Abstract
PURPOSE:
Data supporting neoadjuvant chemotherapy (NAC) in high-grade (HG) upper-tract urothelial carcinoma (UTUC) is scant. This multi-institution prospective phase II trial investigated pathologic complete response (pCR) after NAC for HG UTUC.
METHODS:
Patients with HG UTUC planned for nephroureterectomy (NU) were assigned to 4 NAC cycles of either accelerated methotrexate, vinblastine, doxorubicin, cisplatin (aMVAC) for baseline CrCl>50 mL/min or gemcitabine and carboplatin (GCa) for 30≤CrCl≤50 mL/min. Primary endpoint was pCR (ypT0N0). Accrual goal was 30 patients per arm. Eighteen percent pCR was considered worthy of further study, while 4% pCR would not justify pursuit of this regimen. With 28 eligible patients per arm, success was defined as ≥3 pCRs (10.7%) in a given arm. Secondary endpoints included safety, renal function, and oncologic outcomes.
RESULTS:
From 2015–2017, 30 patients enrolled in the aMVAC arm. Six enrolled in the GCa arm, which closed for poor accrual. Of 29 eligible aMVAC patients (23 M, 6F) with median age 65 (40–84), 80% completed all planned treatments, 3 achieved ypT0N0 (10.3%), and a fourth achieved ypT0Nx (pCR=13.8%, 90% CI [4.9 – 28.8]). One aMVAC patient deferred NU due to grade 4 sepsis. Grade 3–4 toxicity rate was 23% in the aMVAC arm, with no grade 5 events.
CONCLUSION:
aMVAC NAC in patients with HG UTUC and CrCl>50 mL/min was safe and demonstrated pre-defined activity with 14% pCR. Final pathologic stage ≤ypT1 in over 60% of patients is encouraging. Together, the results of this prospective trial support the use of NAC in eligible patients with HG UTUC.
Keywords: upper tract urothelial carcinoma, neoadjuvant chemotherapy, radical nephroureterectomy, pathologic response
INTRODUCTION
Approximately 90% of urothelial cancers occur in the bladder, while 5–10% arise from the renal pelvis or ureter, upper tract urothelial carcinoma (UTUC).1, 2 Like patients with bladder cancer, UTUC are older, predominantly male, with a smoking history and related comorbidities such as acute or chronic kidney injury due to tumor obstruction and or parenchymal renal disease.1,3.
LLevel 1 evidence demonstratesdemonstrates overall survival benefit for neoadjuvant cisplatin-based chemotherapy (NAC) in urothelial bladder cancer.4, 5 For UTUC, the data for NAC is encouraging, based on pathologic downstaging and improved survival outcomes;6–10 studies are in retrospective series. Prospective evaluation of NAC in large populations is challenging, as the cancer is relatively rare, difficult to preoperatively stage due to the technical limitations of upper urinary tract sampling,, and aforementioned comorbid conditions.,
In 2018, the randomized phase III POUT trial of postoperative platinum-based chemotherapy for patients with high risk UTUC (pT2–4 or N+) wasshowed a 2-year disease-free survival (DFS) benefit with the addition of adjuvant chemotherapy, with greatest benefit observed in the cisplatin-treated patients..11 However, delivery of chemotherapy in the adjuvant setting for urothelial cancer has significant limitations.12 Though cisplatin-based NAC improves OS for bladder cancer, results from adjuvant trials show modest benefit and are often underpowered.13–16 In UTUC, post nephroureterectomy renal function impairment following nephroureterectomyoften precludes the use of cisplatin,3, 17, 18 which is not recommended for creatinine clearance (CrCl) below 50–60 ml/min. Thus, morethe neoadjuvant setting is optimal to prospectively investigate perioperative chemotherapy in UTUC. These unmet needs and factors influenced the design of the ECOG-ACRIN 8141 NAC trial reported herein.
METHODS
Patient Eligibility
Eligible patients with high-grade (HG) UTUC in the upper urinary tract (renal pelvis or proximal ureter) planned for NU prospectively enrolled in this two-arm phase II study. Institutional Review Board (IRB) approval was obtained at each study site. Diagnosis of HG UTUC could be confirmed by biopsy, cytology with a mass on cross-sectional imaging, or cytology with ureteroscopic mass visualization. Patients with radiographic metastases or any lymph nodes >1 cm were excluded. Baseline serum creatinine and 24-hour urine CrCl was measured, CrCl ≥30 was required for eligiblity. ECOG performance status (PS) of 0–1 was required.
A history of prior resectable urothelial bladder cancer was allowed (see Supplementary Methods). Concomitant primary bladder cancer was also allowed, as long as these sites were surgically resected and non-muscle-invasive (≤cT1N0). Patients with any active second malignancy (defined as > 30% risk of relapse after completion of all necessary therapy) other than non-melanoma skin cancers and biochemical relapsed prostate cancer were ineligible. Additional eligibility criteria are provided in the Supplementary Methods.
Treatment
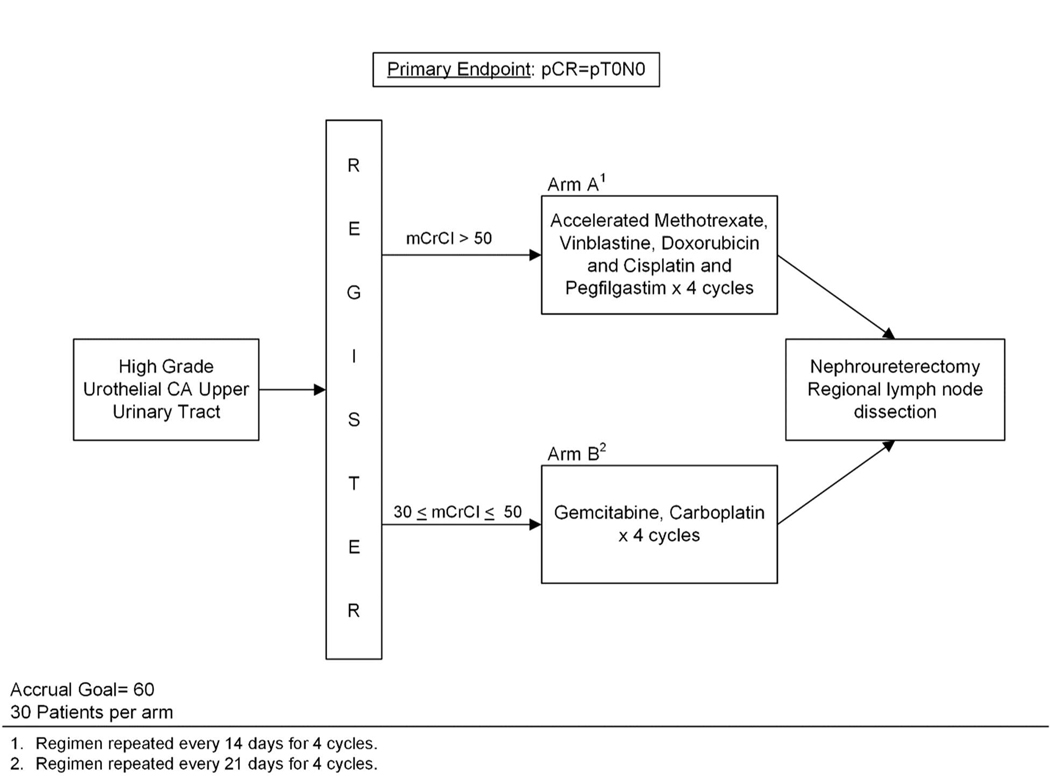
Patients were assigned to one of two treatment arms of four cycles of NAC according to baseline renal function. Cisplatin-eligible patients with CrCl >50 mL/min were assigned to arm A - accelerated methotrexate, vinblastine, doxorubicin, and cisplatin (aMVAC) cycled every 14 days. Cisplatin-ineligible patients with CrCl 30–50 mL/min were assigned to arm B - carboplatin and gemcitabine (GCa) cycled every 21 days.Growth factor support was permitted per ASCO guidelines.5 Treatment regimens are provided in the Supplementary Methods. Post-chemotherapy, preoperative imaging was performed to ensure that there was no metastatic progression during chemotherapy. Radical NU and regional lymph node dissection were to be performed between 21 and 60 days after completion of chemotherapy. Surgical approach (open, laparoscopic, or robotic assistance) was at surgeon’s discretion. Urinary bladder cuff was routinely excised, and regional lymph node dissection was performed according to tumor location.19 Given little prospective data on the utility of routine lymph node dissection in UTUC, template-based lymphadenectomy was encouraged, but not mandated. Figure 1 shows the study schema
Figure 1.
Study schema. Accrual goal for this two-arm phase II trial was 60 patients (30 per arm) with high-grade upper tract urothelial carcinoma (HG UTUC). Patients were assigned to one of two treatment arms depending on their baseline creatinine clearance (CrCl). Primary endpoint was complete pathologic response (pCR), defined as pT0N0.
Outcome Measures and Statistical Analysis
The primary outcome measure was the rate of complete pathologic response (pCR, ypT0N0) attained by NAC, assessed by standard pathologic review.following. The primary analysis was performed in all eligible patients who started any part of protocol therapy. A sensitivity analysis was conducted among eligible patients who received at least one dose of chemotherapy. There was no planned direct comparison between treatment arms. Accrual goal was 30 per arm.
Based on prior studies, a pCR rate ≥18% was deemed worthy of further study, while a pCR rate of ≤4% would not justify further pursuit of either regimen.6–9 With 28 eligible patients per arm, a treatment was considered promising if ≥3 pCRs were observed in an arm. Using this design, there was a 10% probability of declaring a treatment worthy of further study if the true pCR rate was 4%, and a 90% probability of declaring a treatment worthy of further study if the true pCR rate was 18%, using a one-sided exact binomial test.
Secondary endpoints included safety, renal functional outcomes, recurrence-free survival (RFS, defined as the time from the date of surgery to disease recurrence or death from any cause), bladder cancer-free survival, cancer-specific mortality (CSM), and event-free survival (EFS, defined as the time to first recurrence, disease progression, new invasive primary cancer, or death from any cause). All patients who received any part of the treatment were included in the toxicity analysis, graded per CTCAE v4.0. Patients undergoing NU beyond 6 months of completion of chemotherapy were followed for secondary endpoints but not evaluable for pCR.
RESULTS
Study Conduct and Patient Characteristics
The study activated in April 2015. In May 2017, the aMVAC arm reached accrual goal of 30 patients. The GCa was closed in January 2018 after only 6 patients enrolled. OOne patient in the aMVAC arm underwent NU for an infiltrative renal mass with urinary cytology concerning for urothelial carcinoma; however, ultimately had a renal cell carcinoma and was excluded from the final pCR efficacy analysis. The CONSORT diagram is shown in Figure 2.
Figure 2.
EA8141 CONSORT diagram. 36 patients were allocated to arm A (n=30) or B (n=6). 29 and 6 patients in arms A and B, respectively, were included in final analysis.
Among the 29 eligible patients in the aMVAC arm (23 males, 6 females), median age was 65 years (range 40–84). ECOG PS was 0 in 17 patients (59%) and 1 in 12 patients (41%). Among the 6 patients in the GCa arm (4 males, 2 females), median age was 74 years (range 53–78). ECOG PS was 0 in 2 patients (33%) and 1 in 4 patients (67%). Across both groups, the population was predominantly white (94%). Seventeen patients harbored isolated renal pelvic tumors, 5 harbored isolated ureteral tumors, and the remainder had multifocal tumors. Patient demographics and baseline disease characteristics are shown in Table 1.
Table 1.
Eligible patient demographics and disease characteristics by treatment arm.
| Arm A | Arm B | Total | |
|---|---|---|---|
| n = 29 | n = 6 | n = 35 | |
| n (%) | n (%) | n (%) | |
| Sex | |||
| Female Gender | 6 (21) | 2 (33) | 8 (23) |
| Male Gender | 23 (79) | 4 (67) | 27 (77) |
| Age - median (range) | 65 (40 – 84) | 75 (53 – 78) | 66 (40 – 84) |
| Race | |||
| Asian | 1 (4) | 0 (0) | 1 (3) |
| Black or African American | 1 (4) | 0 (0) | 1 (3) |
| White | 26 (93) | 4 (100) | 30 (94) |
| Unknown/Not Reported | 1 | 2 | 3 |
| Ethnicity | |||
| Hispanic or Latino | 0 (0) | 2 (33) | 2 (6) |
| Not Hispanic or Latino | 27 (100) | 4 (67) | 31 (94) |
| Not Reported | 2 | 0 | 2 |
| PS | |||
| 0 | 17 (59) | 2 (33) | 19 (54) |
| 1 | 12 (41) | 4 (67) | 16 (46) |
| Clinical T stage (as reported by investigator) | |||
| T1 | 4 (14) | 1 (17) | 5 (14) |
| T2 | 8 (28) | 1 (17) | 9 (26) |
| T3 | 2 (7) | 0 (0) | 2 (6) |
| Ta | 8 (28) | 4 (67) | 12 (34) |
| TX | 7 (24) | 0 (0) | 7 (20) |
| Clinical N stage (as reported by investigator) | |||
| N0 | 22 (76) | 6 (100) | 28 (80) |
| N1 | 1 (3) | 0 (0) | 1 (3) |
| NX | 6 (21) | 0 (0) | 6 (17) |
| Prior radiation therapy (any) | 1 (3) | 1 (17) | 2 (6) |
| Prior anthracyclines/doxorubicin | 0 (0) | 0 (0) | 0 (0) |
| Prior surgery (any) | 2 (7) | 1 (17) | 3 (9) |
| Prior NAC | 1 (3) | 0 (0) | 1 (3) |
| Other prior therapy (any) | 2 (7) | 0 (0) | 2 (6) |
Study Treatments
The total number of NAC cycles received per patient is summarized in Supplemental Table S1. In each arm, the median number of chemotherapy cycles received was 4 (range 1–4 for aMVAC, range 3–4 for GCa). Twenty-four of 30 eligible patients (80%) completed all 4 cycles of aMVAC, and 5/6 patients (83%) completed all 4 cycles of GCa. No progressed or developed metastatic disease on post-chemotherapy imaging. One eligible patient refused surgery following toxicity from NAC. Median time to NU from completion of NAC for the aMVAC arm was 42 days (range 28–106). Two patients underwent delayed NU outside of the protocol 60-day window (at 70 and 106 days). Median time to NU from completion of NAC for the GCa arm was 36 days (range 29–48).
Efficacy
For the primary endpoint, 3/29 patients in the aMVAC arm achieved ypT0N0 at NU (10.3%, 90% CI [2.9–24.6]). After including one additional patient with clinical CR on preoperative imaging, who did not undergo lymph node dissection at NU at surgeon discretion (ypT0Nx), the pCR rate in the aMVAC arm was 4/29 (14%, 90% CI [4.9–28.8]). Final pathologic stage ≤pT1 was also of interest, given its association with improved long-term outcomes in retrospective UTUC series.6–8 In total, 18/29 patients (62%) in the aMVAC arm achieved final pathologic stage ≤pT1. In the GCa arm, 1 patient (17%) achieved ypT0N0 at NU, and 3/6 patients (50%) achieved final pathologic stage ≤pT1. Initial investigator-assessed clinical stage and final pathologic stage at NU by arm are shown in Table 2(A-B).
Table 2.
Initial investigator assessed clinical stage and final pathologic stage at nephroureterectomy for A) aMVAC and B) GCa arms.
| A) aMVAC arm | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Clinical stage | Pathologic stage | ||||||||||
| T0N0 | T0NX | T1N0 | T1NX | T2N0 | T3N0 | T3NX | TaN0 | TaN2 | TisN0 | Unevaluable | |
| T1N0 | - | 1 | - | - | - | - | - | - | - | 1 | - |
| T1N1 | - | - | - | - | - | 1 | - | - | - | - | - |
| T1NX | - | - | - | - | - | - | 1 | - | - | - | - |
| T2N0 | 1 | - | 3 | - | - | 1 | - | 1 | - | 1 | - |
| T2NX | - | - | - | - | - | 1 | - | - | - | - | - |
| T3N0 | - | - | - | - | - | - | 1 | - | - | 1 | - |
| TaN0 | 1 | - | 2 | - | 1 | - | - | 1* | 1 | - | - |
| TaNX | - | - | 1 | - | - | 1 | - | - | - | - | - |
| TXN0 | 1 | - | 1 | 1 | - | 1 | - | 1 | - | - | - |
| TXNX | - | - | - | - | - | 1 | - | - | - | - | 1** |
| B) GCa arm | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Clinical stage | Pathologic stage | ||||||||||
| T0N0 | T2NX | T3N0 | T3N2 | TaN0 | |||||||
| T1N0 | - | 1 | - | - | - | ||||||
| T2N0 | - | - | - | 1 | - | ||||||
| TaN0 | 1 | - | 1 | - | 2 | ||||||
One patient in Arm B had R1 disease.
Reason unevaluable: Patient did not have NU
Oncologic outcomes are displayed in Supplemental Figures S1-S4. There was one patient in arm A who did not undergo NU and another in arm B who had positive margins after surgery and were excluded from RFS and bladder cancer-free survival (BCFS) analyses. Patients without postoperative follow-up were censored at day 0. RFS curves are shown in Figure S1. The median RFS has not been reached on arm A with a median follow-up of 21.1 months. The median (90% CI) RFS in arm B was 8.5 months (3.3, NA). EFS curves are shown in Figure S2. In arm A, the median has not been reached; in arm B, the median EFS was 14.5 months (10.2, NA). BCFS is displayed in Figure S3. There have been two bladder recurrences in arm A and one in arm B. A median BCFS has not been reached in either arm. Eighteen-month BCFS rates (90% CI) for arms A and B were 91.3% (82.1, 100.0) and 75.0% (46.6, 100.0), respectively. CSM is shown in Figure S4. There were no competing mortality events, as all deaths were due to urothelial cancer. A median CSM has not been reached in either arm. The cumulative incidence (90% CI) of cancer-specific deaths at 24 months was 0.09 (0.02, 0.21) in arm A and 0.20 (0.01, 0.56) in arm B.
Safety
All treated patients were included in the safety analysis. There were no lethal (grade 5) eventssubjects. Grade 3–4 toxicity rate was 23% (7/30 patients) in the aMVAC arm and 50% (3/6 subjects) in the GCa arm. One patient in the aMVAC arm with cycle 1 grade 4 sepsis deferred further NAC and NU. Toxicities are summarized by treatment arm in Table 3.
Table 3.
Treatment-related toxicities by CTCAE v4.0 per treatment arm.
| Toxicity Type | Treatment Arm | |||||||
|---|---|---|---|---|---|---|---|---|
| A (n=30) | B (n=6) | |||||||
| Grade | Grade | |||||||
| 1,2 | 3 | 4 | 5 | 1,2 | 3 | 4 | 5 | |
| (n) | (n) | (n) | (n) | (n) | (n) | (n) | (n) | |
| Anemia | 20 | 2 | - | - | 3 | 2 | - | - |
| Fatigue | 22 | 1 | - | - | 5 | - | - | - |
| Diarrhea | - | 1 | - | - | - | - | - | - |
| Gastrointestinal disorders - Other | - | 1 | - | - | - | - | - | - |
| Mucositis oral | - | 1 | - | - | - | - | - | - |
| Nausea | 23 | - | - | - | 4 | - | - | - |
| Vomiting | 6 | - | - | - | 2 | - | - | - |
| Lower gastrointestinal bleed hemorrhage | - | 1 | - | - | - | - | - | - |
| Sepsis | - | - | 1 | - | - | - | - | - |
| Fall | - | 1 | - | - | - | - | - | - |
| Vascular access complication | - | 1 | - | - | - | - | - | - |
| Alanine aminotransferase increased | - | 1 | - | - | - | - | - | - |
| Creatinine increased | 10 | - | - | - | 2 | - | - | - |
| Elevated D-dimer | - | - | 1 | - | - | - | - | - |
| Neutrophil count decreased | 1 | 1 | 1 | - | 2 | 1 | 1 | - |
| Platelet count decreased | 8 | 1 | - | - | 2 | 1 | - | - |
| White blood cell decreased | 2 | 1 | 1 | - | 2 | 2 | - | - |
| Dehydration | - | 3 | - | - | - | - | - | - |
| Hypokalemia | - | 1 | - | - | - | - | - | - |
| Peripheral sensory neuropathy | 4 | - | - | - | - | - | - | - |
| Pharyngolaryngeal pain | - | 1 | - | - | - | - | - | - |
| Pneumonitis | - | 1 | - | - | - | - | - | - |
| Thromboembolic event | - | 1 | - | - | - | - | - | - |
| WORST DEGREE | 23 | 5 | 2 | - | 3 | 2 | 1 | - |
Renal Functional Outcomes
Renal function at baseline, following chemotherapy, and following surgery is summarized in Table 4 and Figure S5, stratified by treatment arm. Pre-chemotherapy, median CrCl in the aMVAC arm was 82.0 mL/min (range 53.7–170.0). Two patients in this arm had renal insufficiency prior to chemotherapy (CrCl <60 mL/min) (6.7%, 90% CI [1.2–19.5]). Following NAC, 6 patients (20.0%, 90% CI [9.1–35.7]) had renal insufficiency (median 1.4% decrease from baseline). After surgery, 20/29 patients (69.0%, 90% CI [52.1–82.8]) had renal insufficiency (median 40.8% decrease from baseline). Seventeen of 29 (59%) of patients were cisplatin-ineligible (CrCl ≤50 mL/min) after surgery.
Table 4.
Renal function outcomes at baseline, following neoadjuvant chemotherapy, and following surgery for each treatment arm.
| Baseline | Post-chemo | Post-surgery | |
|---|---|---|---|
| Arm A (aMVAC) | |||
| Measured | 30 | 30 | 29 |
| Median (range) CrCl (mL/min) | 82.0 (53.7, 170.0) | 75.5 (23.6, 203.0) | 48.0 (25.9, 173.4) |
| N, CrCl < 60 mL/min | 2 | 6 | 20 |
| % (90% CI), CrCl < 60 mL/min | 6.7 (1.2, 19.5) | 20.0 (9.1, 35.7) | 69.0 (52.1, 82.8) |
| N, CrCl < 30mL/min | 0 | 1 | 2 |
| % (90% CI), CrCl < 30 mL/min | 0.0 (0.0, 9.5) | 3.3 (0.2, 14.9) | 6.9 (1.2, 20.2) |
| Median (range) % change from baseline | - | −1.4 (−64.5, 71.1) | −40.8 (−61.8, 17.2) |
| Arm B (GCa) | |||
| Measured | 6 | 6 | 6 |
| Median (range) CrCl (mL/min) | 45.5 (37.0, 50.0) | 48.1 (35.3, 91.4) | 38.7 (18.9, 68.3) |
| N, CrCl < 60 mL/min | 6 | 5 | 5 |
| % (90% CI), CrCl < 60 mL/min | 100 (60.7, 100.0) | 83.3 (41.8, 99.1) | 83.3 (41.8, 99.1) |
| N, CrCl < 30 mL/min | 0 | 0 | 2 |
| % (90% CI), CrCl < 30 mL/min | 0.0 (0.0, 39.3) | 0.0 (0.0, 39.3) | 33.3 (6.3, 72.9) |
| Median (range) % change from baseline | - | 10.6 (−4.6, 82.8) | −12.9 (−61.5, 84.6) |
The six subjects in the GCa arm had pre-chemotherapy baseline CrCl median 45.5 mL/min (range 37.0–50.0). Following NAC, CrCl notably increased by median 10.6% from baseline. Following surgery, however, median change in CrCl was −12.9% from baseline, and 2/6 (33%) patients had CrCl <30 mL/min.
DISCUSSION
This multicenter ECOG-ACRIN-led intergroup prospective trial shows that neoadjuvant aMVAC for HG UTUC is safe in patients with adequate renal function and leads to complete responses in 14% and non-muscle-invasive final pathologic stage in 62% of patients. These findings are congruent with previously published retrospective studies of NAC in UTUC showing similar rates of complete response and non-muscle-invasive disease.6–9 Postoperative complication rates were comparable to series reporting NU outcomes without chemotherapy,20 and no patient died or progressed during chemotherapy. Renal function was relatively stable following aMVAC, with 6.7% of patients having pre-chemotherapy baseline CrCl <60 mL/min, compared to 20.0% post-aMVAC. The proportion of patients with CrCl <60 mL/min increased substantially to 69.0% after aMVAC and NU, providing clear contemporary prospective evidence of the impact of nephrectomy upon renal function.
Together with the phase III POUT study,11 evidence is mounting in support of perioperative chemotherapy for HG UTUC. Questions regarding use and optimal perioperative chemotherapy timing for patients with UTUC remain. Concerns of overtreatment with NAC should be dispelled, as retrospective comparisons of UTUC populations treated with and without NACovertreatment show final pathologic stage is lower in groups who received chemotherapy, suggesting clinical benefit from treatment6–9. Overtreatment concern must be balanced by the reality of under-staging, apparent in our cohort as 10 subjects had non-organ-confined disease (≥pT3 and/or pN+) on final pathology, yet only 3 patients were were assigned ≥pT3 on investigator-assessed preoperative clinical staging (Table 2). Unfortunately, a significant proportion of patients are not candidates for adjuvant chemotherapy based on renal function declines, possible postoperative complications or other eligibility limiting comorbidities.3, 17, 18, 21 The 2-year survival data from the current study is superior to that reported by the POUT trial; while comparing results from 2 different studies may be difficult, the totality of evidence strongly supports the NAC approach. .22
Every 14 day (accelerated) MVAC was adopted from, and shown superior to multi-day MVAC in metastatic UC. Several studies have shown it is a safe and effiecnt neoadjuvant regimen, reducing the time from chemotherapy to extirpative surgery. Furthermore, in a retrospective multicenter study, neoadjuvant aMVAC was associated with a 28% pCR rate compared to 15% for neoadjuvant cisplatin and gemcitabine.7 Thus aMVAC has become the preferred standard at many academic centers, is the backbone of several modern urothelial trials, and guideline endorsed. The cisplatin-ineligible arm (GCa) did not accrue, we believe due to the,likely due to narrow CrCl (30–50 mL/min), and and lack of enthusiasm among referring urologists to defer NU for 12 weeks of GCa protocol therapy) eligibility, low. Learning the impact of the constraints, our team has been mindful of these limitations along with the lack of a control group of similar patients who did not receive aMVAC in the present single-arm design as we pursue novel UTUC protocol designs.
Questions remain whether biological differences between cancers in the upper tract compared to bladder cancer have different sensitivity to chemotherapy.23 The Cancer Genome Atlas (TCGA) and other annotated bladder cancer sequencing datasets show in bladder cancer, tumors inof the luminal-papillary subset have high FGFR3 mutations, fusions and amplifications, and low chemotherapy response.24 These subtypes are postulated but not validated in UTUC.but. UTUC has higher FGFR3 aberrations rate than bladder cancer, and therefore possibly more of the papillary luminal subset unlikely to respond to chemotherapy.23, 25 Consistent in neoadjuvant UTUC studies a lower pCR rate −9–18%, compared to approximately 38% pCR rate in bladder cancer following NAC.4–9 Standard debulking transurethral bladder resection (TURBT) prior to NAC can produce pCR rate as high as 15% \ alone.26–28 Challenge of performing debulking endoscopy in UTUC may account for some of this discrepancy, and the difficulty in the clinical staging of UTUC.
Adjuvant chemotherapy in the Phase III POUT study demonstrated increased 2-year DFS compared to observation, most pronounced in the cisplatin-eligible arm.11 As eligible patients were screened following surgeryPOUT did not capture high risk patients who were ineligible.by decline. In their study, 66% of chemotherapy-treated patients received gemcitabine/cisplatin, and 68% successfully completed the 4 planned chemotherapy cycles, while 80% and 83% of patients completed all cycles of aMVAC and GCa, respectively, in our study. Furthermore, the grade ≥3 toxicities werediedwere considerably higher than those in our smaller trial, as well as in bladder NAC aMVAC and modern gemcitabine/cisplatin studies, reinforcing observations that chemotherapy for urothelial cancers may be better tolerated in the neoadjuvant versus adjuvant setting.29, 30 Ongoing perioperative Perioperative practice informing urothelial studies \ (NCT01261728, NCT02876861, NCT02969083), and a chemo-immunotherapy NAC UTUC intergroup trial is in late-stage planning. We encourage enrollment of patients with UTUC to clinical trials, an understudied, but clinically aggressive form of urothelial cancer.
Supplementary Material
Supplemental Figure S1. Kaplan-Meier analysis of recurrence-free survival (RFS: time from the date of surgery to disease recurrence or death from any cause) stratified by treatment arm. One patient in arm A who did not undergo NU and another in arm B who had positive margins after surgery were excluded from RFS analysis. The median RFS has not been reached on arm A with a median follow-up of 21.1 months. The median (90% CI) RFS on arm B was 8.5 months (3.3, NA). Online only.
Supplemental Figure S2. Kaplan-Meier analysis of event-free survival (EFS: time to first recurrence, disease progression, new invasive primary cancer, or death from any cause) stratified by treatment arm. Median EFS has not been reached in arm A; in arm B, the median EFS was 10.2 (7.1, NA). Online only.
Supplemental Figure S3. Kaplan-Meier analysis of bladder cancer-free survival stratified by treatment arm. One patient in arm A who did not undergo NU and another in arm B who had positive margins after surgery were excluded from analysis. Median survival has not been reached in either arm. There have been two bladder cancer-related deaths on arm A and one on arm B. Online only.
Supplemental Figure S4. Kaplan-Meier analysis of urothelial cancer-specific mortality (CSM) stratified by treatment arm. Median CSM has not been reached in either arm. The cumulative incidence (90% CI) of cancer-specific deaths at 24 months was 0.09 (0.02, 0.21) in arm A and 0.20 (0.01, 0.56) in arm B. Online only.
Supplemental Figure S5. CrCl for each patient, shown by the black lines, at baseline, following receipt of NAC, and following NU for aMVAC (arm A) and GCa (arm B) groups. Red line represents CrCl 60 mL/min (threshold for renal insufficiency). Cisplatin eligibility was defined as CrCl ≥50 mL/min.
ACKNOWLEDGEMENTS
We would like to thank the patients and their families for participation in this trial.
FUNDING
This study was coordinated by the ECOG-ACRIN Cancer Research Group (Petr J. O’Dwyer, MD and Mitchell D. Schnall, MD, PhD, Group Co-Chairs) with study ID EA8141 and supported by the National Cancer Institute of the National Institutes of Health under the following award numbers: CA180820, CA180794, CA180834, CA180870, CA180801, CA180802, CA180858, CA180888. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. government. This trial is registered with the U.S. National Library of Medicine under ClinicalTrials.gov identifier NCT02412670.
This study was coordinated by the ECOG-ACRIN Cancer Research Group and supported by the National Cancer Institute of the National Institutes of Health: CA180820, CA180794, CA180834, CA180870, CA180801, CA180802, CA180858, CA180888
First presented as a late breaking abstract at the American Urologic Association (AUA) annual meeting May 21, 2018
Key of abbreviations
- NAC
neoadjuvant chemotherapy
- HG
high-grade
- UTUC
upper-tract urothelial carcinoma
- CKD
chronic kidney disease
- pCR
pathologic complete response
- DFS
disease-free survival
- EFS
event-free survival
- CSM
cancer specific mortality
- RFS
recurrence-free survival
- BCFS
bladder cancer-free survival
- NU
nephroureterectomy
- aMVAC
accelerated methotrexate, vinblastine, doxorubicin, cisplatin
- GCa
gemcitabine and carboplatin
- CrCl
creatinine clearance
- TCGA
The Cancer Genome Atlas
- TURBT
transurethral resection of bladder tumor
APPENDIX
All of the following collaborating co-authors contributed to this study:
-
-
Nirmish Singla, MD, MSCS, University of Texas Southwestern Medical Center, Dallas, TX
-
-
Antony Ruggeri, MD, Aurora Cancer Care-Southern Lakes VLCC, Burlington, WI
-
-
Leslie Howard, MD, Baystate Medical Center, Springfield, MA
-
-
John McCann, MD, Baystate Medical Center, Springfield, MA
-
-
Scott Delacroix, MD, East Jefferson General Hospital, Metairie, LA
-
-
Matthew Zibelman, MD, Fox Chase Cancer Center, Philadelphia, PA
-
-
Yagnesh Oza, MD, Good Samaritan Regional Health Center, Mount Vernon, IL
-
-
Jennifer Wang, MD, The University of Texas MD Anderson Cancer Center, Houston, TX
-
-
Benjamin Gartrell, MD, Montefiore Medical Center, Albert Einstein College of Medicine, Bronx, NY
-
-
Maha Hussain, MD, Northwestern University, Chicago, IL
-
-
Marc Matrana, MD, Ochsner Medical Center Jefferson, New Orleans, LA
-
-
Sam Benjamin, MD, State University of New York Upstate Medical University, Syracuse, NY
-
-
Guru Sonpavde, MD, University of Alabama at Birmingham Cancer Center, Birmingham, AL
-
-
Elaine Lam, MD, University of Colorado Hospital, Aurora, CO
-
-
Brandon Bernard, MD, University of Colorado Hospital, Aurora, CO
-
-
Yousef Zakharia, MD, Holden Comprehensive Cancer Center, University of Iowa, Iowa City, IA
-
-
Sarah Taylor, MD, University of Kansas Cancer Center, Westwood, KS
-
-
Matthew Milowsky, MD, University of North Carolina at Chapel Hill, Chapel Hill, NC
-
-
Sofia Ghani, MD, University of Oklahoma Health Sciences Center, Oklahoma City, OK
-
-
Sindhu Singh, MD, University of Oklahoma Health Sciences Center, Oklahoma City, OK
-
-
Kevin Kane, MD, University of Pittsburgh Cancer Institute, Pittsburgh, PA
-
-
Yull Arriaga, MD, University of Texas Southwestern Medical Center, Dallas, TX
-
-
Alicia Morgans, MD, MPH, Ingram Cancer Center, Vanderbilt University, Nashville, TN
-
-
David Chism, MD, MS, Ingram Cancer Center, Vanderbilt University, Nashville, TN
Footnotes
CONFLICTS OF INTEREST
The authors declare no relevant conflicts of interest.
REFERENCES
- 1.Roupret M, Babjuk M, Comperat E et al. : European Association of Urology Guidelines on Upper Urinary Tract Urothelial Carcinoma: 2017 Update. Eur Urol, 73: 111, 2018 [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A: Cancer statistics, 2018. CA Cancer J Clin, 68: 7, 2018 [DOI] [PubMed] [Google Scholar]
- 3.Singla N, Hutchinson R, Menegaz C et al. : Comparing Changes in Renal Function After Radical Surgery for Upper Tract Urothelial Carcinoma and Renal Cell Carcinoma. Urology, 96: 44, 2016 [DOI] [PubMed] [Google Scholar]
- 4.Grossman HB, Natale RB, Tangen CM et al. : Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med, 349: 859, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Milowsky MI, Rumble RB, Booth CM et al. : Guideline on Muscle-Invasive and Metastatic Bladder Cancer (European Association of Urology Guideline): American Society of Clinical Oncology Clinical Practice Guideline Endorsement. J Clin Oncol, 34: 1945, 2016 [DOI] [PubMed] [Google Scholar]
- 6.Almassi N, Gao T, Lee B et al. : Impact of Neoadjuvant Chemotherapy on Pathologic Response in Patients With Upper Tract Urothelial Carcinoma Undergoing Extirpative Surgery. Clin Genitourin Cancer 10.1158/1078-0432.CCR-18-2039 [DOI] [PubMed] [Google Scholar]
- 7.Aziz A, Dobruch J, Hendricksen K et al. : Perioperative chemotherapy in upper tract urothelial carcinoma: a comprehensive review. World J Urol, 35: 1401, 2017 [DOI] [PubMed] [Google Scholar]
- 8.Leow JJ, Martin-Doyle W, Fay AP et al. : A systematic review and meta-analysis of adjuvant and neoadjuvant chemotherapy for upper tract urothelial carcinoma. Eur Urol, 66: 529, 2014 [DOI] [PubMed] [Google Scholar]
- 9.Matin SF, Margulis V, Kamat A et al. : Incidence of downstaging and complete remission after neoadjuvant chemotherapy for high-risk upper tract transitional cell carcinoma. Cancer, 116: 3127, 2010 [DOI] [PubMed] [Google Scholar]
- 10.Porten S, Siefker-Radtke AO, Xiao L et al. : Neoadjuvant chemotherapy improves survival of patients with upper tract urothelial carcinoma. Cancer, 120: 1794, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Birtle AJ, Chester JD, Jones RJ, et al. : Results of POUT: A phase III randomised trial of perioperative chemotherapy versus surveillance in upper tract urothelial cancer (UTUC). J Clin Oncol, 36:6s: 407, 2018. (suppl; abstr 407) [Google Scholar]
- 12.Necchi A, Lo Vullo S, Mariani L et al. : Adjuvant chemotherapy after radical nephroureterectomy does not improve survival in patients with upper tract urothelial carcinoma: a joint study by the European Association of Urology-Young Academic Urologists and the Upper Tract Urothelial Carcinoma Collaboration. BJU Int, 121: 252, 2018 [DOI] [PubMed] [Google Scholar]
- 13.Galsky MD, Stensland KD, Moshier E et al. : Effectiveness of Adjuvant Chemotherapy for Locally Advanced Bladder Cancer. J Clin Oncol, 34: 825, 2016 [DOI] [PubMed] [Google Scholar]
- 14.Leow JJ, Martin-Doyle W, Rajagopal PS et al. : Adjuvant chemotherapy for invasive bladder cancer: a 2013 updated systematic review and meta-analysis of randomized trials. Eur Urol, 66: 42, 2014 [DOI] [PubMed] [Google Scholar]
- 15.Sternberg CN, Skoneczna I, Kerst JM et al. : Immediate versus deferred chemotherapy after radical cystectomy in patients with pT3–pT4 or N+ M0 urothelial carcinoma of the bladder (EORTC 30994): an intergroup, open-label, randomised phase 3 trial. The Lancet Oncology, 16: 76, 2015 [DOI] [PubMed] [Google Scholar]
- 16.Stadler WM, Lerner SP, Groshen S et al. : Phase III study of molecularly targeted adjuvant therapy in locally advanced urothelial cancer of the bladder based on p53 status. J Clin Oncol, 29: 3443, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singla N, Gayed BA, Bagrodia A et al. : Multi-institutional analysis of renal function outcomes following radical nephroureterectomy and partial ureterectomy for upper tract urothelial carcinoma. Urol Oncol, 33: 268 e1, 2015 [DOI] [PubMed] [Google Scholar]
- 18.Xylinas E, Rink M, Margulis V et al. : Impact of renal function on eligibility for chemotherapy and survival in patients who have undergone radical nephro-ureterectomy. BJU Int, 112: 453, 2013 [DOI] [PubMed] [Google Scholar]
- 19.Matin SF, Sfakianos JP, Espiritu PN et al. : Patterns of Lymphatic Metastases in Upper Tract Urothelial Carcinoma and Proposed Dissection Templates. J Urol, 194: 1567, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Raman JD, Jafri SM: Complications Following Radical Nephroureterectomy. Curr Urol Rep, 17: 36, 2016 [DOI] [PubMed] [Google Scholar]
- 21.Dash A, Galsky MD, Vickers AJ et al. : Impact of renal impairment on eligibility for adjuvant cisplatin-based chemotherapy in patients with urothelial carcinoma of the bladder. Cancer, 107: 506, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Petros FG, Qiao W, Singla N et al. : Preoperative multiplex nomogram for prediction of high-risk nonorgan-confined upper-tract urothelial carcinoma. Urol Oncol, 2018 [DOI] [PubMed] [Google Scholar]
- 23.Audenet F, Isharwal S, Cha EK et al. : Clonal relatedness and mutational differences between upper tract and bladder urothelial carcinoma. Clin Cancer Res 10.1158/1078-0432.CCR-18-2039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Robertson AG, Kim J, Al-Ahmadie H et al. : Comprehensive Molecular Characterization of Muscle-Invasive Bladder Cancer. Cell, 171: 540, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moss TJ, Qi Y, Xi L et al. : Comprehensive Genomic Characterization of Upper Tract Urothelial Carcinoma. Eur Urol, 72: 641, 2017 [DOI] [PubMed] [Google Scholar]
- 26.Chang SS, Bochner BH, Chou R et al. : Treatment of Non-Metastatic Muscle-Invasive Bladder Cancer: AUA/ASCO/ASTRO/SUO Guideline. J Urol, 198: 552, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leibovici D, Kassouf W, Pisters LL et al. : Organ preservation for muscle-invasive bladder cancer by transurethral resection. Urology, 70: 473, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Mazza P, Moran GW, Li G et al. : Conservative Management Following Complete Clinical Response to Neoadjuvant Chemotherapy of Muscle Invasive Bladder Cancer: Contemporary Outcomes of a Multi-Institutional Cohort Study. J Urol, 200: 1005, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Plimack ER, Hoffman-Censits JH, Viterbo R et al. : Accelerated methotrexate, vinblastine, doxorubicin, and cisplatin is safe, effective, and efficient neoadjuvant treatment for muscle-invasive bladder cancer: results of a multicenter phase II study with molecular correlates of response and toxicity. J Clin Oncol, 32: 1895, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Choueiri TK, Jacobus S, Bellmunt J et al. : Neoadjuvant dose-dense methotrexate, vinblastine, doxorubicin, and cisplatin with pegfilgrastim support in muscle-invasive urothelial cancer: pathologic, radiologic, and biomarker correlates. J Clin Oncol, 32: 1889, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure S1. Kaplan-Meier analysis of recurrence-free survival (RFS: time from the date of surgery to disease recurrence or death from any cause) stratified by treatment arm. One patient in arm A who did not undergo NU and another in arm B who had positive margins after surgery were excluded from RFS analysis. The median RFS has not been reached on arm A with a median follow-up of 21.1 months. The median (90% CI) RFS on arm B was 8.5 months (3.3, NA). Online only.
Supplemental Figure S2. Kaplan-Meier analysis of event-free survival (EFS: time to first recurrence, disease progression, new invasive primary cancer, or death from any cause) stratified by treatment arm. Median EFS has not been reached in arm A; in arm B, the median EFS was 10.2 (7.1, NA). Online only.
Supplemental Figure S3. Kaplan-Meier analysis of bladder cancer-free survival stratified by treatment arm. One patient in arm A who did not undergo NU and another in arm B who had positive margins after surgery were excluded from analysis. Median survival has not been reached in either arm. There have been two bladder cancer-related deaths on arm A and one on arm B. Online only.
Supplemental Figure S4. Kaplan-Meier analysis of urothelial cancer-specific mortality (CSM) stratified by treatment arm. Median CSM has not been reached in either arm. The cumulative incidence (90% CI) of cancer-specific deaths at 24 months was 0.09 (0.02, 0.21) in arm A and 0.20 (0.01, 0.56) in arm B. Online only.
Supplemental Figure S5. CrCl for each patient, shown by the black lines, at baseline, following receipt of NAC, and following NU for aMVAC (arm A) and GCa (arm B) groups. Red line represents CrCl 60 mL/min (threshold for renal insufficiency). Cisplatin eligibility was defined as CrCl ≥50 mL/min.