Abstract
Background
Observational studies suggest that low 25-hydroxyvitamin D status is common and has been associated with higher mortality in critically ill patients. This study aim to investigate whether vitamin D supplementation is associated with lower mortality in critically ill patients.
Method
We searched Medline, Embase, and Cochrane databases from inception to January 12, 2020, without language restrictions, for randomized controlled trials comparing the effect of vitamin D supplementation with placebo in critically ill patients. Two authors independently performed data extraction and assessed study quality. The primary outcome was all-cause mortality at the longest follow-up.
Result
We identified nine trials with a total of 2066 patients. Vitamin D supplementation was not associated with reduced all-cause mortality at the longest follow-up (RR 0.90, 95% CI 0.74 to 1.09, I2 = 20%), at 30 days (RR 0.81, 95% CI 0.56 to 1.15), at 90 days (RR 1.15, 95% CI 0.92 to 1.44), and at 180 days (RR 0.82, 95% CI 0.65 to 1.03). Results were similar in the sensitivity analysis. The sample size met the optimum size in trial sequential analysis. Similarly, supplemental vitamin D was not associated with length of ICU stay, hospital stay, or mechanical ventilation.
Conclusion
Vitamin D supplement was not associated with reduced all-cause mortality in critically ill patients.
Systematic review registration
Open Science Framework https://osf.io/bgsjq
Background
Vitamin D plays an important role in maintaining the normal function of neurologic, cardiovascular, respiratory, and immune system [1, 2]. Observational studies have indicated that vitamin D deficiency is common in critically ill patients and is associated with mortality and length of ICU stay [3, 4]. Early randomized clinical trials (RCTs) individual showed lower observed mortality than placebo, although the differences were not significant [5–10]. In 2017 and 2018, three systematic reviews [11–13] have discussed the association between vitamin D supplementation and the most important clinical outcomes: all-cause mortality. One review [12] has shown benefit of vitamin D on survival, while two reviews [11, 13] have not shown the benefit. The ongoing debate has been fueled by the recent publications, two large RCTs [5, 14]. Thus, we performed a systematic review and meta-analysis to assess the effect of vitamin D compared to placebo on mortality.
Method
Protocol and guidance
This systematic review and meta-analysis has been reported in accordance with the preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement (eTable 1 in S1 Appendix) [15]. We registered a protocol for the review in Open Science Framework https://osf.io/bgsjq.
Eligibility criteria
Eligible studies met the following PICOS (participants, interventions, comparators, outcomes, and study design) criteria: (1) Population: adults admitted to the intensive care unit. (2) Intervention: administration of vitamin D, without restrictions in the type, dose, duration, or route of administration. (3) Comparison: placebo or no treatment. (4) Outcome: The primary outcome was all-cause mortality at the longest follow-up; second outcomes included mortality at 30 days, mortality at 90 days, mortality at 180 days, ICU mortality, in-hospital mortality, length of hospital stay, length of ICU stay, and length of mechanical ventilation. (5) Study design: RCT.
Search strategy
We did computerized literature searches of Medline, Embase, and Cochrane CENTRAL Register of Controlled Trials from inception through January 12, 2020, without any language restrictions. The reference lists of included studies and reviews were searched for additional studies. Finally, we searched the World Health Organization’s International Clinical Trials Registry Platform to identify ongoing trials and evaluate the possibility of publication bias. The details of the search strategy are in eTable 2 in S1 Appendix.
Study selection
After removal of duplicates, two investigators (LP and PW) screen each title and abstract independently and in duplicate. The full texts of the remaining studies were also assessed independently and in duplicate by the two authors (LP and PW). Discrepancies were resolved through discussion among the study team.
Data extraction
Two investigators (HD and HF) independently and in duplicate extracted data about study characteristics, outcomes, and funding sources from the eligible trials using a predesigned spreadsheet for further analysis. Discrepancies were resolved through discussion among the study team. Correspondent authors of trials were contacted for unclear information and additional information that did not report outcomes of interest.
Risk of bias
Risk of bias assessment was conducted by two investigators (LP and PW) using Cochrane Collaboration risk of bias tool 2 across seven domains. (https://methods.cochrane.org/bias/news/rob-2-tool) The individual domains included (1) random-sequence generation, (2) allocation sequence concealment, (3) blinding of participants and personnel, (4) blinding of outcome assessment, (5) completeness of outcome data, (6) selective reporting, and (7) other sources of bias.
Data synthesis
All meta-analyses were conducted using Review Manager version 5.4 (Cochrane Collaboration). We calculated the relative risk (RR) with 95% CI for dichotomous outcomes and the mean difference with 95% CI for continuous outcomes. I2 values were calculated to estimate variation among studies attributable to heterogeneity. We calculated pooled effect sizes using random-effects models regardless of the value of I2 in a meta-analysis. We planned to examine publication bias via funnel plots (visually) and more formally with the Begg test and Egger test.
Subgroup analysis
We conducted some subgroup analyses to test interactions according to the dose (≥300000IU or <300000IU), baseline 25 hydroxyvitamin D (≥20 or <20 ng/ml) and the route of administration (oral, intravenous, or intramuscular).
Sensitivity analyses
We conducted sensitivity analyses to test the robustness of the findings included the following: using fixed-effect models, using absolute risk, and excluding trials at each time.
Trial sequential analysis
We conducted a trial sequential analysis for the primary outcome. An optimal information size set to an overall 5% risk of type I error, 80% power, and relative risk reduction of 20%. Trial sequential analysis was done using Trial Sequential Analysis v.0.9.5.10 beta (Copenhagen Trial Unit, Copenhagen, Denmark).
Quality of evidence
The grading of recommendations assessment, development and evaluation (GRADE) methodology was used for assessing the quality of evidence by two investigators (LP and PW) [16].
Result
Study selection and study characteristics
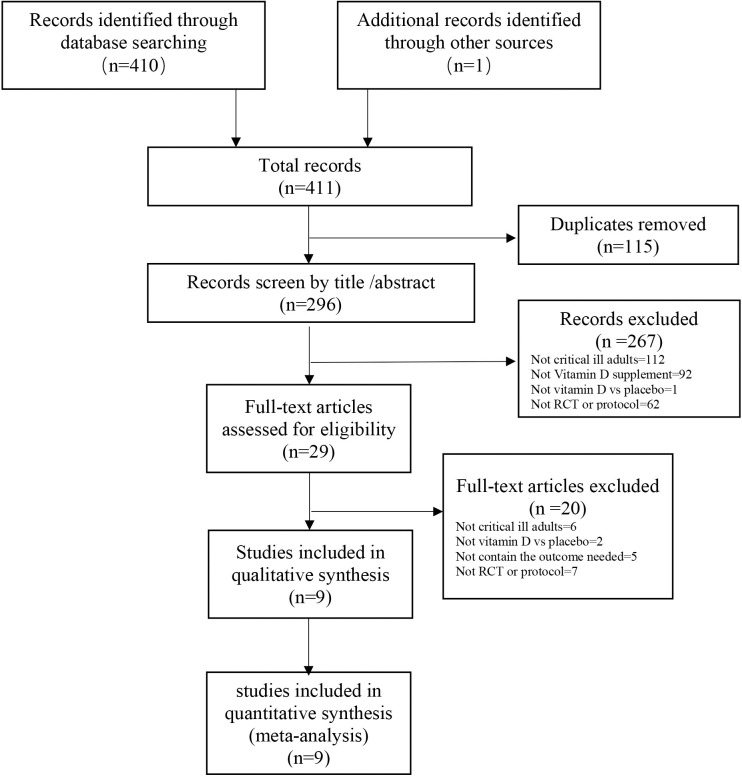
Our systematic electronic literature search identified a total of 411 reports (Fig 1). After the exclusion of incomplete reports, nine trials [5–8, 10, 17–20] were included in the systematic reviews and meta-analyses.
Fig 1. Search strategy and final included and excluded studies.
Details of the trials are summarized in Table 1. All studies were published between 2011 and 2020. The number of recipients ranged from 25 to 1078 patients. The route of administration of vitamin D varied across trials, with six trials [5, 8, 10, 17–19] using oral vitamin D, and one [20] intravenous, two [6, 7] intramuscular. All trials used placebo as a control.
Table 1. Characteristics of studies included in the systematic review of vitamin D supplement in critically ill patients.
| Trials | Site | patient | BMI mean (SD) | 25-hydroxyvitamin D level mean (SD), ng/ml | Patients, N | Mean age, mean (SD), years | Female % | Initial dosages of Vitamin D3(IU) | Longest follow-up for mortality |
|---|---|---|---|---|---|---|---|---|---|
| Amrein 2011 | 1 | Multidisciplinary ICU patients, | 29(8.9) | 13.6(0.5) | 25 | 62.7(16.3) | 24 | 540000 orally | 7 days |
| Amrein 2014 | 1 | Multidisciplinary ICU patients | 27.1(5.3) | 13.1(4.1) | 475 | 64.6(14.8) | 34 | 540000 orally | 180 days |
| Ginde 2019 | 45 | Multidisciplinary ICU patients | 30.4(10.8) | 11.1(4.7) | 1078 | 55.5(16.3) | 44 | 540000 orally | 90 days |
| Han 2016 | 2 | Ventilated ICU patients | NR | 21.4(9.1) | 31 | 63.4(17.4) | 37 | 250000 or 500000 orally | 84 days |
| Karsy 2019 | 1 | Neurocritical care patients | NR | 14.3(4.4) | 274 | 54(17.2) | 43 | 540000 orally | 30 days |
| Leaf 2014 | 2 | Severe sepsis and septic shock ICU patients | NR | NR | 67 | 63.4(14.1) | 45 | 80 intravenous | 28 days |
| Miri 2019 | 1 | Mechanically ventilated patients | NR | 9.7(13.1) | 40 | 53.8(21.9) | 27 | 300000 intramuscular | 28 days |
| Miroliaee 2018 | 2 | Mechanically ventilated patients with pneumonia | NR | 18.3(5.5) | 51 | 57.1(19.6) | 43 | 300000 intramuscular | 28 days |
| Quraishi 2015 | 1 | Multidisciplinary ICU patients | 28.7(5.9) | 17(7.2) | 30 | 63.7(7.6) | 40 | 200000 or 400000 orally | 30 days |
Risk of bias and quality of evidence
eFigs 1 and 2 in S1 Appendix present risk-of-bias assessments. eTable 6 in S1 Appendix shows the support for judgment for included trials rated as high or unclear risk of bias. All trials had a low risk of bias [10, 17, 21–27]. Key findings of GRADE assessment of certainty for all outcomes are presented in eTable 4 in S1 Appendix. The quality of evidence of primary outcome was ranked as high.
Mortality
The association between vitamin D and all-cause mortality is shown in Fig 2. The RR revealed no association between vitamin D and reduced all-cause mortality at the longest follow-up (RR 0.90; 95% CI, 0.74 to 1.09; I2 = 20%; 26 fewer events per 1000 [95% CI, -69 to 23]; high-quality evidence).
Fig 2. Association of vitamin D versus placebo with mortality.
In trial sequential analyses of all-cause mortality at the longest follow-up, the effect estimated has reached the futility boundary (eFig 3 in S1 Appendix). Sensitivity analyses by excluding trials at each time and using random-effect models yielded similar results (eTable 5 in S1 Appendix). Funnel plot analysis showed no asymmetry (eFig 4 in S1 Appendix), as well as Egger test (p = 0.397) and Begg test (p = 0.602) detected no significant small-study effects.
Subgroup analyses found that there is no significant difference between vitamin D supplementation and placebo in the subgroup analysis of all-cause mortality on dose (≥300000IU or <300000IU) (eFig 5 in S1 Appendix), the baseline 25 hydroxyvitamin D (≥20 and <20 ng/ml) (eFig 6 in S1 Appendix) and the route of administration (oral, intravenous, or intramuscular).
Similarly, the use of vitamin D was not associated with reduced all-cause mortality at 30 days, all-cause mortality at 90 days, all-cause mortality at 180 days, all-cause ICU mortality, or all-cause in-hospital mortality (Fig 2).
Other outcomes
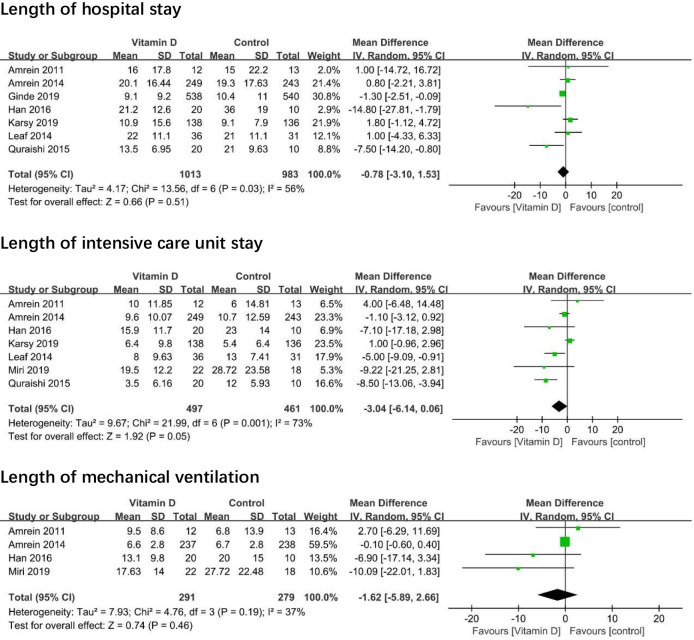
Seven trials [5, 8, 10, 17–20] reported length of hospital stay, and seven trials [5, 6, 8, 10, 17, 19, 20] reported the length of ICU stay. One thousand thirteen patients take vitamin D and 983 patients did not take vitamin D. The mean difference (MD) between the vitamin D group and placebo group revealed no association between vitamin D and length of hospital stay (MD -0.78; 95% CI -3.10 to 1.53; I2 = 56%; moderate-quality evidence; Fig 3) or length of ICU stay (MD -3.04; 95% CI, -6.14 to 0.06; I2 = 73%; moderate-quality evidence). Four trials [6, 8, 17, 19] reported the length of mechanical ventilation. Two hundred ninety-one patients take vitamin D and 279 patients not take vitamin D. There was no association between vitamin D and length of mechanical ventilation (MD -1.62; 95%Cl, -5.89 to 2.66; I2 = 37%).
Fig 3. Association of vitamin D versus placebo with length of hospital stay, length of intensive care unit stay, and length of mechanical ventilation.
Discussion
Principal findings
In this meta-analysis of 9 trials with a total of 2066, we did not detect a significant difference between vitamin D and placebo as the treatment of critically ill patients for all-cause mortality. Similarly, vitamin D supplementation does not reduce the length of ICU stay, hospital stay or mechanical ventilation.
Comparison with other studies
Three systematic reviews and meta-analyses [11–13] have assessed the effect of vitamin D on mortality in critically ill patients in 2017. One review [12] found that vitamin D supplements decreased all-cause mortality at the longest follow-up in analyses of 5 trails [8–10, 17, 20] with a total of 627 patients(odd ratios 0.70, 95% confidence interval 0.50 to 0.98, p = 0.04). The review probably reached more optimistic conclusions as to the use of odd ratios to estimate the effect size. The other two systematic reviews [11, 13] found that vitamin D supplement was trending to decrease all-cause mortality at the longest follow-up in critically ill, but the finding was not statistically significant. The RRs were 0.84 (95%CI 0.66 to 1.06) and 0.77(95%CI 0.58 to 1.03), respectively.
Compared with previous meta-analyses, this study got three times the sample size to make the results more credible. This data improved the precision concerning the treatment effects and provided enough power to the optimum sample size in trial sequential analysis. Moreover, this study has presented absolute as well as relative risks and rank of the quality of evidence of main outcomes. In addition, risk of bias assessment was conducted using the new RoB 2 tool to make the result reliable.
Limitations
The present study has the following limitations that must be taken into account. First, there were differences across all trials in baseline characteristics (e.g., 25‑hydroxyvitamin D level), the definition of outcomes, and treatment (douse of vitamin D), leading to clinical heterogeneity. Secondly, due to the small number of trials, funnel plots, Egger test, and Begg’s test were non-significant. We cannot rule out the possibility of small-study effects. Thirdly, some trials included patients who personal supplement with vitamin D regularly. For example, in the VIOLET trial [18], 5% of participants in the control group used vitamin D supplementation in the past week. This made it more difficult to distinguish the effect between the treatment and control groups.
Conclusion
Current evidence shows that vitamin D supplement was not associated with reduced all-cause mortality in critically ill patients. Similarly, vitamin D supplementation does not reduce the length of ICU stay, hospital stay, or mechanical ventilation.
Supporting information
(DOCX)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.Bikle DD. Vitamin D Metabolism, Mechanism of Action, and Clinical Applications. Chemistry & Biology. 2014;21(3):319–29. 10.1016/j.chembiol.2013.12.016 WOS:000333407000004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thacher TD, Clarke BL. Vitamin D Insufficiency. Mayo Clinic Proceedings. 2011;86(1):50–60. 10.4065/mcp.2010.0567 WOS:000286015400009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sriram K, Perumal K, Alemzadeh G, Osei A, Voronov G. The relationship between immediate preoperative serum 25-hydroxy-vitamin D₃ levels and cardiac function, dysglycemia, length of stay, and 30-d readmissions in cardiac surgery patients. Nutrition (Burbank, Los Angeles County, Calif). 2015;31(6):820–6. Epub 2015/02/28. 10.1016/j.nut.2014.11.022 . [DOI] [PubMed] [Google Scholar]
- 4.Upala S, Sanguankeo A, Permpalung N. Significant association between vitamin D deficiency and sepsis: a systematic review and meta-analysis. BMC anesthesiology. 2015;15:84 Epub 2015/06/05. 10.1186/s12871-015-0063-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karsy M, Guan J, Eli I, Brock AA, Menacho ST, Park MS. The effect of supplementation of vitamin D in neurocritical care patients: RandomizEd Clinical TrIal oF hYpovitaminosis D (RECTIFY). Journal of neurosurgery. 2019:1–10. 10.3171/2018.11.JNS182713. [DOI] [PubMed] [Google Scholar]
- 6.Miri M, Kouchek M, Dahmardeh AR, Sistanizad M. Effect of high-dose vitamin D on duration of mechanical ventilation in ICU patients. Iranian Journal of Pharmaceutical Research. 2019;18(2):1067–72. 10.22037/ijpr.2019.1100647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miroliaee AE, Salamzadeh J, Shokouhi S, Sahraei Z. The study of vitamin D administration effect on CRP and Interleukin-6 as prognostic biomarkers of ventilator associated pneumonia. Journal of critical care. 2018;44:300–5. CN-01465975 NEW. 10.1016/j.jcrc.2017.08.040 [DOI] [PubMed] [Google Scholar]
- 8.Amrein K, Schnedl C, Holl A, Riedl R, Christopher KB, Pachler C, et al. Effect of high-dose vitamin D3 on hospital length of stay in critically ill patients with vitamin D deficiency: the VITdAL-ICU randomized clinical trial. JAMA. 2014;312(15):1520–30. 10.1001/jama.2014.13204 [DOI] [PubMed] [Google Scholar]
- 9.Grossmann RE, Zughaier SM, Kumari M, Seydafkan S, Lyles RH, Liu S, et al. Pilot study of vitamin D supplementation in adults with cystic fibrosis pulmonary exacerbation: A randomized, controlled trial. Dermatoendocrinol. 2012;4(2):191–7. Epub 2012/08/29. 10.4161/derm.20332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Quraishi SA, De Pascale G, Needleman JS, Nakazawa H, Kaneki M, Bajwa EK, et al. Effect of Cholecalciferol Supplementation on Vitamin D Status and Cathelicidin Levels in Sepsis. Critical Care Medicine. 2015;43(9):1928–37. 10.1097/CCM.0000000000001148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Langlois PL, Szwec C, D'Aragon F, Heyland DK, Manzanares W. Vitamin D supplementation in the critically ill: A systematic review and meta-analysis. Clinical Nutrition. 2018;37(4):1238–46. 10.1016/j.clnu.2017.05.006 WOS:000436204200019. [DOI] [PubMed] [Google Scholar]
- 12.Putzu A, Belletti A, Cassina T, Clivio S, Monti G, Zangrillo A, et al. Vitamin D and outcomes in adult critically ill patients. A systematic review and meta-analysis of randomized trials. Journal of Critical Care. 2017;38:109–14. 10.1016/j.jcrc.2016.10.029 [DOI] [PubMed] [Google Scholar]
- 13.Weng H, Li J-G, Mao Z, Zeng X-T. Randomised trials of vitamin D3 for critically ill patients in adults: systematic review and meta-analysis with trial sequential analysis. Intensive care medicine. 2017;43(2):277–8. 10.1007/s00134-016-4591-1 [DOI] [PubMed] [Google Scholar]
- 14.National Heart L, Blood Institute PCTN, Ginde AA, Brower RG, Caterino JM, Finck L, et al. Early High-Dose Vitamin D3 for Critically Ill, Vitamin D-Deficient Patients. The New England journal of medicine. 2019;381(26):2529–40. Epub 2019/12/12. 10.1056/NEJMoa1911124 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;349:g7647 Epub 2015/01/04. 10.1136/bmj.g7647 . [DOI] [PubMed] [Google Scholar]
- 16.Balshem H, Helfand M, Schunemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64(4):401–6. Epub 2011/01/07. 10.1016/j.jclinepi.2010.07.015 . [DOI] [PubMed] [Google Scholar]
- 17.Amrein K, Sourij H, Wagner G, Holl A, Pieber TR, Smolle KH, et al. Short-term effects of high-dose oral vitamin D3 in critically ill vitamin D deficient patients: a randomized, double-blind, placebo-controlled pilot study. Critical care (London, England). 2011;15(2):R104 10.1186/cc10120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ginde AA, Brower RG, Caterino JM, Finck L, Banner-Goodspeed VM, Grissom CK, et al. Early high-dose Vitamin D3 for critically ill, Vitamin D-deficient patients. New England Journal of Medicine. 2019;381(26):2529–40. 10.1056/NEJMoa1911124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Han JE, Jones JL, Tangpricha V, Brown MA, Hao L, Hebbar G, et al. High dose Vitamin D administration in ventilated intensive care unit patients: A pilot double blind randomized controlled trial. Journal of Clinical and Translational Endocrinology. 2016;4:59–65. 10.1016/j.jcte.2016.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leaf DE, Raed A, Donnino MW, Ginde AA, Waikar SS. Randomized controlled trial of calcitriol in severe sepsis. American journal of respiratory and critical care medicine. 2014;190(5):533–41. 10.1164/rccm.201405-0988OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Amrein K, Schnedl C, Holl A, Riedl R, Christopher KB, Pachler C, et al. Effect of high-dose vitamin D3 on hospital length of stay in critically ill patients with vitamin D deficiency: the VITdAL-ICU randomized clinical trial. JAMA. 2014;312(15):1520–30. Epub 2014/10/01. 10.1001/jama.2014.13204 . [DOI] [PubMed] [Google Scholar]
- 22.Ginde AA, Brower RG, Caterino JM, Finck L, Banner-Goodspeed VM, Grissom CK, et al. Early High-Dose Vitamin D3 for Critically Ill, Vitamin D-Deficient Patients. N Engl J Med. 2019;381(26):2529–40. Epub 2019/12/12. 10.1056/NEJMoa1911124 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Han JE, Jones JL, Tangpricha V, Brown MA, Brown LAS, Hao L, et al. High Dose Vitamin D Administration in Ventilated Intensive Care Unit Patients: A Pilot Double Blind Randomized Controlled Trial. J Clin Transl Endocrinol. 2016;4:59–65. Epub 2016/07/16. 10.1016/j.jcte.2016.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karsy M, Guan J, Eli I, Brock AA, Menacho ST, Park MS. The effect of supplementation of vitamin D in neurocritical care patients: RandomizEd Clinical TrIal oF hYpovitaminosis D (RECTIFY). Journal of neurosurgery. 2019:1–10. Epub 2019/09/14. 10.3171/2018.11.Jns182713 . [DOI] [PubMed] [Google Scholar]
- 25.Leaf DE, Raed A, Donnino MW, Ginde AA, Waikar SS. Randomized controlled trial of calcitriol in severe sepsis. Am J Respir Crit Care Med. 2014;190(5):533–41. Epub 2014/07/17. 10.1164/rccm.201405-0988OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miri M, Kouchek M, Rahat Dahmardeh A, Sistanizad M. Effect of High-Dose Vitamin D on Duration of Mechanical Ventilation in ICU Patients. Iranian journal of pharmaceutical research: IJPR. 2019;18(2):1067–72. Epub 2019/09/19. 10.22037/ijpr.2019.1100647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miroliaee AE, Salamzadeh J, Shokouhi S, Sahraei Z. The study of vitamin D administration effect on CRP and Interleukin-6 as prognostic biomarkers of ventilator associated pneumonia. J Crit Care. 2018;44:300–5. Epub 2017/12/19. 10.1016/j.jcrc.2017.08.040 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.