Abstract
The intestine is an essential physical and immunological barrier comprised of a monolayer of diverse and specialized epithelial cells that perform functions ranging from nutrient absorption to pathogen sensing and intestinal homeostasis. The intestinal barrier prevents translocation of intestinal microbes into internal compartments. The microbiota is comprised of a complex community largely populated by diverse bacterial species that provide metabolites, nutrients, and immune stimuli that promote intestinal and organismal health. Although commensal organisms promote health, enteric pathogens, including a diverse plethora of enteric viruses cause acute and chronic diseases. The barrier epithelium plays fundamental roles in immune defenses against enteric viral infections by integrating diverse signals, including those from the microbiota, to prevent disease. Importantly, many model systems have contributed to our understanding of this complex interface. This review will focus on the antiviral mechanisms at play within the intestinal epithelium and how these responses are shaped by the microbiota.
Keywords: Enterocytes, enteric viruses, microbiota, intestine, antiviral, innate immunology, Drosophila, C.elegans
Introduction
Enteric pathogens cause significant human disease and an estimated 2 million deaths yearly (Kirk et al., 2015). Enteric viruses are largely transmitted through the fecal-oral route by entering and infecting the gastrointestinal tract. Norovirus is the leading cause of viral gastroenteritis worldwide (Bányai et al., 2018; Kirk et al., 2015). Other significant human enteric viral pathogens include rotavirus, coxsackieviruses, reovirus, enteroviruses, and some adenoviruses (Bányai et al., 2018). These viruses typically cause acute infections, characterized by diarrheal disease, which is thought to facilitate transmission. Enteric viruses are ubiquitous, and thus all organisms must possess mechanisms to overcome or co-exist with these pathogens. Indeed, enteric viruses in insects resemble those in mammals, and studies in model systems have revealed important, conserved facets of intestinal immunity.
The gut presents a formidable barrier to infection by enteric viruses (Gill et al., 2011; Nagler-Anderson, 2001; Pfeiffer, 2010). Indeed, the co-evolution of enteric viruses with their hosts has allowed viruses to evade barrier immunity. This is exemplified by the fact that it is difficult to infect mice with human enteric viruses orally. Thus, most studies of human enteric viruses in mice rely on alternative routes of infection (e.g., i.v.), use of immunocompromised mice (e.g., type I interferon [IFN] knockout) for oral transmission (Bopegamage et al., 2005; Lancaster and Pfeiffer, 2010; Ohka et al., 2007), or study of murine viruses with similarity to human pathogens (Baldridge et al., 2015; Cadwell et al., 2010; Grau et al., 2019; Hernández et al., 2015; Jones et al., 2014; Kernbauer et al., 2014; Nice et al., 2015; Pott et al., 2011; Pott et al., 2012; Shi et al., 2019; Uchiyama et al., 2014; Wilen et al., 2018; Zhou et al., 2007).
Arthropod-borne viruses (arboviruses) are a unique group of viruses because they are transmitted to vertebrates through the blood; in contrast, they orally infect the insect host. Once arboviruses establish infection in the insect intestinal epithelium, they ultimately spread to the salivary glands for transmission to the next vertebrate host (Weaver and Barrett, 2004). Even in insects, the intestine presents a high barrier to infection; oral transmission to mosquitoes that are not the natural vector is usually non-productive. However, inoculating virus directly into the insect body cavity and bypassing the gut leads to productive infection that can be transmitted to vertebrates (Bennett et al., 2002; Salazar et al., 2007). Although epithelial cells are not normally considered immune cells, these cells are highly responsive to infection and play a fundamental role in establishing immune defenses using both autonomous and non-autonomous mechanisms in the gut.
Metazoans have an intestinal tract with an evolutionarily conserved structure. The intestine is comprised of a monolayer of polarized intestinal epithelial cells where the apical side faces the lumen, and the basolateral side faces the basement membrane and the internal structures of the organism. Across evolution, absorptive enterocytes comprise the vast majority of epithelial cells in this tissue. All metazoans harbor a microbiota within the lumen of the intestine, mainly composed of commensal bacteria. This symbiotic relationship likely arose to provide a nutrient source to increasingly complex organisms and to provide instructive cues to developing immune systems. Although the density and species of the microbiota are distinct depending on the host, the microbiota provides metabolites and micronutrients, stimulates immunity, and promotes intestinal homeostasis across evolution (Blacher et al., 2017; Broderick et al., 2014; Buchon et al., 2014). Indeed, germ-free flies and mice present with many of the same abnormalities (Buchon et al., 2013; Kitajima et al., 2001), including in immune development and pathogen defense (Round and Mazmanian, 2009; Sansone et al., 2015; Shin et al., 2011).
We are only beginning to understand these interactions on a molecular basis. Therefore, this review will focus on the diverse roles the intestinal epithelium and the microbiota play in shaping antiviral enteric immunity. We will first discuss the basic and shared physiology of the intestine. Next, we will outline different model systems used to study antiviral immunity in the intestine and examine mechanisms by which the microbiota influences enteric virus infection. We suggest that simple model organisms, including flies and worms, provide an important reductionist view, because it remains difficult to dissect the contribution of individual commensals and their products on innate antiviral immunity in higher organisms such as mice. Therefore, we will begin by discussing studies using these simpler organisms and subsequently discuss more complex mammalian models.
Intestinal Structure and Biology
The intestine is comprised of an epithelial monolayer that acts as a barrier between the outside, including the microbiota, and the host (Table 1). Absorptive enterocytes make up the vast majority of the surface area of the intestine across evolution given that absorption of nutrients and micronutrients is the most important basal function of the intestine. These nutrients are derived from the food we eat as well as from the microbiota; thus, one of the major contributions the microbiota makes to host-microbe mutualism is nutrient provision (Rowland et al., 2018). For example, the microbiota synthesizes essential vitamins and breaks down dietary fiber and complex polysaccharides into digestible products (Blacher et al., 2017; Rowland et al., 2018).
Table 1. Intestinal Structures across Diverse Organisms.
This table reviews the cell types and physical barriers present in the intestine of nematodes, Drosophila, and mammals. It also lists the homeostatic mechanisms present in the intestine and the 3D structures the intestine is organized into across species.
| Epithelial Cell types | Homeostatic Maintenance | Physical Barriers | 3D structures | |
|---|---|---|---|---|
| Nematodes | Enterocytes | None | Glycocalyx | None |
| Drosophila melanogaster | Enterocytes Intestinal Stem Cells Enteroendocrine Cells |
Local Stem Cell Proliferation (turnover ~1 week) | Peritrophic matrix Mucus |
Looping |
| Mammals | Enterocytes Intestinal Stem Cells Enteroendocrine Cells Paneth Cells Tuft Cells Goblet Cells M Cells |
Local Stem Cell Proliferation (turnover ~ 3–5 days) | Mucus | Looping Crypts Villi |
Our knowledge of the spectrum of metabolites and micronutrients produced by the microbiota is incomplete and of great interest. Moreover, how microbial-derived metabolites affect infection and immunity is an emerging field. Recent studies have revealed new insights, including roles for short-chain fatty acids in regulating enterocyte biology and immunity in the gut (Thaiss et al., 2016). However, studies on how microbial metabolites affect enteric viral infections are at their infancy (Blacher et al., 2017). These studies will shed light on antiviral mechanisms influenced by microbiota-derived metabolites and products.
To facilitate nutrient uptake while maintaining an impermeable monolayer, enterocytes are highly specialized. They have apical microvilli that form a brush border, which increases the surface area of the intestine for increased absorption. In addition, the cells maintain a tightly regulated barrier harboring both apical and tight junctions to control the movement of the microbiota, nutrients, and material larger than 4 Å from the lumen into the organism (Peterson and Artis, 2014; Watson et al., 2001). Enterocytes both sense and respond to pathogens by producing antimicrobial products including secreted factors that block local infection. Moreover, enterocytes produce cytokines and chemokines that orchestrate immune responses by tissueresident immune cells and induce systemic immunity (Stadnyk, 2002).
Insects and other more complex animals have evolved diverse intestinal epithelial cell types including enteroendocrine cells. Enteroendocrine cells represent 1% of the mammalian epithelium and are best known for their roles in nutrient sensing and hormone release (Worthington et al., 2018). However, these cells have recently been found to act as key sensors of microbial metabolites and immune responses (Worthington et al., 2018). Mammals have evolved additional epithelial cell types such as tuft cells, which are chemosensory cells linked to immunity in the intestine through the production of cytokines such as IL-25 (Ting and von Moltke, 2019).
Organisms have developed different strategies to maintain a separation of the microbiota from the intestinal epithelium. The vertebrate intestine evolved a mucus layer produced by goblet cells that separates the lumen of the intestine from the enterocytes. Mucus is largely made up of the heavily glycosylated protein mucin-2 that forms a gelatinous matrix acting as a semi-permeable barrier and, for some microbes, a food source (Birchenough et al., 2015; Johansson et al., 2008; Peterson and Artis, 2014). Insects produce a peritrophic matrix that forms a barrier between the lumen and epithelium and is composed of peritrophins and chitins that create a semipermeable separation, like mucus (Apidianakis and Rahme, 2011). Peritrophins and chitins are secreted by specialized epithelial cells at the anterior of the intestinal tract in Drosophila (Hegedus et al., 2009). In addition, insects have mucin-like proteins that form a thin mucus layer that also likely provides protection from enteric pathogens (Syed et al., 2008; Vodovar et al., 2005; Wu et al., 2019). C. elegans enterocytes produce a glycocalyx, which is a lipid and glycoprotein rich matrix that protects the intestine from the microbiota and other luminal contents (McGee et al., 2011).
The secretion of antimicrobial peptides (AMPs) by the intestine provides another barrier to maintain separation between the microbiota and the host (Holly and Smith, 2018; Peterson and Artis, 2014). AMPs are a large family of small, usually positively charged peptides that can permeabilize membranes from bacteria, fungi, and other microorganisms (Mahlapuu et al., 2016). The production of antimicrobial peptides is evolutionarily conserved, and in lower organisms AMPs are produced by enterocytes (Buchon et al., 2014; Dierking et al., 2016). In higher organisms, a specialized epithelial cell type, called paneth cells, are the major AMP producers in the intestine (Bar Shira and Friedman, 2018; Holly and Smith, 2018).
An important facet of the intestine is that it regenerates, and homeostatic mechanisms maintain an intact barrier in the face of stressors, including infection. From insects to mammals, enterocytes slough off and resident intestinal stem cells differentiate to maintain the barrier and intestinal homeostasis (Gehart and Clevers, 2019). This regenerative process is induced upon damage to the epithelium and when dysregulated can lead to cancer. Interestingly, the turnover rate in insects and humans is quite similar: enterocytes are replaced approximately weekly (Micchelli and Perrimon, 2006; Peterson and Artis, 2014).
Another conserved strategy to increase the enteric surface area of the gut involves the 3D architecture of the intestine. Whereas the intestine of worms is a linear tube, in more complex organisms, including insects, the intestine is looped, which increases the length of the intestine (Buchon et al., 2013). Moreover, these structures create niches where the contents are slowed, regulating interactions between the luminal contents and the epithelium. In addition to looping, fish and mammals have further evolved villi, which are projections of the monolayer from the small intestine into the lumen. Additionally, mammals and some reptilia have evolved crypts, which are invaginations of the monolayer from the intestine into the lamina propria (Bar Shira and Friedman, 2018; Brugman, 2016). The presence of villi and microvilli in higher organisms increases the surface area of the intestine at least 30-fold (Helander and Fandriks, 2014€). In addition, the base of a crypt is further from the luminal contents and this more sterile environment houses the intestinal stem cells, which are further protected by AMPs produced by nearby paneth cells (Gehart and Clevers, 2019). A basement membrane is located on the basolateral side of the intestine. Below the basement membrane in mammals is the lamina propria, which houses an extensive immune compartment and gut-associated lymphoid tissue (Faria et al., 2012). These organized immune structures are absent in non-chordate species, although resident immune cells might play similar roles.
The microbiota is important for many facets of health and can contribute to disease. Most animals acquire their microbiota from the environment, and the density of the microbiota varies greatly between species and across regions of the intestinal tract. For example, the colon houses the highest bacterial density in mammals and has fewer species and lower total density as you move apically (Donaldson et al., 2016). The complexity and specific bacterial families found in different organisms are generally distinct. Simpler organisms tend to have simpler communities, and lab-raised animals also tend to have simpler communities (Kreisinger et al., 2014; Rosshart et al., 2017; Staubach et al., 2013). Moreover, there is large variability between organisms within the same species; each human harbors a community distinct from every other person (Moya and Ferrer, 2016). We and others suggest that because many distinct bacterial communities provide similar products (e.g., metabolites and immune ligands) necessary to promote a healthy state, the function but not the species of bacteria are what defines health (Moya and Ferrer, 2016). A clearer understanding of what functions provided by the microbial community are beneficial will allow us to more accurately describe a healthy and pathogenic state and to ultimately modulate the microbiota to promote health.
Enteric Antiviral Immunity
The physical barrier of the gut plays an important role in protection from microbes. Innate immune defenses provide a second essential layer of protection and are initiated through the recognition of foreign or non-self ligands. Foreign recognition leads to the activation of signaling cascades, which induce antimicrobial transcriptional programs, the production of antimicrobial effector proteins, and the production of secreted factors that can create an antimicrobial state as well as alert both innate and adaptive immune cells. Although many studies focus on innate responses in professional immune cells (e.g., myeloid cells), all cells, and in particular barrier epithelial cells, recognize and respond to foreign invaders. The intestinal epithelium, with its complex array of cell types, plays a fundamental role in recognizing microbes and shaping downstream immune responses. Moreover, although it is clear intestinal epithelial cells are essential players, we are only beginning to unravel the immune mechanisms involved. This is in large part due to challenges with oral infection models.
This is particularly true for enteric viral infections. Although many studies have explored antiviral immune mechanisms active against enteric viruses, most experiments have been performed either in vitro or during systemic infection. Studies during bona fide enteric infection of an intact intestine are essential to uncover the unique pathways and players engaged in this complex tissue. For example, whereas type I IFNs are essential antiviral players in diverse cell types, type III IFNs play a more important role in barriers including the gut epithelium (Baldridge et al., 2017; MahlakÕ iv et al., 2015; Pott et al., 2011). We will overview our understanding of enteric viral infection in diverse models such as mice and human intestinal organoids as well as simpler model organisms including insects and worms. We will focus on our understanding of intestinal antiviral immunity active in the epithelium and how the microbiota influences intestinal immune defenses.
A Simplified Intestinal Model: Caenorhabditis elegans
C. elegans presents the most biologically simple model organism where enteric viral infections have been explored. C. elegans immunity is driven by cell-intrinsic defenses because they lack innate and adaptive immune cells. Moreover, C. elegans lacks many innate signaling pathways that are considered canonical in mammals including the IFN response, nuclear factor k-light-chain-enhancer of activated B cells (NFkB), and nucleotide-binding oligomerization domain (NOD)-like receptors (Irazoqui et al., 2010). The intestine of C.elegans is a simple tube comprised of 20 clonal enterocytes and does not self-renew, perhaps because their lifespan is short (schematic Figure 1A). C. elegans feeds on bacteria and thus harbors bacteria in the gut. However, the role of the microbiota in shaping immunity to enteric viruses has not yet been explored (Félix and Wang, 2019; Zhang et al., 2017).
Figure 1. The C. elegans intestine.
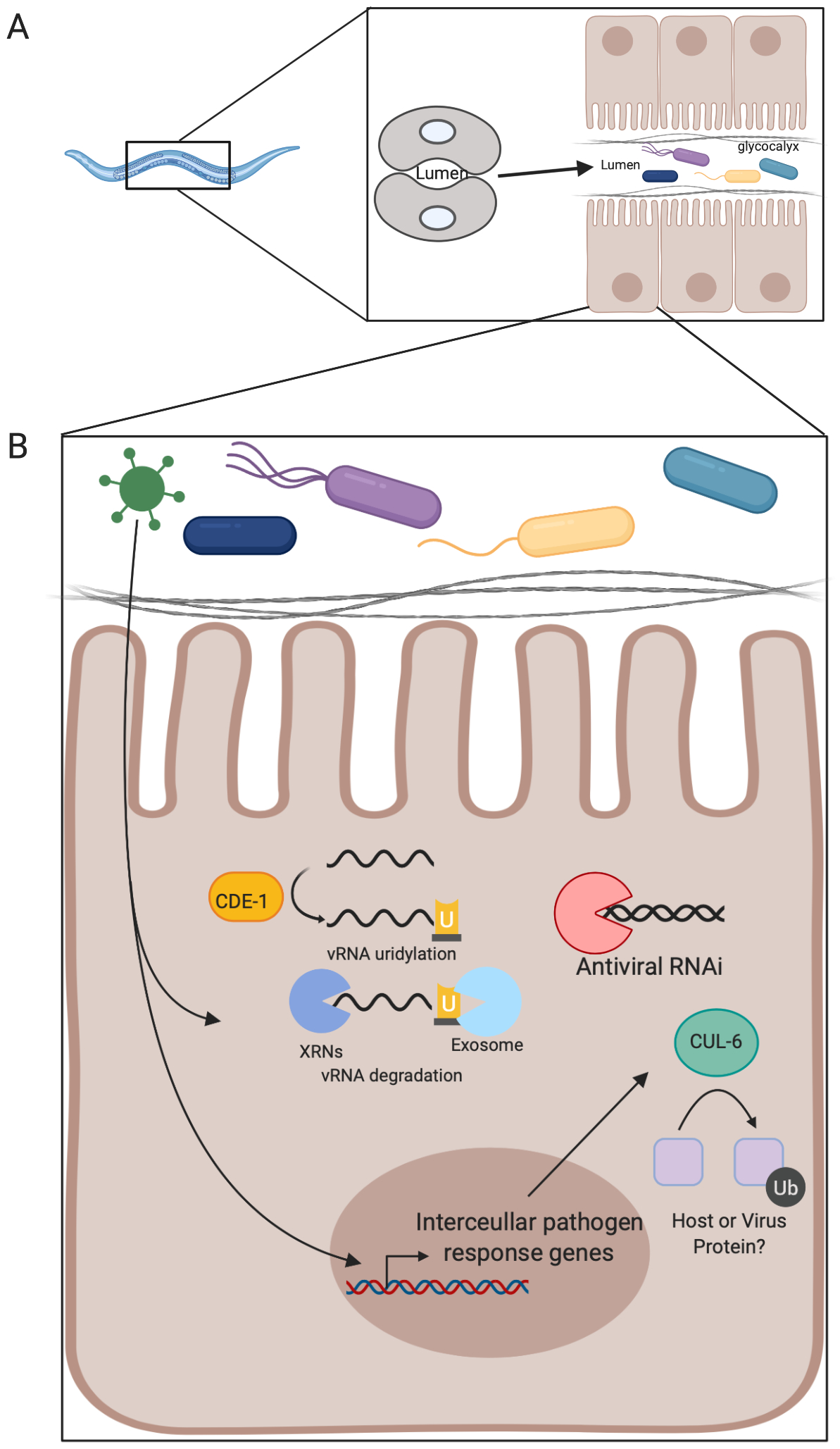
(A) Shown is the organization of the nematode intestine and the components present in the intestine. The intestine is a monolayer tube composed of 20 clonal enterocytes, 10 cells long. The glycocalyx protects the enterocytes from luminal contents. (B) Depiction of the antiviral pathways known to protect enterocytes against Orsay virus. Briefly, after Orsay virus enters a cell an intracellular pathogen response transcriptional program is triggered and results in production of several antiviral factors. One of these is cul-6, which is a ubiquitin ligase with antiviral activity. Cytoplasmic Orsay virus RNA can be uridylated and then degraded by the exosome as a means to control infection. Additionally, RNAi is active and antiviral in enterocytes.
Orsay virus was discovered in 2011 as a non-enveloped RNA virus that infects and replicates in nematode intestinal cells, leading to intestinal pathology (Félix et al., 2011; Félix and Wang, 2019; Franz et al., 2014). Innate immune recognition of many RNA viruses is through RNA binding proteins, and this recognition can result in viral RNA decay. Genetic screens in worms indicate that RNA decay pathways are antiviral in the gut. For example, Cde-1 uridylates the 30 end of Orsay virus RNA, which likely leads to its degradation by the RNA exosome, which is the major 30-50 exonuclease in the cell (Le Pen et al., 2018). Moreover, in worms, RNA interference (RNAi) is a well-characterized antiviral RNA decay pathway that controls systemic infection (Guo et al., 2019). However, in the intestine both genetic studies and a genome-wide association study (GWAS) found the RNAi machinery is active but does not provide strong antiviral protection (Ashe et al., 2013; Félix et al., 2011). This is an emerging theme; there are clearly intestinal-specific responses that are distinct from systemic virus control.
Many antiviral effector pathways are transcriptionally induced by infection. Gene expression studies found that Orsay virus infection was sensed by the epithelium and led to the induction of a gene expression program distinct from bacterial or fungal infection but overlapping with genes induced by the intracellular parasite Nematocida parisii (Bakowski et al., 2014). The transcriptional program induced by intracellular viruses and parasites was termed the intracellular pathogen response. This suggests that there are some innate responses that are more general, and other facets that are more pathogen specific. This is reminiscent of mammalian systems where infections by diverse organisms lead to overlapping but unique patterns of innate immune gene expression.
Autophagy is a degradative pathway that can target pathogens, including viruses, for destruction and is known to play important roles in enteric homeostasis in higher organisms (Aden et al., 2018; Cadwell et al., 2010; Haq et al., 2019; Liu et al., 2018; Moy et al., 2014b; Orvedahl et al., 2011; Shelly et al., 2009). Many cargos destined for decay by autophagy are marked by ubiquitylation. Interestingly, Orsay virus infection results in an accumulation of ubiquitinated proteins, and several ubiquitin ligases, including cul-6, are antiviral (Bakowski et al., 2014). Autophagy can be monitored by the accumulation of punctae marked with the ubiquitin-like molecule LC3. Orsay virus infection of enterocytes leads to the induction of LGG-1 (a homolog of the mammalian LC3 protein) puncta, raising the possibility that ubiquitin-mediated autophagy could be a conserved antiviral pathway in the intestine. An overview of antiviral pathways present in enterocytes in C. elegans can be found in Figure 1B.
Enteric Immunity in Insects: Drosophila melanogaster and Mosquitoes
Insects are a diverse and abundant group of organisms that are infected by enteric pathogens, including viruses. Arboviruses are an interesting group of arthropod-borne viruses because they are blood-borne in vertebrates, but they are orally transmitted to the vector insect. These viruses infect the gastrointestinal tract of vectors, such as mosquitoes, when they are ingested during a blood meal from a viremic vertebrate. Thus, arboviruses must establish infection of the intestine and subsequently breach the intestinal barrier to ultimately infect the salivary glands to be transmitted to the next vertebrate host. The intestine is a major barrier to infection as arboviruses that cannot establish infection of the insect gut can readily infect other tissues when delivered systemically (Kingsolver et al., 2013; Tabachnick, 2013; Xu and Cherry, 2014). These viruses cause significant global disease leading to morbidity and mortality (Labeaud et al., 2011). Indeed, 2/5 of the world population is at risk for dengue, and arboviruses are emerging and re-emerging (Brady et al., 2012).
Because arboviruses infect the intestine of insects, there is a need to understand the enteric antiviral mechanisms at play and whether we can boost these responses to prevent the cycle of transmission. The insect intestine is an epithelial monolayer with enterocytes and enteroendocrine cells that are repopulated by intestinal stem cells that reside in a niche at the basement membrane (Figure 2A). Insects lack acquired immunity but have innate immune cells and many of the same innate immune signaling pathways in higher organisms. Indeed, studies in Drosophila have revealed many facets of innate immunity, including mechanisms conserved in mosquitoes and mammals (e.g., Toll-like receptors [TLRs]) (Lemaitre, 1996; Moy et al., 2014a; Moy et al., 2014b; Sansone et al., 2015; Xu et al., 2013).
Figure 2. The insect intestine.

(A) The 3-D structure and looping of the fly intestine is shown, as is the organization of the intestine including the epithelium, basement membrane, and visceral muscle. An inset shows the cell types including enterocytes, enteroendocrine cells, differentiating enteroblasts, and intestinal stem cells that compose the epithelium. The peritrophic matrix and mucus layer separate the epithelium from the lumen. An apoptotic enterocyte is depicted to indicate the constant process of epithelial sloughing and regeneration that occurs to maintain intestinal homeostasis. (B) Represents the known antiviral pathways active in enterocytes and how these pathways are influenced by the microbiota in Drosophila. Gram-negative peptidoglycan recognition and subsequent NF-kB signaling combined with a virus-induced Cdk9-dependent RNA polymerase pause release results in transcriptional induction of antiviral cytokines including Pvf2. Pvf2 binds its receptor PVR and results in downstream activation of ERK which induces transcription of antiviral effectors. Insulin feeding results in ERK activation and protection from diverse enteric viruses. The epithelium also has active RNAi machinery that offers protection from infection. (C) Depicted is the antiviral defenses present in mosquito enterocytes and how the microbiota influences enteric infection. RNAi machinery is active in the epithelium and protects against some arboviruses. Additionally, NF-kB signaling protects the intestine from arbovirus infection by inducing expression of AMPs. It is unclear whether the virus or the microbiota induces NF-kB signaling in the context of infection. JAK-STAT signaling is induced and activated by virus infection and results in transcriptional induction of antiviral AMPs. An apoptotic infected enterocyte is depicted to indicate the association between apoptosis and protection from virus infection in the intestine. The enhancin enzyme secreted by Serratia marcescens degrades the mucus layer of the intestine which increases susceptibility to arbovirus infection. The fungi Talaromyces releases products that inhibit endogenous trypsin and lead to increased infection of the intestine.
Moreover, studies in insects have revealed interesting interactions between components of the microbiota, intestinal responses, and antiviral activities. Most human arboviruses are transmitted by Aedes mosquitoes (Brady et al., 2012). The Aedes mosquito microbiota contains gram-positive and gram-negative bacteria including Actinobacteria and Proteobacteria (Muturi et al., 2019). Because only the female mosquito takes blood meals, the female microbiota is more resilient and rapidly expands upon ingestion of the nutrient-rich blood meal. Therefore, there are major differences in the microbiota between male and female mosquitoes.
The Drosophila system has powerful genetic tools and their microbiota is simple and easily manipulatable because germfree animals and gnotobiotic animals are readily produced. The Drosophila microbiota is primarily dominated by Lactobacillus and Acetobacter species that are readily cultured, and 5 species make up >90% of the gut in the laboratory setting (Buchon et al., 2014; Wong et al., 2011). This simplicity allows for mechanistic understanding of what each species contributes to nutrition, intestinal homeostasis, and pathogen control.
Although mosquitoes harbor a large number of human arboviruses (Brady et al., 2012), epidemiological studies have also identified a number of natural enteric viruses of insects including Drosophila. Drosophila C virus is related to mammalian enteric picornaviruses and serve as models for natural enteric infections of flies (Mondotte et al., 2018; Sansone et al., 2015). Additionally, Drosophila can be orally infected by human arboviruses to model mosquito enteric virus infection (Sansone et al., 2015; Xu et al., 2013). Indeed, the fly gut also presents a high barrier to infection; although many arboviruses readily replicate in flies upon systemic infection, infection is not readily detected upon oral challenge (Sansone et al., 2015; Xu et al., 2013). Therefore, this system can be used to identify innate pathways and mechanisms by which the intestinal epithelium and the microbiota shapes enteric infections.
Many innate signaling pathways control viral infection in insects. One major pathway, as in worms, is antiviral RNA silencing (Campbell et al., 2008; McFarlane et al., 2014). However, in insects, most antiviral RNAi studies have relied on systemic infection. Recent studies in flies found that the RNAi machinery is not needed for clearance of virus from the intestine but does help protect from subsequent reinfections (Mondotte et al., 2018). In mosquitoes, during oral infection, the RNAi machinery inhibits Sindbis virus and chikungunya virus replication in the epithelium and attenuates Sindbis virus dissemination from the intestine to the rest of the mosquito (Campbell et al., 2008; McFarlane et al., 2014). Likewise, transgenic mosquitoes specifically expressing the RNAi suppressor B2 from the insect virus, Flock House virus, in the midgut had increased Sindbis virus and dengue virus replication in the gut (Khoo et al., 2013). However, in these experiments, there was no effect on dissemination from the midgut to the body, and it has become increasingly clear that RNAi is not the main antiviral defense pathway in the intestine (Khoo et al., 2013; Mondotte et al., 2018). Moreover, despite its antiviral effect against some viruses, RNAi doesn’t appear to be universally antiviral in the mosquito intestine because loss of RNAi signaling had no effect on o’nyong’nyong virus infection in Anopheles gambiae (Angleró-Rodriguez et al., 2017a; Carissimo et al., 2015). Altogether, these data suggest that RNAi is particularly important during systemic infections, and that there are distinct intestinal responses to virus infection.
In mosquitoes, the Janus kinase-signal transducer and activator of transcription (JAK-STAT) pathway is both induced by and restricts infection of the gut (Angleró-Rodriguez et al., 2017a; Carissimo et al., 2015; Souza-Neto et al., 2009). JAK-STAT signaling results in transcriptional induction of antiviral genes, such as dengue virus restriction factors 1 and 2 (DVRF1 and DVRF2), that protect the midgut from dengue virus infection (Souza-Neto et al., 2009). Inflammatory NF-kB signaling is an important antiviral response in the mosquito intestine as well (Carissimo et al., 2015; Xi et al., 2008). It is known that the microbiota influences NF-kB signaling (Xi et al., 2008), but during infection, it is unclear whether it is sensing of the microbiota or virus that results in activation of NF-kB pathways that canonically sense bacteria or fungi. Moreover, infection of mosquitoes occurs in the context of a blood meal, which both affects the nutrient status of the animals and leads to significant changes in the microbiota (Muturi et al., 2019; Zhu et al., 2017). How these signals are integrated is also unclear.
Emerging studies have explored the role of the microbiota during arbovirus infection of the mosquito. Antibiotic treatment led to decreased infection of Anopheles gambiae by o’nyong’nyong virus (Carissimo et al., 2015). In contrast, antibiotic treatment of Culex mosquitoes led to increased infection by Japanese encephalitis virus and antibiotic treatment of Aedes albopictus mosquitoes resulted in increased Sindbis virus infection (Barletta et al., 2017; Mourya and Soman, 1985). These studies suggest that signals from the microbiota can affect infection in diverse ways.
Indeed, studies on dengue virus infection of Aedes aegypti highlight the fact that there is no one “microbiota” but rather different mosquitoes fed distinct diets in different labs likely have different microbial communities, and these communities provide distinct stimuli that can affect infection in different ways. Xi et al., (2008) found that antibiotic treatment led to increased dengue infection and they linked this to microbiotadependent activation of innate signaling through Toll receptors and NF-kB. In contrast, Wu et al., (2019) found that antibiotic treatment led to decreased dengue virus infection and that this was due to the presence of Serratia marcescens within their mosquito microbiota. Serratia marcescens secretes an enhancin enzyme that degrades membrane-bound mucins and loss of this mucin protection increases susceptibility to viral infection. Therefore, it is important to characterize the constituents of the microbiota before and after antibiotic treatments. A simple explanation for the different outcomes in these studies is that the composition of the microbiota in these labs is different; Serratia marcescens might have been absent in the mosquitoes from the first study. Moreover, these findings underscore that the products produced by the microbiota can directly shape immunity and pathogenesis.
Although fungi are clearly part of the microbiota, their roles in immune defense are less studied. A filamentous fungal Talaromyces species isolated from wild Aedes aegypti mosquitoes inhibits host trypsin activity, which promotes dengue virus infection of the gut (Angleró-Rodriguez et al., 2017b). Currently, efforts are underway to identify particular species of fungi or bacteria that can prevent arbovirus acquisition by mosquitoes and could thus be used as probiotics to block transmission. A clear example of this strategy involves the use of the intracellular bacteria Wolbachia. Although not a part of the exogenous microbiota, they are vertically transmitted symbionts and are found in 60%–65% of insects (Rasgon, 2008). Interestingly, the major mosquito vectors for human viruses lack this symbiont. Studies first performed in flies demonstrated that Drosophila harboring Wolbachia were resistant to diverse viral infections (Teixeira et al., 2008). This led to the introduction of Wolbachia into vector mosquitoes, which can experimentally protect vectors from diverse arboviruses including dengue virus and chikungunya virus (Moreira et al., 2009). Although this protection is not universal, trials are underway to determine whether this strategy can be used to block the spread of these arboviral pathogens (Dodson et al., 2014; Glaser and Meola, 2010; Mains et al., 2019).
Studies in Drosophila have also revealed an interplay between the microbiota and antiviral responses in enterocytes. Antibiotic treatment of flies led to increased infection by diverse enteric viruses and this pathway was dependent on inflammatory NFkB signaling (Sansone et al., 2015). Moreover, gram-negative commensals and peptidoglycan recognition were required to induce this antiviral response in the enterocytes. Enterocyte NF-kB signaling was needed for the induction of an antiviral cytokine, Pvf2, that binds the cytokine receptor poliovirus receptor (PVR) on enterocytes to activate antiviral extracellularsignal-regulated kinase (ERK) signaling. Thus, microbiota dependent priming is essential for barrier immune defenses. The spectrum of microbiota-derived products that prime immunity and confer protection from enteric viral infections are unknown.
Nutrient availability also influences virus infections, and this is likely even more important in the intestinal tract, where nutrient amounts change dramatically (Xu et al., 2013). One extreme example of this variation in nutrient availability is a mosquito taking a blood meal, which is a huge nutrient bolus and influences arbovirus infection (Zhu et al., 2017). Moreover, the blood contains human growth factors including insulin, and studies have shown that insulin in vertebrate blood affects enteric biology in mosquitoes (Kang et al., 2008). Moreover, studies found that flies fed vertebrate insulin at the amounts present in a blood meal led to ERK-dependent antiviral signaling in enterocytes, and conferred immune protection from viruses (Ahlers et al., 2019; Xu et al., 2013). The spectrum of nutrients that affect viral infection is unknown and clearly important.
Another facet of intestinal biology that affects infection and immunity is intestinal turnover. From insects to humans, the intestinal epithelium is dynamically regenerated, and this is regulated by locally resident intestinal stem cells that differentiate to maintain homeostasis. This turnover is tightly regulated and the interplay between enteric virus infection and intestinal turnover is likely important. Dengue virus infection affects the maturation of enterocytes in the Aedes aegypti midgut (Serrato-Salas et al., 2018). In insects, maturation involves both differentiation and endoreplication to create larger absorptive cells. Inhibition of this maturation pathway leads to increased susceptibility to enteric infection (Serrato-Salas et al., 2018). Although there is no intestinal turnover in C. elegans, endoreplication is needed for the intestine to grow with the organism. Endoreplication, and thus barrier integrity, also plays a role in infection of the nematode intestine (Félix and Wang, 2019). In addition, death of enterocytes can drive intestinal turnover from insects to humans. In mosquitoes there is a correlation between the expression of cell death caspase genes and resistance to dengue virus infection, because loss of caspase activity increases dengue virus susceptibility. Moreover, dengue-virus resistant Aedes aegypti have higher caspase gene expression in the midgut compared with dengue-virus-susceptible strains (Ocampo et al., 2013). Altogether, these data suggest the increased turnover of enterocytes might be a protective mechanism that purges the intestine of infected cells. An overview of antiviral pathways present in enterocytes in Drosophila and mosquitoes can be found in Figures 2B–2C.
Mammalian Enteric Studies
Human Enteroids and Organoids
The mammalian gut is complex, and Figure 3A depicts the organization of the mammalian intestine. Because many human enteric viruses do not readily infect mice orally, a variety of mini-intestine models have been developed to study human enteric viruses in human intestinal cultures. In addition, although many facets of innate immunity are conserved, there are potentially important differences between mice and humans including differences in TLR localization. These differences likely affect the responsiveness of the epithelium to the microbiota and result in differences in microbiota-mediated tonic signaling between the mouse and human intestine (Hamonic et al., 2018; Price et al., 2018). Indeed, studies in mice have shown that TLR stimulation of enterocytes apically versus basolaterally results in different responses (Lee et al., 2006; Stanifer et al., 2019). Moreover, systemic flagellin, but not LPS, protected mice against enteric rotavirus in a TLR5- and NLR family CARD domain-containing 4 (NLRC4)dependent manner (Uchiyama et al., 2014). This is likely due to the different immune responses as is seen for apical versus basolateral stimulation of TLRs on intestinal epithelial cells (IECs) (Lee et al., 2006; Stanifer et al., 2019). For example, TLR5 is located on the basolateral side of IECs, which would make it only responsive to systemic flagellin (Rhee et al., 2005). Moreover, the development of non-transformed intestinal cell systems is particularly important because there are clear differences in innate immune signaling in transformed cell lines compared with that in non-transformed systems (Drummond et al., 2017; Hare et al., 2016; Krishnamurthy et al., 2006). For example, traditional in vitro studies in cancer cell lines described a-defensins as antiviral against adenovirus, but a mouse enteroid model found a-defensins enhance mouse adenovirus infection, and many cancer cell lines are defective in stimulator of interferon genes (STING) signaling (de Queiroz et al., 2019; Wilson et al., 2017; Xia et al., 2016). Thus, human studies in intestinal models are essential.
Figure 3. The mammalian intestine.
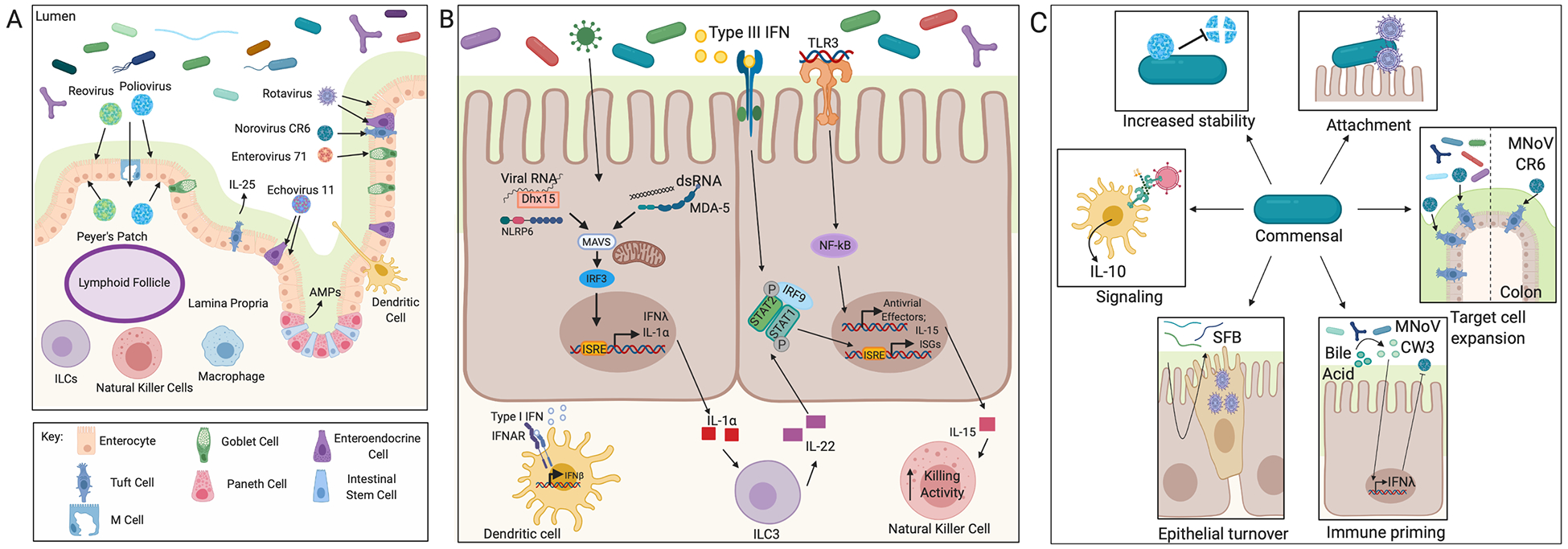
(A) Presented is the organization of the epithelium into villi and crypts and the cell types present in the mammalian intestine. Peyer’s patches with lymphoid follicles and immune cells in the lamina propria are also shown. The mucus layer separates the epithelium from the luminal contents. The cellular tropism of mammalian enteric viruses is depicted by arrows. (B) Shows antiviral pathways active in IECs. The sensors MDA-5 and NLRP6 are antiviral in the intestine and activate type III IFNs. Viral sensing can induce IL-1α which activates ILC3s in the lamina propria to produce IL-22. IL-22 is protective against enteric virus infection and acts synergistically with type III IFNs to induce expression of ISGs. TLR3 is another virus sensor that is present in the epithelium and leads to NF-kB signaling and production of IL-15. IL-15 activates NK cell killing in the lamina propria. Macrophages in the lamina propria produce type I IFNs in response to virus infection which escapes the epithelium to protect from disseminated infection. (C) Depicts mechanisms by which the microbiota or microbiota-derived products interact with enteric viruses and influence infection. Enteric viruses can bind to bacteria which can increase virion stability. Bacteria can bind virions and promote attachment to IECs. Viruses can incorporate products derived from the microbiota like LPS into their envelopes which then induces immune signaling upon entry. The microbiota increases the number of tuft cells present in the colon which increases MNoV infection. Segmented filamentous bacteria (SFB) increase epithelial turnover, which might protect from infection by expulsing infected IECs. The microbiota metabolized bile acids prime type III IFN signaling in the small intestine which is protective from acute MNoV infection.
Organoids, derived from induced pluripotent stem cells, and enteroids, derived from biopsies, are three-dimensional epithelial cultures with apical-basolateral polarity that have a lumen and can develop crypts and villus-like structures (Sato et al., 2009; Spence et al., 2011). Compared with enterocyte-onlyderived cell lines, these models can contain multiple epithelial cell lineages, which allows for added complexity. Moreover, these complex structures have revealed cell-type-specific functions and have been used to study diverse human enteric viruses (Brown et al., 2018; Drummond et al., 2017; Ettayebi et al., 2016; Good et al., 2019; Kolawole et al., 2019; Pervolaraki et al., 2017; Saxena et al., 2017; Wilson et al., 2017).
Human mini-intestine models have uncovered the cellular tropism of human enteric viruses, revealing that these viruses infect distinct cell types (overview in Figure 3A). Enterovirus 71 specifically infects goblet cells through the apical membrane and this preference might be due to high expression of scavenger receptor class B member 2 (SCARB2), the primary enterovirus 71 receptor, in these cells (Good et al., 2019). Echovirus 11 infects enterocytes and enteroendocrine cells (Drummond et al., 2017). Echovirus11 infects enteroids more efficiently from the basolateral side of the epithelium rather than the apical side, which is surprising given the pan-echovirus receptor FcRn is present at the apical side of enterocytes (Hornby et al., 2014; Morosky et al., 2019). Moreover, high expression of FcRn on enteroendocrine cells might indicate a particularly important role of enteroendocrine cells for the establishment of echovirus 11 infection of the intestinal epithelium (Hornby et al., 2014).
Enteroid models have also challenged current hypotheses regarding poliovirus and reovirus infection in the intestine. For example, reovirus and poliovirus were thought to enter and transcytose through M cells in Peyer’s patches and infect the basolateral side of enterocytes in the lamina propria. The viruses are thought to shed into the lumen of the intestine, which enables transmission to the next host (Pfeiffer, 2010; Rubin et al., 1985). However, recent studies found that reovirus infects human enteroids apically, which suggests that they might directly infect enterocytes rather than requiring transit through M cells (Brown et al., 2018). Indeed, poliovirus was visualized by microscopy at microvilli tips of CD155-transgenic mice (Ohka et al., 2007). Altogether, these complex in vitro model systems can reveal important aspects of cell biology.
Type I and type III IFN responses are important innate antiviral pathways. Treatment of enteroids or other cell types that express the IFN-lR with type 1 and type III IFNs can induce similar genes, suggesting that the location and temporal control of these cytokines can affect antiviral protection (Drummond et al., 2017; Forero et al., 2019). Indeed, these cytokines clearly play distinct roles within the intestine because the type I IFN pathway is active in the lamina propria, whereas the type III IFN pathway is active in IECs (Baldridge et al., 2017; Mahlakõiv et al., 2015; Pott et al., 2011). In IECs, the IFN-lR is expressed and viral infection induces IFN-l expression to induce production of antiviral interferon-stimulated genes (ISGs) (Drummond et al., 2017; Good et al., 2019; Pervolaraki et al., 2017; Saxena et al., 2017). Studies using enteroids and organoids have established the antiviral role of type III IFN in IECs and have begun to uncover the pathways controlling interferon induction in these cells. Indeed, whereas reovirus and rotavirus infection induces both IFN-b and IFN-l expression, there is no accompanying increase in type I IFN protein amounts in colon organoids or human enteroids, respectively (Pervolaraki et al., 2017; Saxena et al., 2017). Moreover, pre-treatment with type III IFN confers protection from rotavirus, enterovirus 71, and reovirus infection (Good et al., 2019; Pervolaraki et al., 2017; Saxena et al., 2017). Although pre-treatment with IFN-l can block rotavirus infection, rotavirus can also antagonize expression of type III IFNs (Saxena et al., 2017). Therefore, the temporal control of infection by IFNs might be complex. Moreover, IFN-l mediated protection from reovirus is JAK-, ERK-, p38-, and c-Jun N-terminal kinase (JNK)-dependent in organoids (Saxena et al., 2017). This data, along with the importance of MAPK signaling in the Drosophila intestine, suggests MAPK signaling has an evolutionarily conserved role of controlling many antiviral responses in the intestine.
Despite the lack of IFN-b protein production during enteric virus infection enteroid, studies have investigated whether an exogenous source of type I IFNs offers protection from enteric virus infection. Pre-treatment of enteroids with type 1 IFNs can protect from some infections (rotavirus and reovirus) but not others (enterovirus 71) (Good et al., 2019; Pervolaraki et al., 2017; Saxena et al., 2017). This is perhaps due to differences in virus tropism, given that enterovirus 71 infects goblet cells and rotavirus and reovirus primarily infect enterocytes. These data also suggest that Type I IFN is not produced by the epithelium itself, but rather by the surrounding lamina propria, and that only subsets of human IECs are appropriately responsive. Single-cell sequencing studies might reveal cell-type-specific IFN responses.
Enteroids have also shown a role for cell death in the control of virus infection. Reovirus induces apoptosis of mouse enteroids, and viral strains that induced less apoptosis had higher titers, suggesting that IEC-induced apoptosis is protective (Brown et al., 2018). This is supported by an in vitro study that found inhibition of caspases increases rotavirus infection (Frias et al., 2012). Epithelial cells produce the cytokine interleukin-22 (IL-22), which stimulates intestinal stem cell (ISC) regeneration, promotes barrier integrity, and maintains intestinal homeostasis (Lindemans et al., 2015). IL-22 pre-treatment of human enteroids blocks human rotavirus growth (Saxena et al., 2017).
Recent work with these mini-intestine systems studied the interaction between specific commensals or bacterial-derived products and IECs, but no organoid or enteroid system with a microbiota has been developed (Blutt et al., 2017). Microinjection of a single commensal E. coli strain led to bacterial replication that was contained with the apical space of enteroids (Karve et al., 2017). This suggests enteroids can be colonized with commensal bacteria and opens the way for microbiota studies in mini-intestines.
Murine Models
Although enteroids are useful models, particularly in the study of human enteric viruses, they also have limitations. First, not all of the specialized epithelial cells present in the mammalian intestine are present in organoids or enteroids. Second, these in vitro cultures lack the lamina propria and the complement of immune cells present in the intestine. And third, these systems lack an intact microbiota. Thus, organismal models are essential to uncover important facets of enteric antiviral immunity. Because most human enteric viruses cannot orally infect wild-type mice, orthologous mouse viruses have been employed. For example, mouse norovirus (MNoV) strains rather than human norovirus (HuNoV) and murine, simian, and rheseus rotavirus are used to model human rotaviruses (Baumler€ and Sperandio, 2016; Cadwell et al., 2010; Nice et al., 2015).
Enteric Virus Tropism
Studies in vivo have shown that the reovirus serotype 1 Lang strain replicates primarily in M cells and Peyer’s patches in the ileum (Rubin et al., 1985), although there are M cells and Peyer’s patches throughout the jejunum and duodenum suggesting that there are region-specific factors that influence infection of the small intestine. Rotavirus infects enterocytes of the villi of the small intestine (Starkey et al., 1986). In rodents there is a sialic acid gradient where the highest levels are at the villus tip and decrease as you move deeper into the crypt. (Jaswal et al., 1988). Given that rotaviruses use sialic acid as a co-receptor for entry, these findings might explain why rotavirus preferentially infects enterocytes at the tip of the villi.
Persistent MNoV strains preferentially infect tuft cells in the mouse small intestine and colon because the MNoV receptor CD300lf is exclusively expressed in these cells (Wilen et al., 2018). Both acute and persistent MNoV strains are reported to infect B cells and other innate and adaptive immune cells in gut-associated lymphoid tissue (Grau et al., 2017; Jones et al., 2014). B-cell-deficient mice had less acute and persistent MNoV infection in the intestine relative to controls, but the acute and persistent viruses could still establish infection of the intestine, which suggests infection of additional cell types.
Tuft cell numbers are controlled by the microbiota, and animals lacking a microbiota display fewer tuft cells and are less permissive to infection by persistent MNoV (Baldridge et al., 2015). Moreover, in vitro studies have shown that particular commensals enhance HuNoV attachment to B cells and that in vivo enteric infection by diverse MNoV strains was enhanced by the microbiota (Jones et al., 2014). Conversely, antibiotic treatment has no effect on acute MNoV virulence and actually increased MNoV infection of the proximal small intestine while decreasing infection of the distal small intestine and colon (Grau et al., 2019). These data demonstrate the complexity of the diverse interactions between the microbiota, microbial ligands, and infection and highlight the region-specific nature of these interactions.
Enteric Antiviral Response
Consistent with the essential role of type III IFNs in the intestinal epithelium, treatment with type III IFNs protects adult and suckling mice against MNoV infection and can protect mice from persistent MNoV infection independent of adaptive immunity (Baldridge et al., 2017; Mahlakõiv et al., 2015). The microbiota metabolizes bile acids, which then prime type III IFN production in the small intestine and protects the small intestine, but not the colon from acute MNoV infection (Grau et al., 2019). Type III IFNs also protect adult and suckling mice against reovirus infection and reovirus-induced mortality (Baldridge et al., 2017; Mahlakõiv et al., 2015; Nice et al., 2015). Additionally, whereas type I interferon receptor knockout mice had increased susceptibility to reovirusinduced mortality, increased infection was seen in the lamina propria, but not in the IECs (Mahlakõiv et al., 2015). This is expected given that myeloid cells in Peyer’s patches produce type I IFNs during reovirus infection (Johansson et al., 2007). Type III IFNs protect against rotavirus replication and pathology in the intestine (Pott et al., 2011). Mouse rotavirus induces IFN-l expression and IL-1a production in IECs and IECderived IL-1a activates IL-22 expression in the innate lymphoid cell 3 (ILC3) population in the lamina propria (Hernéndez et al., 2015). IL-22 acts synergistically with IFN-l to activate STAT1 to decrease rotavirus infection in the intestine. Importantly, IL-22 and IL-18 combination therapy protects mice from rotavirus infection and pathology (Zhang et al., 2014).
Although Type III interferons are essential components of the epithelial response to infection, little is known about how viruses are sensed to induce this pathway in the gut. Melanoma differentiation-associated protein 5 (MDA5) and retinoic acidinducible gene I (RIG-I) are cytoplasmic viral RNA sensors that bind mitochondrial antiviral-signaling protein (MAVS) to induce interferons (Kato et al., 2006). Compared to control mice, MDA5 knockout mice had increased MNoV titers in the proximal intestine, but not the distal intestine (McCartney et al., 2008). Heme-oxidized IRP2 ubiquitin ligase 1 (HOIL1), a component of the linear ubiquitin chain assembly complex, is antiviral against acute and persistent MNoV infection in the intestine, (MacDuff et al., 2018). In vitro data suggest HOIL1 is required for MDA5-dependent induction of type I and type III IFNs during infection with positive-sense single-stranded RNA viruses. It is unknown whether MDA5 or HOIL1 are required in the epithelium or the lamina propria.
Compared with control mice, MAVS knockout mice had more rotavirus infection in intestinal IECs (Broquet et al., 2011). Nlrp6 is another cytoplasmic pathogen sensor that was canonically described as antibacterial (Anand et al., 2012). Recent work has shown that Nlrp6 mutant mice displayed increased mortality and increased MNoV and encephalomyocarditis virus infection in the intestine after oral challenge (Wang et al., 2015). This antiviral function is inflammasome-independent and likely induces interferons downstream of MAVS.
The epithelial barrier is maintained in part through the presence of tight junctions. TLRs are extracellular pathogen recognition receptors and have been found to play an important role in regulating barrier function through the control of tight junction proteins (Gibson et al., 2008; Rakoff-Nahoum et al., 2004). TLR3 signaling in the epithelium protects adult, but not suckling, mice from rotavirus infection (Pott et al., 2012). Suckling mice have lower amounts of TLR3 than adult mice, suggesting that there might be age-dependent changes in innate signaling. The mechanism by which TLR3 signaling controls infection remains unclear; however, TLR3-induced expression of regulated on activation, normal T cell expressed and secreted (RANTES) or IL-15 might be involved (Pott et al., 2012; Zhou et al., 2007). Simian rotavirus induces IL-15 expression in a TLR3-dependent manner in mouse IEC ex vivo cultures. IL-15 increases lamina propria natural killer (NK) cell killing activity suggesting a clear circuit (Zhou et al., 2007). However, mice mutant for the TLR3 signaling adaptor TIR-domain-containing adapter-inducing interferon-b (TRIF) did not display altered infection by rhesus or monkey rotavirus, making interpretations less clear (Broquet et al., 2011). Figure 3B presents an overview of the antiviral pathways defined in epithelial cells in mammals.
The Microbiota and Virus Infection
One aspect of enteric immunity that can lead to confounding results are differences in the composition of the microbiota. A number of studies have clearly demonstrated that diverse enteric viruses, including enteroviruses, rotaviruses, and reoviruses, can bind particular members of the microbiota and particular bacterial surface structures, including LPS, peptidoglycan, or sialic acid. Poliovirus and reovirus physically bind LPS from particular strains of bacteria, and this interaction can increase cell surface attachment and virion thermostability, which might aid transmission of the virus (Berger et al., 2017; Kuss et al., 2011; Robinson et al., 2014). Additionally, certain species of the microbiota, but not all, can bind to multiple poliovirus virions and can promote genetic recombination (Erickson et al., 2018). LPS also binds coxsackie B virus but does not promote virion thermostability, and the functionality of this interaction is unknown (Aguilera et al., 2019).
In support of the positive role for virus-commensal interactions, mice that received antibiotic treatment showed decreased poliovirus, coxsackie B virus, persistent MNoV, rotavirus, mouse mammary tumor virus (MMTV), and reovirus infection in the stool, compared with the stool of conventional mice (Baldridge et al., 2015; Jones et al., 2014; Kane et al., 2011; Robinson et al., 2014; Uchiyama et al., 2014). However, although antibiotic treatment results in protection from both coxsackie B virus and poliovirus, the shift in community structure necessary to confer protection from enteric infection is different between the two viruses (Robinson et al., 2019). This adds an extra layer of complexity to microbiota-virus interaction studies. It is also clear that there are differences in the relationship between enteric infection, the microbiota, and regional differences across the gut. Indeed, recent studies have revealed that the microbiota promotes MNoV infection distally while restricting infection in the proximal small intestine (Grau et al., 2019).
Antibiotic treatment attenuates rotavirus infection most likely through influencing entry (Uchiyama et al., 2014). Many viruses, including rotaviruses, bind sialic acid to facilitate cellular entry. Commensals can affect enteric sialic acid availability in diverse ways, including degrading sialic acid or expressing sialic acid on their surface, suggesting a complexity in these interactions. The expression of sialic acid on the surface of certain commensals might enhance rotavirus infection of the intestine. Moreover, virion binding to bacteria expressing sialic acid could enhance virion thermostability as in the case of reovirus or poliovirus.
Recently, it was reported that a specific group of commensals, segmented filamentous bacteria, provide resistance to rotavirus infection (Shi et al., 2019). Rotavirus resistance was due to an increase in epithelial cell turnover caused by the segmented filamentous bacteria. This, along with data in insect systems suggests intestinal turnover is a conserved pathway that can be used in intestinal defense. Additionally, this further highlights the commensal-specific functions that differentially affect virus infection and the need for definition of the microbiota and standardization across experiments.
MMTV is an enteric virus transmitted vertically between a mother and her pups through her milk. An intact microbiota is needed for vertical transmission (Kane et al., 2011). The MMTV envelope contains CD14, TLR4, myeloid differentiation protein 2 (MD2), and LPS-binding protein, which enables virions to bind LPS from the microbiota (Wilks et al., 2015). The LPS bound to MMTV virions activates TLR4 signaling on splenocytes or dendritic cells (DCs), which induces IL-10 production that leads to suppression of an antiviral immune response and MMTV persistence. Therefore, MMTV co-opted the TLR4-LPS interaction between the microbiota and host cells to promote infection. A summary of the indirect and direct ways the microbiota influences enteric virus infection can be found in Figure 3C.
The microbiota has an important role in tonic immune signaling and sets a threshold for a healthy state. In the absence of a microbiota, the intestine has abnormal morphology, impaired cellular immune responses, and is more susceptible to injury (Kernbauer et al., 2014; Kitajima et al., 2001). This basal level of immune activation allows the host to launch a productive response during virus infection (Abt et al., 2012; Ichinohe et al., 2011). Germ-free mice have increased susceptibility to influenza A virus and lymphocytic choriomeningitis virus because the microbiota acts as a tonic signal that helps set the immune threshold in the gut to appropriately respond to infections (Abt et al., 2012; Ichinohe et al., 2011). Macrophages from antibiotic-treated mice had dampened responsiveness to type I and type II IFN signaling, and restoration of this responsiveness was sufficient to protect from influenza virus infection. However, the continual stimulation of IECs and immune cells by the microbiota has to be tightly regulated to prevent persistent inflammation and autoimmunity. Interestingly, the exact ligands needed for setting immune thresholds seem fluid. Fungal colonization of antibiotic-treated mice protects them from influenza-virusor dextran sulfate sodium (DSS)-induced mortality (Jiang et al., 2017). MNoV infection of germ-free mice is sufficient to act as a “microbiota” because this viral infection protects against intestinal injury and pathogenic bacterial infection (Kernbauer et al., 2014). Astroviruses can also mimic the role of the microbiota in setting an immune threshold by inducing type III IFN in IECs that protects the epithelium from enteric pathogens (Ingle et al., 2019). The instructive nature of the microbiota affects both innate and adaptive cells locally and systemically. Loss of the microbiota decreased survival during subcutaneous infection with the flaviviruses West Nile virus, dengue virus, and Zika virus because of impaired CD8+ T cell responses (Thackray et al., 2018)
Perhaps counterintuitively, loss of the microbiota can also lead to an enhanced production of pathogen-specific antibodies. In es the case of rotavirus, antibiotic treatment protects mice against infection and increases rotavirus-specific immunoglobulin G (IgG) and IgA titers, which are a correlate of protection (Uchiyama et al., 2014). Altogether, these studies suggest that stimuli from the microbiota are part of the healthy network between commensals and the epithelium that maintains homeostasis of the epithelium and surrounding adaptive immune cells for health.
Future Directions
In conclusion, the intestine and the diverse spectrum of IECs have specialized functions that help maintain intestinal homeostasis, sustain the epithelial barrier, and protect against enteric viral infections. There is much to be discovered and each of the diverse models discussed in this review are contributing to our understanding of this complex environment. Future development of these systems, and a more mechanistic understanding of how the microbiota interfaces with the epithelium will go a long way toward our discovery of the molecular underpinnings of innate-cell-intrinsic immunity in the intestine. Simple models such as flies and worms provide a reductionist view, which is essential because it remains difficult to dissect the contribution of individual commensals and their products on innate antiviral immunity in higher organisms such as mice. We suggest that developing systems with intermediate complexity such as zebrafish, which have interferon signaling and an adaptive immune response but lack certain elements of the mammalian intestine, could shed further light onto the interplay between innate and adaptive immunity in interacting with the microbiota and controlling infections of the gut (Brugman, 2016). The first natural picornavirus to infect zebrafish, zebrafish picornavirus 1 (ZfPV-1), was recently identified, but the effect of the virus on the intestine remains unclear (Altan et al., 2019). Additionally, zebrafish might serve as a good model organism to study human enteric pathogens given that zebrafish larvae can be infected with human norovirus strains GI and GII (Van Dycke et al., 2019). It will be interesting from an evolutionary perspective to identify deeply conserved antiviral systems using diverse nonmammalian model systems.
From this review we hope that it has become clear that there is no such thing as a single or uniform “microbiota,” rather diverse species producing diverse ligands produce different outcomes. This explains why the microbiota does not have a universally protective or restrictive role during enteric virus infection. In insects it appears that the microbiota is largely protective, at least in young healthy animals and as long as a pathobiont is not present. It is less clear in mouse models; there are likely opposing forces at work, and it is the balance of the signals that affect outcomes. A better understanding of how the immune threshold that is set by the microbiota is beneficial or harmful in different disease contexts is needed. Of note, most antibiotic experiments in mice merely shift the community structure rather than ablating the microbiota (Abt et al., 2012; Ichinohe et al., 2011; Thackray et al., 2018; Uchiyama et al., 2014). This makes it hard to know whether loss of a specific commensal or gain or increased abundance of a different commensal is what affects enteric virus infection rather than a decrease in commensal burden. This might explain how the microbiota is protective against non-enteric viruses like influenza and flaviviruses but can be harmful during enteric virus infection. Use of germ-free organisms is complementary given that there is a complete loss of the community; however, developmental issues might confound interpretations. Nevertheless, gnotobiotic animals are essential to define the role of a particular commensal in intestinal homeostasis or pathogen defense.
Human clinical trials and in vitro data from human IEC experiments suggests that specific commensals or microbiota-derived products restrict rotavirus infection (Ahmadi et al., 2015; Uchiyama et al., 2014). Additionally, probiotics such as Lactobacillus rhamnosus GG decrease the duration of diarrhea in rotavirus-positive children (Ahmadi et al., 2015). However, there have been several randomized control trials with differing results, which could be attributable to species- or strain-level differences between the bacteria or human cohorts used (Brussow, 2019€). Although randomized control trials have found Lactobacillus rhamnosus GG to reduce duration of diarrhea, other studies have found it has no effect. In one case, this seemed to be due to the display of different pili genes between the reference strain and the Lactobacillus rhamnosus GG used in the trial (Freedman et al., 2018). This further highlights the need for increased definition and specificity in research on the microbiota and its interaction with the intestine.
If we could understand the microbial ligands and microbial metabolites that contribute to immunomodulation of the epithelium, we could use this information to generate informed therapeutics and pre-biotics. Metabolites and ligands clearly affect IECs and immune cells, and the spectrum of activities present in the gut are unknown (Blacher et al., 2017; Thaiss et al., 2016). Moreover, most metabolites or bacterial components have to bind or enter IECs to be sensed by cellular innate immune or adaptive immune cells, but the mechanisms involved are largely unknown. Non-mammalian models with simpler microbiota and robust genetics will likely reveal conserved mechanisms. Altogether the future will redefine the microbiota in molecular terms incorporating food, drugs, and the other ligands to define the circuitry within the intestine, which will hopefully allow us to develop new therapeutic approaches to promote human health.
Acknowledgments
This publication was made possible by NIH grants T32-AI-007324 and F31 AI147415-01 to E.S., as well as NIH grants (R01AI122749, R01AI150246, R01AI152362, R01AI140539) to S.C. S.C. is a recipient of the Burroughs Wellcome Investigators in the Pathogenesis of Infectious Disease Award.
REFERENCES
- Abt MC, Osborne LC, Monticelli LA, Doering TA, Alenghat T, Sonnenberg GF, Paley MA, Antenus M, Williams KL, Erikson J, et al. (2012). Commensal bacteria calibrate the activation threshold of innate antiviral immunity. Immunity 37, 158–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aden K, Tran F, Ito G, Sheibani-Tezerji R, Lipinski S, Kuiper JW, Tschurtschenthaler M, Saveljeva S, Bhattacharyya J, Hasler R, et al. (2018). ATG16L1 orchestrates interleukin-22 signaling in the intestinal epithelium via cGAS-STING. J Exp Med 215, 2868–2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilera ER, Nguyen Y, Sasaki J, and Pfeiffer JK (2019). Bacterial Stabilization of a Panel of Picornaviruses. mSphere 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahlers LRH, Trammell CE, Carrell GF, Mackinnon S, Torrevillas BK, Chow CY, Luckhart S, and Goodman AG (2019). Insulin Potentiates JAK/STAT Signaling to Broadly Inhibit Flavivirus Replication in Insect Vectors. Cell Rep 29, 1946–1960.e1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmadi E, Alizadeh-Navaei R, and Rezai MS (2015). Efficacy of probiotic use in acute rotavirus diarrhea in children: A systematic review and meta-analysis. Caspian J Intern Med 6, 187–195. [PMC free article] [PubMed] [Google Scholar]
- Altan E, Kubiski SV, Boros A, Reuter G, Sadeghi M, Deng X, Creighton EK, Crim MJ, and Delwart E (2019). A Highly Divergent Picornavirus Infecting the Gut Epithelia of Zebrafish (Danio rerio) in Research Institutions Worldwide. Zebrafish 16, 291–299. [DOI] [PubMed] [Google Scholar]
- Anand PK, Malireddi RK, Lukens JR, Vogel P, Bertin J, Lamkanfi M, and Kanneganti TD (2012). NLRP6 negatively regulates innate immunity and host defence against bacterial pathogens. Nature 488, 389–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anglero-Rodriguez YI, MacLeod HJ, Kang S, Carlson JS, Jupatanakul N, and Dimopoulos G (2017a). Aedes aegypti Molecular Responses to Zika Virus: Modulation of Infection by the Toll and Jak/Stat Immune Pathways and Virus Host Factors. Front Microbiol 8, 2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anglero-Rodriguez YI, Talyuli OA, Blumberg BJ, Kang S, Demby C, Shields A, Carlson J, Jupatanakul N, and Dimopoulos G (2017b). An Aedes aegypti-associated fungus increases susceptibility to dengue virus by modulating gut trypsin activity. Elife 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apidianakis Y, and Rahme LG (2011). Drosophila melanogaster as a model for human intestinal infection and pathology. Dis Model Mech 4, 21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashe A, Belicard T, Le Pen J, Sarkies P, Frezal L, Lehrbach NJ, Felix MA, and Miska EA (2013). A deletion polymorphism in the Caenorhabditis elegans RIG-I homolog disables viral RNA dicing and antiviral immunity. Elife 2, e00994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakowski MA, Desjardins CA, Smelkinson MG, Dunbar TL, Lopez-Moyado IF, Rifkin SA, Cuomo CA, and Troemel ER (2014). Ubiquitin-mediated response to microsporidia and virus infection in C. elegans. PLoS Pathog 10, e1004200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldridge MT, Lee S, Brown JJ, McAllister N, Urbanek K, Dermody TS, Nice TJ, and Virgin HW (2017). Expression of Ifnlr1 on Intestinal Epithelial Cells Is Critical to the Antiviral Effects of Interferon Lambda against Norovirus and Reovirus. J Virol 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldridge MT, Nice TJ, McCune BT, Yokoyama CC, Kambal A, Wheadon M, Diamond MS, Ivanova Y, Artyomov M, and Virgin HW (2015). Commensal microbes and interferon-lambda determine persistence of enteric murine norovirus infection. Science 347, 266–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banyai K, Estes MK, Martella V, and Parashar UD (2018). Viral gastroenteritis. Lancet 392, 175–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar Shira E, and Friedman A (2018). Innate immune functions of avian intestinal epithelial cells: Response to bacterial stimuli and localization of responding cells in the developing avian digestive tract. PLoS One 13, e0200393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barletta AB, Nascimento-Silva MC, Talyuli OA, Oliveira JH, Pereira LO, Oliveira PL, and Sorgine MH (2017). Microbiota activates IMD pathway and limits Sindbis infection in Aedes aegypti. Parasit Vectors 10, 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumler AJ, and Sperandio V (2016). Interactions between the microbiota and pathogenic bacteria in the gut. Nature 535, 85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett KE, Olson KE, Munoz Mde L, Fernandez-Salas I, Farfan-Ale JA, Higgs S, Black W.C.t., and Beaty BJ (2002). Variation in vector competence for dengue 2 virus among 24 collections of Aedes aegypti from Mexico and the United States. The American journal of tropical medicine and hygiene 67, 85–92. [DOI] [PubMed] [Google Scholar]
- Berger AK, Yi H, Kearns DB, and Mainou BA (2017). Bacteria and bacterial envelope components enhance mammalian reovirus thermostability. PLoS Pathog 13, e1006768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birchenough GM, Johansson ME, Gustafsson JK, Bergstrom JH, and Hansson GC (2015). New developments in goblet cell mucus secretion and function. Mucosal Immunol 8, 712–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blacher E, Levy M, Tatirovsky E, and Elinav E (2017). Microbiome-Modulated Metabolites at the Interface of Host Immunity. J Immunol 198, 572–580. [DOI] [PubMed] [Google Scholar]
- Blutt SE, Crawford SE, Ramani S, Zou WY, and Estes MK (2018). Engineered Human Gastrointestinal Cultures to Study the Microbiome and Infectious Diseases. Cell Mol Gastroenterol Hepatol 5, 241–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bopegamage S, Kovacova J, Vargova A, Motusova J, Petrovicova A, Benkovicova M, Gomolcak P, Bakkers J, van Kuppeveld F, Melchers WJ, et al. (2005). Coxsackie B virus infection of mice: inoculation by the oral route protects the pancreas from damage, but not from infection. J Gen Virol 86, 3271–3280. [DOI] [PubMed] [Google Scholar]
- Brady OJ, Gething PW, Bhatt S, Messina JP, Brownstein JS, Hoen AG, Moyes CL, Farlow AW, Scott TW, and Hay SI (2012). Refining the global spatial limits of dengue virus transmission by evidence-based consensus. PLoS Negl Trop Dis 6, e1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broderick NA, and al., e. (2014). Microbiota-induced changes in drosophila melanogaster host gene expression and gut morphology. MBio 5, e01117–01114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broquet AH, Hirata Y, McAllister CS, and Kagnoff MF (2011). RIG-I/MDA5/MAVS are required to signal a protective IFN response in rotavirus-infected intestinal epithelium. J Immunol 186, 1618–1626. [DOI] [PubMed] [Google Scholar]
- Brown JJ, Short SP, Stencel-Baerenwald J, Urbanek K, Pruijssers AJ, McAllister N, Ikizler M, Taylor G, Aravamudhan P, Khomandiak S, et al. (2018). Reovirus-Induced Apoptosis in the Intestine Limits Establishment of Enteric Infection. J Virol 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugman S (2016). The zebrafish as a model to study intestinal inflammation. Dev Comp Immunol 64, 82–92. [DOI] [PubMed] [Google Scholar]
- Brüssow H (2019). Probiotics and prebiotics in clinical tests: an update. F1000Res 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchon N, Osman D, David FP, Fang HY, Boquete JP, Deplancke B, and Lemaitre B (2013). Morphological and molecular characterization of adult midgut compartmentalization in Drosophila. Cell Rep 3, 1725–1738. [DOI] [PubMed] [Google Scholar]
- Buchon N, Silverman N, and Cherry S (2014). Immunity in Drosophila melanogaster--from microbial recognition to whole-organism physiology. Nat Rev Immunol 14, 796–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadwell K, Patel KK, Maloney NS, Liu TC, Ng AC, Storer CE, Head RD, Xavier R, Stappenbeck TS, and Virgin HW (2010). Virus-plus-susceptibility gene interaction determines Crohn’s disease gene Atg16L1 phenotypes in intestine. Cell 141, 1135–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell CL, Keene KM, Brackney DE, Olson KE, Blair CD, Wilusz J, and Foy BD (2008). Aedes aegypti uses RNA interference in defense against Sindbis virus infection. BMC Microbiol 8, 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carissimo G, Pondeville E, McFarlane M, Dietrich I, Mitri C, Bischoff E, Antoniewski C, Bourgouin C, Failloux AB, Kohl A, et al. (2015). Antiviral immunity of Anopheles gambiae is highly compartmentalized, with distinct roles for RNA interference and gut microbiota. Proc Natl Acad Sci U S A 112, E176–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Queiroz NMGP, Xia T, Konno H, and Barber GN (2019). Ovarian Cancer Cells Commonly Exhibit Defective STING Signaling Which Affects Sensitivity to Viral Oncolysis. Mol Cancer Res 17, 974–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dierking K, Yang W, and Schulenburg H (2016). Antimicrobial effectors in the nematode Caenorhabditis elegans: an outgroup to the Arthropoda. Philos Trans R Soc Lond B Biol Sci 371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodson BL, Hughes GL, Paul O, Matacchiero AC, Kramer LD, and Rasgon JL (2014). Wolbachia enhances West Nile virus (WNV) infection in the mosquito Culex tarsalis. PLoS Negl Trop Dis 8, e2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson GP, Lee SM, and Mazmanian SK (2016). Gut biogeography of the bacterial microbiota. Nat Rev Microbiol 14, 20–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond CG, Bolock AM, Ma C, Luke CJ, Good M, and Coyne CB (2017). Enteroviruses infect human enteroids and induce antiviral signaling in a cell lineage-specific manner. Proc Natl Acad Sci U S A 114, 1672–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson AK, Jesudhasan PR, Mayer MJ, Narbad A, Winter SE, and Pfeiffer JK (2018). Bacteria Facilitate Enteric Virus Co-infection of Mammalian Cells and Promote Genetic Recombination. Cell Host Microbe 23, 77–88 e75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettayebi K, Crawford SE, Murakami K, Broughman JR, Karandikar U, Tenge VR, Neill FH, Blutt SE, Zeng XL, Qu L, et al. (2016). Replication of human noroviruses in stem cell-derived human enteroids. Science 353, 1387–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faria AM, Mucida D, McCafferty DM, Tsuji NM, and Verhasselt V (2012). Tolerance and inflammation at the gut mucosa. Clin Dev Immunol 2012, 738475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix MA, Ashe A, Piffaretti J, Wu G, Nuez I, Belicard T, Jiang Y, Zhao G, Franz CJ, Goldstein LD, et al. (2011). Natural and experimental infection of Caenorhabditis nematodes by novel viruses related to nodaviruses. PLoS Biol 9, e1000586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix MA, and Wang D (2019). Natural Viruses of Caenorhabditis Nematodes. Annu Rev Genet. [DOI] [PubMed] [Google Scholar]
- Forero A, Ozarkar S, Li H, Lee CH, Hemann EA, Nadjsombati MS, Hendricks MR, So L, Green R, Roy CN, et al. (2019). Differential Activation of the Transcription Factor IRF1 Underlies the Distinct Immune Responses Elicited by Type I and Type III Interferons. Immunity 51, 451–464.e456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franz CJ, Renshaw H, Frezal L, Jiang Y, Felix MA, and Wang D (2014). Orsay, Santeuil and Le Blanc viruses primarily infect intestinal cells in Caenorhabditis nematodes. Virology 448, 255–264. [DOI] [PubMed] [Google Scholar]
- Freedman SB, Williamson-Urquhart S, Farion KJ, Gouin S, Willan AR, Poonai N, Hurley K, Sherman PM, Finkelstein Y, Lee BE, et al. (2018). Multicenter Trial of a Combination Probiotic for Children with Gastroenteritis. N Engl J Med 379, 2015–2026. [DOI] [PubMed] [Google Scholar]
- Frias AH, Jones RM, Fifadara NH, Vijay-Kumar M, and Gewirtz AT (2012). Rotavirus-induced IFN-beta promotes anti-viral signaling and apoptosis that modulate viral replication in intestinal epithelial cells. Innate Immun 18, 294–306. [DOI] [PubMed] [Google Scholar]
- Gehart H, and Clevers H (2019). Tales from the crypt: new insights into intestinal stem cells. Nat Rev Gastroenterol Hepatol 16, 19–34. [DOI] [PubMed] [Google Scholar]
- Gibson DL, Ma C, Rosenberger CM, Bergstrom KS, Valdez Y, Huang JT, Khan MA, and Vallance BA (2008). Toll-like receptor 2 plays a critical role in maintaining mucosal integrity during Citrobacter rodentium-induced colitis. Cell Microbiol 10, 388–403. [DOI] [PubMed] [Google Scholar]
- Gill N, Wlodarska M, and Finlay BB (2011). Roadblocks in the gut: barriers to enteric infection. Cellular microbiology 13, 660–669. [DOI] [PubMed] [Google Scholar]
- Glaser RL, and Meola MA (2010). The native Wolbachia endosymbionts of Drosophila melanogaster and Culex quinquefasciatus increase host resistance to West Nile virus infection. PLoS One 5, e11977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good C, Wells AI, and Coyne CB (2019). Type III interferon signaling restricts enterovirus 71 infection of goblet cells. Sci Adv 5, eaau4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grau KR, Roth AN, Zhu S, Hernandez A, Colliou N, DiVita BB, Philip DT, Riffe C, Giasson B, Wallet SM, et al. (2017). The major targets of acute norovirus infection are immune cells in the gut-associated lymphoid tissue. Nat Microbiol 2, 1586–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grau KR, Zhu S, Peterson ST, Helm EW, Philip D, Phillips M, Hernandez A, Turula H, Frasse P, Graziano VR, et al. (2019). The intestinal regionalization of acute norovirus infection is regulated by the microbiota via bile acid-mediated priming of type III interferon. Nature Microbiology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z, Li Y, and Ding SW (2019). Small RNA-based antimicrobial immunity. Nat Rev Immunol 19, 31–44. [DOI] [PubMed] [Google Scholar]
- Hamonic G, Pasternak JA, and Wilson HL (2018). Recognizing conserved non-canonical localization patterns of toll-like receptors in tissues and across species. Cell Tissue Res 372, 1–11. [DOI] [PubMed] [Google Scholar]
- Haq S, Grondin J, Banskota S, and Khan WI (2019). Autophagy: roles in intestinal mucosal homeostasis and inflammation. J Biomed Sci 26, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare D, Collins S, Cuddington B, and Mossman K (2016). The Importance of Physiologically Relevant Cell Lines for Studying Virus-Host Interactions. Viruses 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegedus D, Erlandson M, Gillott C, and Toprak U (2009). New insights into peritrophic matrix synthesis, architecture, and function. Annu Rev Entomol 54, 285–302. [DOI] [PubMed] [Google Scholar]
- Helander HF, and Fändriks L (2014). Surface area of the digestive tract - revisited. Scand J Gastroenterol 49, 681–689. [DOI] [PubMed] [Google Scholar]
- Hernandez PP, Mahlakoiv T, Yang I, Schwierzeck V, Nguyen N, Guendel F, Gronke K, Ryffel B, Hoelscher C, Dumoutier L, et al. (2015). Interferon-lambda and interleukin 22 act synergistically for the induction of interferon-stimulated genes and control of rotavirus infection. Nat Immunol 16, 698–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holly MK, and Smith JG (2018). Paneth Cells during Viral Infection and Pathogenesis. Viruses 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornby PJ, Cooper PR, Kliwinski C, Ragwan E, Mabus JR, Harman B, Thompson S, Kauffman AL, Yan Z, Tam SH, et al. (2014). Human and non-human primate intestinal FcRn expression and immunoglobulin G transcytosis. Pharm Res 31, 908–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichinohe T, Pang IK, Kumamoto Y, Peaper DR, Ho JH, Murray TS, and Iwasaki A (2011). Microbiota regulates immune defense against respiratory tract influenza A virus infection. Proc Natl Acad Sci U S A 108, 5354–5359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingle H, Lee S, Ai T, Orvedahl A, Rodgers R, Zhao G, Sullender M, Peterson ST, Locke M, Liu TC, et al. (2019). Viral complementation of immunodeficiency confers protection against enteric pathogens via interferon-λ. Nat Microbiol 4, 1120–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irazoqui JE, Urbach JM, and Ausubel FM (2010). Evolution of host innate defence: insights from Caenorhabditis elegans and primitive invertebrates. Nat Rev Immunol 10, 47–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaswal VM, Babbar HS, and Mahmood A (1988). Changes in sialic acid and fucose contents of enterocytes across the crypt-villus axis in developing rat intestine. Biochem Med Metab Biol 39, 105–110. [DOI] [PubMed] [Google Scholar]
- Jiang TT, Shao TY, Ang WXG, Kinder JM, Turner LH, Pham G, Whitt J, Alenghat T, and Way SS (2017). Commensal Fungi Recapitulate the Protective Benefits of Intestinal Bacteria. Cell Host Microbe 22, 809–816.e804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson C, Wetzel JD, He J, Mikacenic C, Dermody TS, and Kelsall BL (2007). Type I interferons produced by hematopoietic cells protect mice against lethal infection by mammalian reovirus. J Exp Med 204, 1349–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson ME, Phillipson M, Petersson J, Velcich A, Holm L, and Hansson GC (2008). The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc Natl Acad Sci U S A 105, 15064–15069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones MK, Watanabe M, Zhu S, Graves CL, Keyes LR, Grau KR, Gonzalez-Hernandez MB, Iovine NM, Wobus CE, Vinje J, et al. (2014). Enteric bacteria promote human and mouse norovirus infection of B cells. Science 346, 755–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane M, Case LK, Kopaskie K, Kozlova A, MacDearmid C, Chervonsky AV, and Golovkina TV (2011). Successful transmission of a retrovirus depends on the commensal microbiota. Science 334, 245–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang MA, Mott TM, Tapley EC, Lewis EE, and Luckhart S (2008). Insulin regulates aging and oxidative stress in Anopheles stephensi. J Exp Biol 211, 741–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karve SS, Pradhan S, Ward DV, and Weiss AA (2017). Intestinal organoids model human responses to infection by commensal and Shiga toxin producing Escherichia coli. PLoS One 12, e0178966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato H, Takeuchi O, Sato S, Yoneyama M, Yamamoto M, Matsui K, Uematsu S, Jung A, Kawai T, Ishii KJ, et al. (2006). Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature 441, 101–105. [DOI] [PubMed] [Google Scholar]
- Kernbauer E, Ding Y, and Cadwell K (2014). An enteric virus can replace the beneficial function of commensal bacteria. Nature 516, 94–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoo CC, Doty JB, Heersink MS, Olson KE, and Franz AW (2013). Transgene-mediated suppression of the RNA interference pathway in Aedes aegypti interferes with gene silencing and enhances Sindbis virus and dengue virus type 2 replication. Insect Mol Biol 22, 104–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsolver MB, Huang Z, and Hardy RW (2013). Insect antiviral innate immunity: pathways, effectors, and connections. J Mol Biol 425, 4921–4936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk MD, Pires SM, Black RE, Caipo M, Crump JA, Devleesschauwer B, Dopfer D, Fazil A, Fischer-Walker CL, Hald T, et al. (2015). World Health Organization Estimates of the Global and Regional Disease Burden of 22 Foodborne Bacterial, Protozoal, and Viral Diseases, 2010: A Data Synthesis. PLoS Med 12, e1001921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitajima S, Morimoto M, Sagara E, Shimizu C, and Ikeda Y (2001). Dextran sodium sulfate-induced colitis in germ-free IQI/Jic mice. Exp Anim 50, 387–395. [DOI] [PubMed] [Google Scholar]
- Kolawole AO, Mirabelli C, Hill DR, Svoboda SA, Janowski AB, Passalacqua KD, Rodriguez BN, Dame MK, Freiden P, Berger RP, et al. (2019). Astrovirus replication in human intestinal enteroids reveals multi-cellular tropism and an intricate host innate immune landscape. PLoS Pathog 15, e1008057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreisinger J, Cížková D, Vohánka J, and Piálek J (2014). Gastrointestinal microbiota of wild and inbred individuals of two house mouse subspecies assessed using high-throughput parallel pyrosequencing. Mol Ecol 23, 5048–5060. [DOI] [PubMed] [Google Scholar]
- Krishnamurthy S, Takimoto T, Scroggs RA, and Portner A (2006). Differentially regulated interferon response determines the outcome of Newcastle disease virus infection in normal and tumor cell lines. J Virol 80, 5145–5155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuss SK, Best GT, Etheredge CA, Pruijssers AJ, Frierson JM, Hooper LV, Dermody TS, and Pfeiffer JK (2011). Intestinal microbiota promote enteric virus replication and systemic pathogenesis. Science 334, 249–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labeaud AD, Bashir F, and King CH (2011). Measuring the burden of arboviral diseases: the spectrum of morbidity and mortality from four prevalent infections. Popul Health Metr 9, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster KZ, and Pfeiffer JK (2010). Limited trafficking of a neurotropic virus through inefficient retrograde axonal transport and the type I interferon response. PLoS pathogens 6, e1000791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Pen J, Jiang H, Di Domenico T, Kneuss E, Kosalka J, Leung C, Morgan M, Much C, Rudolph KLM, Enright AJ, et al. (2018). Terminal uridylyltransferases target RNA viruses as part of the innate immune system. Nat Struct Mol Biol 25, 778–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Mo JH, Katakura K, Alkalay I, Rucker AN, Liu YT, Lee HK, Shen C, Cojocaru G, Shenouda S, et al. (2006). Maintenance of colonic homeostasis by distinctive apical TLR9 signalling in intestinal epithelial cells. Nat Cell Biol 8, 1327–1336. [DOI] [PubMed] [Google Scholar]
- Lemaitre B (1996). The Dorsoventral Regulatory Gene Cassette spätzle/Toll/cactus Controls The DorsoventralRegulatory Gene Cassette spätzle/Toll/cactus Controls the Potent Antifungal Response in Drosophila Adults the Potent Antifungal Response in Drosophila Adults, Nicolas E, ed. (Cell), pp. 973–983. [DOI] [PubMed] [Google Scholar]
- Lindemans CA, Calafiore M, Mertelsmann AM, O’Connor MH, Dudakov JA, Jenq RR, Velardi E, Young LF, Smith OM, Lawrence G, et al. (2015). Interleukin-22 promotes intestinal-stem-cell-mediated epithelial regeneration. Nature 528, 560–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Gordesky-Gold B, Leney-Greene M, Weinbren NL, Tudor M, and Cherry S (2018). Inflammation-Induced, STING-Dependent Autophagy Restricts Zika Virus Infection in the Drosophila Brain. Cell Host Microbe 24, 57–68 e53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDuff DA, Baldridge MT, Qaqish AM, Nice TJ, Darbandi AD, Hartley VL, Peterson ST, Miner JJ, Iwai K, and Virgin HW (2018). HOIL1 Is Essential for the Induction of Type I and III Interferons by MDA5 and Regulates Persistent Murine Norovirus Infection. J Virol 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahlakoiv T, Hernandez P, Gronke K, Diefenbach A, and Staeheli P (2015). Leukocyte-derived IFN-alpha/beta and epithelial IFN-lambda constitute a compartmentalized mucosal defense system that restricts enteric virus infections. PLoS Pathog 11, e1004782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahlapuu M, Håkansson J, Ringstad L, and Björn C (2016). Antimicrobial Peptides: An Emerging Category of Therapeutic Agents. Front Cell Infect Microbiol 6, 194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mains JW, Kelly PH, Dobson KL, Petrie WD, and Dobson SL (2019). Localized Control of Aedes aegypti (Diptera: Culicidae) in Miami, FL, via Inundative Releases of Wolbachia-Infected Male Mosquitoes. J Med Entomol 56, 1296–1303. [DOI] [PubMed] [Google Scholar]
- McCartney SA, Thackray LB, Gitlin L, Gilfillan S, Virgin HW, and Colonna M (2008). MDA-5 recognition of a murine norovirus. PLoS Pathog 4, e1000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarlane M, Arias-Goeta C, Martin E, O’Hara Z, Lulla A, Mousson L, Rainey SM, Misbah S, Schnettler E, Donald CL, et al. (2014). Characterization of Aedes aegypti innate-immune pathways that limit Chikungunya virus replication. PLoS Negl Trop Dis 8, e2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee MD, Weber D, Day N, Vitelli C, Crippen D, Herndon LA, Hall DH, and Melov S (2011). Loss of intestinal nuclei and intestinal integrity in aging C. elegans. Aging Cell 10, 699–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micchelli CA, and Perrimon N (2006). Evidence that stem cells reside in the adult Drosophila midgut epithelium. Nature 439, 475–479. [DOI] [PubMed] [Google Scholar]
- Mondotte JA, Gausson V, Frangeul L, Blanc H, Lambrechts L, and Saleh MC (2018). Immune priming and clearance of orally acquired RNA viruses in Drosophila. Nat Microbiol 3, 1394–1403. [DOI] [PubMed] [Google Scholar]
- Moreira LA, Iturbe-Ormaetxe I, Jeffery JA, Lu G, Pyke AT, Hedges LM, Rocha BC, Hall-Mendelin S, Day A, Riegler M, et al. (2009). A Wolbachia symbiont in Aedes aegypti limits infection with dengue, Chikungunya, and Plasmodium. Cell 139, 1268–1278. [DOI] [PubMed] [Google Scholar]
- Morosky S, Wells AI, Lemon K, Evans AS, Schamus S, Bakkenist CJ, and Coyne CB (2019). The neonatal Fc receptor is a pan-echovirus receptor. Proc Natl Acad Sci U S A 116, 3758–3763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mourya DT, and Soman RS (1985). Effect of gregarine parasite, Ascogregarina culicis & tetracycline on the susceptibility of Culex bitaeniorhynchus to JE virus. Indian J Med Res 81, 247–250. [PubMed] [Google Scholar]
- Moy RH, Cole BS, Yasunaga A, Gold B, Shankarling G, Varble A, Molleston JM, tenOever BR, Lynch KW, and Cherry S (2014a). Stem-loop recognition by DDX17 facilitates miRNA processing and antiviral defense. Cell 158, 764–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moy RH, Gold B, Molleston JM, Schad V, Yanger K, Salzano MV, Yagi Y, Fitzgerald KA, Stanger BZ, Soldan SS, et al. (2014b). Antiviral autophagy restrictsRift Valley fever virus infection and is conserved from flies to mammals. Immunity 40, 51–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moya A, and Ferrer M (2016). Functional Redundancy-Induced Stability of Gut Microbiota Subjected to Disturbance. Trends Microbiol 24, 402–413. [DOI] [PubMed] [Google Scholar]
- Muturi EJ, Dunlap C, Ramirez JL, Rooney AP, and Kim CH (2019). Host blood-meal source has a strong impact on gut microbiota of Aedes aegypti. FEMS Microbiol Ecol 95. [DOI] [PubMed] [Google Scholar]
- Nagler-Anderson C (2001). Man the barrier! Strategic defences in the intestinal mucosa. Nature reviews Immunology 1, 59–67. [DOI] [PubMed] [Google Scholar]
- Nice TJ, Baldridge MT, McCune BT, Norman JM, Lazear HM, Artyomov M, Diamond MS, and Virgin HW (2015). Interferon-lambda cures persistent murine norovirus infection in the absence of adaptive immunity. Science 347, 269–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ocampo CB, Caicedo PA, Jaramillo G, Ursic Bedoya R, Baron O, Serrato IM, Cooper DM, and Lowenberger C (2013). Differential expression of apoptosis related genes in selected strains of Aedes aegypti with different susceptibilities to dengue virus. PLoS One 8, e61187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohka S, Igarashi H, Nagata N, Sakai M, Koike S, Nochi T, Kiyono H, and Nomoto A (2007). Establishment of a poliovirus oral infection system in human poliovirus receptor-expressing transgenic mice that are deficient in alpha/beta interferon receptor. Journal of virology 81, 7902–7912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orvedahl A, Sumpter R Jr., Xiao G, Ng A, Zou Z, Tang Y, Narimatsu M, Gilpin C, Sun Q, Roth M, et al. (2011). Image-based genome-wide siRNA screen identifies selective autophagy factors. Nature 480, 113–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pervolaraki K, Stanifer ML, Munchau S, Renn LA, Albrecht D, Kurzhals S, Senis E, Grimm D, Schroder-Braunstein J, Rabin RL, et al. (2017). Type I and Type III Interferons Display Different Dependency on Mitogen-Activated Protein Kinases to Mount an Antiviral State in the Human Gut. Front Immunol 8, 459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson LW, and Artis D (2014). Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nat Rev Immunol 14, 141–153. [DOI] [PubMed] [Google Scholar]
- Pfeiffer JK (2010). Innate host barriers to viral trafficking and population diversity: lessons learned from poliovirus. Adv Virus Res 77, 85–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pott J, Mahlakoiv T, Mordstein M, Duerr CU, Michiels T, Stockinger S, Staeheli P, and Hornef MW (2011). IFN-lambda determines the intestinal epithelial antiviral host defense. Proc Natl Acad Sci U S A 108, 7944–7949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pott J, Stockinger S, Torow N, Smoczek A, Lindner C, McInerney G, Backhed F, Baumann U, Pabst O, Bleich A, et al. (2012). Age-dependent TLR3 expression of the intestinal epithelium contributes to rotavirus susceptibility. PLoS Pathog 8, e1002670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price AE, Shamardani K, Lugo KA, Deguine J, Roberts AW, Lee BL, and Barton GM (2018). A Map of Toll-like Receptor Expression in the Intestinal Epithelium Reveals Distinct Spatial, Cell Type-Specific, and Temporal Patterns. Immunity 49, 560–575.e566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, and Medzhitov R (2004). Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell 118, 229–241. [DOI] [PubMed] [Google Scholar]
- Rasgon JL (2008). Using predictive models to optimize Wolbachia-based strategies for vector-borne disease control. Adv Exp Med Biol 627, 114–125. [DOI] [PubMed] [Google Scholar]
- Rhee SH, Im E, Riegler M, Kokkotou E, O’Brien M, and Pothoulakis C (2005). Pathophysiological role of Toll-like receptor 5 engagement by bacterial flagellin in colonic inflammation. Proc Natl Acad Sci U S A 102, 13610–13615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson CM, Jesudhasan PR, and Pfeiffer JK (2014). Bacterial lipopolysaccharide binding enhances virion stability and promotes environmental fitness of an enteric virus. Cell Host Microbe 15, 36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson CM, Woods Acevedo MA, McCune BT, and Pfeiffer JK (2019). Related Enteric Viruses Have Different Requirements for Host Microbiota in Mice. J Virol 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosshart SP, Vassallo BG, Angeletti D, Hutchinson DS, Morgan AP, Takeda K, Hickman HD, McCulloch JA, Badger JH, Ajami NJ, et al. (2017). Wild Mouse Gut Microbiota Promotes Host Fitness and Improves Disease Resistance. Cell 171, 1015–1028.e1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Round JL, and Mazmanian SK (2009). The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol 9, 313–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland I, Gibson G, Heinken A, Scott K, Swann J, Thiele I, and Tuohy K (2018). Gut microbiota functions: metabolism of nutrients and other food components. Eur J Nutr 57, 1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin DH, Kornstein MJ, and Anderson AO (1985). Reovirus serotype 1 intestinal infection: a novel replicative cycle with ileal disease. J Virol 53, 391–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar MI, Richardson JH, Sanchez-Vargas I, Olson KE, and Beaty BJ (2007). Dengue virus type 2: replication and tropisms in orally infected Aedes aegypti mosquitoes. BMC microbiology 7, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansone CL, Cohen J, Yasunaga A, Xu J, Osborn G, Subramanian H, Gold B, Buchon N, and Cherry S (2015). Microbiota-Dependent Priming of Antiviral Intestinal Immunity in Drosophila. Cell Host Microbe 18, 571–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters PJ, et al. (2009). Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459, 262–265. [DOI] [PubMed] [Google Scholar]
- Saxena K, Simon LM, Zeng XL, Blutt SE, Crawford SE, Sastri NP, Karandikar UC, Ajami NJ, Zachos NC, Kovbasnjuk O, et al. (2017). A paradox of transcriptional and functional innate interferon responses of human intestinal enteroids to enteric virus infection. Proc Natl Acad Sci U S A 114, E570–E579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrato-Salas J, Hernandez-Martinez S, Martinez-Barnetche J, Conde R, Alvarado-Delgado A, Zumaya-Estrada F, and Lanz-Mendoza H (2018). De Novo DNA Synthesis in Aedes aegypti Midgut Cells as a Complementary Strategy to Limit Dengue Viral Replication. Front Microbiol 9, 801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelly S, Lukinova N, Bambina S, Berman A, and Cherry S (2009). Autophagy is an essential component of Drosophila immunity against vesicular stomatitis virus. Immunity 30, 588–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Z, Zou J, Zhang Z, Zhao X, Noriega J, Zhang B, Zhao C, Ingle H, Bittinger K, Mattei LM, et al. (2019). Segmented Filamentous Bacteria Prevent and Cure Rotavirus Infection. Cell 179, 644–658.e613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin SC, Kim SH, You H, Kim B, Kim AC, Lee KA, Yoon JH, Ryu JH, and Lee WJ (2011). Drosophila microbiome modulates host developmental and metabolic homeostasis via insulin signaling. Science 334, 670–674. [DOI] [PubMed] [Google Scholar]
- Souza-Neto JA, Sim S, and Dimopoulos G (2009). An evolutionary conserved function of the JAK-STAT pathway in anti-dengue defense. Proc Natl Acad Sci U S A 106, 17841–17846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence JR, Mayhew CN, Rankin SA, Kuhar MF, Vallance JE, Tolle K, Hoskins EE, Kalinichenko VV, Wells SI, Zorn AM, et al. (2011). Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature 470, 105–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadnyk AW (2002). Intestinal epithelial cells as a source of inflammatory cytokines and chemokines. Can J Gastroenterol 16, 241–246. [DOI] [PubMed] [Google Scholar]
- Stanifer ML, Mukenhirn M, Muenchau S, Pervolaraki K, Kanaya T, Albrecht D, Odendall C, Hielscher T, Haucke V, Kagan JC, et al. (2019). Asymmetric distribution of TLR3 leads to a polarized immune response in human intestinal epithelial cells. Nat Microbiol. [DOI] [PubMed] [Google Scholar]
- Starkey WG, Collins J, Wallis TS, Clarke GJ, Spencer AJ, Haddon SJ, Osborne MP, Candy DC, and Stephen J (1986). Kinetics, tissue specificity and pathological changes in murine rotavirus infection of mice. J Gen Virol 67 (Pt 12), 2625–2634. [DOI] [PubMed] [Google Scholar]
- Staubach F, Baines JF, Künzel S, Bik EM, and Petrov DA (2013). Host species and environmental effects on bacterial communities associated with Drosophila in the laboratory and in the natural environment. PLoS One 8, e70749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syed ZA, Hard T, Uv A, and van Dijk-Hard IF (2008). A potential role for Drosophila mucins in development and physiology. PLoS One 3, e3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabachnick WJ (2013). Nature, nurture and evolution of intra-species variation in mosquito arbovirus transmission competence. Int J Environ Res Public Health 10, 249–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira L, Ferreira A, and Ashburner M (2008). The bacterial symbiont Wolbachia induces resistance to RNA viral infections in Drosophila melanogaster. PLoS Biol 6, e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thackray LB, Handley SA, Gorman MJ, Poddar S, Bagadia P, Briseno CG, Theisen DJ, Tan Q, Hykes BL Jr., Lin H, et al. (2018). Oral Antibiotic Treatment of Mice Exacerbates the Disease Severity of Multiple Flavivirus Infections. Cell Rep 22, 3440–3453 e3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaiss CA, Zmora N, Levy M, and Elinav E (2016). The microbiome and innate immunity. Nature 535, 65–74. [DOI] [PubMed] [Google Scholar]
- Ting HA, and von Moltke J (2019). The Immune Function of Tuft Cells at Gut Mucosal Surfaces and Beyond. J Immunol 202, 1321–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchiyama R, Chassaing B, Zhang B, and Gewirtz AT (2014). Antibiotic treatment suppresses rotavirus infection and enhances specific humoral immunity. J Infect Dis 210, 171–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dycke J, Ny A, Conceição-Neto N, Maes J, Hosmillo M, Cuvry A, Goodfellow I, Nogueira TC, Verbeken E, Matthijnssens J, et al. (2019). A robust human norovirus replication model in zebrafish larvae. PLoS Pathog 15, e1008009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vodovar N, Vinals M, Liehl P, Basset A, Degrouard J, Spellman P, Boccard F, and Lemaitre B (2005). Drosophila host defense after oral infection by an entomopathogenic Pseudomonas species. Proc Natl Acad Sci U S A 102, 11414–11419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Zhu S, Yang L, Cui S, Pan W, Jackson R, Zheng Y, Rongvaux A, Sun Q, Yang G, et al. (2015). Nlrp6 regulates intestinal antiviral innate immunity. Science 350, 826–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson CJ, Rowland M, and Warhurst G (2001). Functional modeling of tight junctions in intestinal cell monolayers using polyethylene glycol oligomers. Am J Physiol Cell Physiol 281, C388–397. [DOI] [PubMed] [Google Scholar]
- Weaver SC, and Barrett AD (2004). Transmission cycles, host range, evolution and emergence of arboviral disease. Nature reviews Microbiology 2, 789–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilen CB, Lee S, Hsieh LL, Orchard RC, Desai C, Hykes BL Jr., McAllaster MR, Balce DR, Feehley T, Brestoff JR, et al. (2018). Tropism for tuft cells determines immune promotion of norovirus pathogenesis. Science 360, 204–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilks J, Lien E, Jacobson AN, Fischbach MA, Qureshi N, Chervonsky AV, and Golovkina TV (2015). Mammalian Lipopolysaccharide Receptors Incorporated into the Retroviral Envelope Augment Virus Transmission. Cell Host Microbe 18, 456–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SS, Bromme BA, Holly MK, Wiens ME, Gounder AP, Sul Y, and Smith JG (2017). Alpha-defensin-dependent enhancement of enteric viral infection. PLoS Pathog 13, e1006446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong CN, Ng P, and Douglas AE (2011). Low-diversity bacterial community in the gut of the fruitfly Drosophila melanogaster. Environ Microbiol 13, 1889–1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worthington JJ, Reimann F, and Gribble FM (2018). Enteroendocrine cells-sensory sentinels of the intestinal environment and orchestrators of mucosal immunity. Mucosal Immunol 11, 3–20. [DOI] [PubMed] [Google Scholar]
- Wu P, Sun P, Nie K, Zhu Y, Shi M, Xiao C, Liu H, Liu Q, Zhao T, Chen X, et al. (2019). A Gut Commensal Bacterium Promotes Mosquito Permissiveness to Arboviruses. Cell Host Microbe 25, 101–112 e105. [DOI] [PubMed] [Google Scholar]
- Xi Z, Ramirez JL, and Dimopoulos G (2008). The Aedes aegypti toll pathway controls dengue virus infection. PLoS Pathog 4, e1000098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia T, Konno H, Ahn J, and Barber GN (2016). Deregulation of STING Signaling in Colorectal Carcinoma Constrains DNA Damage Responses and Correlates With Tumorigenesis. Cell Rep 14, 282–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, and Cherry S (2014). Viruses and antiviral immunity in Drosophila. Dev Comp Immunol 42, 67–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Hopkins K, Sabin L, Yasunaga A, Subramanian H, Lamborn I, Gordesky-Gold B, and Cherry S (2013). ERK signaling couples nutrient status to antiviral defense in the insect gut. Proc Natl Acad Sci U S A 110, 15025–15030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Chassaing B, Shi Z, Uchiyama R, Zhang Z, Denning TL, Crawford SE, Pruijssers AJ, Iskarpatyoti JA, Estes MK, et al. (2014). Viral infection. Prevention and cure of rotavirus infection via TLR5/NLRC4-mediated production of IL-22 and IL-18. Science 346, 861–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Berg M, Dierking K, Felix MA, Shapira M, Samuel BS, and Schulenburg H (2017). Caenorhabditis elegans as a Model for Microbiome Research. Front Microbiol 8, 485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou R, Wei H, Sun R, and Tian Z (2007). Recognition of double-stranded RNA by TLR3 induces severe small intestinal injury in mice. J Immunol 178, 4548–4556. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Zhang R, Zhang B, Zhao T, Wang P, Liang G, and Cheng G (2017). Blood meal acquisition enhances arbovirus replication in mosquitoes through activation of the GABAergic system. Nat Commun 8, 1262. [DOI] [PMC free article] [PubMed] [Google Scholar]


