditis, arrythmias and sudden cardiac death, elevated troponin, elevated aspartate aminotransferase, elevated alanine aminotransferase, and rhabdomyolysis. Covid-19 was also associated with increased risk of death, need for mechanical ventilation, and admission to ICU and prolonged length of hospital stay. Excess rates of death for covid-19 (in excess of those for seasonal influenza) were highest in adults older than 75 years with chronic kidney disease and dementia and in black patients with obesity, diabetes mellitus, and chronic kidney disease. The results were robust to challenge in multiple sensitivity analyses.
Contextual evaluation of findings
As our understanding of covid-19 is evolving, the fact that, although the virus preferentially infects cells in the respiratory tract, it may also be detected in multiple organs, suggesting broad extrapulmonary organotropism, is becoming increasingly clear.22 23 Covid-19 seems to have a kidney tropic effect; early observations suggested high rates of acute kidney injury and need for renal replacement therapy among patients with covid-19.22 23 24 25 Here, we show that risks of acute kidney injury and incident renal replacement therapy were higher with covid-19 than seasonal influenza. In our cohort, 15.0% of patients with covid-19 needed mechanical ventilation, generally consistent with the range of 12% to 24% reported in other US studies of covid-19.26 27 28 Recent reports suggested a bidirectional relation between covid-19 and diabetes mellitus, in that diabetes may predispose to covid-19 and that a diagnosis of covid-19 may result in derangement in insulin and glucose homeostasis and new onset of diabetes.29 30 Our findings of higher risk of incident insulin use among patients who were not taking any treatment for diabetes mellitus before hospital admission with covid-19 suggests the need for greater attention to management of glucose in patients with covid-19 and the need for long term follow-up to gain a better understanding of the chronicity of these acute disturbances in glucose homeostasis.29 Risks of severe septic shock and vasopressor use were also higher in the covid-19 group, consistent with reports of septic shock in patients with covid-19.31 We also report increased risk of pulmonary embolism, deep venous thrombosis, and stroke compared with seasonal influenza; clarity about the underlying biologic mechanism of a prothrombotic hypercoagulable state in covid-19 is evolving.32 33 34 35 36 The findings of increased risk of acute myocarditis, arrythmias and sudden cardiac death, and elevated troponin suggest that covid-19 may portend serious cardiac complications. Risk of elevated transaminases and rhabdomyolysis was also increased among patients with covid-19; the underlying pathophysiology of these abnormalities is not yet entirely clear. Our approach for the comparative evaluation of the acute clinical manifestations of covid-19 versus seasonal influenza was based on a priori knowledge; however, as our understanding of the clinical manifestations of covid-19 deepens, additional important differences in the clinical sequalae of these two diseases may come to light and may be investigated in future research.
Many of the acute clinical manifestations of covid-19 described in this report are disease states that need chronic management (for example, new onset diabetes, stroke). This is compounded by emerging evidence suggesting that some acute symptoms of covid-19 may linger far beyond the acute illness and that recovery may be protracted and incomplete in many “long haulers.” Taken together, these observations suggest that, beyond the acute setting, a greater understanding of the long term health trajectories of patients with “long covid-19” and care strategies to optimize wellness and reduce the burden of chronic and permanent health loss are needed to lessen the overall health, societal, and economic toll of this pandemic.
We observed much higher rates of death among patients admitted to hospital with covid-19 than with seasonal influenza. We also report that in adjusted models using the doubly robust approach, the risk of death in covid-19 patients was nearly fivefold higher than in those in the seasonal influenza group. We estimated that compared with seasonal influenza, covid-19 was associated with 16.85 (95% confidence interval 14.85 to 18.99) excess deaths per 100 patients. Our results also suggest significantly increased risk of mechanical ventilation use, ICU admission, and prolonged length of stay. Our estimates provide an “apples with apples” comparative quantitative assessment of the magnitude of risk of death (and other health outcomes) in patients admitted to hospital with covid-19 and seasonal influenza. The estimates may help to inform efforts to manage the ongoing covid-19 global pandemic in which some surge in activity (wave) may coincide with seasonal influenza.
Our heatmap approach, which provides adjusted estimates of the rates of death by age, race, and comorbid condition, shows that differences in rates of death between covid-19 and seasonal influenza (excess rates of death in covid-19) are most pronounced in older adults, among black people, and in those with dementia, diabetes mellitus, obesity, and kidney disease, suggesting that a more targeted prevention effort aimed at these high risk populations may be needed to reduce mortality rates.
Several other considerations merit discussion. Although we balanced the two groups according to demographic and health characteristics to optimize the comparative evaluation of the clinical characteristics and outcomes of covid-19 versus seasonal influenza, increased awareness of covid-19 may have resulted in greater ascertainment of covid-19 infections, whereby less severe cases of covid-19 relative to seasonal influenza would be diagnosed and possibly admitted to hospital, and relatively less historical ascertainment of seasonal influenza, whereby milder influenza cases may not have been diagnosed, enriching the seasonal influenza group with relatively sicker patients and resulting in underestimation of the risk of outcomes in covid-19 compared with seasonal influenza. Also, relaxed admission criteria and reduced threshold for admission in the covid-19 era—primarily driven by heightened concerns about the virus—may have resulted in admission of less sick people with covid-19, which may have resulted in underestimation of risk of covid-19 versus seasonal influenza. Health outcomes of patients admitted to hospital may deteriorate as hospitals become overwhelmed; if this existed in our data, it may have resulted in overestimation of the risk of adverse outcomes in covid-19 versus seasonal influenza. However, although the pandemic exacted a serious challenge to the VA hospital system, our examination of weekly average bed occupancy and weekly average ICU bed occupancy did not suggest that the system was substantially strained (supplementary figure S6), and we consistently observed that covid-19 was associated with increased risk of adverse outcomes regardless of level of occupancy and the excess risk was evident even in low occupancy. In our comparative evaluation, we considered several seasons of influenza, and the predominant subtype of seasonal influenza may vary by season; influenza A (H3N2) predominated in 2016-17 and 2017-18, influenza A(H1N1)pdm09 predominated in 2018-19, and influenza B predominated early in the 2019-20 season. H3N2 predominant seasons have been associated with more severe illness and mortality, especially in older adults, relative to seasons during which H1N1 or B viruses predominated.37 38 In our evaluation, we did not identify appreciable differences in risk of covid-19 versus seasonal influenza by season or by influenza type (A or B). However, our data did not contain enough information to allow a comparative evaluation according to influenza subtype; evaluation of the risks of death, mechanical ventilation, and ICU admission for covid-19 versus influenza type A by season yielded consistently higher risk of adverse events for covid-19. Effect of sex, body mass index, and smoking status on health outcomes may be different in covid-19 and seasonal influenza.39 40 41 42 43 44 45 Our analyses of subgroups based on sex, body mass index category, and smoking status showed consistently that covid-19 was associated with worse outcomes than seasonal influenza, but further studies are needed to gain a fuller understanding of how these characteristics may differentially influence health outcomes in patients with covid-19 and seasonal influenza.
Limitations and strengths of study
This study has several limitations. We relied on data from electronic health records to do this comparative evaluation, and, although we used validated definitions, inaccurate measurements of variables and misclassification bias may not be completely eliminated. We used a doubly robust approach to examine the risk of clinical manifestations and health outcomes for covid-19 versus seasonal influenza,19 but we cannot completely exclude the possibility of residual confounding. Our cohort comprised US veterans who were admitted to hospital for seasonal influenza or covid-19 and included mostly older white males, so our results may not be generalizable to broader populations such as younger and healthier people. Although we provide estimates of the adjusted absolute rates of death according to age, race, and comorbid condition to facilitate comparative evaluation of risk in these groups, absolute rates may be lower in younger and healthier populations. Our evaluation included a comparative assessment of clinical manifestations and outcomes of seasonal influenza and covid-19; however, a pandemic influenza may have different clinical manifestations and outcome profile. Finally, as this pandemic continues to evolve, our understanding of the epidemiology, clinical characteristics, and outcomes of covid-19 will continue to evolve; in particular, we note that care of covid-19 patients is evolving rapidly, including discovery and use of new therapeutics, and a vaccine is anticipated sometime soon. Both drivers (improved care and vaccine availability) will likely result in amelioration of health outcomes among people with covid-19.
The study also has several strengths. We designed the study to evaluate risk of death among people admitted to hospital with covid-19 and seasonal influenza in the same nationally integrated healthcare delivery system designed to give equal access to care for eligible veterans, which enhances the ability to establish a comparative evaluation of risk. The multidimensional nature and depth of VA data facilitate ascertainment of medical conditions not only through diagnostic or procedure codes but also through laboratory data (for example, serum creatinine to define acute kidney injury, estimated glomerular filtration rate to define chronic kidney disease, creatine phosphokinase to define rhabdomyolysis), vital signs (including body mass index), vital status, and medication records (for example, vasopressor use, insulin requirements). Finally, we used a doubly robust approach to examine the association between exposures and outcomes, and our results were robust to challenge in multiple sensitivity analyses including the application of two alternative methods (overlap weighting method and inverse probability weighting method).
Conclusions
In sum, our results suggest that compared with patients admitted to hospital with seasonal influenza, those admitted with covid-19 have increased risk of systemic clinical manifestations (including higher risk of acute kidney injury, incident renal replacement therapy, incident insulin use, severe septic shock, vasopressor use, pulmonary embolism, deep venous thrombosis, stroke, acute myocarditis, arrythmias and sudden cardiac death, elevated troponin, elevated aspartate aminotransferase, elevated alanine aminotransferase, and rhabdomyolysis), increased risk of death, and higher need for healthcare resources (mechanical ventilation, ICU admission, length of hospital stay). Rates of death for covid-19 in excess of rates for seasonal influenza were highest in adults aged over 75 years with chronic kidney disease and dementia and in black people with obesity, diabetes, and chronic kidney disease. Our results inform the global discussion about the comparative risks of covid-19 and seasonal influenza and may guide efforts to manage this ongoing global pandemic.
What is already known on this topic
Covid-19 is an emerging infectious disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), of which the predominant clinical presentation is respiratory disease
Evidence is accumulating that it is associated with extrapulmonary manifestations and high risk of healthcare use and death
Comparisons of clinical manifestations and mortality between covid-19 and seasonal influenza have been made using disparate data and statistical methods and do not represent “apples with apples” comparisons
What this study adds
Among people admitted to hospital, compared with seasonal influenza, covid-19 was associated with increased risk of various extrapulmonary clinical manifestations
Covid-19 was also associated with increased health resource use, including mechanical ventilator use, admission to intensive care, and length of hospital stay, and nearly five times the risk of death
The differences in death rates between covid-19 and seasonal influenza were most manifest in older adults with kidney disease or dementia and black people with obesity, diabetes, or kidney disease
Web extra.
Extra material supplied by authors
Web appendix: Supplementary materials
Contributors: YX, BB, and ZAA contributed to the development of the study concept and design. YX and BB contributed to data acquisition. YX, BB, and ZAA contributed to data analysis and interpretation. YX and BB contributed to statistical analysis. YX and ZAA drafted the manuscript. XY, BB, GM, and ZAA contributed to critical revision of the manuscript. ZAA provided administrative, technical, and material support, as well as supervision and mentorship. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved. All authors approved the final version of the report. The corresponding author attests that all the listed authors meet the authorship criteria and that no others meeting the criteria have been omitted. ZAA is the guarantor.
Funding: This research was funded by the United States Department of Veterans Affairs and the Institute for Public Health at Washington University in Saint Louis, MO, USA (for ZAA), and the American Society of Nephrology and KidneyCure pre-doctoral fellowship awards (for YX and BB). The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication. The contents do not represent the views of the US Department of Veterans Affairs or the US government.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: support from the United States Department of Veterans Affairs, the Institute for Public Health at Washington University in Saint Louis, MO, USA, the American Society of Nephrology, and KidneyCure; no financial relationships with any organizations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Ethical approval: This research project was reviewed and approved by the Institutional Review Board of the Department of Veterans Affairs Saint Louis Health Care System.
Data sharing: All data are available through the United States Department of Veterans Affairs.
The lead author (the manuscript’s guarantor) affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Provenance and peer review: Not commissioned; externally peer reviewed.
Dissemination to participants and related patient and public communities: There are no plans to disseminate the results of the research to study participants. Study results will be shared with the public via press release, social media, and conference presentations.
References
- 1.Johns Hopkins Coronavirus Resource Center. COVID-19 map. 2020. https://coronavirus.jhu.edu/map.html.
- 2. Faust JS, Del Rio C. Assessment of Deaths From COVID-19 and From Seasonal Influenza. JAMA Intern Med 2020;180:1045-6. 10.1001/jamainternmed.2020.2306 [DOI] [PubMed] [Google Scholar]
- 3. Xie Y, Bowe B, Li T, Xian H, Balasubramanian S, Al-Aly Z. Proton Pump Inhibitors and Risk of Incident CKD and Progression to ESRD. J Am Soc Nephrol 2016;27:3153-63. 10.1681/ASN.2015121377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Xie Y, Bowe B, Li T, Xian H, Yan Y, Al-Aly Z. Risk of death among users of Proton Pump Inhibitors: a longitudinal observational cohort study of United States veterans. BMJ Open 2017;7:e015735. 10.1136/bmjopen-2016-015735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Xie Y, Bowe B, Li T, Xian H, Yan Y, Al-Aly Z. Long-term kidney outcomes among users of proton pump inhibitors without intervening acute kidney injury. Kidney Int 2017;91:1482-94. 10.1016/j.kint.2016.12.021 [DOI] [PubMed] [Google Scholar]
- 6. Xie Y, Bowe B, Li T, Xian H, Yan Y, Al-Aly Z. Higher blood urea nitrogen is associated with increased risk of incident diabetes mellitus. Kidney Int 2018;93:741-52. 10.1016/j.kint.2017.08.033 [DOI] [PubMed] [Google Scholar]
- 7. Vincent BM, Wiitala WL, Burns JA, Iwashyna TJ, Prescott HC. Using veterans affairs corporate data warehouse to identify 30-day hospital readmissions. Health Serv Outcomes Res Methodol 2018;18:143-54 10.1007/s10742-018-0178-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maynard C. Ascertaining veterans' vital status: VA data sources for mortality ascertainment and cause of death. 2017. https://www.hsrd.research.va.gov/for_researchers/cyber_seminars/archives/video_archive.cfm?SessionID=1242.
- 9. Xie Y, Bowe B, Gibson AK, et al. Comparative Effectiveness of SGLT2 Inhibitors, GLP-1 Receptor Agonists, DPP-4 Inhibitors, and Sulfonylureas on Risk of Kidney Outcomes: Emulation of a Target Trial Using Health Care Databases. Diabetes Care 2020;43:2859-69. 10.2337/dc20-1890 [DOI] [PubMed] [Google Scholar]
- 10. Xie Y, Bowe B, Gibson AK, et al. Comparative Effectiveness of the Sodium-Glucose Cotransporter 2 Inhibitor Empagliflozin Versus Other Antihyperglycemics on Risk of Major Adverse Kidney Events. Diabetes Care 2020;43:2785-95. 10.2337/dc20-1231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract 2012;120:c179-84. [DOI] [PubMed] [Google Scholar]
- 12. Bowe B, Xie Y, Li T, Yan Y, Xian H, Al-Aly Z. Associations of ambient coarse particulate matter, nitrogen dioxide, and carbon monoxide with the risk of kidney disease: a cohort study. Lancet Planet Health 2017;1:e267-76. 10.1016/S2542-5196(17)30117-1 [DOI] [PubMed] [Google Scholar]
- 13. Xie Y, Bowe B, Yan Y, Xian H, Li T, Al-Aly Z. Estimates of all cause mortality and cause specific mortality associated with proton pump inhibitors among US veterans: cohort study. BMJ 2019;365:l1580. 10.1136/bmj.l1580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bowe B, Xie Y, Xian H, Li T, Al-Aly Z. Association between Monocyte Count and Risk of Incident CKD and Progression to ESRD. Clin J Am Soc Nephrol 2017;12:603-13. 10.2215/CJN.09710916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bowe B, Xie Y, Li T, Yan Y, Xian H, Al-Aly Z. Estimates of the 2016 global burden of kidney disease attributable to ambient fine particulate matter air pollution. BMJ Open 2019;9:e022450. 10.1136/bmjopen-2018-022450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bowe B, Xie Y, Yan Y, Al-Aly Z. Burden of Cause-Specific Mortality Associated With PM2.5 Air Pollution in the United States. JAMA Netw Open 2019;2:e1915834. 10.1001/jamanetworkopen.2019.15834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Palevsky PM, Liu KD, Brophy PD, et al. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for acute kidney injury. Am J Kidney Dis 2013;61:649-72. 10.1053/j.ajkd.2013.02.349 [DOI] [PubMed] [Google Scholar]
- 18. Bowe B, Xie Y, Li T, et al. Changes in the us burden of chronic kidney disease from 2002 to 2016: An analysis of the global burden of disease study. JAMA Netw Open 2018;1:e184412. 10.1001/jamanetworkopen.2018.4412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Funk MJ, Westreich D, Wiesen C, Stürmer T, Brookhart MA, Davidian M. Doubly robust estimation of causal effects. Am J Epidemiol 2011;173:761-7. 10.1093/aje/kwq439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Linden A. Improving causal inference with a doubly robust estimator that combines propensity score stratification and weighting. J Eval Clin Pract 2017;23:697-702. 10.1111/jep.12714 [DOI] [PubMed] [Google Scholar]
- 21. Fine JP, Gray RJ. A Proportional Hazards Model for the Subdistribution of a Competing Risk. J Am Stat Assoc 1999;94:496-509 10.1080/01621459.1999.10474144. [DOI] [Google Scholar]
- 22. Puelles VG, Lütgehetmann M, Lindenmeyer MT, et al. Multiorgan and Renal Tropism of SARS-CoV-2. N Engl J Med 2020;383:590-2. 10.1056/NEJMc2011400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bowe B, Cai M, Xie Y, Gibson AK, Maddukuri G, Al-Aly Z. Acute Kidney Injury in a National Cohort of Hospitalized US Veterans with COVID-19. Clin J Am Soc Nephrol 2020;CJN.09610620. 10.2215/CJN.09610620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chan L, Coca S. Acute Kidney Injury in the time of COVID-19. Kidney360 2020;1:588-90 10.34067/KID.0003722020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Arentz M, Yim E, Klaff L, et al. Characteristics and Outcomes of 21 Critically Ill Patients With COVID-19 in Washington State. JAMA 2020;323:1612-4. 10.1001/jama.2020.4326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang D, Hu B, Hu C, et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA 2020;323:1061-9. 10.1001/jama.2020.1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Richardson S, Hirsch JS, Narasimhan M, et al. the Northwell COVID-19 Research Consortium Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA 2020;323:2052-9. 10.1001/jama.2020.6775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Petrilli CM, Jones SA, Yang J, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ 2020;369:m1966. 10.1136/bmj.m1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rubino F, Amiel SA, Zimmet P, et al. New-Onset Diabetes in Covid-19. N Engl J Med 2020;383:789-90. 10.1056/NEJMc2018688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ren H, Yang Y, Wang F, et al. Association of the insulin resistance marker TyG index with the severity and mortality of COVID-19. Cardiovasc Diabetol 2020;19:58. 10.1186/s12933-020-01035-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dallan C, Romano F, Siebert J, Politi S, Lacroix L, Sahyoun C. Septic shock presentation in adolescents with COVID-19. Lancet Child Adolesc Health 2020;4:e21-3. 10.1016/S2352-4642(20)30164-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Middeldorp S, Coppens M, van Haaps TF, et al. Incidence of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost 2020;18:1995-2002. 10.1111/jth.14888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhang Y, Xiao M, Zhang S, et al. Coagulopathy and Antiphospholipid Antibodies in Patients with Covid-19. N Engl J Med 2020;382:e38. 10.1056/NEJMc2007575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mao L, Jin H, Wang M, et al. Neurologic Manifestations of Hospitalized Patients With Coronavirus Disease 2019 in Wuhan, China. JAMA Neurol 2020;77:683-90. 10.1001/jamaneurol.2020.1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Romero-Sánchez CM, Díaz-Maroto I, Fernández-Díaz E, et al. Neurologic manifestations in hospitalized patients with COVID-19: The ALBACOVID registry. Neurology 2020;95:e1060-70. 10.1212/WNL.0000000000009937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Klok FA, Kruip MJHA, van der Meer NJM, et al. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: An updated analysis. Thromb Res 2020;191:148-50. 10.1016/j.thromres.2020.04.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Salgado CD, Farr BM, Hall KK, Hayden FG. Influenza in the acute hospital setting. Lancet Infect Dis 2002;2:145-55. 10.1016/S1473-3099(02)00221-9 [DOI] [PubMed] [Google Scholar]
- 38. Lytras T, Pantavou K, Mouratidou E, Tsiodras S. Mortality attributable to seasonal influenza in Greece, 2013 to 2017: variation by type/subtype and age, and a possible harvesting effect. Euro Surveill 2019;24:1800118. 10.2807/1560-7917.ES.2019.24.14.1800118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bhopal SS, Bhopal R. Sex differential in COVID-19 mortality varies markedly by age. Lancet 2020;396:532-3. 10.1016/S0140-6736(20)31748-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Grasselli G, Greco M, Zanella A, et al. COVID-19 Lombardy ICU Network Risk Factors Associated With Mortality Among Patients With COVID-19 in Intensive Care Units in Lombardy, Italy. JAMA Intern Med 2020;180:1345-55. 10.1001/jamainternmed.2020.3539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Han L, Ran J, Mak YW, et al. Smoking and Influenza-associated Morbidity and Mortality: A Systematic Review and Meta-analysis. Epidemiology 2019;30:405-17. 10.1097/EDE.0000000000000984 [DOI] [PubMed] [Google Scholar]
- 42. Morgan OW, Bramley A, Fowlkes A, et al. Morbid obesity as a risk factor for hospitalization and death due to 2009 pandemic influenza A(H1N1) disease. PLoS One 2010;5:e9694. 10.1371/journal.pone.0009694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pettit NN, MacKenzie EL, Ridgway JP, et al. Obesity is Associated with Increased Risk for Mortality Among Hospitalized Patients with COVID-19. Obesity (Silver Spring) 2020;28:1806-10. 10.1002/oby.22941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tsigaris P, Teixeira da Silva JA. Smoking Prevalence and COVID-19 in Europe. Nicotine Tob Res 2020;22:1646-9. 10.1093/ntr/ntaa121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. van Zyl-Smit RN, Richards G, Leone FT. Tobacco smoking and COVID-19 infection. Lancet Respir Med 2020;8:664-5. 10.1016/S2213-2600(20)30239-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Web appendix: Supplementary materials