Abstract
Introduction:
The millenarian breathing exercises from Yoga, commonly called Pranayamas, are known to induce meditative states, reduce stress, and increase lung capacity. However, the physiological mechanisms by which these practices modulate the human nervous system still need to be unveiled.
Objectives:
The aim of this work was to review studies describing the influence of breathing exercises on the brain/mind of humans.
Methodology:
We reviewed articles written in English and published between 2008 and 2018. Inclusion and exclusion criteria were based on the PRISMA recommendations to filter articles from Science Direct, PubMed, and Virtual Health Library databases. Patient/Population, Intervention, Comparison, and Outcome technique and Prospective Register of Systematic Reviews registration were also considered.
Results:
From a total of 1588 articles, 14 attended the criteria. They were critically compared to each other and presented in a table divided into study; country; sample size; gender; age; objective; technique; outcome.
Discussion:
In general, the 14 papers highlight the impact of yogic breathing techniques on emotional and cognitive performance.
Conclusion:
In-depth studies focusing on specific aspects of the practices such as retentions, prolonged expiration, attention on fluid respiration, and abdominal/thoracic respiration should better elucidate the effects of Yogic Breathing Techniques (YBT).
Keywords: Brain oscillations, breathing techniques, Pranayamas, systematic review
Introduction
Simple breathing exercises such as slow and controlled breathing are known to calm the mind and are clinically used to reduce excessive arousal. However, the understanding of the relationship between mind and breath is still incomplete.[1] According to the Ancient Yoga Tradition, the breath and the mind are closely interconnected and their influence is bidirectional.[2] Modern science has just started to confirm and analyze this fact in more detail. Yogic breathing exercises are known as Pranayamas and are considered a form of meditation in itself, as well as a preparation for deep meditation. They promote physical well-being and self-awareness, improve lung and cognitive capacities, reduce blood pressure, anxiety, and other psychosomatic patterns, probably by increasing the parasympathetic tone.[3,4,5,6,7]
A critical study showed that nasal breathing generates phase-locked oscillations in the local field potential activity of the piriform cortex, amygdala, and hippocampus of humans.[8] Interestingly, breathing through the mouth does not induce the same pattern of synchronization and is correlated with sleep disturbances and attention deficit hyperactive disorder.[8,9] Some studies also suggested that asymmetry in nasal airflow – in which most of the flow occurs through one of the nasal passages – may be associated with asymmetry in brain activity.[10,11] Of note, increases in respiration rate are synchronized to amygdala activation during states of anxiety or fear[12] and have a modulatory role in neuronal activity of the neocortex, directly linking breathing to cognitive processes.[13] In addition, one recent study highlighted that conscious breathing might promote top-down emotional regulation through the prefrontal cortex network with the limbic system.[14]
However, the mechanism by which yogic breathing techniques (YBT) interact with the autonomic and cognitive functions of the human nervous system still needs to be better elucidated.[15] Here, we sought to review recent studies on this subject and discuss their results based on previous knowledge on neural plasticity and respiratory modulation of emotional and cognitive functions.
Methodology
What kind of changes can breathing exercises exert in the brain/mind of humans? This research question was based on the Patient/Population, Intervention, Comparison, and Outcome technique, used to search for evidence-based information. This study was carried out between August and September of 2018 and followed the PRISMA recommendations.[16] To provide transparency and best-quality evidence, the protocol for this systematic review was registered on Prospective Register of Systematic Reviews (Unique ID number: CRD42018107624).
Articles were initially collected in PubMed, Science Direct, and Virtual Health Library electronic databases using the following predictors: “BREATH” OR “BREATHING” OR “PRANAYAMA” AND “BRAIN” OR “MIND.” Data extraction, analysis, and the selection of articles were performed in pairs. The search strategies were customized according to filters available in each database [Table 1]. After the process of the identification of the eligible literature, studies were systematically filtered based on inclusion and exclusion criteria. Subsequently, the final papers were read in full text for a critical analysis of the content. The inclusion criterium was to select papers with at least 2 of 5 of the predictors in the text.
Table 1.
Scientific databases and predictors used to screen for eligible articles
| Academic databases | Strategy |
|---|---|
| PubMed | ((BREATHING[Title/Abstract]) OR BREATH [Title/Abstract] OR PRANAYAMA[Title/Abstract]) AND (MIND [Title/Abstract] OR BRAIN [Title/Abstract]). Filters: Full text, during the last 10 years, in humans, written in English. |
| Science direct | ((BREATHING[Title/Abstract]) OR BREATH [Title/Abstract] OR PRANAYAMA[Title/Abstract]) AND (MIND[Title/Abstract] OR BRAIN [Title/Abstract]). Filters: During the last 10 years, research articles only. |
| Virtual health library | (tw:(BREATHING)) OR (tw:(BREATH)) OR (tw:(PRANAYAMA)) AND (tw:(MIND)) OR (tw:(BRAIN)). Filters: During the last 10 years, written in English. |
Exclusion criteria were divided into subsets: (a) Nonresearch articles (e.g., books, reviews, editorials, commentaries, case studies, and case reports); (b) duplicated; (c) breathing exercises focused on the treatment of respiratory diseases; (d) brain-breathing analysis in sleep disorders or during sleep; (e) breathing exercises combined but not compared to each other, or breathing exercises combined but not compared to different techniques; and (f) only nonbreathing exercises (e.g., drug administration, manipulation of air concentration and/or pressure, mechanical ventilation, invasive methods, and clinical protocols).
Results
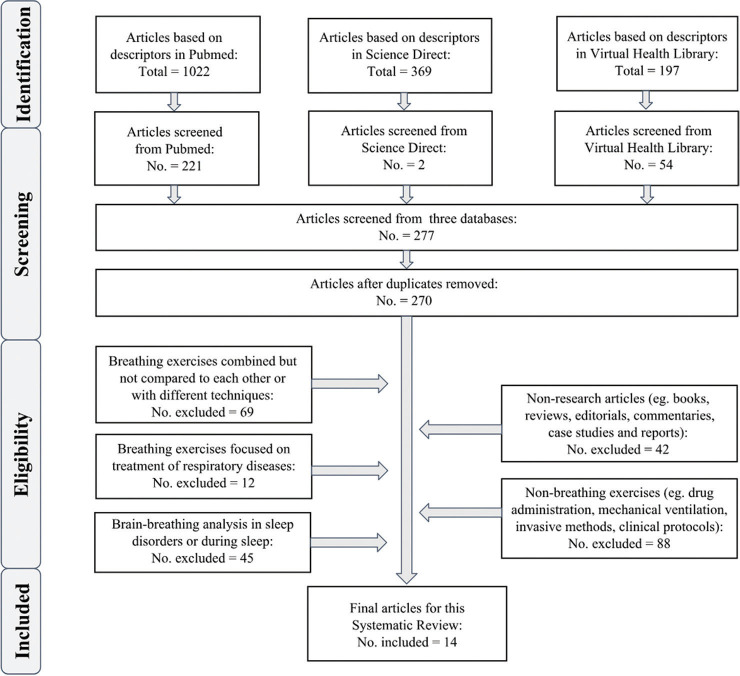
From 1.588 papers screened, 277 attended the inclusion criterium for further analysis. After filtering based on exclusion criteria, 14 were selected for this review [Figure 1]. The final articles were organized chronologically [Table 2] with the following information: study, country, sample size, gender (♂♀), age of volunteers, objective, technique, and outcome.
Figure 1.
Flow diagram based on PRISMA recommendations
Table 2.
Selected articles published between January/2008 and August/2018
| Study/experience | Country | Sample size, gender and (age) | Objective | Technique | Outcome |
|---|---|---|---|---|---|
| Vialatte et al., 2009/1 month | Japan | 8♂ (age not specified) | To obtain new insights into the nature of the EEG during BhPr | EEG and tomography | PGW were observed during BhPr. This EEG activity is most probably nonepileptic |
| Ren et al., 2011/1 day (naïve) | China | 23♂ (mean: 23.3) 25♂(mean: 23.3) | To determine if breathing meditation could promote insight | EEG and problem-solving training | Watchfulness in meditation, rather than relaxation, contributed to insight. The percentage of alpha waves (a brain index of mental relaxation) was negatively correlated with insights |
| Hasenkamp et al., 2012/1 year or more | USA | 3♂ (28-66) 11♂ (28-66) | To understand fluctuating cognitive state (mind wandering, awareness of mind wandering, shifting of attention and sustained attention) | fMRI | Regions associated with the DMN were activated during mind wandering, and in salience network regions during awareness of mind wandering. Elements of the executive network were active during shifting and sustained attention |
| Bhavanani et al., 2012/3 months or more | India | 22♂ (13-16) | To determine if Mukh Bhastrika affects central neural processing by studying RT | RT measure-ments: VRT and ART | Mukh Bhastrika may be used as an effective means of training to improve the RT in such group |
| Pradhan, 2013/ 3 months | India | 21♂ (mean: 25.71) 15♂ (mean: 24.13) |
To study the effect of 1 min KB1 and 5-min (KB5) on the SLCT and DLST | SLCT and DLST | Both KB1 and KB5 increased error scores for DLST but not for SLCT |
| Arsenault et al., 2013/1 day (naïve) | Canada | 11♂ (21-42) 9♂ (21-42) |
To examine how breathing frequency and phase affect pain perception, spinal nociceptive activity, and brain activity | EEG, painful electrical stimulation, plethysmograph pulse oxymeter strain-gauge belt transducer and anxiety questionnaire | Pain-related brain activity may be reduced during inspiration, but these changes are dissociated from the spinal nociceptive transmission. Factors other than respiration contribute to the analgesic effects of relaxation and meditation techniques |
| Marshall et al., 2014/10 weeks | USA | 9♂ (31-74) 2♂ (31-74) |
To explore whether UNB influences language ability in poststroke individuals, with and without aphasia | Attention, language, spatial abilities, depression, and anxiety tasks before, during, and after UNB treatment | UNB treatment may be used alongside traditional speech -language therapy in poststroke individuals |
| Rajesh et al., 2014/6 months-6 years | India | 31♂ (19-31) | To examine the effect of BhPr on response inhibition in healthy individuals | SST | BhPr reduced SSRT, suggesting enhanced response inhibition, related to a flexible cognitive control |
| Ju et al., 2016/1 day (naïve) | Taiwan | 36♂ (18-29) 46♂ (18-29) |
To compare the effectiveness of FBS and FDS on working memory capacities | Automated ope-rating span, concentration, and thought suppression tasks | FBS is more effective than FDS in reducing mind wandering and thought intrusions. In ddition, in contrast to FDS, the effect of FBS is independent of users’ mental resources |
| Doll et al., 2016/2 weeks | Germany | 10♂ (22-31) 16♂ (22-31) |
To analyze the effects of ATB as a basic mindfulness practice on aversive emotions at behavioral and brain levels | fMRI, respiration belt transducer and attention questionnaire | ATB decreases amygdala activation and increases prefrontal integration with the amygdala during aversive emotions, independently of effects on breathing frequency |
| Bidgoli et al., 2016/1 day (naïve) | Iran | 40♂ (48-68) 40♂ (48-68) |
To examine the effects of Sukha Pranayama on anxiety of coronary angiography candidates | Demographic questionnaire and the Spielberger State Anxiety Inventory | Sukha Pranayama reduced the mean anxiety score in the experimental group |
| Nivethitha et al., 2017A/1 year | India and Singapore | 15♂ (22-32) | To evaluate the effect of Bhastrika and Kumbhaka Pranayamas on cerebral hemodynamics | Continuous TCD | Bhastrika reduced end- diastolic velocity and mean flow velocity while increased pulsatility index between 15 and 60 s. Kumbhaka had the opposite effect during 30-60 s |
| Khng, 2017/1 day (naïve) | Singapore | 63♂ (10-11) 59♂ (10-11) |
To examine the effects of deep breathing on anxiety and test performance in children, and their possible mechanisms and moderators | Anxiety and adapted math tests, children’s cognitive assessment questionnaire and distractor interference task | Deep breathing significantly reduced self-reported feelings of anxiety and improved test performance. There was a statistical trend towards greater effectiveness in reducing state anxiety for boys compared to girls |
| Nivethitha et al., 2017B/1 year | India | 17♂ (20-26) 1♂ (20-26) |
To evaluate the effect of BhPr, KB and Bahir-Kumbhaka Pra- nayamas on brain hemodynamics | Continuous TCD | Various types of Pranayama techniques produce different brain hemodynamic changes in healthy volunteers |
EEG=Electroencephalography, PGW=Paroxysmal gamma waves, BhPr=Bhramari Pranayama, fMRI=Functional magnetic resonance imaging, DMN=Default mode network, RT=Reaction time, VRT=Visual reaction time, ART=Auditory reaction time, SLCT=Six-letter cancellation task, DLST=Digit-letter substitution task, TCD=Transcranial Doppler, UNB=Unilateral nostril breathing, SST=Stop signal task, SSRT=Stop signal RT, FBS=Focused-breathing strategy, FDS=Focused-distraction strategy, ATB=Attention-to-breath, KB=Kapalabhati
From the 14 papers, ten were from east countries and four had only male volunteers. Among studies, the age of the subjects varied from 10 to 74 years old. Regarding their methodology, four studies used electroencephalography (EEG), two used functional magnetic resonance imaging (fMRI), two used continuous transcranial Doppler (TCD), and one used tomography low-resolution brain electromagnetic tomography. Four studies employed questionnaires.
Vialatte et al.[17] described Bhramari Pranayama (BhPr) as a vibrating, buzzing, and constant sound of bumblebees produced while exhaling strictly through the nasal airways. After the start of the exhalation, the EEG of all subjects exhibited a dramatic increase in the power of high frequencies (>15 Hz), which was not observed during false BhPr. This increase was due to the appearance of a particular pattern of EEG activity, which they called “paroxysmal gamma wave” (PGW). The EEG waveform remained stable across time, and PGW activity peaked in the left middle temporal lobe, not propagating to other brain areas during the practice. Subjectively, the subjects reported only a feeling of peacefulness. The authors argued that although displaying an epileptiform-like waveform, the PGW activity measured during BhPr would not be pathological but represent nonepileptic hypersynchrony.
Rajesh et al.[18] analyzed the stop-signal reaction time (SSRT), which estimates the ability to suppress motor responses, before and after two respiration techniques: deep breathing and BhPr, with the latter described in the same way as in Vialatte et al.: a humming sound produced during exhalation. Their results indicate a significant reduction in SSRT only for BhPr, suggesting that the practice enhances response inhibition, which means a flexible cognitive control.
Bhavanani et al.[19] also studied reaction time (RT), but focusing on Mukh Bhastrika, described as having the breath actively blasted out in multiple “whooshes” with forced abdominal contractions. Working with a sample of mentally retarded children, they showed that Mukh Bhastrika produces an immediate reduction in both visual and auditory RT, indicating improved sensorimotor performance. Their hypothesis is that afferent inputs from abdominal and thoracic regions probably modulate the ascending reticular activating system and thalamocortical processing, resulting in greater arousal.
Ren et al.,[20] on the other hand, analyzed how breathing meditation promotes insight. Participants were required to raise the hand to report every ten breaths (M10) or every 100 breaths (M100). Before the experimental session, they were taught Susoku meditation, which requires mindful control and focuses on their deep abdominal breathing. After meditation, five examples of insight problems were given to all three groups (M10, M100, and rest/control). Participants were not aware that they would be given the unsolved problems later to try to solve them again. Results showed that participants who engaged in meditation solved more previously unsolved problems compared to participants in the control condition, thereby providing direct evidence for the role of meditation in promoting insight. Moreover, the M10 group had better performance than the M100 group and they also found a significant negative correlation between insightful problem-solving in the final session and the percentage of alpha waves (known to be related to mental relaxation).
Mobini Bidgoli et al.[21] described Sukha Pranayama as a conscious slow breath done regularly through the nostrils, which uses all parts of the lungs during inhalation and exhalation. Working with a sample of patients undergoing coronary angiography (CA), they showed that anxiety levels decreased five times more in the experimental than the control group. Thus, the authors concluded that Sukha Pranayama is effective in alleviating the anxiety of CA candidates and suggested its use as close as possible to the CA procedure.
Ju and Lien[22] compared the focused-breathing strategy (FBS) to the focused-distraction strategy (FDS) and found that the first is generally more effective than the second in reducing mind wandering (MW) and intrusions of unwanted thoughts. They concluded that FBS allows users to have better control over unwanted thoughts and with less effort than with FDS. The performance of the FBS group was independent of working memory capacities, which indicates that the effect of FBS does not come from effortful top-down control.
Hasenkamp et al.[23] noticed that during focused attention (FA) meditation, nonadvanced practitioners experiment a dynamic fluctuation between states such as MW, awareness of mind wandering (AWARE), shifting of focus back to the breath (SHIFT), and attentional focus on the breath (FOCUS). During the MW phase, they detected activity in the posterior cingulate cortex, medial prefrontal cortex, posterior parietal/temporal cortex, and parahippocampal gyrus (brain regions frequently associated with the default mode network). The AWARE phase revealed robust activations in the bilateral anterior insula and dorsal part of the anterior cingulate cortex (brain regions associated with conflict monitoring and self-regulation). During the SHIFT phase, significant activation was observed in the lateral prefrontal cortex (dorsal and ventral) and lateral inferior parietal cortex, with more robust activation in the right hemisphere (related to the executive network). Moreover, in the FOCUS phase, there was a cluster in the dorsolateral prefrontal region and a lack of activation in parietal elements of the executive network (both findings were reminiscent from the SHIFT phase).
Pradhan[24] defined Kapalabhati (KB) as active exhalation by a rapid contraction of the abdominal muscles with passive inhalation. Participants were divided into two groups depending on the length of the KB session (KB1: 1 min and KB5: 5 min) and subjected to the six-letter cancellation task (SLCT) and the digit letter substitution task (DLST) test. Of note, even though these two are usually considered equivalent for measuring sustained attention, DLST involves mental flexibility (since it requires reflective consideration when substituting digits for letters), which is not present in SLCT (since it only requires a simple reaction to seeing the selected letters). Surprisingly, the study found more errors in DLST after either KB1 or KB5, while performance in SLCT was not affected. The author concluded that KB does not improve motor skills and may interfere with the reflection period present in DLST.
Arsenault et al.[25] hypothesized that slow breathing would produce analgesic effects on shock pain, especially at the end of inspiration, due to the activation of descending inhibitory pathways by respiration-sensitive parasympathetic processes. However, the amplitude of the nociceptive flexor reflex (RIII-reflex) was greater during inspiration compared with expiration. They suggested that this breathing hypoalgesia may be due to central processes independent of descending modulation.
Marshall et al.[26] hypothesized that right unilateral nostril breathing (UNB) would improve attention and language as well as decrease anxiety and depression in poststroke patients. These individuals are at an increased risk for depression, especially those with language impairment due to aphasia. Participants were asked to close the left nostril and breath with the right nostril, extending the exhalation to twice longer than inhalation (ratio 1:2). Improvements in several metrics of anxiety and language were observed after a 4-week guided breathing practice; however, many of these improvements did not remain significant after the full course of the 10-week breathing program, which required an extra 6 weeks of independent practice by the patients. The authors concluded that UNB may be a low-cost prospective complementary technique and may need a more extended period of guidance before independent practice for poststroke patients to continue improving affective or linguistic abilities.
Doll et al.[27] investigated the neural mechanisms of attention to breath (ATB) in emotion regulation. Their results provide evidence that, independently of the effects on breathing frequency, ATB decreases amygdala activation while increasing the integration between the amygdala and prefrontal cortex during aversive emotions. In particular, an increase in emotion-related functional connectivity was found between the right amygdala and the left prefrontal and cingulate areas during ATB.
Khng,[28] working with a sample of children, suggested that taking deep breaths before a test can help reduce feelings of anxiety and enhance (math) performance. Deep breathing can become a self-regulatory tool to be applied at a child's disposal to bring about a better state-of-mind and performance in anxiety-inducing test-like situations. The results also showed that deep breathing may be more helpful for boys than girls and that this technique can be quickly learned (<10 min) and effectively used by children.
Nivethitha et al.[29] defined Antar-Kumbhaka Pranayama as breath retention after inhalation and argued that this technique increases carbon dioxide (CO2) levels and consequently leads to vasodilatation and increased cerebral blood flow (CBF). On the contrary, Bhastrika Pranayama, described as forceful inhalation and exhalation, would increase heart rate and blood pressure reducing CO2 levels, producing vasoconstriction, and reducing CBF in the intracranial arteries. Consistent with this scenario, the authors showed that these pranayamas produce opposite effects in cerebral hemodynamics when analyzed through continuous TCD. Months later, the same group[30] analyzed BhPr, KB, and Bahir-Kumbhaka (breath retention after exhalation) also using TCD. They found that the practices of KB and Bahir-Kumbhaka produced opposite cerebrovascular hemodynamic changes: the first increased sympathetic activity, whereas the second, parasympathetic activity. No significant changes in the cerebral hemodynamic parameters were observed during normal breathing nor during BhPr.
Discussion
In this systematic review, all 14 selected studies highlight the behavioral and neurophysiological changes after the practice of YBT. Marshall et al.[26] indicate that Surya Bedhana pranayama would improve language in individuals with aphasia. Of note, most people have the left hemisphere responsible for language. From a neuroscientific perspective, the nostrils have a cross-wised interaction with the brain hemispheres[31,32,33] and Surya Bedhana means to breathe in through the right nostril and breath out through the left nostril (with or without Kumbhaka or air retention). However, in this research, they combined a breathing technique called Rechaka, when exhalation and inhalation have a proportion of 2:1, respectively, which is known to calm the mind (in fact, they noticed reduced anxiety and depression among participants). These results are consistent with others suggesting that alternation of nasal airflow through the nostrils modulates contralateral activation of brain hemispheres and that prolonged expiration compared to inspiration may increase parasympathetic activity.[34,35,36]
Literature has shown that theta oscillation in the frontal cortex correlates to mindfulness meditation and parasympathetic dominance.[37,38] Vialatte et al.[17] found a significant decrease of theta activity during BhPr, although, in their discussion, they claimed that it was increased. They also found a hypersynchronous activity in the high gamma range in the left medial temporal lobe after the practice of BhPr, which was defined as high-frequency biphasic ripples. A previous study reported that ripples in the medial temporal lobe are associated with neuroplasticity and human memory consolidation,[39] but this is a polemic topic. While some authors claim that fast oscillations could predispose meditators to seizures and that gamma oscillation is related to default mode network in frontal areas,[40,41] others show that long-term practice of meditation bilaterally induces high-amplitude gamma synchrony in EEG recordings over the parieto-temporal and midfrontal cortical areas.[42,43] In humans, the intracortical theta (4–8 Hz) oscillation is associated with attention modulation and verbal and spatial memory tasks.[44,45]
Rajesh et al.[18] showed that BhPr may enhance response inhibition, suggesting flexible cognitive control. Bhavanani et al.[19] also found an immediate reduction in visual and auditory RTs, but for Mukh Bhastrika. They hypothesize that afferent inputs from abdominal and thoracic regions modulate the ascending reticular activating system and thalamocortical processing, resulting in greater arousal. However, they claimed that this technique is similar to KB because of an active and strong expiration and that KB has been previously shown to increase mental activity. However, this is also debatable since Pradhan[24] noted that both 1 min or 5 min of KB practice did not improve motor skills after two tasks assessing sustained attention. In addition, they found that KB may negatively interfere with the reflection ability of participants. In this context, on the Internet Mukh Bhastrika has been more linked to Bhastrika (active inhalation and active exhalation) than to KB/Kapalbhati (passive inhalation and active exhalation), which highlights the lack of consensus about the definition of these techniques.
In parallel, Nivethitha et al.[29,30] showed that Bahir-Kumbhaka-or the pause after expiration-changes the cerebrovascular hemodynamic by increasing parasympathetic activity, in contrast to KB, which increased sympathetic activity. Antar-Kumbhaka-or air retention after inspiration-may produces hypercapnia (high CO2 levels) and leads to vasodilatation and increased CBF. On the contrary, Bhastrika Pranayama-described as forceful inhalation and exhalation-reduces CBF and increases the heart rate and blood pressure reducing CO2 levels (hypocapnia). A relevant question is a difference between KB and Bhastrika with regard to cerebrovascular hemodynamics and EEG signal, since both may stimulate sympathetic activity. This is an interesting phenomenon since meditation and YBT are usually related to parasympathetic dominance.[46,47,48]
Hence how could these two techniques promote meditation? A supposition would be that Bhastrika or KB combined to Kumbhaka (retention), may potentialize the effect of retention through the increase of cortical control over breathing centers in the brainstem. These two types of Kumbhaka may lead to a better neuronal adaptation to abnormal situations since sublethal hypoxic or ischemic events can improve the tolerance for subsequent injury caused by hypoxia or ischemia, what is called, respectively, hypoxic and ischemic preconditioning.[49]
However, neuroplasticity in the pre-Bötzinger complex induced by peripheral chemoreceptor activation following hypoxia may contribute to the development of sympathetic overactivity and hypertension.[50] This gives rise to an open question: Could conscious air retention produce hypoxia that stimulates the parasympathetic activity, whereas involuntary hypoxia would stimulate the sympathetic nervous system? Moreover, could a prolonged practice of conscious air retention make the practitioner less reactive to involuntary hypoxia?
Four reviewed papers did not explicitly call the techniques “Pranayamas,” but they were included since their description of deep and slow breathing can be considered Sukha Pranayama. Mobini Bidgoli et al.[21] showed that “a conscious slow and deep breathing” done regularly through nostrils could reduce anxiety in patients before a CA procedure. Similarly, Ren et al.[20] noticed that participants who controlled and focused on deep abdominal breathing (called by the author Susoku meditation) resolved more unsolved problems compared to participants in the control condition. This result indicates that the practice of this technique could promote insights. In addition, there was a significant negative correlation between insightful problem solving and the percentage of alpha waves (known to be related to mental relaxation).
Arsenault et al.[25] hypothesized that this type of breathing technique would produce analgesic effects on shock pain, especially at the end of inspiration, due to the activation of parasympathetic processes. However, the amplitude of nociceptive flexion reflex was higher during inspiration than expiration, suggesting that the breathing hypoalgesia may be due to cerebral processes independent of descending modulation. This apparent contradiction may perhaps be better analyzed in the light of a possible increase in body awareness during the performance of the technique, which may cause better responsiveness to nociceptive stimulus but not necessarily a greater painful sensation (explaining the higher amplitude). Khng,[28] in turn, studied the benefits of taking deep abdominal breaths before a test with groups of children. They found that the practice can help to reduce anxiety and enhance math performance. Curiously, deep breathing was effective for students prone to autonomic reactions during tests/examinations, but not for those who were not.
Three papers dealt with paying attention to the breath rather than controlling its rhythm. This may be considered either an initial or advanced step in the Pranayama pathway. It is an initial step if the practitioner wants to observe the qualities of his/her breath (regularity, flow, time), and an advanced step if he/she uses it to meditate with a part of the body as an anchor (e.g., Nasagre or the space between the nostril). In the case of Ju et al.,[22] they called it FBS and discovered that nonadvanced practitioners had better control over unwanted thoughts and with less effort than the group with FDS, which means paying attention to one's flow of thoughts. Doll et al.[27] found that ATB decreases amygdala activation and increases prefrontal functional connectivity with the amygdala during aversive emotions. Their results suggest the amygdala-prefrontal cortex integration as a potential neural pathway of emotion regulation.
Finally, Hasenkamp et al.[23] noticed that during FA on the breath, nonadvanced practitioners experiment a dynamic fluctuation among brain states defined as MW, AWARE, shifting of focus back to the breath (SHIFT) and attentional focus on the breath (FOCUS). They further showed particular patterns of activated brain regions in each state. Consistent with these, other studies also show differences of brain activity during different mental states such as MW and breathing-focused meditation using fMRI, highlighting that meditation training associates with distinct patterns of the default mode network[51] and with an increase in the activity of the medial prefrontal cortex and anterior cingulate cortex in meditators, brain areas known to be relevant for attention and emotion regulation.[52]
To better situate the various YBT used in these 14 studies, it is also important to discuss the origins of pranayama definitions in classical yogic traditions. Some of the selected papers had not explicitly considered or defined their techniques as Pranayamas. However, judging by their description, they fit perfectly as traditional pranayama practices. This is an issue when trying to unify all these practices from different yoga lineages for a scientific purpose. So how can one classify a breathing technique as a Pranayama? In this context, it is justifiable to look at the classical approaches and definitions on the subject. In the millenary text Yoga Sutras of Patãnjali, there is a specific state that explains the spiritual purpose of Pranayama practice: “Mastery in pranayama removes the veil that covers the lamp of intelligence and heralds the dawn of wisdom.”[53] For Patãnjali, there are four aspects to be considered in breathing: Inhalation (Puraka), exhalation (Rechaka), the retention of air (Kumbhaka), and prana itself, which means primary energy or vital energy. Prana has different names in other cultures, such as “Ki” in Japan and “Chi” in China, and is through the practice of this pranayama that the practitioner (Sadhaka) can prepare his/her mind to truly meditate. Additionally, these four aspects can be regulated by space (where to focus the breath on your body [e.g., diaphragm or nostrils]), time (the balance between inhalation and exhalation) and count (the length of inhalation and exhalation).
Patãnjali does not name any breathing technique; he only explains the state of Pranayama and its aspects. In contrast, the Hatha Yoga Pradipika, written by Svātmārāma enumerates names eight types: Surya Bhedana Kumbhaka, Ujjayi Kumbhaka, Siktari Kumbhaka, Sitali Kumbhaka, Bhastrika Kumbhaka, Bhramari Kumbhaka, Murccha Kumbhaka, and Plavini Kumbhaka, highlighting the importance of air retention. The late Swami Kuvalayananda includes KB, one of the ShatKarmas or the six classical cleansers in this list and B. K. S Iyengar claims that Murccha and Plavini are no longer in vogue. From the perspective of the Himalayan Lineage of Samaya Srividya Tradition, Nadi Shodhana (alternate nostril breathing) is considered a Pranayama and Kumbhaka, an advanced technique.[53,54,55,56]
In this scenario, it is challenging to have a consensus on how to name and to practice Pranayama since the different lineages of Yoga teach the techniques in distinct ways and for different purposes. We suggest that all forms of techniques may be included as long as a clear and detailed description is provided, and hence, one can understand the physiology behind the technique. Complementarily, since the word pranayama can be either a mental state and a technique to achieve this state, we suggest that in scientific texts, the term YBT should be favored instead of the traditional word Pranayama.
In general, neuroplasticity could be involved in physiological and behavioral alterations related to the practice of YBT. Through the increase of respiration control, attentional circuits could be recruited and stimulated to maintain self-centered focus[57] as observed, for example, in hyperactivity disorder patients.[58] According to a recent study in humans, the phase of natural breathing can be employed to promote oscillatory synchrony and to optimize information processing in brain areas involved with goal-directed behaviors.[8] In rodents, researchers characterized respiration-entrained oscillations with origin in the olfactory bulb that also occur in the hippocampus and frontal lobe, being thus implicated in widespread communication in the brain.[59] This influence of olfactory bulb reflects delta, theta, and beta-range band oscillations that are associated with attention, memory, and goal-directed behaviors, thus possibly allowing the coupling between sensory and memory networks.[60,61]
Another recent paper described a specific neuronal type in the pre-Bötzinger complex (pre-BötC) regulating calm and arousal behaviors in rodents. The inactivation or ablation of those neurons left breathing intact but promoted an increase in calm behaviors and a decrease in arousal.[1] In addition, some researchers have found that the prolonged practice of pranayama (4 weeks) also modulates the activity of brain regions involved in emotional processing, such as the amygdala, anterior cingulate, anterior insula, and prefrontal cortex.[6] Besides, through fMRI, it was possible to detect in these volunteers a reduction of connectivity between the anterior insula and lateral portions of the prefrontal cortex, which is a network associated with anxiety.[6] In this context, we argue that the sustained practice of YBT could promote neuroplasticity mechanisms underlying these findings. That is, specific neurons from pre-BötC could be affected by the voluntary control of respiration during YBT, thus stimulating less the locus coeruleus and reducing anxiety-related states. In addition, considering the prolongation of the respiratory cycle or the increase in nasal airflow during these practices, we argue that the olfactory bulb could exert a modulatory influence on the frontal lobe and limbic structures during YBT, resulting in long-term plasticity changes.
Future studies should employ high-quality methodologies to accurately analyze the mechanisms involved in Pranayamas/YBT practices. These include a detailed description of the effect of single techniques since many studies combine them with each other or with asanas (postures). In this sense, the problem of mixed approaches is that we cannot understand in detail the neuroplasticity impacts of each specific technique to apply them in modern-day life or a clinical context. Finally, the underlying neural mechanisms could be better characterized by focusing on studying the behavioral and structural changes promoted by intensive training schedules. This can be accomplished with the aid of technologies such as fMRI for neuroplasticity analysis, respiratory plethysmography combined with EEG to understand the relationship between brain oscillations and breathing patterns in real-time, electrocardiogram to highlight the parasympathetic/sympathetic dominance through heart rate variability, besides anxiety/interception scales through blinded, randomized and controlled trials.
Conclusion
Yoga-based respiratory techniques are millenary practices, and quite widespread through oral and written tradition until present days. However, much yet has to be done for a better scientific understanding of their effects on the human brain. In-depth studies focusing on specific aspects of the practices such as retentions, prolonged expiration, attention on fluid respiration, and abdominal/thoracic respiration should better elucidate the effects of YBT.
Financial support and sponsorship
This research was supported by INCT 2014: Translational Medicine (n° 14/50891-1) and CNPq grants n° 308653/2018-1 and n° 408508/2018-3.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
This work was supported by CNPq, CAPES, and the National Institute of Translational Medicine in Brazil.
References
- 1.Yackle K, Schwarz LA, Kam K, Sorokin JM, Huguenard JR, Feldman JL, et al. Breathing control center neurons that promote arousal in mice. Science. 2017;355:1411–5. doi: 10.1126/science.aai7984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gilbert C. Yoga and breathing. J Bodyw Mov Ther. 1999;3:44–54. [Google Scholar]
- 3.Brown RP, Gerbarg PL. Yoga breathing, meditation, and longevity. Ann N Y Acad Sci. 2009;1172:54–62. doi: 10.1111/j.1749-6632.2009.04394.x. [DOI] [PubMed] [Google Scholar]
- 4.Rocha KK, Ribeiro AM, Rocha KC, Sousa MB, Albuquerque FS, Ribeiro S, et al. Improvement in physiological and psychological parameters after 6 months of yoga practice. Conscious Cogn. 2012;21:843–50. doi: 10.1016/j.concog.2012.01.014. [DOI] [PubMed] [Google Scholar]
- 5.Sharma VK, Rajajeyakumar M, Velkumary S, Subramanian SK, Bhavanani AB, Madanmohan AS, et al. Effect of fast and slow pranayama practice on cognitive functions in healthy volunteers. J Clin Diagn Res. 2014;8:10–3. doi: 10.7860/JCDR/2014/7256.3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Novaes MM, Palhano-Fontes F, Onias H, Andrade KC, Lobão-Soares B, Arruda-Sanchez T, et al. Effects of yoga respiratory practice (Bhastrika pranayama) on anxiety, affect, and brain functional connectivity and activity: A randomized controlled trial. Front Psychiatry. 2020;11:467. doi: 10.3389/fpsyt.2020.00467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thanalakshmi J, Maheshkumar K, Kannan R, Sundareswaran L, Venugopal V, Poonguzhali S. Effect of Sheetali pranayama on cardiac autonomic function among patients with primary hypertension – A randomized controlled trial. Complement Ther Clin Pract. 2020;39:101138. doi: 10.1016/j.ctcp.2020.101138. [DOI] [PubMed] [Google Scholar]
- 8.Zelano C, Jiang H, Zhou G, Arora N, Schuele S, Rosenow J, et al. Nasal respiration entrains human limbic oscillations and modulates cognitive function. J Neurosci. 2016;36:12448–67. doi: 10.1523/JNEUROSCI.2586-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sano M, Sano S, Oka N, Yoshino K, Kato T. Increased oxygen load in the prefrontal cortex from mouth breathing: A vector-based near-infrared spectroscopy study. Neuroreport. 2013;24:935–40. doi: 10.1097/WNR.0000000000000008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kahana-Zweig R, Geva-Sagiv M, Weissbrod A, Secundo L, Soroker N, Sobel N. Measuring and characterizing the human nasal cycle. PLoS One. 2016;11:e0162918. doi: 10.1371/journal.pone.0162918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Price A, Eccles R. Nasal airflow and brain activity: Is there a link? J Laryngol Otol. 2016;130:794–9. doi: 10.1017/S0022215116008537. [DOI] [PubMed] [Google Scholar]
- 12.Masaoka Y, Izumizaki M, Homma I. Where is the rhythm generator for emotional breathing? Prog Brain Res. 2014;209:367–77. doi: 10.1016/B978-0-444-63274-6.00019-9. [DOI] [PubMed] [Google Scholar]
- 13.Heck DH, McAfee SS, Liu Y, Babajani-Feremi A, Rezaie R, Freeman WJ, et al. Breathing as a fundamental rhythm of brain function. Front Neural Circuits. 2016;10:115. doi: 10.3389/fncir.2016.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang W, Ouyang Y, Tang F, Chen J, Li H. Breath-focused mindfulness alters early and late components during emotion regulation. Brain Cogn. 2019;135:103585. doi: 10.1016/j.bandc.2019.103585. [DOI] [PubMed] [Google Scholar]
- 15.Jerath R, Edry JW, Barnes VA, Jerath V. Physiology of long pranayamic breathing: Neural respiratory elements may provide a mechanism that explains how slow deep breathing shifts the autonomic nervous system. Med Hypotheses. 2006;67:566–71. doi: 10.1016/j.mehy.2006.02.042. [DOI] [PubMed] [Google Scholar]
- 16.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Med. 2009;6:e1000100. doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vialatte FB, Bakardjian H, Prasad R, Cichocki A. EEG paroxysmal gamma waves during Bhramari Pranayama: A yoga breathing technique. Conscious Cogn. 2009;18:977–88. doi: 10.1016/j.concog.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 18.Rajesh SK, Ilavarasu JV, Srinivasan TM. Effect of Bhramari Pranayama on response inhibition: Evidence from the stop signal task. Int J Yoga. 2014;7:138–41. doi: 10.4103/0973-6131.133896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhavanani AB, Ramanathan M, Kt H. Immediate effect of Mukha Bhastrika (a bellows type pranayama) on reaction time in mentally challenged adolescents. Indian J Physiol Pharmacol. 2012;56:174–80. [PubMed] [Google Scholar]
- 20.Ren J, Huang Z, Luo J, Wei G, Ying X, Ding Z, et al. Meditation promotes insightful problem-solving by keeping people in a mindful and alert conscious state. Sci China Life Sci. 2011;54:961–5. doi: 10.1007/s11427-011-4233-3. [DOI] [PubMed] [Google Scholar]
- 21.Mobini Bidgoli M, Taghadosi M, Gilasi H, Farokhian A. The effect of sukha pranayama on anxiety in patients undergoing coronary angiography: A single -blind randomized controlled trial. J Cardiovasc Thorac Res. 2016;8:170–5. doi: 10.15171/jcvtr.2016.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ju YJ, Lien YW. Better control with less effort: The advantage of using focused-breathing strategy over focused-distraction strategy on thought suppression. Conscious Cogn. 2016;40:9–16. doi: 10.1016/j.concog.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 23.Hasenkamp W, Wilson-Mendenhall CD, Duncan E, Barsalou LW. Mind wandering and attention during focused meditation: A fine-grained temporal analysis of fluctuating cognitive states. Neuroimage. 2012;59:750–60. doi: 10.1016/j.neuroimage.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 24.Pradhan B. Effect of kapalabhati on performance of six-letter cancellation and digit letter substitution task in adults. Int J Yoga. 2013;6:128–30. doi: 10.4103/0973-6131.113415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arsenault M, Ladouceur A, Lehmann A, Rainville P, Piché M. Pain modulation induced by respiration: Phase and frequency effects. Neuroscience. 2013;252:501–11. doi: 10.1016/j.neuroscience.2013.07.048. [DOI] [PubMed] [Google Scholar]
- 26.Marshall RS, Basilakos A, Williams T, Love-Myers K. Exploring the benefits of unilateral nostril breathing practice post-stroke: Attention, language, spatial abilities, depression, and anxiety. J Altern Complement Med. 2014;20:185–94. doi: 10.1089/acm.2013.0019. [DOI] [PubMed] [Google Scholar]
- 27.Doll A, Hölzel BK, Mulej Bratec S, Boucard CC, Xie X, Wohlschläger AM, et al. Mindful attention to breath regulates emotions via increased amygdala-prefrontal cortex connectivity. Neuroimage. 2016;134:305–13. doi: 10.1016/j.neuroimage.2016.03.041. [DOI] [PubMed] [Google Scholar]
- 28.Khng KH. A better state-of-mind: Deep breathing reduces state anxiety and enhances test performance through regulating test cognitions in children. Cogn Emot. 2017;31:1502–10. doi: 10.1080/02699931.2016.1233095. [DOI] [PubMed] [Google Scholar]
- 29.Nivethitha L, Mooventhan A, Manjunath NK, Bathala L, Sharma VK. Cerebrovascular hemodynamics during pranayama techniques. J Neurosci Rural Pract. 2017;8:60–3. doi: 10.4103/0976-3147.193532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nivethitha L, Mooventhan A, Manjunath NK, Bathala L, Sharma VK. Cerebrovascular hemodynamics during the practice of Bhramari Pranayama, Kapalbhati and Bahir-Kumbhaka: An exploratory study. Appl Psychophysiol Biofeedback. 2018;43:87–92. doi: 10.1007/s10484-017-9387-8. [DOI] [PubMed] [Google Scholar]
- 31.Werntz DA, Bickford RG, Bloom FE, Shannahoff-Khalsa DS. Alternating cerebral hemispheric activity and the lateralization of autonomic nervous function. Hum Neurobiol. 1983;2:39–43. [PubMed] [Google Scholar]
- 32.Klein R, Pilon D, Prosser S, Shannahoff-Khalsa D. Nasal airflow asymmetries and human performance. Biol Psychol. 1986;23:127–37. doi: 10.1016/0301-0511(86)90077-3. [DOI] [PubMed] [Google Scholar]
- 33.Upadhyay-Dhungel K, Sohal A. Physiology of nostril breathing exercises and its probable relation with nostril and cerebral dominance: A theoretical research on literature. J Med Sci. 2013;1:38–47. [Google Scholar]
- 34.Camm AJ, Malik M, Bigger JT, Breithardt G, Cerutti S, Cohen RJ, et al. Heart rate variability: Standards of measurement, physiological interpretation and clinical use.Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation. 1996;93:1043–65. [PubMed] [Google Scholar]
- 35.Berntson GG, Bigger JT, Jr, Eckberg DL, Grossman P, Kaufmann PG, Malik M, et al. Heart rate variability: Origins, methods, and interpretive caveats. Psychophysiology. 1997;34:623–48. doi: 10.1111/j.1469-8986.1997.tb02140.x. [DOI] [PubMed] [Google Scholar]
- 36.Yasuma F, Hayano J. Respiratory sinus arrhythmia: Why does the heartbeat synchronize with respiratory rhythm? Chest. 2004;125:683–90. doi: 10.1378/chest.125.2.683. [DOI] [PubMed] [Google Scholar]
- 37.Takahashi T, Murata T, Hamada T, Omori M, Kosaka H, Kikuchi M, et al. Changes in EEG and autonomic nervous activity during meditation and their association with personality traits. Int J Psychophysiol. 2005;55:199–207. doi: 10.1016/j.ijpsycho.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 38.Ivanovski B, Malhi GS. The psychological and neurophysiological concomitants of mindfulness forms of meditation. Acta Neuropsychiatr. 2007;19:76–91. doi: 10.1111/j.1601-5215.2007.00175.x. [DOI] [PubMed] [Google Scholar]
- 39.Axmacher N, Elger CE, Fell J. Ripples in the medial temporal lobe are relevant for human memory consolidation. Brain. 2008;131:1806–17. doi: 10.1093/brain/awn103. [DOI] [PubMed] [Google Scholar]
- 40.Jaseja H. Potential role of self-induced EEG fast oscillations in predisposition to seizures in meditators. Epilepsy Behav. 2010;17:124–5. doi: 10.1016/j.yebeh.2009.10.022. [DOI] [PubMed] [Google Scholar]
- 41.Berkovich-Ohana A, Glicksohn J, Goldstein A. Mindfulness-induced changes in gamma band activity-implications for the default mode network, self-reference and attention. Clin Neurophysiol. 2012;123:700–10. doi: 10.1016/j.clinph.2011.07.048. [DOI] [PubMed] [Google Scholar]
- 42.Lutz A, Greischar LL, Rawlings NB, Ricard M, Davidson RJ. Long-term meditators self-induce high-amplitude gamma synchrony during mental practice. Proc Natl Acad Sci U S A. 2004;101:16369–73. doi: 10.1073/pnas.0407401101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Travis F, Shear J. Focused attention, open monitoring and automatic self-transcending: Categories to organize meditations from Vedic, Buddhist and Chinese traditions. Conscious Cogn. 2010;19:1110–8. doi: 10.1016/j.concog.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 44.O'Keefe J, Burgess N. Theta activity, virtual navigation and the human hippocampus. Trends Cogn Sci. 1999;3:403–6. doi: 10.1016/s1364-6613(99)01396-0. [DOI] [PubMed] [Google Scholar]
- 45.Raghavachari S, Kahana MJ, Rizzuto DS, Caplan JB, Kirschen MP, Bourgeois B, et al. Gating of human theta oscillations by a working memory task. J Neurosci. 2001;21:3175–83. doi: 10.1523/JNEUROSCI.21-09-03175.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Raghuraj P, Telles S. Effect of yoga-based and forced uninostril breathing on the autonomic nervous system. Percept Mot Skills. 2003;96:79–80. doi: 10.2466/pms.2003.96.1.79. [DOI] [PubMed] [Google Scholar]
- 47.Wu SD, Lo PC. Inward-attention meditation increases parasympathetic activity: A study based on heart rate variability. Biomed Res. 2008;29:245–50. doi: 10.2220/biomedres.29.245. [DOI] [PubMed] [Google Scholar]
- 48.Tang YY, Ma Y, Fan Y, Feng H, Wang J, Feng S, et al. Central and autonomic nervous system interaction is altered by short-term meditation. Proc Natl Acad Sci U S A. 2009;106:8865–70. doi: 10.1073/pnas.0904031106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li S, Hafeez A, Noorulla F, Geng X, Shao G, Ren C, et al. Preconditioning in neuroprotection: From hypoxia to ischemia. Prog Neurobiol. 2017;157:79–91. doi: 10.1016/j.pneurobio.2017.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Barnett WH, Abdala AP, Paton JF, Rybak IA, Zoccal DB, Molkov YI. Chemoreception and neuroplasticity in respiratory circuits. Exp Neurol. 2017;287:153–64. doi: 10.1016/j.expneurol.2016.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brewer JA, Worhunsky PD, Gray JR, Tang YY, Weber J, Kober H. Meditation experience is associated with differences in default mode network activity and connectivity. Proc Natl Acad Sci U S A. 2011;108:20254–9. doi: 10.1073/pnas.1112029108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hölzel BK, Ott U, Hempel H, Hackl A, Wolf K, Stark R, et al. Differential engagement of anterior cingulate and adjacent medial frontal cortex in adept meditators and non-meditators. Neurosci Lett. 2007;421:16–21. doi: 10.1016/j.neulet.2007.04.074. [DOI] [PubMed] [Google Scholar]
- 53.Svatmarama YS. Foreword by B K S Iyengar. In: Buchanan H, editor. Hatha Yoga Pradipika. 1st ed. Detroit: The Aquarian Press; 1992. p. 6. [Google Scholar]
- 54.Kuvalayananda S, Chapter V. In: Prāāāyāma. 1st ed. Maheshananda S, editor. Lonavala: Kaivalyadhama Ashram Publications; 1966. pp. 83–105. [Google Scholar]
- 55.Iyengar BK. In: Light on Pranayama. 1st ed. Chaudhuri SR, editor. Ch 21 New Delhi: HarperCollins Publishers India; 2005. p. 152. [Google Scholar]
- 56.Grouven SR. Mastering Pranayama From Breathing Techniques to Kundalini Awakening. 1st ed. Frankfurt: THAT First Publishing; 2018. Kumbhaka, the elusive breathless state; pp. 171–9. [Google Scholar]
- 57.Ma X, Yue ZQ, Gong ZQ, Zhang H, Duan NY, Shi YT, et al. The effect of diaphragmatic breathing on attention, negative affect and stress in healthy adults. Front Psychol. 2017;8:874. doi: 10.3389/fpsyg.2017.00874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Simpson DD, Nelson AE. Attention training through breathing control to modify hyperactivity. J Learn Disabil. 1974;7:274–83. [Google Scholar]
- 59.Tort AB, Brankačk J, Draguhn A. Respiration-Entrained Brain Rhythms Are Global but Often Overlooked. Trends Neurosci. 2018;41:186–97. doi: 10.1016/j.tins.2018.01.007. [DOI] [PubMed] [Google Scholar]
- 60.Nguyen Chi V, Müller C, Wolfenstetter T, Yanovsky Y, Draguhn A, Tort AB, et al. Hippocampal respiration-driven rhythm distinct from theta oscillations in awake mice. J Neurosci. 2016;36:162–77. doi: 10.1523/JNEUROSCI.2848-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lockmann AL, Tort AB. Nasal respiration entrains delta-frequency oscillations in the prefrontal cortex and hippocampus of rodents. Brain Struct Funct. 2018;223:1–3. doi: 10.1007/s00429-017-1573-1. [DOI] [PubMed] [Google Scholar]