Abstract
Objectives:
To measure the diagnostic accuracy, timeliness, and ease of use of Ceribell rapid response electroencephalography. We assessed physicians’ diagnostic assessments and treatment plans before and after rapid response electroencephalography assessment. Primary outcomes were changes in physicians’ diagnostic and therapeutic decision making and their confidence in these decisions based on the use of the rapid response electroencephalography system. Secondary outcomes were time to electroencephalography, setup time, ease of use, and quality of electroencephalography data.
Design:
Prospective multicenter nonrandomized observational study.
Setting:
ICUs in five academic hospitals in the United States.
Subjects:
Patients with encephalopathy suspected of having nonconvulsive seizures and physicians evaluating these patients.
Interventions:
Physician bedside assessment of sonified electroencephalography (30 s from each hemisphere) and visual electroencephalography (60 s) using rapid response electroencephalography.
Measurements and Main Results:
Physicians (29 fellows or residents, eight attending neurologists) evaluated 181 ICU patients; complete clinical and electroencephalography data were available in 164 patients (average 58.6 ± 18.7 yr old, 45% females). Relying on rapid response electroencephalography information at the bedside improved the sensitivity (95% CI) of physicians’ seizure diagnosis from 77.8% (40.0%, 97.2%) to 100% (66.4%, 100%) and the specificity (95% CI) of their diagnosis from 63.9% (55.8%, 71.4%) to 89% (83.0%, 93.5%). Physicians’ confidence in their own diagnosis and treatment plan were also improved. Time to electroencephalography (median [interquartile range]) was 5 minutes (4–10 min) with rapid response electroencephalography while the conventional electroencephalography was delayed by several hours (median [interquartile range] delay = 239 minutes [134–471 min] [p < 0.0001 using Wilcoxon signed rank test]). The device was rated as easy to use (mean ± sd: 4.7 ± 0.6 [1 = difficult, 5 = easy]) and was without serious adverse effects.
Conclusions:
Rapid response electroencephalography enabled timely and more accurate assessment of patients in the critical care setting. The use of rapid response electroencephalography may be clinically beneficial in the assessment of patients with high suspicion for nonconvulsive seizures and status epilepticus.
Keywords: Ceribell electroencephalography, diagnostic device evaluation, electroencephalography, epilepsy, nonconvulsive status epilepticus, rapid response electroencephalography, seizure
A substantial number of critically ill patients with altered mental status have nonconvulsive seizures. For instance, 48% of patients after convulsive status epilepticus (1), 18–33% of comatose patients with traumatic brain injury (2, 3), 20% of patients with subarachnoid hemorrhage (4–6), 3–17% of patients with intraparenchymal hemorrhage (7–9), 7% of patients with ischemic stroke (8), about 6–12% of patients with brain infections (10, 11), and 10–30% patients with cardiac arrest (12–15) are estimated to have nonconvulsive seizures.
Electroencephalography (EEG) is the standard method for diagnosing nonconvulsive seizures (16), and existing guidelines recommend that EEG monitoring should be initiated within 1 hour of suspicion for nonconvulsive seizures (17). However, many hospitals do not have the capacity to offer EEG (18, 19), and in those with 24/7 capacity, conventional EEG is delayed well beyond the time window recommended by current guidelines (20, 21).
Evidence from studies in patients (22–31) and animal models (32–35) suggests a clear association between prolonged nonconvulsive seizures and neuronal damage and poor neurologic outcomes. Early access to EEG leads to early detection, and hence, more effective treatment of seizures (36), which will in turn prevent neuronal injury, and potentially deleterious impacts on patient morbidity, mortality, and long-term cognitive disability.
Here, we conducted a multicenter prospective nonrandomized clinical study to measure the potential impact of a new EEG device, the rapid response electroencephalography system (Rapid-EEG; Fig. 1) developed by Ceribell (Mountain View, CA) and cleared by the U.S. Food and Drug Administration. This new system not only enables Rapid-EEG acquisition but also provides actionable diagnostic information in the form of EEG sonification. Multiple prior studies have validated its signal quality in a head-to-head comparison with two conventional EEG systems (37), its diagnostic utility for detecting seizures by sound (38), its feasibility in the ICU setting in academic (39) and community (40) hospitals, and its diagnostic utility compared with standard 16–20 electrode EEG systems (41–43).
Figure 1.
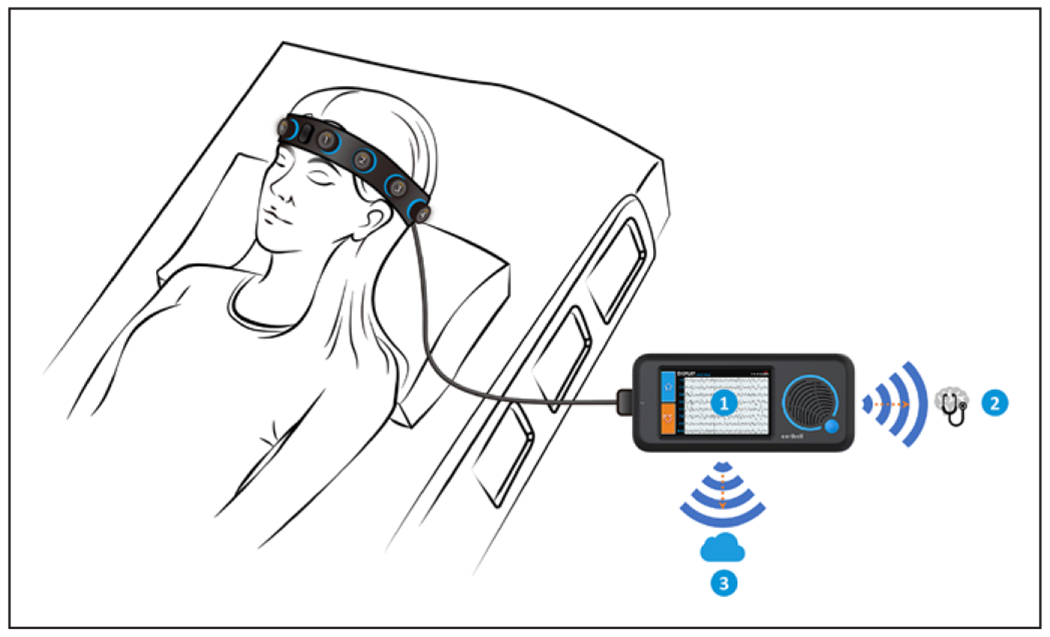
Rapid Response Electroencephalogram (Rapid-EEG) System. Rapid-EEG developed by Ceribell enables electroencephalogram (EEG) acquisition without trained EEG technologists and provides EEG diagnostic information in real time by three means: 1) the visual display on the device screen, 2) the “Brain Stethoscope” (38) function activated by a button press on the device that enables the user to “listen” to the sound of the brain (for samples, see “Brain Stethoscope Training” at https://ceribell.com/training.html), and 3) real-time wireless transmission of the EEG data to a cloud server for remote evaluation (either in real time or retrospectively) by neurologists using a web browser interface. The electrodes are configured in a bipolar montage with five electrodes (four electrode pairs) on each hemisphere. The EEG channels correspond approximately to the Fp1–F7, F7–T3, T3–T5, and T5–O1 sites on the left and the Fp2–F8, F8–T4, T4–T6, and T6–O2 sites on the right according to the International 10–20 System. Data are acquired as digital samples at a rate of 250 Hz with a frequency response of 0.5–100 Hz.
The current study was designed to test the hypothesis that “Rapid-EEG would provide immediate and accurate assessment of nonconvulsive seizures and would change physicians’ diagnostic suspicions and treatment decisions and increase their confidence in diagnostic and therapeutic decision making compared to clinical suspicion alone.” We also anticipated that the Rapid-EEG would be easy to set up by physicians at the bedside.
MATERIALS AND METHODS
Sites, Participants, and Informed Consent
Participating medical centers in the Does Use of Rapid Response EEG Impact Clinical Decision Making (DECIDE) trial were: Massachusetts General Hospital, Rush University Medical Center, University of California Los Angeles, University of Texas Southwestern, and Wake Forest Baptist Health. Sites were chosen based on their geographical locations to represent five distinct regions of the United States with 24/7 onsite conventional EEG technologists. Each site followed the requirements of its local Institutional Review Board regarding informed consent procedures. Eligible patients were identified by physicians based on clinical presentation (primarily altered mental status). Recruited patients formed a random series.
Study Procedure
This study was designed to compare the conventional practice of seizure management (i.e., physicians relying solely on their own clinical judgment) versus EEG-guided diagnostic and therapeutic decision making based on Rapid-EEG data obtained at the bedside by the physicians themselves and without the presence of EEG technologists.
The details of the study are shown in the study protocol (Fig. 2) and in eMethods 1 (Supplemental Digital Content 1, http://links.lww.com/CCM/F569). In brief, physicians determined the clinical need for EEG monitoring given their patient’s clinical presentation and ordered conventional EEG to be applied by trained EEG technologists. After ordering the conventional EEG, and before the arrival of conventional EEG, each physician completed a brief four-item questionnaire ascertaining their: 1) diagnostic suspicion for seizures (yes/ no); 2) treatment plan to escalate treatment with antiseizure medications (yes/no), and confidence in their own; 3) diagnostic; and 4) therapeutic assessments (5-point Likert scale for each: 1 = very low, 5 = very high) (eMethods 2, Supplemental Digital Content 1, http://links.lww.com/CCM/F569). Then, the same physician set up the Rapid-EEG system at the bedside (without the help of EEG technologists) and performed a 2-minute assessment of the Rapid-EEG data. This bedside EEG assessment consisted of listening to EEG sound for 30 seconds from each hemisphere using the “Brain Stethoscope” function (Fig. 1) and reviewing visual EEG waveforms on the Rapid-EEG device for 60 seconds. Rapid-EEG data were automatically time stamped when physicians performed their bedside assessment. After gaining Rapid-EEG diagnostic information, physicians completed the same four-item questionnaire. In addition, they rated the ease of use of the headband and device (5-point Likert scale for each: 1 = difficult, 5 = easy). Rapid-EEG recording continued until the conventional EEG system arrived, at which point, the Rapid-EEG was disconnected instantly and the conventional EEG setup started.
Figure 2.
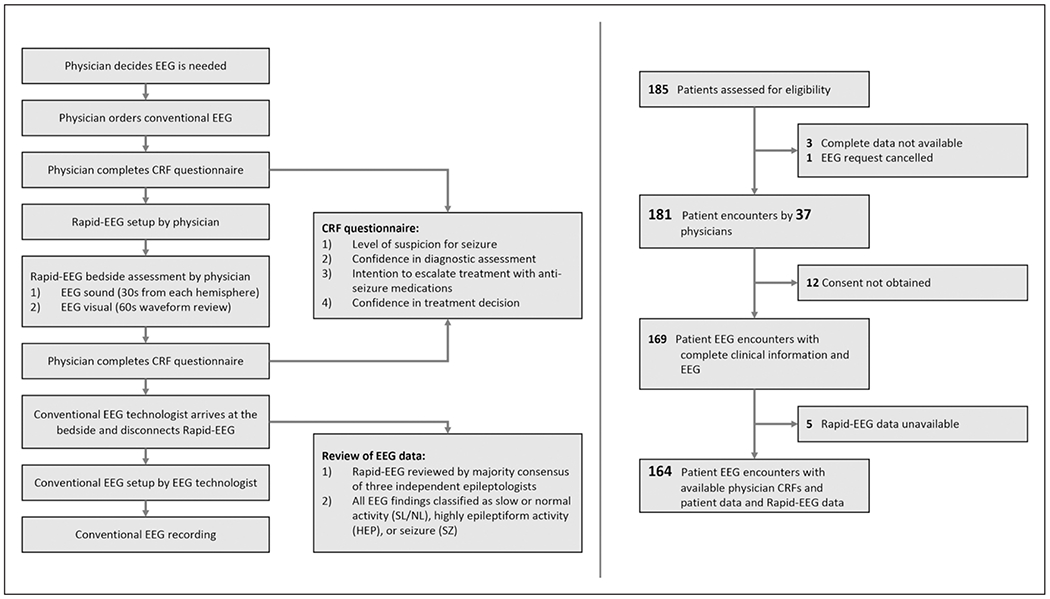
Flow diagram and study protocol. The results of the study are based on 181 patient encounters with questionnaire data completed by 37 physicians and 164 patients with Rapid Response Electroencephalogram (Rapid-EEG) data (left). The study protocol (right) demonstrates the timing of physician questionnaires before and after Rapid-EEG assessment and the electroencephalogram (EEG) review process. Physicians were instructed not to delay the conventional EEG system in the process of acquiring Rapid-EEG data. Clinical Report Forms (CRF) were used.
EEG Diagnosis
We asked three independent EEG expert neurologists to retrospectively review each Rapid-EEG recording while being blind to participating physicians’ responses. These experts provided a diagnosis for the portion of the Rapid-EEG that was reviewed by physicians during their 2-minutes long bedside Rapid-EEG assessment (60 s of sonification and 60 s of visual review). They also provided an EEG diagnosis for the entire recording using 2012 Standardized Critical Care EEG Terminology defined by the American Clinical Neurophysiology Society (44). To account for the well-recognized problem of inter-rater variability in EEG interpretation even among expert neurologists (45, 46), we used a majority consensus (2/3) among the three expert readers to define their final diagnosis for each recording.
Final Rapid-EEG diagnoses were subsequently classified into one of three categories: 1) seizures, 2) slow or normal activity, or 3) highly epileptiform patterns (HEPs). The third group includes patterns that do not fully meet the Salzburg criteria (47) for electrographic seizure activity but do represent abnormal electrographic epileptiform activity such as periodic discharges of any kind or lateralized rhythmic delta activity. See eFig. 1 (Supplemental Digital Content 1, http://links.lww.com/CCM/F569) for a representative sample of each of these categories, and eMethods 3 and eFig. 2 (Supplemental Digital Content 1, http://links.lww.com/CCM/F569) for information and data regarding signal quality evaluation.
In those patients in whom Rapid-EEG recordings were followed by a conventional EEG study, we collected a copy of the EEG report for the first day of the conventional EEG monitoring. The diagnosis documented in the EEG report by the patient’s clinical team was categorized into one of the three patterns (seizures, slow or normal activity, or HEP) and was used as the final diagnosis for the conventional EEG recording.
Sensitivity and Specificity of Physician Diagnoses With and Without Rapid-EEG
We compared the sensitivity and specificity of physicians’ seizure diagnosis before Rapid-EEG condition (baseline) and after reviewing Rapid-EEG data. We used expert EEG readers’ interpretations of the Rapid-EEG (during physician bedside assessment) as the reference standard. To err on the conservative side, nonseizure activity included both slow or normal activity and HEP conditions. Mindful of the problem of “incorporation bias” (48), the purpose of this analysis was not to measure the accuracy of Rapid-EEG since the device does not provide a final seizure diagnosis. It only sonifies and visualizes the raw EEG data and the final diagnosis is made by the physician interpreting the data by listening to the sound of the EEG and looking at the tracings. As such, the analysis aimed to compare the accuracy of nonexpert physicians’ interpretation of Rapid-EEG compared with expert readers’ interpretation of the same EEG. In other words, did physicians without formal EEG training make wrong EEG diagnostic assessments compared with expert neurologists with formal training in clinical neurophysiology?
Statistical Analysis
For details of statistical analysis, please refer to eMethod 4 (Supplemental Digital Content 1, http://links.lww.com/CCM/F569). The primary analysis of the clinical impact of Rapid-EEG was performed by calculating the change at the individual patient level in physicians’ diagnostic suspicion for seizure, decision to escalate treatment with antiseizure medications, confidence in diagnostic assessment, and confidence in treatment plan.
RESULTS
Across all five sites, we enrolled a total of 37 physicians who participated in the care of 181 patients suspected to have nonconvulsive seizures for which conventional EEG was ordered; complete information was available for 164 patients (Fig. 2).
Physician and Patient Characteristics
All physicians were neurology-trained (attending, n = 8; neurocritical care fellow, n = 22; and resident, n = 7) with varying years of ICU experience (median: 1 yr [interquartile range, IQR 2 yr], range: 0–11 yr) and minimal EEG experience (median: 0 yr [IQR 3 yr], range: 0–10 yr). About 60% of encounters involved physicians who had used the device less than 3 times, while only 12% of encounters involved physicians who had used the device greater than 10 times. The majority of patients (87%) had some degree of encephalopathy either with (32%) or without (55%) witnessed seizure or seizure-like activity. Most patients were already on antiseizure medications (69%) and 56% were intubated (eTables 1 and 2, Supplemental Digital Content 1, http://links.lww.com/CCM/F569).
EEG Findings
Retrospective review of the entire Rapid-EEG recordings (n = 164) by three independent EEG experts showed that 17 patients (11%) had seizures, 19 (12%) had HEPs without seizures, and 128 (78%) had only slow or normal activity. Of the 17 patients with electrographic seizures at some point during the Rapid-EEG monitoring, nine occurred at the time of physicians’ bedside assessments and eight were captured after the physicians had completed their bedside assessment (but before arrival of the conventional EEG system).
Review of conventional EEG reports revealed that of 17 patients with seizures during Rapid-EEG monitoring, 11 also had seizures during the conventional EEG monitoring while six did not. Additionally, five patients without seizures on Rapid-EEG eventually had seizures within the next 24 hours on conventional EEG. In all of these cases, seizures occurred more than 2 hours after the Rapid-EEG recording had ended, and the seizures were not localized to parasagittal regions.
Impact of Rapid-EEG on Physician Diagnostic and Therapeutic Decision Making and Confidence
For this analysis, data from two patients were missing. In 179 patient cases, access to Rapid-EEG data at the bedside changed physicians’ diagnostic suspicion for seizures in 72 cases (40.2%) and treatment decision in 36 cases (20.1%) (Fig. 3A, Table 1). There were 59 patients (32.6%; 95% CI, 25.8–40.0%) whose treating physician changed their suspicion for seizure from “yes” to “no” after Rapid-EEG, compared with 13 patients (7.3%; 95% CI, 3.9–12.1%) whose treating physician increased their suspicion for seizure from “no” to “yes” after Rapid-EEG (p < 0.0001 using McNemar test). Treating physicians’ inclination to escalate treatment with antiseizure medications decreased (from “yes” to “no”) after Rapid-EEG for 23 patients (12.9%; 95% CI, 8.3–18.7%), compared with 13 patients (7.3%; 95% CI, 3.9–12.1%) for whom physicians’ treatment decisions changed to escalate antiseizure medications (from “no” to “yes) (p = 0.10 using McNemar test).
Figure 3.
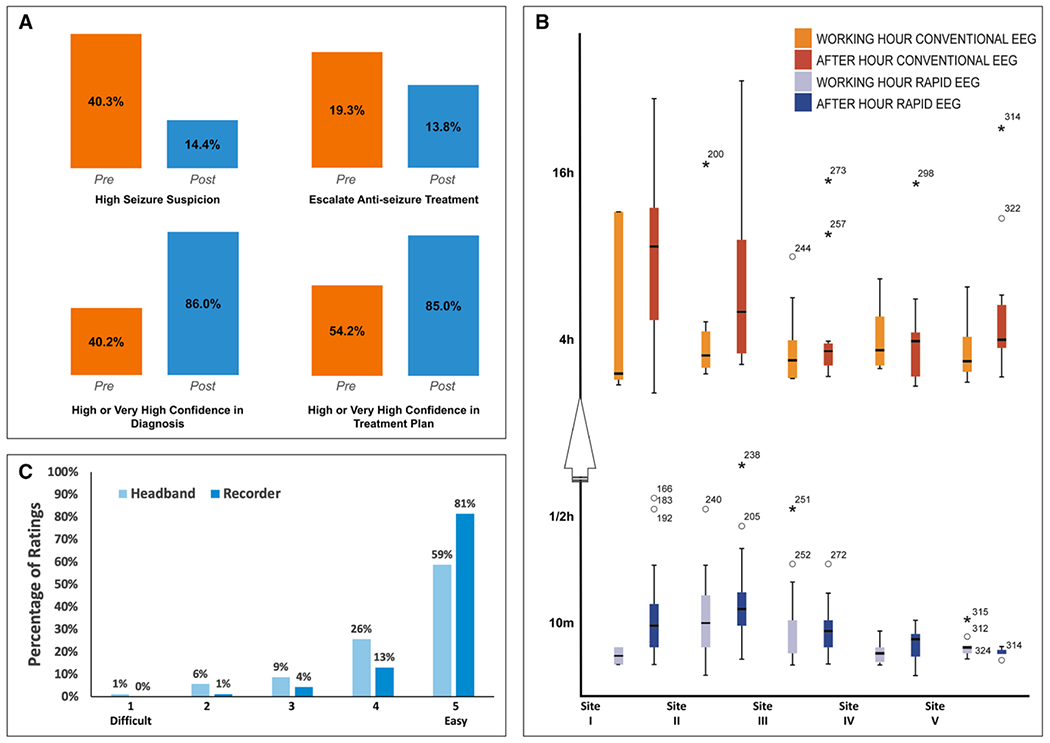
Primary and secondary study outcomes. A, Clinical impact: Bar graphs comparing physicians’ diagnostic and treatment decisions and confidence level for all 181 cases (two values missing). High confidence combined “high” and “very high” categories (ratings of 4 and 5). B, Time to EEG: Delays in obtaining Rapid-EEG (blue) compared with conventional EEG (orange) during business hours (lighter shade) and after-hours (darker shade). Note the high variance in hours to conventional EEG across sites compared with minutes to obtain Rapid-EEG. C, Ease of use: Perceived ease of use of the headband sensors (light blue) and recording device (dark blue) was high.
TABLE 1.
Clinical Impact of Rapid Response Electroencephalography
|
After Rapid-EEG, n (%) |
|||||||
|---|---|---|---|---|---|---|---|
| Diagnostic Suspicion | Before Rapid-EEG | Seizure | Nonseizure | Totala | |||
| Seizure | 13 (7.2) | 59 (32.6) | 72 (40.2) | ||||
| Nonseizure | 13 (7.2) | 94 (51.9) | 107 (59.8) | ||||
| Total | 26 (14.4) | 153 (84.5) | 179a (100.0) | ||||
| Treatment Decision | Before Rapid-EEG | Escalate treatment | Do not escalate | Total | |||
| Escalate treatment | 12 (6.6) | 23 (12.7) | 35 (19.3) | ||||
| Do not escalate | 13 (7.2) | 131 (72.4) | 146 (80.7) | ||||
| Total | 25 (13.8) | 154 (85.1) | 181 (100.0) | ||||
| Confidence in Diagnosis | Before Rapid-EEG | 1 | 2 | 3 | 4 | 5 | Total |
| 1 (very low) | 0 | 0 | 0 | 2 | 3 | 5 | |
| 2 (low) | 0 | 0 | 5 | 10 | 6 | 21 | |
| 3 (medium) | 0 | 3 | 7 | 37 | 34 | 81 | |
| 4 (high) | 0 | 1 | 7 | 16 | 21 | 45 | |
| 5 (very high) | 0 | 0 | 2 | 6 | 19 | 27 | |
| Total | 0 | 4 | 21 | 71 | 83 | 179 | |
| Confidence in Treatment Decision | Before Rapid-EEG | 1 | 2 | 3 | 4 | 5 | Total |
| 1 (very low) | 0 | 0 | 0 | 1 | 2 | 3 | |
| 2 (low) | 0 | 1 | 4 | 8 | 4 | 17 | |
| 3 (medium) | 0 | 2 | 9 | 28 | 23 | 62 | |
| 4 (high) | 0 | 2 | 6 | 25 | 21 | 54 | |
| 5 (very high) | 0 | 0 | 3 | 5 | 35 | 43 | |
| Total | 0 | 5 | 22 | 67 | 85 | 179 | |
Rapid-EEG = rapid response electroencephalography.
Two cases were excluded because of missing data.
We then assessed the accuracy of physicians’ diagnostic assessments by comparing their suspicion for seizure before and after Rapid-EEG to the majority consensus of three epileptologists on the presence of seizure at the time of physicians’ bedside assessments (seizure: n = 9; nonseizure: n = 155). We found that the sensitivity of physicians’ diagnosis of seizure increased from 77.8% (95% CI, 40.0–97.2%) to 100.0% (66.4–100.0%) (p = 0.16 using Cochran-Mantel-Haenszel test stratified by individual patient; difference of 22.2% [95% CI, −4.9 to 49.4%]) and their specificity increased from 63.9% (95% CI, 55.8–71.4%) to 89% (95% CI, 83.0–93.5%) (p < 0.0001 using Cochran-Mantel-Haenszel test stratified by individual patient; difference: 25.2% [95% CI, 16.1–34.2%]). In a secondary analysis where seizures and HEP cases were grouped together as seizures and slow or normal activity as non-seizures, we found a significant increase in specificity from 62.9% (95% CI, 54.0–71.1%) to 90.2% (95% CI, 83.7–94.7%) (p < 0.0001 using Cochran-Mantel-Haenszel test stratified by individual patient).) but no significant difference in sensitivity from pre-(43.8% and 95% CI, 26.4%−62.3%) to post-Rapid EEG (40.6% and 95% CI, 23.7–59.4%) (p = 0.76 using Cochran-Mantel-Haenszel test stratified by individual patient).
Median confidence in diagnosis improved from 3.0 (IQR 3–4) before Rapid-EEG to 4.0 (IQR 4–5) after Rapid-EEG (p < 0.0001 using Wilcoxon signed rank test), as did median confidence in treatment (pre: 4.0 [IQR 3–4], post: 4.0 [4–5], p < 0.0001 using Wilcoxon signed rank test) (Table 1; and eFig. 3, Supplemental Digital Content 1, http://links.lww.com/CCM/F569). Subgroup and exploratory analyses on the association of primary outcomes with prior treatment with antiseizure medication, intubation status, and physician experience in ICU and EEG are provided in eTables 3 and 4 (Supplemental Digital Content 1, http://links.lww.com/CCM/F569). These analyses revealed no noticeable differences for any of the outcomes between the group of patients who were empirically treated for seizures or intubated prior to EEG compared with the group of patients who were not. Greater years of ICU experience (but not years of EEG experience) was associated with higher rate of drop in seizure suspicion (p = 0.015), higher confidence levels in diagnostic assessments (p = 0.019), and treatment plan (p = 0.049) as a result of using Rapid-EEG.
Time to EEG
Rapid-EEG acquisition was relatively fast (median 5 min [IQR,4–10 min], n = 143 due to missing data in 21 cases from site III). By comparison, conventional EEG arrival and set up was delayed by hours during both business hours (165 min [IQR, 99–274 min], n = 56 due to 11 missing records) and during after-hours (288 min [IQR, 165–582 min], n = 87 due to 10 missing records) (Table 2 and Fig. 3B).
TABLE 2.
Time to Electroencephalography
| Time to Set Up Rapid-EEG | All, n = 163 | Site I, n = 32 | Site II, n = 45 | Site III, n = 46 | Site IV, n = 15 | Site V, n = 25 |
|---|---|---|---|---|---|---|
| Median (IQR) (in min) | 5 (4–10) | 5 (4–10) | 11 (5–15) | 6 (4–10) | 5 (2–7) | 4 (4–5) |
| Time to Conventional EEG | All, n = 142 | Site I, n = 32 | Site II, n = 43 | Site III, n = 31 | Site IV, n = 15 | Site V, n = 21 |
| Median (IQR) (in min) | 239 (134–471) | 498 (157–684) | 245 (136–435) | 165 (107–216) | 269 (125–517) | 253 (94–380) |
| Ratio of after-hour cases, % | 61 | 88 | 56 | 52 | 44 | 46 |
| Number of onsite EEG technologists during after-hours | 0–3 | 0–1a | 2 | 2–3 | 1 | 1 |
EEG = electroencephalography, IQR = interquartile range, Rapid-EEG = rapid response EEG.
At the time of the study, site I had on-call (but not in-house) EEG technologists during after-hours. Physicians participating in the study ordered conventional EEG and started setting up the Rapid-EEG device. Here, we show how long it took for them to set up Rapid-EEG once they had decided that the patient needed an EEG and how long it took for the conventional EEG technologists to arrive at the bedside and complete the conventional EEG set up. We measured the delay to conventional EEG from the start of Rapid-EEG recording to the start of conventional EEG recording. We chose these time points to calculate delay because they could be accurately obtained from the digital recording systems rather than from a subjective report by physicians or from EEG order times in the electronic medical records (which are often not reliable indicators of the actual EEG orders (e.g., verbal order or through direct paging of EEG team). It should be noted that this delay does not account for additional potential delays from the time of the conventional EEG recording to the time of obtaining EEG interpretation from the hospital’s EEG specialists.
In 36 patients whose seizures (n = 17) or HEP (n = 19) were captured by Rapid-EEG within minutes, the conventional EEG recordings were delayed by about 260 min (IQR, 140–515 min). Of note, 87.5% of these 36 patients had been empirically treated with antiseizure medications and 56% were already intubated.
Ease of Use and Safety
Physicians found Rapid-EEG easy to use. Average ratings (on a scale of 1–5, higher scores indicating greater ease of use) for the headband and device were 4.4 ± 0.9 and 4.7 ± 0.6, respectively (Fig. 3C). Patient-level and physician-level analysis of ease of use ratings by site are presented in eTable 5 (Supplemental Digital Content 1, http://links.lww.com/CCM/F569).
The use of Rapid-EEG was without any reportable adverse event. Of 181 patient encounters, only one case of scalp irritation and bruising was reported in a patient who had thrombocytopenia and was treated with anticoagulants; the patient’s scalp healed without the need for additional care at the skin site.
DISCUSSION
In this multicenter clinical study, Rapid-EEG resulted in substantial changes in physician decision making compared with clinical judgment alone and improved the sensitivity and specificity of physician judgments regarding the presence or absence of nonconvulsive seizure activity. In addition, Rapid-EEG increased physicians’ confidence in their diagnostic and therapeutic decisions, dramatically shortened time to EEG acquisition, was easy to use by physicians without the help of EEG technologists, and was well-tolerated by patients.
Our findings indicate that even in large academic medical centers with 24/7 in-house EEG technologists, access to conventional EEG is often delayed by several hours, a finding that is consistent with prior studies (20, 21). Such delays in EEG acquisition can contribute to delays in the diagnosis and treatment of nonconvulsive seizures. Early access to EEG will lead to early detection, and hence, more effective treatment of seizures (36), which will in turn prevent refractory status epilepticus; neuronal injury; and potentially deleterious impacts on patient morbidity, mortality, and long-term outcome in terms of cognitive disability, overall neurologic function, and development of chronic epilepsy (22–31).
A critical implication of our findings was that, for patients found not to be seizing, Rapid-EEG often would have appropriately reduced physician’s suspicion for nonconvulsive seizures and their inclination to escalate antiseizure treatment. This suggests that another potential benefit of the Rapid-EEG system is in the prevention of unnecessary escalation of antiseizure medications or “overtreatment” of suspected cases in, for instance, emergency department settings. This could lead to fewer medication-related adverse events (including sedation), intubations and ICU admissions, and thus lower healthcare costs. This should be tested in future studies.
The current study possibly underestimated the potential clinical impact of Rapid-EEG. Physicians in the study used the investigational device only 1–3 times after a brief hands-on training. Therefore, they could not be expected to be very familiar with the reduced (eight-channel) EEG display or the Brain Stethoscope function. In addition, they only listened to EEG sound for 1 minute and reviewed visual EEG epochs rolling in real time for another 1 minute. In real-life practice, we expect the Rapid-EEG data to be reviewed on the Cloud portal (Fig. 1) in consultation with EEG experts scrolling back and forth between EEG epochs spanning longer durations of recording to obtain a better sense of the character and evolution of pathologic activity. Thus, the real-life uses of the Rapid-EEG might yield even better utility for detecting epileptiform abnormalities or distinguishing HEPs from seizures (16, 49).
We are mindful that our study was not without limitations. First, our evaluation was limited to theoretical diagnostic and therapeutic decision making and, as such, evaluation of actual treatment decisions based on Rapid-EEG data and patients’ clinical outcomes were not studied. Future randomized clinical trials will be better suited to address the important issue of clinical outcome. Second, the study protocol did not mandate specific physician responses to acquired information, such as a treatment algorithm for HEPs on the ictal-interictal continuum. As such, variability in physicians’ treatment decisions reflect at least in part local variations in the standard of care. In addition, most patients were already being treated with antiseizure medications prior to obtaining EEG, and therefore, the lack of change in treatment decisions may reflect the high percentage of patients being empirically treated prior to ordering and acquiring conventional EEG. Third, the duration of Rapid-EEG monitoring was variable and determined by the delay in the conventional EEG system; hence, the detection rate and optimal duration of monitoring with Rapid-EEG remain unclear. Last, the current study measured the impact of Rapid-EEG system used at the bedside by residents, fellows, and attending physicians who had varying degrees of neurology training. Prior pilot studies have found a positive impact when Rapid-EEG was used by general intensivisits in a community hospital (40) and emergency physicians in an academic hospital (50). However, further studies are needed to make the findings of our current study generalizable across different disciplines of medicine.
CONCLUSIONS
This study supports the claim that Rapid-EEG is feasible and easy to use in critical care settings, can be done more expediently than conventional EEG, and provides additional valuable diagnostic information to physicians, and by doing so, increases the sensitivity and specificity of their seizure diagnosis as well as their confidence in their diagnosis and treatment plan.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to research coordinators for their diligent contributions: Ryan Tesh, BSc (Massachusetts General Hospital); Lydia Raquel Garcia-Cano, BA (Rush University Medical Center); Courtney R. Real, RN, MSN, AGACNP-BC (University of California, Los Angeles); Anjali Catherine Perera, BSN, RN, and Hend T. Nadim, MBA (University of Texas Southwestern Medical Center); and Cara P. Everhart (Wake Forest Baptist Health), Peng Yang (Clindata Insight), David McArthur (University of California, Los Angeles), and Steve Goodman (Stanford University) for their assistance with statistical analysis of the data and review of the statistical plan and methods; and Kunal Sampat, Eleanor Shen, Gina Barga, and other members of Ceribell for their role in the successful execution of this trial.
This trial was funded and supported by Ceribell.
Drs. Vespa’s, Olson’s, Hobb’s, and Westover’s institutions received funding from Ceribell. Dr. John disclosed that he is an Advisory Committee member for Gift of Hope, Organ Procurement Organization, IL. Dr. Markert received funding from expert testimony in critical care electroencephalography and from Lotus and Oak Venture Studio. Dr. Bleck received funding from Marinus Pharmaceuticals and BioProducts Labs. Dr. Hirsch’s institution received funding from Eisai, Proximagen, Sunovion, and The Daniel Raymond Wong Neurology Research Fund at Yale; he received funding from Neuropace, Medtronic, Adamas, Aquestive, Ceribell, Eisai, Marinus, Monteris, UCB Pharma, and UpToDate-Neurology (royalties), and from Wiley for co-authoring the book “Atlas of EEG in Critical Care” by Drs. Hirsch and Brenner. The remaining authors have disclosed that they do not have any potential conflicts of interest.
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccmjournal).
Trial Registration: ClinicalTrials.gov Identifier: NCT03534258.
REFERENCES
- 1.DeLorenzo RJ, Waterhouse EJ, Towne AR, et al. : Persistent nonconvulsive status epilepticus after the control of convulsive status epilepticus. Epilepsia 1998; 39:833–840 [DOI] [PubMed] [Google Scholar]
- 2.Vespa PM, Nuwer MR, Nenov V, et al. : Increased incidence and impact of nonconvulsive and convulsive seizures after traumatic brain injury as detected by continuous electroencephalographic monitoring. J Neurosurg 1999; 91:750–760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ronne-Engstrom E, Winkler T: Continuous EEG monitoring in patients with traumatic brain injury reveals a high incidence of epileptiform activity. Acta Neurol Scand 2006; 114:47–53 [DOI] [PubMed] [Google Scholar]
- 4.Little AS, Kerrigan JF, McDougall CG, et al. : Nonconvulsive status epilepticus in patients suffering spontaneous subarachnoid hemorrhage. J Neurosurg 2007; 106:805–811 [DOI] [PubMed] [Google Scholar]
- 5.Claassen J, Peery S, Kreiter KT, et al. : Predictors and clinical impact of epilepsy after subarachnoid hemorrhage. Neurology 2003; 60:208–214 [DOI] [PubMed] [Google Scholar]
- 6.Dennis LJ, Claassen J, Hirsch LJ, et al. : Nonconvulsive status epilepticus after subarachnoid hemorrhage. Neurosurgery 2002; 51:1136–1143; XXXdiscussion 1144 [DOI] [PubMed] [Google Scholar]
- 7.Claassen J, Jetté N, Chum F, et al. : Electrographic seizures and periodic discharges after intracerebral hemorrhage. Neurology 2007; 69:1356–1365 [DOI] [PubMed] [Google Scholar]
- 8.Mecarelli O, Pro S, Randi F, et al. : EEG patterns and epileptic seizures in acute phase stroke. Cerebrovasc Dis 2011; 31:191–198 [DOI] [PubMed] [Google Scholar]
- 9.Vespa PM, O’Phelan K, Shah M, et al. : Acute seizures after intracerebral hemorrhage: A factor in progressive midline shift and outcome. Neurology 2003; 60:1441–1446 [DOI] [PubMed] [Google Scholar]
- 10.Flores-Cordero JM, Amaya-Villar R, Rincón-Ferrari MD, et al. : Acute community-acquired bacterial meningitis in adults admitted to the intensive care unit: Clinical manifestations, management and prognostic factors. Intensive Care Med 2003; 29:1967–1973 [DOI] [PubMed] [Google Scholar]
- 11.Zoons E, Weisfelt M, de Gans J, et al. : Seizures in adults with bacterial meningitis. Neurology 2008; 70:2109–2115 [DOI] [PubMed] [Google Scholar]
- 12.Legriel S, Bruneel F, Sediri H, et al. : Early EEG monitoring for detecting postanoxic status epilepticus during therapeutic hypothermia: A pilot study. Neurocrit Care 2009; 11:338–344 [DOI] [PubMed] [Google Scholar]
- 13.Rittenberger JC, Popescu A, Brenner RP, et al. : Frequency and timing of nonconvulsive status epilepticus in comatose post-cardiac arrest subjects treated with hypothermia. Neurocrit Care 2012; 16: 114–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rossetti AO, Logroscino G, Liaudet L, et al. : Status epilepticus: An independent outcome predictor after cerebral anoxia. Neurology 2007; 69:255–260 [DOI] [PubMed] [Google Scholar]
- 15.Rundgren M, Rosén I, Friberg H: Amplitude-integrated EEG (aEEG) predicts outcome after cardiac arrest and induced hypothermia. Intensive Care Med 2006; 32:836–842 [DOI] [PubMed] [Google Scholar]
- 16.Claassen J, Mayer SA, Kowalski RG, et al. : Detection of electrographic seizures with continuous EEG monitoring in critically ill patients. Neurology 2004; 62:1743–1748 [DOI] [PubMed] [Google Scholar]
- 17.Brophy GM, Bell R, Claassen J, et al. ; Neurocritical Care Society Status Epilepticus Guideline Writing Committee: Guidelines for the evaluation and management of status epilepticus. Neurocrit Care 2012; 17:3–23 [DOI] [PubMed] [Google Scholar]
- 18.Kolls BJ, Mace BE, Dombrowski KE: Implementation of continuous video-electroencephalography at a community hospital enhances care and reduces costs. Neurocrit Care 2018; 28:229–238 [DOI] [PubMed] [Google Scholar]
- 19.Hill CE, Blank LJ, Thibault D, et al. : Continuous EEG is associated with favorable hospitalization outcomes for critically ill patients. Neurology 2019; 92:e9–e18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Quigg M, Shneker B, Domer P: Current practice in administration and clinical criteria of emergent EEG. J Clin Neurophysiol 2001; 18:162–165 [DOI] [PubMed] [Google Scholar]
- 21.Gururangan K, Razavi B, Parvizi J: Utility of electroencephalography: Experience from a U.S. tertiary care medical center. Clin Neurophysiol 2016; 127:3335–3340 [DOI] [PubMed] [Google Scholar]
- 22.Vespa P, Tubi M, Claassen J, et al. : Metabolic crisis occurs with seizures and periodic discharges after brain trauma. Ann Neurol 2016; 79:579–590 [DOI] [PubMed] [Google Scholar]
- 23.Vespa P, Prins M, Ronne-Engstrom E, et al. : Increase in extracellular glutamate caused by reduced cerebral perfusion pressure and seizures after human traumatic brain injury: A microdialysis study. J Neurosurg 1998; 89:971–982 [DOI] [PubMed] [Google Scholar]
- 24.Vespa PM, McArthur DL, Xu Y, et al. : Nonconvulsive seizures after traumatic brain injury are associated with hippocampal atrophy. Neurology 2010; 75:792–798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Young GB, Jordan KG, Doig GS: An assessment of nonconvulsive seizures in the intensive care unit using continuous EEG monitoring: An investigation of variables associated with mortality. Neurology 1996; 47:83–89 [DOI] [PubMed] [Google Scholar]
- 26.Krumholz A: Epidemiology and evidence for morbidity of nonconvulsive status epilepticus. J Clin Neurophysiol 1999; 16:314–322; discussion 353 [DOI] [PubMed] [Google Scholar]
- 27.Abend NS, Wusthoff CJ, Goldberg EM, et al. : Electrographic seizures and status epilepticus in critically ill children and neonates with encephalopathy. Lancet Neurol 2013; 12:1170–1179 [DOI] [PubMed] [Google Scholar]
- 28.Topjian AA, Gutierrez-Colina AM, Sanchez SM, et al. : Electrographic status epilepticus is associated with mortality and worse short-term outcome in critically ill children. Crit Care Med 2013; 41:215–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Payne ET, Zhao XY, Frndova H, et al. : Seizure burden is independently associated with short term outcome in critically ill children. Brain 2014; 137:1429–1438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wagenman KL, Blake TP, Sanchez SM, et al. : Electrographic status epilepticus and long-term outcome in critically ill children. Neurology 2014; 82:396–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Marchis GM, Pugin D, Meyers E, et al. : Seizure burden in subarachnoid hemorrhage associated with functional and cognitive outcome. Neurology 2016; 86:253–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meldrum BS, Vigouroux RA, Brierley JB: Systemic factors and epileptic brain damage. Prolonged seizures in paralyzed, artificially ventilated baboons. Arch Neurol 1973; 29:82–87 [DOI] [PubMed] [Google Scholar]
- 33.Hartings JA, Rolli ML, Lu XC, et al. : Delayed secondary phase of periinfarct depolarizations after focal cerebral ischemia: Relation to infarct growth and neuroprotection. J Neurosci 2003; 23:11602–11610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krsek P, Mikulecká A, Druga R, et al. : Long-term behavioral and morphological consequences of nonconvulsive status epilepticus in rats. Epilepsy Behav 2004; 5:180–191 [DOI] [PubMed] [Google Scholar]
- 35.Avdic U, Ahl M, Chugh D, et al. : Nonconvulsive status epilepticus in rats leads to brain pathology. Epilepsia 2018; 59:945–958 [DOI] [PubMed] [Google Scholar]
- 36.Lowenstein DH, Alldredge BK: Status epilepticus at an urban public hospital in the 1980s. Neurology 1993; 43:483–488 [DOI] [PubMed] [Google Scholar]
- 37.Kamousi B, Grant AM, Bachelder B, et al. : Comparing the quality of signals recorded with a rapid response EEG and conventional clinical EEG systems. Clin Neurophysiol Pract 2019; 4:69–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Parvizi J, Gururangan K, Razavi B, et al. : Detecting silent seizures by their sound. Epilepsia 2018; 59:877–884 [DOI] [PubMed] [Google Scholar]
- 39.Hobbs K, Krishnamohan P, Legault C, et al. : Rapid bedside evaluation of seizures in the ICU by listening to the sound of brainwaves: A prospective observational clinical trial of ceribell’s brain stethoscope function. Neurocrit Care 2018; 29:302–312 [DOI] [PubMed] [Google Scholar]
- 40.Yazbeck M, Sra P, Parvizi J: Rapid response electroencephalography for urgent evaluation of patients in community hospital intensive care practice. J Neurosci Nurs 2019; 51:308–312 [DOI] [PubMed] [Google Scholar]
- 41.Gururangan K, Razavi B, Parvizi J: Diagnostic utility of eight-channel EEG for detecting generalized or hemispheric seizures and rhythmic periodic patterns. Clin Neurophysiol Pract 2018; 3:65–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Westover MB, Gururangan K, Markert MS, et al. : Diagnostic value of electroencephalography with ten electrodes in critically ill patients. Neurocrit Care 2020. February 7 [online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gururangan K, Parvizi J: Midline and parasagittal seizures are rare in adult patients. Neurocrit Care 2020; 32:193–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hirsch LJ, LaRoche SM, Gaspard N, et al. : American Clinical Neurophysiology Society’s Standardized Critical Care EEG Terminology: 2012 version. J Clin Neurophysiol 2013; 30:1–27 [DOI] [PubMed] [Google Scholar]
- 45.Ronner HE, Ponten SC, Stam CJ, et al. : Inter-observer variability of the EEG diagnosis of seizures in comatose patients. Seizure 2009; 18:257–263 [DOI] [PubMed] [Google Scholar]
- 46.Tu B, Young GB, Kokoszka A, et al. : Diagnostic accuracy between readers for identifying electrographic seizures in critically ill adults. Epilepsia Open 2017; 2:67–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Leitinger M, Beniczky S, Rohracher A, et al. : Salzburg consensus criteria for non-convulsive status epilepticus–approach to clinical application. Epilepsy Behav 2015; 49:158–163 [DOI] [PubMed] [Google Scholar]
- 48.Reitsma JB, Rutjes AW, Khan KS, et al. : A review of solutions for diagnostic accuracy studies with an imperfect or missing reference standard. J Clin Epidemiol 2009; 62:797–806 [DOI] [PubMed] [Google Scholar]
- 49.Struck AF, Osman G, Rampal N, et al. : Time-dependent risk of seizures in critically ill patients on continuous electroencephalogram. Ann Neurol 2017; 82:177–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Govindarajan P, Madill E: Clinical utility of Ceribell rapid response electroencephalography in emergency medicine: A proof of concept study. Society of Academic Emergency Medicine, Denver, CO (virtual), May 12-15, 2020 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


